Abstract
Increased peripheral resistance is a hallmark of chronic heart failure and has been primarily attributed to neurohumoral pathways involving both the renin–angiotensin and sympathetic nervous systems. The increased resistance is thought to serve as a compensatory mechanism to help maintain perfusion to the vital organs by sustaining blood pressure in the fate of a failing heart. Local mechanisms, and in particular endothelial dysfunction, have also been shown to be important contributors in regulating arterial resistance and vascular remodeling in this disease. In this issue of the British Journal of Pharmacology, Gschwend et al. (2003) present new data suggesting that in the absence of a functional endothelium, myogenic constriction of small pressurized mesenteric arteries, an intrinsic property of vascular smooth muscle cells, is enhanced in a coronary artery ligation-induced myocardial infarction model of congestive heart failure (CHF) in the rat. The increased myogenic tone appears to be tightly linked to angiotensin II type 1 receptors (AT1). The possibility that CHF-induced stimulation of myogenic constriction is due to the local release of preformed angiotensin II or constitutive upregulation of the AT1 receptor signaling pathways are discussed along with other potential cellular and molecular mechanisms previously suggested to play a role in myogenic reactivity.
Keywords: Myogenic tone, renin–angiotensin system (RAS), angiotensin receptors, angiotensin-converting enzyme (ACE), constitutive receptor activation, phospholipase C, G-protein-coupled receptors (GPCR), blood volume regulation, ion channels, intracellular calcium
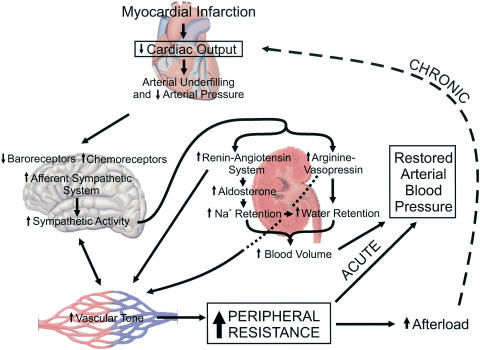
Following myocardial infarction, the gradual progression from compensated cardiac hypertrophy to decompensated heart failure is associated with complex neurohumoral interactions originating from the heart, brain, kidney and peripheral vasculature (Figure 1). Weakened heart function in congestive heart failure (CHF) results in reduced cardiac output and arterial underfilling. Decreased stretching of the high- and low-pressure baroreceptors and hypoperfusion-mediated stimulation of chemoreceptors enhance sympathetic tone. This increases peripheral vascular resistance by direct stimulation of smooth muscle contraction, and indirectly through stimulation of the renin–angiotensin system (RAS). Peripheral resistance is increased further by RAS through angiotensin Il-induced vasoconstriction and an increase in blood volume caused by stimulation of aldosterone production, which promotes sodium retention and water retention, and sympathetic stimulation of nonosmotic arginine–vasopressin (AVP) release limiting diuresis. AVP may also help in elevating peripheral resistance by enhancing vascular smooth muscle contractility primarily through activation of vasopressin V1 receptors, although a role for V2 receptors has also been suggested. The increased resistance is thought to serve as a compensatory mechanism to help maintain perfusion to the vital organs by sustaining blood pressure in the fate of a failing heart. These factors over the long term, however, can prove to be deleterious, exacerbating the already impaired cardiac pump (Schrier & Abraham, 1999).
Figure 1.
Sequence of major neurohumoral systems activated in CHF following myocardial infarction. Reduction in cardiac output and resulting arterial underfilling trigger a cascade of reflexes via various afferent pathways that signal the cardiovascular centers in the brain to increase sympathetic tone. The increase in central sympathetic activity is also enhanced by immune and stress signaling molecules (cytokines, Ang II) released locally in the peripheral vasculature. The RAS is stimulated in response to enhanced sympathetic tone that directly increases peripheral resistance by enhancing vascular smooth muscle contraction (mainly via angiotensin type 1 receptors), and indirectly by increasing blood volume in response to stimulation of aldosterone production and Na+ retention by the kidney, and enhanced water retention due indirectly to Na+ reuptake by the kidney and directly through stimulation of antidiuretic hormone production (AVP). AVP may also participate in increasing vascular resistance by stimulating vascular smooth muscle contraction. In the short term (ACUTE), the increase in peripheral resistance allows for restoration of arterial blood pressure to near-normal levels. However, as heart function slowly progresses towards decompensation, enhanced peripheral resistance and afterload may further exacerbate cardiac mechanics (CHRONIC) leading to congestion of the left and right ventricles and pulmonary circulation.
Local mechanisms have also been shown to be important contributors in regulating arterial resistance. Constricting (e.g. thromboxane A2, angiotensin II (Ang II), endothelin-1), vasodilating (e.g. NO) and inflammatory hormones (cytokines) released locally by the endothelium, nerve terminals, platelets and leukocytes have also been suggested to participate in heart failure-induced vascular remodeling (Fang & Marwick, 2002) and shown to feedback to the forebrain and hypothalamic centers to stimulate further sympathetic tone (Felder et al., 2003; Figure 1). Endothelial nitric oxide synthase is downregulated (Supaporn et al., 1996) and NO-induced vasorelaxation is blunted in heart failure (Fang & Marwick, 2002). Reduced production of NO and other endothelium-derived relaxing factors is thought to increase vascular resistance by promoting the action of circulating and locally produced vasoconstrictors (Fang & Marwick, 2002).
Most studies, carried out so far, have primarily focused on CHF-induced alterations in vascular tone by extrinsic factors. In this issue of the British Journal of Pharmacology, Gschwend et al. (2003) provide convincing evidence that arterial smooth muscle tone may also be altered in a rat myocardial infarction model of CHF. In addition to responding to endogenous constricting agonists, smooth muscle cells of small arteries and arterioles contract in response to an increase in transmural pressure, an endothelium-independent response referred as ‘myogenic tone' (Davis & Hill, 1999). This mechanism is thought to play an important role in autoregulation of blood flow in several vascular beds and to limit the deleterious impact of excessive blood pressures on perfused tissues. Their report shows that myogenic constriction of small pressurized mesenteric arteries was significantly augmented in CHF relative to sham-operated controls. In the absence of any agonist, it was selectively antagonized by Ang II type 1 (AT1) but not AT2 receptor blockade. Gschwend et al. (2003) also showed that at 60 mmHg, myogenic constrictions were more sensitive to exogenous application of Ang II in CHF vs control arteries, while endothelium-mediated inhibition of myogenic constriction appeared similar in the two groups. Pharmacological interventions to investigate the contribution of several potential mechanisms including angiotensin-converting enzyme (ACE) all failed to reveal a significant change in mesenteric arteries from CHF rats. The lack of effect of ACE inhibition was surprising and led the authors to propose two hypotheses: (1) preformed and stored Ang II is released locally in response to stretch, thereby increasing myogenic tone; (2) the AT1 receptor, due to changes in receptor density and/or coupling with its G-protein, is constitutively active in the absence of agonist and primes myogenic reactivity. The former hypothesis is based on studies in cultured cardiac myocytes showing that stretch stimulates the release of preformed endogenous Ang II. Although an interesting possibility that warrants to be tested, this hypothesis seems unlikely as AT1-dependent myogenic constriction would likely be variable from preparation to preparation depending on the cellular storage levels of Ang II and would be predicted to decline with time as Ang II molecules are broken down and storage levels fall. Indeed, release of Ang II by stretch in cardiac myocytes was transient (Leri et al., 1998).
The second hypothesis seems more attractive in view of several lines of evidence. There are many examples of agonist-independent constitutive activation of several classes of G-protein-coupled receptors, which have served as a conceptual framework to understand the molecular basis of several human disorders (Leurs et al., 1998). More pertinent to the study by Gschwend et al. (2003) are reports showing that mutations of asparagine residues 111 (Groblewski et al., 1997) and 295 (Balmforth et al., 1997) of the AT1 receptor, increase IP3 synthesis in the absence of Ang II. Pressure-dependent myogenic reactivity is augmented by subthreshold concentrations of constricting agonist (Davis & Hill, 1999). Moreover, phospholipase C activity is increased by stretch of aortic smooth muscle (Matsumoto et al., 1995). Constitutive activation of the AT1 receptor could produce a similar effect. In the study by Gschwend et al. (2003; see their Figure 1), arteries from CHF rats were more sensitive to pressure and developed larger myogenic constrictions than those of sham-operated animals. Arteries from CHF rats were also more sensitive to exogenous Ang II exposure than control arteries, but the maximal constriction elicited by Ang II was similar in both groups (their Figure 3). This would be consistent with CHF-induced constitutive AT1 receptor activation resulting in agonist-independent displacement on the dose–response curve due to elevated basal PLC activity.
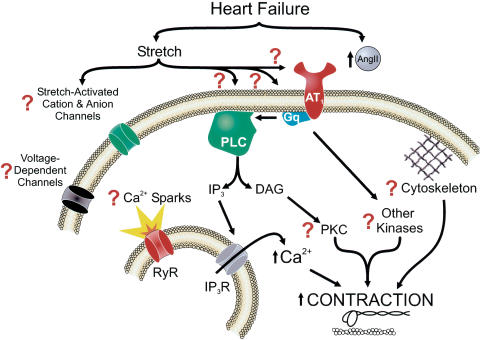
Regardless of the exact mechanism responsible for upregulation of the AT1 receptor in CHF, the study by Gschwend et al. (2003) raises a number of interesting questions regarding the signaling pathways involved in the altered myogenic response. A number of potential targets (Figure 2) will require further investigation at the cellular and molecular levels. These include changes in smooth muscle membrane potential, voltage-dependent (Ca2+ and K+ channels) and stretch-sensitive ion channels (nonselective cation or anion channels), global and local (Ca2+ sparks) Ca2+ signaling pathways, integrins and cytoskeletal proteins, and a number of protein kinases (e.g. PKC, Rho, tyrosine and MAP kinases), which have been suggested to modulate myogenic tone by phosphorylating ion channels, exchangers and transporters, as well as contractile proteins (Davis & Hill, 1999). As carefully stated by the authors, we must interpret their results with caution as different CHF models could lead to different conclusions. Nevertheless, their study provides compelling evidence that an important contractile property of smooth muscle in resistance arteries is modified in CHF and may contribute in elevating peripheral vascular resistance. Enhanced myogenic constriction may thus represent a new therapeutic target in CHF patients.
Figure 2.
Signaling pathways potentially involved in altered AT1 receptor function and enhanced myogenic reactivity in CHF. Evidence provided by Gschwend et al. (2003) suggests that myogenic constriction is enhanced in heart failure and appears to be linked to altered AT1 receptor function. These authors suggest that preformed and stored Ang II is released and local elevated Ang II levels increase myogenic tone. An alternative hypothesis is that the AT1 receptor itself, its coupling to Gq and/or phospholipase C (PLC) is (are) modified in heart failure leading to constitutive activation of this pathway. Future studies should be designed to investigate the possible role of downstream events such as smooth muscle membrane potential, voltage-dependent and stretch-activated cation and anion channels, mechanisms regulating global intracellular Ca2+ levels, the ryanodine receptor (RyR) which is responsible for generating Ca2+ sparks and inositol-tris-phosphate (IP3)-Ca2+ release channels, protein kinase C (PKC) and other kinases (Rho, Ras, MAPK, Erk, etc.), cytoskeletal and contractile proteins, all of which have been implicated to play role in generating myogenic tone (Davis & Hill, 1999).
Acknowledgments
J.L. was supported by a Doctoral Studentship Award from the Canadian Institutes of Health Research (CIHR). D.M.G. was supported by a Medical Student Summer Fellowship from the Nevada NIH BRIN. The work was also supported by grants to N.L. from the CIHR (MOP-10863), the Quebéc Heart and Stroke Foundation (NL-08-FMCQ), The Montréal Heart Institute Fund and the Center of Biomedical Research Excellence (NIH NCRR P2015581), University of Nevada School of Medicine, Reno, Nevada.
Abbreviations
- ACE
angiotensin converting enzyme
- Ang II
angiotensin II
- AT1
angiotensin type 1 receptor
- AT2
angiotensin type 2 receptor
- AVP
nonosmotic arginine-vasopressin
- CHF
congestive heart failure
- DAG
diacylglycerol
- Erk
extracellular signal-regulated kinase
- GPCR
G-protein-coupled receptors
- Gq
G-protein type q
- IP3
inositol-tris-phosphate
- IP3R
IP3 receptor
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- PKC
protein kinase C
- PLC
phospholipase C
- RAS
renin-angiotensin system
- RyR
ryanodine receptor
References
- BALMFORTH A.J., LEE A.J., WARBURTON P., DONNELLY D., BALL S.G. The conformational change responsible for AT1 receptor activation is dependent upon two juxtaposed asparagine residues on transmembrane helices III and VII. J. Biol. Chem. 1997;272:4245–4251. doi: 10.1074/jbc.272.7.4245. [DOI] [PubMed] [Google Scholar]
- DAVIS M.J., HILL M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- FANG Z.Y., MARWICK T.H. Vascular dysfunction and heart failure: epiphenomenon or etiologic agent. Am. Heart J. 2002;143:383–390. doi: 10.1067/mhj.2002.120780. [DOI] [PubMed] [Google Scholar]
- FELDER R.B., FRANCIS J., ZHANG Z.H., WEI S.G., WEISS R.M., JOHNSON A.K. Heart failure and the brain: new perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R259–R276. doi: 10.1152/ajpregu.00317.2002. [DOI] [PubMed] [Google Scholar]
- GROBLEWSKI T., MAIGRET B., LARGUIER R., LOMBARD C., BONNAFOUS J.C., MARIE J. Mutation of Asn111 in the third transmembrane domain of the AT1A angiotensin II receptor induces its constitutive activation. J. Biol. Chem. 1997;272:1822–1826. doi: 10.1074/jbc.272.3.1822. [DOI] [PubMed] [Google Scholar]
- GSCHWEND S., HENNING R.H., PINTO Y.M., de ZEEUW D., van GILST W.H., BUIKEMA H. Myogenic constriction is increased in mesenteric resistance arteries from rats with chronic heart failure: instantaneous counteraction by acute AT1 receptor blockade. Br. J. Pharmacol. 2003;139:1317–1325. doi: 10.1038/sj.bjp.0705367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERI A., CLAUDIO P.P., LI Q., WANG X., REISS K., WANG S., MALHOTRA A., KAJSTURA J., ANVERSA P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin–angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J. Clin. Invest. 1998;101:1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEURS R., SMIT M.J., ALEWIJNSE A.E., TIMMERMAN H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem. Sci. 1998;23:418–422. doi: 10.1016/s0968-0004(98)01287-0. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO H., BARON C.B., COBURN R.F. Smooth muscle stretch-activated phospholipase C activity. Am. J. Physiol. 1995;268:C458–C465. doi: 10.1152/ajpcell.1995.268.2.C458. [DOI] [PubMed] [Google Scholar]
- SCHRIER R.W., ABRAHAM W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- SUPAPORN T., SANDBERG S.M., BORGESON D.D., HEUBLEIN D.M., LUCHNER A., WEI C.M., DOUSA T.P., BURNETT J.C., JR Blunted cGMP response to agonists and enhanced glomerular cyclic 3′,5′-nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int. 1996;50:1718–1725. doi: 10.1038/ki.1996.491. [DOI] [PubMed] [Google Scholar]