Abstract
Fentanyl is a μ-opioid analgesic that might disinhibit 5-HT neurons and thus increase 5-HT efflux. However, fentanyl also binds to 5-HT1A receptors, and if it activates 5-HT1A somatodendritic autoreceptors, the resultant inhibition might offset opioid-mediated increases in 5-HT efflux. To test this hypothesis, we used microdialysis to study effects of fentanyl on extracellular 5-HT in the dorsal raphe nucleus (DRN) of unanesthetized rats.
Systemic administration of fentanyl (0.01–0.2 mg kg−1, s.c.) increased 5-HT efflux in the DRN. An intermediate dose of fentanyl (0.05 mg kg−1) produced the maximum increase in 5-HT to ∼180% of baseline levels in the DRN. Naltrexone (10 mg kg−1, s.c.) blocked the increase in response to systemic fentanyl (0.05 mg kg−1).
In contrast, during infusion into the DRN, fentanyl (10–1000 μM) induced a dose-dependent decrease in 5-HT. Naltrexone and nor-binaltorphimine failed to block the decrease suggesting that μ- and κ-opioid receptors did not mediate this effect.
Systemic (−)-pindolol (8 mg kg−1, s.c.) or infusion of WAY-100635 (100 μM) into the DRN blocked the decrease, and instead 5-HT increased in response to local infusion of fentanyl (100 μM). WAY-100635 (0.3 mg kg−1, s.c.) also potentiated the effect of systemic fentanyl (0.2 mg kg−1, s.c.). (−)-Pindolol and WAY-100635 block 5HT1A receptors, indicating that inhibition of 5-HT neuronal activity resulting from fentanyl binding to somatodendritic autoreceptors attenuated opioid-mediated increases in 5-HT efflux.
These results provide novel evidence that besides stimulating μ-opioid receptors, fentanyl is a 5-HT1A receptor agonist. Possibly, this contributes to lethality of fentanyl overdose.
Keywords: Dorsal raphe nucleus, serotonin, fentanyl, opioid receptor, 5-HT1A receptor, microdialysis
Introduction
Fentanyl is a potent synthetic opioid frequently used as a short-acting surgical anesthetic. Similar to morphine, fentanyl acts primarily at μ-opioid receptors, and its analgesic effect is blocked by naltrexone (Maguire et al., 1992). In the CNS, 5-hydroxytryptamine (5-HT) mediates some physiological effects of opioids, and in particular, the analgesia produced by fentanyl is attenuated by 5-HT1A receptor antagonists (Xu et al., 1994; Clarke & Ward, 2000). One possible explanation for this observation is that opioids elicit increases in 5-HT release and that the analgesic effect depends partly upon the consequent activation of 5-HT1A receptors in postsynaptic sites involved in modulating nociception. However, fentanyl itself has affinity for 5-HT1A receptors in the low micromolar range (Martin et al., 1991). These in vitro data suggest that fentanyl binds to 5-HT1A receptors at clinically relevant doses and thus, its analgesic and other physiological effects might be mediated in part by direct interaction of fentanyl with 5-HT1A receptors in postsynaptic sites. The 5-HT1A receptor subtype also serves as a somatodendritic autoreceptor and thus, fentanyl-induced changes in 5-HT neuronal activity also might influence physiological processes such as nociception.
The dorsal raphe nucleus (DRN) is important in mediating the analgesic effects of opioids in the midbrain (Wang & Nakai, 1994). Moreover, earlier studies suggest that opioids in the midbrain disinhibit 5-HT neurons in the DRN (Tao & Auerbach, 1994; Tao & Auerbach, 1995; Jolas & Aghajanian, 1997). In this study, we used microdialysis to investigate the effects of fentanyl on extracellular 5-HT in the DRN of freely behaving rats. Our previous results support the conclusion that 5-HT efflux in the DRN depends on depolarization-induced exocytosis, presumably from axon collaterals or dendrites in the raphe. Thus, extracellular 5-HT in the DRN usually reflects changes in 5-HT neuronal activity and release in forebrain projection sites (Tao & Auerbach, 2000). Moreover, during infusion of the μ-opioid peptide DAMGO into the DRN, 5-HT influx increased in the DRN and the forebrain (Tao & Auerbach, 2002a). However, the μ-opioid fentanyl might have a complex influence on 5-HT efflux as a result of its affinity for the 5-HT1A receptor (Martin et al., 1991). Thus, we examined the ability of opioid and 5-HT receptor antagonists to block the effect of fentanyl on 5-HT efflux. These experiments tested the hypothesis that increased 5-HT efflux mediated by fentanyl binding to opioid receptors might be offset by stimulation of 5-HT1A autoreceptors.
Methods
Animal preparation
Sprague–Dawley albino male rats were obtained from Harlan Sprague–Dawley Inc. (Indianapolis, IN, U.S.A.) and Charles River Labs (Wilmington, MA, U.S.A.). Rats were individually housed on a reversed light–dark cycle (lights off from 09.30 to 21.30 h). Food and water were available ad libitum. All procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Rutgers University Institutional Review Board and Harvard University VAMC Animal Studies Subcommittee.
Rats weighing 300–350 g were anesthetized with a combination of xylazine (4 mg kg−1, i.p.) and ketamine (80 mg kg−1, i.p.). The operations were performed in a Kopf stereotaxic frame in the flat skull position. Guide cannulae (22-gauge stainless-steel tubing) were implanted above the dura (2 mm ventral below the skull surface). Based on a rat brain atlas (Paxinos & Watson, 1986), the stereotaxic coordinates for the DRN guide cannulae relative to interaural zero were anterior–posterior (AP) 1.2 and medial–lateral (ML) 4.0 at an angle 32° lateral to midline. Experiments began no sooner than 1 week after surgery.
Microdialysis procedures
Concentric style (I-shaped) microdialysis probes were constructed from 26-gauge stainless-steel tubing and glass silica. The dialysis tubing was hollow nitrocellulose fiber (0.2 mm outer diameter., 6000 MW cutoff; Spectrum Medical Industries, Los Angeles, CA, U.S.A.). The length of the steel shaft was adjusted to place a 1.0-mm-long segment of dialysis tubing in the DRN (DV 5.5–6.4, 32° angle). The night before an experiment, rats were briefly anesthetized with ether, and the dialysis probes were inserted through the guide cannulae and secured with dental cement. Rats were then attached to a fluid swivel to allow relatively free movement. Before collecting samples, dialysis probes were perfused with artificial cerebrospinal fluid (aCSF) containing 140 mM NaCl, 3.0 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 1.2 mM Na2HPO4, 0.27 mM NaH2PO4, pH 7.4. Except where noted, the aCSF contained the selective serotonin reuptake inhibitor citalopram (1.0 μM) to enhance detection of 5-HT. The aCSF was pumped at a rate of 1.0 μl min−1. Collection of samples began at the start of the lights-off period under dim red light conditions. Samples were collected at 30 min intervals and analyzed within 1 h by high-performance liquid chromatography with electrochemical detection (h.p.l.c.-ED). Mobile phase (0.15 M chloroacetic acid, 0.12 M NaOH, 0.18 mM EDTA, 1.0 mM sodium octane sulfonic acid and 56 ml l−1 acetonitrile) was pumped at a rate of 0.90 ml min−1. HPLC-ED methods for analysis of 5-HT were previously described in detail (Tao et al., 1996). Drugs were administered after 5-HT levels in four successive samples were stable (<±10% fluctuation of baseline).
After completion of experiments, rats were deeply anesthetized and a solution of 1% methylene blue was infused through the dialysis probe for approximately 20 min. The rats were then decapitated and the brains were removed, frozen, and sliced by hand using a razor blade. The slices were then examined to determine if the dialysis probes were located in the DRN. Data were excluded from analysis when the probes were outside of the target boundaries.
Experimental design and data analysis
Fentanyl was administered systemically by subcutaneous injection, or by local infusion for 2 h via reverse dialysis in the DRN. Receptor antagonists were injected subcutaneously 30 min before fentanyl, or by reverse dialysis infusion beginning 30 min before and continuing through a 2 h period of fentanyl infusion. Mean baseline 5-HT levels were calculated as the average of the four successive samples before drug administration and reported in the figure legends as pg sample−1 (uncorrected for probe recovery). Also, the data were normalized and presented in figures as mean (±s.e.mean) percent change from the averaged baseline measurements. Statistical analysis was carried out by repeated measure analysis of variance with normalized data, and if significant (P<0.05), individual time points were compared using Scheffe's post hoc test.
Drugs
Fentanyl (N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]propanamide) citrate salt, (−)-pindolol, naltrexone and WAY-100635 maleate salt (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexane carboxamide) were purchased from Sigma (St Louis, MO, U.S.A.). Nor-binaltorphimine (nor-BNI) was purchased from RBI (Sigma/RBI, Natick, MA, U.S.A.). Drugs were dissolved in saline (0.9% NaCl) for subcutaneous injection and in the aCSF for reverse dialysis infusion. Doses for systemic injection of fentanyl citrate and WAY-100635 maleate are for the salt forms. Doses of naltrexone and (−)-pindolol, or concentrations of nor-BNI and WAY-100635 were sufficient for blocking receptor agonists based on earlier work (Sharp et al., 1989; Tao & Auerbach, 1995).
Results
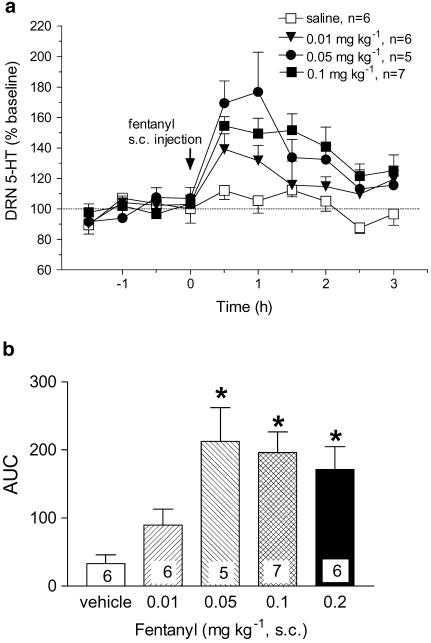
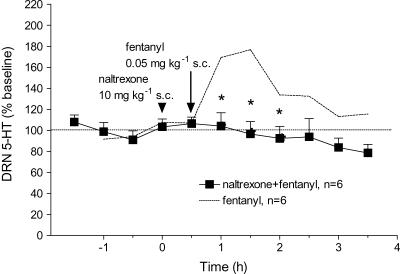
Systemic injection of fentanyl (0.01–0.2 mg kg−1, s.c.) produced relatively short-lasting increases in 5-HT in the DRN with return to baseline levels within ∼2–3 h after administration (Figure 1a). The maximum response to fentanyl was an ∼80% increase above baseline levels of 5-HT. As illustrated by ‘area under the curve' (AUC) values, the increase was dose-dependent with the largest effect obtained at 0.05 mg kg−1 fentanyl (Figure 1b). At higher doses of fentanyl, increases in 5-HT tended to be lower although the differences were not statistically significant (Figure 1b). The opioid antagonist, naltrexone (10 mg kg−1, s.c.) injected 30 min before fentanyl (0.05 mg kg−1, s.c.) blocked the increase in 5-HT in the DRN (Figure 2).
Figure 1.
Effect of systemic fentanyl on extracellular 5-HT in the DRN of freely behaving rats: mean baseline level of extracellular 5-HT was 5.6±0.8 pg sample−1 (n=25). (a) Fentanyl produced a dose-dependent increase in 5-HT: F(3,20)=4.95, P=0.0094. The 0.2 mg kg−1 dose and asterisks indicating significance were omitted from the graph for the sake of clarity. (b) AUC values expressed as the cumulative increase above baseline from 0 to 2 h after fentanyl administration. The effect was significant as determined by analysis of AUC values (F(4,23)=6.37, P=0.0013). *P<0.05, compared to vehicle.
Figure 2.
Naltrexone blocked the effect of systemic fentanyl on extracellular 5-HT: Mean baseline level of extracellular 5-HT in the DRN was 7.0±3.0 pg sample−1 (n=6). Arrowhead and arrow indicate injection of naltrexone and fentanyl, respectively. The dotted line shows data replotted from Figure 1 for the effect of fentanyl (0.05 mg kg−1, s.c.) alone. Naltrexone (10 mg kg−1, s.c.) blocked the effect of systemic fentanyl (0.05 mg kg−1, s.c.): F(1,10)=8.89, P=0.0137. *P<0.05 compared to fentanyl alone.
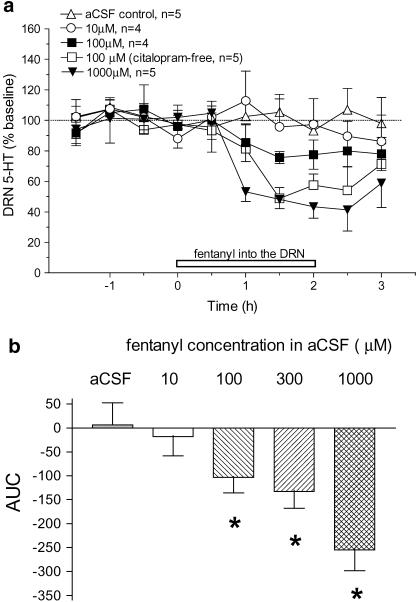
Opposite to the effect of systemic injection, reverse dialysis administration of fentanyl in the DRN decreased extracellular levels of 5-HT in the DRN. At concentrations of 10, 100, 300 and 1000 μM in the aCSF, fentanyl decreased 5-HT ∼2, 20, 35 and 50%, respectively (Figure 3a). In most experiments, a reuptake inhibitor, citalopram (1 μM), was added to the aCSF to improve detection of baseline levels of 5-HT. To investigate the influence of inhibiting reuptake, we omitted citalopram and re-examined the effect of fentanyl. As shown in Figure 3a, fentanyl (100 μM) infusion into the DRN elicited an ∼50% decrease in 5-HT in the absence of citalopram. As determined by AUC values, the decrease in 5-HT was dose-dependent with significant effects obtained at fentanyl concentrations of 100 μM and greater (Figure 3b).
Figure 3.
Effect on extracellular 5-HT of reverse dialysis infusion of fentanyl in the DRN: (a) Mean baseline level of extracellular 5-HT was 0.6±0.1 pg sample−1 (n=11) in the rats infused with citalopram-free aCSF and 7.0±0.8 pg sample−1 (n=23) with citalopram in the aCSF. The open horizontal bar indicates the period of fentanyl infusion. Fentanyl produced a concentration-dependent decrease in extracellular 5-HT in the DRN: F(3,14)=3.56, P=0.0421. Similarly, in response to local fentanyl infusion, 5-HT was decreased in rats infused with the citalopram-free aCSF (F(1,9)=18.01, P=0.0022). Plots of 300 μM fentanyl and citalopram-free aCSF control data and asterisks indicating statistical significance were omitted for the sake of clarity. (b) AUC values expressed as the cumulative decrease below baseline from 0 to 2 h after reverse dialysis infusion of fentanyl. The effect was significant (F(4,19)=6.53, P=0.0018). *P<0.05, compared to vehicle.
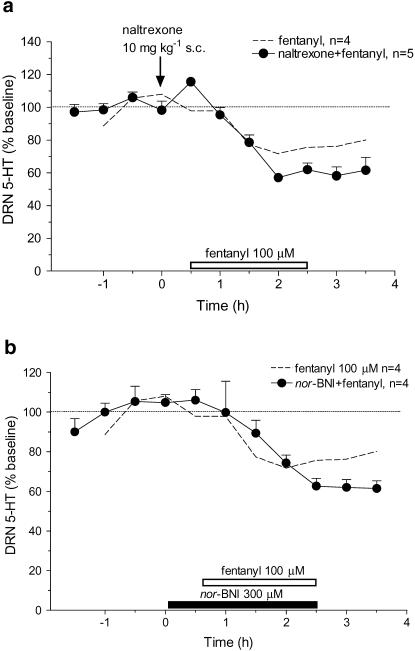
Based on the effects of systemic injection of fentanyl and of other opioid receptor agonists, we expected that local infusion of fentanyl would have increased 5-HT influx. To determine if opioid receptors were involved in the decrease, naltrexone (10 mg kg−1, s.c.) was administered 30 min before infusing fentanyl (100 μM) into the DRN. Fentanyl in the presence of naltrexone produced an ∼40% decrease in 5-HT compared to an ∼20% decrease in response to fentanyl alone (Figure 4a). Thus, the response to local fentanyl in the presence of an opioid receptor antagonist was not blocked but instead was larger, although the difference was not statistically significant (F(1,9)=2.95, P=0.12).
Figure 4.
Effect of opioid receptor antagonists on local fentanyl-induced decrease in extracellular 5-HT in the DRN. The mean baseline level of extracellular 5-HT was 6.6±0.8 pg sample−1 (n=9). The open horizontal bar indicates the period of fentanyl infusion. Dotted lines show data replotted from Figure 3 for the effect of infusing fentanyl (100 μM) alone. (a) Arrow indicates the time of naltrexone injection. Systemic naltrexone (10 mg kg−1, s.c.) did not block the effect of local fentanyl (100 μM: F(1,9)=2.95, P=0.12). (b) The solid horizontal bar represents infusion of nor-BNI (300 μM). Pretreatment with nor-BNI had no significant effect on the fentanyl-induced decrease in 5-HT: F(1,8)=0.13, P=0.7299.
Naltrexone is relatively selective for μ-opioid receptors, suggesting that this subtype was not involved in mediating fentanyl-induced decreases in 5-HT. However, fentanyl may act also at κ-opioid receptors (Maguire et al., 1992), which have an inhibitory influence on 5-HT neurons in the DRN (Pinnock, 1992; Monroe et al., 1995; Tao & Auerbach, 2002b). To test this hypothesis, we infused a κ receptor antagonist, nor-BNI (300 μM), into the DRN 30 min before fentanyl (100 μM). In the presence of nor-BNI, fentanyl produced an ∼40% decrease in 5-HT in the DRN (Figure 4b).
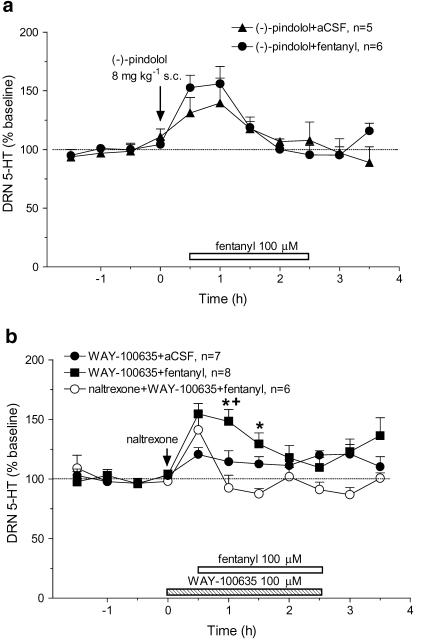
Based on evidence that fentanyl also has affinity for 5-HT1A receptors (Martin et al., 1991), we examined the possibility that fentanyl inhibits 5-HT release by activating autoreceptors in the DRN. To test this hypothesis, 30 min before local infusion of fentanyl (100 μM), rats were injected with (−)-pindolol (8 mg kg−1, s.c.) to block 5-HTIA receptors (Sharp et al., 1989). Consistent with the evidence that it blocks somatodendritic autoreceptors, (−)-pindolol alone induced a small increase in 5-HT in the control group (Figure 5a). Moreover, instead of eliciting a decrease in 5-HT, fentanyl infusion following (−)-pindolol increased 5-HT. The response to local fentanyl after pretreatment with (−)-pindolol was larger than the effect of (−)-pindolol alone, but this difference was not significant.
Figure 5.
Effect of 5-HT1A receptor antagonists on fentanyl-induced decreases in extracellular 5-HT in the DRN. Mean baseline level of extracellular 5-HT was 4.7±0.6 pg sample−1 (n=32). The open horizontal bar indicates the period of fentanyl infusion (a) The arrow indicates (−)-pindolol injection (8 mg kg−1, s.c.). After pretreatment with (−)-pindolol, fentanyl infusion did not significantly affect 5-HT as compared to (−)-pindolol alone (F(1,9)=0.41, P=0.5390). (b) The arrow indicates naltrexone (10 mg kg−1, s.c.) injection. The stipled bar indicates the period of WAY-100635 (100 μM) infusion. There were significant differences between groups (F(2,18)=10.25, P=0.0011). For the WAY-100635 pretreatment group, fentanyl infusion induced a significant increase in 5-HT (F(1,13)=5.63, P=0.034; WAY-100635+fentanyl compared to WAY-100635+aCSF). This effect was blocked by naltrexone (F(1,12)=18.82, P=0.001; naltrexone+WAY-100635+fentanyl compared to WAY-100635+fentanyl). *P<0.05 WAY-100635+fentanyl vs WAY-100635+aCSF; +P<0.05 WAY-100635+fentanyl vs naltrexone+WAY-100635+fentanyl.
To further test the role of autoreceptors in the effect of local fentanyl, we used the more selective 5-HT1A receptor antagonist WAY-100635 (Figure 5b). In this experiment, WAY-100635 (100 μM) was locally infused into the DRN beginning 30 min before fentanyl (100 μM). Similar to (−)-pindolol, WAY-100635 alone (100 μM) produced a small increase in 5-HT, and also blocked the fentanyl-induced decrease in 5-HT. Moreover, 5-HT was further elevated by fentanyl in the rats pretreated with WAY-100635. To determine if activation of opioid receptors by fentanyl was involved in this effect, rats were pretreated with naltrexone (10 mg kg−1, s.c.) in addition to WAY-100635 (100 μM). As shown in Figure 5b, after combined pretreatment with naltrexone and WAY-100635, fentanyl (100 μM) infusion into the DRN did not significantly increase or decrease extracellular 5-HT in the DRN.
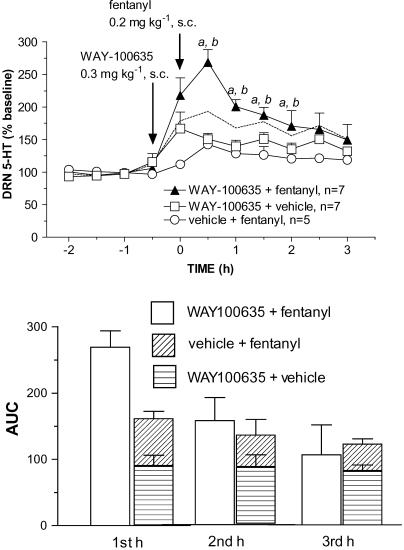
As another test of the possibility that fentanyl activates 5-HT1A receptors, WAY-100635 was administered 30 min before a high systemic dose of fentanyl. As shown in Figure 6a, after pretreatment with WAY-100635 (0.3 mg kg−1, s.c.), fentanyl (0.2 mg kg−1, s.c.) produced a significantly larger increase in 5-HT compared to WAY-100635+vehicle or vehicle+fentanyl. As illustrated by AUC values, the effect of fentanyl administration after pretreatment with WAY-100635 was substantially greater than the sum of the effects of WAY-100635 alone and fentanyl alone (Figure 6b). This was apparent in the first hour after fentanyl injection, the period when the increase in 5-HT peaked, but not during the second and third hours when extracellular 5-HT was returning to baseline levels. Thus, in the first hour the sum of the effects of fentanyl alone and WAY-100635 alone was an ∼175% increase compared to an ∼275% increase in response to fentanyl administration after pretreatment with WAY-100635. During the second and third hours, the sum of the effect of WAY-100635 alone and fentanyl alone was equivalent to the increase produced by combined administration.
Figure 6.
WAY-100635 enhanced the effect of systemic fentanyl. (a) Arrows indicate injection of WAY-100635 (0.3 mg kg−1, s.c.) at t=−0.5 h and fentanyl (0.2 mg kg−1, s.c.) 30 min later (t=0). The effect of WAY-100635 followed by fentanyl was compared to control groups injected with WAY-100635 followed by vehicle (1 ml kg−1; 0.9% NaCl) or vehicle followed by fentanyl. The dashed line indicates the sum of the increases for the two control groups (WAY-100635+vehicle and vehicle+fentanyl). The increase in 5-HT was significantly greater in rats treated with WAY-100635 followed by fentanyl compared to WAY-100635+vehicle (F(1,12)=9.016, aP=0.01, p<0.05 ) and compared to vehicle+fentanyl (F(1,10)=11.982, P=0.0061, bp<0.05). (b) Calculated AUC values during the first, second and third hour after fentanyl injection. In the first hour, the AUC value for the effect of fentanyl following WAY-100635 pretreatment (open bar; WAY+fentanyl) is greater than the AUC value shown by the height of the stacked bar, which represents the sum of the effects of WAY-100635 alone and fentanyl alone (horizontal striped bar; WAY+vehicle; obliquely striped bar; vehicle+fentanyl). For the second and third hours, AUC values for the WAY+fentanyl group were equivalent to the sum of the effects of the two control groups.
Discussion
This study investigated the mechanisms involved in the effects of fentanyl on 5-HT efflux in the rat CNS. Systemic administration of fentanyl increased extracellular 5-HT in the DRN, and this effect was blocked by the opioid receptor antagonist naltrexone. In contrast, fentanyl infusion into the DRN decreased 5-HT. Neither naltrexone, which is a μ-preferring antagonist, nor a κ-opioid receptor antagonist nor-BNI prevented this effect. However, both (−)-pindolol and WAY-100635, which block 5-HT1A receptors, reversed the decrease in 5-HT during fentanyl infusion into the DRN. Also, WAY-100635 pretreatment enhanced the increase in 5-HT produced by a high systemic dose of fentanyl. These results suggest that fentanyl acts both as an opioid receptor agonist and as a 5-HT1A receptor agonist. Via stimulation of μ-opioid receptors fentanyl may increase 5-HT efflux by a disinhibitory mechanism (Jolas & Aghajanian, 1997; Tao & Auerbach, 2002a), but at higher doses, fentanyl can activate the 5-HT1A somatodendritic autoreceptor and directly inhibit 5-HT efflux.
μ-Opioid receptors involved in fentanyl-induced increase in 5-HT
Systemic administration of fentanyl citrate increased extracellular 5-HT in the DRN with a maximal effect at a dose of 0.05 mg kg−1. Thus, fentanyl was ∼200-fold more potent than morphine sulfate, which produced a similar increase in extracellular 5-HT at a dose of 10 mg kg−1 (Tao & Auerbach, 1995). This is consistent with evidence that the maximally effective dose of fentanyl in the rat tail flick test was 0.03 mg kg−1, nearly 300-fold more potent than morphine, which produced maximal analgesia at a dose of 8 mg kg-1 (Megens et al., 1998). Partly accounting for its potency compared to morphine, fentanyl has ∼5-fold higher affinity for μ-opioid receptors (Chaturvedi et al., 2000). Also, fentanyl is highly lipophilic, which facilitates penetration of the blood–brain barrier and rapid concentration in the brain (Hug & Murphy, 1981).
The increase in 5-HT in response to systemic fentanyl was fully blocked by naltrexone, indicating that opioid receptors were involved in this effect. μ-Opioids may act in the DRN and surrounding periaqueductal gray to disinhibit 5-HT neurons. GABAergic afferents tonically inhibit 5-HT neurons in the DRN (Tao et al., 1996; Gervasoni et al., 2000; Tao & Auerbach, 2003). Moreover, μ-opioids attenuate GABA-mediated inhibition of 5-HT neurons in the DRN and thus, may increase 5-HT efflux (Tao & Auerbach, 1994, 2002a; Jolas & Aghajanian, 1997).
5-HT1A autoreceptors involved in decreased 5-HT during fentanyl infusion into the DRN
Reverse dialysis infusion of fentanyl at a concentration of 100 μM in the aCSF decreased 5-HT efflux. Fentanyl has analgesic effects at much lower concentrations, ∼1–10 μM in brain tissue (Hug & Murphy, 1981). The dialysis membrane serves as a barrier to free diffusion and thus, drug concentrations in extracellular fluid near the probe drop to ∼10–20% of the level in the aCSF infusion medium (Dykstra et al., 1992). Together, this evidence suggests that the extracellular concentration necessary for fentanyl-induced decreases in 5-HT efflux is at the high end of the analgesic range. Moreover, naltrexone did not attenuate the decrease, suggesting that μ-opioid receptors were not involved. It is possible that this effect was mediated by κ-opioids, which inhibit 5-HT neuronal activity and efflux (Pinnock, 1992; Monroe et al., 1995; Tao & Auerbach, 2002b). Fentanyl binds to κ-opioid receptors with moderate affinity (Maguire et al., 1992), and this may play a role in the analgesic effect of fentanyl (Endoh et al., 1992). However, the selective κ-antagonist nor-BNI did not block the decrease in 5-HT efflux during fentanyl infusion, indicating that κ-opioid receptors were not involved.
We used the reuptake inhibitor citalopram in the aCSF and this may have interacted with the effect of fentanyl (Singh et al., 2001). However, fentanyl in the absence of citalopram still produced a reduction in 5-HT in the DRN. Thus, the differential effect of systemic and local administration of fentanyl cannot be attributed to citalopram. We also tested the possibility that infusion of fentanyl at much lower concentrations might increase 5-HT efflux. However, infusion of fentanyl at 100 nM or 1 μM in the aCSF had no effect on 5-HT in the DRN (data not shown).
Based on evidence that fentanyl binds to the 5-HT1A receptor subtype (Martin et al., 1991), which functions as an autoreceptor in the DRN, we tested the ability of (−)-pindolol and WAY-100635 to block the decrease in 5-HT. (−)-Pindolol is a mixed 5HT1A/β-adrenoceptor antagonist that blocks the 5-HT1A autoreceptor at the dose we used (Sharp et al., 1989). (−)-Pindolol may also act as a weak partial agonist at the 5-HTIA receptor subtype (Clifford et al., 1998; Fornal et al., 1999). However, (−)-pindolol alone under our experimental conditions produced a small increase in extracellular 5-HT in the DRN and furthermore, (−)-pindolol prevented the reduction in 5-HT during infusion of fentanyl. The more selective 5-HT1A receptor antagonist WAY-100635 (Fletcher et al, 1996) also blocked the decrease in 5-HT. Importantly, WAY-100635 in this experiment was administered by reverse dialysis in combination with fentanyl and thus, the two drugs perfused the same area of the CNS. These results support the hypothesis that fentanyl at high concentrations in the DRN activated 5-HT1A autoreceptors and thus inhibited 5-HT efflux.
With autoreceptors blocked, instead of decreasing, extracellular 5-HT increased in response to fentanyl infusion. Naltrexone blocked this increase, suggesting that the influence of fentanyl binding to opioid receptors was unmasked by blocking autoreceptor-mediated inhibition of 5-HT neuronal activity. This raises the question of why the effect of systemic fentanyl was preferentially mediated by opioid receptors, but the predominant effect of fentanyl infusion into the DRN was mediated by 5-HT1A receptors. We suggest that this may be related to the location of opioid receptors on GABAergic afferents to 5-HT neurons (Kalyuzhny & Wessendorf, 1997). Although some GABAergic afferents are interneurons within the DRN, the cell bodies for many GABAergic afferents are in adjacent areas of the brainstem and in forebrain sites (Gervasoni et al., 2000; Celada et al., 2001). If the opioid receptors were located on cell bodies, these GABAergic neurons would be outside the area perfused during reverse dialysis administration of fentanyl. In contrast, because 5-HT1A receptors are present on 5-HT cell bodies and dendrites within the DRN, local fentanyl infusion may result in strong activation of these autoreceptors. With subcutaneous administration, opioid receptors on the entire population of GABAergic afferents to the DRN would be stimulated and presumably, this was the major influence on 5-HT at low systemic doses of fentanyl. This conclusion is further supported by our observation that WAY-100635 enhanced the increase in 5-HT in the first hour after systemic administration of fentanyl, but not at later times when presumably, the concentration of fentanyl in the brain fell below the level that activates 5-HT1A receptors in the DRN. In summary, the inhibitory effect of local fentanyl infusion may be the result of stimulation of autoreceptors on 5-HT neurons, and the disinhibitory effect of systemic fentanyl may be mediated predominately by opioid receptors on GABAergic afferent neurons both within and outside of the DRN.
In conclusion, these results provide novel evidence that fentanyl can act in vivo as a 5-HT1A receptor agonist. Thus, fentanyl could influence 5-HT efflux by both a direct and an indirect mechanism. At low, clinically effective doses, systemic administration of fentanyl may stimulate μ-opioid receptors on GABAergic neurons that inhibit 5-HT neurons in the DRN. This in turn could disinhibit 5-HT neurons (Jolas & Aghajanian, 1997; Tao & Auerbach, 2002b) and thus, contribute to the analgesic effect of fentanyl (Wang & Nakai, 1994). In contrast, the reduction in 5-HT efflux during local infusion of fentanyl into the DRN can be ascribed to direct activation of 5-HT1A autoreceptors. Fentanyl at high systemic doses might also bind to postsynaptic 5-HT1A receptors and thus influence physiological functions such as regulation of blood pressure and respiration. Central control of respiratory function is modulated by 5-HT receptors in a complex manner. At low doses that primarily stimulate somatodendritic autoreceptors in the raphe, systemic administration of 8-OH-DPAT enhanced respiratory function (Sahibzada et al., 2000). In contrast, 8-OH-DPAT inhibited breathing at doses that presumably stimulated postsynaptic 5-HT1A receptors in a medullary respiratory center (Lalley et al., 1994; Richter et al., 1999). Moreover, several opioid receptor antagonists at doses that blocked the analgesic effect of fentanyl only slightly attenuated fentanyl-induced lethality (Jang & Yoburn, 1991). Together, these observations suggest that binding to 5-HT1A receptors might contribute to the lethality of fentanyl overdose.
Acknowledgments
This research was supported by National Institute of Health Grants MH51080 (SBA) and DA14541(RT).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AP
anterior–posterior
- AUC
area under the curve
- nor-BNI
nor-binaltorphimine
- CNS
central nervous system
- DRN
dorsal raphe nucleus
- EDTA
ethylenediaminetetraacetic acid
- h.p.l.c.-ED
high performance liquid chromatography with electrochemical detection
- 5-HT
5-hydroxytryptamine
- ML
medial–lateral
- WAY-100635
(N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexane carboxamide
References
- CELADA P., PUIG M.V., CASANOVAS J.M., GUILLAZO G., ARTIGAS F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin1A, GABAA, and glutamate receptors. J. Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATURVEDI K., SHAHRESTANIFAR M., HOWELLS R. μ Opioid receptor: role for the amino terminus as a determinant of ligand binding affinity. Mol. Brain Res. 2000;76:64–72. doi: 10.1016/s0169-328x(99)00332-0. [DOI] [PubMed] [Google Scholar]
- CLARKE R.W., WARD R.E. The role of 5-HT1A receptors in fentanyl-induced bulbospinal inhibition of a spinal withdrawal reflex in the rabbit. Pain. 2000;85:239–245. doi: 10.1016/s0304-3959(99)00272-9. [DOI] [PubMed] [Google Scholar]
- CLIFFORD E.M., GARTSIDE S.E., UMBERS V., COWEN P.J., HAJOS M., SHARP T. Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A autoreceptor invivo. Br. J. Pharmacol. 1998;124:206–212. doi: 10.1038/sj.bjp.0701796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYKSTRA K.H., HSIAO J.K., MORRISON P.P., BUNGAY P.M., MEFFORD I.N., SCULLY M.M., DEDRICK R.L. Quantitative examination of tissue concentration profiles associated with microdialysis. J. Neurochem. 1992;58:931–940. doi: 10.1111/j.1471-4159.1992.tb09346.x. [DOI] [PubMed] [Google Scholar]
- ENDOH T., MATSUURA H., TANAKA C., NAGASE H. Nor-binaltorphimine: a potent and selective κ-opioid receptor antagonist with long-lasting activity invivo. Arch. Int. Pharmacodyn. Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- FLETCHER A., FORSTER E.A., BILL D.J., BROWN G., CLIFFE I.A., HARTLEY J.E., JONES D.E., MCLENACHAN A., STANHOPE K.J., CRITCHLEY D.J., CHILDS K.J., MIDDLEFELL V.C., LANFUMEY L., CORRADETTI R., LAPORTE A.M., GOZLAN H., HAMON M., DOURISH C.T. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- FORNAL C.A., MARTIN F.J., METZLER C.W., JACOBS B.L. Pindolol suppresses serotonergic neuronal activity and does not block the inhibition of serotonergic neurons produced by 8-hydroxy-2-(di-n-propylamino)tetralin in awake cats. J. Pharmacol. Exp. Ther. 1999;291:229–238. [PubMed] [Google Scholar]
- GERVASONI D., PEYRON C., RAMPON C., BARBAGLI B., CHOUVET G., URBAIN N., FORT P., LUPPI P.H. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J. Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUG C.C., JR, MURPHY M.R. Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology. 1981;55:369–375. doi: 10.1097/00000542-198110000-00006. [DOI] [PubMed] [Google Scholar]
- JANG Y., YOBURN B.C. Evaluation of receptor mechanism mediating fentanyl analgesia and toxicity. Eur. J. Pharmacol. 1991;197:135–141. doi: 10.1016/0014-2999(91)90512-o. [DOI] [PubMed] [Google Scholar]
- JOLAS T., AGHAJANIAN G.K. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat invitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- KALYUZHNY A.E., WESSENDORF M.W. CNS GABA neurons express the μ-opioid receptor: immunocytochemical studies. Neuroreport. 1997;8:3367–3372. doi: 10.1097/00001756-199710200-00035. [DOI] [PubMed] [Google Scholar]
- LALLEY P.M., BISCHOFF A.M., RICHTER D.W. 5-HT1A receptor-mediated modulation of medullary expiratory neurones in the cat. J. Physiol. (Lond.) 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE P., TSAI N., KAMAL J., COMETTA-MORINI C., UPTON C., LOEW G. Pharmacological profiles of fentanyl analogs at μ, δ and κ opiate receptors. Eur. J. Pharmacol. 1992;213:219–225. doi: 10.1016/0014-2999(92)90685-w. [DOI] [PubMed] [Google Scholar]
- MARTIN D.C., INTRONA R.P., ARONSTAM R.S. Fentanyl and sufentanil inhibit agonist binding to 5-HT1A receptors in membranes from the rat brain. Neuropharmacology. 1991;30:323–327. doi: 10.1016/0028-3908(91)90056-h. [DOI] [PubMed] [Google Scholar]
- MEGENS A.A., ARTOIS K., VERMEIRE J., MEERT T., AWOUTERS F.H. Comparison of the analgesic and intestinal effects of fentanyl and morphine in rats. J. Pain Symptom Manage. 1998;15:253–257. doi: 10.1016/s0885-3924(97)00371-0. [DOI] [PubMed] [Google Scholar]
- MONROE P.J., KRADEL B.K., SMITH D.L., SMITH D.J. Opioid effects on spinal [3H]5-hydroxytryptamine release are not related to their antinociceptive action. Eur. J. Pharmacol. 1995;272:51–56. doi: 10.1016/0014-2999(94)00623-f. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. 1986Sydney: Academic Press; 2nd edn [Google Scholar]
- PINNOCK R.D. Activation of kappa-opioid receptors depresses electrically evoked excitatory postsynaptic potentials on 5-HT-sensitive neurones in the rat dorsal raphe nucleus invitro. Brain Res. 1992;583:237–246. doi: 10.1016/s0006-8993(10)80029-0. [DOI] [PubMed] [Google Scholar]
- RICHTER D.W., SCHMIDT-GARCON P., PIERREFICHE O., BISCHOFF A.M., LALLEY P.M. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J. Physiol. (Lond.) 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHIBZADA N., FERREIRA M., WASSERMAN A.M., TAVEIRA-DASILVA A.M., GILLIS R.A. Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine1A receptors. J. Pharmacol. Exp. Ther. 2000;292:704–713. [PubMed] [Google Scholar]
- SHARP T., BRAMWELL S.R., HJORTH S., GRAHAME-SMITH D.G. Pharmacological characterization of 8-OH-DPAT-induced inhibition of rat hippocampal 5-HT release invivo as measured by microdialysis. Br. J. Pharmacol. 1989;98:989–997. doi: 10.1111/j.1476-5381.1989.tb14630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH V.P., JAIN N.K., KULKARNI S.K. On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res. 2001;915:218–226. doi: 10.1016/s0006-8993(01)02854-2. [DOI] [PubMed] [Google Scholar]
- TAO R., AUERBACH S.B. Anesthetics block morphine-induced increases in serotonin release in rat CNS. Synapse. 1994;18:307–314. doi: 10.1002/syn.890180406. [DOI] [PubMed] [Google Scholar]
- TAO R., AUERBACH S.B. Involvement of the dorsal raphe but not median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neuroscience. 1995;68:553–561. doi: 10.1016/0306-4522(95)00154-b. [DOI] [PubMed] [Google Scholar]
- TAO R., AUERBACH S.B. Regulation of serotonin release by GABA and excitatory amino acids. J. Psychopharmacol. 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- TAO R., AUERBACH S.B. GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J. Pharmacol. Exp. Ther. 2002a;303:704–710. doi: 10.1124/jpet.102.038133. [DOI] [PubMed] [Google Scholar]
- TAO R., AUERBACH S.B. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J. Pharmacol. Exp. Ther. 2002b;303:549–556. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- TAO R., AUERBACH S.B. Influence of inhibitory and excitatory inputs on serotonin differs in the dorsal and median raphe nuclei. Brain Res. 2003;961:109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- TAO R., MA Z., AUERBACH S.B. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br. J. Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q.P., NAKAI Y. The dorsal raphe: an important nucleus in pain modulation. Brain Res. Bull. 1994;34:575–585. doi: 10.1016/0361-9230(94)90143-0. [DOI] [PubMed] [Google Scholar]
- XU W., CUI X., HAN J.S. Spinal serotonin 1A and 1C/2 receptors mediate supraspinal mu opioid-induced analgesia. Neuroreport. 1994;5:2665–2668. doi: 10.1097/00001756-199412000-00065. [DOI] [PubMed] [Google Scholar]