Abstract
The 2-(p-chlorophenoxy)propionic acid (CPP) modulates in a stereoselective manner the macroscopic chloride conductance (gCl), the electrical parameter sustained by the CLC-1 channel, of skeletal muscle. In order to determine the structural requirements for modulating native gCl and to identify high-affinity ligands, the effects of newly synthesised CPP analogues have been evaluated on gCl of rat EDL muscle fibres by means of the two-microelectrode current-clamp technique.
Each type of the following independent modification of CPP structure led to a three- to 10-fold decrease or to a complete lack of gCl-blocking activity: replacement of the electron-attractive chlorine atom of the aromatic ring, substitution of the oxygen atom of the phenoxy group, modification at the chiral centre and substitution of the carboxylic function with a phosphonate one.
The analogues bearing a second chlorophenoxy group on the asymmetric carbon atom showed a significant gCl-blocking activity. Similar to racemate CPP, the analogue with this group, spaced by an alkyl chain formed by three methylenic groups, blocked gCl by 45% at 100 μM.
These latter derivatives were tested on heterelogously expressed CLC-1 performing inside-out patch-clamp recordings to further define how interaction between drug and channel protein could take place. Depending on the exact chemical nature of modification, these derivatives strongly blocked CLC-1 with KD values at −140 mV ranging from about 4 to 180 μM.
In conclusion, we identified four molecular determinants pivotal for the interaction with the binding site on muscle CLC-1 channels: (a) the carboxylic group that confers the optimal acidity and the negative charge; (b) the chlorophenoxy moiety that might interact with a hydrophobic pocket; (c) the chiral centre that allows the proper spatial disposition of the molecule; (d) an additional phenoxy group that remarkably stabilises the binding by interacting with a second hydrophobic pocket.
Keywords: Skeletal muscle, CLC-1 chloride channel, chloride conductance, clofibric acid analogues
Introduction
CLC-1 is a voltage-dependent Cl− channel selectively expressed in skeletal muscle, where it is responsible for the maintenance of the membrane potential at rest and consequently for the electrical stability of the sarcolemma. Genetic defects of CLC-1 lead to inheritable myotonia, a disease characterised by abnormal membrane hyperexcitability and muscle stiffness, in mice, goats and humans (Steinmeyer et al., 1991; Koch et al., 1992). Both the small single-channel conductance (Pusch et al., 1994) and the probable localisation of the channel in the transverse tubules (Chua & Betz, 1991) do not allow the study of this muscle Cl− channel by direct inspection with the patch-clamp technique on native fibres. The measurement of the macroscopic Cl− conductance (gCl), the electrical parameter sustained by CLC-1, allows to gain insight into the activity and pharmacology of the Cl− channel in its native environment.
The 2-(p-chlorophenoxy) propionic acid (CPP) and the 9-anthracenecarboxylic acid (9-AC) are two specific ligands of the CLC-1 channel (Jentsch et al., 2002; Pusch et al., 2002). Pioneering studies conducted in our laboratory on native skeletal muscle have demonstrated that CPP, having an asymmetric carbon atom α to the carboxylic group, is capable of stereoselectively modulating the native gCl (Conte Camerino et al., 1988; De Luca et al., 1992b). In particular, S(−)CPP blocks gCl in a concentration-dependent manner with a half-maximal concentration in the range of 10–20 μM, whereas the R(+) enantiomer produces a typical biphasic effect, enhancing gCl slightly at low concentrations (1–10 μM) and decreasing it at higher concentrations (>10 μM). The opposite effects produced by the individual enantiomers allowed us to hypothesise the presence of two different receptors or binding sites able, respectively, to enhance and decrease channel activity. The mechanism of the inhibition mediated by CPP has been studied in great detail using cellular lines or Xenopus oocytes as heterologous expression systems (Aromataris et al., 1999; Pusch et al., 2000). S(−)CPP blocks CLC-1 current in an apparently complex voltage-dependent manner and interferes with channel gating acting from the intracellular side. In particular, as demonstrated by single-channel recording of homologous CLC-0 chloride currents, S(−)CPP inhibits the individual protopores of the double-barrelled channel by binding to the pore preferentially when the channel is closed (Pusch et al., 2001). Furthermore, CPP is a specific ligand for the muscle CLC-1 channel, being much less effective on the ubiquitous CLC-2 channel and completely ineffective on renal CLC-5 and CLC-K channels from the extracellular side (Pusch et al., 2000; Liantonio et al., 2002). The phosphorylation of the CLC-1 protein by Ca-dependent protein kinase C (PKC) modulates channel activity in native skeletal muscle and in heterologous expression systems (De Luca et al., 1998; Rosenbohm et al., 1999). Furthermore, the sensitivity of CLC-1 to CPP enantiomers changes with the PKC-dependent phosphorylation state of the channel protein (De Luca et al., 1994; 1992a). These findings corroborate the idea that the measurement of gCl of native skeletal muscle fibres represents a useful system to obtain information about the modulation of CLC-1 activity, preserving the mechanisms of biochemical constituents that are fundamental for the channel physiology. On the other hand, taking into account the intracellular localisation of the hypothetical binding site of CPP (Pusch et al., 2000; 2001), inside-out patch-clamp recordings on heterologously expressed CLC-1 channel allow to study the interaction between the drugs and the amino-acid residues involved in the binding site independently from the capability of the molecule to cross the plasma membrane.
The stereoselective effect of CPP and the reduced potency of clofibric acid and p-chlorophenoxyacetic acid (CPA), two achiral CPP-like compounds, in blocking native gCl (Conte Camerino et al., 1988; Loiodice et al., 1993), suggest that the asymmetric carbon atom of the drug molecule represents an important pharmacophore moiety. In order to determine the molecular requisites for the modulation of the native gCl and consequently the activity of CLC-1 channel, and with the goal of identifying high-affinity ligands, we performed a structure–activity relationship study by synthesising and evaluating the effects of a series of CPP derivatives (Figure 1). We initially screened the CPP-like compounds on the native chloride conductance of rat skeletal muscle using the two-microelectrode current-clamp technique. All tested derivatives resulted less potent with respect to CPP. Taking into account that membrane permeability can be a limiting step for the effect on gCl, the derivatives having physical–chemical properties different from that of CPP, as the phosphonate derivatives and bis-phenoxy derivatives (N.23 and N.25–33), were also tested on heterologously expressed CLC-1 performing inside-out patch-clamp recordings. This approach allowed to better define the interaction between drug and channel protein, corroborating a high-affinity interaction of the bis-phenoxy derivatives, in spite of their limited permeability.
Figure 1.
Chemical structures of different analogues of CPP.
Methods
Experimental procedure of muscle preparation
Adult male Wistar rats of 350–400 g were used for the experiments. The electrophysiological experiments were made on isolated extensor digitorum longus (EDL) muscle. The muscle was removed under urethane anaesthesia (1.2 g kg−1, i.p.). Soon after the biopsy, the EDL muscle was stretched to about 1.5 times its resting length on a 3 mm plastic rod in a temperature-controlled muscle chamber at 30°C and perfused with a physiological solution (see below) in the absence and in the presence of tested compounds (De Luca et al., 1992b).
Measurements of macroscopic Cl− conductance on native muscle fibres
The macroscopic Cl− conductance (gCl) of EDL muscle fibres in the absence and in the presence of the tested compounds was calculated from the cable parameters, and in particular from the membrane resistance (Rm) values, measured by standard cable analysis with the two-intracellular-microelectrode technique. In brief, a voltage-sensitive microelectrode (3 M KCl) was used to measure the membrane potential and the voltage deflection (electrotonic potential), monitored at two distances (0.5 mm and about 1 mm), in response to a hyperpolarising square wave current pulse passed through a second electrode (2 M Kcitrate). In any experimental condition, the fibres sampled for recording of the cable parameters had membrane potential values ranging from −65 to −75 mV; in this condition, no electronic setting of potential was necessary (Bryant & Conte Camerino, 1991). The bath electrode was a salt bridge made with 3 M KCl in agar. Current pulse generation, acquisition of the voltage records and calculation of fibre constants (fibre diameter, membrane capacitance and membrane resistance) were carried out under computer control, as detailed elsewhere (Bryant & Conte Camerino, 1991; De Luca et al., 1992b; 1998). In each fibre, the total membrane conductance (gm) was 1/Rm in the normal physiological solution, whereas potassium conductance (gK) was 1/Rm in the Cl−-free physiological solution. The mean gCl was calculated as the mean gm minus the mean gK (De Luca et al., 1992a, 1992b). The data are expressed as mean±standard error of the mean (s.e.m.). The s.e.m. for gCl was calculated as previously described (Green & Margerison, 1978; De Luca et al., 1992a, 1992b). Significance between groups of means was evaluated by Student's unpaired t-test.
Expression of CLC-1 in Xenopus laevis oocytes
Expression of human CLC-1 channel in Xenopus oocytes was performed as described previously (Pusch et al., 2000). Patch-clamp measurements were acquired at 18±1°C using the inside-out configuration with an EPC-7 amplifier (HEKA, Lambrecht, Germany) and the following pulse protocol: from a holding potential of 0 mV, after a prepulse to +60 mV for 100 ms, the voltage was stepped to various test values (from −140 to +60 mV in 20 mV increments) for 200 ms and followed by a constant tail voltage to −140 mV. Apparent dissociation constants, KD, were determined by calculating the ratio of the steady-state current in the presence and absence of the drug and fitting the ratios at a fixed voltage by using the equation: I(c)/I(0)=1/(1+c/KD), where c is the concentration. Errors in figures are indicated as s.e.m.
Solutions and drugs
For measurements in native rat skeletal muscle fibres, the normal physiological solution contained: 148 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 0.44 mM NaH2PO4, 12 mM NaHCO3 and 5.5 mM glucose. The Cl−-free solution was made by equimolar substitution of methylsulphate salt for NaCl and KCl and nitrate salts for CaCl2 and MgCl2. The physiological solution was continuously bubbled with 95% O2 and 5% CO2, pH 7.2.
For patch-clamp measurements on heterologously expressed CLC-1, the following solutions were used: intracellular solution, 100 mM N-methyl-D-glucamine-chloride, 2 mM MgCl2, 10 mM HEPES, 2 mM EGTA at pH 7.3; extracellular solution, 100 mM N-methyl-D-glucamine-chloride, 5 mM MgCl2 and 10 mM HEPES at pH 7.3. The effect of CPP derivatives is dependent on the intracellular chloride concentration, being more potent in low than in high Cl (Pusch et al., 2001). In order to maximise the inward currents at negative voltages, we performed the inside-out patch-clamp measurements with a high intracellular chloride concentration, allowing a precise determination of apparent inhibition constants (KD). With the exception of analogues N.2 (CPA) and N.11 that were purchased from Sigma-Aldrich (Milano-Italy), all the tested compounds were synthesised in our laboratory according to procedures previously reported: analogues N.3 and N.4 (Bettoni et al., 1987); analogues N.5 and N.6 (Bettoni et al., 1992); analogue N.7 (Calleri et al., 2002); analogues N.8–N.10 (Bettoni et al., 1992); analogue N.12 (Massolini et al., 1990); analogue N.13 (Romstedt et al., 1996); analogue N.14 (Kuchar et al., 1979); analogue N.15 (Ferorelli et al., 1997); analogues N.16 and N.17 (Ferorelli et al., 2001); analogues N.18 and N.19 (Loiodice et al., 1993); analogue N.20 (Witiak et al., 1971b); analogue N.21 (Witiak et al., 1971a); analogues N.22–N.24 (Liantonio et al., 2002); analogues N.25–N.30 (Carbonara et al., 2001); analogues 31–33 were prepared according to the procedure reported for compound 27 starting from the suitable diethyl 2-(4-substituted phenoxy)malonates and 4-chlorophenoxy- or phenoxy propyl-bromides, respectively.
The tested compounds were used as racemate or as pure enantiomers if these were available. For the optically active compounds, the enantiomeric excess, determined by polarimetric and HPLC analyses, resulted always >95%. This value was not determined for racemic compounds because they were obtained not by simple mixing of equimolar amounts of both enantiomers, but by synthetic procedures which only lead to racemate products (see references above). Each compound was daily prepared in aqueous bicarbonate stock solutions and the final concentrations were obtained by appropriate dilution with normal or Cl−-free physiological solution as needed. On each preparation, not more than three concentrations were tested and recordings were performed starting after 20 min of incubation, to be sure that the steady state of drug effect was reached (De Luca et al., 1992b). As well as CPP, all tested compounds did not show any effect on potassium conductance (gK), nor produced remarkable modifications of the resting membrane potential of the sampled fibres.
Results
CPP analogues
To clarify the role of the different parts of the molecule for the interaction with the binding site on the CLC-1 channel, the following modifications of the CPP structure have been accomplished (Figure 1): (1) substitution of the methyl group on the chiral centre with a longer alkyl chain, or a phenyl or a benzyl group (analogues N.4–N.7) to confirm the hypothesised pivotal role of the asymmetric carbon atom to direct the drug at the binding site; (2) isosteric substitution (analogues N.13–N.15) to evaluate the function of the oxygen of the phenoxy group; (3) removal or substitution of the chlorine atom on the aromatic ring with other halogen atoms, or a methyl or a benzoyl group (analogues N.8–N.12) and (4) introduction of one or two methyl groups ortho to the oxygen atom of the phenoxy group (analogues N.16 and N.17) to evaluate the role of the electric cloud and of the steric hindrance of the ring; (5) insertion of a methylenic group increasing the distance between the phenoxy group and the carboxylic function (analogues N.18 and N.19); (6) introduction of the chiral carbon atom in a six- or five-membered ring (analogues N.20–N.21) to evaluate the effect of the increased molecular rigidity; (7) substitution of the carboxylic moiety with a bioisosteric phosphonate group (analogues N.22–N.24) to clarify the role of the acid function; (8) introduction of a second phenoxy group at variable distance from the chiral centre (analogues N.25–N.33).
Effect of CPP analogues on the macroscopic Cl− conductance (gCl) of rat skeletal muscle fibres
Effect of the substitution of the methyl group on the asymmetric carbon atom of CPP
In agreement with previous results obtained on native gCl and on expressed CLC-1 (Pusch et al., 2000), in this study it was confirmed that, with the exception of the ethyl group (analogue N.4), the substitution of the methyl group with a phenyl or an n-propyl group (analogues N.6 and N.5) on the chiral centre of CPP is detrimental for gCl-blocking activity. In fact the S(−)-enantiomers that are the most active isomers of these derivatives, show higher values of IC50, 66±1.9 and 72±2.1 μM, respectively, compared with S(−)CPP (10–20 μM). Also, the introduction of a benzyl group decreased the drug effect, with 300 μM of derivative N.7 as racemate being necessary to produce a 49% block of gCl (data not shown).
Effect of the isosteric substitution of the oxygen atom of the phenoxy group of CPP
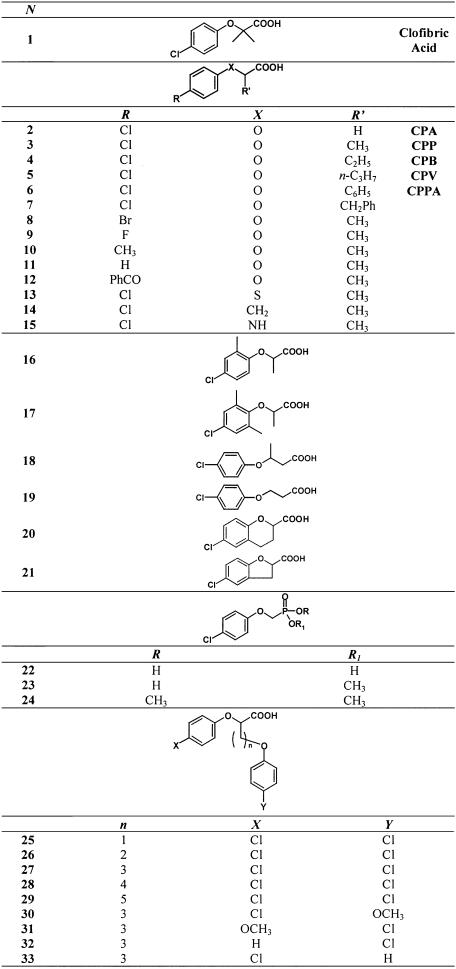
In order to define the role of the oxygen atom of the phenoxy group of CPP for the interaction with the binding site of the native Cl− channel, we substituted it with a sulphur, an amino and a methylenic group. Figure 2a shows the concentration–response curves of these isosteric analogues as racemates. As can be seen from the completely overlapping dose–response curves of CPP and derivative N.13, the substitution of the oxygen atom with sulphur (N.13) did not change the blocking activity. On the other hand, analogues in which an amino (N.15) or a methylenic group (N.14) replaced the oxygen atom were poorly effective, producing at the dose of 100 μM, which is a concentration close to the IC50 of racemate CPP, a block of native gCl of 11 and 3%, respectively (Figure 2a).
Figure 2.
Concentration–response curves for the block of gCl of native skeletal muscle fibres by (a) racemate CPP and isosteric analogues, (b) racemate CPP and aromatic ring-substituted analogues and (c) S(−)-enantiomer of CPP and of analogue N.18. For each compound, the mean value of gCl obtained after application of each concentration (17–30 fibres from two to three preparation) has been normalised to the related mean value of gCl recorded in the absence of drug. Thus, each point represents the normalised percent block of gCl±s.e.m. in the presence of drug.
Effect of the substitution of the chlorine atom on the aromatic ring of CPP
The substitution of the chlorine atom in the para position on the aromatic ring with other halogen atoms did not change the inhibitory activity of the lead compound. As shown in Figure 2b, the dose–response curves of racemate analogues N.8 and N.9 in which the chlorine atom was substituted with a bromine or fluorine atom, respectively, almost overlapped that of racemate CPP, with values of IC50 ranging from 80 to 90 μM. By contrast, the elimination of the electron-attractive halogen atoms in para position led to a drastic decrease of blocking activity. Indeed, as shown in Figure 2b, the concentration–response curves of analogues N.10 and N.11 in which the chlorine atom was substituted with a methyl group or a hydrogen atom, respectively, are clearly shifted to higher concentrations with respect to that of CPP (Figure 2b). Additionally, also the para substitution with a benzoyl moiety, a bulky group capable of realising an electron-attractive effect on the aromatic ring, drastically reduced the drug activity. In fact, application of racemate derivative N.12 at a dose of 100 μM produced a gCl block of only 7.5%.
Effect of the introduction of one or two methyl groups on the aromatic ring of CPP
In agreement with the results obtained previously (Ferorelli et al., 2001), the introduction of substituents on the aromatic ring in addition to the para chlorine atom, drastically reduced the ability of the drug to inhibit native gCl. Indeed, the S(−) enantiomers of the analogues N.16 and N.17, having one or two methyl groups on the aromatic ring in ortho position with respect to the oxygen atom, were poorly effective producing at 10 μM concentration a gCl block below 20% (data not shown).
Effect of the increase of the distance between the phenoxy group and the carboxylic function of CPP
To evaluate if an increase in the distance between the aromatic ring and the carboxylic function could affect the drug potency, a methylenic group between the chiral centre and the carboxylic moiety was introduced (N.18). As shown in Figure 2c, the most active S(−) enantiomer of derivative N.18 resulted much less potent with respect to S(−)CPP at all tested concentrations, producing an appreciable decrease of gCl only at concentrations higher than 300 μM. The decrease of the potency due to this chemical modification is independent on the presence of the chiral centre. Indeed, also the derivative N.19, lacking the asymmetric carbon atom, showed a reduced inhibitory potency with respect to its relative parent compound CPA (data not shown).
Effect of cyclic analogues of CPP
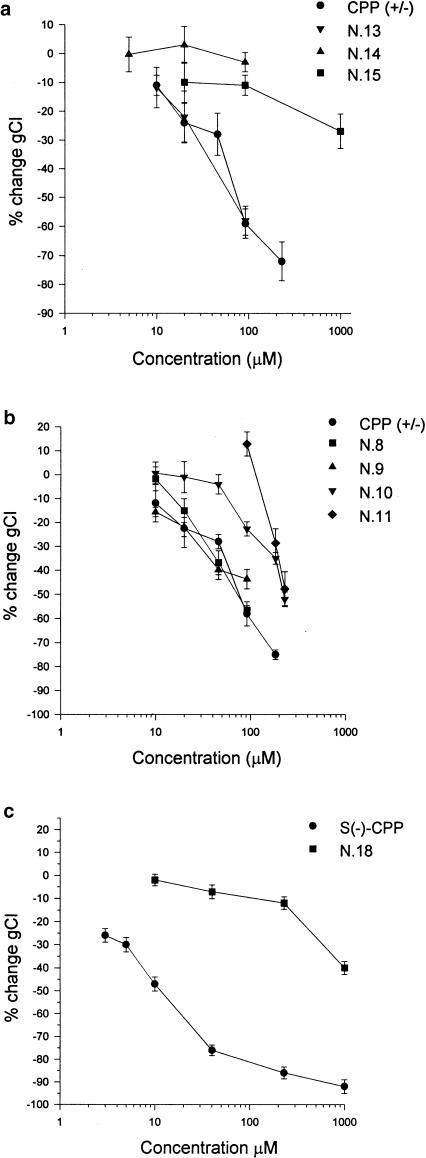
By constraining the CPP structure in a six- (N.20) or five-membered (N.21) ring, we evaluated whether an increase in the rigidity of the resulting molecules could affect the interaction of the drug with the binding sites. The most active S(−) enantiomers of both derivatives (N.20 and N.21) showed a reduced potency with respect to CPP, producing at a dose of 10 μM only a 16 and 6.9% block of gCl, respectively (Figure 3a).
Figure 3.
Effect of (a) cyclic analogues, (b) phosphonate analogues and (c) bis-chlorophenoxy analogues of CPP on gCl of EDL muscle fibres of adult rats. In particular, in (a) the effect produced by S(−) enantiomer of CPP and of analogues N.20 and N.21, at a concentration of 10 μM, in (b) the effect produced by CPA and analogues N.22, N.23, N.24 at a concentration of 500 μM and in (c) the effect of racemate CPP and of racemate bis-chlorophenoxy analogues N.25–N.29 at a concentration of 100 μM are shown. For each compound, the mean value of gCl obtained after application of drug (23–35 fibres from two to three preparations) has been normalised to the related mean value of gCl recorded in the absence of drug. Each bar represents the normalised percent block of gCl±s.e.m. in the presence of drug. Significantly different with respect to: * the relative control value and **CPP with P<0.05 and less.
Effect of the substitution of the carboxylic moiety of CPP with a phosphonate group
To clarify the role of the acidic function, we substituted the carboxylic group with a phosphonate group having different acidity. As the phosphonate analogues used in this study are devoid of the chiral centre, their blocking activity was compared to CPA. As shown in Figure 3b, the analogue with one (N.23) or two (N.22) acidic functions on the phosphonate moiety resulted much less potent with respect to CPA, producing at 500 μM a decrease of gCl of 20 and 17%, respectively. The elimination of any acidic function obtained by preparing the corresponding diester N.24, completely abolished the gCl-blocking activity (Figure 3b), corroborating the mechanism of action previously postulated for CPP (Pusch et al., 2001).
Effect of the introduction of a second chlorophenoxy group at variable distance from the chiral centre of CPP
Since the chlorophenoxy group is pivotal for drug activity (as demonstrated by the above results), we tested a series of CPP analogues in which a second chlorophenoxy function was introduced on the chiral centre at different distances. In Figure 3c, the effects of 100 μM of racemate bis-p-chloro phenoxy analogues (N.25–N.29) and of racemate CPP are compared. All the tested products blocked gCl less potently, compared to the parent compound. However, it is interesting to notice that the effect of these analogues was dependent on the length of the alkyl chain that separates the phenoxy group from the chiral centre. In particular, analogue N.27, with three methylenic groups, resulted as the most potent one producing a 45% block of gCl at 100 μM. To determine whether also the bis-p-chloro phenoxy analogues modulate the native gCl in a stereoselective manner as well as CPP, we tested the effect of the readily available enantiomers of analogue N.25. Application of the S(−) enantiomer produced a 28% block of gCl at 100 μM, reducing it from the control value of 2836±126 to 2030±166 μS cm−2. On the other hand, the R(+) enantiomer was less potent, producing a block of gCl of only 10% at 100 μM and even at higher concentrations (200–300 μM). We further determined the role of the chlorine atom of both the aromatic moieties. The chlorine atom was alternatively removed or substituted with a methoxy group (N.30–N.33). As expected from the pivotal role of the chlorine atom for CPP activity (see above), the removal or substitution of the chlorine atom of the phenoxy group directly linked to the chiral centre with a methoxy group (analogues N.32 and N.31) completely abolished or remarkably reduced the gCl-blocking activity (Table 1 ). On the contrary, when the same chemical modification was carried out on the phenoxy group of the side chain (analogues N.33 and N.30), no change in the blocking activity was obtained. Indeed analogues N.30 and N.33, when applied on EDL muscle fibres at 100 μM, produced a block of gCl of about 45–48% (Table 1) similar to that of the parent compound, N.27.
Table 1.
Effect of the chlorine atom on the aromatic rings of bis-phenoxy analogues of CPP
| Compound | n | gCl (μS cm−2) | % block of gCl |
|---|---|---|---|
| Control | 74 | 2736±63 | – |
| N.27 | 37 | 1493±102* | 45±3.4 |
| N.30 | 21 | 1420±79* | 48±2.4 |
| N.33 | 10 | 1498±97* | 45±2.7 |
| N.31 | 22 | 2713±76 | 0.8±2.4 |
| N.32 | 24 | 2393±77* | 13±2.0 |
The compounds shown in the table have been tested on gCl of EDL muscle fibres of adult rats at a concentration of 100 μM. The columns from left to right are as follows: drug used; n, number of sampled fibres; gCl, macroscopic chloride conductance expressed as mean±s.e.m. (from n fibres) obtained in control condition or after application of each compound; % block of gCl, normalised percent block produced by each compound.
Significantly different with respect to the control value with P<0.05.
Effect of CPP analogues on heterologously expressed CLC-1
The effect of each derivative on the gCl of native skeletal muscle fibres is the result of both the drug ability to cross the plasma membrane and its affinity toward the binding site. To evaluate the changes in the drug activity independently on the plasma membrane diffusion, we performed inside-out patch-clamp recordings on CLC-1 expressed in Xenopus oocytes. In particular, we focused our attention on the phosphonate and the bis-phenoxy derivatives because the physical–chemical properties associated to these drugs might hamper the diffusion through the plasma membrane and could mask a high affinity for binding site.
Effect of the phosphonate derivative N.23
As shown in Figure 4, the application of derivative N.23 on expressed CLC-1 at a dose of 100 μM produced a slight reduction of the steady-state current at negative voltages. The apparent KD at −140 mV had a mean value of 284±38 μM (n=4), a value four-fold greater than that of the lead compound CPA.
Figure 4.
Effect of internally applied phosphonate derivative N.23 on heterologously expressed CLC-1. The inside-out patch-clamp traces were elicited using the pulse-protocol described in Methods. For clarity, only the current traces corresponding to −140, −80 and +60 mV are shown.
Effect of bis-phenoxy derivatives
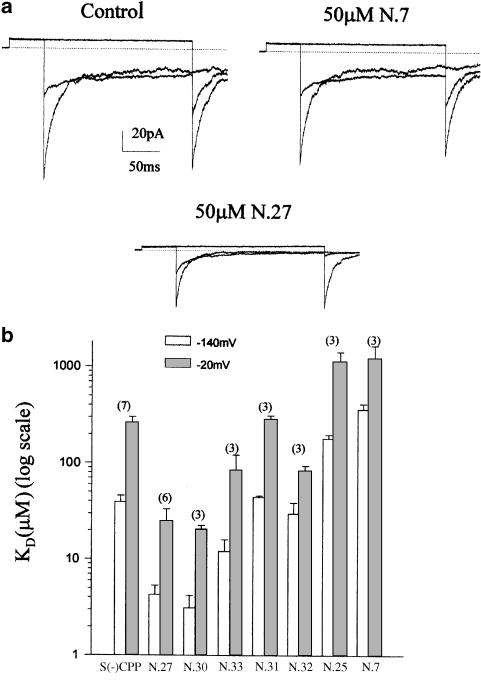
Since the bis-phenoxy analogues were the unique class, among the CPP-like compounds tested in this study, capable of producing a block of native gCl with a potency similar to CPP, we tested the effect of these derivatives also on expressed CLC-1, to better evaluate the role of the second chlorophenoxy group in drug affinity. The mechanism of action of the tested compounds thus appeared to be very similar to that previously described for CPP (Pusch et al., 2000; 2001). Although with a different potency, each tested compound produced a voltage-dependent block, as is also seen for CPP (Pusch et al., 2000). The inhibition was indeed strong at negative voltages, while only a small reduction of the current was observed at positive voltages. In Figure 5a, samples of current traces obtained after application of both the most and the least potent derivatives among the series are shown. In agreement with a previous study (Liantonio et al., 2002), racemate derivative N.27 produced a 10-fold stronger inhibition of CLC-1-mediated current with respect to S(−)CPP, showing at −140 mV a KD value of 4.3±0.8 μM (Figure 5b). The analogue N.25, in which only one methylenic group separates the phenoxy group of the side alkyl chain from the chiral centre, was 40-fold less potent with respect to the lead derivative N.27 (Figure 5b). The substitution of the chlorine atom of the aromatic ring of the side chain with a methoxy group (analogue N.30) or its removal (analogue N.33) produced none or a slight decrease of drug potency, respectively. By contrast, the same chemical substitutions on the phenoxy group directly linked to the chiral centre, altered the interaction between drug and binding site. Indeed, in this case, the scale of the drug potency was chlorine atom (analogue N.27)>hydrogen atom (analogue N.32)>methoxy group (analogue N.31) (Figure 5b).
Figure 5.
(a) Effect of internally applied derivatives N.7 and N.27 on heterologously expressed CLC-1. The inside-out patch-clamp traces were elicited using the pulse-protocol described in Methods. For clarity, only the current traces corresponding to −140, −80 and +60 mV are shown. The scale bars apply to the entire figure; (b) mean values of the apparent inhibition constant at −140 and −20 mV, obtained by fitting the equation reported in Methods to the inhibition data, for each indicated derivative. The number in brackets represents the number of patches for each condition.
To verify if the simple introduction of a second phenyl group on the chiral centre of CPP is sufficient for increasing the drug affinity to the binding site, we also tested on the heterologously expressed CLC-1 the effect of derivative N.7 having on the chiral centre a benzyl function in the place of the phenoxy one. Application of derivative N.7 at a dose of 50 μM led only to a small reduction in the steady-state current at negative voltages, indicating that the affinity for the binding site was significantly reduced (Figure 5a). This derivative was also much less potent with respect to analogue N.25, suggesting that the concomitant lack of the oxygen atom and of the two methylenic groups, between the chiral centre and the aromatic ring, produced a drastic reduction of drug potency (Figure 5b).
As already stated above, the drug affinity for each derivative decreased at more positive potentials. In fact, for each derivative, the KD values obtained at −20 mV were larger than those at −140 mV (Figure 5b).
Discussion
In the present study, we investigated a large array of derivatives of CPP, with modifications at several strategic positions of the molecule, for their inhibitory effect on the muscle chloride channel, CLC-1. Some of these molecules have been studied earlier (Liantonio et al., 2002). Here, we re-evaluated these known substances using different concentrations and we synthesised many new, rationally designed, derivatives based on the initial results.
The data obtained in this structure–activity relationship study allowed us to identify several molecular features of CPP-like molecules that are essential for an effective block of the CLC-1 channel, the muscle chloride channel underlying native gCl. From these molecular determinants, we can hypothesise how the interaction of the drug with its binding site on the CLC-1 protein could take place.
In accordance with previous findings (Conte Camerino et al., 1988; Pusch et al., 2000), the absolute configuration of the asymmetric carbon atom adjacent to the carboxylic function, combined with the presence of a methyl or ethyl group, is important for the drug activity. In fact, the S(−) enantiomers show a stronger blocking activity than the corresponding R(+) isomers, corroborating the idea that the chiral centre strongly influences the disposition of the molecule at the receptor site. The replacement of the methyl group with an n-propyl or phenyl group (derivatives N.5 and N.6) reduced the potency. This is in line with previous observations that the removal of the methyl groups (CPA) or the addition of a second methyl group (clofibric acid) led to a decrease in potency (Loiodice et al., 1993).
The nature of the substituents in para position of the aromatic ring of CPP can also modulate the interaction between drug and binding site. The kind of halogen substituent did not affect the drug potency. In fact, the analogue with a bromine (N.8) or a fluorine (N.9) atom is as potent as CPP that has a chlorine atom in the para position, indicating that the presence of an electron-attractive substituent in para position of the aromatic ring is an important requisite for drug activity. It is reasonable to hypothesise that a dipole–dipole interaction occurs between this part of the molecule and the Cl− channel binding site. To test this hypothesis, we replaced the chlorine atom by a hydrogen (N.11) and by a methyl group (N.10). As expected, both analogues resulted less potent compared to CPP. Furthermore, we have also evaluated the effect of derivative N.12, having in para position a benzoyl group, an electron-attractive substituent that is additionally capable of forming hydrogen bonds. This derivative had a poor activity, suggesting that the para substituent is not involved in other types of interaction and that actually its steric hindrance overwhelms the ability to depauperate the electronic cloud of the ring.
In line with the important role of the electronic density of the aromatic ring, the derivatives having one (N.16) or two (N.17) methyl groups on the aromatic ring in ortho position to the oxygen atom resulted less potent in blocking gCl with respect to CPP. This can be explained by considering that the introduction of these substituents, besides increasing the steric hindrance of the resulting molecules, hampers the coplanarity and the consequent conjugation effect between the π-electronic cloud of the aromatic ring and the lone electron pairs of the oxygen. Thus, the concomitant presence of an electron-attractive group, such as the halogen atoms, in para position and, on the other hand, of a conjugation effect between the oxygen atom and the aromatic ring, could induce a favourable distribution of electric charge in this portion of the molecules allowing an optimal interaction with the corresponding pocket of the receptor site. Accordingly, the oxygen atom of the phenoxy group of CPP also represents a functionally important component of the molecule. Indeed, among the isosteric derivatives, only the substitution of oxygen with sulphur atom (N.13) having similar physicochemical properties, did not alter the drug potency, further corroborating the role of conjugation effect between these atoms and the π-electronic cloud of the aromatic ring.
The increase of rigidity of the CPP structure, obtained constraining the chiral centre in a cycle (N.20 and N.21) led to a drastic decrease of the inhibitory activity. Thus, we hypothesise that the reduced flexibility of the resulting compounds could reduce the capability of the drug to interact with the binding site.
The carboxylic group represents the anionic part of the CPP structure. The drastic decrease of blocking activity observed, by replacing it with a phosphonate group, suggests that the negative charge and the chemical nature of the carboxylic group of the molecule are also critical features for the binding with the receptor site. Additionally, the smaller values of the pKa of phosphonate analogues N.22 and N.23 could account for their reduced potency. The presumed binding site for CPP is located at the intracellular side of the CLC-1 channel (Pusch et al., 2000). When CPP is applied from the extracellular side, as in our experimental condition, it is necessary that a certain amount of undissociated drug is available to cross the plasma membrane and reach the binding site. At the physiological pH value at which we recorded native gCl, the phosponate analogues would exist, with respect to CPP, almost completely in the charged form that is much less able to diffuse through the membrane. We ruled out any diffusion problem by evaluating the effect of the derivative N.23 on expressed CLC-1 in inside-out patches. In fact, when applied internally, derivative N.23 still maintained a lower potency with respect to CPA, corroborating that the reduced affinity for the binding site mainly accounts for the reduced potency of this derivative observed on native skeletal muscle fibres. Moreover, the anion charge carried by the carboxylic group is also important for drug mechanism of action. Indeed, evaluating the effect of 2-(p-chlorophenoxy) butyric acid (CPB), a derivative that acts in a very similar manner to CPP, on Xenopus oocytes expressing the Torpedo CLC-0 channel using the patch-clamp technique, we have already demonstrated that CPP could compete with Cl− ions for the occupation of the same or a closely located binding site (Pusch et al., 2001). The results obtained with the phosphonate analogue lacking the negatively charged acidic group (analogue N.24), could indirectly confirm this mechanism of action. In fact this compound, although more membrane permeable, is ineffective in blocking gCl, suggesting that the elimination of the acidic function does not allow a competition between Cl− ions and drug, thus reducing the capability of the drug to interfere with channel gating. Furthermore, as evidenced by the slight reduction of native gCl produced by the analogue N.18 in which a methylenic group has been introduced between the carboxylic moiety and the chiral centre, the carboxylic group needs to be at a certain distance from the phenoxy group, the optimal spacing being one C atom.
Thus, together with the carboxylic function, the chlorophenoxy group is absolutely necessary to produce the block of native gCl, since the modification of any part of this moiety on CPP structure leads to less or noneffective drugs. Interestingly, although the introduction of a second chlorophenoxy moiety (analogues N.25–N.29) did not enhance gCl-blocking activity with respect to CPP, the effect of the bis-chlorophenoxy analogues depends on the length of the aliphatic chain spacing the introduced chlorophenoxy moiety from the chiral centre. Indeed, the analogue with a spacer length of three methylenic groups (N.27) was the most potent one in this class of derivatives.
In contrast to what we observed for native gCl, we presently confirmed the previous observation (Liantonio et al., 2002) that the application of the analogue N.27 to the intracellular side during patch-clamp recordings produced a block of expressed CLC-1 channel with a 10-fold increased affinity with respect to S(−)CPP, indicating that the second chlorophenoxy group on the side chain adds a functionally important component to the molecule.
The mechanism of action of compound N.27 and of the other bis-chlorophenoxy derivatives tested on expressed CLC-1 is probably very similar to that previously described for CPP (Pusch et al., 2000; 2001). However, from a preliminary estimation, the binding/unbinding kinetics is slower than that of CPP, corroborating the stabilising role of the second chlorophenoxy moiety. The greater accessibility in the inside-out configuration would lead one to predict a greater potency of CPP derivatives with respect to native muscle. In contrast, we found that the KD for the reduction of CLC-1 inward current by S(−)CPP was of ∼40 μM, a value that is larger than the KD (10–20 μM) obtained for the inhibition of native gCl. An explanation for this apparent discrepancy could be that we have performed inside-out patch-clamp recordings of CLC-1 currents using a high intracellular chloride concentration of about 100 mM in order to achieve large inward currents and a precise estimate of the drug potency. In contrast, it is well known that the intracellular chloride concentration in skeletal muscle fibres is about 4 mM (Hille, 2001). As stated above, detailed investigation of the effect of CPB on CLC-0 showed that by lowering the intracellular chloride concentration from 104 to 14 mM, the apparent KD decreased by an almost constant factor of five (Pusch et al., 2001). Furthermore, in contrast to patch-clamp recordings from expressed CLC-1, the native gCl is measured from intact native muscle fibres in which potential effects of the metabolic environment on the biophysical and pharmacological properties of the channel are preserved (De Luca et al., 1998).
On the other hand, the lower potency of bis-chlorophenoxy derivatives on native gCl may be explained considering the pKa and log P values of these compounds. Indeed the elevated acidity and the bulky conformation associated with these compounds could reduce their capability of crossing the plasma membrane, an effect that might be less evident with a more simple structure like that of CPP and derivative N.7.
Even though the absolute efficacy was different, the order of drug potency of the bis-phenoxy derivatives measured on expressed CLC-1 was similar to that obtained for native gCl. The effect of bis-phenoxy analogues on CLC-1 applied directly to the intracellular side was clearly dependent on the number of methylenic groups of the side alkyl chain. In particular, the potency in inhibiting CLC-1 channel was strongly decreased for derivative N.25, having only one methylenic group forming the side alkyl chain, with respect to derivative N.27. These data suggest that the phenoxy group of the alkyl chain must be at a certain distance from the chiral centre and that the optimal distance is three C atoms.
The use of derivatives N.30–N.33 allowed us to investigate also the influence of substituents on the aromatic rings in modulating drug affinity. As expected from the pivotal role that the para chlorine atom plays on the molecule of CPP (explained above), its removal (analogue N.32) or its substitution with a methoxy group (analogue N.31) led to a reduction of blocking activity of native gCl as well as of CLC-1 currents.
The lead derivative N.27 bears a chlorine atom in para position of the additional aromatic ring. Substituting the chlorine with a methoxy group, that has different electronic properties (analogue N.30), did not alter drug activity, suggesting that the electronic characteristics of the second ring are of minor importance for the blocking potency. On the other hand, substituting the chlorine atom of the second ring with a hydrogen (N.33) significantly decreased the block of heterologously expressed CLC-1. It is thus possible to hypothesise that an important property of the additional phenoxy group is a proper bulkiness required to fit in the putative hydrophobic pocket.
Derivatives N.27, N.30 and N.33 resulted thus the most potent inhibitors of the CLC-1 channel identified by this study. Consequently, the presence of a second phenoxy group on the chiral centre of CPP is able to produce a significantly enhanced drug-binding affinity. This evidence is further supported by the results obtained with derivative N.7. This latter, having a benzyl group in the place of a phenoxy moiety, was drastically less potent than derivative N.27 in both the experimental systems used here.
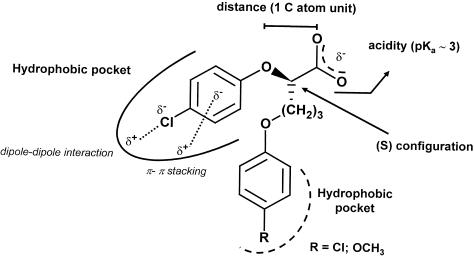
Collecting all the data obtained in this study, and taking into account the previously published results, we finally propose the structural molecular requisites necessary to potently inhibit the muscle CLC-1 channel (Figure 6). Four parts of the molecular structure seem to be fundamental for the interaction with the hypothesised binding site: (1) the carboxylic group that confers to the molecule the optimal acidity and the negative charge: the first influences the diffusion through the plasma membrane, the latter is necessary for the channel block; (2) the chlorophenoxy moiety that could interact with a hydrophobic pocket and, at the same time, could realise a π−π interaction; (3) the absolute configuration of the chiral centre which permits the adequate spatial disposition of the molecule; (4) an additional phenoxy group that interacting with a second hydrophobic pocket stabilises the binding, remarkably increasing drug affinity. The presence of a substituent in para position confers to this aromatic ring a bulkiness that could improve such an interaction.
Figure 6.
Schematic representation of the parts of a CPP-like molecule that are pivotal for drug interaction with the binding site.
The obtained results may be a starting point for the design of drugs with possible therapeutic benefit, especially taking into account the involvement of CLC channels in genetic diseases. Additionally, CPP-like compounds may represent useful tools for the understanding of structure and function of muscle CLC-1 and of other CLC channels, especially now that the 3D structure of bacterial CLC homologues is available and could allow molecular modelling studies (Dutzler et al., 2002).
Abbreviations
- CLC-1
voltage-dependent chloride channel of skeletal muscle
- CPA
p-chlorophenoxyacetic acid
- CPP
2-(p-chlorophenoxy)propionic acid
- EDL
extensor digitorum longus
- gCl
resting chloride conductance
- PKC
Ca-dependent protein kinase C
References
- AROMATARIS E.C., ASTILL D.St.J., RYCHKOV G.Y., BRYANT S.H., BRETAG A.H., ROBERTS M.L. Modulation of the gating of ClC-1 by S-(−) 2-(4-chlorophenoxy) propionic acid. Br. J. Pharmacol. 1999;126:1375–1382. doi: 10.1038/sj.bjp.0702459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETTONI G., FERORELLI S., LOIODICE F., TANGARI N., TORTORELLA V., GASPARRINI F., MISITI D., VILLANI C. Chiral α-substituted α-aryloxyacetic acids: synthesis, absolute configuration, chemical resolution, and direct separation by HPLC. Chirality. 1992;4:193–203. [Google Scholar]
- BETTONI G., LOIODICE F., TORTORELLA V., CONTE CAMERINO D., MAMBRINI M., FERRANNINI E., BRYANT S.H. Stereospecificity of the chloride ion channel: the action of chiral clofibric acid analogues. J. Med. Chem. 1987;30:1267–1270. doi: 10.1021/jm00391a002. [DOI] [PubMed] [Google Scholar]
- BRYANT S.H., CONTE CAMERINO D. Chloride channel regulation in the skeletal muscle of normal and myotonic goats. Pflügers Arch. 1991;417:605–610. doi: 10.1007/BF00372958. [DOI] [PubMed] [Google Scholar]
- CALLERI E., MASSOLINI G., LOIODICE F., FRACCHIOLLA G., TEMPORINI C., FELIX G., TORTORELLA P., CACCIALANZA G. Evaluation of a penicillin G acylase-based chiral stationary phase towards a series of 2-aryloxyalkanoic acids, isosteric analogs and 2-arylpropionic acids. J. Chromatogr. A. 2002;958:131–140. doi: 10.1016/s0021-9673(02)00403-x. [DOI] [PubMed] [Google Scholar]
- CARBONARA G., FRACCHIOLLA G., LOIODICE F., TORTORELLA P., CONTE CAMERINO D., DE LUCA A., LIANTONIO A. Carboxylic acids and skeletal muscle chloride channel conductance: effects on the biological activity induced by the introduction of an aryloxyalkyl group α to the carboxylic function of 4-chloro-phenoxyacetic acid. Farmaco. 2001;56:749–754. doi: 10.1016/s0014-827x(01)01127-2. [DOI] [PubMed] [Google Scholar]
- CHUA M., BETZ W.I. Characterization of ion channels on the surface membrane of adult rat skeletal muscle. Biophys. J. 1991;59:1251–1260. doi: 10.1016/S0006-3495(91)82340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTE CAMERINO D., MAMBRINI M., DE LUCA A., TRICARICO D., BRYANT S.H., TORTORELLA V., BETTONI G. Enantiomers of clofibric acid analogs have opposite actions on rat skeletal muscle chloride channels. Pflügers Arch. 1988;413:105–107. doi: 10.1007/BF00581238. [DOI] [PubMed] [Google Scholar]
- DE LUCA A., PIERNO S., LIANTONIO A., CAMERINO C., CONTE CAMERINO D. Phosphorylation and IGF-1-mediated dephosphorylation pathways control the activity and the pharmacological properties of skeletal muscle chloride channels. Br. J. Pharmacol. 1998;125:477–482. doi: 10.1038/sj.bjp.0702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LUCA A., TORTORELLA V., CONTE CAMERINO D. Chloride channels of skeletal muscle from developing, adult and aged rats are differently affected by enantiomers of 2-(p-chlorophenoxy) propionic acid. Naunyn-Schmiedeberg's. Arch. Pharmacol. 1992a;346:601–606. doi: 10.1007/BF00168731. [DOI] [PubMed] [Google Scholar]
- DE LUCA A., TRICARICO D., PIERNO S., CONTE CAMERINO D. Aging and chloride channel regulation in rat fast-twitch muscle fibres. Pflügers Arch. 1994;427:80–85. doi: 10.1007/BF00585945. [DOI] [PubMed] [Google Scholar]
- DE LUCA A., TRICARICO D., WAGNER R., BRYANT S.H., TORTORELLA V., CONTE CAMERINO D. Opposite effect of enantiomers of clofibric acid derivative on rat skeletal muscle chloride conductance: antagonism studies and theoretical modelling of two different receptor site interactions. J. Pharmacol. Exp. Ther. 1992b;260:364–368. [PubMed] [Google Scholar]
- DUTZLER R., CAMPBELL E.B., CADENE M., CHAIT B.T., MACKINNON R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature (Lond.) 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- FERORELLI S., LOIODICE F., TORTORELLA V., AMOROSO R., BETTONI G., CONTE CAMERINO D., DE LUCA A. Isosteres of chiral clofibric acid analogs: synthesis, resolution, absolute configuration and HPLC detection of the optical purity. Farmaco. 1997;52:367–374. [PubMed] [Google Scholar]
- FERORELLI S., LOIODICE F., TORTORELLA V., CONTE CAMERINO D., DE LUCA A. Carboxylic acids and skeletal muscle chloride channel conductance: effects on the biological activity induced by the introduction of methyl groups on the aromatic ring of chiral α-(4-chloro-phenoxy)alkanoic acids. Farmaco. 2001;56:239–246. doi: 10.1016/s0014-827x(01)01041-2. [DOI] [PubMed] [Google Scholar]
- GREEN J.R., MARGERISON D. Statistical Treatment of Experimental Data. New York: Elsevier; 1978. pp. 86–88. [Google Scholar]
- HILLE B. Ion Channels of Excitable Membranes. 2001Sunderland, MA: Sinauer Associates; 3rd edn [Google Scholar]
- JENTSCH T.J., STEIN V., WEINREICH F., ZDEBIK A.A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- KOCH M.C., STEINMEYER K., LORENZ C., RICKER K., WOLF F., OTTO M., ZOLL B., LEHMANN-HORN F., GRZESCHIK K-H., JENTSCH T.J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- KUCHAR M., REJHOLEC V., ROUBAL Z., NEMECEK O. Quantitative relationships between structure and fibrinolytic activity in the series of α-methyl-β-arylpropionic acids. Collect. Czechoslov. Chem. Commun. 1979;44:183–193. [Google Scholar]
- LIANTONIO A., ACCARDI A., CARBONARA G., FRACCHIOLLA G., LOIODICE F., TORTORELLA P., TRAVERSO S., GUIDA P., PIERNO S., DE LUCA A., CONTE CAMERINO D., PUSCH M. Molecular requisites for drug binding to muscle CLC-1 and renal CLC-K channel revealed by the use of phenoxy-alkyl derivatives of 2-(p-chlorophenoxy)propionic acid. Mol. Pharmacol. 2002;62:265–271. doi: 10.1124/mol.62.2.265. [DOI] [PubMed] [Google Scholar]
- LOIODICE F., FERORELLI S., TANGARI N., BETTONI G., TORTORELLA V., PIERNO S., DE LUCA A., TRICARICO D., CONTE-CAMERINO D. Carboxylic acids and chloride conductance in skeletal muscle: influence on the pharmacological activity induced by the chain substituents and the distance between the phenolic group and the carboxylic function in 4-chloro-phenoxyalkanoic acids. Farmaco. 1993;48:45–63. [PubMed] [Google Scholar]
- MASSOLINI G., CARMELLINO M.L., BORGNA P. Herbicidal activity of some 4′-substituted 2-(4-benzoylphenoxy)alkanoic acids. Farmaco. 1990;45:263–268. [Google Scholar]
- PUSCH M., ACCARDI A., LIANTONIO A., FERRERA L., DE LUCA A., CONTE CAMERINO D., CONTI F. Mechanism of block of single protopores of the Torpedo chloride channel ClC-0 by 2-(p-chlorophenoxy) butyric acid. J. Gen. Physiol. 2001;118:45–62. doi: 10.1085/jgp.118.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUSCH M., ACCARDI A., LIANTONIO A., GUIDA P., TRAVERSO S., CONTE CAMERINO D., CONTI F. Mechanisms of block of muscle type CLC chloride channels. Mol. Membr. Biol. 2002;19:285–292. doi: 10.1080/09687680210166938. [DOI] [PubMed] [Google Scholar]
- PUSCH M., LIANTONIO A., BERTORELLO L., ACCARDI A., DE LUCA A., PIERNO S., TORTORELLA V., CONTE CAMERINO D. Pharmacological characterization of the chloride channels belonging to the ClC family by the use of chiral clofibric acid derivatives. Mol. Pharmacol. 2000;58:498–507. doi: 10.1124/mol.58.3.498. [DOI] [PubMed] [Google Scholar]
- PUSCH M., STEINMEYER K., JENTSCH T.J. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophys. J. 1994;66:149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMSTEDT K.J., LEI L.-P., FELLER D.R., WITIAK D.T., LOIODICE F., TORTORELLA V. Differential eudismic ratios in the antagonism of human platelet function by phenoxy- and thiophenoxyacetic acids. Farmaco. 1996;51:107–114. [PubMed] [Google Scholar]
- ROSENBOHM A., RÜDEL R., FAHLKE C. Regulation of the human skeletal muscle chloride channel hClC-1 by protein kinase C. J. Physiol. 1999;514:677–685. doi: 10.1111/j.1469-7793.1999.677ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINMEYER K., ORTLAND C., JENTSCH T.J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature (Lond.) 1991;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- WITIAK D.T., FELLER D.R., STRATFORD E.S., HACKNEY R.E., NAZARETH R., WAGNER G. Inhibitory action of α-(4-chlorophenoxy)-α-methylpropionic acid analogs on cholesterol biosynthesis and lipolysis in vitro. J. Med. Chem. 1971a;14:754–757. doi: 10.1021/jm00290a020. [DOI] [PubMed] [Google Scholar]
- WITIAK D.T., STRATFORD E.S., NAZARETH R., WAGNER G., FELLER D.R. 6-Chlorochroman-2-carboxylic acids. Synthesis and biological evaluation as antagonists for cholesterol biosynthesis and lipolysis in vitro. J. Med. Chem. 1971b;14:754–766. doi: 10.1021/jm00290a021. [DOI] [PubMed] [Google Scholar]