Abstract
Increased vascular resistance in chronic heart failure (CHF) has been attributed to stimulated neurohumoral systems. However, local mechanisms may also importantly contribute to set arterial tone. Our aim, therefore, was to test whether pressure-induced myogenic constriction of resistance arteries in vitro – devoid of acute effects of circulating factors – is increased in CHF and to explore underlying mechanisms.
At 12 weeks after coronary ligation-induced myocardial infarction or SHAM-operations in rats, we studied isolated mesenteric arteries for myogenic constriction, determined as the active constriction (% of passive diameter) in response to stepwise increase in intraluminal pressure (20 – 160 mmHg), in the absence and presence of inhibitors of potentially involved modulators of myogenic constriction.
We found that myogenic constriction in mesenteric arteries from CHF rats was markedly increased compared to SHAM over the whole pressure range, the difference being most pronounced at 60 mmHg (24±2 versus 4±3%, respectively, P<0.001).
Both removal of the endothelium as well as inhibition of NO production (L-NG-monomethylarginine, 100 μM) significantly increased myogenic constriction (+16 and +25%, respectively), the increase being similar in CHF- and SHAM-arteries (P=NS). Neither endothelin type A (ETA)-receptor blockade (BQ123, 1 μM) nor inhibition of perivascular (sympathetic) nerve conduction (tetrodotoxin, 100 nM) affected the myogenic response in either group.
Interestingly, increased myogenic constriction in CHF was fully reversed after angiotensin II type I (AT1)-receptor blockade (candesartan, 100 nM; losartan, 10 μM), which was without effect in SHAM. In contrast, neither angiotensin-converting enzyme (ACE) inhibition (lisinopril, 1 μM; captopril, 10 μM) or AT2-receptor blockade (PD123319, 1 μM), nor inhibition of superoxide production (superoxide dismutase, 50 U ml−1), TXA2-receptor blockade (SQ29,548, 1 μM) or inhibition of cyclooxygenase-derived prostaglandins (indomethacin, 10 μM) affected myogenic constriction.
Sensitivity of mesenteric arteries to angiotensin II (10 nM – 100 μM) was increased (P<0.05) in CHF (pD2 7.1±0.4) compared to SHAM (pD2 6.2±0.3), while the sensitivity to KCl and phenylephrine was not different.
Our results demonstrate increased myogenic constriction in small mesenteric arteries of rats with CHF, potentially making it an important target for therapy in counteracting increased vascular resistance in CHF. Our results further suggest active and instantaneous participation of AT1-receptors in increased myogenic constriction in CHF, involving increased sensitivity of AT1-receptors rather than apparent ACE-mediated local angiotensin II production.
Keywords: Rat, myocardial infarction, heart failure, small mesenteric artery, myogenic constriction, AT1 receptor blockade, angiotensin II
Introduction
Increased peripheral resistance is a hallmark of chronic heart failure (CHF) (Zelis & Flaim, 1982; Schrier & Abraham, 1999). Initially, increased vasoconstriction may serve as a compensatory mechanism for decreased cardiac output to maintain optimal circulatory efficiency, to redistribute blood flow between and within organs, and to maintain normal levels of blood pressure. In the long-term, however, chronic vasoconstriction may become excessive and may contribute towards further progression of CHF (Zelis & Flaim, 1982). Therefore, it seems of significant importance to assess the underlying mechanisms of increased vasoconstriction in CHF.
Increased peripheral resistance in CHF is generally thought to be conveyed by stimulation of neurohumoral systems such as the renin – angiotensin system (RAS) and sympathetic activation (Schrier & Abraham, 1999). Apart from systemic factors, however, local mechanisms also importantly contribute to set arterial tone. Arteries respond to an increase in intraluminal pressure with an active constriction of vascular smooth muscle cells (VSMCs). This active constriction has been termed myogenic and may involve not only membrane depolarization and intracellular calcium increase but also changes in calcium sensitivity and alterations of cell structure (Davis & Hill, 1999). The myogenic response is an intrinsic property of VSMCs, but several factors may modulate its intensity. In this respect, locally produced factors/hormones with constrictive (i.e. endothelin-1, angiotensin II) (Nguyen et al., 1999; Nurkiewicz & Boegehold, 2000) as well as dilative ability (i.e. nitric oxide (NO)) (Skarsgard et al., 1997; Garcia & Bund, 1998) may be important and have been shown to differentially modulate myogenic constriction in several forms of cardiovascular disease (Huang & Koller, 1997; Falcone & Meininger, 1999; Wang & Cupples, 2001).
The aim of the current study was to test whether myogenic constriction of small resistance arteries may be increased in CHF and to explore what may be the underlying mechanisms. To this end, we employed the rat coronary occlusion – myocardial infarction (MI) model of experimental heart failure. At 12 weeks after MI, small mesenteric arteries were obtained and studied in an in vitro model for pressurized arteries, that is, devoid of acute effects of circulatory factors, in comparison with arteries of SHAM-operated rats. Special attention was given to the question whether alterations in the myogenic response may be due to locally produced modulators of myogenic constriction or due to changes in constrictive ability of VSMCs.
Methods
Male Wistar rats (250 – 300 g) were obtained from Harlan (Zeist, The Netherlands) and housed groupwise at the Central Animal Laboratory, University of Groningen (The Netherlands), with free access to food (regular rat chow; Hope Farms, Woerden, The Netherlands) and drinking water. After a 10-day acclimatization period, rats underwent surgery for induction of experimental MI. In short, rats were placed on a homeothermic blanket, anaesthetized with isoflurane (2.0 – 2.5%) in a mixture of N2O/O2 (2 : 1), intubated and mechanically ventilated (Amsterdam Infant Ventilator, Hoek/Loos, Schiedam, The Netherlands). A left-sided thoracotomy was made, and the left anterior descending coronary artery was occluded with a 6-0 silk suture 1 – 2 mm after its origin (Pfeffer et al., 1979); in SHAM-operated rats, the ligation was not tightened. Subsequently, the thorax was closed and rats were extubated upon spontaneous respiration. Afterwards, Temgesic® (4 μg kg−1) was administered intraperitoneally for analgesic purposes. A total number of 30 rats underwent SHAM-operations (n=12) or surgery for MI (n=18). All SHAM-operated rats survived the surgical procedure and the post-surgical period, whereas total mortality among MI rats was 28% (five out of 18). All procedures were reviewed and approved by the Animal Research Committee at the University of Groningen.
Determination of LV function
At 12 weeks after infarction, rats were anaesthetized as described above. The right carotid artery was cannulated with a pressure transducer catheter (Micro-Tip 3French, Millar Instruments, Germany) connected to a 486-PC equipped with an analog-to-digital converter and appropriate software (Millar Instruments, Germany). A zero-pressure baseline was obtained by placing the pressure sensor in 38°C saline before the catheter was advanced into the aorta and the left ventricle (LV). After a 3-min period of stabilization, maximal LV end-systolic pressure (LVESP), LV end-diastolic pressure (LVEDP), and heart rate were recorded. As indices of global contractility and relaxation, the maximal rates of increase and decrease in LVP (systolic dP dt−1 and diastolic dP dt−l) were determined. Hereafter, the catheter was withdrawn to measure systolic and diastolic blood pressure in the aortic root.
Cardiac morphometry
Hearts were then removed from the rat, arrested in diastole in 2 mol l−1 KCl and weighed. The atria and great vessels were dissected from the ventricles and the right ventricular free wall was separated from the left LV, before ventricular weights were obtained. A transverse slice through the midst of the LV containing the infarcted area was fixed in Bouin's fluid, embedded in paraffin and 10 μm slices were cut and stained for histological analysis. Total epicardial and endocardial circumferences of the left ventricle and the epicardial and endocardial scar length of the infarcted areas were determined by means of a computerized planimeter (Quantimet 520, Cambridge Instruments). Infarct size was then calculated by dividing the sum of the scar lengths by the sum of the total circumferences and was expressed as percentage of scare to total LV circumference, as described in detail elsewhere (van Gilst et al., 1994).
Preparation and cannulation of mesenteric arteries
The mesenterium was removed and put in cold Krebs solution. Third-order branches of the superior mesenteric artery were isolated from the surrounding perivascular tissue and transferred to an arteriograph system for pressurized arteries (Halpern et al., 1984) (Living System Instrumentation, Burlington, VT, U.S.A.). Arteries were cannulated at both ends on glass micropipettes, secured, and the lumen of the vessel was filled with Krebs solution through the micropipettes. The intraluminal pressure was set to 60 mmHg and was held constant (without flow) by a pressure servo system (Living System Instrumentation, Burlington, VT, U.S.A.). The vessel chamber was continuously recirculated with warmed (37°C) and oxygenated (5% CO2 in O2) Krebs with a pH of 7.4. The vessel chamber was transferred to the stage of an inverted light microscope with a video camera attached to a viewing tube. The video dimension analyser (Living System Instrumentation, Burlington, VT, U.S.A.) was used to analyse the signal obtained from the video image and to continuously register lumen diameter and wall thickness. Arteries were allowed to equilibrate for 40 min. To test for viability, arteries were then constricted with phenylephrine (PE, 1 μmol l−1) and arteries that did not respond to PE were discarded.
Determination of pressure – diameter curves and experimental conditions
Active pressure – diameter curves in calcium-containing Krebs solution were obtained for mesenteric arteries from SHAM (n=9) and CHF rats (n=9) over a pressure range of 20 – 160 mmHg in steps of 20 mmHg. After each step increase in intraluminal pressure, lumen diameter was registered when stable diameter/contraction was reached (normally after 5 min). After exchanging calcium-containing Krebs solution by calcium-free Krebs solution supplemented with ethylene glycol-bis-(b-amino ethyl ether) tetraacetic acid (EGTA, 2 mmol l−1) passive pressure – diameter curves were obtained over the same pressure range. To investigate potentially involved modulators of the myogenic response, additional artery segments from each rat were used to determine pressure – diameter curves after removal of the endothelium or in the presence of different inhibitors of potentially involved pathways given to the superfusion bath solution 20 min before determination of pressure – diameter curves. To remove the endothelium, a hair was inserted into the artery and gently moved for several times. Successful removal of the endothelium was checked by the response to acetylcholine. Only arteries in which the relaxation to acetylcholine, 100 μmol l−1, was completely absent were used for analysis. The myogenic response was also studied in arteries pretreated with L-NG-monomethylarginine (L-NMMA, 100 μmol l−1), an inhibitor of the production of endothelium-derived NO (Rees et al., 1990). The involvement of endothelin type A (ETA)receptor-mediated effects in the myogenic reponse was tested by pretreatment of arteries with BQ123, 1 μmol l−1. Any influence of perivascular (sympathetic) nerve conduction on the myogenic response was investigated by treatment of arteries with tetrodotoxin, 100 nmol l−1.
To investigate the potential involvement of the RAS in modulating the myogenic response, active pressure – diameter curves were obtained after pretreatment with two different angiotensin II type 1 (AT2) receptor blockers, that is candesartan, 100 nmol l−1, losartan, 10 μmol l−1, the angiotensin II type 2 (AT2) receptor blocker PD123319, 1 μmol l−1, and after inhibition of angiotensin-converting enzyme (ACE)-mediated angiotensin II (Ang II) production with two different ACE inhibitors, that is, lisinopril, 1 μmol l−1 and captopril, 10 μmol l−1. Taking into account, the localization of ACE at the luminal side of the endothelium (Ryan et al., 1976; Cockcroft et al., 1995), additional experiments were performed given the ACE inhibitors also into the lumen of the arteries. Neither incubation of arteries with AT1 and AT2 receptor blockers nor ACE inhibition had a significant effect on resting control diameters (data not shown).
Based on reports describing interactions of Ang II with several other (contractile) substances (Touyz & Schiffrin, 2000), we further investigated the potential involvement of superoxide production, and cyclooxygenase (COX)-derived prostaglandins, including thromboxane A2 (TXA2), on the myogenic response by pretreatment of arteries, respectively, with superoxide dismutase (SOD) 50 U ml−1, the COX inhibitor indomethacin, 10 μmol l−1, and the TXA2 receptor antagonist SQ 29,548, 1 μmol l−1.
Contractile responses to exogenous vasoconstrictors
In a separate set of experiments, we additionally obtained full concentration – response curves for Ang II (10 nmol l−1 – 100 μmol l−1), the α-adrenoceptor agonist PE (10 nmol l−1 – 10 μmol l−1), and KCl (20 – 80 mmol l−1). Contractile responses to these exogenously administered vasoconstrictors were obtained at an intraluminal pressure of 60 mmHg (the pressure-step at which the differences in myogenic constriction between CHF and SHAM arteries were most pronounced, see also Results section).
To determine pressure – diameter curves (in the absence or presence of inhibitors) and contractile responses to the different agonists, six to eight mesenteric artery segments were obtained from one rat, but importantly, only one artery segment per rat was used for one specific protocol.
Solutions and drugs
The Krebs bicarbonate solution had the following composition (mmol l−1): NaCl 120.4, KCl 5.9, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, glucose 11.5, NaHCO3 25.0 and was freshly prepared daily. These compounds were purchased from Merck, Darmstadt, Germany. Candesartan (CV11974) was a kind gift from Astra Pharmaceutica BV (Zoetermeer, The Netherlands). All other compounds were purchased from Sigma (St Louis, MO, U.S.A.). Stock solution for indomethacin and SQ 29,548 (10 mmol l−1) was prepared in 96% ethanol.
Data analysis
Myogenic constriction is expressed as per cent decrease in diameter from the maximally dilated (passive) diameter determined at the same pressure in calcium-free solution supplemented with EGTA (2 mmol l−1), that is myogenic constriction (%)=100[(DCa−free−DCa)DCa−free−1], where D is the diameter in calcium-free (DCa-free) or calcium-containing (DCa) Krebs. Constriction to increasing concentrations of Ang II, PE, and KCl is expressed as per cent decrease in diameter from the maximally dilated (passive) diameter of the same vessel determined at the same pressure. The concentrations of agonists causing half-maximal responses (EC50 values) are expressed as the negative logarithm of the molar concentrations (pD2 values). Data are expressed as mean±s.e.mean; n-values represent the number of investigated rats as well as the number of investigated arteries, as not more than one artery segment per rat was used for the same protocol. Differences in levels of myogenic constriction at 60 mmHg (Table 2 ) were tested using Student's unpaired t-test. Differences in pressure – diameter curves (Figure 1a), and differences in myogenic constriction between arteries from CHF and SHAM rats (Figure 1b and c), and between arteries with and without endothelium (Figure 2) were tested using ANOVA for repeated measures, and corrected for multiple comparisons by Duncan's multiple range test where appropriate (SPSS for Windows, Standard Version 8.0). Differences were considered significant at P<0.05 (two-tailed).
Table 2.
Overview of the level of myogenic constriction at 60 mmHg in small mesenteric arteries of rats with and without CHF, before and after several different acute interventions
| Level of myogenic constriction (%) (significant changes versus control) | |||
|---|---|---|---|
| Intervention | SHAM | CHF | P-value |
| Control (n=9) | 4±3 | 24±2 | <0.001 |
| Endothelial removal (n=5) | 21±2* (+17) | 38±3* (+15) | 0.001 |
| L-NMMA (100 μmol/l−1, n=5) | 30±5* (+26) | 49±5* (+25) | 0.035 |
| BQ123 (1 μmol l−1, n=5) | 8±4 | 26±4 | 0.019 |
| Tetrodotoxin (100 nmol l−1, n=5) | 3±2 | 23±4 | 0.001 |
| Candesartan (100 nmol l−1, n=5) | 8±4 | 4±3* (−20) | NS |
| Losartan (10 μmol l−1, n=6)# | — | 8±3* (−16) | — |
| PD123319 (1 μmol l−1, n=5) | 6±2 | 26±2 | <0.001 |
| Lisinopril (1 μmol l−1, n=4) | 5±5 | 26±5 | 0.022 |
| Captopril (10 μmol l−1, n=4) | 6±2 | 23±4 | 0.009 |
| SQ 29,548 (1 μmol l−1, n=5) | 7±6 | 27±5 | 0.04 |
| Indomethacin (10 μmol l−1, n=5) | 6±5 | 25±6 | 0.038 |
| SOD (50 U ml−1, n=5) | 3±2 | 22±2 | <0.001 |
The level of myogenic constriction was determined as the degree of active constriction (i.e. decrease in lumen diameter) comparing arteries in calcium-free (passive diameter) and calcium-containing medium (active diameter) at the same intraluminal pressure, and expressed in per cent constriction of passive lumen diameter (see also Methods and Figure 1a and b). Data are mean±s.e.mean and represent the level of myogenic constriction at 60 mmHg. n-Values indicate number of investigated rats. P-values indicate statistical significance for differences in myogenic constriction for arteries of SHAM versus CHF rats during different conditions of acute intervention.
Indicates P<0.05 for myogenic constriction during acute intervention versus the appropriate control; when significant, the net effect of intervention is given between brackets.
Losartan was only tested in CHF rats.
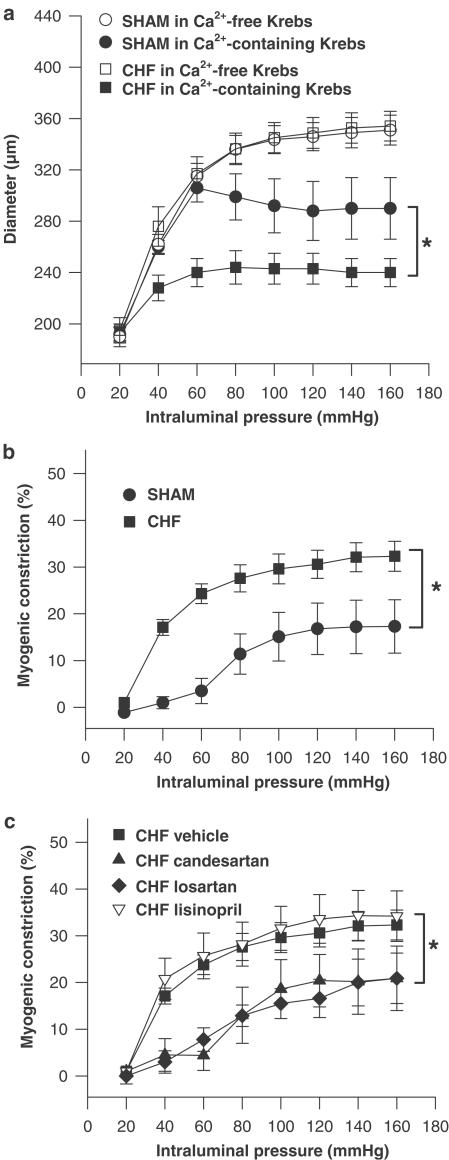
Figure 1.
(a) Pressure – diameter curves of mesenteric arteries from CHF rats (n=9) and SHAM-operated rats (n=9) determined in calcium-free Krebs solution (passive diameter) and calcium-containing Krebs solution (active diameter), respectively. (b) Myogenic constriction of mesenteric arteries from CHF rats (n=9) and SHAM-operated rats (n=9) determined as the degree of active constriction (i.e. decrease in lumen diameter) comparing arteries in calcium-free (passive diameter) and calcium-containing Krebs solution (active diameter) and expressed in per cent constriction compared to passive lumen diameter. (c) Myogenic constriction of mesenteric arteries from CHF rats in the absence of any drug (CHF vehicle, n=9) and in the presence of the AT1 receptor blocker candesartan, 100 nmol l−1 (CHF candesartan, n=5), losartan, 10 μmol l−1 (CHF losartan, n=5), or the ACE inhibitor lisinopril, 1 μmol l−1 (CHF lisinopril, n=4). *P<0.05.
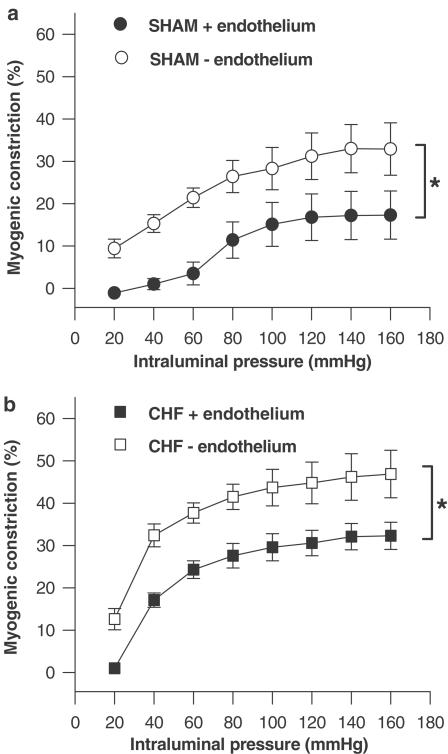
Figure 2.
(a) Myogenic constriction of mesenteric arteries from SHAM-operated rats in the presence of the endothelium (SHAM +endothelium, n=5) or in the absence of the endothelium (SHAM -endothelium, n=5). (b) Myogenic constriction of mesenteric arteries from CHF rats in the presence of the endothelium (CHF +endothelium, n=5) or in the absence of the endothelium (CHF -endothelium, n=5). *P<0.05.
Results
Rat characteristics and cardiac function
In rats with myocardial infarction (infarct size 28±2%, n=13) heart weight and heart to body weight ratio were increased, while systolic and diastolic dP dt−1 and LVESP were decreased compared to SHAM-operated rats (n=12, Table 1 ). Mean aortic blood pressure did not differ between CHF (80±5 mmHg) and SHAM rats (78±4 mmHg) (Table 1).
Table 1.
Rat characteristics and cardiac function
| SHAM | CHF | |
|---|---|---|
| (n=12) | (n=13) | |
| Rat characteristics | ||
| Infarct size (%) | — | 28±2* |
| Body weight (g) | 467±12 | 470±9 |
| Heart weight (g) | 1.28±0.02 | 1.61±0.07* |
| Heart/body weight ratio (mg g−1) | 2.78±0.05 | 3.43±0.13* |
| Aortic systolic pressure (mmHg) | 96±4 | 93±5 |
| Aortic diastolic pressure (mmHg) | 72±4 | 71±5 |
| Cardiac function | ||
| LVESP (mmHg) | 99±4 | 88±3* |
| LVEDP (mmHg) | 9±1 | 12±2 |
| Contractility (mmHg s−1) | 6451±317 | 5297±130* |
| Relaxation (mmHg s−1) | 6073±317 | 4770±166* |
| Heart rate (beats min−1) | 348±6 | 347±7 |
Data are mean±s.e.mean.
P<0.05 compared to SHAM.
Myogenic response in mesenteric arteries of CHF rats compared to SHAM controls
Passive diameters in calcium-free Krebs solution did not differ between mesenteric arteries of SHAM (n=9) and CHF rats (n=9) over the whole pressure range studied (20 – 160 mmHg; see Figure 1a). Active diameters in calcium-containing Krebs solution were significantly smaller in both groups (Figure 1a), indicating the development of myogenic constriction. The level of myogenic constriction was determined as the degree of active constriction (i.e. decrease in lumen diameter) comparing arteries in calcium-free solution (passive diameter) and calcium-containing solution (active diameter) at the same intraluminal pressure (for a total pressure-range of 20 – 160 mmHg), and expressed as per cent constriction of passive lumen diameter. As seen in Figure 1b myogenic constriction increased with increasing pressure steps reaching stable values at approximately 90 – 100 mmHg. Note that myogenic constriction was significantly increased in arteries of CHF rats as compared to SHAM over the whole pressure range (Figure 1b), most pronounced at intraluminal pressures between 40 and 80 mmHg, which lies in the range of approximated mean in vivo blood pressure values for small mesenteric arteries in the current study. These approximated values were calculated based on reports of in vivo blood pressure measurements in small arteries (250 – 400 μm) and aorta, which describe a blood pressure ratio of 0.5 – 0.96 between small arteries and aorta (Christensen & Mulvany, 2001).
The difference in myogenic constriction between arteries from SHAM and CHF rats was most pronounced at 60 mmHg. At this pressure passive diameter, wall thickness and wall-to-lumen ratio of mesenteric arteries in the current study were 315±10, 32±3, and 0.101±0.009 μm for SHAM rats (n=9); and 317±13, 28±2, and 0.092±0.010 μm for CHF rats (n=9) (P=NS for all). Active diameters decreased to 306±11 and 240±11 μm, representing myogenic constriction at 60 mmHg of 4±3 and 24±2% in arteries of SHAM and CHF rats, respectively, see Table 2. Also summarized in Table 2 is the effect of several acute interventions on the level of myogenic constriction at 60 mmHg.
Involvement of endothelium-derived factors and perivascular sympathetic nerves
Inhibition of the production of endothelium-derived relaxing factor NO using L-NMMA (100 μmol l−1, Table 2) as well as removal of the endothelium (Table 2, Figure 2) increased myogenic constriction in CHF and SHAM rats to a similar extent. Blockade of ETA receptors with BQ123 (1 μmol l−1) had no effect on the myogenic response in either group (Table 2). These results suggest that endothelium-derived constrictive/dilative mediators may not account for the difference in the myogenic response between CHF and SHAM. Furthermore, blockade of perivascular (sympathetic) nerve conduction with tetrodotoxin (100 nmol l−1) did not alter the extent of myogenic constriction in CHF arteries as well as in SHAM arteries (Table 2).
Involvement of the RAS
Blockade of AT1 receptors with candesartan (100 nmol l−1) fully reversed increased myogenic constriction in CHF arteries (Figure 1c, Table 2), whereas AT1 receptor blockade was without effect in SHAM arteries (Table 2). In contrast, acute ACE inhibition with lisinopril (1 μmol l−1, Figure 1c, Table 2) did not have any effect on myogenic constriction, and also when given intraluminally (n=3 for SHAM and CHF rats, respectively, data not shown). The same results were obtained after using a different AT1 receptor blocker (i.e., losartan, 10 μmol l−1, Figure 1c, Table 2), and a different ACE inhibitor (captopril, 10 μmol l−1, Table 2), which seems to exclude the possibility that the reversal of increased myogenic constriction in CHF arteries was due to a nonspecific action of candesartan, or that the inability to reverse increased myogenic constriction was drug specifically related to the properties of lisinopril. Furthermore, AT2 receptor blockade with PD123319 (1 μmol l−1) had no effect on myogenic constriction in either group (Table 2), suggesting that AT2 receptor-mediated dilative effects may not account for the difference in myogenic constriction. Collectively, these data suggest the active and instantaneous participation of AT1 receptors in increased myogenic constriction in CHF but without the apparent involvement of acute, ACE-mediated production of endogenous Ang II.
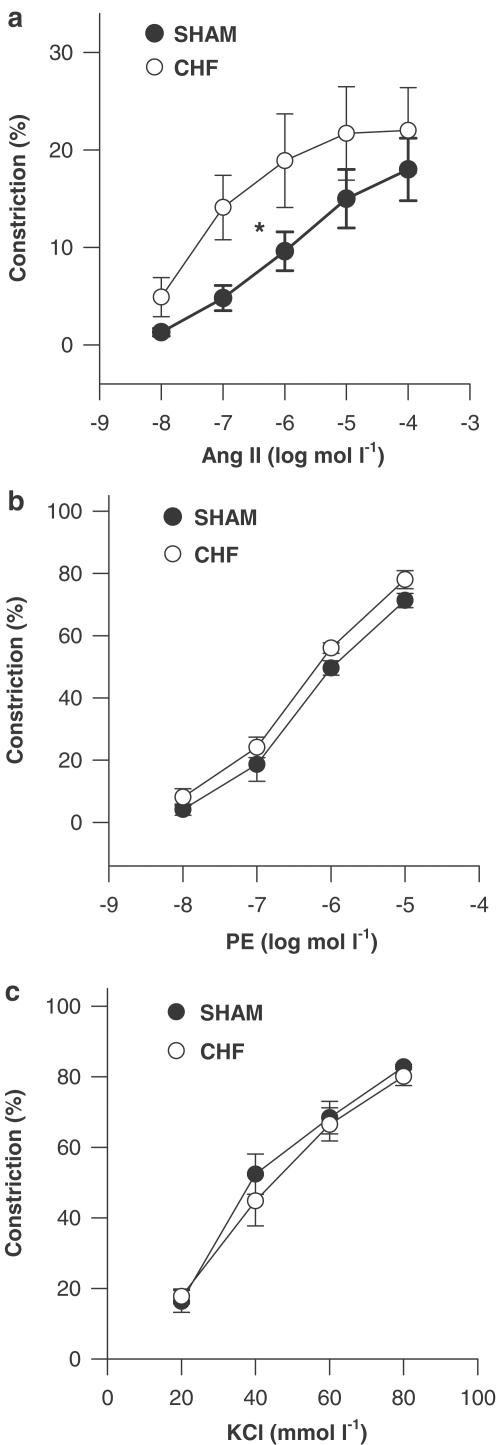
Interestingly, concentration – response curves to Ang II (performed at the intraluminal pressure at which the difference in myogenic constriction between CHF and SHAM was most pronounced, i.e. 60 mmHg) showed that the sensitivity for contractile responses to exogenous Ang II was significantly (P<0.05) increased in CHF mesenteric arteries (pD2 7.1±0.4) compared to SHAM (pD2 6.2±0.3; Figure 3a). Moreover, contractile responses to PE and KCl were similar in both groups (Figure 3b and c), thus suggesting a selective increase in sensitivity for AT1 receptor-mediated contractility instead of general alterations in contractility of arterial smooth muscle cells in CHF arteries.
Figure 3.
Concentration-dependent constriction to (a) Ang II, (b) phenylephrine (PE) and (c) KCl for mesenteric arteries of CHF rats (n=6 for each) and SHAM-operated rats (n=6 for each) determined at an intraluminal pressure of 60 mmHg and expressed in per cent constriction compared to the maximally dilated (passive) diameter of the same vessel at the same pressure. *P<0.05 for pD2 values.
Inhibition of prostaglandin production with the COX inhibitor indomethacin (10 μmol l−1) as well as TXA2 receptor blockade with SQ 29,548 (1 μmol l−1), and inhibition of superoxide production with SOD (50 U ml−1) had no effect on myogenic responses in either group (Table 2), suggesting that prostaglandins as well as superoxide production may not account for increased myogenic response in CHF mesenteric arteries in the present study.
Discussion
In the present study, we found increased pressure-induced myogenic constriction in isolated mesenteric arteries of rats with CHF as compared to SHAM controls. Furthermore, increased myogenic constriction in CHF was fully reversed by acute AT1 receptor blockade.
Mechanisms of increased vasoconstriction in CHF: role of myogenic constriction
Increased peripheral vascular resistance is a hallmark of CHF, contributing to its progressive nature and is therefore considered an important target for therapy (Zelis & Flaim, 1982; Schrier & Abraham, 1999). Several mechanisms acting at different levels may be involved, each of them having the potential to facilitate increased vasoconstriction independently but in practice acting together. First of all, neurohumoral systems at the systemic level such as the RAS, endothelin-1 (ET-1) and the sympathetic nerve system become increasingly stimulated in heart failure (Schrier & Abraham, 1999) and may act directly on VSMCs to cause vasoconstriction. Although the contribution of neurohumoral mechanisms may be of major importance in increased vasoconstriction in CHF in vivo, acute systemic interference was excluded in the present study by the fact that isolated artery preparations were studied in vitro. Furthermore, blockade of perivascular sympathetic nerve conduction with tetrodotoxin had no effect on myogenic responses in both groups.
Apart from the stimulation of neurohumoral systems, several studies have provided evidence that in CHF the vasodilative function of the endothelium is impaired (Ferrari et al., 1998). As an intact endothelium is believed to be important in limiting vasoconstrictive responses (Teerlink et al., 1994; Didion et al., 1997), that is, through the release of vasodilative NO (Boulanger & Vanhoutte, 1998), an impaired endothelial function could contribute to increased vasoconstriction in CHF. In the present study, we investigated small mesenteric artery segments, which spontaneously develop some degree of myogenic constriction when subjected to intraluminal pressure in a set-up for pressurized arteries. Removal of the endothelium or NO inhibition using L-NMMA resulted in increased myogenic constriction. Similar observations have also been reported by others for several artery types (Sun et al., 1994; Huang & Koller, 1997; Nurkiewicz & Boegehold, 1999), and may be indicative of a role of endothelial vasodilator influences in normal limitation of myogenic constriction. In contrast, however, several other studies could not demonstrate any role of the endothelium in the modulation of the myogenic response (Sun et al., 1992; Falcone & Meininger, 1999). Overall, this controversy may be due to the use of different experimental procedures to remove the endothelium, but also due to differences in the investigated species and artery type (Meininger & Davis, 1992). Importantly, however, in the current study the difference between endothelium-intact arteries of SHAM compared to CHF rats in development of myogenic constriction also persisted after endothelial removal and inhibition of NO, thus suggesting that basal endothelial function/NO-activity did not account for the observed differences between small mesenteric arteries of SHAM and CHF rats. As this basal endothelial function/NO activity measured in vitro in the absence of flow may be different from endothelial function after stimulation with substances such as acetylcholine and/or flow in vivo, we cannot exclude the possibility that endothelial dilative influences on the myogenic response may be differently pronounced under actual in vivo conditions.
Endothelial dysfunction in CHF may also involve enhanced production of vasoconstrictor compounds such as ET-1 (Kiowski et al., 1995, 1998). Circulating ET-1 levels may be chronically increased in CHF (Wei et al., 1994), but ET-1 may also be induced locally after pressure increase (Hishikawa et al., 1995; Hasdai et al., 1997; Lauth et al., 2000). Although this would leave the possibility that potentially enhanced ET-1 release in isolated CHF arteries in response to intraluminal pressure increase might have contributed to increased myogenic constriction, the lack of effect of ETA receptor blockade using BQ123 seems to exclude the involvement of ETA receptor-mediated (constrictive) effects of ET-1 in the present study. Taken together our results for the first time provide evidence of increased myogenic constriction in small resistance arteries in CHF rats as an additional (independent) mechanism causing increased vasoconstriction in CHF, potentially contributing to increased vascular resistance.
Increased myogenic constriction in CHF arteries: acute involvement of AT1 receptors
Increased myogenic constriction in CHF mesenteric arteries was fully reversed in the presence of the AT1 receptor blockers candesartan as well as losartan, whereas AT2 receptor blockade with PD123319 was without effect in both groups. Furthermore, the effect of AT1 receptor blockade was specific for CHF mesenteric arteries, as candesartan had no effect in SHAM arteries. These results suggest the specific involvement of AT1 receptors in increased pressure-induced myogenic constriction in CHF mesenteric arteries. Previously, subthreshold concentrations of Ang II have been shown to potentiate myogenic constriction in (renal) arteries of normal rats in vitro, possibly involving stimulation of shared signalling pathways for AT1 receptor-mediated and myogenic constriction (Kirton & Loutzenhiser, 1998). Moreover, alterations in (local) endogenous Ang II during dietary sodium restriction and subsequent RAS-stimulation (hence, as may also be the case in CHF) have been described to modulate myogenic constriction via AT1 receptors in arteries of normal rats (Nurkiewicz & Boegehold, 2000). We therefore wished to further investigate whether increased local Ang II production or increased sensitivity of AT1 receptors for contractile responses may underlie increased (AT1-mediated) myogenic constriction in CHF.
Neither lisinopril nor captopril significantly affected myogenic constriction in either group, even when applied to the lumen of the arteries. The latter may exclude the possibility that differences between ACE inhibitors and AT1 receptor blockers were due to the inability of the ACE inhibitors to reach ACE on the endothelial surface. The lack of effect of ACE inhibition on increased myogenic constriction in CHF arteries seems to deny the involvement of locally produced Ang II through ACE, although the potential involvement of other enzymes metabolizing angiotensins (chymase, neutral endopeptidases, etc.) was not tested in the current study. On the other hand, concentration – response curves showed a significantly increased contractile response of CHF mesenteric arteries specifically for exogenous Ang II, but not KCl and PE. The contractile response of CHF arteries has already been investigated in previous studies. Increased as well as unaltered or impaired responses to Ang II, PE and other contractile substances have been described, and the results may not provide a consistent picture (Didion et al., 1997; Stassen et al., 1997; Ikenaga et al., 1999; Miller et al., 2000). In this respect, the results may considerably depend on the postinfarct time studied and the arterial bed investigated as the vascular adaptation mechanisms may differ depending on duration and severity of CHF and in different vascular beds among which blood flow may be redistributed. Our present findings demonstrate a selective increase in the contractile response to Ang II in CHF arteries, and seem to rule out that the constrictive ability of VSMCs may have been generally altered compared to SHAM. This may indicate an increased AT1 receptor sensitivity in CHF arteries, although the potential involvement of AT2 receptor-mediated modulation of Ang II-induced constriction cannot be excluded. Several AT1 mediated interactions with other contractile mediators, that is, COX-derived prostaglandins, including thromboxane, and superoxide production, have been demonstrated to be enhanced in several forms of cardiovascular diseases such as hypertension (Wilcox & Lin, 1993; Kawazoe et al., 2000; Touyz & Schiffrin, 2000). To test if these interactions may also be present in CHF mesenteric arteries and may thereby account for increased myogenic constriction, we investigated the effect of the COX inhibitor indomethacin, the TXA2 receptor blocker SQ 29,548, and prevention of superoxide production with SOD, but none of these compounds had an effect on myogenic constriction in both groups.
Our results thus far provide evidence (1) for the selective involvement of AT1 receptors in increased myogenic constriction in CHF mesenteric arteries, (2) for the lack of involvement of ACE-derived Ang II in this response, and (3) for an increased sensitivity of CHF mesenteric arteries selectively for contractile responses to Ang II. A quantitative and/or functional upregulation of AT1 receptors in CHF arteries could account for the increased contractile response to Ang II. However, this only mildly increased AT1 receptor sensitivity (demonstrated at a concentration range well above any expected levels of tissue Ang II) may not be sufficient to explain the much more pronounced increase in myogenic response in these arteries. Although the exact mechanism underlying the AT1 receptor-mediated increase in myogenic constriction in CHF arteries cannot be determined from the present study, it seems that two hypotheses may emerge, both of which not excluding each other. First, it could be possible that in CHF arteries AT1 receptors are constitutively active in the absence of an agonist, perhaps by enhanced coupling to its G protein, as it has been described for several other G protein-coupled receptors (Leurs et al., 1998). Secondly, preformed and stored Ang II may be released upon stretch, stimulating the more sensitive AT1 receptors in CHF arteries. In this respect, stretch-mediated release of (preformed) Ang II has previously been demonstrated for cultured cardiomyocytes (Sadoshima et al., 1993; Leri et al., 1998). In both hypotheses, however, increased AT1 receptor activity in CHF arteries could potentiate the myogenic response via interactions of shared signal transduction pathways such as protein kinase C and Rho-kinase-dependent mechanisms (Kirton & Loutzenhiser, 1998; Matrougui et al., 2001; Massett et al., 2002), thereby sensitizing the smooth muscle contractile apparatus to Ca2+. Similar mechanisms of myogenic enhancement have also been described for other substances such as ET-1, TXA2, and norepinephrine (Liu et al., 1994; Ungvari & Koller, 2000).
In conclusion, we found a considerable increase in myogenic constriction in isolated mesenteric arteries from rats with CHF compared to SHAM controls, which pertained to removal of the endothelium. This emphasizes that apart from stimulated neurohumoral systems and endothelial alterations also local myogenic mechanisms are disturbed in this model of CHF and may thereby contribute to increased vascular resistance. Furthermore, we found that increased myogenic constriction was fully reversable by acute AT1 receptor blockade but not by acute ACE inhibition, and the sensitivity to contractile responses to Ang II was selectively increased in CHF mesenteric arteries compared to SHAM. This suggests active and instantaneous participation of AT1 receptors in increased myogenic constriction in CHF, involving increased sensitivity/activity of AT1 receptors per se rather than apparent ACE-mediated local Ang II production. Further studies are needed to explore exact alterations in AT1 receptors and/or postreceptor signalling underlying increased myogenic response in CHF – including the role of stored/preformed tissue Ang II – and to specify potential interactions between AT1-mediated and myogenic signal transduction mechanisms.
Acknowledgments
S.G. is a recipient of an Ubbo Emmius Grant from the Groningen University Institute of Drug Exploration (GUIDE).
Abbreviations
- ACE
angiotensin-converting enzyme
- Ang II
angiotensin II
- AT1 receptor
angiotensin II type 1 receptor
- AT2 receptor
angiotensin II type 2 receptor
- CHF
chronic heart failure
- COX
cyclooxygenase
- EGTA
ethylene glycol-bis-(b-amino ethyl ether) tetraacetic acid
- ET-1
endothelin-1
- ETA receptor
endothelin type A receptor
- L-NMMA
L-NG-monomethylarginine
- LV
left ventricle
- LVEDP
left ventricular end-diastolic pressure
- LVESP
left ventricular end-systolic pressure
- MI
myocardial infarction
- NO
nitric oxide
- PE
phenylephrine
- RAS
Renin – angiotensin system
- s.e.mean
standard error of the mean
- SOD
superoxide dismutase
- TXA2
thromboxane A2
- VSMC
vascular smooth muscle cell
References
- BOULANGER C.M., VANHOUTTE P.M. The endothelium: a modulator of cardiovascular health and disease. Dialogues Cardiovasc. Med. 1998;3:187–200. [Google Scholar]
- CHRISTENSEN K.L., MULVANY M.J. Location of resistance arteries. J. Vasc. Res. 2001;38:1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- COCKCROFT J.R., O'KANE K.P.J., WEBB D.J. Tissue angiotensin generation and regulation of vascular tone. Pharmacol. Ther. 1995;65:193–213. doi: 10.1016/0163-7258(94)00062-8. [DOI] [PubMed] [Google Scholar]
- DAVIS M.J., HILL M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- DIDION S.P., CARMINES P.K., IKENAGA H., MAYHAN W.G. Enhanced constrictor responses of skeletal muscle arterioles during chronic myocardial infarction. Am. J. Physiol. 1997;273:H1502–H1508. doi: 10.1152/ajpheart.1997.273.3.H1502. [DOI] [PubMed] [Google Scholar]
- FALCONE J.C., MEININGER G.A. Endothelin mediates a component of the enhanced myogenic responsiveness of arterioles from hypertensive rats. Microcirculation. 1999;6:305–313. [PubMed] [Google Scholar]
- FERRARI R., BACHETTI T., AGNOLETTI L., COMINI L., CURELLO S. Endothelial function and dysfunction in heart failure. Eur. Heart J. 1998;19:G41–G47. [PubMed] [Google Scholar]
- GARCIA S.R., BUND S.J. Nitric oxide modulation of coronary artery myogenic tone in spontaneously hypertensive and Wistar – Kyoto rats. Clin. Sci. 1998;94:225–229. doi: 10.1042/cs0940225. [DOI] [PubMed] [Google Scholar]
- HALPERN M., OSOL G., COY G.S. Mechanical behaviour of pressurized in vitro prearteriolar vessels determined with a video system. Ann. Biomed. Eng. 1984;12:463–479. doi: 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- HASDAI D., HOLMES D.R., JR, GARRATT K.N., EDWARDS W.D., LERMAN A. Mechanical pressure and stretch release endothelin-1 from human atherosclerotic coronary arteries in vivo. Circulation. 1997;95:357–362. doi: 10.1161/01.cir.95.2.357. [DOI] [PubMed] [Google Scholar]
- HISHIKAWA K., NAKAKI T., MARUMO T., SUZUKI H., KATO R., SARUTA T. Pressure enhances endothelin-1 release from cultured endothelial cells. Hypertension. 1995;25:449–452. doi: 10.1161/01.hyp.25.3.449. [DOI] [PubMed] [Google Scholar]
- HUANG A., KOLLER A. Endothelin and prostaglandin H2 enhance arteriolar myogenic tone in hypertension. Hypertension. 1997;30:1210–1215. doi: 10.1161/01.hyp.30.5.1210. [DOI] [PubMed] [Google Scholar]
- IKENAGA H., ISHII N., DIDION S.P., ZHANG K., CORNISH K.G., PATEL K.P., MAYHAN W.G., CARMINES P.K. Suppressed impact of nitric oxide on renal arteriolar function in rats with chronic heart failure. Am. J. Physiol. Renal. Physiol. 1999;276:F79–F87. doi: 10.1152/ajprenal.1999.276.1.F79. [DOI] [PubMed] [Google Scholar]
- KAWAZOE T., KOSAKA H., YONEYAMA H., HATA Y. Acute production of vascular superoxide by angiotensin II but not by catecholamines. J. Hypertens. 2000;18:179–185. doi: 10.1097/00004872-200018020-00008. [DOI] [PubMed] [Google Scholar]
- KIOWSKI W., SUTSCH G., HUNZIKER P., MULLER P., KIM J., OECHSLIN E., SCHMITT R., JONES R., BERTEL O. Evidence for endothelin-1 mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- KIOWSKI W., SUTSCH G., SCHALCHER C., BRUNNER H.P., OECHSLIN E. Endothelial control of vascular tone in chronic heart failure. J. Cardiovasc. Pharmacol. 1998;32:S67–S73. [PubMed] [Google Scholar]
- KIRTON C.A., LOUTZENHISER R. Alterations in basal protein kinase C activity modulate renal afferent arteriolar myogenic reactivity. Am. J. Physiol. Heart Circ. Physiol. 1998;44:H467–H475. doi: 10.1152/ajpheart.1998.275.2.H467. [DOI] [PubMed] [Google Scholar]
- LAUTH M., BERGER M.M., CATTARUZZA M., HECKER M. Elevated perfusion pressure upregulates endothelin-1 and endothelin B receptor expression in the rabbit carotid artery. Hypertension. 2000;35:648–654. doi: 10.1161/01.hyp.35.2.648. [DOI] [PubMed] [Google Scholar]
- LERI A., CLAUDIO P.P., LI Q., WANG X., REISS K., WANG S., MALHOTRA A., KAJSTURA J., ANVERSA P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin – angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J. Clin. Invest. 1998;101:1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEURS R., SMIT M.J., ALEWIJNSE A.E., TIMMERMAN H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem. Sci. 1998;23:418–422. doi: 10.1016/s0968-0004(98)01287-0. [DOI] [PubMed] [Google Scholar]
- LIU J., HILL M.A., MEININGER G.A. Mechanisms of myogenic enhancement by norepinephrine. Am. J. Physiol. 1994;266:H440–H446. doi: 10.1152/ajpheart.1994.266.2.H440. [DOI] [PubMed] [Google Scholar]
- MASSETT M.P., UNGVARI Z., CSISZAR A., KALEY G., KOLLER A. Different roles of PKC and MAP kinases in arteriolar constriction to pressure and agonists. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2282–H2287. doi: 10.1152/ajpheart.00544.2002. [DOI] [PubMed] [Google Scholar]
- MATROUGUI K., TANKO L.B., LOUFRANI L., GORNY D., LEVY B.I., TEDGUI A., HENRION D. Involvement of Rho-kinase and the actin filament network in angiotensin II-induced contraction and extracellular signal-regulated kinase activity in intact rat mesenteric resistance arteries. Arterioscler. Thromb. Vasc. Biol. 2001;21:1288–1293. doi: 10.1161/hq0801.093653. [DOI] [PubMed] [Google Scholar]
- MEININGER G.A., DAVIS M.J. Cellular mechanisms involved in the vascular myogenic response. Am. J. Physiol. Heart Circ. Physiol. 1992;263:H647–H659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- MILLER A.A., MEGSON I.L., GRAY G.A. Inducible nitric oxide synthase-derived superoxide contributes to hyperreactivity in small mesenteric arteries from a rat model of chronic heart failure. Br. J. Pharmacol. 2000;131:29–36. doi: 10.1038/sj.bjp.0703528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN T.D., VEQUAUD P., THORIN E. Effects of endothelin receptor antagonists and nitric oxide on myogenic tone and α-adrenergic-dependent contractions of rabbit resistance arteries. Cardiovasc. Res. 1999;43:755–761. doi: 10.1016/s0008-6363(99)00170-4. [DOI] [PubMed] [Google Scholar]
- NURKIEWICZ T.R., BOEGEHOLD M.A. Limitation of arteriolar myogenic activity by local nitric oxide: segment specific effect of dietary salt. Am. J. Physiol. Heart Circ. Physiol. 1999;277:H1946–H1955. doi: 10.1152/ajpheart.1999.277.5.H1946. [DOI] [PubMed] [Google Scholar]
- NURKIEWICZ T.R., BOEGEHOLD M.A. Reinforcement of arteriolar myogenic activity by endogenous Ang II: susceptibility to dietary salt. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H269–H278. doi: 10.1152/ajpheart.2000.279.1.H269. [DOI] [PubMed] [Google Scholar]
- PFEFFER M.A., PFEFFER J.M., FISHBEIN M.C., FLETCHER P.J., SPADARO J., KLONER R.A., BRAUNWALD E. Myocardial infarct size and ventricular function in rats. Circ. Res. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., SCHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYAN U.S., RYAN J.W., WHITAKER C., CHIU A. Localization of angiotensin converting enzyme (kininase II). Immunocytochemistry and immunofluorescence. Tissue Cell. 1976;8:125–145. doi: 10.1016/0040-8166(76)90025-2. [DOI] [PubMed] [Google Scholar]
- SADOSHIMA J., XU Y., SLAYTER H.S., IZUMO S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- SCHRIER R.W., ABRAHAM W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- SKARSGARD P., VAN BREEMEN C., LAHER I. Estrogen regulates myogenic tone in pressurized cerebral arteries by enhanced basal release of nitric oxide. Am. J. Physiol. 1997;273:H2248–H2256. doi: 10.1152/ajpheart.1997.273.5.H2248. [DOI] [PubMed] [Google Scholar]
- STASSEN F.R., FAZZI G.E., LEENDERS P.J., SMITS J.F., DE MEY J.G. Coronary arterial hyperreactivity and mesenteric arterial hyporeactivity after myocardial infarction in the rat. J. Cardiovasc. Pharmacol. 1997;29:780–788. doi: 10.1097/00005344-199706000-00011. [DOI] [PubMed] [Google Scholar]
- SUN D., KALEY G., KOLLER A. Characteristics and origin of myogenic response in isolated gracilis muscle arterioles. Am. J. Physiol. 1994;266:H1177–H1183. doi: 10.1152/ajpheart.1994.266.3.H1177. [DOI] [PubMed] [Google Scholar]
- SUN D., MESSINA E.J., KALEY G., KOLLER A. Characteristics and origin of myogenic response in isolated mesenteric arteries. Am. J. Physiol. 1992;263:H1486–H1491. doi: 10.1152/ajpheart.1992.263.5.H1486. [DOI] [PubMed] [Google Scholar]
- TEERLINK J.R., GRAY G.A., CLOZEL M., CLOZEL J.P. Increased vascular responsiveness to norepinephrine in rats with heart failure is endothelium dependent. Dissociation of basal and stimulated nitric oxide release. Circulation. 1994;89:393–401. doi: 10.1161/01.cir.89.1.393. [DOI] [PubMed] [Google Scholar]
- TOUYZ R.M., SCHIFFRIN E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- UNGVARI Z., KOLLER A. Endothelin and prostaglandin H2/thromboxane A2 enhance myogenic constriction in hypertension by increasing Ca2+ sensitivity of arterial smooth muscle. Hypertension. 2000;36:856–861. doi: 10.1161/01.hyp.36.5.856. [DOI] [PubMed] [Google Scholar]
- VAN GILST W.H., VAN VELDHUISEN D.J., HEGEMAN H., BUIKEMA H., SCHOLTENS E., DE GRAEFF P.A., LIE K.I. Effect of ibopamine on ventricular remodeling after experimental myocardial infarction: a comparison with captopril. J. Cardiovasc. Pharmacol. 1994;24:171–174. doi: 10.1097/00005344-199407000-00026. [DOI] [PubMed] [Google Scholar]
- WANG X., CUPPLES W.A. Interaction between nitric oxide and renal myogenic autoregulation in normotensive and hypertensive rats. Can. J. Physiol. Pharmacol. 2001;79:238–245. [PubMed] [Google Scholar]
- WEI C.M., LERMAN A., RODEHEFFER R.J., MCGREGOR C.G., BRANDT R.R., WRIGHT S., HEUBLEIN D.M., KAO P.C., EDWARDS W.D., BURNETT J.C., JR Endothelin in human congestive heart failure. Circulation. 1994;89:1580–1586. doi: 10.1161/01.cir.89.4.1580. [DOI] [PubMed] [Google Scholar]
- WILCOX C.S., LIN L. Vasoconstrictor prostaglandins and angiotensin-dependent renovascular hypertension. J. Nephrol. 1993;6:124–133. [Google Scholar]
- ZELIS R., FLAIM S.F. Alterations in vasomotor tone in congestive heart failure. Prog. Cardiovasc. Dis. 1982;14:437–459. doi: 10.1016/0033-0620(82)90012-3. [DOI] [PubMed] [Google Scholar]