Abstract
Endothelin-1 (ET-1) activates two types of Ca2+-permeable nonselective cation channels (designated NSCC-1 and NSCC-2) and a store-operated Ca2+ channel (SOCC) in vascular smooth muscle cells (VSMCs). These channels can be distinguished by their sensitivity to Ca2+-channel blockers, SK&F 96365 and LOE 908. LOE 908 is sensitive to NSCC-1 and NSCC-2, and SK&F 96365 is sensitive to NSCC-2 and SOCC. Moreover, these channels play essential roles in ET-1-induced epidermal growth factor receptor protein tyrosine kinase (EGFR PTK) transactivation. The main purpose of the present study was to demonstrate the involvement of EGFR PTK transactivation in ET-1-induced arachidonic acid release in VSMCs.
Both SK&F 96365 and LOE 908 inhibited ET-1-induced arachidonic acid release with the IC50 values correlated to those of ET-1-induced Ca2+ influx. Moreover, combined treatment with these blockers abolished ET-1-induced arachidonic acid release.
AG1478, a specific inhibitor of EGFR PTK, inhibited ET-1-induced arachidonic acid release and extracellular signal-regulated kinase 1 and 2 (ERK1/2). The IC50 values of AG1478 for ET-1-induced arachidonic acid release and ERK1/2 correlated well with those for ET-1-induced EGFR PTK transactivation.
Mitogen-activated protein kinase kinase inhibitor, PD 98059, inhibited ET-1-induced arachidonic acid release. The IC50 values of PD 98059 for ET-1-induced arachidonic acid release were similar to those for ET-1-induced ERK1/2 activity. In contrast, PD 98059 failed to inhibit ET-1-induced EGFR PTK transactivation.
These results indicate that (1) extracellular Ca2+ influx through NSCCs and SOCC plays important roles for ET-1-induced arachidonic acid release, (2) EGFR PTK transactivation/ERK1/2 pathways are involved in ET-1-induced arachidonic acid release.
Keywords: Endothelin-1, arachidonic acid release, Ca2+ channel, epidermal growth factor receptor
Introduction
Activation of phospholipase A2 (PLA2) liberates arachidonic acid from phospholipids. Arachidonic acid metabolites, including prostaglandins, leukotrienes, lipoxins, and hydroxy derivatives, have been implicated in numerous physiological and pathophysiological processes (Brady & Serhan, 1996; Makita et al., 1996; Vane & Botting, 1997). This arachidonic acid is preferentially released by the 85-kDa cytosolic PLA2 (cPLA2) (Sharp et al., 1991; Trevisi et al., 2002). Both Ca2+ and phosphorylation regulate cPLA2 activity. Ca2+ is required for cPLA2 to translocate from the cytosol to phospholipid, a membrane that is mediated by its Ca2+-dependent phospholipid binding domain (Nalefski et al., 1994). Endothelin-1 (ET-1) induces arachidonic acid release through the activation of cPLA2 in vascular smooth muscle cells (Resnik et al., 1989; Trevisi et al., 2002). In addition, extracellular Ca2+ influx plays critical roles in ET-1-induced arachidonic acid release (Dunican et al., 1996; Wu-Wong et al., 1996). However, it remains unclear as to what types of Ca2+ channels are involved in ET-1-induced arachidonic acid release in vascular smooth muscle cells (VSMCs). These uncertainties are mainly attributable to the lack of specific Ca2+-channel blockers. We have recently shown that ET-1 activates three types of voltage-independent Ca2+ channel (VICC), as well as voltage-operated Ca2+ channels (VOCCs) in rabbit internal carotid artery (ICA) VSMCs. The VICCs include two types of Ca2+-permeable nonselective cation channel (designated NSCC-1 and NSCC-2) and a store-operated Ca2+ channel (SOCC) (Kawanabe et al., 2002a). Importantly, we have also shown that these channels can be distinguished by their sensitivity to blockers of the receptor-operated Ca2+ channel such as SK&F 96365 and LOE 908 (Meritt et al., 1990; Encabo et al., 1996). NSCC-1 is sensitive to LOE 908 and resistant to SK&F 96365, NSCC-2 is sensitive to both LOE 908 and SK&F 96365, and SOCC is resistant to LOE 908 and sensitive to SK&F 96365 (Kawanabe et al., 2002a). Based on these results, we tried to elucidate which Ca2+ channels are involved in ET-1-induced arachidonic acid release using SK&F 96365 and LOE 908 in this study.
Next, we investigated the intracellular mechanisms of the ET-1-induced arachidonic acid release. Stimulation of cells by epidermal growth factor (EGF) results in the activation of cPLA2 (Bonventre et al., 1990). Moreover, EGF-induced extracellular signal-regulated kinase 1 and 2 (ERK1/2) phosphorylation results in cPLA2 activation (van Rossum et al., 2001). ET-1 transactivates EGFR protein tyrosine kinase (PTK) in VSMCs (Iwasaki et al., 1999; Kawanabe et al., 2002b). Therefore, we focused on investigating whether ET-1-induced EGFR PTK transactivation was involved in the stimulation of arachidonic acid release by ET-1.
Methods
Cell culture
Isolated VSMCs were prepared from rabbit ICA, as described previously (Kawanabe et al., 2002a). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum supplemented with 100 U ml−1 penicillin G and 100 μg ml−1 streptomycin, under a humidified 5% CO2/95% air atmosphere.
[3H] arachidonic acid release
The level of [3H] arachidonic acid release was determined as described previously (Perez et al., 1993). Briefly, cells in 100-mm dishes were incubated overnight with [3H] arachidonic acid (final concentration, 1 μCi ml−1). After washing, ET-1 was added and, after 5 min, the medium was removed, acidified with 100 μl of 1 N formic acid, and extracted with 3 ml of chloroform. The extracts were evaporated to dryness, resuspended in 50 μl chloroform, and applied to silica gel plates for thin-layer chromatography (Merck, Darmstadt, Germany). The plates were developed in heptane/diethyl ether/acetic acid (v v−1; 75 : 25 : 4). The distance of movement was visualized with iodine vapor. The plate was scraped, and the radioactivity was counted with a liquid scintillation counter.
Measurement of EGFR PTK transactivation
Measurement of EGFR PTK transactivation was performed using a Universal Tyrosine Kinase Assay Kit (Takara, Tokyo, Japan), as described previously (Kawanabe et al., 2002b). Extraction buffer and kinase reacting solution were equipped with this kit. Cells seeded at 5 × 106 cells well−1 in six-well plates were starved for 24 h and then stimulated with 10 nM ET-1 for 2 min. The reaction was terminated by washing three times with phosphate-buffered saline (PBS). After the addition of 1 ml of extraction buffer, the cells were scraped off with a scraper and centrifuged at 14,500 rpm for 10 min at 4°C. The supernatant was incubated with mouse monoclonal anti-EGFR antibody (Takara, Tokyo, Japan) for 2 h at room temperature and subsequently incubated with Protein A–agarose for an additional 1 h. The mixture was centrifuged at 10,000 × g for 1 min at 4°C, and the pellets were washed three times with PBS. The washed pellets were resuspended in 150 μl of kinase reaction buffer. EGFR PTK transactivation was determined according to the manufacturer's instructions. The absorbance of the lysate at 450 nm was measured with an EL340 Microtiter Plate Reader (Bio-Tek Instruments, Winooski, VT, USA).
Measurement of ERK1/2 activity
Measurement of ERK1/2 activity was performed as described previously (Sugawara et al., 1996; Kawanabe et al., 2001). Briefly, ICA VSMCs at 50% confluency in 10-cm dishes were starved for 24 h before being stimulated by 10 nM ET-1 for 5 min in serum-free DMEM. The reaction was terminated by washing once with PBS and twice with 20 mM Tris-HCl (pH 7.4). After the addition of 1 ml of ice-cold extraction buffer (10 mM Tris-HCl, 0.5 mM EDTA, 0.5 mM EGTA, 5 mM MgCl2, 1 mM dithiothreitol, 5 mg ml−1 aprotinin, 0.05 mM NaF, 0.5 mM Na3PO4, 0.5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, and 5 mM β-glycerophosphate; pH 7.4), the cells were scraped off using a scraper. For partial purification of ERK, the cell suspension was transferred to a 15-ml conical tube, sonicated for 10 s × 3 at 10-s intervals, and centrifuged at 25,000 × g for 20 min. The supernatant was applied to a DEAE-Sephadex column (bed volume, 0.5 ml) pre-equilibrated with equilibration buffer (extraction buffer containing 100 mM NaCl). The enzyme was eluted with the elution buffer (extraction buffer containing 500 mM NaCl) and concentrated using Centricon YM-30 (Millipore Corporation, Bedford, MA, U.S.A.). The protein concentration of the partially purified enzyme in each sample was determined with a BCA Microprotein Assay Kit (Pierce, Rockford, IL, U.S.A.), and 5 μg of the enzyme was used for each assay. ERK1/2 activity was determined using a MAP Kinase Assay Kit (Amersham, Buckinghamshire, U.K.) according to the manufacturer's instructions.
Drugs
Boehringer Ingelheim K.G. (Ingelheim, Germany) kindly provided LOE 908. Other chemicals were commercially obtained.
Statistical analysis
All results were expressed as mean±s.e.m. The data were subjected to a two-way analysis of variance, and when a significant F-value was encountered, the Newman–Keuls' multiplerange test was used to test for significant differences between treatment groups. A probability level of P<0.05 was considered statistically significant.
Results
Effects of ET-1 on arachidonic acid release in VSMCs
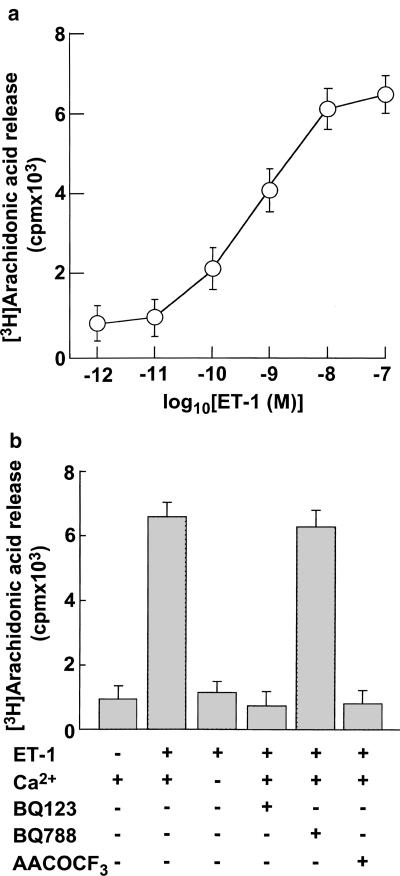
ET-1 induced arachidonic acid release in a concentration-dependent manner with an EC50 value of around 1 nM, and maximal effects were observed at concentrations of ⩾10 nM (Figure 1a). In the absence of extracellular Ca2+, the magnitudes of ET-1-induced arachidonic acid release were near the basal level (Figure 1b). Therefore, extracellular Ca2+ influx appears to play an important role in ET-1-induced arachidonic acid release. ET-1-induced arachidonic acid release was abolished by BQ123, a specific antagonist of endothelinA receptor, but it was unaffected by BQ788, a specific antagonist of endothelinB receptor (Figure 1b). Moreover, ET-1-induced arachidonic acid release was inhibited by arachydonyl trifluoromethyl ketone (AACOCF3), a selective inhibitor of cPLA2.
Figure 1.
(a) Effects of various concentrations of ET-1 on arachidonic acid release in vascular smooth muscle cells. The cells were stimulated with increasing concentrations of ET-1 for 5 min. (b) Effects of extracellular Ca2+, BQ123, BQ788, and AACOCF3 on ET-1-induced arachidonic acid release in VSMCs. The cells were pretreated with or without 5 μM BQ123, 5 μM BQ788, or 50 μM AACOCF3 for 30 min and incubated with 10 nM ET-1 for 5 min. Arachidonic acid release was determined as described in Methods. Data presented are the mean±s.e.m. of six determinations, each done in triplicate.
Effects of SK&F 96365 and LOE 908 on ET-1-induced arachidonic acid release
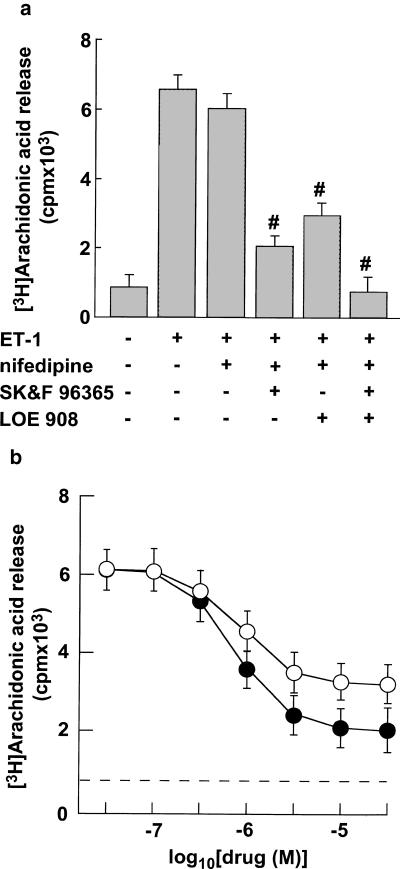
We examined the effects of extracellular Ca2+ influx through VOCCs on ET-1-induced arachidonic acid release using nifedipine, a specific blocker of L-type VOCCs. Nifedipine at 1 μM completely inhibited 10 nM ET-1-induced extracellular Ca2+ influx through VOCCs in VSMC (data not shown). In contrast, it inhibited 10 nM ET-1-induced arachidonic acid release by a maximum of only about 10% (Figure 2a). Using SK&F 96365 and LOE 908, we attempted to determine the effects of extracellular Ca2+ influx through VICCs on ET-1-induced arachidonic acid release. In the following experiments, nifedipine was added to the incubation media at a final concentration of 1 μM to analyze the role of Ca2+ channels other than L-type VOCC. SK&F 96365 inhibited 10 nM ET-1-induced arachidonic acid release in a concentration-dependent manner with IC50 values of around 1 μM (Figure 2b). Maximal inhibition was observed at concentrations ⩾10 μM. The extent of maximal inhibition was around 80% of 10 nM ET-1-induced arachidonic acid release (Figure 2). Similarly, LOE 908 inhibited 10 nM ET-1-induced arachidonic acid release in a concentration-dependent manner with IC50 values of around 1 μM, and maximal inhibition was observed at concentrations ⩾10 μM (Figure 2). The extent of maximal inhibition was around 60% of 10 nM ET-1-induced arachidonic acid release (Figure 2b). Moreover, the combined treatment with maximal effective concentration (10 μM) of SK&F 96365 and LOE 908 completely inhibited 10 nM ET-1-induced arachidonic acid release (Figure 2a).
Figure 2.
(a) Effects of a maximal effective concentration of nifedipine (1 μM), SK&F 96365 (10 μM), and LOE 908 (10 μM) on ET-1-induced arachidonic acid release in VSMCs. (b) Effects of various concentrations of SK&F 96365 and LOE 908 on ET-1-induced arachidonic acid release in VSMCs. The cells were incubated for 15 min with various concentrations of SK&F 96365 (closed circles) or LOE 908 (open circles) and then stimulated with 10 nM ET-1 for 5 min in the presence of 1 μM nifedipine. Arachidonic acid release was determined as described in Methods. Data presented are the mean±s.e.m. of six determinations, each done in triplicate. #P<0.05; significantly different from the control values stimulated by ET-1 in each experiment.
Effects of AG1478 on ET-1-induced arachidonic acid release and ERK1/2 stimulation
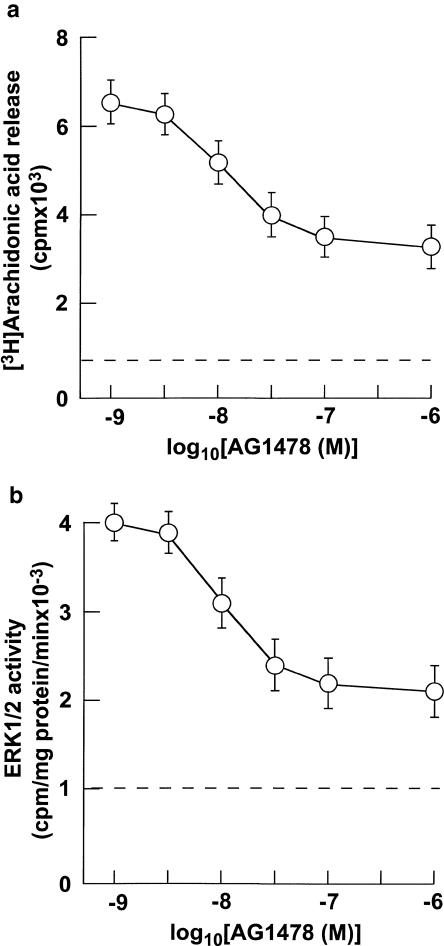
AG1478, a specific inhibitor of EGFR PTK, inhibited 10 nM ET-1-induced arachidonic acid release (Figure 3a). The inhibitory effect of AG1478 on ET-1-induced arachidonic acid release was in a concentration-dependent manner with IC50 values of around 10 nM (Figure 3a). Maximal inhibition was observed at concentrations ⩾1 μM (Figure 3a). The extent of maximal inhibition was around 55% of ET-1-induced arachidonic acid release (Figure 3a). Similarly, AG1478 inhibited 10 nM ET-1-induced ERK1/2 stimulation (Figure 3b). The inhibitory effect of AG1478 on ET-1-induced ERK1/2 was in a concentration-dependent manner with IC50 values of around 10 nM (Figure 3b). Maximal inhibition was observed at concentrations ⩾1 μM (Figure 3b). The extent of maximal inhibition was around 60% of ET-1-induced ERK1/2 stimulation (Figure 3a).
Figure 3.
(a) Effects of various concentrations of AG1478 on ET-1-induced arachidonic acid release in VSMCs. The cells were incubated for 15 min with various concentrations of AG1478 and then stimulated with 10 nM ET-1 for 5 min. Arachidonic acid release was determined as described in Methods. (b) Effects of various concentrations of AG1478 on ET-1-induced ERK1/2 in VSMCs. ERK1/2 activity was determined as described in Methods. Data presented are the mean±s.e.m. of six determinations, each done in triplicate.
Effects of PD 98059 on ET-1-induced arachidonic acid release and EGFR PTK transactivation
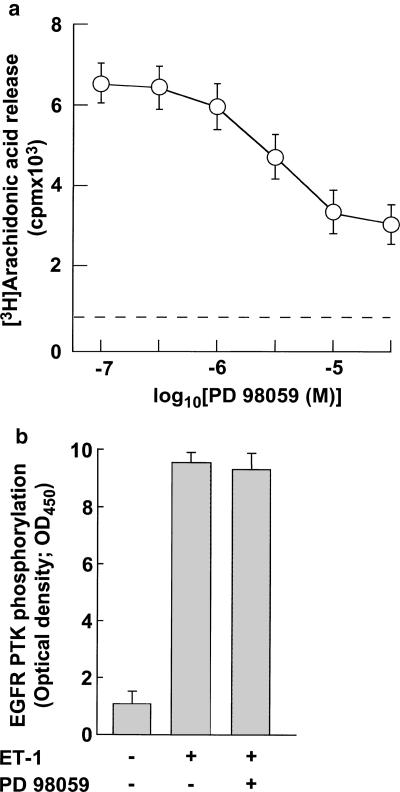
PD 98059, an inhibitor of mitogen-activated protein kinase kinase, inhibited 10 nM ET-1-induced arachidonic acid release (Figure 4a). The inhibitory effect of PD 98059 on ET-1-induced arachidonic acid release was in a concentration-dependent manner with IC50 values of around 3 μM (Figure 4a). Maximal inhibition was observed at concentrations ⩾30 μM (Figure 4a). The extent of maximal inhibition was around 60% of ET-1-induced arachidonic acid release (Figure 4a). In contrast, 30 μM PD 98059 failed to inhibit 10 nM ET-1-induced EGFR PTK transactivation (Figure 4b).
Figure 4.
(a) Effects of PD 98059 on ET-1-induced arachidonic acid release in VSMCs. The cells were incubated for 15 min with various concentrations of PD 98059 and then stimulated with 10 nM ET-1 for 5 min. ERK1/2 activity was determined as described in Methods. Data presented are the mean±s.e.m. of six determinations, each done in triplicate. (b) Effects of PD 98059 on ET-1-induced EGFR PTK transactivation. The cells were incubated for 15 min with or without 30 μM PD 98059 and then stimulated with 10 nM ET-1 for 2 min. EGFR PTK activity was determined as described in Methods. Data presented are the mean±s.e.m. of six determinations, each done in triplicate.
Discussion
Based on the sensitivity to BQ123 and BQ788, endothelinA receptor plays essential roles in the ET-1-induced arachidonic acid release in ICA VSMCs (Figure 1b). Based on the sensitivity to AACOCF3 (Figure 1b), ET-1 induces arachidonic acid release through cPLA2 activation. These results are in agreement with the observations that agonist-induced AA release is mainly mediated by cPLA2 in many cell types (Wu-Wong et al., 1996; Trevisi et al., 2002). Previous reports demonstrated that extracellular Ca2+ influx plays important roles in the arachidonic acid release (Dunican et al., 1996; Wu-Wong et al., 1996). We tried to characterize the Ca2+ channels involved in the ET-1-induced arachidonic acid release in ICA VSMCs. The magnitudes of ET-1-induced arachidonic acid release in the absence of extracellular Ca2+ were near the basal level (Figure 1b). These results indicate that extracellular Ca2+ influx plays important roles in ET-1-induced arachidonic acid release in ICA VSMCs. Our recent study indicated that NSCC-1, NSCC-2, and SOCC play a major part in the ET-1-induced extracellular Ca2+ influx in ICA VSMCs (Kawanabe et al., 2002a). Moreover, extracellular Ca2+ influx through these Ca2+ channels plays essential roles in the ET-1-induced PYK2 protein tyrosine kinase activation and cell proliferation (Kawanabe et al., 2002a, 2002c; 2003). Thus, we examined the involvement of NSCC-1, NSCC-2, and SOCC in the ET-1-induced arachidonic acid release using SK&F 96365 and LOE 908. According to the nifedipine sensitivity of ET-1-induced arachidonic acid release, involvement of VOCC in this response was estimated to be minor, at around only 10% (Figure 1b). We demonstrated in a recent report that nifedipine suppressed the 10 nM ET-1-induced sustained increase in [Ca2+]i by a maximum of no more than 10% (Kawanabe et al., 2002a). Therefore, Ca2+ channels other than VOCC may play important roles in ET-1-induced arachidonic acid release in addition to extracellular Ca2+ influx in ICA VSMCs. The inhibitory action of SK&F 96365 and LOE 908 on the ET-1-induced arachidonic acid release is considered to be mediated by blockade of Ca2+ entry through VICCs for the following reasons. (1) In our recent work using patch-clamp and [Ca2+]i monitoring, ET-1 was found to activate three types of VICCs in VSMCs, namely NSCC-1, NSCC-2, and SOCC. In addition, LOE 908 was found to be a blocker of both NSCC-1 and NSCC-2, whereas SK&F 96365 was found to be a blocker of NSCC-2 and SOCC (Kawanabe et al., 2002a). (2) The IC50 values of these blockers for the ET-1-induced arachidonic acid release (Figure 2b) correlated well with those for the ET-1-induced extracellular Ca2+ influx (Kawanabe et al., 2002a). Moreover, because SK&F 96365 and LOE 908 failed to inhibit ET-1-induced transient increase in [Ca2+]i due to the release of intracellular Ca2+ store (Kawanabe et al., 2002a), the release of salcoplasmic reticulum Ca2+ is not sufficient to stimulate arachidonic acid release. Three types of VICC seem to be involved in the ET-1-induced arachidonic acid release in terms of its sensitivity to SK&F 96365 and LOE 908 (Figure 5). One type of Ca2+ channel is sensitive to LOE 908 and resistant to SK&F 96365, another type is sensitive to both LOE 908 and SK&F 96365, and the third type is resistant to LOE 908 and sensitive to SK&F 96365. Based on pharmacological criteria, these channels are considered to be NSCC-1, NSCC-2, and SOCC, respectively. Moreover, the percent contribution of NSCC-1, NSCC-2, and SOCC to the ET-1-induced arachidonic acid release is calculated to be about 20, 40, and 40%, respectively, of nifedipine-resistant part of arachidonic acid release caused by 10 nM ET-1 (Figure 5). The magnitudes of the ET-1-induced arachidonic acid release that were inhibited by the combined treatment with nifedipine, SK&F 96365, and LOE 908 were similar to those in the absence of extracellular Ca2+ (Figures 1b, 2a). Therefore, extracellular Ca2+ influx through NSCC-1, NSCC-2, and SOCC plays important roles for ET-1-induced arachidonic acid release in ICA VSMCs.
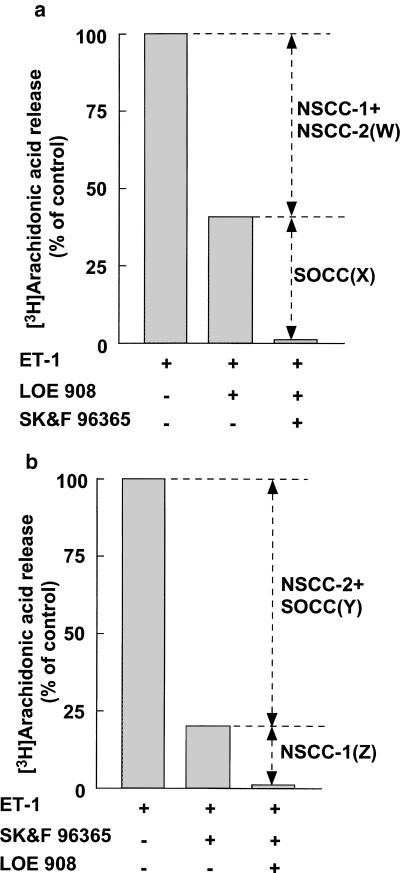
Figure 5.
(a, b) Calculation for contribution of Ca2+ influx through three types of VICCs to ET-1-induced arachidonic acid release in VSMCs in the presence of 1 μM nifedipine. The ET-1-induced arachidonic acid release in the presence of 10 μM LOE 908 and/or 10 μM SK&F 96365 is represented as a percentage of values in its absence. The contributions of SOCC and NSCC-1 are represented as X and Z, respectively. The contribution of NSCC-2 is represented as W–Z or Y–X.
EGF stimulates arachidonic acid release (Bonventre et al., 1990). Based on the data that ET-1 transactivates EGFR PTK in VSMCs (Iwasaki et al., 1999; Kawanabe et al., 2002b), and both EGFR PTK transactivation and arachidonic acid release by ET-1 are dependent on extracellular Ca2+ influx through NSCC-1, NSCC-2, and SOCC (Kawanabe et al., 2002b; Figure 5), we hypothesized that EGFR PTK transactivation was involved in ET-1-induced arachidonic acid release. The inhibitory effects of AG1478 on ET-1-induced arachidonic acid release may be due to its inhibitory effects on EGFR PTK transactivation, judging from the following data: (1) AG 1478 is generally accepted as a EGFR PTK inhibitor (Iwasaki et al., 1999). (2) The IC50 values (around 10 nM) and maximal effective concentration (1 μM) of AG1478 for ET-1-induced arachidonic acid release (Figure 3a) were similar to those for ET-1-induced EGFR PTK transactivation (Kawanabe et al., 2002b). These results indicate that EGFR PTK transactivation plays important roles in ET-1-induced arachidonic acid release. As we mentioned above, extracellular Ca2+ influx through voltage-independent Ca2+ channels is involved in ET-1-induced arachidonic acid release. Modulation of ET-1-induced EGFR PTK transactivation may be at least one target of extracellular Ca2+ influx. Based on the sensitivity to AG1478 (Figure 3), ET-1 induces arachidonic acid release via EGFR PTK transactivation-independent pathway, as well as EGFR PTK transactivation-dependent pathway. Recent reports demonstrate that protein kinase C and phosphoinositide 3-kinase play important roles for arachidonic acid release (Silfani & Freeman, 2002; Trevisi et al., 2002). Protein kinase C and phosphoinositide 3-kinase pathways may be involved in EGFR PTK transactivation-independent parts of ET-1-induced arachidonic acid release. ET-1-induced arachidonic acid release by cPLA2 activation is dependent on ERK1/2 pathway in VSMCs (Trevisi et al., 2002). PD 98059 inhibited ET-1-induced arachidonic acid release (Figure 4). The IC50 values (around 3 μM) of PD 98059 for ET-1-induced arachidonic acid release (Figure 4a) were similar to those for ET-1-induced ERK1/2 activation (Kawanabe et al., 2002c). In contrast, PD 98059 failed to inhibit ET-1-induced EGFR PTK transactivation (Figure 4b). These results indicate that ERK1/2 plays some roles in ET-1-induced arachidonic acid release downstream of EGFR PTK transactivation in ICA VSMCs. Some reports demonstrated that extracellular Ca2+ influx plays important roles for phosphorylation and translocation of cPLA2 (Crawford & Jacobson, 1998; Fatima et al., 2001). We have preliminary data that Ca2+ influx through NSCCs and SOCCs plays important roles for translocation of cPLA2. The effects of EGFR PTK and ERK1/2 on ET-1-induced phosphorylation and translocation of cPLA2 are now under investigation in our laboratory.
In summary, extracellular Ca2+ influx through voltage-independent Ca2+ channels such as NSCC-1, NSCC-2, and SOCC plays important roles for ET-1-induced arachidonic acid release. In addition, EGFR PTK transactivation is involved in ET-1-induced arachidonic acid release. Finally, ERK1/2 has important roles in the EGFR PTK transactivation-dependent component of ET-1 -induced arachidonic acid release.
Acknowledgments
We thank Boehringer Ingelheim K.G. for the kind donation of LOE 908. This study was supported by a grant from the Smoking Research Foundation, Japan and by the Uehara Memorial Foundation Fellowship, Tokyo, Japan.
Abbreviations
- AACOCF3
arachydonyl trifluoromethyl ketone
- cPLA2
cytosolic PLA2
- DMEM
Dulbecco's modified Eagle's medium
- EGFR
epidermal growth factor receptor
- ERK 1/2
extracellular signal-regulated kinase 1 and 2
- ET-1
endothelin-1
- ICA
internal carotid artery
- NSCC
nonselective cation channel
- PBS
phosphate-buffered saline
- PLA2
phospholipase A2
- PTK
protein tyrosine kinase
- SOCC
store-operated Ca2+ channel
- VICC
voltage-independent Ca2+ channel
- VOCC
voltage-operated Ca2+ channel
- VSMC
vascular smooth muscle cell
References
- BONVENTRE J.V., GRONICH J.H., NEMENOFF R.A. Epidermal growth factor enhances glomerular mesangial cell soluble phospholipase A2 activity. J. Biol. Chem. 1990;265:4934–4938. [PubMed] [Google Scholar]
- BRADY H.R., SERHAN C.N. Lipoxins: putative braking signals in host defense, inflammation and hypersensitivity. Curr. Opin. Nephrol. Hypertens. 1996;5:20–27. [PubMed] [Google Scholar]
- CRAWFORD J.R., JACOBSON B.S. Extracellular calcium regulates HeLa cell morphology during adhesion to gelatin: role of translocation and phosphorylation of cytosolic phospholipase A2. Mol. Biol. Cell. 1998;9:3429–3443. doi: 10.1091/mbc.9.12.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNICAN D.J., GRIFFITHS R., WILLIAMS D.C., O'NEILL L.A. Endothelin-1 increases arachidonic acid release in C6 glioma cells through a potassium-modulated influx of calcium. J. Neurochem. 1996;67:830–837. doi: 10.1046/j.1471-4159.1996.67020830.x. [DOI] [PubMed] [Google Scholar]
- ENCABO A., ROMANIN C., BIRKE F.W., KUKOVETZ W.R., GROSCHINER K. Inhibition of a store-operated Ca2+ entry pathway in human endothelial cells by the isoquinoline derivative LOE 908. Br. J. Pharmacol. 1996;119:702–706. doi: 10.1111/j.1476-5381.1996.tb15729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATIMA S., KHANDEKAR Z., PARMENTIER J.H., MALIK K.U. Cytosolic phospholipase A2 activation by the p38 kinase inhibitor SB203580 in rabbit aortic smooth muscle cells. J. Pharmacol. Exp. Ther. 2001;298:331–338. [PubMed] [Google Scholar]
- IWASAKI H., EGUCHI S., UENO H., MARUMO F., HIRATA Y. Endothelin-mediated vascular growth requires p42/p44 mitogen-activated protein kinase and p70 S6 kinase cascades via transactivation of epidermal growth factor receptor. Endocrinology. 1999;140:4659–4668. doi: 10.1210/endo.140.10.7023. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., HASHIMOTO N., MASAKI T. B103 neuroblastoma cells predominantly express endothelin ETB receptor; effects of extracellular Ca2+ influx on endothelin-1-induced mitogenesis. Eur. J. Pharmacol. 2001;425:173–179. doi: 10.1016/s0014-2999(01)01150-5. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., HASHIMOTO N., MASAKI T. Ca2+ channels involved in endothelin-induced mitogenic response in carotid artery vascular smooth muscle cells. Am. J. Physiol. Cell. Physiol. 2002a;282:C330–C337. doi: 10.1152/ajpcell.00227.2001. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., HASHIMOTO N., MASAKI T. Characterization of Ca2+ channels involved in ET-1-induced transactivation of EGF receptors. Am. J. Physiol. Heart Circ. Physiol. 2002b;283:H2671–H2675. doi: 10.1152/ajpheart.00350.2002. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., HASHIMOTO N., MASAKI T. Extracellular Ca2+ influx and endothelin-1-induced intracellular mitogenic cascades in rabbit internal carotid artery vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2002c;40:307–314. doi: 10.1097/00005344-200208000-00016. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., HASHIMOTO N., MASAKI T. Involvements of voltage-independent Ca2+ channels and phosphoinositide 3-kinase in endothelin-1-induced PYK2 tyrosine phosphorylation. Mol. Pharmacol. 2003;63:808–813. doi: 10.1124/mol.63.4.808. [DOI] [PubMed] [Google Scholar]
- MAKITA K., FALCK J.R., CAPDEVILA J.H. Cytochrome P450, the arachidonic acid cascade, and hypertension: new vistas for an old enzyme system. FASEB J. 1996;10:1456–1463. doi: 10.1096/fasebj.10.13.8940291. [DOI] [PubMed] [Google Scholar]
- MERITT J.E., AIRMSTRONG W.P, BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.A., MOORES K.E., RINK T.J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NALEFSKI E.A., SULTZMAN L.A., MARTIN D.M., KRIZ R.W., TOWLER PS., KNOPF J.L., CLARK J.D. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipid-binding domain and a Ca2+-independent catalytic domain. J. Biol. Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- PEREZ D.M., DEYOUNG M.B., GRAHAM R.M. Coupling of expressed alpha 1B- and alpha 1D-adrenergic receptor to multiple signaling pathways is both G protein and cell type specific. Mol. Pharmacol. 1993;44:784–795. [PubMed] [Google Scholar]
- RESNIK T.J., SCOTT-BURDEN T., BUHLER F.R. Activation of phospholipase A2 by endothelin in cultured vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1989;158:279–286. doi: 10.1016/s0006-291x(89)80209-8. [DOI] [PubMed] [Google Scholar]
- SHARP J.D., WHITE D.L., CHIOU X.G., GOODSON T., GAMBOA G.C., MCCLURE D., BURGETT S., HOSKINS J., SKATRUD P.L., SPORTSMAN J.R. Molecular cloning and expression of human Ca2+-sensitive cytosolic phospholipase A2. J. Biol. Chem. 1991;266:14850–14853. [PubMed] [Google Scholar]
- SILFANI T.N., FREEMAN E.J. Phosphatidylinositoide 3-kinase regulates angiotensin II-induced cytosolic phospholipase A2 activity and growth in vascular smooth muscle cells. Arch. Biochem. Biophys. 2002;402:84–93. doi: 10.1016/S0003-9861(02)00066-8. [DOI] [PubMed] [Google Scholar]
- SUGAWARA F., NINOMIYA H., OKAMOTO Y., MIWA S., MAZDA O., KATSURA Y., MASAKI T. Endothelin-I-induced mitogenic responses of Chinese hamster ovary cells expressing human endothelin A: the role of a wortmannin-sensitive signaling pathway. Mol. Pharmacol. 1996;49:447–457. [PubMed] [Google Scholar]
- TREVISI L., BOVA S., CARGNELI G., CEOLOTTO G., LUCIANI S. Endothelin-1-induced arachidonic acid release by cytosolic phospholipase A2 activation in rat vascular smooth muscle via extracellular signal-regulated kinases pathway. Biochem. Pharmacol. 2002;64:425–431. doi: 10.1016/s0006-2952(02)01066-3. [DOI] [PubMed] [Google Scholar]
- VANE J.R., BOTTING R.M. Mechanism of action of aspirin-like drugs. Semin. Arthritis. Rheum. 1997;26 Suppl 1:2–10. doi: 10.1016/s0049-0172(97)80046-7. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM G.S., KLOOSTER R., VAN DEN BOSCH H., VERKLEIJ A.J., BOONSTRA J. Phosphorylation of p42/44MAPK by various signal transduction pathways activates cytosolic phospholipase A2 to variable degrees. J. Biol. Chem. 2001;276:28976–28983. doi: 10.1074/jbc.M101361200. [DOI] [PubMed] [Google Scholar]
- WU-WONG J.R., DAYTON B.D., OPGENORTH T.J. Endothelin-1-evoked arachidonic acid release: a Ca2+-dependent pathway. Am. J. Physiol. 1996;271:C869–C877. doi: 10.1152/ajpcell.1996.271.3.C869. [DOI] [PubMed] [Google Scholar]