Abstract
Diadenosine polyphosphates have been described to be present in the myocardium and exert purinergic- and nonreceptor-mediated effects. Since the electrophysiological properties of atrial myocardium are effectively regulated by A1 receptors, we investigated the effect of diadenosine pentaphosphate (Ap5A) in rabbit myocardium.
Parameters of supraventricular electrophysiology and atrial vulnerability were measured in Langendorff-perfused rabbit hearts. Muscarinic potassium current (IK(ACh/Ado)) and ATP-sensitive potassium current (IK(ATP)) were measured by using the whole-cell voltage clamp method.
Ap5A prolonged the cycle length of spontaneously beating Langendorff perfused hearts from 225±14 (control) to 1823±400 ms (Ap5A 50 μM; n=6; P<0.05). This effect was paralleled by higher degree of atrio-ventricular block. Atrial effective refractory period (AERP) in control hearts was 84±14 ms (n=6). Ap5A⩾1 μM reduced AERP (100 μM, 58±11 ms; n=6).
Extrastimuli delivered to hearts perfused with Ap5A- or adenosine (⩾ μM)-induced atrial fibrillation, the incidence of which correlated to the concentration added to the perfusate. The selective A1-receptor antagonist CPX (20 μM) inhibited the Ap5A- and adenosine-induced decrease of AERP. Atrial fibrillation was no longer observed in the presence of CPX.
The described Ap5A-induced effects in the multicellular preparation were enhanced by dipyridamole (10 μM), which is a cellular adenosine uptake inhibitor. Dipyridamole-induced enhancement was inhibited by CPX.
Ap5A (⩽1 mM) did neither induce IK(Ado) nor IK(ATP). No effect on activated IK(Ado/ATP) was observed in myocytes superfused with Ap5A. However, effluents from Langendorff hearts perfused with Ap5A 100 μM activated IK(Ado) by using A1 receptors.
Ap5A did not activate A1 receptors in rabbit atrial myocytes. The Ap5A induced A1-receptor-mediated effects on supraventricular electrophysiology and vulnerability suggest that in the multicellular preparation Ap5A is hydrolyzed to yield adenosine, which acts via A1 receptors. An influence on atrial electrophysiology or a facilitation of atrial fibrillation under conditions resulting in increased interstitial Ap5A concentrations might be of physiological/pathophysiological relevance.
Keywords: Cardiac cell, atrial myocyte, atrial fibrillation, diadenosine polyphosphates, electrophysiology, muscarinic K+ current, adenosine, A1 receptor
Introduction
Diadenosine polyphosphates (ApnA) act as mediators in various tissues via different subtypes of purinergic receptors (for reviews see Ogilvie et al., 1996; Flores et al., 1999; Luo et al., 1999). APnA have been described to be present in the myocardium and are released by activated platelets (Jovanovic et al., 1998; Flores et al., 1999). Not least because of these observations, the effect of ApnA on the myocardium has been the subject of investigation. Nonreceptor-mediated regulation of ATP-sensitive myocardial potassium current has been shown (Jovanovic et al., 1996a, 1996b; Brandts et al., 1998). Stavrou et al. (2001) performed experiments in isolated Langendorff-perfused hearts where diadenosine pentaphosphate (Ap5A) induced ventricular action potential prolongation. Negative chronotropic actions and modulation of inotropy in the heart have been described and were sensitive to specific A1-receptor antagonists (Hoyle et al., 1996; Rubino & Burnstock, 1996; Vahlensieck 1996; Neumann et al., 1999). It was concluded that ApnA acts as direct A1 adenosine-receptor agonists.
All studies performed in the multicellular preparation (isolated tissue, isolated heart) so far focused on the effect of ApnA on nodal and/or ventricular myocardium. However, the electrophysiological property of atrial myocardium is known to be very effectively influenced by the activation of A1 receptors. These atrial A1 receptors are coupled via β/γ-subunits of heterotrimeric pertussis toxin sensitive G-proteins to an ion channel composed of GIRK1/GIRK4 subunits (Huang et al., 1995). Main cellular electrophysiological effects of the activation of these channels are hyperpolarization and shortening of action potential duration (Belardinelli et al., 1995). Furthermore, activation of this muscarinic potassium current (IK(Ado/ACh) facilitates the occurrence of atrial fibrillation due to a marked reduction of the atrial effective refractory period (AERP; Nunain et al., 1992). Hence an A1-receptor agonist, like ApnA, is expected to exert significant effects in the atrial myocardium and in particular, the atrial myocardium is an ideal subject to investigate A1-receptor-mediated signal transduction of ApnA.
We studied the effect of Ap5A in Langendorff-perfused rabbit atria (conventional electrophysiology) and in isolated rabbit atrial myocytes (whole-cell voltage clamp) in order (1) to evaluate the potency of Ap5A in the regulation of atrial cardiac electrophysiology and (2) to investigate the cellular basis for the observed electrophysiological phenomena.
Methods
Isolation of myocytes
After stunning, White New Zealand rabbits (1200–1600 g) were killed by cervical dislocation. Single atrial myocytes were isolated by using an enzyme solution in a modified Langendorff setup (Banach et al., 1993). Atrial myocytes were used for measurements for up to 24 h. No difference was found between freshly isolated and cultured atrial myocytes in the phenomena to be studied. Cells were plated at a density of several hundred per culture dish (36 mm).
Solutions for whole-cell voltage clamp experiments
If not otherwise mentioned, for measurements in isolated myocytes, enzyme solution was replaced by a solution containing (mM): NaCl 120; KCl 20; CaCl2 2.0; MgCl2 1.0; HEPES/NaOH 10.0; pH 7.4. Pipettes were filled with a solution containing (mM): potassium aspartate 110; KCl 20; MgATP 5.0; MgCl2 1.0; EGTA 2.0; GTP 0.01; HEPES/NaOH 10.0; pH 7.4. The chosen potassium concentrations yielded an equilibrium potential for K+ of −48 mV. Ap5A, adenosine, acetylcholine, HEPES, EGTA, MgATP, GTP were from Sigma (Deisenhofen, Germany). CPX and glibenclamide were from RBI. Standard salts were from Merck (Darmstadt, Germany). Ap5A was dissolved in buffer (Tyrode).
Current measurements
Membrane currents were measured under voltage clamp by means of patch clamp pipettes (Hamill et al., 1981). Pipettes were fabricated from borosilicate glass with filament (Clark Electromedical, Pangbourne, U.K.) and were filled with the solution listed above. DC resistance of the filled pipettes ranged from 3 to 6 MΩ. Currents were measured by means of a patch clamp amplifier (List LM/EPC 7, Gieβen, Germany). Signals were analog filtered (cutoff frequency 1–3 kHz) and were digitally stored on the hard disk of an IBM compatible AT computer. The computer was equipped with a hardware/software package for voltage control, data acquisition and data evaluation (ISO-2 by MFK, Frankfurt, Germany). Rapid superfusion of the cells for application and withdrawal of different solutions was performed by means of a solenoid operated flow system that permitted switching between up to six different solutions within 500 ms. The cell under study was continuously superfused at approximately 0.3 ml × min−1. Experiments were performed at ambient temperature (21–24°C) to avoid artefacts due to temperature changes with rapid switching between different superfusion solutions. If not otherwise stated, cells were voltage clamped at a holding potential of −90 mV. Under these conditions evoked potassium currents were in the inward direction. This protocol was chosen because of the strong inward rectifying properties of the muscarinic potassium current. Fast voltage ramps (−120 to +60 mV; 500 ms) were performed to measure current–voltage (I–V) relations and monitor constancy of electrical access to the cell. Physiological outward current was measured during these fast voltage ramps. No qualitative or quantitative difference between inward and outward currents was detected in the phenomena to be studied. Cell capacitance was measured by means of short (9 ms) de- and hyperpolarizing voltage ramps (−100 to −80 to −100 mV).
Isolated heart experiments
White New Zealand rabbits (1200–1600 g) were killed as described above. The aorta was cannulated and mounted on a Langendorff apparatus. Perfusion was at constant pressure (60 mmHg). The temperature was 39°C. Perfusion fluid contained (mM): NaCl 130; KCl 4; CaCl2 2.2; MgCl2 0.5; NaHCO3 24.2; Na2HPO4 1.2; glucose 12. The solution was gassed with O2/CO2=95%/5%. Two silver wires, one placed on the right atrium, the other on the free wall of the right ventricle, were used to record surface ECG. Spontaneous atrial cycle length, AERP and atrial vulnerability were measured in the atria of Langendorff-perfused rabbit hearts. If part of the protocol right atrium was stimulated by means of an external bipolar silver electrode (S1S1 220 ms), a similar electrode positioned on the left atrium was used for the measurement of local atrial activation. AERP was measured by increasing stepwise the S1S2 interval from failure to capture until capture of the atria was achieved (steps of 2 ms). The last noncaptured coupling interval was defined as the AERP. For evaluation of atrial vulnerability, an atrial extrastimulus was delivered, the coupling interval of which was chosen 4 ms longer than the measured AERP during a given experimental condition. This procedure was repeated 10 times to obtain the mean incidence of atrial fibrillation. If induced atrial fibrillation did not stop after a period of 5 min, agonist was washed out until sinus rhythm was achieved. Between every episode of atrial fibrillation, a period of 3 min of pacing (S1S1 220 ms) was introduced for equilibration. Induction of atrial fibrillation was considered if a train of >10 consecutive premature atrial depolarizations occurred.
Data analysis
If not otherwise stated, data are presented as mean±standard deviation (s.d.). Ion current densities were calculated at a holding potential of −90 mV. The t-test was used for comparison of means. A P-value less than 0.05 was considered statistically significant.
Results
Effect of Ap5A on supraventricular electrophysiology
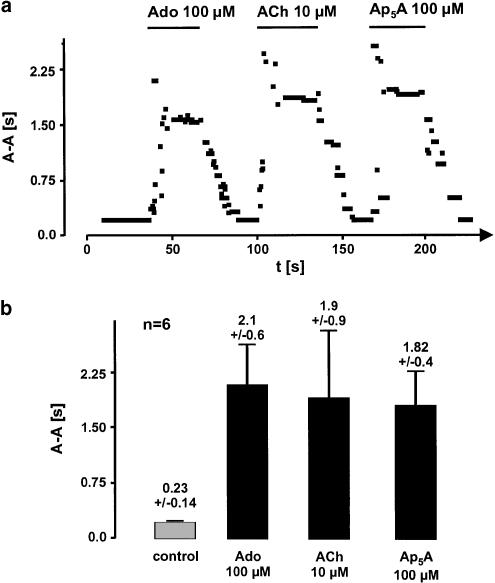
To determine the electrophysiological effects of Ap5A, we used the isolated Langendorff-perfused heart method. Acetylcholine and adenosine were used as reference agonists. A representative experiment is shown in Figure 1a. Adenosine (100 μM) prolonged the mean spontaneous cycle length of sinus rhythm from about 220 to almost 2000 ms. Initial negative chronotropism was due to inhibition of sinus node activity and higher degrees of atrio-ventricular block. A similar pattern was observed due to perfusion with Ap5A (100 μM) and acetylcholine (10 μM). After prolonged perfusion of the hearts with one of the agonists, prolongation of cycle length after some tens of seconds reached a plateau of slow sinus node activity with 1 : 1 conduction to the ventricles. From this stable plateau, mean atrial cycle length was calculated out of six hearts for control (0.22±0.01 s) adenosine (2.07 s), acetylcholine (1.92±0.6 s) and Ap5A (1.79±0.4 s) (Figure 1b).
Figure 1.
Effects of adenosine (Ado, 100 μM), acetylcholine (ACh, 10 μM) and diadenosine pentaphosphate (Ap5A, 100 μM) on spontaneous cycle length of rabbit Langendorff-perfused hearts. (a) Illustrates a typical recording representative for six isolated hearts. Perfusion with either agonist induced a prolonging of spontaneous atrial cycle length which was assessed by measurement of A–A interval. (b) Shows the mean cycle length during control and during agonist perfusion after a stable plateau of bradycardia was reached.
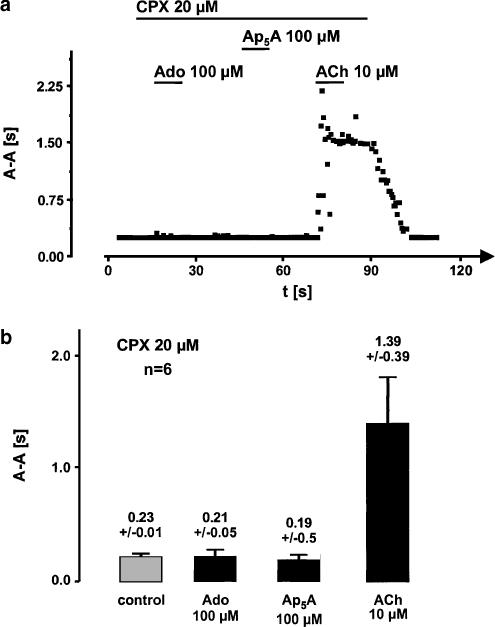
Ap5A- and adenosine-induced effects were completely inhibited by CPX (20 μM), which is a selective A1-receptor antagonist. The acetylcholine-mediated effects were not sensitive to CPX. An experiment which is qualitatively representative for six hearts is shown in Figure 2a. The statistics of this data are summarized in Figure 2b.
Figure 2.
Inhibition of adenosine (Ado) and diadenosine pentaphosphate (Ap5A)-induced prolonging of atrial cycle length by the specific A1-receptor antagonist CPX. (a) Shows a representative experiment where Ado- and Ap5A-induced bradycardia as shown in Figure 1 is completely inhibited by CPX, whereas M2-receptor-mediated cycle length prolongation due to acetylcholine (ACh) remains unaffected. (b) Illustrates the mean atrial cycle length of isolated hearts during perfusion with CPX (20 μM). The same hearts were used as in Figure 1. No statistically significant difference was found between control, Ado- and Ap5A-perfused episodes. ACh effect was not inhibited by CPX throughout all hearts as can be seen by comparison of the right most column with the Ach-induced effect in Figure 1.
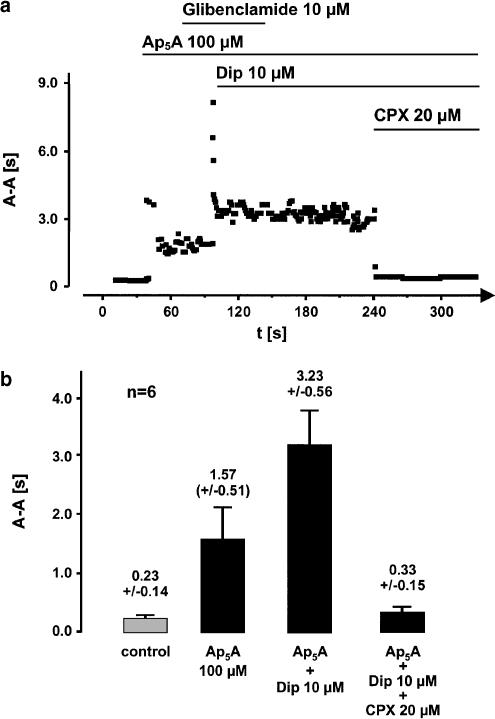
APnA can be degraded by endothelial ectoenzymes to yield AMP (Mateo et al., 1997). This lends itself to the testing of the hypothesis of an A1-receptor-mediated action after breakdown of Ap5A to yield adenosine. It is well known that adenosine-induced effects in the heart can be enhanced by the use of dipyridamole, which is an inhibitor of cellular adenosine uptake. Dipyridamole 10 μM increased the Ap5A effect on cycle length. Figure 3a shows a representative experiment where Ap5A prolonged mean cycle length from about 230–1500 ms Addition of dipyridamole further increased atrial cycle length to more than 3100 ms. The dipyridamole-induced enhancement of the Ap5A effect was also inhibited by CPX, whereas glibenclamide (10 μM), an inhibitor of ATP-sensitive potassium current, did neither influence Ap5A- nor dipyridamole-induced effects. The statistical analysis of the data for this effect is summarized in Figure 3b.
Figure 3.
Enhancement of Ap5A-induced effect on atrial cycle length by the adenosine uptake inhibitor dipyridamole (Dip) and inhibition of the enhancement by the selective A1-receptor antagonist CPX (a) illustrate a trace representative for six experiments with similar results. Prolongation of spontaneous atrial cycle length by Ap5A is more than twice as potent when dipyridamole was added to the perfusate. The IK(ATP) inhibitor glibenclamide showed no influence on Ap5A- or Dip+Ap5A-induced effects which excludes participation of IK(ATP) in the mechanism of bradycardia. CPX inhibited the Dip-dependent enhancement completely. (b) Shows the mean atrial cycle length obtained in the same six hearts used in Figure 1 and Figure 2. CPX induced a complete inhibition of Dip-enhanced Ap5A effect.
Effect of Ap5A on AERP and atrial vulnerability
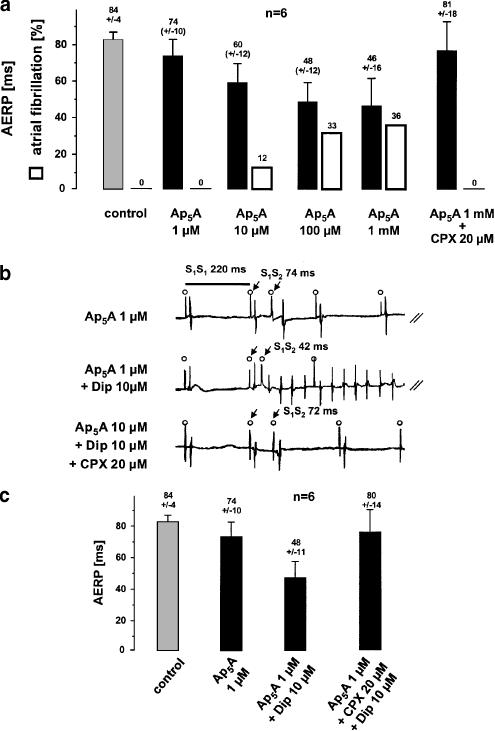
In Langendorff-perfused stimulated control hearts, mean AERP was 84±4 ms (n=6, S1S1 200 ms). Ap5A significantly reduced AERP in a concentration-dependent manner (Figure 4a). The Ap5A-mediated decrease of AERP was enhanced by addition of dipyridamole. An experiment which is representative for six isolated rabbit hearts is shown in Figure 4b. Ap5A (1 μM) had only a minor effect on AERP (74 ms). However, in the presence of dipyridamole, AERP was further reduced from 74 to 42 ms. The extrastimulus induced atrial fibrillation, which persisted for a few seconds. After a period of 180 s of continuous stimulation (S1S1 220 ms), CPX 20 μM was added to the Ap5A and dipyridamole-containing perfusate. CPX reversed the shortening of AERP in this experiment almost completely and no arrhythmia was induced by the extrastimulus protocol. The statistics for these experiments are shown in (Figure 4c).
Figure 4.
Effect of Ap5A on AERP and on atrial vulnerability in isolated rabbit hearts. (a) Mean AERPs and incidence of atrial fibrillation due to perfusion of six isolated hearts with different concentrations of Ap5A. Decrease of AERP and increase of atrial vulnerability were completely reversed by adding CPX to the perfusate. (b) Shows an experiment representative for six isolated hearts. Local atrial activation can be seen directly after the stimulation spike (open circles). During perfusion with Ap5A 1 μM, the AERP was 74 ms (upper trace; indicated by arrows). Addition of dipyridamole (Dip) to the perfusate shortened the AERP to 42 ms and atrial fibrillation was induced in this experiment. The A1-receptor antagonist CPX reversed the AERP to 72 ms. No atrial fibrillation was induced in the presence of CPX. (c) Illustrates the mean AERP out of six Langendorff hearts, the data of which were obtained by using the protocol shown in (b).
Due to these observations, we systematically studied Ap5A-induced atrial vulnerability in the isolated hearts by using an extrastimulus protocol and atrial fibrillation definitions described above. In control hearts, extrastimuli never induced atrial fibrillation (n=6). Extrastimuli delivered to hearts perfused with Ap5A (⩾10 μM) or Ap5A (⩾1 μM) plus dipyridamole- induced atrial fibrillation, the incidence of which correlated to the concentration added to the perfusate and to the shortening of AERP (n=6) (Figure 4a). The selective A1-receptor antagonist CPX (20 μM) inhibited the Ap5A- and adenosine-induced decrease of AERP, and atrial fibrillation was no longer observed in the presence of CPX (Figure 4c, rightmost columns).
Effect of Ap5A and adenosine on muscarinic and ATP-sensitive potassium current in rabbit atrial myocytes
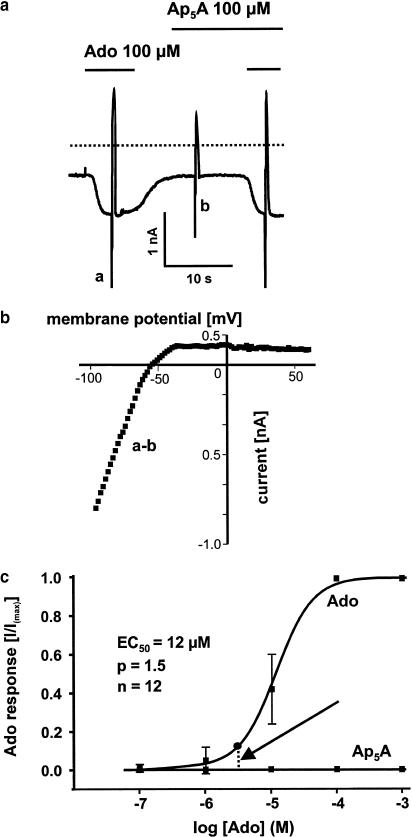
As stated in the Introduction, IK(Ado) is a major target of the signaling pathway linked to A1 receptors. Thus, if Ap5A activates A1 receptors the compound should cause activation of that current. Adenosine 100 μM induced IK(Ado) in isolated rabbit atrial myocytes, the mean current density of which was 52±19 pA/pF (n=6). A representative current recording is shown in Figure 5a. The strong inward rectifying properties of the activated current measured by subtraction of fast voltage ramps (a–b as indicated in Figure 5a) revealed strong inward rectifying properties of the activated current being typical for muscarinic potassium current (Figure 5b). EC50 of the concentration–response curve was 12 μM (n=6) (Figure 1c). Ap5A (⩽1 mM) did not induce IK(Ado) and no effect on background or adenosine-induced currents was observed (Figure 5a+c).
Figure 5.
Adenosine (Ado) induces muscarinic potassium current, whereas Ap5A fails to show an A1-receptor-mediated effect in single rabbit atrial myocytes. (a) Representative whole-cell voltage clamp experiment. Superfusion of an atrial myocytes with Ado 100 μM induces an inward ion current. Ion current activation is reversible within a few seconds after washing off the agonist. Ap5A has no effect on background current and on Ado-induced current. Dashed line indicates zero current level. Rapid deflections are due to fast voltage ramps. Similar results were obtained in 12 single atrial myocytes out of eight different rabbit hearts used for cell isolation. (b) The Ado-induced current is identified to be the muscarinic potassium current (IK(Ado)) because of its typical strong inward rectifying properties. Reversal potential resembles the equilibrium potential for potassium. (c) Concentration–response curves for Ado and Ap5A obtained in 12 isolated atrial myocytes. The vertical dashed line (arrow) indicates the estimated concentration of Ado in the effluent of Ap5A (100 μM) perfused hearts (see Figure 6).
We showed previously that IK(ATP) was induced by prolonged superfusion of the cells with ApnA or intracellular application of the compound in guinea-pig atrial myocytes (Brandts et al., 1998). However, in isolated rabbit atrial myocytes, prolonged superfusion with Ap5A (100 μM, 30 min, n=6) or Ap5A added into the pipette solution (100 μM, 30 min, n=6) showed no activation of IK(ATP) (data not shown).
So far, the experiments in isolated atrial myocytes have excluded a participation of direct activation of IK(ATP) or A1 receptor-dependent IK(Ado) in the observed phenomena of the isolated hearts.
Effect of effluent of Ap5A-perfused Langendorff hearts on muscarinic potassium current
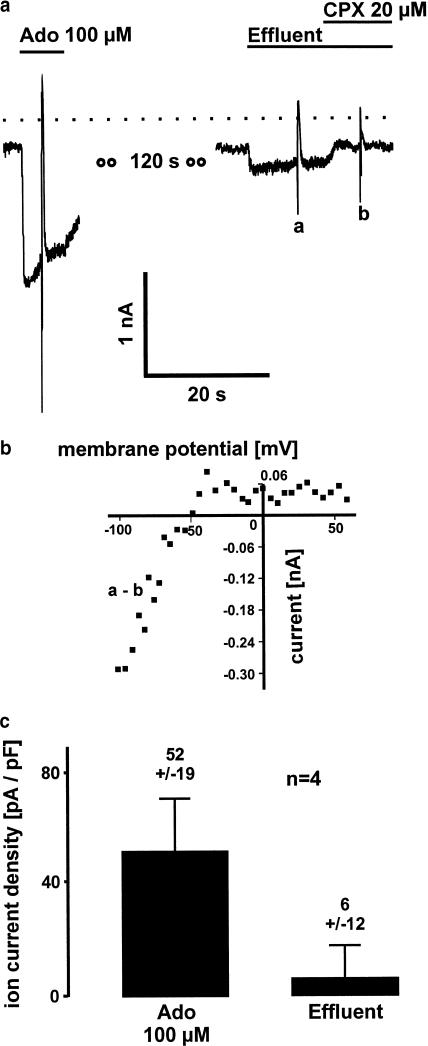
To further study the mechanism of Ap5A effect on supraventricular electrophysiology, we sampled the effluent of Ap5A (100 μM) and control Langendorff-perfused hearts and tested it in the isolated myocytes assay with respect to its potency to activate IK(ACh/Ado). No activation of muscarinic potassium current was found in effluents of control hearts (n=4). The effluent of rabbit hearts perfused with Ap5A showed an activation of muscarinic potassium current, which was identified to be IK(Ado) due to its sensitivity to CPX (20 μM) and due to its strong inward rectifying properties. A representative experiment is shown in Figure 6a+b. Calculated from the concentration–response curve in Figure 5, the mean adenosine concentration in the effluent of this representative experiment was about 3 μM. The data of four hearts each tested in six single atrial myocytes are shown in Figure 6c.
Figure 6.
Activation of A1-receptor-induced muscarinic potassium current by effluents of Ap5A 100 μM perfused rabbit hearts. (a) Ion current trace obtained in a single atrial myocytes by means of the whole-cell voltage clamp technique. Adenosine 100 μM (Ado) induces muscarinic potassium current. The effluent from an Ap5A 100 μM perfused Langendorff heart elicits an inward current which is inhibited by the selective A1-receptor antagonist CPX. (b) I–V curve of the effluent induced ion current obtained by subtraction of voltage ramps as indicated in (a). Strong inward rectification identifies muscarinic potassium current to be active. (c) Mean current densities of Ado-induced muscarinic potassium current and muscarinic potassium current induced by effluents of four Ap5A Langendorff-perfused hearts. Effluents elicits a current being about 12% of Ado 100 μM induced potassium current.
Discussion
ApnA are involved in different signal transduction pathways. The most commonly described property is the activation of purinergic receptors in various tissues (for a review, see Flores et al., 1999). The presence of Ap5A in the myocardium was first shown by Jovanovic et al. (1998). Several reports have been published which describe the ApnA activation of A1 receptors in ventricular myocardium of different species. Vahlensieck et al. (1996) showed an inhibitory effect of Ap5A on isoproterenol-induced L-type calcium current in ventricular myocytes. By common pharmacological criteria, it was shown beyond doubt that A1-receptor activation was responsible for this effect. No studies have been conducted so far investigating the effect of ApnA on the atria, although the atrial myocardium is very suitable for investigation of A1-receptor-mediated effects.
Negative chronotropism and a decrease of AERP are typical for atrial A1-receptor activation and have been extensively described in the literature (for a review, see, Shen & Kurachi, 1995). These effects were also induced by Ap5A in our rabbit heart model. The dependency on A1-receptor activation was confirmed by the complete reversibility of the effects by using a selective A1-receptor antagonist (CPX). The sensitivity of the heart to Ap5A was comparable to the sensitivity described by other investigators to cardiac effects of different ApnA (micromolar range). The shortening of AERP as well as the negative chronotropic effect was increased by dipyridamole. Dipyridamole inhibits the uptake of adenosine but not the uptake of Ap5A into guinea-pig atrial myocytes (Brandts et al., 1998). Thus, it is likely that Ap5A in the present model increases extracellular adenosine but not extracellular Ap5A concentration. These observations led to the hypothesis that adenosine is involved in the documented Ap5A-dependent A1-receptor activation. To evaluate the potency of Ap5A to directly activate A1 receptors, we performed experiments in isolated rabbit myocytes. The main effect of A1-receptor activation in atrial myocytes is the induction of the muscarinic potassium current IK(Ado; see Kurachi, 1995 for a review). In rabbit atrial myocytes, we detected no activation of IK(Ado) due to superfusion with Ap5A⩽1 mM. This excludes an A1-receptor agonism of the compound in the model used for this study. Moreover, the effluent of Ap5A 100 μM-perfused rabbit hearts exerted A1 agonistic actions at the cellular level as can be seen from the activation of IK(Ado) in Figure 6. Although any A1-receptor agonist might be present in this effluent, previous studies in guinea-pig hearts using HPLC analysis suggest adenosine to be active (Brandts et al., 1998). By using the concentration–response curve for adenosine-induced IK(Ado) (Figure 5c), the mean adenosine concentration in the effluent of four isolated rabbit hearts is estimated to be about ∼7 μM. Adenosine 7 μM is high enough to induce electrophysiological effects in the atria. Furthermore, this concentration is rather underestimated and not a true reflection of interstitial adenosine concentrations since an adenosine uptake into the cells during interstitial washout can be expected.
Thus, we suggest the Ap5A effect of negative chronotropism and decrease of AERP in rabbit atrial myocytes to be mediated by adenosine which activates A1 receptors. Interstitial adenosine concentration might be increased due to breakdown of Ap5A or due to a release of the compound after activation of a previously unknown mechanism being activated by Ap5A. Further studies should elucidate the mechanism. Recently, Stavrou et al. (2001) described an action potential prolongation in ventricular myocytes due to perfusion of isolated guinea-pig hearts with APnA. Since it is well known that muscarinic potassium current is quantitatively not involved in regulation of ventricular electrophysiology due to a low expression level in guinea-pigs (Koumi & Wasserstrom, 1994), these data are not contradictory to our results in atrial myocytes where IK(Ado) plays a major role in the determination of action potential duration and refractoriness (Belardinelli et al., 1995). The mechanisms of the Ap5A-induced action potential prolongation in the ventricle remain unclear.
Ap5A exerted arrhythmogenic effects in the isolated heart preparation at the atrial level. During perfusion with Ap5A ⩾10 μM, premature depolarization sometimes induced atrial fibrillation. The incidence of atrial fibrillation was dependent on the concentration of Ap5A in the perfusate and correlated to the shortening of AERP. Like negative chronotropism, atrial vulnerability mediated by Ap5A was completely reversed by the A1-receptor antagonist CPX. This was paralleled by an almost complete prolonging of the AERP to the baseline level. Moreover, vulnerability of the atria at a given concentration of Ap5A in the perfusate was enhanced by adding dipyridamole. Thus, like negative chronotropism and shortening of AERP, the increased atrial vulnerability during Ap5A perfusion meet the criteria of an adenosine-mediated effect. Induction of atrial fibrillation due to activation of A1 receptors is a well-known phenomenon. Adenosine-induced activation of muscarinic potassium current with consecutive shortening of action potential duration and effective refractory period are the basis for this proarrhythmic effect (Nunain et al., 1992; Kabell et al., 1994).
In contrast to our previous results in isolated guinea-pig atrial myocytes, an activation of IK(ATP) in rabbit atrial myocytes was never observed. A difference in the metabolical properties of rabbit atrial myocytes compared to guinea-pig atrial myocytes might be the reason, because it was shown that IK(ATP) activation in guinea-pig atrial myocytes is due to interference with the equilibrium of intracellular adenosine phosphates (Brandts et al., 1998). The lack of Ap5A-induced IK(ATP) activation excludes a participation of that current in shortening of AERP in rabbit atrial myocytes. This is supported by the fact that AERP shortening was reversed by CPX, but not by the IK(ATP) inhibitor glibenclamide. On the other hand, Jovanovic et al. (1996a), (1996b) described an inhibitory effect of Ap5A on IK(ATP) in guinea-pig ventricular myocytes which could be due to an inhibition of adenylate kinase by the compound (Carrasco et al., 2001). Such a desensitization of IK(ATP) might counteract a metabolic-induced activation of IK(ATP) in rabbit atrial myocytes and therefore cannot be excluded in our model.
Hence, it is suggested that the described Ap5A-induced vulnerability, like the effects on spontaneous cycle length and AERP, is adenosine mediated. Preliminary experiments on AP6A-induced prolongation of atrial cycle length in isolated rabbit hearts show similar results in respect of dipyridamole and CPX sensitivity, suggesting that the mechanisms described in this study are not specific for Ap5A.
Conclusions
Ap5A exerts indirect A1-receptor-dependent effects in the atria of isolated rabbit hearts, most likely by increasing interstitial adenosine concentrations. Ap5A itself has no direct A1-receptor-activating properties. Ap5A is present in the heart and is released from activated platelets. Mechanisms have been described to yield ApnA concentrations of at least 1 μM (Jovanovic et al., 1998; Flores et al., 1999). Thus, the phenomena presented in this study occur in relevant concentrations. The relevance of the Ap5A-induced electrophysiological and proarrhythmic effects on the atria in vivo remains unclear. A shortening of AERP and a consecutive increased atrial vulnerability might be one mechanism of arrhythmogenesis when elevated interstitial Ap5A concentrations occur.
Abbreviations
- ApnA
diadenosine polyphosphates
- Ap5A
diadenosine pentaphosphate
- AERP
atrial effective refractory period
- IK(ATP)
ATP-dependent potassium current
- IK(Ado)
adenosine-induced muscarinic potassium current
- IK(ACh)
acetylcholine-induced muscarinic potassium current
References
- BANACH K., HUESER J., LIPP P., WELLNER M.C., POTT L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by a serum factor. J. Physiol. 1993;461:263–281. doi: 10.1113/jphysiol.1993.sp019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELARDINELLI L., SHRYOCK J.C., SONG Y., WANG D., SRINIVAS M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995;9:359–365. doi: 10.1096/fasebj.9.5.7896004. [DOI] [PubMed] [Google Scholar]
- BRANDTS B., BRANDTS A., WELLNER-KIENITZ M.C., ZIDEK W., SCHLUTER H., POTT L.Non-receptor-mediated activation of IK(ATP) and inhibition of IK(Ach) by diadenosine polyphosphates in guinea-pig atrial myocytes J. Physiol. 1998512407–420.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRASCO A.J., DZEJA P.P., ALEKSEEV A.E., PUCAR D., ZINGMAN L.V., ABRAHAM M.R., HODGSON D., BIENENGRAEBER M., PUCEAT M., JANSSEN E., WIERINGA B., TERZIC A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLORES N.A., STAVROU B.M., SHERIDAN D.J. The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc. Res. 1999;42:15–26. doi: 10.1016/s0008-6363(99)00004-8. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H., ZIGANSHIN A.U., PINTOR J., BURNSTOCK G. The activation of P1 and P2-purinoceptors in the guinea-pig left atrium by diadenosine polyphosphates. Br. J. Pharmacol. 1996;118:1294–1300. doi: 10.1111/j.1476-5381.1996.tb15536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG C.L., SLESINGER P.A., CASEY P.J., JAN Y.N., Jan L.Y. Evidence that direct binding of Gβ/γ to the GIRK 1 G protein gated inwardly rectifying K channels is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- JOVANOVIC A., ALEKSEEV A.E., TERZIC A. Cardiac ATP-sensitive K+ channel, a target for diadenosine 5′,5″-P1,P5-pentaphosphate. Naunyn-Schmiedebergs Arch. Pharmacol. 1996a;353:241–244. doi: 10.1007/BF00168763. [DOI] [PubMed] [Google Scholar]
- JOVANOVIC A., JOVANOVIC S., MAYS D.C., LIPSKY J.J., TERZIC A. Diadenosine 5′,5″-P1,P5-pentaphosphate harbors the properties of a signaling molecule in the heart. FEBS Lett. 1998;27,423:314–318. doi: 10.1016/s0014-5793(98)00114-8. [DOI] [PubMed] [Google Scholar]
- JOVANOVIC A., ZHANG S., ALEKSEEV A.E., TERZIC A. Diadenosine polyphosphate-induced inhibition of cardiac KATP channels, operative state-dependent regulation by a nucleoside diphosphate. Pflugers Arch. 1996b;431:800–802. doi: 10.1007/BF02253848. [DOI] [PubMed] [Google Scholar]
- KABELL G., BUCHANAN L.V., GIBSON J.K., BELARDINELLI L. Effects of adenosine on atrial refractoriness and arrhythmias. Cardiovasc. Res. 1994;28:1385–1389. doi: 10.1093/cvr/28.9.1385. [DOI] [PubMed] [Google Scholar]
- KOUMI S., WASSERSTROM J.A.Acetylcholine-sensitive muscarinic K+ channels in mammalian ventricular myocytes Am. J. Physiol. 1994266H1812–H1821.(Part 2) [DOI] [PubMed] [Google Scholar]
- KURACHI Y.G protein regulation of cardiac muscarinic potassium channel Am. J. Physiol. 1995269C821–C830.(Part 1) [DOI] [PubMed] [Google Scholar]
- LUO J., JANKOWSKI J., KNOBLOCH M., VAN DER GIET M., GARDANIS K., RUSS T., VAHLENSIECK U., NEUMANN J., SCHMITZ W., TEPEL M., DENG M.C., ZIDEK W., SCHLUTER H. Identification and characterization of diadenosine 5′,5″-P1,P2-diphosphate and diadenosine 5′,5″-P1,P3-triphosphate in human myocardial tissue. FASEB J. 1999;13:695–705. doi: 10.1096/fasebj.13.6.695. [DOI] [PubMed] [Google Scholar]
- MATEO J., NIRAS-POTUGAL M.T., ROTLLAN P. Ectoenzymatic hydrolysis of diadenosine polyphosphates by cultured adrenomedullary vascular endothelial cell. Am. J. Physiol. 1997;273:C918–C927. doi: 10.1152/ajpcell.1997.273.3.C918. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., MEISSNER A., BOKNIK P., GOMBOSOVA I., KNAPP J., LUSS H., MULLER F.U., SCHLUTER H., ZIDEK W., ROLF N., VAN AKEN H., VAHLENSIECK U., SCHMITZ W. Inotropic effects of diadenosine tetraphosphate in isolated canine cardiac preparations. J. Cardiovasc. Pharmacol. 1999;33:151–156. doi: 10.1097/00005344-199901000-00023. [DOI] [PubMed] [Google Scholar]
- NUNAIN S.O., GARRATT C., PAUL V., DEBBAS N., WARD D.E., CAMM A.J. Effect of intravenous adenosine on human atrial and ventricular repolarisation. Cardiovasc. Res. 1992;26:939–943. doi: 10.1093/cvr/26.10.939. [DOI] [PubMed] [Google Scholar]
- OGILVIE A., BLASIUS R., SCHULZE-LOHOFF E., STERZEL R.B. Adenine dinucleotides, a novel class of signalling molecules. J. Auton. Pharmacol. 1996;16:325–328. doi: 10.1111/j.1474-8673.1996.tb00045.x. [DOI] [PubMed] [Google Scholar]
- RUBINO A., BURNSTOCK G.Possible role of diadenosine polyphosphates as modulators of cardiac sensory–motor neurotransmission in guinea-pigs J. Physiol. 19961495515–523.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN W.K., KURACHI Y. Mechanisms of adenosine-mediated actions on cellular and clinical cardiac electrophysiology. Mayo Clin. Proc. 1995;70:274–291. doi: 10.4065/70.3.274. [DOI] [PubMed] [Google Scholar]
- STAVROU B.M., BECK C., FLORES N.A. Changes in extracellular pH and myocardial ischaemia alter the cardiac effects of diadenosine tetraphosphate and pentaphosphate. Br. J. Pharmacol. 2001;134:639–647. doi: 10.1038/sj.bjp.0704288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAHLENSIECK U., BOKNIK P., GOMBOSOVA I., HUKE S., KNAPP J., LINCK B., LUSS H., MULLER H.L., NEUMANN J., DENG M.C., SCHELD H.H., JANKOWSKI H., SCHLUTER H., ZIDEK W., ZIMMERMANN N., SCHMITZ W. Inotropic effects of diadenosine tetraphosphate (Ap4A) in human and animal cardiac preparations. J. Pharmacol. Exp. Ther. 1999;288:805–813. [PubMed] [Google Scholar]
- VAHLENSIECK U., BOKNIK P., KNAPP J., LINCK B., MULLER F.U., NEUMANN J., HERZIG S., SCHLUTER H., ZIDEK W., DENG M.C., SCHELD H.H., SCHMITZ W. Negative chronotropic and inotropic effects exerted by diadenosine hexaphosphate (Ap6A) via A1-adenosine receptors. Br. J. Pharmacol. 1996;119:835–844. doi: 10.1111/j.1476-5381.1996.tb15748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]