Abstract
Increased oxidative stress plays a significant role in the etiology of cardiovascular disease. Lipid peroxidation, initiated in the presence of hydroxy radicals resulting in the production of malondialdehyde, directly produces oxidative stress. This study was designed to examine the direct impact of malondialdehyde on ventricular contractile function at the single cardiac myocyte level. Ventricular myocytes from adult rat hearts were stimulated to contract at 0.5 Hz, and mechanical and intracellular Ca2+ properties were evaluated using an IonOptix Myocam® system. Contractile properties analyzed included peak shortening amplitude (PS), time-to-PS (TPS), time-to-90% relengthening (TR90), maximal velocity of shortening/relengthening (±dLdt), and Ca2+-induced intracellular Ca2+ fluorescence release (CICR) and intracellular Ca2+ decay (τ). p38 mitogen-activated protein (MAP) kinase phosphorylation was assessed with Western blot.
Our results indicated that malondialdehyde directly depressed PS, ±dLdt and CICR in a concentration-dependent manner and shortened TPS without affecting TR90 and τ. Interestingly, the malondialdehyde-induced cardiac mechanical effect was abolished by both the p38 MAP kinase inhibitor SB203580 (1 and 10 μM) and the antioxidant vitamin C (100 μM). Western blot analysis confirmed direct phosphorylation of p38 MAP kinase by malondialdehyde.
These findings revealed a novel role of malondialdehyde and p38 MAP kinase in lipid peroxidation and oxidative stress-associated cardiac dysfunction.
Keywords: Malondialdehyde, myocyte shortening, intracellular Ca2+, MAP kinase, antioxidant
Introduction
Oxidation of polyunsaturated fatty acids of membranes is a common process in the living organism, since polyunsaturated fatty acids in the sarcolemma and sarcoplasmic reticulum are extremely vulnerable to the oxygen-derived free radicals produced during mitochondrial electron transport, which initiates lipid peroxidation (Porter et al., 1995). Hydroxy radicals extract an electron from polyunsaturated lipids generating high-energy lipid compounds. Lipid radicals then react with oxygen to form lipid peroxy radicals (LOO•) and lipid peroxide (LOOH). The peroxy radicals react and transfer electrons with neighboring lipids initiating a chain reaction that destroys the integrity of the membrane. Lipid peroxidation affects membrane permeability and alters membrane-bound enzymes/ion channels, which disturbs ion transport and can lead to Ca2+ overload (Kaneko et al., 1994; Dhalla et al., 1996; Buffon et al., 2000). Lipid peroxidation has been implicated in the pathogenesis of cardiovascular diseases such as heart failure and atherosclerosis (Holvoet et al., 1995; Diaz-Velez et al., 1996). Both enzymatic and nonenzymatic antioxidant mechanisms including α-tocopherol, ascorbate (vitamin C), uric acid, catalase, superoxide dismutase (SOD) and glutathione peroxidase have been demonstrated to protect against the deleterious effects of free radicals and lipid peroxidation. Nevertheless, the precise nature involving lipid peroxidation-related ventricular dysfunction or the antioxidant-elicited protection against heart failure has not been elucidated.
Malondialdehyde is a terminal product of lipid peroxidation, which can be measured in plasma and serves as an effective lipid peroxidation marker and an indirect index of reactive oxygen species (ROS) activity (Gutteridge et al., 1990). Although enhanced malondialdehyde levels have been reported in patients with compromised heart condition (Diaz-Velez et al., 1996), its direct impact on the ventricular function has been masked somewhat by the fact that other concomitant factors such as enhanced production of free fatty acids (FFAs) or harmful FFA metabolites (Hendrickson et al., 1997), localized cytokine secretion (interacting with NO synthase) (Katz et al., 1994; Torre-Aminone et al., 1996) and altered protein metabolism (Berlett et al., 1997; Kooy et al., 1997) appear to play a role in decreased myocardial contractility and/or intracellular Ca2+ homeostasis. Therefore, the aim of this present study is to elucidate the role of malondialdehyde on cardiac contractile function at the cellular level by evaluating myocyte shortening and intracellular Ca2+ properties in isolated rat ventricular myocytes.
Methods
Isolation of ventricular myocytes
The experimental procedures described in this study were approved by the institutional animal care and use committee of University of North Dakota (Grand Forks, ND, U.S.A.). Single ventricular myocytes were isolated from adult male Sprague–Dawley rats (200–225 g) as described previously (Wold et al., 2002). Briefly, hearts were rapidly removed and perfused (at 37°C) with oxygenated (5% CO2–95% O2) Krebs–Henseleit bicarbonate (KHB) buffer (mm: NaCl 118, KCl 4.7, CaCl2 1.25, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, N-[2-hydro-ethyl]-piperazine-N′-[2-ethanesulfonic acid] (HEPES) 10, glucose 11.1, pH 7.4). Hearts were subsequently perfused with a nominally Ca2+-free KHB buffer for 2–3 min followed by a 20 min perfusion with Ca2+-free KHB containing 223 U/ml-1 type II collagenase (Worthington Biochemical Corporation, Freehold, NJ, U.S.A.) and 0.1 mg/ml−1 hyaluronidase (Sigma Chemical, St Louis, MO, U.S.A.). After perfusion, the left ventricle was removed, minced and further digested with trypsin (Sigma) before being filtered through a nylon mesh (300 μm) and collected by centrifugation. Cells were initially washed with Ca2+-free KHB buffer to remove remnant enzyme and extracellular Ca2+ was added incrementally back to 1.25 mM.
Myocyte shortening and relengthening
Mechanical properties of ventricular myocytes were assessed by an IonOptix Myocam system (IonOptix Inc., Milton, MA, U.S.A.). Cells were placed in a chamber mounted on the stage of an inverted microscope and superfused (at 25°C) with a buffer containing (in mM): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, at pH 7.4. The cells were field stimulated at a frequency of 0.5 Hz. Cell shortening and relengthening were assessed using the following indices: peak shortening (PS), time-to-90% PS (TPS) and time-to-90% re-lengthening (TR90), maximal velocities of shortening (+dLdt) and relengthening (−dLdt) (Wold et al., 2002). To test the effect of malondialdehyde on cardiac contraction, cell shortening was recorded before and 5 min after malondialdehyde application at concentrations 10−9–10−3 M) encompassing the expected physiological range. In some experiments, the antioxidant vitamin C (100 μM,) or p38 mitogen-activated protein (MAP) kinase inhibitor SB203580 (1 and 10 μM) was present prior to and during malondialdehyde application to the myocytes.
Intracellular Ca2+ fluorescence measurement
Myocytes were loaded with fura-2/AM (0.5 μM) for 10 min and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (Ionoptix) as described (Wold et al., 2002). Myocytes were plated on glass cover slips on an Olympus IX-70 inverted microscope and imaged through a Fluor × 40 oil objective. Cells were exposed to light emitted by a 75 W lamp and passed through either a 360 or a 380 nm filter (bandwidths were±15 nm), while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480 and 520 nm after first illuminating cells at 360 nm for 0.5 s then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 nm excitation scan was repeated at the end of the protocol. Qualitative changes in intracellular Ca2+ levels were inferred from the ratio of the fluorescence intensity at two wavelengths (360*380−1) and were presented as changes of fura-2 fluorescence intensity (ΔFFI). Intracellular Ca2+ removal was evaluated as the rate of fluorescence decay (τ).
Immunoblot analysis of p38 MAP kinase phosphorylation
To determine phosphorylation of p38 MAP kinase, immunoblot analysis was performed using the method as described by Pollock et al. (1993). Briefly, cell lysates of cardiac myocyte (25 μg) were electrophoresed on 10% denaturing sodium dodecyl sulfate polyacrylamide gels. The proteins were transferred electrophoretically to polyvinylidene difluoride (PVDF) membrane (Gelman Sciences, Pierce, Ann Arbor, MI, U.S.A.). Nonspecific binding sites on the membrane were blocked with 5% nonfat dry milk in Tris-HCl containing 0.2% Tween-20 buffer (TBST) overnight at 4°C. The membranes were then probed with phospho-p-38-MAP kinase antibody (1 : 2000; Santa Cruz Biotech, Santa Cruz, CA, U.S.A.) for 1 h at room temperature. After five washings in TBST, the membranes were incubated with the secondary anti-body (1 : 4000) for 1 h at room temperature. Finally, membranes were washed three times with TBST. The specific proteins were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, U.S.A.). The blots were analyzed using Un-Scan-It software to estimate the density of the bands in pixels. The positive control used was NIH -3T3 WCL.
Data analysis
Data were presented as mean±s.e.m. Statistical significance (P<0.05) for each variable was estimated by analysis of variance (ANOVA).
Results
Effect of malondialdehyde on myocyte shortening (PS)
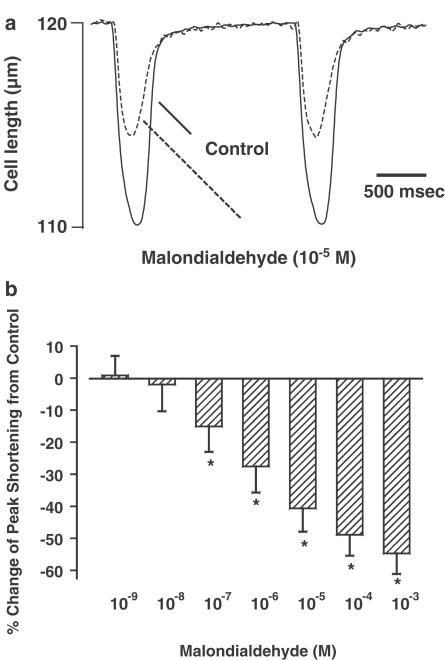
The average cell length used in this study was 103±4 μM (n=73). Acute exposure of malondialdehyde did not affect resting myocyte cell length over the concentration range tested. A representative trace depicting the effect of malondialdehyde (10−5 M) on myocyte shortening (PS) is shown in Figure 1a. At the end of a 5 min exposure to this concentration of malondialdehyde, PS was inhibited by 44.6%. Malondialdehyde (10−9–10−3 M) elicited a concentration-dependent decrease in PS, with a maximal inhibition of 54.7%. The threshold of response was between 10−8 and 10−7 M (Figure 1b). The malondialdehyde-induced inhibition in cell shortening was maximal within 4 min of exposure, maintainable up to 20 min and reversible upon a 5 min washout (data not shown). The inhibitory effect of malondialdehyde on myocyte contractility was associated with reduced maximal velocity of shortening/relengthening (±dLdt), although the threshold of depression was between 10−8 and 10−7 M. The duration of shortening (TPS) but not that of relengthening (TR90) was shortened by malondialdehyde (Table 1 ).
Figure 1.
(a) Representative traces depicting the effect of malondialdehyde (10−5 M) on cell shortening in ventricular myocytes. (b) Concentration-dependent response of malondialdehyde (10−9–10−3 M) on peak cell shortening. Data are presented as percent change from basal PS, which was 9.22±0.83%. Mean±s.e.m., n=22 per data group, *P< 0.05 vs baseline value.
Table 1.
Effect of malondialdehyde (MDA) on duration (TPS and TR90) and maximal velocity (±dLdt) of myocyte shortening and relengthening in the absence and presence of the p38 MAP kinase inhibitor SB203580 (10 μm)
| TPS (ms) | TR90 (ms) | +dLdt (μm s-1) | −dLdt (μm s−1) | |
|---|---|---|---|---|
| Control | 169±7 | 244±15 | 126.6±8.0 | −107.5±8.2 |
| MDA 10−9 M | 153±7 | 272±16 | 124.6±10.4 | −90.4±8.0 |
| MDA 10−8 M | 164±9 | 268±17 | 113.7±11.3 | −91.7±10.6 |
| MDA 10−7 M | 163±8 | 255±18 | 96.9±9.2* | −75.2±7.9* |
| MDA 10−6 M | 163±7 | 268±21 | 78.9±7.7* | −61.8±6.9* |
| MDA 10−5 M | 148±5* | 252±20 | 67.7±7.8* | −52.3±8.4* |
| MDA 10−4 M | 143±7* | 249±20 | 57.7±6.4* | −46.5±6.2* |
| MDA 10−3 M | 144±6* | 248±30 | 53.4±6.5* | −53.0±8.5* |
| Control+SB203580 | 156±7 | 245±25 | 139.3±16.6 | −115.3±13.2 |
| MDA 10−4 M+SB203580 | 155±7 | 257±28 | 132.3±13.9 | −129.2±19.9 |
| MDA 10−3 M+SB203580 | 155±6 | 259±24 | 122.7±13.9 | −106.0±12.7 |
TPS, Time-to-peak shortening: TR90, time-to-90% relengthening: ±dLdt, maximal velocities of shortening and relengthening. Data represent mean±s.e.m., n=20–22 cells
P<0.05 vs control value.
Effect of malondialdehyde on intracellular Ca2+ transients
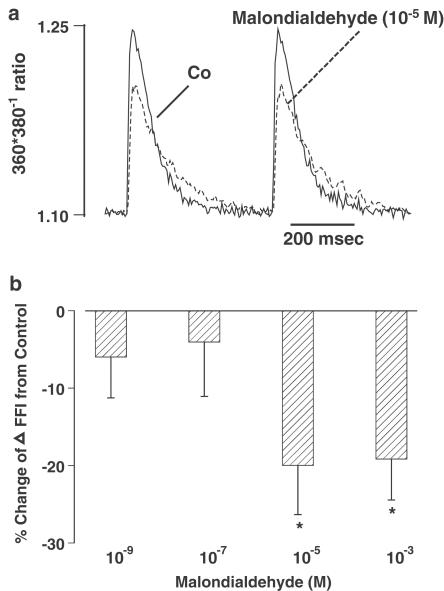
To determine whether malondialdehyde-induced inhibition of PS was due to reduced availability of intracellular Ca2+, the influence of malondialdehyde on electrically stimulated increases in intracellular Ca2+ (ΔFFI) was examined. Representative traces of intracellular Ca2+ transients shown in Figure 2a depict that malondialdehyde (10−5 M) significantly depressed ΔFFI. Bar graphs in Figure 2b further revealed that malondialdehyde elicited a concentration-dependent (10−9–10−3 M) inhibition on ΔFFI, with a threshold between 10−7 and 10−5 M. The difference in the threshold between malondialdehyde-induced inhibition of myocyte shortening and that of intracellular Ca2+ transients suggests possible involvement of Ca2+-independent mechanisms. In addition, resting intracellular Ca2+ levels and intracellular Ca2+ decay rate were unaffected by malondialdehyde (Table 2 ).
Figure 2.
(a) Representative traces depicting effect of malondialdehyde (10−5 M) on intracellular Ca2+ transient changes (ΔFFI) in ventricular myocytes. (b) Concentration-dependent response of malondialdehyde (10−9–10−3 M) on ΔFFI. Data are presented as percent change from respective basal ΔFFI value. Mean±s.e.m., n=29 per data group, *P<0.05 vs baseline value.
Table 2.
Effect of malondialdehyde (MDA) on baseline intracellular Ca2+ levels and intracellular Ca2+ decay in myocytes from adult rat hearts
| Baseline intracellular Ca2+ (360*380-1 ratio) | Intracellular Ca2+ decay (ms) | |
|---|---|---|
| Control | 1.10±0.01 | 448±14 |
| MDA 10−9 M | 1.11±0.01 | 489±12 |
| MDA 10−7 M | 1.10±0.01 | 478±17 |
| MDA 10−5 M | 1.09±0.01 | 478±17 |
| MDA 10−3 M | 1.08±0.01 | 504±15 |
Data represent mean±s.e.m., n=28–29 cells.
Effect of malondialdehyde on myocyte shortening in the presence of the antioxidant vitamin C or the p38 MAP kinase inhibitor SB203580
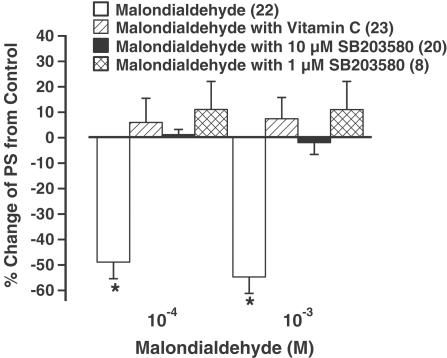
Lipid peroxidation often deteriorates cellular oxidative damage and may trigger activation of MAP kinase (Leonarduzzi et al., 2000). To examine the role of oxidative stress and the involvement of MAP kinase signaling pathway in malondialdehyde-induced cardiac depression, the effect of malondialdehyde on myocyte shortening was re-examined in the presence of either the antioxidant vitamin C (100 μM) or the p38 MAP kinase inhibitor SB203580 (1 and 10 μM). Vitamin C or SB203580 alone had no effect on cell shortening over 30 min (Wold et al., 2002). Interestingly, as shown in Figure 3, the malondialdehyde-induced depression in PS was completely abolished by either vitamin C or SB203580, suggesting involvements of oxidative stress and MAP kinase activation in malondialdehyde-induced cardiac contractile depression.
Figure 3.
Effect of the antioxidant vitamin C and p38 MAP kinase inhibitor SB203580 on malondialdehyde-induced depression of myocyte shortening. Vitamin C (100 μM) or SB203580 (1 and 10 μM) was applied prior to the addition of malondialdehyde (10−4 and 10−3 M). Mean±s.e.m., n=20–23 per data group, *P<0.05 vs baseline value.
Effect of malondialdehyde on p38 MAP kinase activation in the absence and presence of the p38 MAP kinase inhibitor SB203580
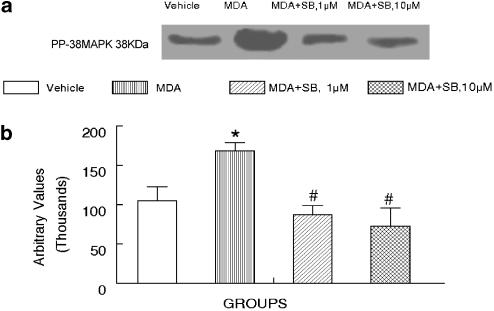
Our result suggested that p38 MAP kinase may be involved in malondialdehyde-induced cardiac depression. To examine if malondialdehyde directly activates p38 MAP kinase, Western blot analysis was performed to determine the phosphorylation of p38 MAP kinase in response to malondialdehyde (10−4 M). Result in Figure 4 exhibits that malondialdehyde directly enhanced phosphorylation of p38 MAP kinase, which can be blocked by either 1 or 10 μM of SB203580. These data supported the notion of direct involvement of p38 MAP kinase in malondialdehyde-induced cardiac depression.
Figure 4.
Effect of malondialdehyde (MDA, 10−4 M) on phosphorylation of p38 MAP kinase in ventricular myocytes in absence or presence of the p38 MAP kinase inhibitor SB203580 (SB, 1 and 10 μM). (a) Representative gel blot showing p38 MAP kinase phosphorylation (pp-MAPK). (b) Bar graph depicting summary of three different isolations. Mean±s.e.m., *P<0.05 vs control (vehicle) group, P<0.05 vs malondialdehyde group.
Discussion
Our study provided evidence for the first time that the lipid peroxidation end product malondialdehyde directly depresses cardiac contraction and maximal velocity of contraction and relaxation associated with a reduction of intracellular Ca2+ transients in isolated ventricular myocytes. The malondialdehyde-induced cardiac depression may involve enhanced oxidative stress and activation of MAP kinase indicated by our pharmacological and immunoblotting studies. In addition, malondialdehyde shortened duration of shortening and had little effect on resting intracellular Ca2+ levels and intracellular Ca2+ clearing rate. These results may suggest a convincing link between the elevated lipid peroxidation end product malondialdehyde and impaired ventricular contractile function under pathological conditions such as heart failure.
Peroxidation of membrane phospholipids has been suspected to be a major mechanism of oxidant injury and impaired cardiac contractile function (Braughler et al., 1986; Durot et al., 2000). Levels of 25–140 nM malondialdehyde have been reported in human plasma following certain cardiac injury such as ischemia–reperfusion (Yeo et al., 1994; Cighetti et al., 1999), consistent with the effective concentration range found in our study. Oxidation of membrane lipid is a process generated by ROS and is responsible for the membrane dysfunction under various disease states (Kaneko et al., 1994; Porter et al., 1995; Dhalla et al., 1996; Buffon et al., 2000;). The ROS radicals may initiate a rapid self-propagating chain reaction by attacking the polyunsaturated fatty acids in the membrane. Polyunsaturated fatty acids display the highest sensitivity among cellular macromolecules to ROS-induced damage (Porter et al., 1995; Dhalla et al., 1996). Lower degree of fatty acid unsaturation of cell membrane is believed to be protective against lipid oxidation-derived damage. Membrane damage due to ROS-induced lipid peroxidation has been considered the predominant mechanism for cellular membrane dysfunction and subsequently, alteration of cellular function (Porter et al., 1995; Dhalla et al., 1996). Malondialdehyde is generated through disruption of membrane lipids following lipid peroxidation and is especially dangerous for the viability of cells or tissues. It may be responsible for the compromised ventricular function under conditions where there is elevated lipid peroxidation. It may be speculated that enhanced oxidative stress may trigger the lipid peroxidation-induced ventricular dysfunction. Despite extensive effort in the field of lipid peroxidation, it has not yet been determined if it is a cause or a consequence of several pathological conditions. Results from the current study indicate that malondialdehyde may be a trigger for cardiac dysfunction. The fact that peak cardiac contraction, duration and velocity of contraction and relaxation were altered by malondialdehyde indicated that lipid peroxidation may affect different cardiac contractile or regulatory components.
Although the mechanism(s) of action underlying reduced myocyte contraction in response to malondialdehyde is not fully clear at this time, several speculations may be made. First, the observation that the antioxidant vitamin C abolished the malondialdehyde-induced negative contractile response suggests that malondialdehyde may elicit its inhibitory effect associated with enhanced oxidative stress in the heart. Oxidative stress and damage are known to impair cardiac contractile function (Goldharber & Qayyum, 2000). Mediators or signaling of oxidative stress such as superoxide anion, hydroxyl radical and peroxynitrite have been reported to depress cardiac contractile function directly (Satoh & Matsui, 1997; Ferdinandy et al., 2000). Vitamin C (ascorbate) has been shown to scavenge free radicals, thus preventing cardiac contractile failure. It is a water-soluble antioxidant and plays an indirect role in terminating lipid peroxidation. It reduces tocopheroxyl radicals thereby elevating the levels of the reduced tocopherol within the tissues (Leung et al., 1981). Tocopherol, on the other hand, is also a lipid-soluble antioxidant with the sole physiological role of directly terminating lipid peroxidation through stabilization of lipid radical by donating a single electron (Burton et al., 1985; Klein et al., 1989). Although direct action of antioxidant such as vitamin C on malondialdehyde-induced membrane damage has not been elucidated, antioxidants have been demonstrated to quench effectively ROS-induced lipid peroxidation and ameliorate certain aspects of biomolecular damage (Dietrich et al., 2002). Secondly, the observation that malondialdehyde may inhibit myocyte shortening at concentrations (such as 10−7 M) where no obvious effect on intracellular Ca2+ transients was observed indicates that malondialdehyde may alter myofilament Ca2+ sensitivity, or other Ca2+-independent machineries.
p38 MAP kinase has been shown to be involved in cellular responses to various assaults such as UV light, osmotic stress, mechanical, oxidative (ROS) and chemical stress (Martin-Blanco, 2000; Obata et al., 2000; Kulisz et al., 2002). Results from our current study provided evidence that p38 MAP kinase activation may mediate malondialdehyde-elicited cardiac contractile inhibition. This is consistent with the finding that malondialdehyde directly facilitate p38 MAP kinase phosphorylation. p38 MAP kinase activation has been shown to elicit a robust negative inotropic effect in intact cardiac myocytes (Liao et al., 2002). As increased p38 MAPK activation is associated with the onset of heart failure (Adams et al., 1998), ischemic or reperfusion injury, and in vivo pressure overload (Wang et al., 1998; Ma et al., 1999), the negative inotropic effect of p38 MAPK may contribute, at least in part, to the diminished cardiac contractility under those pathological circumstances. It is speculated that accumulation of ROS may lead to lipid peroxidation and production of malondialdehyde en route to p38 MAP kinase activation and cardiac depression. Indeed, a number of studies have demonstrated that inhibiting p38 MAP kinase signaling improves contractile function in ischemia/reperfusion-injured hearts (Cain et al., 1999; Ma et al., 1999). This notion not only reveals an unappreciated function of p38 MAP kinase but also demonstrates a novel mechanism for stress-activated signals in the regulation of cardiac contractility. Interestingly, the negative inotropic effect of p38 MAP kinase is believed to be mediated by reducing the responsiveness of myofilaments to Ca2+ (Liao et al., 2002), consistent with our findings in the discrepant threshold of malondialdehyde-induced inhibition between myocyte shortening and intracellular Ca2+ transient. Another interesting observation from our current study is that SB203580 inhibits the dual phosphorylation of p38 MAP kinase. These data may suggest that the malondialdehyde-induced activation of p38 MAP kinase could possibly arise from an autophosphorylation reaction of p38 MAP kinase. Autophosphorylation has been indicated to be an alternative activation pathway contributing to the biological response of p38 MAP kinase (Ge et al., 2002). Nevertheless, with the limited information from this study we can only speculate but cannot suggest such autophosphorylation mechanisms involved in malondialdehyde-induced cardiac response.
In conclusion, our study demonstrates direct cardiac depression by malondialdehyde at the ventricular myocyte level, possibly through oxidative stress. These findings not only reveal a novel function for p38 MAPK but also provide new insights for better understanding of enhanced p38 MAPK signaling in cardiac dysfunction under certain pathophysiological conditions, such as cardiac ischemic/reperfusion injury or chronic heart failure. The precise nature of the cardiac contractile effects of malondialdehyde is still not clear. Future studies should focus on its action on cardiac excitation–contraction coupling and membrane ion channels. These approaches will be essential to further our understanding of the cellular effects, pharmacological profiles as well as clinical applications of malondialdehyde and lipid peroxidation.
Acknowledgments
We acknowledge Faye L. Norby, Kadon Hintz, Loren Wold and Feng Dong for their assistance. DVF was supported by a medical student research fellowship from the University of North Dakota School of Medicine Associate Dean for Research's Office.
Abbreviations
- CICR
Ca2+-induced intracellular Ca2+ fluorescence release
- ±dLdt
maximal velocity of shortening/relengthening
- MAP
p38 mitogen-activated protein
- MDA
malondialdehyde
- PS
peak shortening
- ROS
reactive oxygen species
- TPS
time-to-PS
- TR90
time-to-90% relengthening
- τ
intracellular Ca2+ decay rate
References
- ADAMS J.W., SAKATA Y., DAVIS M.G., SAH V.P., WANG Y., LIGGETT S.B., CHIEN K.R., BROWN J.H., DORN G.W., II Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERLETT B.S., STADTMAN E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- BRAUGHLER J.M., DUNCAN L.A., CHASE R.L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 1986;261:10282–10289. [PubMed] [Google Scholar]
- BUFFON A., SANTINI S.A., RAMAZZOTTI V., RIGATTIERI S., LIUZZO G., BIASUCCI L.M., CREA F., GIARDINA B., MASERI A. Large, sustained cardiac lipid peroxidation and reduced antioxidant capacity in the coronary circulation after brief episodes of myocardial ischemia. J. Am. Coll. Cardiol. 2000;35:633–639. doi: 10.1016/s0735-1097(99)00581-1. [DOI] [PubMed] [Google Scholar]
- BURTON G.W., FOSTER D.O., PERLY B., SLATER T.F., SMITH I.C.P., INGOLD K.U. Biological antioxidants. Philos Trans. R. Soc. London B. 1985;311:565–578. doi: 10.1098/rstb.1985.0164. [DOI] [PubMed] [Google Scholar]
- CAIN B.S., MELDRUM D.R., MENG X., DINARELLO C.A., SHAMES B.D., BANERJEE A., HARKEN A.H. p38-MAPK inhibition decreases TNF-α production and enhances postischemic human myocardial function. J. Surg. Res. 1999;83:7–12. [Google Scholar]
- CIGHETTI G., DEBIASI S., PARONI R., ALLEVI P. Free and total malondialdehyde assessment in biological matrices by gas chromatography–mass spectrometry: what is needed for an accurate detection. Anal. Biochem. 1999;266:222–229. doi: 10.1006/abio.1998.2952. [DOI] [PubMed] [Google Scholar]
- DHALLA A.K., HILL M.F., SINGAL P.K. Role of oxidative stress in transition of hypertrophy in heart failure. J. Am. Coll. Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- DIAZ-VELEZ C.R., GARCIA-CASTINEIRAS S., MENDOZA-RAMOS E., HERNANDEZ-LOPEZ E. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am. Heart J. 1996;131:146–152. doi: 10.1016/s0002-8703(96)90063-0. [DOI] [PubMed] [Google Scholar]
- DIETRICH M., BLOCK G., HUDES M., MORROW J.D., NORKUS E.P., TRABER M.G., CROSS C.E., PACKER L. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol. Biomarkers Prev. 2002;11:7–13. [PubMed] [Google Scholar]
- DUROT I., MAUPOIL V., PONSARD B., CORDELET C., VERGELY-VANDRIESSE C., ROCHETTE L., ATHIAS P. Oxidative injury of isolated cardiomyocytes: dependence on free radical species. Free Radic. Biol. Med. 2000;29:846–857. doi: 10.1016/s0891-5849(00)00382-8. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., DANIAL H., AMBRUS I., ROTHERY R.A., SCHULZ R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ. Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- GE B., GRAM H., DI PADOVA F., HUANG B., NEW L., ULEVITCH R.J., LUO Y., HAN J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38α. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- GOLDHABER J.I., QAYYUM M.S. Oxygen free radicals and excitation–contraction coupling. Antioxid. Redox. Signal. 2000;2:55–64. doi: 10.1089/ars.2000.2.1-55. [DOI] [PubMed] [Google Scholar]
- GUTTERIDGE J.M.C., HALLIWELL B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- HENDRICKSON S.C., ST LOUIS J.D., LOWE J.E., ABDEL-ALEEM S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol. Cell. Biochem. 1997;166:85–94. doi: 10.1023/a:1006886601825. [DOI] [PubMed] [Google Scholar]
- HOLVOET P., PEREZ G., ZHAO Z., BROUWERS E., BERNAR H., COLLEN D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J. Clin. Invest. 1995;95:2611–2619. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEKO M., MATSUMOTO Y., HAYASHI H., KOBAYASHI A., YAMAZAKI N. Oxygen free radicals and calcium homeostasis in the heart. Mol. Cell. Biochem. 1994;139:91–100. doi: 10.1007/BF00944207. [DOI] [PubMed] [Google Scholar]
- KATZ S.D., RAO R., BERMAN J.W., SCHWARZ M., DEMOPOULOS L., BIJOU R., LEJEMTEL T.H. Pathophysiological correlates of increased serum tumor necrosis factor in patients with congestive heart failureRelation to nitric oxide-dependent vasodilation in the forearm circulation. Circulation. 1994;90:12–16. doi: 10.1161/01.cir.90.1.12. [DOI] [PubMed] [Google Scholar]
- KLEIN H.H., PICH S., LINDERT S., NELENDAHL K., NIEDMAGNN P., KNEUZER H. Combination treatment with vitamin E and C in experimental myocardial infarction in pigs. Am. Heart J. 1989;118:667–673. doi: 10.1016/0002-8703(89)90577-2. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., LEWIS S.J., ROYALL J.A., YE Y.Z., KELLY D.R., BECKMAN J.S. Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Crit. Care. Med. 1997;25:812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- KULISZ A., CHEN N., CHANDEL N.S., SHAO Z., SCHUMACKER P.T. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2002;282:L1324–L1329. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- LEONARDUZZI G., ARKAN M.C., BASAGA H., CHIARPOTTO E., SEVANIAN A., POLI G. Lipid oxidation products in cell signaling. Free Radic. Biol. Med. 2000;28:1370–1378. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- LEUNG H-W., VANG M.J., MAVIS R.D. The cooperative interaction between vitamin E and vitamin C in suppression of peroxidation of membrane phospholipids. Biochem. Biophys. Acta. 1981;664:266–272. doi: 10.1016/0005-2760(81)90049-7. [DOI] [PubMed] [Google Scholar]
- LIAO P., WANG S.Q., WANG S., ZHENG M., ZHANG S.J., CHENG H., WANG Y., XIAO R.P. p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ. Res. 2002;90:190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA X.L., KUMAR S., GAO F., LOUDEN C.S., LOPEZ B.L., CHRISTOPHER T.A., WANG C., LEE J.C., FEUERSTEIN G.Z., YUE T.L. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- MARTIN-BLANCO E. MAPK signalling cascades: ancient roles and new functions. Bioessays. 2000;22:637–645. doi: 10.1002/1521-1878(200007)22:7<637::AID-BIES6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- OBATA T., BROWN G.E., YAFFE M.B. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit. Care Med. 2000;28:N67–N77. doi: 10.1097/00003246-200004001-00008. [DOI] [PubMed] [Google Scholar]
- POLLOCK J.S., NAKANE M., BUTTERY L.D., MARTINEZ A., SPRINGALL D., POLAK J.M., FORSTERMANN U., MURAD F. Characterization and localization of endothelial nitric oxide synthase using specific monoclonal antibodies. Am J. Physiol. 1993;265:C1379–C1387. doi: 10.1152/ajpcell.1993.265.5.C1379. [DOI] [PubMed] [Google Scholar]
- PORTER N.A., CALDWELL S.E., MILLS K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- SATOH H., MATSUI K. Electrical and mechanical modulations by oxygen-derived free-radical generating systems in guinea-pig heart muscles. J. Pharm. Pharmacol. 1997;49:505–510. doi: 10.1111/j.2042-7158.1997.tb06832.x. [DOI] [PubMed] [Google Scholar]
- TORRE-AMINONE G., KAPADIA S., BENEDICT C., ORAL H., YOUNG J.B., MANN D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J. Am. Coll. Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- WANG Y., HUANG S., SAH V.P., ROSS J., JR, BROWN J.H., HAN J., CHIEN K.R. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- WOLD L.E., RELLING D.P., DUAN J., NORBY F.L., REN J. Abrogated leptin-induced cardiac contractile response in ventricular myocytes under spontaneous hypertension: role of Jak/STAT pathway. Hypertension. 2002;39:69–74. doi: 10.1161/hy0102.100777. [DOI] [PubMed] [Google Scholar]
- YEO H.C., HELBOCK H.J., CHYU D.W., AMES B.N. Assay of malondialdehyde in biological fluids by gas chromatography-mass spectrometry. Anal. Biochem. 1994;220:391–396. doi: 10.1006/abio.1994.1355. [DOI] [PubMed] [Google Scholar]