Abstract
The C-5 halogenation of the vanillyl moiety of resiniferatoxin, an ultrapotent agonist of vanilloid TRPV1 receptors, results in a potent antagonist for these receptors. Here, we have synthesized a series of halogenated derivatives of ‘synthetic capsaicin' (nonanoyl vanillamide=nordihydrocapsaicin) differing for the nature (iodine, bromine–chlorine) and the regiochemistry (C-5, C-6) of the halogenation.
The activity of these compounds was investigated on recombinant human TRPV1 receptors overexpressed in HEK-293 cells. None of the six compounds exerted any significant agonist activity, as assessed by measuring their effect on TRPV1-mediated calcium mobilization. Instead, all compounds antagonized, to various extents, the effect of capsaicin in this assay.
All 6-halo-nordihydrocapsaicins behaved as competitive antagonists against human TRPV1 according to the corresponding Schild's plots, and were more potent than the corresponding 5-halogenated analogues. The iodo-derivatives were more potent than the bromo- and chloro-derivatives.
Using human recombinant TRPV1, 6-iodo-nordihydrocapsaicin (IC50=10 nM against 100 nM capsaicin) was about four times more potent than the prototypical TRPV1 antagonist, capsazepine, and was tested against capsaicin also on native TRPV1 in: (i) rat dorsal root ganglion neurons in culture; (ii) guinea-pig urinary bladder; and (iii) guinea-pig bronchi. In all cases, except for the guinea-pig bronchi, the compound was significantly more potent than capsazepine as a TRPV1 antagonist.
In conclusion, 6-iodo-nordihydrocapsaicin, a stable and easily prepared compound, is a potent TRPV1 antagonist and a convenient replacement for capsazepine in most of the in vitro preparations currently used to assess the activity of putative vanilloid receptor agonists.
Keywords: TRPV1, capsaicin, receptor, pain, resiniferatoxin, vanilloid, halogenated derivatives
Introduction
The vanilloid TRPV1 receptor, also known as VR1 receptor (Szallasi & Blumberg, 1999), belongs to the large family of ‘transient receptor potential' (TRP), six trans-membrane domain, cation channels. Among the subfamily of the TRPV receptors (Gunthorpe et al., 2002), TRPV1 is the only one discovered to date that responds also to stimulation by natural products, with capsaicin and resiniferatoxin being the best known and most thoroughly studied examples (Sterner & Szallasi, 1999). It is now recognized that TRPV1 functions as a molecular integrator of nociceptive stimuli, including heat, protons and plant toxins, and is most abundant in peripheral sensory fibers of the C and Aδ type. Conversely, other TRPV channels, that is, TRPV2, TRPV3 and TRPV4, also known as VR1-like receptors, respond uniquely to heat or mechanical/osmotic stimuli and have a more homogeneous distribution in the mammalian body (Gunthorpe et al., 2002). Studies carried out with transgenic mice lacking functional TRPV1 receptors implicated this protein in the perception of thermal and inflammatory pain (Caterina et al., 2000; Davis et al., 2000). Other investigations showed that TRPV1 is also involved in inflammatory bowel disorders (Yiangou et al., 2001), neuropathic pain (Walker et al., 2003) and pathological cough (see Chung & Chang, 2002, for review). TRPV1 might play an important role also in physiological conditions, for example, in bladder function (Birder et al., 2002), and its recent finding in several brain nuclei (Mezey et al., 2000; Sanchez et al., 2001) widens its possible biological importance, suggesting that this ion channel is involved in the control of CNS functions, such as neuronal plasticity, body temperature, food intake and movement (Di Marzo et al., 2001; Szallasi, 2002).
Potent, nonpungent synthetic agonists, capable of immediately desensitizing TRPV1, can be used against various conditions, including inflammatory hyperalgesia, bladder hyperactivity and emesis (Szallasi, 2002). On the other hand, the involvement of this receptor in both pathological and physiological conditions suggests that therapeutically useful drugs devoid of undesired side effects are more likely to be developed from TRPV1 antagonists. Furthermore, the increasing evidence in favour of the biological importance of TRPV1 is likely to result in an evergrowing need for selective probes to be used as pharmacological tools for the study of this receptor. Research on vanilloids and vanilloid receptors has long relied on capsazepine, which remained the only known TRPV1 antagonist for a decade (Bevan et al., 1992). It has now been realized that this compound is not selective, and is not always effective in vivo, particularly when ‘central' effects of agonists are investigated (see Di Marzo et al., 2000 for an example). In this context, the recent development of the ultrapotent and selective TRPV1 antagonist, 5-iodo-resiniferatoxin (5-iodo-RTX; Wahl et al., 2001), represents an important breakthrough in vanilloid receptor research. Yet, further studies showed that this compound, while being 40-800 fold more potent than capsazepine in isolated cells and in some tissue preparations, is less efficacious in vivo and in certain important in vitro assays of TRPV1-mediated activity, such as the isolated guinea-pig bronchi (Seabrook et al., 2002; Undem & Kollarik, 2002). These discrepant potencies might be due to the well-documented species-dependent sensitivity of vanilloid receptors to agonists and antagonists (see, for example, McIntyre et al., 2001; Seabrook et al., 2002), as well as to pharmacodynamic factors. Using different strategies, other TRPV1 antagonists have been designed recently: (i) SB-366791 (Davis et al., 2001); (ii) synthetic trialkylglycines (Garcia-Martinez et al., 2002); (iii) two series of thiourea compounds (Lee et al., 2001; Wang et al., 2002); and (iv) two synthetic hexapeptides (Himmel et al., 2002). However, the properties in vitro of most of these compounds, beyond their effects on isolated cells expressing TRPV1 receptors, have not been always thoroughly investigated.
Inspired by the dramatic effect of iodination on the activity of RTX, in this study we synthesized novel TRPV1 antagonists by halogenation of nordihydrocapsaicin on the vanillyl moiety. We investigated the structure–activity relationships of halogenated derivatives of this chemically stable capsaicin analogue, and report that at least one of the six compounds developed might represent a valid alternative to capsazepine in assays in vitro of TRPV1-mediated activities.
Methods
Synthesis and characterization of halogenated nordihydrocapsaicins
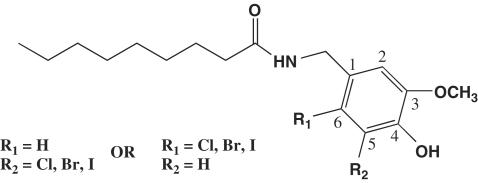
5-Iodo-nordihydrocapsaicin was prepared from commercially available (Aldrich) nordihydrocapsaicin in overall 36% yield by: (a) protection of the phenolic hydroxyl as a mem (=methoxyethoxymethyl) ether; (b) iodination with the iodine–silver trifluoroacetate system; and (c) deprotection with SnCl4 in THF (Figure 1). All the other halogenated capsaicinoids were prepared from vanillin by direct halogenation for the 5-derivatives or by halogenation after protection of the phenolic hydroxyl (mem group), reduction and acetylation for the 6-bromo- and chloro derivatives. Oxygen to nitrogen replacement at the benzyl position was affected by azidation (diphenylphosphoryl azide), followed by Staudinger reduction and acylation with commercially available nonanoyl chloride (mem-protected compounds) or with nonanoyl acid and propylphosphonic anhydride for the compound having a free 4-hydroxyl. Full details of the synthesis and the analytical characterization of the compounds will be reported elsewhere. Each compound was purified chromatographically and characterized by nuclear magnetic resonance.
Figure 1.
General chemical structure of the compounds synthesized and investigated in this study.
Assays of intracellular [Ca2+]i in HEK cells overexpressing the human TRPV1
Overexpression of human TRPV1 cDNA into human hembryonic kidney (HEK) 293 cells was carried out as described previously (Hayes et al., 2000). Cells were grown as monolayers in minimum essential medium supplemented with nonessential amino acids, 10% fetal calf serum and 0.2 mM glutamine, and maintained under 95%/5% O2/CO2 at 37°C. The effect of the substances on [Ca2+]i was determined by using Fluo-3, a selective intracellular fluorescent probe for Ca2+ (De Petrocellis et al., 2000). At 1 day prior to experiments, cells were transferred into six-well dishes coated with poly-L-lysine (Sigma) and grown in the culture medium mentioned above. On the day of the experiment, the cells (50–60,000 per well) were loaded for 2 h at 25°C with 4 μM Fluo-3 methylester (Molecular Probes) in DMSO containing 0.04% Pluoronic. After the loading, cells were washed with Tyrode pH=7.4, trypsinized, resuspended in Tyrode and transferred to the cuvette of the fluorescence detector (Perkin-Elmer LS50B) under continuous stirring. Experiments were carried out by measuring cell fluorescence at 25°C (λEX=488 nm, λEM=540 nm) before and after the addition of the test compounds at various concentrations. Varying doses of 5-iodo-RTX (Tocris Cookson, U.K.), capsazepine (Alexis Biochemicals, Switzerland) or SB-366791 (a kind gift from J.B. Davis, GlaxoSmithKline, U.K.), or of the six novel compounds, were added 10 min prior to capsaicin. Data were expressed, for capsaicin and nordihydrocapsaicin, as the concentration exerting a half-maximal effect (EC50), and, for the antagonists, as the concentration exerting a half-maximal inhibition (IC50), both calculated by using GraphPad®. The efficacy of the agonists was determined by comparing it to the analogous effect observed with 4 μM ionomycin. Response calibration was carried out by measuring the fluorescence intensity of intracellular fluo-3 with known extracellular [Ca2+] (Molecular Probes). The following equation was used to determine an ion dissociation constant (Kd) of 325 nM: [Ca2+]free=Kd [F−Fmin]/[Fmax−F], where Fmin and Fmax are the fluorescence intensities of fluo-3 without or with maximal [Ca2+], and F is the fluorescence intensity with an intermediate [Ca2+]. Average FEM/FEX was 200 and this value was increased by 60±7% in the presence of 4 μM ionomycin.
TRPV1 receptor-binding assays
The affinity of 6-iodo-nor-6,7-dihydrocapsaicin and capsaicin for human TRPV1 receptors was assessed by means of displacement assays carried out with membranes (50 μg per tube) from HEK cells overexpressing the receptors, prepared as described previously (Ross et al., 2001), and the high-affinity TRPV1 ligand RTX (48 Ci mmol−1, NEN-Dupont), using the incubation conditions described previously (Ross et al., 2001). Under these conditions, the Kd and Bmax for RTX were 0.5 nM and 1.39 pmol mg protein−1. The Ki for the displacement of 1 nM RTX by increasing concentrations of the compounds was calculated from the IC50 value (obtained by GraphPad Software) using the Cheng-Prusoff equation. Specific binding was calculated with 1 μM RTX (Alexis Biochemicals) and was 48.1±5.6%.
Assays of [Ca2+]i in rat dorsal root ganglion neurons in culture
Newborn rats (2–3 days old) were terminally anesthetized and decapitated. The dorsal root ganglia (DRG) were removed and rapidly placed in cold phosphate buffered solution (PBS) before being transferred to collagenase/dispase (1 mg ml−1 dissolved in Ca2+–Mg2+-free PBS) for 35 min at 37°C. Enrichment of the fraction of nociceptive neurons was obtained following the methods reported previously (Gilabert & McNaughton, 1997). After the enzymatic treatment, ganglia were rinsed three times with Ca2+–Mg2+-free PBS and then placed in 2 ml of cold DMEM supplemented with 10% fetal bovine serum (FBS, heat inactivated), 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. The ganglia were then dissociated into single cells by several passages through a series of syringe needles (23G down to 25G). Finally, the complex of medium and ganglia cells were sieved through a 40 μM filter to remove debris and topped up with 8 ml of DMEM medium and centrifuged (200 × g for 5 min). The final cell pellet was resuspended in DMEM medium (supplemented with 100 ng ml−1 mouse Nerve Growth Factor (mouse-NGF-7S) and cytosine-b-D-arabinofuranoside free base (ARA-C) 2.5 μM). Cells were plated on poly-L-lysine- (8.3 μM) and laminin (5 μM)-coated 25 mm glass coverslips and kept for 2–5 days at 37°C in a humidified incubator gassed with 5% CO2 and air. Cells were fed on the second day (and subsequent alternate days) with DMEM medium (with 1% FBS instead of 10% FBS).
Experiments were performed as previously reported (Tognetto et al., 2001). Briefly, plated neurons (2–5 days) were loaded with Fura-2-AM-ester (3 μM) in Ca2+ buffer solution of the following composition (mM): CaCl2 1.4, KCl 5.4, MgSO4 0.4, NaCl 135, D-glucose 5, HEPES 10 with BSA 0.1%, at pH 7.4, for 40 min at 37°C, washed twice with the Ca2+ buffer solution and transferred to a chamber on the stage of a Nikon eclipse TE300 microscope. The dye was excited at 340 and 380 nm to indicate relative [Ca2+]i changes by the F340/F380 ratio recorded with a dynamic image analysis system (Laboratory Automation 2.0, RCS, Florence, Italy). Capsaicin (0.1 μM), 6-iodo-nordihydrocapsaicin (0.1 nM–100 μM) or their respective vehicles were added to the chamber. A calibration curve using a buffer containing Fura-2-AM-ester and fixed concentrations of free Ca2+ (Kudo et al., 1986) was used to convert the data obtained from F340/F380 ratio to [Ca2+]i (nM).
Guinea-pig urinary bladder and bronchi assays
Guinea-pigs were killed by cervical dislocation and the airways and urinary bladder were removed. Rings were taken from the main bronchi (approximately 2 mm in width) and suspended with a resting tension of 1.5 × g, while urinary bladders were halved vertically and suspended with a resting tension of 1 × g. The tissues were bathed (37°C) and aerated (95% O2 and 5% CO2) with Krebs' solution of the following composition (mM): NaCl 119, NaHCO3 25, KH2PO4 1.2, MgSO4 1.5, CaCl2 2.5, KCl 4.7 and D-glucose 11, phosphoramidon 0.001 and captopril 0.001. Tissues were allowed to equilibrate for 60 min prior to the beginning and between each set of experiments (washed every 5 min). In all experiments, the tissues were first contracted with carbachol (CCh, 1 μM). Cumulative concentration–response curves were performed with capsaicin (0.1 nM–100 μM) and substance P (SP, 0.1 nM–1 μM) in the presence of either the novel TRPV1 antagonist, 6-iodo-nordihydrocapsaicin (1–10 μM) or its vehicle.
Results
Effect of 5- and 6-halo-nordihydrocapsaicins on intracellular [Ca2+]i
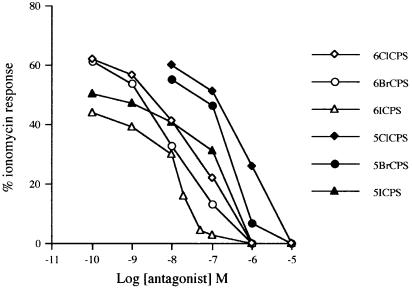
The effect of the six novel compounds synthesized in this study was first tested on TRPV1-mediated increases of [Ca2+]i in HEK-293 cells transfected with the human TRPV1. None of the compounds exerted any significant effect on [Ca2+]i up to a 10 μM concentration. As reported in several previous studies, capsaicin and nordihydrocapsaicin instead induced strong TRPV1-mediated increase of [Ca2+]i with EC50=30.2±4.5 and 61.4±5.6 nM, respectively, and a maximal effect at 10 μM of 72.1±3.3 and 60.1±4.1% of the effect of ionomycin (4 μM). All compounds, given to cells 10 min prior to capsaicin (100 nM), dose-dependently inhibited the effect of capsaicin, which, alone, exerted an effect on Ca2+ that was 62.2±2.5% of that exerted by 4 μM ionomycin. Some of the compounds exerted a significant antagonistic effect even at the lowest concentration tested. The 6-halo-derivatives were always significantly more potent as antagonists than the corresponding 5-halo-derivatives, while, within the same series of compounds, the iodo-derivatives appeared to be more potent than the bromo-derivatives, which in turn appeared to be more potent than the chloro-derivatives. Therefore, the most potent antagonist was 6-iodo-nordihydrocapsaicin (IC50=10.0±2.1 nM, mean±s.e., n=3) (Figure 2). The potency of the 6-bromo-nordihydrocapsaicin (IC50=16.1±2.7 nM, mean±s.e., n=3) was not significantly different from that of 6-iodo-nordihydrocapsaicin, whereas the 6-chloro-nordihydrocapsaicin was significantly less potent (IC50=50.3±3.9 nM, mean±s.e,, n=3, P<0.05 by the Student's t-test). The 5-iodo- and 5-bromo-nordihydrocapsaicin (IC50=126.2±15.4 and 251.6±22.2 nM) were significantly less potent than the 6-halo-derivatives and more potent than the 5-chloro-nordihydrocapsaicin (IC50=631.0±45.5 nM, means±s.e., n=3, P<0.05 by the Student's t-test). Under the same conditions, 5-iodo-RTX (IC50=0.4±0.1 nM) was the most potent antagonist tested, whereas capsazepine (IC50=40.1±3.9 nM) and SB-366791 (IC50=32.0±5.1 nM, means±s.e.m., n=3) were four- and three-fold less potent than 6-iodo-nordihydrocapsaicin.
Figure 2.
Dose-dependent inhibition by the six novel compounds synthesized here on the effect of 100 nM capsaicin on [Ca2+]i in HEK-293 cells overexpressing the human TRPV1 receptor. The effect was expressed as percent of the maximum possible enhancement of [Ca2+]i induced by 4 μM ionomycin. Data are means of at least n=3 separate experiments. standard error. Bars are not shown for the sake of clarity and were never higher than 10% of the means. CPS=nordihydrocapsaicin.
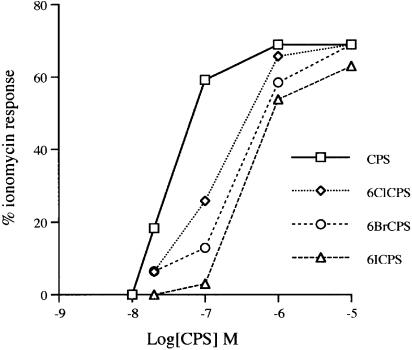
When tested at a fixed concentration against varying concentrations of capsaicin, the most potent 6-halo-nordihydrocapsaicins appeared to behave as competitive antagonists, since the inhibitory effect could not be observed at the highest concentrations of capsaicin (Figure 3). Schild's plots for the three compounds confirmed this suggestion (slope=0.86±0.07, 0.92±0.06 and 0.80±15, for 6-iodo-, 6-bromo- and 6-chloro-nordihydrocapsaicin, respectively, means±s.e.m., n=3). The Kb for 6-iodo-nordihydrocapsaicin (10, 25 and 100 nM) against capsaicin was calculated as 4.3±1.6 nM, whereas those of capsazepine (100 nM) and 5-iodo-RTX (1 nM) were 9.0±2.1 and 0.09±1.1 nM (means±s.e.m., n=3), and that of SB-366791 was previously reported as 25 nM (Davis et al., 2001). Binding experiments with membranes from HEK-293 cells overexpressing human TRPV1 confirmed that 6-iodo-nordihydrocapsaicin and capsaicin are likely to share the same binding site on VR1 receptors, since they were both capable of displacing [3H]RTX from its specific binding sites (Ki=0.33±0.14 and 2.91±0.30 μM, respectively, means±s.e.m., n=3). In similar binding experiments, the Ki values reported for capsazepine and 5-iodo-RTX were 0.58–3.3 μM and 1.7–6.7 nM, respectively (Lee et al., 2001; McDonnell et al., 2002; Seabrook et al., 2002).
Figure 3.
Antagonistic effect of the 6-halo-nordihydrocapsaicins on the dose-dependent effect of capsaicin on [Ca2+]i in HEK-293 cells overexpressing the human TRPV1 receptor. The effect was expressed as percent of the maximum possible enhancement of [Ca2+]i induced by 4 μM ionomycin. Data are means of at least n=3 separate experiments. standard error. Bars are not shown for the sake of clarity and were never higher than 10% of the means. CPS=nordihydrocapsaicin.
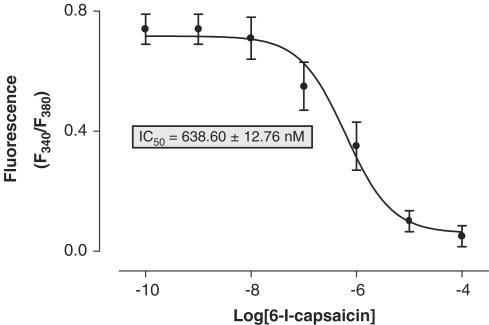
Effect of 6-iodo-nordihydrocapsaicin on [Ca2+]i in rat DRG neurons in culture
The most potent antagonist in the calcium assay, 6-iodo-nordihydrocapsaicin, was also tested on capsaicin induced in rat DRG neurons in culture, which express levels of rat TRPV1 lower than the transfected cells used above, but higher than most of other rat tissues. The compound inhibited the effect of 100 nM capsaicin with an IC50=638.6±12.8 nM (Figure 4). Under the same conditions, 5-iodo-RTX and capsazepine exhibited IC50 values of 0.87±0.01 and 2344.5±53.9 nM, respectively.
Figure 4.
Dose-dependent antagonistic effect of 6-iodo-nordihydrocapsaicin on the effect of 100 nM capsaicin on [Ca2+]i in neonatal rat DRG neurons in culture. The effect was assessed by the calcium-imaging technique described in the Methods section. Data are means±s.e. of n=6 separate determinations.
Effect of 6-iodo-nordihydrocapsaicin on guinea-pig urinary bladder contractions
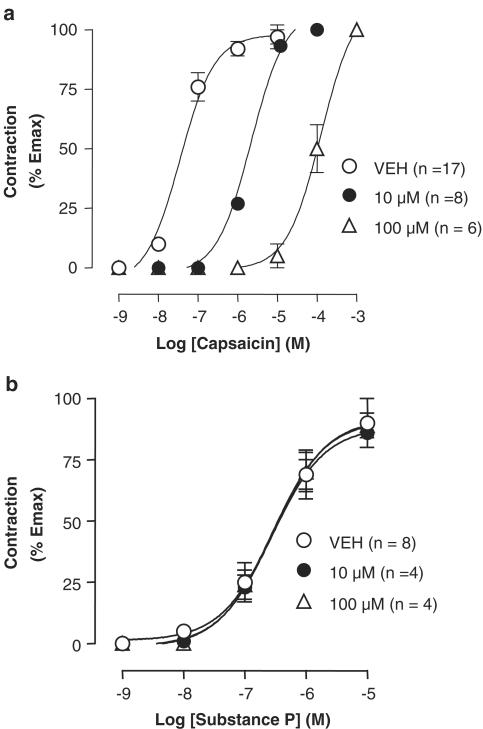
The 6-iodo-nordihydrocapsaicin was tested on TRPV1-mediated contractions in the guinea-pig urinary bladder (Figure 5a), and found to dose-dependently inhibit the effect of capsaicin more potently than capsazepine (pA2=7.17±0.39 and 6.56±0.20, respectively). The novel antagonist was inactive against the contractions induced by substance P (Figure 5b), indicating a possible prejunctional site of action for 6-iodo-nordihydrocapsaicin on the TRPV1-mediated release of substance P, rather than on its postjunctional effect.
Figure 5.
Effect of two concentrations of 6-iodo-nordihydrocapsaicin on the dose-dependent effects of capsaicin (a) or substance P (b) on guinea-pig urinary bladder contractions. The effect was expressed as percent of the maximal contraction induced by 1 μM CCh. Data are means±s.e. of n⩾4 experiments.
Effect of 6-iodo-nordihydrocapsaicin on guinea-pig trachea contractions
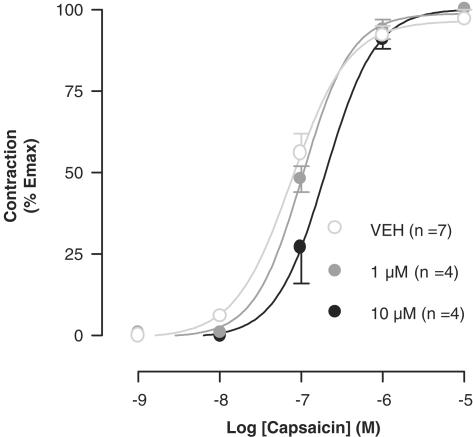
The 6-iodo-nordihydrocapsaicin was tested also on the TRPV1-mediated contractions induced by capsaicin in the guinea-pig trachea preparations (Figure 6). Under these conditions, the compound was significantly less potent than capsazepine (pA2=5.6±0.3 and 7.0±0.3, respectively), whereas 5-iodo-RTX was reported to exhibit a pA2 value of 6.3±0.3 (Rigoni et al., 2003). The 5-iodo-nordihydrocapsaicin was totally inactive in this assay (not shown).
Figure 6.
Effect of two concentrations of 6-iodo-nordihydrocapsaicin on the dose-dependent effect of capsaicin on guinea-pig trachea contractions. The effect was expressed as percent of the maximal contraction induced by 1 μM CCh. Data are means±s.e. of n⩾4 experiments.
Discussion
This study was inspired by the previous finding that iodination of the ultrapotent vanilloid receptor agonist RTX at the 5-position of the vanillyl moiety results in a very potent and selective TRPV1 antagonist (Wahl et al., 2001). Therefore, we wondered whether a similar modification could also revert the activity of capsaicin, the other prototypical vanilloid receptor agonist. To evaluate this point and gain insights on the rationale underlying the agonist–antagonist transformation, we prepared a series of halogenated derivatives of ‘synthetic capsaicin' (nonanoyl vanillamide=nordihydro-capsaicin) differing from the nature (iodine, bromine, chlorine) and the regiochemistry (C-5, C-6) of halogenation.
The activity of these compounds was evaluated in a typical in vitro assay of TRPV1 activity, namely the capability to enhance [Ca2+]i in HEK-293 cells overexpressing the human recombinant receptor. We found that halogenation at either C-5 or C-6 of the vanillyl moiety led to complete loss of activity on [Ca2+]i, even at a concentration (10 μM) at which both capsaicin and nordihydrocapsaicin are maximally active. That both C-5 and C-6 iodination of nordihydrocapsaicin could abolish its capability to gate TRPV1 receptors is at variance with that observed with RTX, whose 6-iodo-derivative behaves as a partial agonist (McDonnell et al., 2002). On the other hand, we found that, as with 5-iodo-RTX, the six compounds behaved as TRPV1 antagonists with varying potency against capsaicin. Unlike 5-iodo-RTX, however, which behaves as a noncompetitive antagonist (Wahl et al., 2001; Undem & Kollarik, 2002), the 6-halo-derivatives developed in this study appeared to behave as competitive antagonists when tested against increasing concentrations of capsaicin. This was confirmed using Schild's plots, which yielded slopes very near to 1. Furthermore, binding studies confirmed that 6-iodo-nordihydrocapsaicin, the most potent antagonist among the six compounds, is very likely to share with capsaicin the same binding site in the human TRPV1, and has 2–10-fold higher affinity than capsazepine for this site. In summary, iodination, and possibly halogenation in general, of the 5-position in the vanillyl moiety of at least two types of TRPV1 agonists is a sufficient chemical modification to render them antagonists. At least in the case of nordihydrocapsaicin, this functional transformation occurs without altering the capability of the compound to interact directly and uniquely with the ligand-binding site in the TRPV1 receptor.
We observed that, independently of the nature of halogenation, the potency of the new antagonists increased sensibly from the 5- to the 6-halo series. Interestingly, 6-iodo-RTX also was previously reported to have higher affinity for the human TRPV1 than the corresponding 5-iodo-homologue (Ki=0.7 and 1.4 nM, respectively (McDonnell et al., 2002)). However, as pointed out above, unlike our 6-halo-derivatives, 6-iodo-RTX behaves as a partial agonist and not as an antagonist (McDonnell et al., 2002). We also found that, particularly with the 5-halo-derivatives, potency increases with the size and polarizability of the halogen introduced. This suggests that: (i) the binding site of TRPV1 for ‘capsaicinoid', and possibly ‘resiniferoid', vanilloid ligands (Szallasi & Blumberg, 1999) is able to better accommodate, at either the 6- or 5-position of the vanillyl moiety, very much hindering halogen atoms, and (ii) the increased polarizability of the halogen, with subsequent increased capability of forming dipolar bonds with acidic amino-acid residues within the binding pocket, might represent one of the possible conformational restraints that impede the gating of the receptor by halogenated ligands.
As mentioned above, 6-iodo-nordihydrocapsaicin was the most potent antagonist developed in the current study, since it was more potent than capsazepine against capsaicin, in the intracellular calcium assay carried out with HEK-293 cells overexpressing the human TRPV1. We wanted to assess if this compound behaved as an antagonist also for native rat TRPV1 expressed by DRG neurons in culture. By using calcium imaging, we confirmed that 6-iodo-nordihydrocapsaicin was about four times more potent than capsazepine, even though the IC50 values of both antagonists in this assay were more than 60 times lower than in HEK cells, whereas the IC50 for 5-iodo-RTX remained almost unchanged. This discrepancy might be due to several factors, including the higher degree of expression of recombinant TRPV1 in transfected HEK-293 cells, or differences in the structure of human and rat TRPV1. Both these factors are indeed likely to produce also differences in the quaternary structure of this receptor (Kedei et al., 2001). Another possible reason for the lower potency of both 6-iodo-nordihydrocapsaicin and capsazepine with respect to 5-iodo-RTX might be the different permeability of intact cells to ‘capsaicinoids' or ‘resiniferanoids'. In fact, the ligand-binding site of TRPV1 for ‘capsaicinoids' and fatty acid-derived ligands is intracellular (Jung et al., 1999; De Petrocellis et al., 2001; Jordt & Julius, 2002; Jung et al., 2002), and requires that the ligands (i.e. agonists or antagonists) pass through the membrane in order to interact with the channel. By contrast, it has been suggested that 5-iodo-RTX, unlike 6-iodo-nordihydrocapsaicin and capsazepine, is not a competitive antagonist for TRPV1 (Wahl et al., 2001; Undem & Kollarik, 2002), which might mean that this compound does not inhibit TRPV1 gating only by competing with agonists for the intracellular binding site, but might have additional sites of interaction with the protein, possibly on its extracellular domains. Finally, it is also possible that 6-iodo-nordihydrocapsaicin and capsazepine are degraded more rapidly by DRG neurons than HEK-293 cells.
In this study, we also assessed the capability of 6-iodo-nordihydrocapsaicin vs capsazepine to antagonize the effects of capsaicin in two different organ preparations. This was important in view of the growing evidence that species and pharmacokinetics play an important role in determining TRPV1 antagonist potency. However, by using two preparations from the same species, we found that the potency of 6-iodo-nordihydrocapsaicin changed considerably depending on whether it was tested against the contractions produced by capsaicin in the guinea-pig urinary bladder, where the compound was still more potent than capsazepine, or guinea-pig trachea, where, by contrast, capsazepine was the most potent of the two compounds. Therefore, the varying potency against capsaicin of 6-iodo-nordihydrocapsaicin vs capsazepine is probably due to the possible different metabolic stability of the compounds or to their different degree of penetration into different tissues, rather than to species-dependent effects. At any rate, it is also noteworthy that 5-iodo-RTX is considerably less potent in the guinea-pig trachea preparation than in isolated DRG neurons (Undem & Kollarik, 2002), and that even this ultrapotent antagonist may become weaker than capsazepine when tested in vivo (Seabrook et al., 2002).
In conclusion, this study provides information on the complex interactions occurring between TRPV1 and its ligands, and on some of the chemical moieties required to transform a potent agonist into an antagonist at this receptor. On the basis of our data, and of results recently reported by other laboratories (Wahl et al., 2001; McDonnell et al., 2002), it is reasonable to hypothesize that halogenation on the 5-position of the vanillyl group of all ‘capsaicinoid' and ‘resiniferoid' TRPV1 agonists results in antagonists. This information might turn out to be very useful if other TRPV1 agonists containing a vanillyl moiety are found. This chemical feature is, in fact, known to be a necessary prerequisite to observe strong agonist activity at vanilloid receptors (Sterner & Szallasi, 1999; Szallasi & Blumberg, 1999). Furthermore, we have described here some of the pharmacological properties of a new TRPV1 antagonist, 6-iodo-nordi-hydrocapsaicin, which, while adding to the growing family of vanilloid antagonists and being definitely less potent than 5-iodo-RTX (Wahl et al., 2001) also appears to be more potent on isolated cells than several of the novel TRPV1 antagonists developed recently (Davis et al., 2001; Lee et al., 2001; Garcia-Martinez et al., 2002; Himmel et al., 2002; Wang et al., 2002). However, it remains to be established whether 6-iodo-nordihydrocapsaicin can be considered as a valid alternative to capsazepine. This latter antagonist is considered not very selective for TRPV1 and can interact also with other ion channels (see, for example, Docherty et al., 1997); however, it was found here to be only four times less potent than 6-iodo-nordihydrocapsaicin when the two compounds were tested in isolated cells under the same conditions. Also, further studies aimed at assessing the efficacy of 6-iodo-nordihydrocapsaicin in vivo will be required before suggesting that this stable and easy-to-synthesize compound may represent a template for the development of more potent TRPV1 antagonists.
Acknowledgments
This study was partly supported by Indena S.p.a. We are grateful to Dr Alessia Ligresti for performing the binding assays, to J.B. Davis (GlaxoSmithKline) for providing HEK-293 cells overexpressing the human TRPV1 and to S. Piantedosi for technical assistance.
Abbreviations
- DMEM
Dulbecco's modified Eagles' medium
- DRG
dorsal root ganglia
- HEK
human hembryonic kidney
- RTX
resiniferatoxin
- TRPV1
transient receptor potential type V1
- VR1
vanilloid receptor type 1
References
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRDER L.A., NAKAMURA Y., KISS S., NEALEN M.L., BARRICK S., KANAI A.J., WANG E., RUIZ G., DE GROAT W.C., APODACA G., WATKINS S., CATERINA M.J. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- CHUNG K.F., CHANG A.B. Therapy for cough: active agents. Pulm. Pharmacol. Ther. 2002;15:335–338. doi: 10.1006/pupt.2002.0342. [DOI] [PubMed] [Google Scholar]
- DAVIS J.B., GRAY J., GUNTHORPE M.J., HATCHER J.P., DAVEY P.T., OVEREND P., HARRIES M.H., LATCHAM J., CLAPHAM C., ATKINSON K., HUGHES S.A., RANCE K., GRAU E., HARPER A.J., PUGH P.L., ROGERS D.C., BINGHAM S., RANDALL A., SHEARDOWN S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DAVIS J.B., GUNTHORPE M.J., JERMAN J.C., GRAY J., SMITH G.D., DAVIES C.H., RANDALL A.D., SMART D., RAMI H.K., WYMAN P.A. Soc. Neurosci. Abs. 2001. p. 1550.
- DE PETROCELLIS L., BISOGNO T., DAVIS J.B., PERTWEE R.G., DI MARZO V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BREIVOGEL C., BISOGNO T., MELCK D., PATRICK G., TAO Q., SZALLASI A., RAZDAN R.K., MARTIN B.R. Neurobehavioral activity in mice of N-vanillyl-arachidonyl-amide. Eur. J. Pharmacol. 2000;406:363–374. doi: 10.1016/s0014-2999(00)00687-7. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., LASTRES-BECKER I., BISOGNO T., DE PETROCELLIS L., MILONE A., DAVIS J.B., FERNANDEZ-RUIZ J.J. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur. J. Pharmacol. 2001;420:123–131. doi: 10.1016/s0014-2999(01)01012-3. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., PIPER A.S. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-MARTINEZ C., HUMET M., PLANELLS-CASES R., GOMIS A., CAPRINI M., VIANA F., DE LA PENA E., SANCHEZ-BAEZA F., CARBONELL T., DE FELIPE C., PEREZ-PAYA E., BELMONTE C., MESSEGUER A., FERRER-MONTIEL A. Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2374–2379. doi: 10.1073/pnas.022285899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILABERT R., MCNAUGHTON P. Enrichment of the fraction of nociceptive neurones in cultures of primary sensory neurones. J. Neurosci. Methods. 1997;71:191–198. doi: 10.1016/s0165-0270(96)00144-6. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., BENHAM C.D., RANDALL A., DAVIS J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- HAYES P., MEADOWS H.J., GUNTHORPE M.J., HARRIES M.H., DUCKWORTH D.M., CAIRNS W., HARRISON D.C., CLARKE C.E., ELLINGTON K., PRINJHA R.K., BARTON A.J., MEDHURST A.D., SMITH G.D., TOPP S., MURDOCK P., SANGER G.J., TERRETT J., JENKINS O., BENHAM C.D., RANDALL A.D., GLOGER I.S., DAVIS J.B. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- HIMMEL H.M., KISS T., BORVENDEG S.J., GILLEN C., ILLES P. The arginine-rich hexapeptide R4W2 is a stereoselective antagonist at the vanilloid receptor 1: a Ca2+ imaging study in adult rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2002;301:981–986. doi: 10.1124/jpet.301.3.981. [DOI] [PubMed] [Google Scholar]
- JORDT S.E., JULIUS D. Molecular basis for species-specific sensitivity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.Y., KANG C.J., KIM W.B., KIM D., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG J., LEE S.Y., HWANG S.W., CHO H., SHIN J., KANG Y.S., KIM S., OH U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J. Biol. Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- KEDEI N., SZABO T., LILE J.D., TREANOR J.J., OLAH Z., IADAROLA M.J., BLUMBERG P.M. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- KUDO Y., OZAKI K., MIYAKAWA A., AMANO T., OGURA A. Monitoring of intracellular Ca2+ elevation in a single neural cell using a fluorescence microscope/video-camera system. Jpn. J. Pharmacol. 1986;41:345–351. doi: 10.1254/jjp.41.345. [DOI] [PubMed] [Google Scholar]
- LEE J., SZABO T., GONZALEZ A.F., WELTER J.D., BLUMBERG P.M. N-(3-acyloxy-2-benzylpropyl)-N'-dihydroxytetrahydrobenzazepine and tetrahydroisoquinoline thiourea analogues as vanilloid receptor ligands. Bioorg. Med. Chem. 2001;9:1713–1720. doi: 10.1016/s0968-0896(01)00068-2. [DOI] [PubMed] [Google Scholar]
- MCDONNELL M.E., ZHANG S.P., DUBIN A.E., DAX S.L. Synthesis and in vitro evaluation of a novel iodinated resiniferatoxin derivative that is an agonist at the human vanilloid VR1 receptor. Bioorg. Med. Chem. Lett. 2002;12:1189–1192. doi: 10.1016/s0960-894x(02)00127-0. [DOI] [PubMed] [Google Scholar]
- MCINTYRE P., MCLATCHIE L.M., CHAMBERS A., PHILLIPS E., CLARKE M., SAVIDGE J., TOMS C., PEACOCK M., SHAH K., WINTER J., WEERASAKERA N., WEBB M., RANG H.P., BEVAN S., JAMES I.F. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br. J. Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEZEY E., TOTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GUO A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGONI M., TREVISANI M., GAZZIERI D., NADALETTO R., TOGNETTO M., CREMINON C., DAVIS J.B., CAMPI B., AMADESI S., GEPPETTI P., HARRISON S. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br. J. Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., DI MARZO V., PERTWEE R.G. Structure–activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ J.F., KRAUSE J.E., CORTRIGHT D.N. The distribution and regulation of vanilloid receptor VR1 and VR1 5' splice variant RNA expression in rat. Neuroscience. 2001;107:373–381. doi: 10.1016/s0306-4522(01)00373-6. [DOI] [PubMed] [Google Scholar]
- SEABROOK G.R., SUTTON K.G., JAROLIMEK W., HOLLINGWORTH G.J., TEAGUE S., WEBB J., CLARK N., BOYCE S., KERBY J., ALI Z., CHOU M., MIDDLETON R., KACZOROWSKI G., JONES A.B. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J. Pharmacol. Exp. Ther. 2002;303:1052–1060. doi: 10.1124/jpet.102.040394. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. Vanilloid (capsaicin) receptors in health and disease. Am. J. Clin. Pathol. 2002;118:110–121. doi: 10.1309/7AYY-VVH1-GQT5-J4R2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- STERNER O., SZALLASI A. Novel natural vanilloid receptor agonists: new therapeutic targets for drug development. Trends Pharmacol. Sci. 1999;20:459–465. doi: 10.1016/s0165-6147(99)01393-0. [DOI] [PubMed] [Google Scholar]
- TOGNETTO T., AMATESI S., HARRISON S., CREMINON C., TREVISANI M., CARRERAS M., MATERA M., GEPPETTI P., BIANCHI A. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J. Neurosci. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDEM B.J., KOLLARIK M. Characterization of the vanilloid receptor 1 antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J. Pharmacol. Exp. Ther. 2002;303:716–722. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]
- WAHL P., FOGED C., TULLIN S., THOMSEN C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- WALKER K.M., URBAN L., MEDHURST S.J., PATEL S., PANESAR M., FOX A.J., MCINTYRE P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- WANG Y., SZABO T., WELTER J.D., TOTH A., TRAN R., LEE J., KANG S.U., SUH Y.G., BLUMBERG P.M. High affinity antagonists of the vanilloid receptor. Mol. Pharmacol. 2002;62:947–956. doi: 10.1124/mol.62.4.947. [DOI] [PubMed] [Google Scholar]
- YIANGOU Y., FACER P., DYER N.H., CHAN C.L., KNOWLES C., WILLIAMS N.S., ANAND P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]