Abstract
We have studied whether curcumin prevents amiodarone-induced lung fibrosis in rats. Intratracheal instillation of amiodarone (6.25 mg kg−1 on days 0 and 2, and then killed on day 3, day 5, week 1, week 3 and week 5 after amiodarone administration) induced increases in total protein and lactate dehydrogenase (LDH) activity on days 3 and 5 in bronchoalveolar lavage fluid (BALF). Total cell counts, alveolar macrophages, neutrophils and eosinophils recovered by BAL, and lung myeloperoxidase (MPO) activity were significantly higher in amiodarone rats.
Tumor necrosis factor-α (TNF-α) release after lipopolysaccharide (LPS) stimulation and superoxide anion generation after phorbol myristate acetate (PMA) stimulation were higher in the alveolar macrophages of amiodarone rats at 3 and 5 weeks postamiodarone instillation than in controls. Amiodarone also induced increases in transforming growth factor-β1 (TGF-β1) expression, collagen deposition, type I collagen expression and c-Jun protein in lungs.
Curcumin (200 mg kg−1 body weight after first amiodarone instillation and daily thereafter for 5 weeks)-treated amiodarone rats had reduced levels of protein, LDH activity, total cell numbers and differential cell counts in BALF. LPS-stimulated TNF-α release and PMA-stimulated superoxide generation were significantly suppressed by curcumin. Furthermore, curcumin inhibited the increases in lung MPO activity, TGF-β1 expression, lung hydroxyproline content, expression of type I collagen and c-Jun protein in amiodarone rats. Our results have important implications for the treatment of amiodarone-induced lung fibrosis.
Keywords: Amiodarone, collagen, curcumin, superoxide anion, tumor necrosis factor-α
Introduction
Treatment with amiodarone, a potent antiarrhythmic agent, is associated with pulmonary injury resulting in fatal pulmonary fibrosis (Gill et al., 1992). The mechanisms for amiodarone pulmonary injury include direct toxicity to lung tissue, hypersensitivity reaction to amiodarone, enhanced oxidative stress, alteration of membrane properties and activation of alveolar macrophages and cytokine release (Kennedy et al., 1988; Wang et al., 1992; Wilson & Lipmann, 1993; Reasor & Kacew, 1996; Reinhart & Gairalo, 1997; Chung et al., 2001). Despite a great deal of information regarding the mechanism of amiodarone-induced lung fibrosis, management of this lung disorder is frequently difficult, and unfortunately, therapeutic approaches to inhibit the development of amiodarone pulmonary fibrosis are scanty. Curcumin (diferuloylmethane), a widely used yellow curry powder from turmeric (Curcuma longa) in Indian and other Asian cuisines, has been used as a therapeutic agent because of its attractive combination of properties that include anti-inflammatory (Rao et al., 1982), antioxidant (Kuchandy & Rao, 1990), antifibrotic (Kang et al., 2002) and anticancer qualities (Gescher et al., 1998). Commercially available curcumin contains three naturally occurring curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin) with curcumin as the main (∼77%) constituent (Ahsan et al., 1999). Recently, we reported the antifibrotic effect of curcumin in bleomycin-induced lung fibrosis in rats (Punithavathi et al., 2000). Emerging evidence that amiodarone administration results in interstitial and/or alveolar inflammation and release of inflammatory mediators (Chung et al., 2001), and reports from our earlier studies that curcumin inhibited the development of pulmonary fibrosis (Punithavathi et al., 2000) are compelling reasons to use curcumin in suppressing amiodarone-induced pulmonary fibrosis. In this study, we demonstrate that curcumin treatment attenuates amiodarone-induced pulmonary fibrosis in rats.
Methods
Induction of lung fibrosis in F344 rats by amiodarone was performed following established methods (Taylor et al. , 2000; Chung et al., 2001). Healthy male Fischer 344 rats (200 – 225 g, purchased from Madras Veterinary College, India) were divided into four groups: (i) a control group, which received two intratracheal (i.t.) instillation of 0.3 ml sterile distilled water on days 0 and 2; (ii) a curcumin group, which received 200 mg kg−1 of curcumin; (iii) the third group received two i.t. instillation of amiodarone (6.25 mg kg−1, in 0.3 ml of water): the day the first instillation was performed was marked as day 0; the second instillation was performed on day 2 and (iv) a curcumin+amiodarone group that received 200 mg kg−1 of curcumin after first amiodarone instillation and daily thereafter throughout the experimental period of 5 weeks. We selected the 200 mg kg−1 concentration of curcumin for our treatment protocol based upon results from dose–response study. Amiodarone hydrochloride was dissolved in sterile triple distilled water at 60°C, cooled and then stored at 4°C. The solution was brought to room temperature before instillation. Curcumin was suspended in corn oil (100 mg ml−2 kg−1 body weight day−1) and administered by gastric intubation. All animals received humane care during the experimental time period.
Preparation of alveolar macrophage-conditioned medium and TNF-α measurement
Six rats per treatment group were killed at day 3, day 5, week 1, week 3 and week 5 after amiodarone administration by an overdose of sodium pentobarbital (75 mg kg−1) following standard ethical procedures. The lungs were then lavaged five times with calcium- and magnesium-free phosphate-buffered saline, pH 7.4 at a volume of 5 ml wash−1. Pooled bronchoalveolar lavage fluid (BALF) was filtered through sterile guaze to remove mucous and particulates and then centrifuged at 300 × g for 10 min. Erythrocytes were lysed, and after washing thrice with Rosewell Park Memorial Institute (RPMI)-1640 media, the pelleted cells were resuspended in RPMI-1640 media containing 25 mM HEPES. Aliquots of the cell suspension were counted by a hemocytometer and cell viability (>95%) was measured by trypan blue exclusion. Differential cell counts were performed on cytocentrifuge preparations that were fixed in methanol and stained with Diff-Quik. The BAL cells were resuspended in RPMI-1640 media supplemented with 2 mM glutamine, antibiotics and 10% heat-inactivated fetal bovine serum. The cells were seeded (1 × 106 cells ml−1) to a 24-well tissue culture plate and allowed to adhere for 2 h at 37°C in a humidified atmosphere in 95% O2 : 5% CO2, after which the nonadherent cells were removed by washing three times with fresh RPMI-1640 media. Alveolar macrophage-enriched monolayers collected at day 3, day 5, week 1, week 3 and week 5 after amiodarone administration were cultured in duplicate at a density of 1 × 106 ml−1 in RPMI-1640 media in the absence or presence of 1 μg ml−1 lipopolysaccharide (LPS; serotype 0111:B4) for 24 h. Macrophage-conditioned medium was collected, centrifuged and cell-free supernatants stored in aliquots at −80°C. Appropriately diluted conditioned media were measured for tumor necrosis factor-α (TNF-α) using commercial rat enzyme-linked immunosorbent assay (ELISA) kit. The assay was performed using the protocol supplied by the manufacturer.
Protein assay
BALF was centrifuged at 300 × g for 10 min to remove cells, and the supernatant was recentrifuged for 20 min at 48,000 × g to remove particulate materials. Protein concentration in the supernatant was then determined by the protein assay system, using bovine serum albumin (BSA) as the standard. The protein assay was performed essentially following the protocol supplied by the manufacturer.
Measurement of lung myeloperoxidase activity
Myeloperoxidase (MPO) activity of lung tissue was measured by the method of Bradley et al. (1982). Briefly, lung tissues were homogenized in 20 mM of potassium phosphate (1 : 10, w v−1; pH 6.0) and aliquots of the homogenates were added to a buffer solution containing 0.5% hexadecyltrimethylammonium bromide dissolved in 50 mM potassium phosphate buffer, pH 6.0. Samples were sonicated (60 s at 4°C) and then centrifuged for 30 min at 40,000 × g at 4°C. Aliquots of the supernatant were allowed to react with a solution of o-dianisidine hydrochloride (0.167 mg ml−1 in phosphate buffer, pH 6.0) and 0.0005% hydrogen peroxide. The rate of change in absorbance at 460 nm was used as an index of MPO activity. One unit of MPO activity is defined as the volume of the supernatant that degrades 1 μmol peroxide min−1 at 37°C.
Measurement of superoxide anion generation
Alveolar macrophage release of superoxide anion was estimated by the ferricytochrome c reduction method in which superoxide dismutase -inhibitable cytochrome c reduction is taken as an index of superoxide generation (Babior et al., 1973). Results of superoxide anion production were expressed as nmol (106 cells)−1 (20 min)−1.
Measurement of lactate dehydrogenase activity
Lactate dehydrogenase (LDH) activity in lung lavage fluid was estimated by monitoring the oxidation of pyruvate coupled with the reduction of NAD at 340 nm with an LDH assay kit. Results were expressed as units l−1. LDH activity (1 U) is defined as the amount of enzyme that converts 1 μmol of lactate to 1 μmol of pyruvate with the concomintant reduction of 1 μmol of NAD to 1 μmol of NADH min−1 l−1 of sample.
Hydroxyproline assay
After BAL, lung tissues were excised, washed free of blood in ice-cold physiological saline and stored at −80°C until further analysis. A portion of the frozen lung tissues was freeze-dried and then hydrolyzed in 6 N HCl at 110°C for 20 h. After hydrolysis, samples were neutralized with 10 N NaOH and assayed for hydroxyproline content (Woessner, 1961) as described previously (Venkatesan et al., 1998). Briefly, 2 ml of the samples containing 2–10 μg of hydroxyproline were placed in test tubes. Hydroxyproline oxidation was initiated by adding 1 ml of chloramine-T to each tube. The tube contents were vortexed for 2 min and allowed to stand for 20 min at room temperature. Adding 1 ml of perchloric acid to each tube then destroyed chloramine-T. The contents were mixed and allowed to stand for 5 min. Finally, 1 ml of p-dimethylbenzaldehyde was added and the mixture was shaken. The tubes were then placed in a 60°C water bath for 20 min, cooled in tap water for 5 min and the color developed was read spectrophotometrically at 557 nm.
Western blot analysis of TGF-β1, type I collagen and c-Jun
Frozen lung samples were finely cut with scissors and then homogenized with a Polytron homogenizer in 0. 05 M Tris-HCl, pH 7.6, 0.15 M NaCl, 0.5% (v v−1) Triton X-100, containing 10 mM EDTA, 1 mM PMSF, 1 μg ml−1 leupeptin and 1 μg ml−1 aprotinin. The homogenate was centrifuged at 10,000 × g for 5 min and the protein content in the supernatant was determined by the protein assay system from Bio-Rad, using BSA as the standard. Protein (50 μg) was run on a 10% SDS–PAGE gel and the separated proteins were transferred onto nitrocellulose membranes. After transfer, the nonspecific protein binding sites on the membranes were blocked with 5% (w vol−1) dried nonfat milk in Tris-buffered saline (TBS; 0.02 M Tris; 0.5 M NaCl; pH 7.6) with 0.1% Tween-20 (TBS-T) for 1 h at room temperature. The membranes were then probed with primary antibodies against transforming growth factor-β1 (TGF-β1) (1 : 1000; specific for the mature form of TGF-β1), type I collagen (1 : 1000; rabbit anti-mouse) or c-Jun (1 : 1000) for 1 h at room temperature. After washing with TBS-T, membranes were incubated with horseradish peroxidase-labeled secondary antibodies (1 : 2000) for 1 h at room temperature, and washed again with TBS – Tween-20. The immunoreactive bands were visualized by enhanced chemiluminescence.
Densitometry
Quantification of Western blots of type-I collagen, TGF-β1 and c-Jun protein was performed with an image analyzer software (Fluorchem, Alpha Innotech Corporation, San Leandro, CA, U.S.A), which measures the sum of all the pixel values after background correction. The mean values of three individual observations are represented in a bar graph.
Materials
Amiodarone hydrochloride, BSA, chloramine-T, curcumin, Diff-Quik, p-dimethylbenzaldehyde, hydroxyproline, LDH assay kit, LPS (serotype 0111:B4), perchloric acid, proteinase inhibitors, Triton X-100, Trizma base, Tween-20 and horseradish peroxidase-labeled secondary antibodies were obtained from Sigma chemicals, St Louis, MO, U.S.A. Protein assay kit was purchased from Bio-Rad, Hercules, CA, U.S.A. TGF-β1 antibody and rat TNF-α ELISA kit were obtained from R&D Systems, MN, U.S.A. Type I collagen antibody was obtained from Research Diagnostics Inc., NJ, U.S.A. Antibody to c-Jun protein was obtained from BD Transduction Laboratories, NJ, U.S.A. Tissue culture media and reagents were purchased from HiMedia Laboratories Pvt. Limited, Mumbai, India. All other reagents were of analytical grade and commercially available.
Statistics
Data are expressed as means±s.d. of six observations from six different rats. Statistical evaluation of the differences between the groups was carried out with two-way analysis of variance and significant differences between experimental groups were determined at P<0.05.
Results
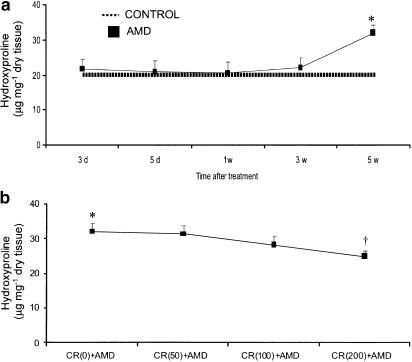
Table 1 shows the values of water- and curcumin-treated control groups for various biochemical and cellular constituents, and the variations in these values were not statistically different within each group over the 5-week study period. Curcumin administration reduced amiodarone-induced lung fibrosis (as measured by lung collagen content, which is a classical biochemical marker of lung fibrosis). Amiodarone significantly enhanced the deposition of lung collagen (Figure 1a) and the highest dose of curcumin (200 mg kg−1 body weight) offered maximal suppression of collagen deposition in fibrotic lungs. However, the two lower concentrations (50 or 100 mg kg−1 body weight) of curcumin did not significantly alter the increased lung collagen content induced by amiodarone (Figure 1b). Therefore, we selected the 200 mg kg−1 dosage as the most effective and used it in further experiments.
Table 1.
time-course anlaysis of various biochemical and cellular constituents in water- and curcumin-treated control groups
| Parameters | Day 3 | Day 5 | Week 1 | Week 3 | Week 5 |
|---|---|---|---|---|---|
| Hydroxyproline | |||||
| Control | 18.65±2.72 | 18.27±2.51 | 18.81±13.13 | 19.99±2.82 | 21.09±2.39 |
| Curcumin | 17.97±1.98 | 17.36±2.53 | 18.40±3.26 | 18.72±3.51 | 19.03±2.84 |
| Total protein | |||||
| Control | 122.48±0.9 | 118.04±13.9 | 128.6±9.10 | 134.13±9.91 | 117.3±20.1 |
| Curcumin | 125.47±12.1 | 119.79±15.3 | 130.82±11.3 | 121.77±14.6 | 96.69±12.5 |
| LDH | |||||
| Control | 41.39±3.98 | 44.29±3.82 | 40.20±4.50 | 41.55±3.04 | 38.78±4.96 |
| Curcumin | 43.32±3.53 | 45.69±2.71 | 39.63±3.94 | 41.92±5.27 | 36.14±5.60 |
| Total cells (× 106) | |||||
| Control | 6.15±0.63 | 5.77±0.40 | 6.41±0.75 | 6.06±0.78 | 6.25±0.73 |
| Curcumin | 6.08±0.70 | 5.96±0.26 | 5.88±0.56 | 5.70±0.48 | 5.36±0.62 |
| AM (× 106) | |||||
| Control | 6.04±0.62 | 5.65±0.40 | 6.11±0.76 | 5.96±0.79 | 6.13±0.74 |
| Curcumin | 5.90±0.66 | 5.80±0.30 | 5.77±0.55 | 5.58±0.48 | 5.32±0.65 |
| Neutrophils (× 105) | |||||
| Control | 0.047±0.007 | 0.042±0.008 | 0.051±0.012 | 0.049±0.011 | 0.043±0.026 |
| Curcumin | 0.046±0.01 | 0.045±0.008 | 0.044±0.015 | 0.040±0.007 | 0.035±0.015 |
| Eosinophils (× 105) | |||||
| Control | 0.021±0.004 | 0.022±0.006 | 0.019±0.006 | 0.023±0.007 | 0.024±0.009 |
| Curcumin | 0.023±0.013 | 0.021±0.008 | 0.024±0.008 | 0.020±0.004 | 0.019±0.008 |
| MPO | |||||
| Control | 6.78±1.36 | 6.90±0.74 | 6.48±0.96 | 7.31±1.39 | 6.30±1.31 |
| Curcumin | 6.15±0.92 | 5.60±0.47 | 5.76±0.58 | 6.39±1.02 | 5.27±0.81 |
| TNF-α | |||||
| Control | 4.46±0.97 | 5.05±1.22 | 4.84±1.31 | 4.63±0.84 | 4.38±0.92 |
| Curcumin | 4.04±1.22 | 3.63±0.61 | 4.39±0.72 | 4.52±0.79 | 3.82±0.68 |
| Superoxide anion | |||||
| Control | 9.30±1.99 | 8.86±1.49 | 9.44±1.67 | 10.4±2.63 | 10.9±3.25 |
| Curcumin | 6.79±0.95 | 7.13±0.79 | 6.95±1.15 | 7.25±0.74 | 7.02±1.75 |
Values are expressed as mean±s.d. (n=6 rats group−1 time point−1). No statistical difference was observed in any of the above parameters between water- and curcumin-treated control groups over the 5-week study period. AM: alveolar macrophages; LDH: lactate dehydrogenase; MPO: myeloperoxidase; TNF-α: tumor necrosis factor-α.
Figure 1.
Time-course analysis of lung hydroxyproline content in amiodarone-treated rats (a) and the protective effects of curcumin on amiodarone-induced increase in lung hydroxyproline content (b). Rats were killed at several time points after i.t. administration of amiodarone and lung hydroxyproline levels were measured as described under the Methods section. All data are expressed as mean±s.d. (n=6 rats group−1 time point−1). Since there was no statistical difference between water- and curcumin-treated control groups, we show the values of water-treated groups as controls. *Significantly (P<0.01) higher than control groups (dotted line); †significantly (P<0.01) lower than CR(0)+AMD group; hydroxyproline values in CR(50)+AMD and CR(100)+AMD groups are not statistically different from CR(0)+AMD group; CR: curcumin; AMD: amiodarone; d: day; w: week.
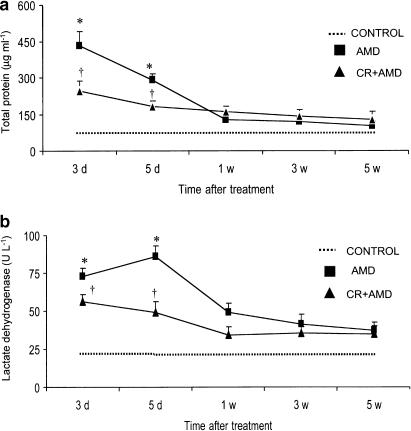
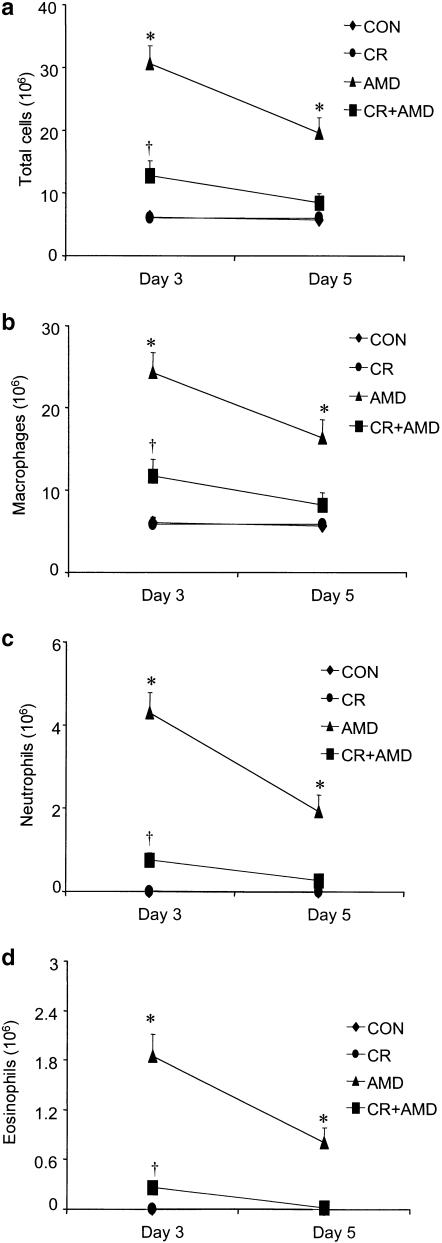
Consistent with the development of pulmonary inflammation and damage of the alveolar integrity, amiodarone treatment resulted in significantly higher levels of total protein in lung lavage fluid on both days 3 and 5, while curcumin was able to reduce the increased content of protein in BALF in amiodarone-treated rats (Figure 2a). A significant increase in LDH activity was observed in BALF of amiodarone rats on days 3 and 5 and treatment with curcumin resulted in blunting of LDH elevation (Figure 2b). The levels of protein and LDH in BALF from amiodarone rats were not different from control groups at 1-, 3- and 5-week postdrug treatment. Amiodarone administration was accompanied by a significant increase in both total cell count and individual cell types recovered from BALF. Compared with control groups, amiodarone caused a 4.8-fold and a 3.1-fold increase in total cell counts at 3 and 5 days, respectively, after amiodarone; however, the total cell counts in the lung lavage fluid were not statistically different from baseline levels at latter time points (Figure 3a). Amiodarone-administered rats demonstrated significantly higher numbers of alveolar macrophages on days 3 and 5 after amiodarone exposure (Figure 3b). Neutrophils (Figure 3c) and eosinophils (Figure 3d) were also significantly increased in a manner similar to alveolar macrophages on days 3 and 5, with a peak increase on day 3 after amiodarone exposure. Interestingly, curcumin treatment significantly reduced the early increases in both total and differential cell counts (Figure 3).
Figure 2.
Effects of curcumin on amiodarone-induced increases in bronchoalveolar lavage BALF total protein (a) and LDH activity (b). BALF was performed at various time points after i.t. administration of amiodarone, and protein concentration and LDH levels in BALF were measured. All data are expressed as mean±s.d. (n=6 rats group−1 time point−1). Since there was no statistical difference between water- and curcumin-treated control groups, we show the values of water-treated groups as controls. *Significantly (P<0.01) higher than control groups (dotted line); †significantly (P<0.01) lower than amiodarone group. CR: curcumin; AMD: amiodarone; d: day; w: week.
Figure 3.
Effects of curcumin on amiodarone-induced increases in lung inflammation as reflected by influx of inflammatory cells into airspaces. BALF was performed at several time points after i.t. administration of amiodarone, and changes in total cells (a) alveolar macrophages (b) neutrophils (c) and eosinophils (d) were measured as described under the Methods section. Since there was no significant difference in total and differential cell counts recovered by BALF at latter time points between control and amiodarone groups, we present the data for 3 and 5 days postamiodarone administration. All data are expressed as mean±s.d. (n=6 rats group−1 time point−1). *Significantly (P<0.01) higher than control groups; †significantly (P<0.01) lower than the amiodarone group. CON: water-treated controls; CR: curcumin; AMD: amiodarone; d: day; w: week.
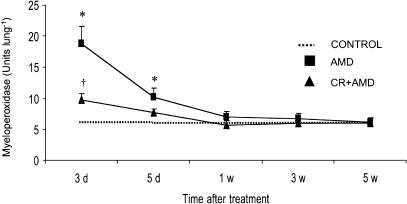
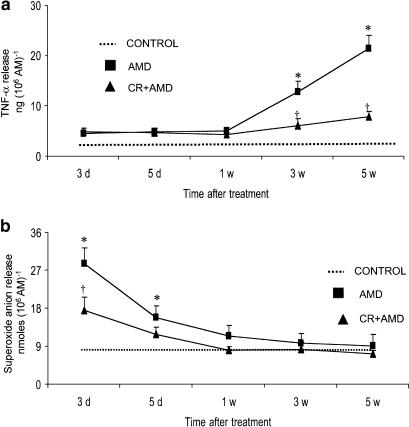
Lung MPO activity was measured as an indicator of neutrophil accumulation in amiodarone-injured tissue. The results demonstrated an increase in lung MPO activity at days 3 and 5 after amiodarone administration and curcumin treatment potentially inhibits amiodarone-induced early increases in lung MPO activity (Figure 4). Compared to control groups, the levels of MPO activity were unchanged at 1-, 3- and 5-week postamiodarone treatment. Baseline release of TNF-α by unstimulated alveolar macrophages was not different in amiodarone and control rats. However, when stimulated with LPS (1 μg ml−1), macrophage release of TNF-α started to increase at 3 weeks after amiodarone treatment and reached higher levels at 5 weeks after amiodarone treatment. Curcumin treatment resulted in significantly lower levels of TNF-α at these time points (Figure 5a). The results also indicate that alveolar macrophage release of TNF-α from amiodarone rats was not changed during the early time points. Under basal conditions, no significant differences in alveolar macrophage release of superoxide anion were observed between amiodarone and control rats. Interestingly, when stimulated with phorbol myristate acetate (PMA; 0.1 μg ml−1), alveolar macrophage from amiodarone rats released significantly increased amounts of superoxide compared to controls. As shown in Figure 5b, superoxide production was significantly higher 3 and 5 days after amiodarone administration, while curcumin treatment resulted in the inhibition of superoxide production in amiodarone rats. The results also show that there was no significant increase in macrophage release of superoxide anion 1- 3-, and 5-week postamiodarone treatment.
Figure 4.
Effects of curcumin on amiodarone-induced increases in lung MPO activity. The lung MPO levels were measured at several time points after i.t. administration of amiodarone as described under the Methods section. All data are expressed as mean±s.d. (n=6 rats group−1 time point−1). Since there was no statistical difference between water- and curcumin-treated control groups, we show the values of water-treated groups as controls. *Significantly (P<0.01) higher than controls (dotted line); †significantly (P<0.01) lower than the amiodarone group. CR: curcumin; AMD: amiodarone; d: day; w: week.
Figure 5.
Effects of curcumin on amiodarone-induced increases in alveolar macrophage release of TNF-α (a) and superoxide anion (b). BALF was performed at several time points after i.t. administration of amiodarone, and the recovered alveolar macrophages were stimulated either by LPS (1 μg ml−1) or PMA (0.1 μg ml−1) for the determination of TNF-α and superoxide anion production, respectively. All data are expressed as mean±s.d. (n=6 rats group−1 time point−1). Since there was no statistical difference between water- and curcumin-treated control groups, we show the values of water-treated groups as controls. *Significantly (P<0.01) higher than control groups (dotted line); †significantly (P<0.01) lower than the amiodarone group. CR: curcumin; AMD: amiodarone; d: day; w: week.
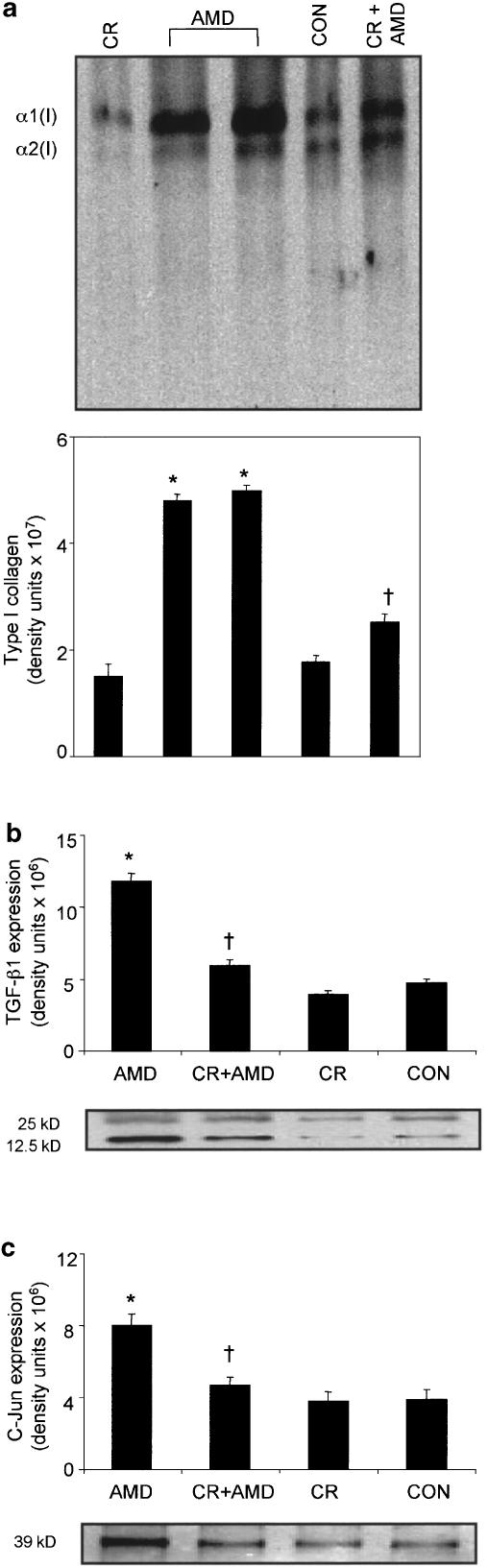
Figure 6a represents the results of experiments involving analysis of type I collagen in the fibrotic lung. It can be clearly seen that immunoblotting with the antibody demonstrates an increase (2.7-fold increase compared to control groups) in the expression of type I collagen in amiodarone lungs, while curcumin-treated amiodarone rats showed a downmodulation of accumulation of type I collagen in fibrotic lungs. We next examined the expression of TGF-β1 in rat lungs at week 1 to week 5 after amiodarone instillation. TGF-β1 protein expression was elevated at week 1, week 2 and week 3 postamiodarone insult, with a peak increase (2.5-fold increase compared to control groups) on week 1. There was no significant change in TGF-β1 protein expression at the latter time-course studied (data not shown). Interestingly, curcumin treatment markedly downregulated amiodarone-induced increases in TGF-β1 expression (Figure 6b). Data from our studies also indicate a significant increase (two-fold increase compared to control groups) in the expression of c-Jun protein in the lungs 5 weeks after amiodarone instillation. Consistent with a previous study (Chung et al., 2001), the expression of c-Jun protein was not affected by amiodarone at earlier time points studied. Curcumin treatment significantly downregulated the amiodarone-induced increases in c-Jun protein expression (Figure 6c).
Figure 6.
Inhibitory effects of curcumin on amiodarone-induced increases in type I collagen (a), TGF-β1 (b) and c-Jun protein (c) expression in the lung tissue. Lung tissue proteins were separated by SDS–PAGE, transferred onto nitrocellulose membranes and then probed using antibodies against type I collagen (a) or TGF-β1 (b) or c-Jun protein (c). Both type I collagen and c-Jun expression were analyzed 5 weeks after amiodarone administration, while TGF-β1 analysis was carried out on 1 week postamiodarone instillation. Quantitative analysis of the respective immunoblots was accomplished by an image analysis system as described under the Methods section. Findings are representative of three separate observations from three different rats. *Significantly (P<0.01) higher than control groups; †significantly (P<0.01) lower than the amiodarone group at the corresponding time. CON: water-treated control; CR: curcumin; AMD: amiodarone.
Discussion
In the present study we show that curcumin, an anti-inflammatory antioxidant compound, inhibits amiodarone-induced lung injury and fibrosis. Characteristically, amiodarone-induced lung injury was associated with biochemical alterations such as increases in BALF total protein (a marker of damage to the alveolar – capillary barrier) and LDH activity (a marker of cell injury). Besides protein and LDH, lung MPO activity (a marker of neutrophil accumulation) is elevated in amiodarone rats. Our data are consistent with previous studies that demonstrated elevated levels of protein, albumin and LDH in amiodarone rats (Wilson et al., 1989; Padmavathy et al., 1992; Taylor et al., 2000). Interestingly, curcumin inhibited amiodarone-induced increases in the inflammatory activity of the lung that may be related to its membrane-stabilizing and anti-inflammatory properties (Mukhopadhyay et al., 1982; Rao et al., 1982; Nirmala & Puvanakrishnan, 1996).
Recent findings show that activation of macrophages and release of inflammatory and cytotoxic mediators drive amiodarone-induced lung fibrosis (Reinhart & Gairola, 1997; Chung et al., 2001). Our results demonstrated that alveolar macrophages from amiodarone rats released increased amounts of TNF-α. Accumulation of TNF-α in increased amounts would modulate fibroblast functions, including chemotaxis and synthesis of collagen, glycosaminoglycans, interleukins, collagenase and prostaglandin E2 (Postlethwaite & Seyer, 1990). Curcumin treatment resulted in marked attenuation of macrophage activation (as evidenced by TNF-α release). Inhibition of TNF-α release may alter the production of other proinflammatory cytokines and adhesion molecules (Kumar et al., 1998), supporting the idea that curcumin inhibition of TNF-α release may be associated with a decrease in fibrotic sequelae. Curcumin has preventive effects on inflammatory cytokine production, including TNF-α, by human peripheral blood monocytes and alveolar macrophages (Abe et al., 1999). Oxidative stress may also be responsible for amiodarone-induced lung injury, which is supported by enhanced production of superoxide anion by stimulated alveolar macrophages derived from amiodarone-treated rats. Our findings are consistent with the findings of Taylor et al. (2000), who reported increased cellular oxidant production in amiodarone rats. Thus, elevated levels of superoxide anion release may represent a pulmonary response to injury and amelioration of this response by curcumin could have therapeutic benefits for amiodarone lung injury. Our data also indicate that amiodarone treatment per se did not result in TNF-α release and superoxide anion generation. Conversely, TNF-α and superoxide release was increased after appropriate stimulation with LPS and PMA, respectively, suggesting that amiodarone primes macrophages for TNF-α and superoxide production. These findings are consistent with previous reports of LPS-stimulated TNF-α release by alveolar macrophages derived from amiodarone-treated rats (Reinhart & Gairola, 1997).
Since TGF-β1 regulates extracellular matrix deposition, including collagens, in the fibrotic tissue (Border & Noble, 1994), we analyzed TGF-β1 and collagen deposition as targets for inhibitory effects of curcumin. In curcumin-treated amiodarone rats, both the production and expression of TGF-β1 protein in the lungs are significantly reduced. We also show that curcumin decreased the elevation in lung hydroxyproline content and type I collagen expression in fibrotic lungs. Curcumin also decreased the excessive deposition of lung collagen in bleomycin-induced pulmonary fibrosis in rats (Punithavathi et al., 2000). Therefore, it is possible that curcumin may modify the fibrotic phenotype of fibroblasts and thus modulate fibrous tissue formation in the lung tissue by altering fibroblast collagen synthesis and deposition. The mechanisms behind curcumin inhibition of collagen deposition may be similar to that in liver injury as reported by a recent study that showed that curcumin inhibits collagen deposition by downregulating collagen mRNA expression (Kang et al., 2002), suggesting that curcumin might inhibit collagen gene transcription in amiodarone-induced lung fibrosis in rats.
c-Jun protein, a nuclear transcription factor belonging to the activator protein-1 (AP-1) family of inducible proteins, has been implicated in inflammatory and fibroproliferative processes (Kim et al., 1990; Angel & Karin, 1991; Chung et al., 1996). An interesting study (Chung et al., 2001) showed that the development of amiodarone lung fibrosis is associated with the accumulation of c-Jun protein. Consistent with this study, we observed an increase in the expression of c-Jun in the lungs of amiodarone rats. Significantly, our data also demonstrate curcumin amelioration of amiodarone-induced increases in c-Jun protein expression. Curcumin-mediated inhibition of c-Jun expression therefore could contribute to downregulation of a battery of genes involved in fibrotic tissue remodeling. It is of great interest to note that curcumin has been shown to be a potent inhibitor of c-Jun induction (Sato et al., 2000).
It is interesting to know that curcumin is effective against lung fibrosis across different experimental models, including amiodarone-induced lung fibrosis. The antifibrotic effect of curcumin against bleomycin lung injury has recently been addressed from our lab (Punithavathi et al., 2000). Although amiodarone and bleomycin are two distinct therapeutic agents, the development of amiodarone-induced pulmonary injury and fibrosis resembles, in many ways, that of bleomycin (Giri & Wang, 1989). Our results disclose remarkable similarities in the antifibrotic effects of curcumin against amiodarone lung injury as it was against bleomycin. Strikingly, in both models, curcumin treatment resulted in the attenuation of several indices of inflammation and fibrosis (a significant reduction in inflammatory activity and macrophage activation, a decrease in oxidative stress and collagen deposition). Results of these studies indicate that the antifibrotic effects of curcumin may be due to its antioxidant and free radical scavenging properties (Ammon & Wahl, 1991). The structural characteristics such as the phenolic and the methoxy group on the phenyl ring and the presence of 1,3-diketone system make curcumin a potent inhibitor of lipid peroxidation (Araujo & Leon, 2001), compared to other antioxidant agents. Whereas the inability of N-acetylcysteine to attenuate amiodarone lung injury in hamsters has been reported (Leeder et al., 1994), the efficacy of ambroxol in inhibiting amiodarone lung injury has not yet been studied. Curcumin may also be beneficial as a candidate for anti-inflammation and antifibrosis, since it not only downregulates nuclear factor kappa B (NF-κB) but also inhibits AP-1, a transcription factor that often acts in concert with NF-κB to regulate the expression of various inflammatory mediators. Our results suggest that dietary supplementation of curcumin may prove beneficial in both these models, a finding of significant clinical interest. The daily intake of curcumin capable of exerting antifibrogenic effects in humans, equivalent to the 200 mg kg−1 day−1 dose that was effective here in rats, when calculated on the basis of equivalent body surface area would be 1.9 g person−1 day−1, when the body weight of an adult is assumed to be 60 kg. Cheng et al. (1998) found in a phase I clinical trial that an oral dose of up to 8 g day−1 of curcumin is safe without toxic side effects. There are reports that high doses of curcumin or turmeric in the diet are relatively safe (Deodhar et al., 1980; Satoskar et al., 1986).
To conclude our results indicate a protective role for curcumin in amiodarone-induced lung fibrosis. Our demonstration that curcumin inhibition of amiodarone-induced elevated production of TNF-α, TGF-β1, collagen deposition and c-Jun protein may help explain the reduction in fibrotic changes seen after amiodarone challenge and the antifibrotic properties of curcumin. Since curcumin has been shown to reduce myocardial infarct size and improve postischemic cardiac function (Sato et al., 2000), our findings may have therapeutic implications for the clinical use of curcumin to attenuate amiodarone-induced lung fibrosis without modifying the therapeutic effect of amiodarone in cardiac disorders.
Acknowledgments
We thank the Director, CLRI for his interest in publishing this work, Mr Elango for his help in animal experiments, Dr V. Arumugam for statistical analysis, and CSIR-UGC, New Delhi for financial assistance to NVN.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- PMA
phorbol myristate acetate
- TGF-β1
transforming growth factor-β1
- TNF-α
tumor necrosis factor-α
References
- ABE Y., HASHIMOTO S., HORIE T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- AHSAN H., PARVEEN N., KHAN N.U., HADI S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem. Biol. Interact. 1999;121:161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- AMMON H.P.T., WAHL M.A. Pharmacology of Curcuma longa. Planta. Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- ANGEL P., KARIN M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- ARAUJO C.A.C., LEON L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz. 2001;96:723–728. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- BABIOR B.M., KIPNES R.S., CURNUTTE J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;2:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORDER W.A., NOBLE N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- CHENG A.L., LIN J.K., HSU M.M., SHEN T.S., KO J.Y., LIN J.T., WU M.S., YU H.S., JEE S.H., CHEN G.S., CHEN T.M., CHEN C.A., LAI M.K., PU Y.S., PAN M.H., WANG U.J., TSAI C.C., HSIEH C.Y. Phase I chemopreventiaon clinical trial of curcumin. Proc. Am. Soc. Clin. Oncol. 1998;17:558. [Google Scholar]
- CHUNG K.Y., AGARWAL A., UITTO J., MAUVIEL A. An AP-1 binding sequence is essential for regulation of the human alpha2(I) collagen (COL1A2) promoter activity by transforming growth factor-beta. J. Biol. Chem. 1996;271:3272–3278. doi: 10.1074/jbc.271.6.3272. [DOI] [PubMed] [Google Scholar]
- CHUNG W.H., BENNETT B.M., RACZ W.J., BRIEN J.F., MASSEY T.E. Induction of c-jun and TGF-beta 1 in Fischer 344 rats during amiodarone-induced pulmonary fibrosis. Am. J. Physiol. 2001;281:L1180–L1188. doi: 10.1152/ajplung.2001.281.5.L1180. [DOI] [PubMed] [Google Scholar]
- DEODHAR S.D., SETHI R., SRIMAL R.C. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J. Med. Res. 1980;71:632–634. [PubMed] [Google Scholar]
- GESCHER A., PASTORINO U., PLUMMER S.M., MANSON M.M. Suppression of tumor development by substances derived from the diet – mechanisms and clinical implications. Br. J. Clin. Pharmacol. 1998;45:1–12. doi: 10.1046/j.1365-2125.1998.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILL J., HEEL R.C., FITTON A. Amiodarone: an overview of its pharmacological properties, and review of its therapeutic use in cardiac arrhythmias. Drugs. 1992;43:69–110. doi: 10.2165/00003495-199243010-00007. [DOI] [PubMed] [Google Scholar]
- GIRI S.N., WANG Q. Mechanisms of bleomycin-induced lung injury. Comments Toxicol. 1989;3:145–176. [Google Scholar]
- KANG H.C., NAN J.X., PARK P.H., KIM J.M., LEE S.H., WOO S.W., ZHAO Y.Z., PARK E.J., SOHN D.H. Curcumin inhibits collagen synthesis and hepatic stellate cell activation in-vivo and in-vitro. J. Pharm. Pharmacol. 2002;54:119–126. doi: 10.1211/0022357021771823. [DOI] [PubMed] [Google Scholar]
- KENNEDY T.P., GORDON G.B., PAKY A., McSHANE A., ADKINSON N.F.J., PETERS S.P., FRIDAY K., JACKMAN W., SCIUTO A.M., GURTNER G.H. Amiodarone causes acute oxidant lung injury in ventillated and perfused rabbit lungs. J. Cardiovasc. Pharmacol. 1988;12:23–36. doi: 10.1097/00005344-198807000-00004. [DOI] [PubMed] [Google Scholar]
- KIM S.J., ANGEL P., LAFYATIS R., HATTORI K., KIM K.Y., SPORN M.B., KARIN M., ROBERTS A.B. Autoinduction of transforming growth factor-β1 is mediated by the AP-1 complex. Mol. Cell. Biol. 1990;10:1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUCHANDY E., RAO M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990;58:237–240. [Google Scholar]
- KUMAR A., DHAWAN S., HARDEGEN N.J., AGGARWAL B.B. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem. Pharmacol. 1998;55:775–783. doi: 10.1016/s0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- LEEDER R.G., BRIEN J.F., MASSEY T.E. Investigation of the role of oxidative stress in amiodarone-induced pulmonary toxicity in the hamster. Can. J. Physiol. Pharmacol. 1994;72:613–621. doi: 10.1139/y94-087. [DOI] [PubMed] [Google Scholar]
- MUKHOPADHYAY A., BASU N., GHATAK N., GUJRAL P.K. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–515. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- NIRMALA C., PUVANAKRISHNAN P. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 1996;159:85–93. doi: 10.1007/BF00420910. [DOI] [PubMed] [Google Scholar]
- PADMAVATHY B., NIRANJALI S., DEVARAJ H. Lung injury by amiodarone, an antiarrhythmic drug, in male rats. Indian J. Exp. Biol. 1992;30:653–654. [PubMed] [Google Scholar]
- POSTLETHWAITE A.E., SEYER J.M. Stimulation of fibroblast chemotaxis by human recombinant tumor necrosis factor alpha (TNF-alpha) and a synthetic TNF-alpha 31–68 peptide. J. Exp. Med. 1990;172:1749–1756. doi: 10.1084/jem.172.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUNITHAVATHI D., VENKATESAN N., BABU M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO T.S., BASU N., SIDDIQUI H.H. Anti-inflammatory activity of curcumin analogues. Indian J. Med. Res. 1982;75:574–578. [PubMed] [Google Scholar]
- REASOR M.J., KACEW S. An evaluation of possible mechanisms underlying amiodarone-induced pulmonary toxicity. Proc. Soc. Exp. Biol. Med. 1996;212:297–304. doi: 10.3181/00379727-212-44019. [DOI] [PubMed] [Google Scholar]
- REINHART P.G., GAIROLA C.G. Amiodarone-induced pulmonary toxicity in Fischer rats: release of tumor necrosis factor alpha and transforming growth factor beta by pulmonary alveolar macrophages. J. Toxicol. Environ. Health. 1997;52:353–365. doi: 10.1080/00984109708984070. [DOI] [PubMed] [Google Scholar]
- SATO M., CORDIS G.A., MAULIK N., DAS D.K. SAPKs regulation of ischemic preconditioning. Am. J. Physiol. 2000;279:H901–H907. doi: 10.1152/ajpheart.2000.279.3.H901. [DOI] [PubMed] [Google Scholar]
- SATOSKAR R.R., SHAH S.J., SHENOY S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with post-operative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986;24:651–656. [PubMed] [Google Scholar]
- TAYLOR M.D., VAN DYKE K., BOWMAN L.L., MILES P.R., HUBBS A.F., MASON R.J., SHANNON K., REASOR M.J. A characterization of amiodarone-induced pulmonary toxicity in F344 rats and identification of surfactant protein-D as a potential biomarker for the development of the toxicity. Toxicol. Appl. Pharmacol. 2000;167:182–190. doi: 10.1006/taap.2000.9000. [DOI] [PubMed] [Google Scholar]
- VENKATESAN N., PUNITHAVATHI D., CHANDRAKASAN G. Glycoprotein composition in cyclophosphamide-induced lung fibrosis. Biochim. Biophys. Acta. 1998;1407:125–134. doi: 10.1016/s0925-4439(98)00035-0. [DOI] [PubMed] [Google Scholar]
- WANG Q., HOLLINGER M.A., GIRI S.N. Attenuation of amiodarone-induced lung fibrosis and phospholipidosis in hamsters by taurine and/or niacin treatment. J. Pharmacol. Exp. Ther. 1992;262:127–132. [PubMed] [Google Scholar]
- WILSON B.D., JAWORSKI A.J., DONNER M.E., LIPPMANN M.L. Amiodarone-induced pulmonary toxicity in the rat. Lung. 1989;167:301–311. doi: 10.1007/BF02714959. [DOI] [PubMed] [Google Scholar]
- WILSON B.D., LIPMANN M.L. Amiodarone pulmonary toxicity in the rat is associated with increased lavage immunoglobulin and alveolar macrophages primed for increased interleukin-1 secretion. Am. J. Respir. Cell Mol. Biol. 1993;9:295–299. doi: 10.1165/ajrcmb/9.3.295. [DOI] [PubMed] [Google Scholar]
- WOESSNER J.F., JR The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]