Abstract
We have studied the acute cardiac electrophysiological effects of KB130015 (KB), a drug structurally related to amiodarone. Membrane currents and action potentials were measured at room temperature or at 37°C during whole-cell patch-clamp recording in ventricular myocytes. Action potentials were also measured at 37°C in multicellular ventricular preparations.
The effects of KB were compared with those of anemone toxin II (ATX-II). Both KB and ATX-II slowed the inactivation of the voltage-gated Na+ current (INa). While KB shifted the steady-state voltage-dependent inactivation to more negative potentials, ATX-II shifted it to more positive potentials. In addition, while inactivation proceeded to completion with KB, a noninactivating current was induced by ATX-II.
KB had no effect on IK1 but decreased ICa-L The drug also did not change Ito in mouse myocytes.
The action potential duration (APD) in pig myocytes or multicellular preparations was not prolonged but often shortened by KB, while marked APD prolongation was obtained with ATX-II. Short APDs in mouse were markedly prolonged by KB, which frequently induced early afterdepolarizations.
A computer simulation confirmed that long action potentials with high plateau are relatively less sensitive to a mere slowing of INa inactivation, not associated with a persisting, noninactivating current. In contrast, simulated short action potentials with marked phase-1 repolarization were markedly modified by slowing INa inactivation.
It is suggested that a prolongation of short action potentials by drugs or mutations that only slow INa inactivation does not necessarily imply identical changes in other species or in different myocardial regions.
Keywords: Cardiac, myocyte, electrophysiology, channel, membrane potential, KB130015, anemone toxi
Introduction
A dysfunction of voltage-dependent Na+ channels is the basis for many congenital cardiac, skeletal muscle and neurological diseases (Balser, 2001; Goldin, 2001). Several genetic mutations in the cardiac Na+ channel have been detected, which result in altered inactivation and may cause a specific type of the long QT syndrome, a condition associated with high propensity to arrhythmias (for recent reviews, see Bennett, 2000; Chiang & Roden, 2000; Li et al., 2000; Bezzina et al., 2001; Grant, 2001; Miyamoto et al., 2001). In addition to slowing inactivation, these mutations are also frequently associated with a persisting, noninactivating opening of a small fraction of Na+ channels. The exact roles played by these two factors in causing the long QT syndrome are not fully clear.
Slowed inactivation of Na+ channels may also be induced by pharmacological agents. Na+-channel modifying agents (e.g. DPI-201-106, BDF-9148, BDF-9196, BDF-9198, etc.) have been proposed as possible new pharmacological tools in the treatment of congestive heart failure (Muller-Ehmsen et al., 1997,1998; Flesch & Erdmann, 2001), since they are expected to increase the intracellular Na+ concentration ([Na+]i), with a consequent positive inotropic action via an effect on Na+ – Ca2+ exchange. However, the beneficial use of these agents is limited by a potential arrhythmogenic action (Stump et al., 2000), attributed to their ability to prolong the action potential duration (APD) and the QT interval on the electrocardiogram (Amos & Ravens, 1994; Stump et al., 2000; Flesch & Erdmann, 2001). Since many Na+-channel modifying agents that slow inactivation also induce a noninactivating current, it would be of interest to identify or design drugs which display either one of these electrophysiological properties, in order to assess their relative importance in the prolongation of the APD and of the QT interval.
KB130015 (KB) is a new drug, synthetically derived by chemical substitution from amiodarone (Carlsson et al., 2002). The drug has been designed with the purpose of retaining the antiarrhythmic effects of amiodarone but without the side effects. We have shown recently that KB causes the following effects on Na+ channels: (1) a marked slowing of the inactivation, (2) a shift of the voltage-dependent inactivation to more negative potentials, (3) a slowing of the recovery from inactivation, and (4) a shift of the threshold for activation to more negative potentials (Macianskiene et al., 2003). The Na+ channel inactivation, although slowed by KB, still preceded to completion during maintained depolarization. Hence the drug could be used to test the consequences of slowed Na+ channel inactivation in the absence of persisting, noninactivating current. In the present study, we examined the extent to which the slowing of Na+ channel inactivation by KB impacts on the APD.
Methods
The measurements were performed on ventricular isolated, single myocytes, and on ventricular multicellular preparations. The study has been carried out in accordance with the Declaration of Helsinki and with the institutional guidelines for the care and use of laboratory animals.
Single cells and multicellular preparations
In most experiments, we used pig or mouse ventricular myocytes. Cardiomyocytes from other species (rabbit, guinea-pig, man) were also obtained occasionally. The methods used for cell dissociation have been described before (Stankovicova et al., 2000; Hernandez-Benito et al., 2001; Macianskiene et al., 2002), and involved the arterial perfusion, at 37°C, of a piece of the (pig or human) left ventricular wall or of the whole (mouse, rabbit, or guinea-pig) heart with a Ca2+-free Tyrode solution containing protease (0.1 mg ml−1; type XIV, Sigma) and collagenase (1.4 mg ml−1; type A, Boehringer, Manheim, Germany). Dissociated cells were stored at room temperature (21–22°C). Experiments on single myocytes were carried out at room temperature or, when explicitly mentioned, at 37°C.
Multicellular preparations consisted of pig left ventricular trabecular muscles or mouse thin papillary muscles. They were mounted in an experimental bath (see Mubagwa et al., 1997) and were superfused with standard Tyrode solution (see composition below) at 37°C.
Ion currents in myocytes
Measurements in single cells were carried out in the whole-cell patch-clamp recording mode (see Macianskiene et al., 2003), using heat-polished borosilicate glass electrodes (1–3 MΩ, when filled with internal solution) connected to an Axopatch 200A or 200B amplifier (Axon Instruments, Foster City, CA, U.S.A.). An analog-to-digital interface controlled by the pClamp 8.1 software (Axon Instruments) was used to generate command pulses and acquire data.
Various ion currents were measured using appropriate voltage protocols. To measure the voltage-dependent Na+ current (INa), step depolarizations were given every 1 s from a holding potential (VH) of −80 mV. To measure the L-type Ca2+ current (ICa-L), a 1-s prepulse to −40 mV was used (to inactivate Na+ and eventual T-type Ca2+ channels) before activating the current and the protocol was repeated every 5 s. During measurements of INa or ICa-L, K+ currents were blocked by substituting intracellular and extracellular K+ with Cs+. The transient outward K+ current (Ito) was measured as described previously (Hernandez-Benito et al., 2001).
Action potentials in myocytes and in multicellular preparations
Action potentials in isolated cardiomyocytes were measured under current-clamp in ruptured or perforated patch recording modes. The stimulus consisted of a 2-ms rectangular current pulse applied at 0.5–3 Hz via the patch electrode.
Action potentials in multicellular preparations were induced by electrically stimulating with the help of a punctate Ag–AgCl electrode placed on the surface of the tissue. Membrane potentials were measured with microelectrodes filled with 3 M KCl (resistance: 20–30 MΩ) connected to a GeneClamp500 (Axon Instruments) amplifier. The signals were digitized at 4 kHz with WindAq (DataQ Instruments; Akron, OH, U.S.A.). APDs were measured at 50% (APD50) and 90% (APD90) repolarization.
Solutions and drugs
The composition of the standard Tyrode solution used during cell isolation and during action potential measurements was (in mM): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.9 MgCl2, 0.33 NaH2PO4, 10 Na-HEPES (pH 7.4) and 10 glucose. The solution was bubbled with 100% O2 during cell dissociation or during experiments on tissues. To measure INa, a low-[Na+]o Tyrode (composition in mM: 10 NaCl, 135.4 CsCl, 2.6 MgCl2, 0.1 CaCl2, 0.33 NaH2PO4, 10 Cs-HEPES and 10 glucose; pH 7.4) was used to optimize voltage control. During measurements of L-type Ca2+ currents (ICa-L), the extracellular solution was identical to the normal Tyrode solution except that K+ was replaced with Cs+. To measure Ito without interference from drug-modified INa, Na+-free solutions were used, in which Na+ was completely replaced by NMDG, while [Ca]o was decreased to 0.18 mM. The patch pipette solution contained (in mM): 155 CsCl or KCl, 1 MgCl2, 5 Na2ATP or MgATP, 1 EGTA, 0.1 Na2GTP, 5 Cs-HEPES or K-HEPES (pH 7.25). For perforated-patch recordings, 325 μM amphotericin B and 1.8 mM CaCl2 (to cause cell death in case of patch rupture) were added to the pipette solution.
KB was obtained from Karo-Bio, Huddingen, Sweden. Anemone toxin II (ATX-II; from Anemonia sulcata) and all other drugs or products were from Sigma Chemie (Bornem, Belgium). Stock solutions (10–100 mM) of KB were prepared in DMSO, which had no effect of its own at the highest concentration used (0.1%, v v−1).
Data analysis
Data were analyzed using Clampfit (Axon Instruments) or Origin (Microcal, Northampton, MA, U.S.A.). Average data are expressed as mean±s.e.m. Statistical comparison was made using a two-tailed t-test or ANOVA followed by Tukey–Kramer multiple comparison test.
Modeling of INa and action potential
The Luo–Rudy computer model of ion channel currents and action potential (Clancy & Rudy, 1999; http://www.cwru.edu/med/CBRTC/LRdOnline/content.htm) was used to test whether the observed action potential changes can be qualitatively consistent with the changes of INa. To avoid division by zero in voltage clamp simulations with settings of the membrane potential to multiples of 10 mV, the original constants −30 and −50 in the expressions of the ‘taud' and ‘tauxs1' variables were replaced by, respectively, −30.1 and −50.1. Unlike in the simulation of action potentials, in voltage clamp mode fixed time steps (0.001 ms) were used. The program was implemented in the Delphi™, version 7.0 (Borland) environment and run on a personal computer. When indicated, the transient outward current Ito was incorporated (Dumaine et al., 1999), using a conductance GIto of 4 mS μF−1. To simulate the effect of the drug, the default kinetic parameters of the fast Na+ channel given in Clancy & Rudy (1999) were modified as indicated in the legend of Figure 9.
Figure 9.
Simulation of the effect of slowed INa inactivation on the action potential. Na+ currents (INa) and action potentials generated using different channel kinetic parameters. Upper panels: INa induced by simulated voltage-clamp from −80 to −30 mV. Middle panels: action potentials generated during simulated pacing at 1 Hz in the absence of the transient outward current (Ito). Lower panels: action potentials in the presence of a large Ito (maximal conductance 4 μS pF−1). (a) Control conditions. Na+ channel with standard kinetics and conductance, as given in the Luo–Rudy model formulated from single channel kinetics (Clancy & Rudy, 1999). (b) Na+ channel with slowed inactivation kinetics but normal maximal conductance. The default rate constants of transitions from the open to the inactivated state (α2) and from the closed to the open state (α13) were decreased by a factor of 20 and 3.8, respectively, whereas the rate constant for the recovery from the inactivated to the closed state (α3) was changed by a factor of 10. α2=0.459 × elV/29.68 m s−1; α13=1/(0.1027 × e−V/12+0.25 × e−V/150) m s−1; α3=3.79 × 10−8 × e−V/5.2 m s−1. (c) Na+ channel with slowed inactivation kinetics and decreased maximal conductance. The rate constant α2 and the maximal conductance (GNa) were decreased. α2=0.2 × eV/29.68 m s−1; GNa=3 instead of 16 μS pF−1.
Results
Slowing of Na+ current inactivation by KB and ATX-II
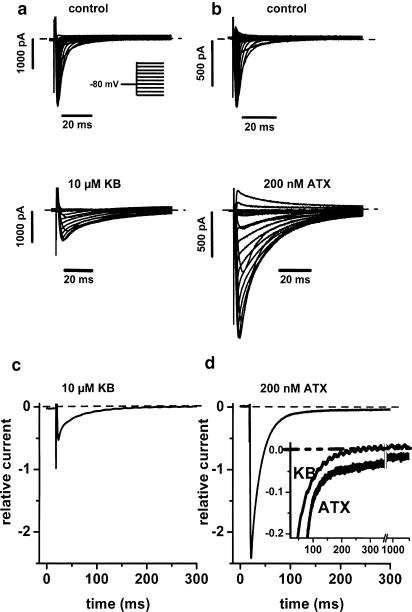
Typical effects of KB on Na+ current (INa) are illustrated in Figure 1a and are compared with those of ATX-II, illustrated in Figure 1b. INa was induced from a holding potential (VH) of −80 mV. Both drugs markedly slowed INa inactivation. However, they had opposite effects on INa peak amplitude: KB markedly decreased, whereas ATX-II increased INa amplitude. Similar results were obtained in five cells with KB (10 μM) and four cells with ATX (200 nM). To illustrate the relative change of INa by these agents, for each cell the current trace in the presence of either KB or ATX was normalized using the peak amplitude of the control current (in the absence of drug). Average traces of the normalized INa at −30 mV are displayed in Figure 1c and d for KB and ATX, respectively. The results confirm the opposite effects of KB and ATX on INa amplitude. The inset in Figure 1d shows that the increase of INa by ATX remained relatively larger than that by KB throughout the whole pulse duration. In addition, while INa inactivated completely after >200 ms in the presence of KB, a noninactivating component persisted in the presence of ATX even after 1 s.
Figure 1.
Comparison of the effects of KB and ATX-II on voltage-dependent Na+ current (INa). (a,b) INa traces elicited by depolarizations to various potentials before (upper panels) and following 10-min exposure (lower panels) to 10 μM KB (a) or 200 nM ATX-II (b). Inset in (a): voltage pulse protocol. Holding potential (VH): −80 mV. Test potentials between −120 and 0 mV (a) or +20 mV (b). (c,d) Average normalized INa traces at −30 mV in the presence of 10 μM KB (c; n=5) or 200 nM ATX (d; n=4). INa in the presence of drug in each cell was normalized relative to the INa peak amplitude before exposure to the drug in the same cell. Inset in (d) superimposed traces from (c) and (d) shown at different current gain. The horizontal dashed lines indicate zero current level. Pig ventricular myocytes. [Na]o=[Na]i=10 mM; K+-free, Cs+-containing external and internal solutions; room temperature.
The changes of peak current amplitude are likely due to the drug effects on voltage-dependent inactivation. Figure 2a, b (upper panels) shows the effects on INa measured at −30 mV following 1-s prepulses to various potentials. Besides slowing the time course of inactivation, no drug had any marked effect on the peak amplitude of INa induced from the most negative potentials. However, the relative decrease of INa with depolarized prepulses was different between the two drugs. Inactivation curves obtained from these data (Figure 2a,b, lower panels) show that KB shifted the steady-state INa inactivation to more negative potentials (see Macianskiene et al., 2003), whereas ATX-II shifted it in the opposite direction. These results help explain the opposite effects of both drugs observed on the INa peak amplitude when using −80 mV as VH (see Figure 1), with no effect on INa amplitude when using a VH of −120 mV (Figure 2c, d).
Figure 2.
Comparison of the effects of KB and ATX-II on voltage-dependent inactivation of INa. (a,b) Superimposed INa traces, elicited by depolarizations to −30 mV following 1-s prepulses to various levels (upper panels), and inactivation curves (lower panels) in the absence (unfilled circles) and in the presence (filled circles) of either 10 μM KB (a) or 200 nM ATX-II (b). Inset in (a): voltage pulse protocol. Holding potential (VH): −80 mV. Horizontal dashed lines indicate zero current level. Fitting of the inactivation curves was made using Boltzmann distribution functions. Average parameters of the Boltzmann functions for ATX: V0.5=−83±2.85 mV, slope=5.7±0.3, in control; V0.5=−72±3.7 mV, slope=7.5±0.4, in the presence of the toxin (n=4; P<0.05 vs control). For average KB data, see Macianskiene et al. (2003). (c,d) Pooled data on peak amplitude of INa at −30 mV using VH of either −120 or −80 mV in control (unfilled columns) and in the presence (hatched columns) of 10 μM KB (c; n=5–6) or 200 nM ATX-II (d; n=4). Pig ventricular myocytes. [Na]o=[Na]i=10 mM; K+-free, Cs+-containing external and internal solutions; room temperature. **P<0.01 for drug vs control (paired t-test).
Different effects of KB and ATX-II on APD in pig preparations
In the above and in previous experiments, extracellular and intracellular Na+ concentrations of 10 mM were used to decrease INa amplitude and improve voltage control. In a few experiments in the present study, we used the normal Tyrode ([Na+]o=150 mM) and a K+-based internal solution to allow recording of both INa and action potential in the same cell. INa and action potentials were elicited at 1 Hz, alternately during steps to −30 mV in the whole-cell voltage clamp mode (VH=−80 mV) or during pacing in current clamp conditions. Figure 3a shows superimposed INa traces obtained during 1-s depolarizations before and after application of 10 μM KB. Despite technical limitations for accurately measuring fast and large currents, the KB effects were essentially similar to those obtained with low [Na]o (see Figure 1). Identical results could be obtained when recording with the perforated-patch method, or when using myocytes from mouse (n=3), rabbit (n=2), guinea-pig (n=1), and human heart (n=3) (not illustrated). These results indicate that KB exerts its effects under physiological conditions and in different animal species. Despite the marked slowing of INa inactivation, there was practically no change of the APD in the same cell (Figure 3c). KB caused a small elevation of the plateau that was followed by a slight APD shortening. In contrast, superimposed traces obtained before and after application of 200 nM ATX-II show that the toxin-induced slowing of INa inactivation was associated with a persisting, noninactivating current (Figure 3b), and a marked APD prolongation in the same cell (Figure 3d).
Figure 3.
Comparison of KB and ATX-II effects on INa and action potentials in pig cardiac myocytes. (a,b) Superimposed INa traces induced at −30 mV in control (unfilled circles) and in the presence (filled circles) of either 10 μM KB (a) or 200 nM ATX-II (b). (c,d) Superimposed action potential recorded under current-clamp conditions in the same cells, in control (unfilled circles) and in the presence (filled circles) of either 10 μM KB (c) or 200 nM ATX-II (d). In (d) action potential labeled with unfilled square was obtained after 10-min washout of ATX-II. Horizontal dashed lines indicate 0 mV level. Stimulation via the patch pipette at 1 Hz. [Na]o=150 mM, [Na]i=10 mM; K+-containing external and internal solutions; ruptured-patch recording; room temperature.
The effects of both drugs were reversible. For KB, reversibility required washout with DMSO (0.1% v v−1) for 40–60 min (see Figure 5a). For ATX-II, a 5–10-min washout in normal solution was sufficient (see Figure 3d).
Figure 5.
Effect of KB on action potentials in mouse myocytes. (a) Action potentials measured in control, at 5 and 10 min after application of 10 μM KB, and following 60-min washout of KB in 0.1% DMSO. Myocyte from the left ventricular free wall; ruptured patch recording. (b,c) Action potential recorded in control and in the presence of 3 or 10 μM KB; perforated patch recordings. Myocytes from the left ventricular free wall (b) and from the septum (c). Horizontal dashed lines indicate zero level; pacing at 1 Hz.
Given that we had expected more prominent action potential changes due to the marked delay of INa inactivation by KB, we examined more extensively the effect of this drug. Figure 4 illustrates effects on action potentials recorded at room temperature in single myocytes (Figure 4a; two different cells), or under more physiological conditions at 37°C in multicellular preparations (Figure 4b; two different muscles). In single cells, some variability in the effect of KB was found between individual preparations. An increase (see Figure 3a) or a marked decrease of the overshoot and plateau, and either no change (Figure 4a, left) or a shortening (Figure 4a, right) of the global APD could be obtained in different cells. In no case was APD significantly prolonged. Pooled measurements of APD50 and APD90 at 1 Hz from 10 cells are given in Figure 4a (rightmost panel). Qualitatively identical results were obtained while pacing at different frequencies, and all measurements in pig myocytes are summarized in Table 1. On the average, a similar lack of APD prolongation was observed (not illustrated) in cells superfused at 37°C with no intracellular Ca2+ buffering by EGTA (APD90 at 1 Hz: 304±22.4 vs 293±30.4 ms, in control vs presence of 10 μM KB, respectively; P>0.05; n=7), although in two of these cells some prolongation was present. In multicellular preparations (Figure 4b), the results were qualitatively identical to those of single myocytes (APD90 at 1 Hz: 298±37.4 vs 265±34.1 ms, in control vs presence of 50 μM KB, respectively; P>0.05; n=5; 37°C). These data indicate that overall KB did not lengthen the APD in pig ventricular preparations.
Figure 4.
Variable effects of KB on action potentials in pig preparations. Superimposed action potential traces (leftmost and middle panels; different cells) recorded in control (unfilled circles) and in the presence of 10 μM KB (filled circles), and average durations (columns; rightmost panels) of the APD at 50% (APD50) and 90% repolarization (APD90). Horizontal dotted lines indicate 0 mV level. Pacing frequency: 1 Hz. (a) Pig ventricular myocytes; room temperature; (b) pig ventricular trabecula; 37°C.
Table 1.
Effect of KB on the resting and action potentials of pig ventricular myocytes
| 1 Hz (n=10) | 2.5 Hz (n=12) | 3 Hz (n=8) | ||||
|---|---|---|---|---|---|---|
| Control | KB | Control | KB | Control | KB | |
| Vrest (mV) | −74±1.5 | −75±1.9 | −74±1.8 | −75±2.1 | −75±1.4 | −75±2.0 |
| APA (mV) | 131±1.4 | 113±3.0 | 117±4.3 | 105±3.8 | 123±2.1 | 99±5.1 |
| APD50 (ms) | 197±15.6 | 149±20.4** | 139±7.0 | 125±13.1.4 | 139±6.2 | 111±15.3* |
| APD90 (ms) | 258±13.4 | 194±22.5** | 195±8.3.4 | 172±15.2 | 185±6.4.4 | 148±17.6* |
Values are given as mean±s.e.m. Same cells used for various frequencies, in the absence and presence of 10 μM KB. Recordings made at room temperature. Vrest: resting potential; APA: amplitude of action potential; action potential duration (APD) measured at 50% (APD50) or at 90% (APD90) repolarization;
P<0.05;
P<0.01; compared to control.
KB lengthens short action potentials in mouse myocytes
The above results suggest that the major difference in the KB and ATX-II effect on APD could be related to their different effects on INa magnitude, and to the absence and presence of a persisting INa, respectively. Since in the absence of a persisting INa, a slowed inactivation is likely to have more prominent effect on very short action potentials, we also examined the effect of KB in mouse ventricular myocytes. Because of a rapid run-down of ICa-L in mouse myocytes during ruptured-patch recording under our experimental conditions (R. Macianskiene & K. Mubagwa, unpublished results), action potentials in this species were recorded under either ruptured patch (n=8; Figure 5a) or perforated patch conditions (n=7; Figure 5b, c). Cells were obtained from the left ventricular free wall (Figure 5a,b) or from the septum (Figure 5c). Action potentials were usually longer in the perforated-patch recording mode. Figure 5a illustrates that in cells from the ventricular free wall, the action potential recorded under ruptured patch mode was very short and that KB caused a progressive prolongation of the action potential and induced early afterdepolarizations (EADs). In some cases, the EADs disappeared as the action potential grew longer and a clear plateau phase developed. Short action potentials recorded with the perforated patch method were also prolonged by KB (Figure 5b). In contrast, when the control APD was long, as during perforated patch recording, the early phase of repolarization could be delayed simultaneously with a shortening of the global APD (Figure 5c). All data from mouse myocytes are summarized in Table 2. Similar results were obtained in papillary muscle preparations (n=2; not illustrated). Altogether these results suggest that KB, which fails to increase the duration of long action potentials, markedly prolongs shorter action potentials.
Table 2.
Effect of KB on the resting and action potentials of mouse ventricular myocytes
| Ruptured patch (n=8) | Perforated patch (n=7) | |||
|---|---|---|---|---|
| Control | KB | Control | KB | |
| Vrest (mV) | −71±1.2 | −72±1.0 | −74±0.6 | −76±1.5 |
| APA (mV) | 100±7.1 | 98±5.7 | 116±3.4 | 118±4.2 |
| APD50 (ms) | 6±0.6 | 48±18.2* | 17±3.7 | 74±13.6* |
| APD90 (ms) | 52±13.4 | 143±13.0* | 155±16.5 | 198±10.4* |
Values are given as mean±s.e.m. Same cells used in the absence and presence of 3 μM KB. Recordings made at room temperature, during pacing at 1 Hz. Vrest: resting potential; APA: amplitude of action potential; action potential duration (APD) measured at 50% (APD50) or at 90% (APD90) repolarization;
P<0.05; compared to control.
Effects of KB on other membrane currents
The failure of KB to prolong the APD in pig preparations and its marked effect on short action potentials in mouse could also be due to changes in other membrane currents that, respectively, counterbalance or enhance the effect of a slowly inactivating INa. We therefore investigated the effect of KB (10 μM) on other membrane currents.
Steady-state currents
Figure 6a shows current traces obtained using K+-containing extra- and intracellular media, upon depolarizing for 1 s to various potentials (VH=−80 mV), and Figure 6c shows the corresponding current–voltage relationships obtained by measuring the current at the end of the voltage step. KB had no effect at potentials where the time-dependent currents are absent (negative to −70 mV), but caused a marked slowing of the initial time-dependent inward currents following steps to potentials positive to −70 mV (i.e. , reflecting INa). At these potentials, there was no change of the steady-state current after the decay of the early inward currents. Steady-state current–voltage relationships obtained under control conditions and in the presence of KB were practically superimposable (Figure 6c), suggesting a lack of effect on IK1.
Figure 6.
Effect of KB on steady-state currents. (a, b) Traces of currents elicited by 1-s steps to various potentials before (upper panels) and after application (lower panels) of either 10 μM KB (a) or 200 nM ATX-II (b). Horizontal dashed lines indicate zero current level. (c, d) Current–voltage relations in control (unfilled circles) and in the presence (filled circles) of either 10 μM KB (c) or 200 nM ATX-II (d). Currents measured at the end of the 1-s pulse. Notice the change of steady-state currents by ATX-II but not KB at potentials positive to −70 mV. Pig ventricular myocytes; K+-containing external and internal solutions; [Na]o=150 mM; [Na]i=10 mM; room temperature.
Corresponding data with ATX-II (200 nM) are shown in Figure 6b and d. ATX-II also did not change the time-independent currents at potentials negative to −70 mV and markedly slowed the initial time-dependent inward currents following steps to more positive potentials. However, steady-state currents were changed at potentials where INa is activated (Figure 6d). This result is also consistent with the existence of a persisting, noninactivating INa in the presence of ATX-II but not in the presence of KB (see also Figure 3b). The lack of ATX-II effect at the most negative potentials (where INa is not activated) indicates that the drug does not affect the inward rectifier IK1.
Effect of KB on ICa-L
Figure 7a shows ICa-L traces recorded in one cell before and after 10-min exposure to 10 μM KB. K+ currents were blocked and INa inactivated by a 1-s prepulse to −40 mV (see Methods). KB caused a small decrease of the peak current amplitude but had no effect on the time course of inactivation (see inset of Figure 7a). Figure 7b and c presents pooled data of current–voltage relations and time constants, respectively. The magnitude of the decrease in peak current varied from cell to cell, but in a total of nine cells, the decrease in peak amplitude at +10 mV amounted to 35% of the control current. Similar effects (not illustrated) were obtained in three pig myocytes superfused at 37°C and not dialyzed with EGTA (decrease of the ICa-L peak amplitude from −4.6±0.7 to −3.3±0.9 pA pF−1; P<0.05) and in three human ventricular myocytes (decrease of ICa-L from −2.1±0.4 to −1.3±0.5 pA pF−1; P<0.05), with no effect on inactivation time course (τfast: 14±4.5 and 12±3.5 ms; τslow: 142±24.9 and 140±16.5 ms; in the human cells, under control conditions and in the presence of 10 μM KB, respectively).
Figure 7.
KB effect on the L-type Ca2+ current (ICa-L). (a) ICa-L currents elicited by steps to various potentials in the absence (left) and in the presence (right) of 10 μM KB. The horizontal dotted line indicates zero current level. Inset in left panel shows the voltage protocol: holding potential (VH) of −80 mV, prestep for 1 s to −40 mV to inactivate INa and any T-type Ca2+ current. Inset in right panel contrasts the failure of KB to change the time course ICa-L (at 0 mV) at a time when it causes slowing of INa (during the prestep to −40 mV). (b) Current–voltage relations in control (unfilled circles) and in the presence (filled circles) of 10 μM KB. Pooled data from various cells (n=9). (c) Time constants (obtained using biexponential fitting) of ICa-L inactivation measured at 0 mV, in control conditions (unfilled columns) and in 10 μM KB (filled columns). Same cells as in (b) pig myocytes; K+-free, Cs+-containing external and internal solutions; room temperature. *P<0.05 vs control.
Lack of effect of KB on the transient outward current (Ito) in mouse
As the marked effect on APD in mouse ventricular preparations could be possibly attributed to a change of Ito, we also examined the effect of KB on this current. Ito was studied under conditions where external Na+ was completely replaced with NMDG to avoid artefacts due to drug-induced change in INa. Figure 8 illustrates typical results, in which 10 μM KB failed to change both the time-independent currents at negative potentials (IK1) and the time-dependent outward currents at potentials positive to −40 mV (Ito).
Figure 8.
Lack of effect of KB on Ito and IK1 in mouse myocytes. (a) Traces of currents elicited by steps to various potentials before (left panel) and after application (right panel) of 10 μM KB. Horizontal dotted lines indicate zero current level. (b) Current–voltage relations in control (unfilled circles) and in the presence (filled circles) of 10 μM KB. Currents measured at peak level. (c) Pooled data on amplitude of currents at −120 mV (largely IK1; n=10) and +60 mV (largely Ito; n=3). Notice lack of KB effect. [Na]o=[Na]i=0 mM; K+-containing external and internal solutions; room temperature.
Discussion
The present study examined the consequences of the KB-induced slowing of INa inactivation on the APD. Under conditions where KB markedly slowed the Na+ channel inactivation, the drug did not lengthen but frequently shortened the APD in pig ventricular myocytes. Since other Na+ channel modifiers are found to be of little clinical usefulness for the treatment of heart failure because they induce an arrhythmogenic prolongation of the QT interval, it was of interest to examine the mechanisms responsible for the lack of APD prolongation by KB.
In our previous study (Macianskiene et al., 2003), slowing of INa inactivation by KB was obtained while using low [Na+]o (to decrease the driving force for Na+) and low [Ca2+]o (to avoid contamination of inward currents by ICa-L). The present study confirms and extends these results by showing that KB causes a qualitatively similar slowing of INa inactivation when physiological [Na+]o and [Ca2+]o are used. In addition, we now show that KB effects are slowly reversible when DMSO is included in the solution during washout. In the early study, we had noticed that washout with normal Tyrode for 20–30 min was not associated with a removal of the drug effect. The slow time course of reversibility, the need for DMSO to remove the drug effect, and our previous observation that intracellular KB was ineffective, all suggest that KB accumulates within the lipid phase of the membrane, from where it is able to interact with its action site on the Na+ channel.
We compared the effects of KB with those of ATX-II, a toxin known to change the inactivation of cardiac Na+ channels (Isenberg & Ravens, 1984). Whereas both drugs slowed INa inactivation, they differed in the ability to induce persisting, noninactivating current (present with ATX-II but not with KB; see Figures 1, 3 and 6) and in their effects on the voltage-dependent steady-state inactivation (shift to more positive potentials with ATX-II, but to more negative potentials with KB; see Figure 2). The different shifts in steady-state inactivation resulted in opposite effects (decrease with KB, increase with ATX-II) on the amplitude of INa induced from −80 mV.
Because of the prolonged opening of Na+ channels by KB and ATX-II, we had expected that either drug would prolong the APD, albeit to different extents. However, we found that in pig ventricular myocytes only ATX-II was able to prolong APD. In most cases, no prolongation or a marked APD shortening was obtained with KB. The difference between KB and ATX-II effects on APD (see Figure 3) is likely due, at least in part, to the differences in their effects on INa. In other studies, differences have been also noticed between the channel modifiers DPI201-2065, BDF9648 and BDF9698 in their ability to prolong the APD and were attributed to different effects on other ion channels (Ravens et al., 1991; Amos et al., 1994; Yuill et al., 2000). Such a situation could also apply in the case of KB and ATX. To account for the absence of an APD prolongation by KB, the following possibilities could be invoked: (1) concomitant direct effects of KB on other ion channels, whose currents counterbalance the effect of slow-inactivating INa; (2) indirect effects on other currents, due to changes in the early part of the action potential, induced by drug-modified INa; (3) relative insensitivity of long action potentials to an effect of slowed INa inactivation in the absence of a persisting current. These possibilities are now examined.
In pig cells, we did not find any effect on steady-state currents. However, there was a significant decrease of ICa-L (Figure 7). The mechanism underlying this decrease and the reason for the observed variability between cells were not elucidated in the present study. A similar decrease of ICa-L has been observed with amiodarone and was attributed to a negative shift of the steady-state voltage-dependent inactivation (Nishimura et al., 1989). Since a marked decrease was not obtained in all cells (see example of Figure 7), it is unlikely to be the sole explanation of the general absence of APD prolongation by KB. In pig cells, the transient outward K+ current (Ito) is not significantly expressed (Macianskiene et al., 2002), and its eventual increase cannot therefore be invoked for a counterbalancing effect relative to INa.
Changes of the early part (overshoot level or initial repolarization) of the action potential due to modified INa may indirectly modify the ion current balance and the repolarization rate during the subsequent part of the plateau. In some pig cells, an elevation of the early part of the plateau was obtained in the presence of KB. Such an effect may lead to more inactivation of INa and ICa-L, and more activation of K+ currents, effects that will tend to shorten the APD. However, in other cells a decrease of the overshoot and a lowering of the plateau level were obtained. These apparently contradictory results are probably accounted for by the opposing consequences of slowed inactivation and of decreased initial INa availability. On the one hand, the slowing of INa inactivation will favor an elevation of the plateau. On the other hand, due to the KB-induced shift of voltage-dependent steady-state inactivation to more negative potentials, fewer Na+ channels are available at the resting potential, and the initial INa may be decreased, favoring both a lower overshoot and a lower plateau. The relative contribution of both effects probably determines the net change in overshoot and plateau.
Finally, it is possible that long action potentials with high plateau are relatively insensitive to a slowly inactivating INa if inactivation can still proceed to completion (i.e., if there is no maintained, noninactivating current). The APD is more likely to increase if INa is increased and contributes a persisting, noninactivating current during the plateau of the action potential, as is the case with ATX-II (see Figure 2; and Boutjdir et al., 1994), while less APD prolongation will occur if INa amplitude is decreased and the current fully inactivates shortly after the beginning of a long plateau. Since plateau levels in pig cells are at potentials above 0 mV, the effect of KB may be minimized by a faster INa inactivation and a decrease of the electrochemical driving force for Na+ at more positive potentials.
The finding that in mouse ventricular cells KB markedly prolonged the APD and could induce EADs is in favor of the hypothesis that slowly inactivating INa is more likely to remodel short action potentials with fast early (phase 1) repolarization. During ruptured patch recording in mouse myocytes, the APD in control conditions is very brief, without clear plateau. Repolarization is terminated through a slow phase 3, that begins at potentials more negative than −50 mV. The most pronounced prolongation of the APD by KB was found in these cells, and was often associated with a decreased overshoot and the appearance of a clear plateau (see Figure 5a). The APD lengthening can be explained by the slowed inactivation of INa, and the decreased overshoot by a decreased INa peak amplitude due to enhanced steady-state inactivation. Our voltage-clamp results indicate that the repolarizing current Ito (which is prominent in mouse cells) is not modified in the presence of KB. Ito is expected to activate maximally at overshoot potentials. During the rapid repolarization due to Ito, INa has not had time to inactivate fully and may actually increase due to an increase in its driving force. This will induce EADs or a clear-cut plateau. The fact that EADs could take off from potentials as negative as −50 mV (see Figure 5a) is consistent with the possibility that they may result from an increased driving force of INa. Less APD remodeling by KB was induced on longer APDs recorded during perforated patch recording in mouse myocytes, in which the most consistent effect was a delay of the phase-1 repolarization. In some preparations, the latter effect was associated with a marked shortening of the APD, probably as a result of increased activation of repolarizing outward currents and inactivation of ICa-L due to the prolonged phase 1 and the elevated plateau.
Our experimental results are also consistent with those of a computer simulation of INa and action potentials, used to test the hypothesis formulated above, that the relative effect of slowed INa inactivation on APD depends on the initial duration and on the plateau level. The results of the simulation are presented in Figure 9. The model has been shown to reproduce electrical changes due to mutations of the voltage-dependent Na+ and HERG K+ channels that cause a prolongation of APD and of the QT interval on the ECG (Clancy & Rudy, 1999,2002). A slowly inactivating INa could be qualitatively reproduced by slowing the rate constants of the transitions between different states. However, the changes were associated with a marked increase in the peak amplitude of INa (Figure 9b), in contrast to our finding when measuring INa under voltage-clamp conditions with a VH of −80 mV. To reduce INa to amplitudes equal to or smaller than control, we either changed the maximal conductance of INa (Figure 9c) or further reduced the rate constants of activation. The parameters used to change INa were then applied in a model of the action potential not incorporating Ito, to simulate the situation in pig cells (Figure 9, middle panels), or in a model incorporating a large Ito, to simulate the situation in mouse cells (Figure 9, lower panels). Action potentials generated by assuming a stimulation at 1 Hz showed no or little effect of slowing INa inactivation on the long, high-plateau action potentials in the absence of Ito (Figure 9, middle panels). In contrast, there was marked APD lengthening and induction of EADs in case of control short, action potentials in the presence of Ito (Figure 9, lower panels). Although the changes in channel kinetics used in the model may not represent a quantitative simulation of those induced by the drug, the results demonstrate the contrasting sensitivity of long or short action potentials to the effect of a slowly inactivating INa.
Conclusions
In contrast to ATX-II and to many other Na+-channel modifying agents that increase APD, KB did not prolong but either did not change or did shorten APD in pig myocytes. It is proposed that APD lengthening is mainly obtained by agents that induce a maintained, noninactivating INa. Following an isolated slowing of inactivation, as may result from drugs such as KB or from mutations on the Na+ channel, cells with short APDs will present more marked APD lengthening even under conditions where insignificant changes are produced in cells with long APDs. These results have practical implications, since they suggest that the consequences of drug- or mutation-induced change in Na+ channel inactivation may differ between animal species with very short (e.g., mouse) or with long (e.g., pig, human) action potentials, and between different regions of the myocardium (e.g., subepicardial vs midmyocardial or subendothelial layers).
Acknowledgments
This work was supported by grants from FWO, the Flemish Foundation for Science. The authors thank Dr F. Rega for supplying pig ventricular tissue.
Abbreviations
- APD
action potential duration
- EAD
early afterdepolarization
- ICa-L
L-type Ca2+ current
- IK1
inward-rectifying background K+ current
- INa
voltage-dependent Na+ current
- Ito
transient outward K+ current
- KB
KB130015 ([2-methyl-3-(3,5-diiodo-4-carboxymethoxybenzyl)benzofuran])
References
- AMOS G.J., RAVENS U. The inotropic agents DPI 201-106 and BDF 9148 differentially affect potassium currents of guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 1994;350:426–433. doi: 10.1007/BF00178962. [DOI] [PubMed] [Google Scholar]
- BALSER J.R. The cardiac sodium channel: gating function and molecular pharmacology. J. Mol. Cell. Cardiol. 2001;33:599–613. doi: 10.1006/jmcc.2000.1346. [DOI] [PubMed] [Google Scholar]
- BENNETT P.B. Long QT syndrome: biophysical and pharmacologic mechanisms in LQT3. J. Cardiovasc. Electrophysiol. 2000;11:819–822. doi: 10.1111/j.1540-8167.2000.tb00055.x. [DOI] [PubMed] [Google Scholar]
- BEZZINA C.R., ROOK M.B., WILDE A.A. Cardiac sodium channel and inherited arrhythmia syndromes. Cardiovasc. Res. 2001;49:257–271. doi: 10.1016/s0008-6363(00)00272-8. [DOI] [PubMed] [Google Scholar]
- BOUTJDIR M., RESTIVO M., WEI Y., STERGIOPOULOS K., EL-SHERIF N. Early afterdepolarization formation in cardiac myocytes: analysis of phase plane patterns, action potential, and membrane currents. J. Cardiovasc. Electrophysiol. 1994;5:609–620. doi: 10.1111/j.1540-8167.1994.tb01302.x. [DOI] [PubMed] [Google Scholar]
- CARLSSON B., SINGH B.N., TEMCIUC M., NILSSON S., LI Y.L., MELLIN C., MALM J. Synthesis and preliminary characterization of a novel antiarrhythmic compound (KB 130015) with an improved toxicity profile compared with amiodarone. J. Med. Chem. 2002;45:623–630. doi: 10.1021/jm001126+. [DOI] [PubMed] [Google Scholar]
- CHIANG C.E., RODEN D.M. The long QT syndromes: genetic basis and clinical implications. J. Am. Coll. Cardiol. 2000;36:1–12. doi: 10.1016/s0735-1097(00)00716-6. [DOI] [PubMed] [Google Scholar]
- CLANCY C.E., RUDY Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999;400:566–569. doi: 10.1038/23034. [DOI] [PubMed] [Google Scholar]
- CLANCY C.E., RUDY Y. Na+ channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation. 2002;105:1208–1213. doi: 10.1161/hc1002.105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMAINE R., TOWBIN J.A., BRUGADA P., VATTA M., NESTERENKO D.V., NESTERENKO V.V., BRUGADA J., BRUGADA R., ANTZELEVITCH C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ. Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- FLESCH M., ERDMANN E. Na+ channel activators as positive inotropic agents for the treatment of chronic heart failure. Cardiovasc. Drugs Ther. 2001;15:379–386. doi: 10.1023/a:1013329203750. [DOI] [PubMed] [Google Scholar]
- GOLDIN A.L. Resurgence of sodium channel research. Annu. Rev. Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- GRANT A.O. Molecular biology of sodium channels and their role in cardiac arrhythmias. Am. J. Med. 2001;110:296–305. doi: 10.1016/s0002-9343(00)00714-2. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ-BENITO M.J., MACIANSKIENE R., SIPIDO K.R., FLAMENG W., MUBAGWA K. Suppression of transient outward potassium currents in mouse ventricular myocytes by imidazole antimycotics and by glybenclamide. J. Pharmacol. Exp. Ther. 2001;298:598–606. [PubMed] [Google Scholar]
- ISENBERG G., RAVENS U. The effects of the Anemonia sulcata toxin (ATX II) on membrane currents of isolated mammalian myocytes. J. Physiol. 1984;357:127–149. doi: 10.1113/jphysiol.1984.sp015493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI H., FUENTES-GARCIA J., TOWBIN J.A. Current concepts in long QT syndrome. Pediatr. Cardiol. 2000;21:542–550. doi: 10.1007/s002460010132. [DOI] [PubMed] [Google Scholar]
- MACIANSKIENE R., MOCCIA F., SIPIDO K.R., FLAMENG W., MUBAGWA K. Channels involved in transient currents unmasked by removal of extracellular calcium in cardiac cells. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1879–H1888. doi: 10.1152/ajpheart.00952.2001. [DOI] [PubMed] [Google Scholar]
- MACIANSKIENE R., VIAPPIANI S., SIPIDO K.R., MUBAGWA K. Slowing of the inactivation of cardiac voltage-dependent sodium channels by the amiodarone derivative 2-methyl-3-(3,5-diiodo-4-carboxymethoxybenzyl)benzofuran ( KB130015) J. Pharmacol. Exp. Ther. 2003;304:130–138. doi: 10.1124/jpet.102.042218. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO S., ZHU B.M., AYE N.N., HASHIMOTO K. Slowing Na+ channel inactivation prolongs QT interval and aggravates adrenaline-induced arrhythmias. Jpn. J. Pharmacol. 2001;86:114–119. doi: 10.1254/jjp.86.114. [DOI] [PubMed] [Google Scholar]
- MUBAGWA K., LIN W., SIPIDO K., BOSTEELS S., FLAMENG W. Monensin-induced reversal of positive force–frequency relationship in cardiac muscle: role of intracellular sodium in rest-dependent potentiation of contraction. J. Mol. Cell. Cardiol. 1997;29:977–989. doi: 10.1006/jmcc.1996.0342. [DOI] [PubMed] [Google Scholar]
- MULLER-EHMSEN J., BRIXIUS K., SCHWINGER R.H. Positive inotropic effects of the novel Na+-channel modulator BDF 9198 in human nonfailing and failing myocardium. J. Cardiovasc. Pharmacol. 1998;31:684–689. doi: 10.1097/00005344-199805000-00006. [DOI] [PubMed] [Google Scholar]
- MULLER-EHMSEN J., FRANK K., BRIXIUS K., SCHWINGER R.H. Increase in force of contraction by activation of the Na+/Ca2+-exchanger in human myocardium. Br. J. Clin. Pharmacol. 1997;43:399–405. doi: 10.1046/j.1365-2125.1997.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMURA M., FOLLMER C.H., SINGER D.H. Amiodarone blocks calcium current in single guinea pig ventricular myocytes. J. Pharmacol. Exp. Ther. 1989;251:650–659. [PubMed] [Google Scholar]
- RAVENS U., WETTWER E., PFEIFER T., HIMMEL H., ARMAH B. Characterization of the effects of the new inotropic agent BDF 9148 in isolated papillary muscles and myocytes of the guinea-pig heart. Br. J. Pharmacol. 1991;104:1019–1023. doi: 10.1111/j.1476-5381.1991.tb12543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANKOVICOVA T., SZILARD M., DE SCHEERDER I., SIPIDO K.R. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc. Res. 2000;45:952–960. doi: 10.1016/s0008-6363(99)00418-6. [DOI] [PubMed] [Google Scholar]
- STUMP G.L., WALLACE A.A., GILBERTO D.B., GEHRET J.R., LYNCH J.J., JR Arrhythmogenic potential of positive inotropic agents. Basic Res. Cardiol. 2000;95:186–198. doi: 10.1007/s003950050181. [DOI] [PubMed] [Google Scholar]
- YUILL K.H., CONVERY M.K., DOOLEY P.C., DOGGRELL S.A., HANCOX J.C. Effects of BDF 9198 on action potentials and ionic currents from guinea-pig isolated ventricular myocytes. Br. J. Pharmacol. 2000;130:1753–1766. doi: 10.1038/sj.bjp.0703476. [DOI] [PMC free article] [PubMed] [Google Scholar]