Abstract
This study shows for the first time that both the putatively selective oestrogen receptor α and oestrogen receptor β agonists PPT (4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) tris-phenol) and DPN (2,3-bis(4-hydroxyphenyl)-propionitrile) can acutely relax precontracted isolated rat mesenteric arteries at pharmacological (i.e. μM) concentrations. When compared to responses observed to similar concentrations of 17β-oestrogen obtained on the same tissues, PPT had a significantly greater vasodilatory effect, while DPN had a significantly smaller effect. All responses were rapid being complete within 5 min exposure time. Thus, both PPT and DPN can acutely relax isolated mesenteric arteries with the relative potency of PPT>17β-oestrogen>DPN.
Keywords: Oestrogen receptor agonists, artery, contractility
Introduction
It is now well established that, in addition to their classical genomic action characteristic of steroid hormones, oestrogens may have more rapid effects that do not involve alterations in protein synthesis. These effects are often seen at high (i.e. μM) concentrations (see Austin, 2000; Levin, 2001). We have previously shown, for example, that acute application of 17β-oestradiol results in a rapid vasodilatory response in isolated small arteries precontracted by a variety of stimuli, responses being independent of the precontracting agent used. This endothelium-independent response was reversible upon washout and was unaffected by inhibitors of protein synthesis (Shaw et al., 2000). Despite much recent interest in these acute actions, the mechanisms involved, particularly in the cellular point of interaction, remain unclear.
The classical genomic actions of oestrogens are mediated via interactions with oestrogen receptors (ERs). The first ER, now known as ERα, was cloned more than 20 years ago (Green et al., 1986); however, it is now apparent that a second receptor subtype, ERβ also exists (Kuiper et al., 1996). Both of these receptor subtypes are found intracellularly where they act to modulate transcription in the nucleus — they are both found in vascular smooth muscle (Register & Adams, 1998). It is unclear, however, whether interactions with one, or both, of these receptor subtypes may also be responsible for the nongenomic effects of oestrogens.
We have previously shown that oestrogens, rendered membrane-impermeant by conjugation to albumin, have a similar effect on isolated arterial contractility to membrane-permeable oestrogens (Shaw et al., 2000). Rapid nongenomic effects of cell-impermeant oestrogens, including effects on calcium homeostasis, have also been reported in other cell types suggesting that they may be modulated by oestrogenic binding with a site and/or receptor on the plasma membrane (for further discussion, see Levin, 2001). Indeed, direct evidence for membraneous binding of oestrogens has been obtained by using fluorescently labelled membrane-impermeant oestrogen conjugates (Pappas et al., 1995). Binding of antibodies directed against various regions of the nuclear ERα has been demonstrated in the plasma membrane of nonvascular cells (Pappas et al., 1995) and transfection of ovary cells with cDNA for both ERα and ERβ demonstrated that both receptor subtypes may be expressed (although significantly less than in the nucleus) in the plasma membrane (Razandi et al., 1999). Taken together, these observations suggest that there may exist cellular membraneous receptors/binding sites for oestrogens that are responsible for their acute nongenomic actions, although nonspecific effects of oestrogens, for example, on Ca2+ influx mechanisms, cannot be discounted.
To date, it has been difficult to investigate directly the involvement of ER subtypes in acute oestrogenic responses such as the effects on arterial contractility due to an absence of ER subtype selective agonists. Although there are anti oestrogens available, these are generally nonspecific and do not differentiate clearly between the two receptor subtypes (for discussion, see Austin, 2000). The mode of action of these anti oestrogens is poorly understood and indeed it has been suggested that their effects on oestrogen-mediated transcription may be due to binding to a high-affinity site, which is distinct from the ER (Parisot et al., 1995). As such, these antioestrogens may not influence nongenomic actions of oestrogens in a similar manner and, in the absence of any clear mechanistic information, their use is thus limited. Recently, however, two putatively selective ER agonists have become available. PPT ((4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) tris-phenol) has been shown to have a 410-fold binding affinity preference for the ERα compared to the ERβ receptor (Kraichely et al., 2000; Stauffer et al., 2000), while DPN (2,3-bis(4-hydroxyphenyl)-propionitrile) exhibits a 70-fold higher relative binding affinity and 170-fold higher relative potency in transciption assays with ERβ than with ERα (Meyers et al., 2001). The vascular actions of these compounds have not been determined. The aim of the present study therefore was to investigate, for the first time, the acute effects of these compounds on arterial contractility.
Methods
Vessel isolation and mounting
Male Wistar rats (200–250 g) were killed by stunning and decapitation. Mesenteric arteries were dissected out and cleared of fat and connective tissue. Small ring segments (2–3 mm length) were mounted between two thin wires on a Mulvany–Halpern myograph for measurement of isometric tension as has previously been described (Mulvany & Halpern, 1977). All vessels were bathed in physiological salt solution (PSS) of the following composition (mM): NaCl 119, KCl 4.7, MgSO4·7H2O 1.2, NaHCO3 25, KH2PO4 1.17, EDTA 0.03, glucose 5.5, calcium 2.5 at pH 7.4 (gassed with 95% air/5% CO2 at 37°C) and were left to equilibrate for 1 h. Subsequently, the resting tension to internal circumference of arteries was determined and the vessels set to a normalized internal circumference of l0, where l0 is 0.9l100 and l100 is the internal circumference of the vessel under an effective transmural pressure of 100 mmHg. Under these conditions, maximum active tension is developed (Mulvany & Halpern, 1977). Following normalization, vessels were subjected to a routine ‘run up' procedure consisting of 2 × 5 min exposures to 60 mM KCl (KPSS) and 1 × 5 min exposure to KPSS+10 μM noradrenaline (NA) (KCl was isosmotically substituted for NaCl).
All vessels relaxed in response to addition of the endothelium-dependent vasodilator acetylcholine (50 μM).
Experimental protocol
Most experiments were carried out on tissues precontracted by addition of KPSS. This produced maintained contractions over the necessary experimental time (−15 min). We have previously shown that the response to 17β-oestradiol is similar in tissues precontracted by a variety of agonists (Shaw et al., 2000).
Cumulative additions of 17β-oestradiol, the putatively selective ERα agonist PPT and to the ERβ agonist DPN over the concentration range 10 nM–30 μM were made. Although we have previously shown that the acute vasodilatory effects of 17β-oestradiol are fully reversible (Otter & Austin, 1998), the reversibility of any effects of DPN and PPT was unknown. To allow direct paired comparisons to be made, concentration-dependent responses to 17β-oestradiol were initially obtained and then, following washing, responses to either DPN or PPT were studied. In initial experiments, the effects of repeated 17β-oestradiol exposure was examined.
After the final concentration–response curve (to either 17β-oestradiol, DPN or to PPT), tissues were washed several times and, following a period of 30 min, then challenged with 2 × 5 min exposures to KPSS alone and 1 × 5 min exposure to KPSS+10 μM NA. Responses obtained to KPSS alone (second exposure) and KPSS+NA were compared to those obtained before agonist exposure to determine reversibility of any effects observed. Some experiments were carried out in tissues precontracted by U46619 (10 μM). This again produced maintained contractions over the necessary experimental time.
Drugs and chemicals
DPN and PPT were dissolved in 100% ethanol to a concentration of 10 mM (17β-oestradiol and DPN) and 1 mM (PPT). Subsequent dilutions of these initial solutions were thus made with KPSS to provide stock solutions of 100 μM. Preliminary experiments revealed that ethanol itself, at similar concentrations used to dissolve the compounds, had no effect on tone. NA and U46619 were dissolved in PSS.
PPT and DPN were obtained from Tocris Cookson Ltd (Bristol, U.K.). All other drugs and chemicals were obtained from Sigma, U.K.
Analysis of data
Responses were recorded on a previously calibrated chart recorder and were expressed as changes in active wall tension (mN mm−1) from resting levels (calculated as force divided by twice the vessel segment length). Responses to 17β-oestradiol, DPN and PPT were normalized as a percentage of the response to 60 mM KCl obtained on each tissue. Responses to DPN or to PPT were compared to similar concentrations of 17β-oestradiol obtained on the same tissue by use of analysis of variance followed by the paired Student's t-test (two-tail). Differences were taken to be statistically significant if P<0.05, n=number of animals. A maximum of two tissues were used from each animal.
Results
The normalized resting diameter of arteries used in these experiments was 326±8 μM (n=18).
Effects of repeated exposures to 17β-oestradiol
As we have previously shown, 17β-oestradiol acutely relaxes isolated rat mesenteric arteries precontracted by KPSS. With cumulative additions of 17β-oestradiol, no significant relaxation was observed until a concentration of 0.3 μM was reached. In the current experiments, near-maximal relaxation (as % KCl) was observed with a concentration of 30 μM. Following washout and further preconstriction with KPSS, a second cumulative concentration–response curve to 17β-oestradiol was constructed. Responses observed in this second curve were almost identical to those observed in the first curve (n=3), relaxations being 16.86±10.27 and 14.30±6.32% for 0.3 μM; 41.52±14.66 and 43.19±8.20% for 1 μM; 66.86±18.83 and 75.67±13.78% for 3 μM, 86.92±11.46 and 84.15±11.11% for 10 μM and 99.21±13.24 (1.20±0.22 mN mm−1) and 101.16±12.16% (1.40±0.38 mN mm−1) KPSS response for 30 μM 17β-oestradiol. All responses were rapid, that is, complete within 5 min. Contractile responses to KPSS were similar in both experiments (1.30±0.23 and 1.29±0.26 mN mm−1 for first and second experiments, respectively). We have previously shown responses to 17β-estradiol in U46619 contracted tissues to be similar to those observed in depolarized tissues (Shaw et al., 2000).
Effects of PPT on vascular tone
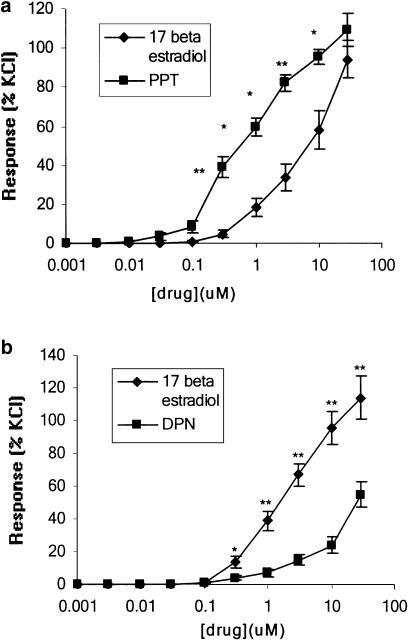
Addition of the putatively selective ERα agonist PPT resulted in a rapid (<5 min for completion) concentration-dependent relaxation of isolated rat mesenteric arteries precontracted with KPSS over the concentration range 0.01–30 μM (Figure 1a). The relaxation of arteries to PPT was significantly greater at concentrations of 0.1–10 μM than those observed to similar concentrations of 17β-oestradiol on the same tissues (n=8) (Figure 1a). Maximum responses obtained to 30 μM of drug were 94.37±9.36% (1.38±0.24 nM/mm) for 17β-oestradiol and 109.60±8.44% (1.64±0.21 nM/mm) for PPT. Contractile responses to KPSS were similar in both experiments (1.36±0.12 and 1.41±0.13 mN mm−1 for first and second experiments, respectively). Responses to PPT were similar in tissues precontracted by U46619 (2.78±2.78% for 0.03 μM; 23.07±7.23% for 0.1 μM; 41.67±4.40% for 0.3 μM; 81.67± 15.90% for 1 μM; 92.77±18.20% for 3 μM; 99.43±17.81% for 10 μM and 100±17.32% U46619 max. for 30 μM PPT) (n=3).
Figure 1.
A comparison of the effects of 17β-oestradiol and (a) PPT and (b) DPN on the contractility of isolated rat mesenteric arteries precontracted with KPSS alone. **P<0.005.
Effects of DPN on vascular tone
Addition of the ERβ agonist DPN to arteries precontracted by KPSS resulted in a rapid (<5 min for completion) concentration-dependent relaxation over the concentration range 0.1–30 μM. This was a similar concentration range to that required to produce a vasodilatory response to 17β-oestradiol in the same tissue (Figure 1b). When compared to responses observed to similar concentrations of 17β-oestradiol, however, responses to DPN were significantly smaller over the concentration range 0.3–30 μM (n=10) (Figure 1b). Responses were 113.83±13.00% (1.67±0.39 mN mm−1) and 54.46± 7.6% (0.76±0.13 mN mm−1) for 30 μM 17β-oestradiol and DPN, respectively. Contractile responses to KPSS were similar in both experiments (1.52±0.32 and 1.51±0.35 mN mm−1 for first and second experiments, respectively). Responses to DPN were again similar in tissues precontracted by U46619 (2.08±2.07% for 1 μM; 24.66±7.15% for 3 μM; 37.10± 4.79% for 10 μM and 49.81±2.80% U46619 max. for 30 μM PPT) (n=4).
Reversibility of PPT and DPN
To examine the reversibility of responses to 17β-oestradiol, PPT and DPN constrictory responses to KPSS and KPSS with NA were compared at the start of the experiment with those obtained after addition of the final agonist concentration after 30 min of washout. Responses to KPSS following exposure to 17β-oestradiol alone at the end of the experiment (1.51± 0.23 mN mm−1) were similar to those obtained initially (1.62±0.40 mN mm−1) (133.12±24.06% of initial responses) (n=4) as were responses following exposure to DPN (95.24±14.05% of initial response) (initial response=1.82± 0.32 mN mm−1; final response=1.62±0.34 mN mm−1) (n=10). Exposure to PPT, however, resulted in a near-complete inhibition of the KPSS response (0.01±0.003% of initial response) (initial response=1.72±0.40 mN mm−1; final response=0.01±0.001 mN mm−1) (n=8).
Similarly, responses to combined addition of KPSS and NA were unaffected by exposure to 17β-oestradiol alone or with DPN over the time course of the experiment responses being 95.32±8.19% for oestradiol alone (initial response=2.53± 0.72 mN mm−1; final response=2.43±0.73 mN mm−1) and 107.70±5.67% for oestradiol and DPN (initial response= 2.72±0.41 mN mm−1; final response=2.81±0.40 mN mm−1) (n=10). Responses were, however, significantly reduced (54.52±8.84%) following exposure to PPT (initial response=2.20±0.34 mN mm−1; final response=1.15± 0.25 mN mm−1).
Discussion
The results of the present study show, for the first time, that the putatively selective ERα and ERβ agonists PPT and DPN can acutely modulate isolated arterial contractility in a concentration-dependent manner. When compared to vasodilatory responses to similar pharmacological concentrations of 17β-oestradiol in the same tissues, responses to PPT were significantly greater, while those to DPN were significantly smaller.
The vasodilatory responses to both PPT and DPN were rapid–as such they are unlikely to be genomic in nature. Indeed, the time course of the responses is similar to that for acute vasodilatory responses to 17β-oestradiol, which we have previously shown to be unaffected by inhibitors of protein synthesis (Shaw et al., 2000). These oestrogenic responses are independent of the endothelium but are accompanied by a reduction in the concentration of smooth muscle intracellular free calcium ([Ca2+]i) (Shaw et al., 2001). This decrease is thought to be due to a direct inhibition of Ca2+ entry via L-type Ca2+ channels (Crews & Khalil, 1999) rather than, in vascular smooth muscle at least, via effects on calcium activated potassium channels (Shaw et al., 2001). In the present study, it was found that contractile responses to KPSS alone (which involves almost entirely Ca2+ influx) were ablated following exposure to PPT but not following exposure to 17β-oestradiol or DPN; responses to KPSS and NA meanwhile (NA contractions being mediated in part via intracellular Ca2+ release) were almost halved following PPT exposure. These findings suggest that at least part of the vasodilatory influence of PPT may be via an inhibition of smooth muscle cell voltage-gated Ca entry. Further studies are clearly required, however, to elucidate the acute effects of PPT and DPN on Ca2+ homeostasis.
The cellular site of interaction that mediates these effects of DPN and PPT remains to be determined. It is difficult to examine directly the involvement of ERs in these responses. We have previously shown that acute effects of 17β-oestradiol are unaffected by the antioestrogen ICI 182,780 (Shaw et al., 2000). Although this may be taken to suggest that ERs are not involved in this response, the mode of action of these compounds is poorly understood. Although they clearly inhibit transcription at the nuclear ER, it is unclear how they actually do this and indeed it has been reported that they may actually bind to a high-affinity site distinct from the ER itself (Parisot et al., 1995). As such it is likely that they do not act by preventing the bind of oestrogen to its receptor(s). It is perhaps not surprising that nongenomic effects of oestrogen are unaffected by such compounds. It has been shown that large arteries from ERβ knockout mice show an enhanced sensitivity to the acute vasodilatory effects of 17β-oestradiol suggesting that the absolute and/or relative expression of the oestrogen receptor subtypes is important in mediating this response (Nilsson et al., 2000). As discussed previously, there is a great deal of evidence to suggest that both ERα and ERβ can be localized to the plasma membrane and that the rapid, acute effects of 17β-oestradiol, including those on Ca entry and contractility, are mediated by membraneous binding (see Austin, 2000 for further discussion). As such, together with the present findings, it is possible that the rapid effects of PPT and DPN on contractility are mediated via actions at the plasma membrane. Although the differences in sensitivity noted in the present study suggests that there may be ER subtype differences in effects on these mechanisms, alternative direct effects of the two agonists, such as an inhibition of Ca2+entry mechanisms, cannot be discounted.
The selectivity of PPT and DPN for the receptor subtypes over the concentrations used in the current experiments is another factor that must be considered. In binding assays in cultured cell lines, PPT has been shown to have a very high relative binding for ERα; 49±12% that of oestradiol compared to 0.12±0.04% for ERβ. Indeed, in transcriptional assays, PPT failed to activate the ERβ-receptor even at micromolar concentrations (Kraichely et al., 2000). DPN has a 70-fold higher relative binding affinity for ERβ than ERα and a 170-fold higher relative potency in transcription assays (Meyers et al., 2001). It is unclear to what concentration this selectivity is evident, but it is worth noting that significant differences in responses to DPN and oestradiol were observed in the present experiment even at the highest ones used.
In conclusion, this study presents the first data concerning the acute effects of the putatively selective ERα and ERβ agonists PPT and DPN. We have shown that both compounds acutely relax isolated rat mesenteric arteries with an order of potency of PPT>17β-oestradiol>DPN. Further studies using these agonists will provide invaluable information regarding the possible ER subtype-specific mechanisms of actions of oestrogens on the vasculature.
Abbreviations
- DPN
(2,3-bis(4-hydroxyphenyl)-propionitrile)
- ERs
oestrogen receptors
- KPSS
high potassium physiological salt solution
- NA
noradrenaline
- PPT
4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) tris-phenol)
- PSS
physiological salt solution
References
- AUSTIN C.E. Chronic and acute effects of oestrogens on vascular contractility. J. Hypertens. 2000;18:1365–1378. doi: 10.1097/00004872-200018100-00003. [DOI] [PubMed] [Google Scholar]
- CREWS J.K., KHALIL R.A. Antagonistic effects of 17β-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 1999;19:1034–1040. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- GREEN S., WALTER P., KUMAR V., KRUST A., BONERT J.M, ARGOS P., CHAMBON P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- KRAICHELY D.M., SUN J., KATZENELLENBOGEN J.A., KATZENELLENBOGEN B.S. Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-α and estrogen receptor-β. Endocrinology. 2000;141:3534–3545. doi: 10.1210/endo.141.10.7698. [DOI] [PubMed] [Google Scholar]
- KUIPER G.G.J.M., ENMARK E., PELTO-HUIKKO M., NILSSON S., GUSTAFSSON J.A. Cloning of a novel estrogen receptor expressed in rat prostrate and ovary. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN E.R. Cell localization, physiology, and nongenomic actions of estrogen receptors. J. Appl. Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- MEYERS M.J., SUN J., CARLSON K.E., MARRINER G.A., KATZENELLENBOGEN B., KATZENELLENBOGEN J.A. Estrogen receptor-beta potency-selective ligands: structure–activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NILSSON B.O., EKBLAD E., HEINE T., GUSTAFSSON J.A. Increased magnitude of relaxation to oestrogen in aorta from oestrogen receptor beta knock-out mice. J. Endocrinol. 2000;166:R5–R9. doi: 10.1677/joe.0.166r005. [DOI] [PubMed] [Google Scholar]
- OTTER D., AUSTIN C. Effects of 17β-oestradiol on rat isolated coronary and mesenteric artery tone. J. Pharm. Pharmacol. 1998;50:531–538. doi: 10.1111/j.2042-7158.1998.tb06195.x. [DOI] [PubMed] [Google Scholar]
- PAPPAS T.C., GAMETCHU B., WATSON C.S. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- PARISOT J.P., HU X.F., SUTHERLAND R.L., WAKELING A., ZALCBERG J.R., DELUISE M. The pure antiestrogen ICI 182,780 binds to a high-affinity site distinct from the estrogen receptor. Int. J. Cancer. 1995;62:480–484. doi: 10.1002/ijc.2910620420. [DOI] [PubMed] [Google Scholar]
- RAZANDI M., PEDRAM A., GREENE G.L., LEVIN E.R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- REGISTER T.C., ADAMS M.R. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor β. Steroid Biochem. Mol. Biol. 1998;64:187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- SHAW L., TAGGART M.J., AUSTIN C. Mechanisms of 17β-oestradiol induced vasodilatation in isolated pressurized rat small arteries. Br. J. Pharmacol. 2000;129:555–565. doi: 10.1038/sj.bjp.0703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW L., TAGGART M.J., AUSTIN C. Acute 17β-oestradiol-induced decrease in intracellular calcium and relaxation in stimulated isolated rat mesenteric arteries. J. Physiol. 2001;92P:533P. [Google Scholar]
- STAUFFER S.R., COLETTA C.J., TEDESCO R., NISHIGUCHI G., CARLSON K., SUN J., KATZENELLENBOGEN B.S., KATZENELLENBOGEN J.A. Pyrazole ligands: structure–affinity/activity relationships and estrogen receptor-alpha-selective agonists. J. Med. Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]