Abstract
Hyperpolarizing voltage steps evoke slowly activating inward currents in a variety of neurones and in cardiac cells. This hyperpolarization-activated inward current (Ih) is thought to play a significant role in cell excitability, firing frequency, or in setting of the resting membrane potential in these cells. We studied the effects of lidocaine, mepivacaine, QX-314 and bupivacaine as well as its enantiomers on Ih in the membrane of dorsal root ganglion neurones (DRG).
The patch-clamp technique was applied to small dorsal root ganglion neurones identified in 200 μM thin slices of young rat DRGs. Under voltage-clamp conditions, the whole-cell Ih current was recorded in the presence of different concentrations of the local anaesthetics. In current-clamp mode the resting membrane potential and the voltage response of DRG neurones to injected current pulses were investigated.
Ih was reversibly blocked by bupivacaine, lidocaine and mepivacaine applied externally in clinically relevant concentrations. Concentration–response curves gave half-maximum inhibiting concentrations of 55, 99 and 190 μM, respectively. Bupivacaine block of the Ih current was not stereoselective. No significant effect was observed when QX-314 was applied to the external surface of the membrane.
In current-clamp experiments 60 μM bupivacaine slightly hyperpolarized the membrane. The membrane stimulation by low-amplitude current pulses in the presence of bupivacaine showed an increase of the hyperpolarizing responses.
Our findings suggest an important role of the Ih-block by local anaesthetics in the complex mechanism of drug action during epidural and spinal anaesthesia.
Keywords: Ion channels, electrophysiology, cationic current, sensory neurones
Introduction
Small dorsal root ganglion (DRG) neurones represent the somata of myelinated Aδ- and unmyelinated C-fibre type neurones (Harper & Lawson, 1985) and possibly participate in processing and transducing sensory information. These neurones are involved in peripheral nerve block when exposed to high concentrations of local anaesthetics during spinal and epidural anaesthesia (Butterworth & Strichartz, 1990).
The blockade of different types of voltage-gated Na+ channels during regional anaesthesia by local anaesthetics has been studied extensively and is of eminent importance for suppressing pain transmission in peripheral nerve, dorsal root ganglion neurones and dorsal horn neurones of the spinal cord. Voltage-gated K+ channels as well as background K+ channels play a major role in firing pattern in different types of neurones (Connor & Stevens, 1971; Llinas, 1988; Christie, 1995; Safronov, 1999; Hess & El Manira, 2001; Hille, 2001; Olschewski et al., 2001) and further have an influence on cell excitability and on resting membrane potential (Koh et al., 1992; Leonoudakis et al., 1998). Local anaesthetics were found to block voltage-gated K+ channels (Olschewski et al., 1998; Komai & McDowell, 2001). It has further been reported that local anaesthetics inhibit a voltage-insensitive K+ channel mainly found in thin, myelinated fibres in Xenopus laevis (Brau et al., 1995; Nau et al., 1999). Similar to this K+ channel, a recently described new family of two pore domain K+ selective channels are sensitive to local anaesthetics (Leonoudakis et al., 1998; Kindler et al., 1999; Buckler et al., 2000; Meadows & Randall, 2001).
The resting potential of the DRG neurones is determined by the counterbalancing action of two different voltage-sensitive conductances, the delayed-rectifier potassium channel and the hyperpolarization-activated inward current (Ih) (Mayer & Westbrook, 1983). Ih is typically seen as a slowly developing inward current activation upon hyperpolarization beyond the resting potential, which makes it a particularly useful mechanism for determining integrative behaviour of these neurones. Ih channels are almost as permeable to Na+ as to K+, blocked by Cs+ and ZD 7288, a specific blocker of Ih, but not strongly blocked by Ba2+ (Pape, 1996). An important and physiologically significant property of Ih channels is their ability to be regulated by neurotransmitters and metabolic stimuli (Ingram & Williams, 1994; Raes et al., 1997; Wang et al., 1997; Cardenas et al., 1999).
Because the modulation of Ih by local anaesthetics has not yet been examined, we studied the effect of lidocaine, mepivacaine, QX-314 and bupivacaine as well as its enantiomers on Ih in visually identified small DRG neurones. The experiments were performed on thin-slice preparations of young rat DRG by means of the patch-clamp technique, in order to record Ih from intact cells in which channel properties and densities had not been modified by enzymatic treatment.
Methods
Preparation
Experiments were performed with the patch-clamp technique (Hamill et al., 1981) on 200 μm thin slices prepared from DRG of 6- to 12-day-old rats as previously described (Safronov et al., 1996). Animals were rapidly decapitated and two or three DRGs from lower thoracic and lumbar regions were carefully cut out in ice-cold preparation solution which was bubbled with O2–CO2 (95–5%). The ganglia were desheathed using fine forceps and embedded in the preparation solution containing 2% (w v−1) agar cooled down to 39°C (Edwards et al., 1989; Takahashi, 1990). After solidification of the agar, small blocks containing the ganglia were cut out and glued to a glass stage fixed in the chamber of the tissue slicer. The ganglia were sliced in ice-cold preparation solution under continuous bubbling with O2–CO2 (95–5%). Thereafter slices were incubated for 30 min at 37°C.
The procedures used for animal decapitation were reported to the local veterinarian authority and are in accordance with the German guidelines.
Identification of neurones in DRG
In the tissue slice of the DRG, a dense population of DRG cells with apparently no connective tissue between the cells was seen under the microscope. The surface of the slice showed cells with a clean membrane. Among these were some small cells with a diameter of 15–25 μm on which we performed our experiments (Safronov et al., 1996).
Solutions
Preparation solution for preparing and maintaining the slices contained (mM): NaCl 115, KCl 5.6, CaCl2 2.2, MgCl2 1, glucose 11, NaH2PO4 1, and NaHCO3 25. The pH was 7.4 when bubbled with 95–5% mixture of O2–CO2 and the final [Na+] was 141 mM. In the experimental chamber the slices were perfused with low-Ca2+ solution (extracellular or bath solution), in order to suppress large-conductance Ca2+-activated K+ channels. This solution comprised the same ion concentrations as the preparation solution except that no CaCl2 was added. The experimental chamber with a volume of 0.6 ml was perfused continuously by extracellular solution at a rate of 2−3 ml min−1. The internal solution (Ki+) contained (mM): NaCl 5, KCl 144.4, MgCl2 1, EGTA 3, HEPES 10 (pH was adjusted to 7.3 by 10.6 mM KOH).
Bupivacaine–HCl and lidocaine–HCl were purchased from Sigma Chemical Co., mepivacaine was Scandicaine 4% from Astra Chemicals (Wedel, Germany). The hydrophilic quaternary derivative of lidocaine QX-314 was obtained from Alomone Labs (Jerusalem, Israel). Bupivacaine enantiomers were provided as crystalline HCl salts from Astra Pain Control (Södertälje, Sweden). The drugs were dissolved in distilled water to give 20-mM stock solutions.
Current recordings
The patch pipettes were pulled in two stages from borosilicate glass tubes (GC 150, Clark Electromedical Instruments, Pangbourne, U.K.). All pipettes were fire-polished directly before the experiments. The pipettes used for whole-cell recording had a resistance of 3–4 MΩ. The patch-clamp amplifiers used in all voltage- and current-clamp experiments were a List EPC-7 (Darmstadt, Germany) and Axopatch 200-B (Axon Instruments, Foster City, CA, U.S.A.). The effective corner frequency of the low-pass filter was 1 kHz. The frequency of digitization was at least twice that of the filter. The data were stored in a computer by using commercially available software (pCLAMP, Axon Instruments, Foster City, CA, U.S.A.). Offset potentials were nulled directly before formation of the seal. Liquid junction potential (<4 mV) was measured during the current-clamp procedure and was not corrected. Whole-cell capacitance and series resistance were corrected (usually 40–60%). No data were included where series resistance resulted in greater than 10 mV error in voltage command.
Hyperpolarization-activated inward cation currents were recorded in whole-cell patch-clamp mode from the somata of dorsal root ganglion neurones in extracellular solution. The pipettes were filled with Ki+ solution. Ih currents were activated from a holding potential of −80 to −160 mV in 10 mV steps. For concentration–effect experiments currents were recorded from a holding potential of −80 mV in hyperpolarizing 60 mV steps to −140 mV in the control and in the presence of local anaesthetics (Figure 4).
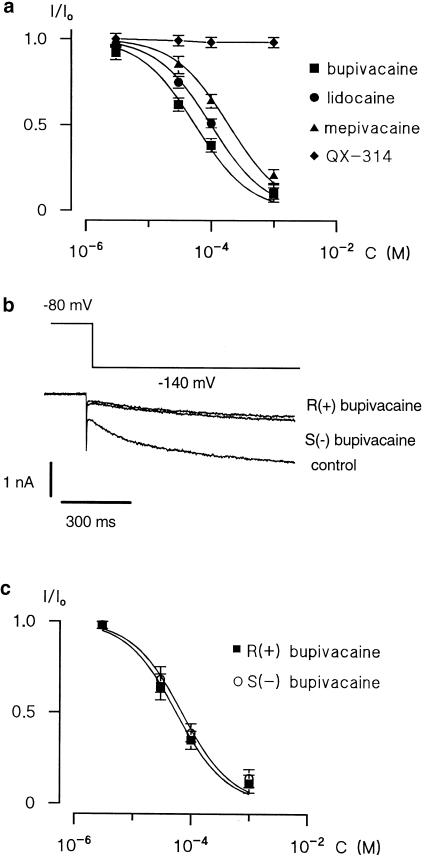
Figure 4.
Effect of local anaesthetics on Ih currents. (a) Concentration-dependence of Ih currents block by bupivacaine (square), lidocaine (circle), mepivacaine (triangle) and QX-314 (diamond). The data points were fitted by means of a nonlinear least-squares procedure using a standard isotherm (equation (1)). The data for the effect of QX-314 were fitted by eye. Error bars indicate±s.e. if exceeding symbol size. (b) The representative current traces shown here were recorded in the presence of 100 μM R (+) and S (−) bupivacaine. (c) Concentration-dependence of Ih current block by R (+) bupivacaine (open circle) and S (−) bupivacaine (filled circle). Curve represents fits of equation (1) to the data points. Error bars indicate±s.e. if exceeding symbol size.
For the comparison of the resting membrane potential (Em) before (control) and after application of bupivacaine, cells were held in current-clamp at their resting Em (without current injection). To compare the response to hyperpolarizing current pulses before (control) and after application of bupivacaine, we kept the membrane potential at the resting membrane potentials without bupivacaine by injecting sustained depolarizing currents through the recording electrode. Traces recorded in current-clamp mode were digitized with an interval of 0.1 ms.
After establishing a whole-cell patch, each slice was perfuse for at least 4–5 min with bath solution alone and with bath solution containing different local anaesthetic concentrations before data were acquired. Under these conditions the steady-state block was reached in all cases.
All experiments were carried out at a room temperature of 22–24°C.
Statistical analysis
The normalized amplitudes of Ih currents in concentration–effect curves were fitted by means of a nonlinear least-squares procedure using a standard isotherm:
where I is the current measured in the presence of a given drug concentration, I0 is the control current measured in the absence of the drug, c is the drug concentration, IC50 is the concentration giving a half-maximum effect and h is the Hill coefficient.
The present study is based on recordings from 57 DRG neurones. All numerical values are given as mean±standard error of the mean (s.e.m.). The parameters obtained by fitting the data points using a nonlinear least-squares procedure are given as mean±standard error (s.e.). Intergroup differences were assessed by analysis of variance with post hoc analysis using Fisher's least-significant difference test. P<0.05 was considered significant.
Result
Ih recording, preliminary experiments
In order to estimate the relative contributions of potassium selective and unspecific leakage conductances in small DRG neurones in the slice preparation the region of minimum membrane conductance was defined. For this purpose, the steady-state currents were measured at different potentials from +10 to −160 mV. By analysing each of the slope conductances the minimum of the membrane conductance was found to be about −80 mV. This voltage range was chosen in voltage-clamp experiments of this study as the holding potential to avoid the activation of depolarization-activated conductances (Safronov et al., 1996). The minimum conductance was decreased by 73% in extracellular solution containing additional 20 mM Cs+, 2 mM Ba2+ and 20 mM TEA suggesting that the greatest part of the conductance at −80 mV is due to delayed rectifier, inward rectifier and Ih currents.
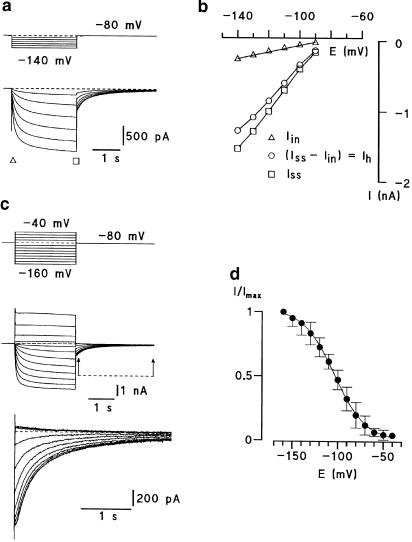
Ih was observed in 57 of 131 (44%) small DRG neurones, a result similar to that reported by Scroggs et al. and by Yagi et al. (Scroggs et al., 1994; Raes et al., 1998). Hyperpolarizing voltage commands elicited an initial current, referred to as the instantaneous current (Iin; Figure 1a, b, triangles), followed by the development of a slow inward current reaching a steady-state level at the end of the voltage command (Iss; Figure 1a, b, squares). The amplitude of Ih was given as the difference of the slow inward and instantaneous current (Ih=Iss−Iin; Figure 1b, circle). The amplitudes of the instantaneous current and the inward relaxation increased with increasing hyperpolarization. Figure 1c shows a typical tail current of a small DRG neurone. Current pulses positive to −60 mV evoked the same tail currents, indicating that no active Ih currents were observed at these potentials. Tail current amplitudes were normalized and plotted against the membrane potential. The activation curve of the Ih current rose between −60 and −160 mV with a potential of half-maximum activation (E50) of −103.8±0.7 mV and a steepness factor (k) of 17.4±0.5 mV (n=5) (Figure 1d).
Figure 1.
Hyperpolarizing-activated inward current (Ih) recording in dorsal root ganglion neurones. (a) Hyperpolarizing voltage commands elicited an initial current, referred to as the instantaneous currents (Iin, triangle) proceeded by a steep capacity current and followed by the development of a slow inward current reaching a steady-state level at the end of the voltage command (Iss, square). (b) Iin, Ih (circle) and Iss are plotted as a function of membrane potential. The amplitude of Ih was given as difference of the slow inward and instantaneous currents (Ih=Iss−Iin). The data were fitted by eye. (c) Activation curves of Ih current. Whole-cell recordings from de- and hyperpolarization-activated currents evoked from −80 mV to different test potentials. Tail-current relaxations are indicated by arrows and are shown at higher resolution in the lower sets of traces. (d) Normalized peak relaxation currents were fitted with the Boltzmann equation: 1/(1+exp(−(E−E50)/k)).
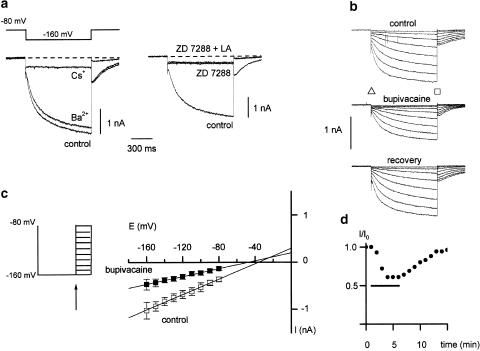
Figure 2a shows the pharmacological properties of the Ih current. The Ih current was sensitive to an extracellular concentration of 2 mM Cs+ (n=9) but almost insensitive to extracellular Ba2+ (2 mM; n=9). Externally applied 100 μM ZD 7288, a selective blocker of Ih channels, blocked the current (n=10). No additional effect was seen after application of 1 mM bupivacaine (LA) in the presence of ZD 7288 (Figure 2a).
Figure 2.
Pharmacology of Ih current. (a) Effect of 2 mM Ba2+, 2 mM Cs+ (left) and 100 μM ZD7288 and 1 mM bupivacaine in the presence of ZD 7288 on Ih (right panel). (b) Response of Ih currents in dorsal root ganglion neurones to bupivacaine. Traces demonstrate whole-cell Ih currents in control and after addition of a local anaesthetic (LA, extracellular 100 μM bupivacaine). The amplitude of Ih was given as difference of the slow inward current (Iin, triangle) and the instantaneous current (Iss, square), with (Ih=Iss−Iin). (c) Estimation of the reversal potential of Ih current in control (open symbols) and after application of 100 μM bupivacaine (filled symbols, n=4). The membrane was hyperpolarized for 1 s to −160 mV, where Ih is fully activated, and is stepped back to different potentials (left). The same protocol was repeated for each neuron in the presence of extracellular 2 mM Cs+ in order to eliminate the contribution of other currents and was subtracted off-line. The instantaneous tail current amplitude (indicated by arrow) of the caesium-sensitive traces were plotted with respect to membrane potential and a linear regression was performed (right panel). (d) Plot of normalized Ih amplitude at steps to −140 mV from the small DRG neuron in (b), in response to bupivacaine. The horizontal bar indicates the period of drug application.
Effects of local anaesthetics on Ih
Ih was sensitive to externally applied bupivacaine. Figure 2b shows the response of a small DRG neurone in voltage-clamp mode. Hyperpolarizing voltage steps evoked both Iin and Ih in this representative cell. About 1 min after changing the extracellular solution to that containing 100 μM bupivacaine, the magnitude of the inward current evoked by hyperpolarization declined slowly, reaching the lowest level after about 4 min (Figure 2d). The recovery was slow and varied from cell to cell: 90% recovery usually occurred between 6 and 12 min after the bupivacaine-containing solution was replaced by extracellular solution.
To estimate the reversal potential of the Ih current in control and after application of the local anaesthetic bupivacaine we used the method described by Doan and Raes (Raes et al., 1998; Doan & Kunze, 1999) (Figure 2c). Bupivacaine 100 μM reversibly reduced the amplitude of Ih (n=4; filled symbols). The extrapolation of the linear regression to 0 pA gave a projected reversal potential of Ih of −35 mV in control and −43 mV after application of bupivacaine (Figure 2c).
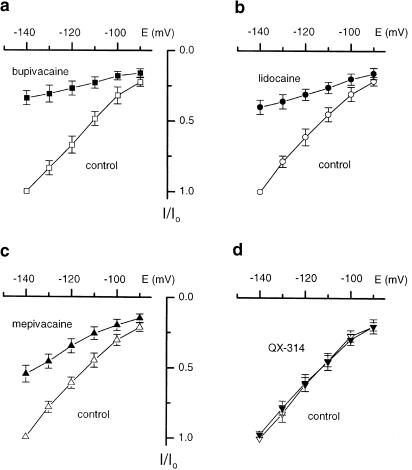
Figure 3 shows the effects of externally applied 100 μM bupivacaine, lidocaine, mepivacaine and QX-314 on Ih. Externally applied QX-314, a hydrophilic quaternary derivative of lidocaine, which permanently carries a positive charge and cannot penetrate the membrane, did not reduce the amplitude of Ih currents at all. The effect of the local anaesthetics on Ih current was reversible.
Figure 3.
Averaged whole-cell I–V plots of Ih currents measured as a difference between the steady-state (Iss) and instantaneous (Iin) currents recorded on stepping to the membrane potential (E), plotted against the membrane potential during the step command. Currents were recorded in extracellular solution (control) and after exposure to 100 μM bupivacaine (a; n=6), 100 μM lidocaine (b; n=6), 100 μM mepivacaine (c; n=6) and 100 μM QX-314 (d; n=6) and normalized to the control Ih current (I/I0). The data were fitted by eye. Values are mean±s.e.
In the following experiments, we estimated the concentration-dependent reduction of Ih current by bupivacaine, lidocaine, mepivacaine and QX-314. Local anaesthetics were applied to small DRG neurones at increasing concentrations (3 μM–1 mM). The amplitude of the Ih current produced by each concentration of the local anaesthetics (I) was normalized to the maximal Ih current in the control (I0, Figure 4a). Nonlinear least-squares fitting of equation (1) to the data points was performed to evaluate half-maximum inhibiting concentrations (IC50). The best fitting was with a Hill coefficient of 1, giving IC50 of 55±5 μM for bupivacaine (n=6), 99±4 μM for lidocaine (n=6) and 190±15 μM for mepivacaine (n=6), respectively. A good quality of fitting obtained with a Hill coefficient of 1 indicated a one-to-one interaction between the channel and the local anaesthetic molecule. The IC50 values of bupivacaine, lidocaine and mepivacaine were significantly different (ANOVA: P<0.05). Blockade of Ih was strongest by bupivacaine (Fisher's test: P<0.01) and weakest by mepivacaine (Fisher's test: P<0.01).
Lack of stereoselective effect of the bupivacaine enantiomers on Ih current
The enantiomers R (+) and S (−) bupivacaine reversibly blocked the Ih current (Figure 4b and c) in a concentration-dependent manner. Concentration-inhibition experiments gave IC50 values of 55±6 μM for R (+) bupivacaine (n=6) and 67±8 μM for S (−) bupivacaine (n=6), revealing a stereopotency ratio (+/−) of 1.21, which indicates that bupivacaine enantiomers do not exert stereoselective effects on the Ih current (ANOVA: P>0.05).
Functional relevance of the Ih current block by bupivacaine
Current-clamp experiments were performed in low-Ca2+ experimental solution, in order to suppress large-conductance Ca2+-activated K+ channels. The mean resting potential measured in small DRG neurones perfused with extracellular solution was −64.3±0.7 mV (57 cells), a result similar to that reported by other groups (Scroggs et al., 1994; Raes et al., 1998; Yagi & Sumino, 1998).
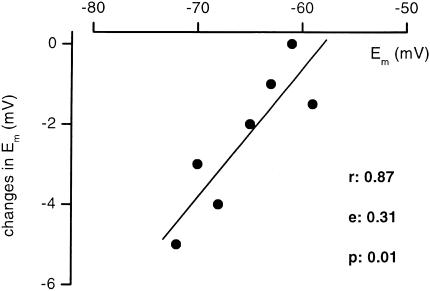
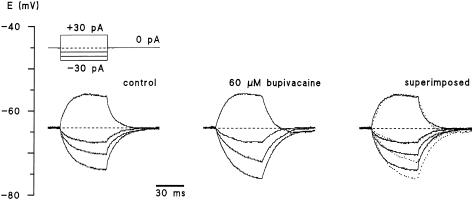
Neurones responded to 60 μM bupivacaine with slight hyperpolarization of 2.4±0.6 mV (n=7). Figure 5 depicts the dependence of the change in membrane potential on the resting membrane potential. Correlation coefficient (r), slope factor and P of the regression line were 0.87, 0.31 and 0.01, respectively. Next, the effects of bupivacaine on the voltage response of DRG neurones to injected current pulses were investigated (Figure 6). The membrane stimulation in current-clamp mode by low-amplitude current pulses of +30, −10, −20 and −30 pA showed an increase in the response to hyperpolarization in the presence of bupivacaine, whereas the response to depolarization was not affected (n=7).
Figure 5.
Changes in membrane potential after application of 60 μM bupivacaine plotted against resting membrane potentials of the neurones.
Figure 6.
Effect of 60 μM bupivacaine on the response to current injection of a dorsal root ganglion neurone. The resting potential of this neurone was −64 mV. Membrane stimulation in current-clamp mode by low-amplitude current pulses of +30, −10, −20 and −30 pA in the absence and presence of 60 μM bupivacaine. The representative traces are shown superimposed on the right (control, continuous line; bupivacaine, dotted line; n=6). Membrane potential was kept at −64 mV in both solutions. Note that bupivacaine abolishes the rectification component and increases the voltage displacement caused by hyperpolarizing current flow.
Discussion
The present experiments show that local anaesthetics block the hyperpolarization-activated inward current in spinal dorsal root ganglion neurones of young rats. This effect is present at clinically relevant concentrations.
The biophysical and pharmacological properties of Ih in our slice preparation are consistent with the characteristics of Ih reported in DRG neurones of mouse embryos in tissue culture (Mayer & Westbrook, 1983) and many other cell types (DiFrancesco et al., 1986; McCormick & Pape, 1990; Hwa & Avoli, 1991; Maccaferri et al., 1993; Wang et al., 1997; Takigawa et al., 1998; Cardenas et al., 1999). These observations are of special interest because the counterbalancing actions of delayed-rectifier outward and hyperpolarization-activated inward potassium conductances determine the resting potential of DRG neurones (Mayer & Westbrook, 1983). Blockade of Ih may thus have a strong impact on excitability of these neurones and many other cells.
The mechanism underlying spinal and epidural anaesthesia by local anaesthetics is generally explained by a blockade of the generation and conduction of nerve impulses by inhibiting ionic current through voltage-gated Na+ channels in the cell membrane (Butterworth & Strichartz, 1990). However, an increasing number of studies additionally describes the action of local anaesthetics on different types of voltage-gated and background K+ channels (Koh et al., 1992; Brau et al., 1995; Olschewski et al., 1996; Leonoudakis et al., 1998; Olschewski et al., 1998; Kindler et al., 1999; Nau et al., 1999; Buckler et al., 2000; Hille, 2001; Komai & McDowell, 2001; Meadows & Randall, 2001), which modulate the resting membrane potential and herewith excitability and firing behaviour of the cell (Connor & Stevens, 1971; Llinas, 1988; Christie, 1995; Safronov, 1999; Hess & El Manira, 2001; Hille, 2001; Olschewski et al., 2001). It could be speculated that the effects of local anaesthetics on small DRG neurones included also other types of conductance like Ih explaining part of their effect on membrane potential and excitability of these neurones.
We found that the externally applied local anaesthetics bupivacaine, lidocaine and mepivacaine lead to reversible and concentration-dependent inhibition of Ih in small DRG neurones. The block exerted by the substances was concentration-dependent but not stereoselective. The effect of local anaesthetics on Ih was largely voltage-independent. The half-maximum inhibiting concentrations were 55, 99 and 190 μM for bupivacaine, lidocaine and mepivacaine, respectively and reflect the potency of analgesia of these drugs. These concentrations are clinically relevant for spinal and epidural anaesthesia (Dennhardt & Konder, 1983; Biscoping, 1986) and they are in a very similar concentration range as for Na+ channel inhibition (Scholz et al., 1998; Brau et al., 2000). In contrast to Na+ current block, this block depends slightly on lipophilicity, probably because interaction between the local anaesthetic molecule and the binding site on the Ih channel is less hydrophobic. Externally applied QX-314, a quaternary derivative of lidocaine, which permanently carries a positive charge and cannot penetrate the membrane, failed to block Ih currents indicating that the binding site on the Ih channel can only be accessed from the internal site. In rat neurocortical neurones it has been reported that following intracellular injection of QX-314, the anomalous inward rectification was completely abolished (Hwa & Avoli, 1991).
Stereoselective interactions of local anaesthetics with different ion channels are of interest because they can reveal three-dimensional relationships of the drug–receptor interaction. The effect of several local anaesthetics on Na+ channels, because of their key role in regional anaesthesia, has been extensively investigated during past decades. In frog peripheral nerve a weak stereoselectivity for blocking of the compound action potential by bupivacaine was demonstrated, revealing a stereopotency ratio R (+)/S (−) of 1.6 (Lee-Son et al., 1992). Similar sensitivity and stereoselectivity of Na+ current to bupivacaine enantiomers was reported by Nau et al. (1999). In addition, in GH3 cells R (+) bupivacaine was shown to be 1.6-fold more potent in inhibition of Na+ channels (Wang & Wang, 1992). In cardiac Na+ channels the block of the inactivated state showed a moderate stereoselectivity with a ratio of 1.7 for R (+) bupivacaine. Interactions of bupivacaine enantiomers with the open and resting states were not stereoselective (Valenzuela et al., 1995b). In TTX-resistant Na+ channels of DRG neurones no stereoselectivity of the inhibition of piperidine local anaesthetics mepivacaine, ropivacaine and bupivacaine was found (Brau et al., 2000). Our knowledge about the effects of local anaesthetic stereoisomers on K+ channels is limited. In a few studies, effects of bupivacaine stereoisomers were investigated on voltage-dependent K+ channels. However, stereoselective bupivacaine block has only been demonstrated on hKv1.5 channels, but not on Kv2.1 or Kv4.3 (Valenzuela et al., 1995a; Franqueza et al., 1997; 1999). Recently, it has been shown that block of the background flicker K+ channel in peripheral nerve fibres has a very high stereoselectivity ratio for bupivacaine (Nau et al., 1999). Our results show that Ih is not stereoselectively blocked by bupivacaine which indicates that the exact three-dimensional structure of the channel may be of minor importance for drug binding. Clinically, peripheral nerve block by bupivacaine also shows little stereoselectivity which is in agreement with our findings of Ih block. S (−) bupivacaine is preferred in clinical use because it has fewer side effects on heart and brain function.
Inward currents activated by hyperpolarizing voltage steps beyond the resting membrane potential play an important role in stabilizing the membrane potential in DRG neurones. Ih counterbalances prolonged membrane hyperpolarization produced by Ca2+ activated K+ channels which are activated by Ca2+ influx during the action potential (Mayer & Westbrook, 1983). Under current-clamp conditions membrane potentials evoked by hyperpolarizing current injections were more negative in 60 μM bupivacaine than in control solution (Figure 6). This is explained by the blockade of Ih channels which results in an increase in membrane resistance. Takigawa and co-workers used ZD 7288, a selective blocker of Ih to demonstrate the presence of a voltage-dependent conductance activated by membrane hyperpolarization in mammalian peripheral nerve fibres (Takigawa et al., 1998). Activation of Ih at the resting membrane potential of small DRG neurones depolarizes the membrane potential to the reversal potential of Ih which is about −35 mV. Inhibition of Ih by local anaesthetics at the resting level will consequently lead to less depolarization when Ih is activated.
In our experiments we used a low extracellular Ca2+ solution in order to suppress large-conductance Ca2+-activated K+ channels. This implies some limitations to our interpretation since it may have caused a shift of the voltage-dependent gating of Ih, as described in the lobster stretch receptor neurones (Edman & Grampp, 1991; Hille, 2001). The negative shift in Ih activation would increase the open probability of Ih of small DRG neurones and hyperpolarize the membrane potential. Thus, the membrane potentials as given in this study may not resemble the true membrane potential. Nevertheless, this would not change the interpretation of the principle local anaesthetics-induced effects on Ih and membrane potential.
Inhibition of Ih by local anaesthetics may contribute to the reduction of excitability in dorsal root ganglia and in peripheral sensory nerve fibres by additive effects. First, at the concentration reported in our study local anaesthetics partly block TTX-sensitive and TTX-2 resistant Na+ channels elevating the firing threshold and thus reducing firing frequency of action potentials evoked by a depolarizing current (Roy & Narahashi, 1992; Scholz et al., 1998; Scholz & Vogel, 2000). Second, local anaesthetic block of Ih hyperpolarizes the membrane potential and therefore shifts it even further from the firing threshold reducing excitability. The blockade of Ih by local anaesthetics therefore may play an important role in the complex mechanisms of drug action during epidural and spinal anaesthesia.
Acknowledgments
This study was supported in part by the Deutsche Forschungsgemeinschaft Grant Vo188/19-1, Bonn, Germany, by the B Braun-Stiftung and by the Justus-Liebig-University Gießen. The authors thank Mary Kay Steen-Muller for carefully reviewing the manuscript and B. Agari and O. Becker for excellent technical assistance.
Abbreviations
- DRG
dorsal root ganglion
- EGTA
ethylene glycol-bis(β-aminoethyle ether) N,N,N′,N′-tetraacetic acid
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)
- IC50
half-maximal inhibiting concentration
- Ih
hyperpolarization-activated inward current
- TEA
tetraethylammonium chloride
- TTX
tetrodotoxin
- ZD 7288
4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)pyrimidinium chloride
References
- BISCOPING J. Effect of glucose concentration in bupivacaine solutions on the distribution of local anesthetics in cerebrospinal fluid during spinal anesthesia. Reg. Anesth. 1986;9:9–14. [PubMed] [Google Scholar]
- BRAU M.E., BRANITZKI P., OLSCHEWSKI A., VOGEL W., HEMPELMANN G. Block of neuronal tetrodotoxin-resistant Na+ currents by stereoisomers of piperidine local anesthetics. Anesth. Analg. 2000;91:1499–1505. doi: 10.1097/00000539-200012000-00038. [DOI] [PubMed] [Google Scholar]
- BRAU M.E., NAU C., HEMPELMANN G., VOGEL W. Local anesthetics potently block a potential insensitive potassium channel in myelinated nerve. J. Gen. Physiol. 1995;105:485–505. doi: 10.1085/jgp.105.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKLER K.J., WILLIAMS B.A., HONORE E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERWORTH J.F., STRICHARTZ G.R. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- CARDENAS C.G., MAR L.P., VYSOKANOV A.V., ARNOLD P.B., CARDENAS L.M., SURMEIER D.J., SCROGGS R.S. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J. Physiol. 1999;518:507–523. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIE M.J. Molecular and functional diversity of K+ channels. Clin. Exp. Pharmacol. Physiol. 1995;22:944–951. doi: 10.1111/j.1440-1681.1995.tb02331.x. [DOI] [PubMed] [Google Scholar]
- CONNOR J.A., STEVENS C.F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J. Physiol. 1971;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNHARDT R., KONDER H. Blood and cerebrospinal fluid levels of bupivacaine in spinal anesthesia. Reg. Anesth. 1983;6:72–75. [PubMed] [Google Scholar]
- DIFRANCESCO D., FERRONI A., MAZZANTI M., TROMBA C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J. Physiol. 1986;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOAN T.N., KUNZE D.L. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J. Physiol. 1999;514:125–138. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMAN A., GRAMPP W. Ion (H+, Ca2+, Co2+) and temperature effects on a hyperpolarization-activated membrane current in the lobster stretch receptor neurone. Acta Physiol. Scand. 1991;141:251–261. doi: 10.1111/j.1748-1716.1991.tb09075.x. [DOI] [PubMed] [Google Scholar]
- EDWARDS F.A., KONNERTH A., SAKMANN B., TAKAHASHI T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- FRANQUEZA L., LONGOBARDO M., VICENTE J., DELPON E., TAMKUN M.M., TAMARGO J., SNYDERS D.J., VALENZUELA C. Molecular determinants of stereoselective bupivacaine block of hKv1.5 channels. Circ. Res. 1997;81:1053–1064. doi: 10.1161/01.res.81.6.1053. [DOI] [PubMed] [Google Scholar]
- FRANQUEZA L., VALENZUELA C., ECK J., TAMKUN M.M., TAMARGO J., SNYDERS D.J. Functional expression of an inactivating potassium channel (Kv4.3) in a mammalian cell line. Cardiovasc. Res. 1999;41:212–219. doi: 10.1016/s0008-6363(98)00220-x. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARPER A.A., LAWSON S.N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J. Physiol. 1985;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS D., EL MANIRA A. Characterization of a high-voltage-activated IA current with a role in spike timing and locomotor pattern generation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5276–5281. doi: 10.1073/pnas.091096198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLE B. Ion Channels of Excitable Membranes 2001MA: Sinauer Associates Inc. Publishers; 3rd edn., ed. Hille, B. Sunderland [Google Scholar]
- HWA G.G., AVOLI M. Hyperpolarizing inward rectification in rat neocortical neurons located in the superficial layers. Neurosci. Lett. 1991;124:65–68. doi: 10.1016/0304-3940(91)90823-c. [DOI] [PubMed] [Google Scholar]
- INGRAM S.L., WILLIAMS J.T. Opioid inhibition of Ih via adenylyl cyclase. Neuron. 1994;13:179–186. doi: 10.1016/0896-6273(94)90468-5. [DOI] [PubMed] [Google Scholar]
- KINDLER C.H., YOST C.S., GRAY A.T. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 1999;90:1092–1102. doi: 10.1097/00000542-199904000-00024. [DOI] [PubMed] [Google Scholar]
- KOH D.S., JONAS P., BRAU M.E., VOGEL W. A TEA-insensitive flickering potassium channel active around the resting potential in myelinated nerve. J. Membr. Biol. 1992;130:149–162. doi: 10.1007/BF00231893. [DOI] [PubMed] [Google Scholar]
- KOMAI H., MCDOWELL T.S. Local anesthetic inhibition of voltage-activated potassium currents in rat dorsal root ganglion neurons. Anesthesiology. 2001;94:1089–1095. doi: 10.1097/00000542-200106000-00025. [DOI] [PubMed] [Google Scholar]
- LEE-SON S., WANG G.K., CONCUS A., CRILL E., STRICHARTZ G. Stereoselective inhibition of neuronal sodium channels by local anesthetics. Evidence for two sites of action. Anesthesiology. 1992;77:324–335. doi: 10.1097/00000542-199208000-00016. [DOI] [PubMed] [Google Scholar]
- LEONOUDAKIS D., GRAY A.T., WINEGAR B.D., KINDLER C.H., HARADA M., TAYLOR D.M., CHAVEZ R.A., FORSAYETH J.R., YOST C.S. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J. Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLINAS R.R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- MACCAFERRI G., MANGONI M., LAZZARI A., DIFRANCESCO D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J. Neurophysiol. 1993;69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- MAYER M.L., WESTBROOK G.L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J. Physiol. 1983;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORMICK D.A., PAPE H.C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEADOWS H.J., RANDALL A.D. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology. 2001;40:551–559. doi: 10.1016/s0028-3908(00)00189-1. [DOI] [PubMed] [Google Scholar]
- NAU C., VOGEL W., HEMPELMANN G., BRAU M.E. Stereoselectivity of bupivacaine in local anesthetic-sensitive ion channels of peripheral nerve. Anesthesiology. 1999;91:786–795. doi: 10.1097/00000542-199909000-00031. [DOI] [PubMed] [Google Scholar]
- OLSCHEWSKI A., BRAU M.E., OLSCHEWSKI H., HEMPELMANN G., VOGEL W. ATP-dependent potassium channel in rat cardiomyocytes is blocked by lidocaine. Possible impact on the antiarrhythmic action of lidocaine. Circulation. 1996;93:656–659. doi: 10.1161/01.cir.93.4.656. [DOI] [PubMed] [Google Scholar]
- OLSCHEWSKI A., HEMPELMANN G., VOGEL W., SAFRONOV B.V. Blockade of Na+ and K+ currents by local anesthetics in the dorsal horn neurons of the spinal cord. Anesthesiology. 1998;88:172–179. doi: 10.1097/00000542-199801000-00025. [DOI] [PubMed] [Google Scholar]
- OLSCHEWSKI A., HEMPELMANN G., VOGEL W., SAFRONOV B.V. Suppression of potassium conductance by droperidol has influence on excitability of spinal sensory neurons. Anesthesiology. 2001;94:280–289. doi: 10.1097/00000542-200102000-00018. [DOI] [PubMed] [Google Scholar]
- PAPE H.C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- RAES A., VAN DE VIJVER G., GOETHALS M., VAN BOGAERT P.P. Use-dependent block of Ih in mouse dorsal root ganglion neurons by sinus node inhibitors. Br. J. Pharmacol. 1998;125:741–750. doi: 10.1038/sj.bjp.0702153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAES A., WANG Z., VAN DEN BERG R.J., GOETHALS M., VAN D., VAN BOGAERT P.P. Effect of cAMP and ATP on the hyperpolarization-activated current in mouse dorsal root ganglion neurons. Pflügers Arch. 1997;434:543–550. doi: 10.1007/s004240050434. [DOI] [PubMed] [Google Scholar]
- ROY M.L., NARAHASHI T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J. Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFRONOV B.V. Spatial distribution of Na+ and K+ channels in spinal dorsal horn neurones: role of the soma, axon and dendrites in spike generation. Prog. Neurobiol. 1999;59:217–241. doi: 10.1016/s0301-0082(98)00051-3. [DOI] [PubMed] [Google Scholar]
- SAFRONOV B.V., BISCHOFF U., VOGEL W. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. J. Physiol. 1996;493:393–408. doi: 10.1113/jphysiol.1996.sp021391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOLZ A., KUBOYAMA N., HEMPELMANN G., VOGEL W. Complex blockade of TTX-resistant Na+ currents by lidocaine and bupivacaine reduce firing frequency in DRG neurons. J. Neurophysiol. 1998;79:1746–1754. doi: 10.1152/jn.1998.79.4.1746. [DOI] [PubMed] [Google Scholar]
- SCHOLZ A., VOGEL W. Tetrodotoxin-resistant action potentials in dorsal root ganglion neurons are blocked by local anesthetics. Pain. 2000;89:47–52. doi: 10.1016/S0304-3959(00)00345-6. [DOI] [PubMed] [Google Scholar]
- SCROGGS R.S., TODOROVIC S.M., ANDERSON E.G., FOX A.P. Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. J. Neurophysiol. 1994;71:271–279. doi: 10.1152/jn.1994.71.1.271. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J. Physiol. 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKIGAWA T., ALZHEIMER C., QUASTHOFF S., GRAFE P. A special blocker reveals the presence and function of the hyperpolarization-activated cation current Ih in peripheral mammalian nerve fibres. Neuroscience. 1998;82:631–634. doi: 10.1016/s0306-4522(97)00383-7. [DOI] [PubMed] [Google Scholar]
- VALENZUELA C., DELPON E., TAMKUN M.M., TAMARGO J., SNYDERS D.J. Stereoselective block of a human cardiac potassium channel (Kv1.5) by bupivacaine enantiomers. Biophys. J. 1995a;69:418–427. doi: 10.1016/S0006-3495(95)79914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENZUELA C., SNYDERS D.J., BENNETT P.B., TAMARGO J., HONDEGHEM L.M. Stereoselective block of cardiac sodium channels by bupivacaine in guinea pig ventricular myocytes. Circulation. 1995b;92:3014–3024. doi: 10.1161/01.cir.92.10.3014. [DOI] [PubMed] [Google Scholar]
- WANG G.K., WANG S.Y. Altered stereoselectivity of cocaine and bupivacaine isomers in normal and batrachotoxin-modified Na+ channels. J. Gen. Physiol. 1992;100:1003–1020. doi: 10.1085/jgp.100.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Z., VAN DEN BERG R.J., YPEY D.L. Hyperpolarization-activated currents in the growth cone and soma of neonatal rat dorsal root ganglion neurons in culture. J. Neurophysiol. 1997;78:177–186. doi: 10.1152/jn.1997.78.1.177. [DOI] [PubMed] [Google Scholar]
- YAGI J., SUMINO R. Inhibition of a hyperpolarization-activated current by clonidine in rat dorsal root ganglion neurons. J. Neurophysiol. 1998;80:1094–1104. doi: 10.1152/jn.1998.80.3.1094. [DOI] [PubMed] [Google Scholar]