Abstract
Atypical β-adrenoceptors resistant to propranolol, but blocked by bupranolol, increase contractile force and/or frequency of the heart in humans and rats. We compared the potencies of the enantiomers of bupranolol and examined the possible effects of seven bupranolol analogues including bevantolol (BEV) at this receptor in pithed and vagotomized rats.
CGP 12177, an agonist of the atypical β-adrenoceptor, increased heart rate dose-dependently. Its dose–response curve was shifted to the right by S-(−)-bupranolol 10 μmol kg−1 by a factor of 8.4, but not affected by the same dose of R-(+)-bupranolol.
Desmethylbupranolol and compounds BK-21, BK-22, BK-23 and BK-25 also increased heart rate dose-dependently. The β1-adrenoceptor antagonist CGP 20712 given in combination with the β2-adrenoceptor antagonist ICI 118,551 (0.1 μmol kg−1 each) reduced the positive chronotropic action of the five bupranolol analogues without affecting that of CGP 12177. The potencies of the bupranolol analogues to increase heart rate were correlated (r=0.91, P<0.05) with their affinities for β1-adrenoceptor binding sites in rat brain cortex membranes labelled with [3H]CGP 12177 (in the presence of ICI 118,551).
BK-26 and BEV, 10 μmol kg−1 each, had only minor effects on heart rate by themselves and did not antagonize the effect of CGP 12177. However, at 1 μmol kg−1, they antagonized the increase in heart rate elicited by the β1-adrenoceptor agonist prenalterol.
In conclusion, bupranolol is a stereoselective antagonist at the atypical cardiostimulant β-adrenoceptor. The effects of the bupranolol analogues are related to the activation or blockade of β1-adrenoceptors, but not of atypical β-adrenoceptors.
Keywords: β1-Adrenoceptor, atypical cardiostimulant β-adrenoceptor, bupranolol enantiomers, bupranolol analogues, CGP 12177, [3H]CGP 12177 binding, CGP 20712, pithed rat, positive chronotropic effect
Introduction
A fourth type of β-adrenoceptors, which causes positive inotropic and chronotropic effects, has been found in the heart of humans (Kaumann, 1996; Bundkirchen et al., 2002; Sarsero et al., 2003), rats (Kaumann & Molenaar, 1996; Malinowska & Schlicker, 1996,1997; Cohen et al., 1999), mice (Kaumann et al., 1998) and ferrets (Lowe et al., 1999,2002), both under in vitro (Kaumann, 1996; Kaumann & Molenaar, 1996; Kaumann et al., 1998; Lowe et al., 1999,2002; Bundkirchen et al., 2002; Sarsero et al., 2003) and in vivo conditions (Malinowska & Schlicker, 1996,1997). This receptor, which will be termed ‘atypical cardiostimulant β-adrenoceptor' here (other names: putative β4-adrenoceptor or low-affinity state of the β1-adrenoceptor), is activated by nonconventional partial agonists like CGP 12177 and cyanopindolol (i.e. drugs that block β1- and/or β2-adrenoceptors at concentrations much lower than those required to activate atypical β-adrenoceptors or β3-adrenoceptors; for a review, see Kaumann & Molenaar, 1997). The cardiostimulant receptor is relatively resistant to many β-adrenoceptor antagonists including propranolol (Kaumann, 1996; Kaumann & Molenaar, 1996; Malinowska & Schlicker, 1996,1997; Sarsero et al., 1999,2003), but is blocked by the nonselective β-adrenoceptor antagonist, bupranolol, and by high concentrations of the β1-adrenoceptor antagonist, CGP 20712 (Malinowska & Schlicker, 1996,1997; Kaumann & Molenaar, 1997).
Recently, it has been suggested that the cardiostimulant atypical β-adrenoceptor represents a special, propranolol-resistant low-affinity state of the β1-adrenoceptor. Thus, the positive inotropic and chronotropic effects of CGP 12177 in atria, although abolished in double β1-/β2-adrenoceptor knockout mice, remained intact in β2-adrenoceptor knockout mice, indicating an obligatory role of the β1-adrenoceptor (Kaumann et al., 2001). A previous study had already revealed that the cardiostimulant atypical β-adrenoceptor, despite some pharmacological similarities, is independent from the β3-adrenoceptor since the cardiostimulant effects of CGP 12177 were not altered in β3-adrenoceptor knockout mice (Kaumann et al., 1998).
A potential clinical significance of the cardiostimulant atypical β-adrenoceptor has been suggested by several reports. First, their activation elicits ventricular and atrial arrhythmias (Lowe et al., 1998; Freestone et al., 1999; Sarsero et al., 1999). Second, not only β1-adrenoceptors but also the atypical β-adrenoceptors undergo downregulation upon failure of the human and rat heart (Kompa & Summers, 1999; Sarsero et al., 2003). Third, there is increasing evidence that activation of the atypical β-adrenoceptor contributes to the intrinsic sympathomimetic activity (ISA) shown by pindolol and alprenolol in the human heart (Lowe et al., 2002). It has been suggested that activation of the atypical cardiostimulant β-adrenoceptor by the latter two compounds and by bucindolol might be implicated in the failure of these β-adrenoceptor antagonists to increase survival when taken by patients after myocardial infarction (Bundkirchen et al., 2002; Lowe et al., 2002).
Detailed studies on the function of the cardiostimulant atypical β-adrenoceptors are, however, still limited by the lack of appropriate pharmacological tools. For this purpose, the potency of 11 clinically used β-blockers at the β1- and the atypical cardiostimulant β-adrenoceptor was compared recently by Lowe et al. (2002). The present study focused on bupranolol, one of the most potent antagonists at the atypical β-adrenoceptor. First, we examined whether the stereoselectivity, a typical property at the β1- and β2-adrenoceptor subtypes (Lemoine & Kaumann, 1983), also extends to the atypical cardiostimulant β-adrenoceptor. Second, we examined whether the atypical β-adrenoceptor is influenced by seven analogues of bupranolol (including bevantolol (BEV)), which differ from the parent compound with respect to the substitution pattern of the phenoxy moiety and/or in which the tertiary butyl moiety at the nitrogen of the side chain is replaced by a 3,4-dimethoxyphenylethyl moiety. The experiments were carried out in the pithed rat model in which a series of related experiments has been performed previously (Malinowska & Schlicker, 1996,1997). In order to have an independent estimate of the affinity of the drugs for the β1-adrenoceptor, the affinities of all compounds for β1-adrenoceptor binding sites labelled by [3H]CGP 12177 (in the presence of the β2-adrenoceptor antagonist ICI 118,551) were determined in rat brain cortex membranes.
Methods
Pithed rats
Male Wistar rats were anaesthetized with pentobarbital 300 μmol kg−1 and then injected with atropine 2 μmol kg−1. After cannulation of the trachea, the animals were pithed and artificially ventilated with air (60 strokes min−1) using a respiratory system (Hugo Sachs Elektronik, March-Hugstetten, Germany). Both vagal nerves were cut. Diastolic blood pressure was measured from the right carotid artery via a pressure transducer (DTX, Spectramed, Bromma, Sweden). Heart rate (HR) was recorded from the ECG by means of subcutaneous electrodes. Body temperature was kept constant at approximately 36°C using a heating table (BIO-SYS-TECH, Białystok, Poland) and monitored by a rectal probe thermometer. The transducers were connected to the monitor Trendscope 8031 (AxMediTec, Białystok, Poland). The left femoral vein was cannulated for i.v. administration of drugs in a volume of 0.5 ml kg−1. Following pithing, vasopressin (0.04–0.4 i.u. kg−1 min−1) was routinely infused into the right femoral vein to raise diastolic blood pressure to about 85 mmHg (like in our previous studies; see Malinowska & Schlicker, 1996,1997).
After 15–20 min of equilibration, during which the cardiovascular parameters were allowed to stabilize, experiments were performed. Like in our previous studies (Malinowska & Schlicker, 1996,1997), agonists were administered in a noncumulative manner. Since recovery from the effects was very slow, each dose of agonist was usually studied in a separate animal. Only in some cases, the two lowest doses of an agonist were injected to the same rat with sufficient time for full recovery to the preinjection value after injection of the lowest dose. The agonist was injected 5 min after administration of the antagonist under study or vehicle.
Binding studies
Cerebral cortex membranes from male Wistar rats were homogenized (Potter-Elvehjem) in 25 volumes of ice-cold Tris-HCl buffer (Tris 50 mM, pH 7.5; EDTA 5 mM; sucrose 10.27%) and centrifuged at 1000 × g for 10 min (4°C). The supernatant was centrifuged at 35,000 × g for 20 min (4°C) and re-centrifuged (35,000 × g for 10 min) twice with 15 ml Tris-HCl buffer (Tris 50 mM, pH 7.5; EDTA 5 mM). The final pellet was resuspended in buffer and frozen at −80°C.
For saturation binding studies, membranes were incubated with Tris-HCl buffer in a final volume of 0.5 ml containing 110–220 μg protein, 70 nM ICI 118,551 (selective β2-adrenoceptor antagonist) and eight concentrations (0.01–0.5 nM) of [3H]CGP 12177. For displacement studies, membranes were incubated with Tris-HCl buffer in a final volume of 0.5 ml containing 80–170 μg protein, 70 nM ICI 118,551 and [3H]CGP 12177 at a concentration of 0.07 nM. The incubation (30°C) was terminated after 30 min by rapid filtration through polyethylenimine (0.3%)-pretreated Whatman GF/C filters. Propranolol (10 μM) was used to determine nonspecific binding (15% for [3H]CGP 12177 0.07 nM). Protein concentration was assayed by the method described by Bradford (1976).
Statistics and calculations
Results are given as means±s.e.m. of n experiments (pithed rats) and n experiments in triplicate (binding studies). To assess the potency (pED50) of the β-adrenoceptor ligands to increase HR, we determined the negative logarithms of the doses (in mol kg−1 body weight, i.v.) causing an increase in HR by 65 (CGP 12177), 50 (BK-25), 45 (desmethylbupranolol (DMB), BK-21, BK-23) or 35 beats min−1 (BK-22). For comparison of the mean values, the t-test for paired and unpaired data was used. When two or more treatment groups were compared to the same control (CON), the one-way analysis of variance (ANOVA) followed by the Dunnett test was used. Differences were considered as significant when P<0.05.
Radioligand binding curves were analysed by nonlinear curve fitting using the computer program GraphPadPrismR (Prism; GraphPad Software, San Diego, CA, U.S.A.) from which the parameters Bmax and KD, Ki and nH were determined. The F-test was applied in order to evaluate whether the inhibition of [3H]CGP 12177 binding by drugs is better fitted by a one- or two-site model.
Drugs used
R,S-(±)-bupranolol hydrochloride, S-(−)-bupranolol hydrochloride, R-(+)-bupranolol hydrochloride, DMB hydrochloride (SchwarzPharma AG, Monheim, Germany); BK-21 (base), and hydrochlorides from BK-22, BK-23, BK-25, BK-26 and BEV (BK-28) (for structures, see Table 1; synthesized at the Department of Chemical Technology of Drugs, Jagiellonian University Medical College, Cracow, Poland); prenalterol hydrochloride (Hässle, Gothenburg, Sweden); [5,7-3H]-(−)-CGP 12177 (specific activity 33 Ci mmol−1) (NEN, Zaventem, Belgium); atropine sulphate, propranolol hydrochloride, urethane, [Lys8]-vasopressin, CGP 20712 ((±)-2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]-phenoxy]propyl]-amino]ethoxy]-benzamide monomethane sulphonate) (Sigma, Deisenhofen, Germany); CGP 12177 ((±)-4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazole-2-one), ICI 118,551 (erythro-(±)-1-(7-methylindan-4-yloxy)-3-isopropylaminobutan-2-ol) (Tocris Cookson, Bristol, U.K. ); pentobarbitone sodium (Biowet, Puławy, Poland). Drugs were dissolved in saline with the following exceptions: BK-21 and BK-26 were dissolved in dimethyl sulphoxide (DMSO); CGP 20712 was dissolved in a mixture of DMSO and saline (6 : 100). These three stock solutions were further diluted with saline. None of the vehicles affected the cardiovascular parameters.
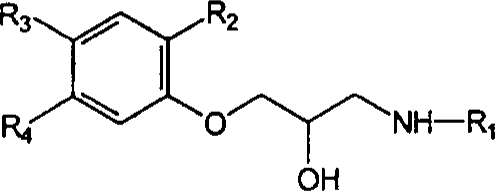
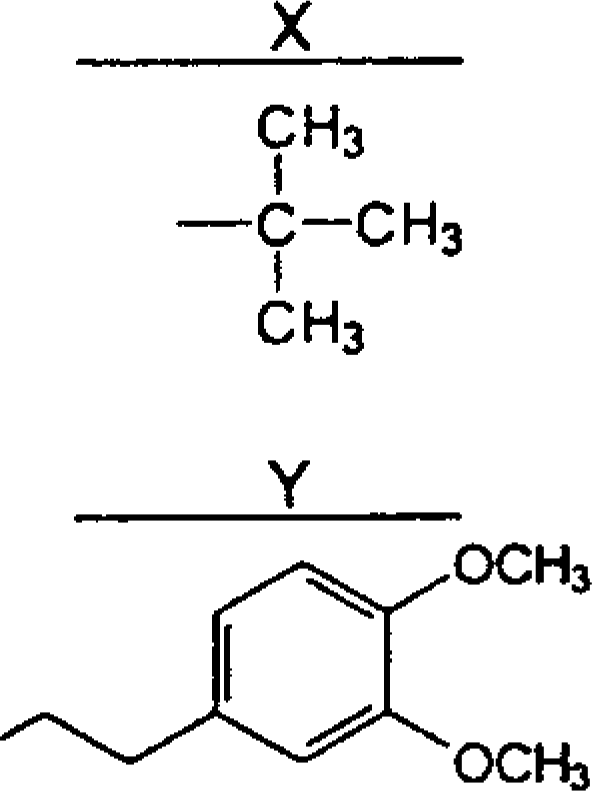
Table 1.
Chemical structures, potencies (to induce tachycardia in pithed rats) and affinities (for β1-adrenoceptor binding sites) of bupranolol, its enantiomers and seven analogues
|
|
Increase in heart rate | Inhibition of [3H]CGP 12177 binding (studied in the presence of ICI 118,551 70 nM) | |||||
|---|---|---|---|---|---|---|---|---|
| Compounds | R1 | R2 | R3 | R4 | pED50a | pKib | ||
| (±)-Bupranolol | – | 8.66±0.07 | ||||||
| (−)-Bupranolol | X | Cl | H | CH3 | – | 8.83±0.21 | ||
| (+)-Bupranolol | – | 7.19±0.12 | ||||||
| Desmethylbupranolol | X | Cl | H | H | 8.4 | 8.98±0.04 | ||
| BK-21 | X | H | H3C-O | H | 6.4 | 5.76±0.13 | ||
| BK-22 | X | CH3 | H | H | 8.3 | 8.60±0.08 | ||
| BK-25 | X | H | H | H | 7.7 | 8.10±0.12 | ||
| BK-26 | X | H | Cl | CH3 | – | 7.24±0.06 | ||
| BK-23 | Y | CH3 | H | H | 7.3 | 8.37±0.06 | ||
| Bevantolol | Y | H | H | CH3 | – | 8.00±0.08 | ||
Based on the increase in heart rate by 45 (desmethylbupranolol, BK-21 and BK-23), 35 (BK-22) and 50 beats min−1 (BK-25). Graphically determined from Figure 2a.
Results are given as means±s.e.m. of four experiments in triplicate.
Results
Pithed rat: diastolic blood pressure
Diastolic blood pressure was maintained at about 85 mmHg by i.v. infusion of vasopressin (0.04–0.4 i.u. kg−1 min−1). (±), (−), (+)-bupranolol, BK-23, BK-26 and BEV (at doses of 10 μmol kg−1 each) given i.v. reduced diastolic blood pressure by 23.4±1.0 (n=9), 24.2±1.8 (n=14), 34.8±1.6 (n=5), 13.6±2.4 (n=5), 14.7±1.5 (n=11) and 37.0±1.8 mmHg (n=9), respectively. The maximal effect occurred 15–30 s after administration of the drug and fully recovered within 3 min. Only in the case of (−)-bupranolol, the fall in blood pressure was still observed 5 min after its injection (6.3±1.7 mmHg; n=14; P<0.01). Lower doses of the above drugs and all other drugs used in the present study did not affect diastolic blood pressure.
Pithed rat: influence of bupranolol enantiomers on HR
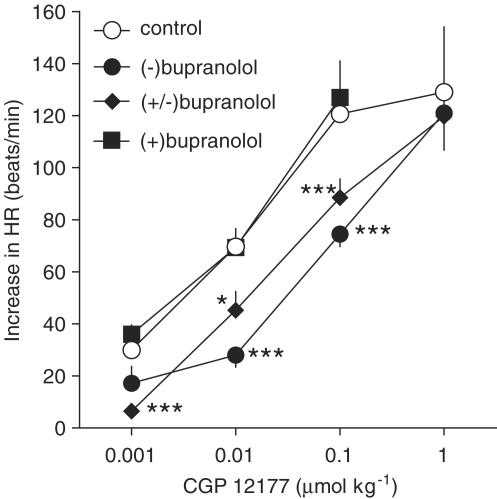
At the beginning of the experiments, HR was 343±1beats min−1 (n=251). (±), (−) and (+)-bupranolol (at a dose of 10 μmol kg−1 each) given i.v. diminished HR by 43.6±4.9 (n=9), 50.6±3.2 (n=14) and 46.6±13.7 beats min−1 (n=5), respectively. The maximal effect occurred after 15–30 s. At 5 min after administration of the drug (i.e. immediately before injection of CGP 12177; see later), HR had fully recovered (in the case of (+)-bupranolol), but was still reduced by about 1.5% [(±)-bupranolol] or 5% [(−)-bupranolol]. As shown in Figure 1, CGP 12177 increased HR in a dose-dependent manner (Emax about 130 beats min−1; pED50=8.1, determined as the negative logarithm of the dose increasing HR by 65 beats min−1). (±)- and (−)-bupranolol (10 μmol kg−1 each) caused 3.4- and 8.4-fold shifts to the right of the dose–response curve for CGP 12177, respectively, with no reduction in the maximum response. By contrast, (+)-bupranolol (10 μmol kg−1) failed to modify the positive chronotropic effect of CGP 12177.
Figure 1.
Influence of (−), (+) and (±)-bupranolol on the CGP 12177-induced increase in HR in pithed and vagotomized rats. Each dose of CGP 12177 was studied in separate rats. In some cases, the two lower doses were applied to one animal. The first or only dose was given 5 min after injection of vehicle (CON) or (−), (+) and (±)-bupranolol (10 μmol kg−1 each). Means of 3–13 rats. *P<0.05; ***P<0.001 compared to the corresponding CON.
Pithed rat: influence of bupranolol analogues on HR
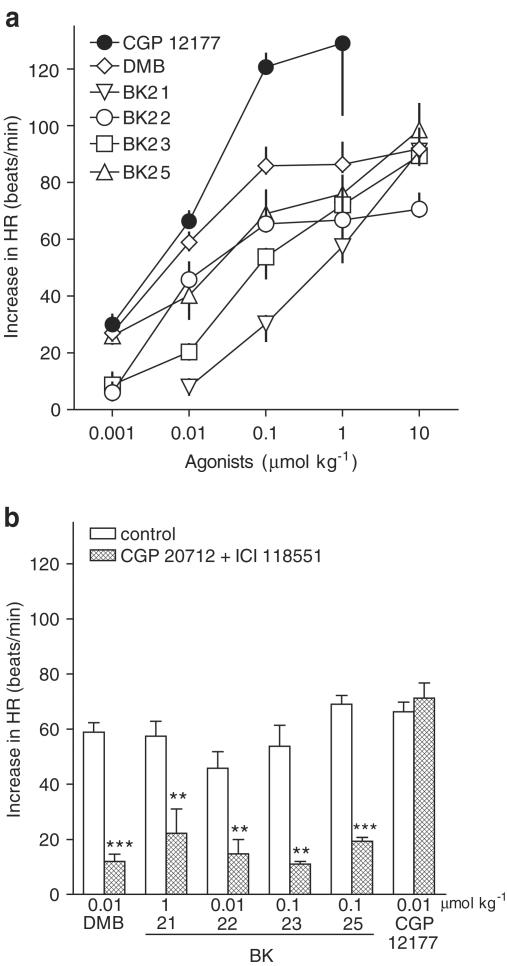
DMB and compounds BK-21, BK-22, BK-23 and BK-25 increased HR in a dose-dependent manner (Figure 2a). The tachycardic effect evoked by the highest doses of agonists was reached within 10 (BK-25) or 15 min (DMB, BK-21, BK-22 and BK-23) and had still the same level 30 min after administration of the drugs. The maximal effect of DMB and BK-22, obtained at 0.1 μmol kg−1, was an increase by about 90 and 70 beats min−1, respectively. With respect to BK-21, BK-23 and BK-25, the exact maximum effect could not be determined since a complete dose–response curve could not be constructed (lack of solubility of high doses of these drugs). The highest dose of these agonists (10 μmol kg−1) increased HR by about 90, 90 and 100 beats min−1, respectively. To assess the agonistic potencies (ED50) of the β-adrenoceptor ligands under study, the doses causing an increase in HR by 50 (BK-25), 45 (DMB, BK-21, BK-23) or 35 beats min−1 (BK-22) were determined graphically from Figure 2a. The pED50 values are given in Table 1.
Figure 2.
Influence of CGP 12177, DMB, BK-21, BK-22, BK-23 and BK-25 on HR (a) and their interaction with CGP 20712 and ICI 118,551 (b). Experiments were performed in pithed and vagotomized rats. Each dose of agonist was studied in separate rats. In some cases, the two lower doses were applied to one animal. The first or only dose was given 5 min after injection of vehicle (CON) or CGP 20712 given in combination with ICI 118,551 (0.1 μmol kg−1 each). Means of 3–13 rats. **P<0.01; ***P<0.001 compared to the corresponding CON. For some data points, s.e.m. is contained within the symbol.
In order to examine whether these positive chronotropic actions were mediated via atypical or classical β-adrenoceptors, we performed additional experiments in the presence of the β1-adrenoceptor antagonist, CGP 20712, given in combination with the β2-adrenoceptor antagonist ICI 118,551 (at doses of 0.1 μmol kg−1 each). In these experiments, the bupranolol analogues and CGP 12177 were given at doses approximately corresponding to their ED50 values. As shown in Figure 2b, the β-adrenoceptor antagonists reduced the positive chronotropic action of the five bupranolol analogues by 60–80%, but not that elicited by CGP 12177. Note that the β-adrenoceptor blockade did not alter a short-lasting (<1 min) fall in HR (by 34.2±9.4 beats min−1; n=5), elicited by the highest dose of BK-23 (10 μmol kg−1) and preceding the increase in HR.
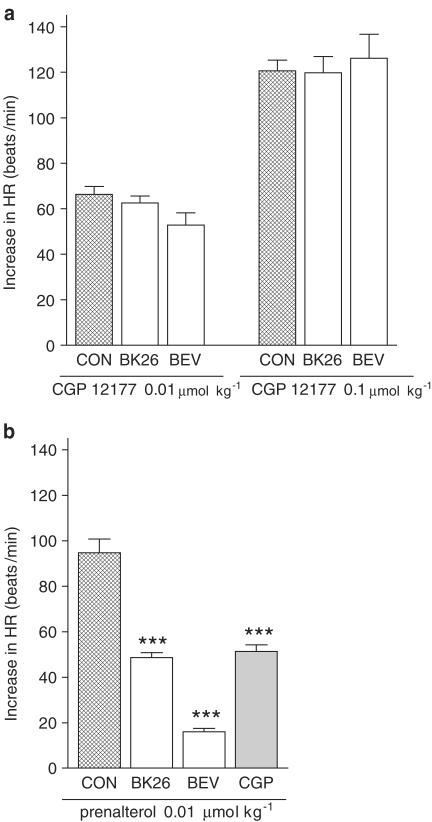
In contrast to the five bupranolol analogues mentioned above, BK-26 and BEV did not display any positive chronotropic activity, but at 10 μmol kg−1 diminished HR by 46.4±3.4 (n=11) and 46.2±1.6 beats min−1 (n=9), respectively. The maximal effect occurred after 15–30 s. At 5 min after administration of the drug, HR was still reduced by about 1.5% (BK-26) or 5% (BEV). Lower doses of both compounds did not affect HR. In order to reveal possible antagonistic properties of these substances against responses mediated via atypical β- and β1-adrenoceptors, we examined their interaction with CGP 12177 and prenalterol, respectively. A high dose of BK-26 and BEV (10 μmol kg−1) failed to affect the increase in HR evoked by the agonist of atypical β-adrenoceptors, CGP 12177, given at doses of 0.01 and 0.1 μmol kg−1 (Figure 3a). By contrast, a lower dose of BK-26 and BEV (1 μmol kg−1) reduced the positive chronotropic effect of the β1-adrenoceptor agonist prenalterol (given at a dose, 0.01 μmol kg−1, approximately equalling its ED50 value; Malinowska & Schlicker, 1996) by about 50 and 80%, respectively. The β1-adrenoceptor antagonist CGP 20712 (at a dose of 0.1 μmol kg−1), which was examined for the sake of comparison, diminished the same response by about 50% (Figure 3b).
Figure 3.
Influence of BK-26 and BEV on the increase in HR induced by CGP 12177 (a) and prenalterol (b). Experiments were performed in pithed and vagotomized rats. BK-26 or BEV was given at a dose of 10 μmol kg−1 5 min before CGP 12177 and at a dose of 1 μmol kg−1 5 min before prenalterol. For comparison, the influence of CGP 20712 (CGP; 0.1 μmol kg−1) on the tachycardia evoked by prenalterol is shown as well (from Malinowska & Schlicker, 1996). Each dose of agonist was studied in separate rats. Means of 3–13 rats. ***P<0.001 compared to the corresponding CON.
Affinities of the test compounds for β1-adrenoceptor sites
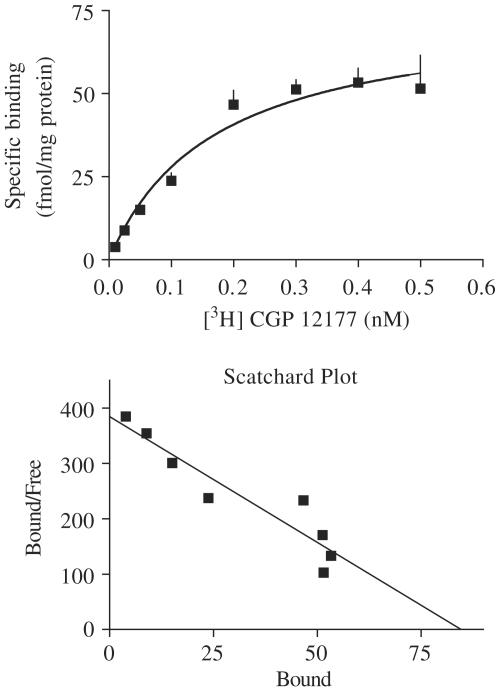
In saturation binding studies on rat brain cortex membranes, using [3H]CGP 12177 at eight concentrations and ICI 118,551 (70 nM) (to block β2-adrenoceptors), a KD value of 0.20±0.05 nM with a maximum number of binding sites (Bmax) of 79±8 fmol mg−1 protein was determined; Scatchard analysis revealed a straight line with a Hill coefficient (nH) not different from unity (Figure 4). In competition binding experiments, binding of [3H]CGP 12177 (0.07 nM) (studied in the presence of ICI 118,551 (70 nM)) was inhibited monophasically (nH near unity) by the 10 compounds, yielding pKi values from 5.76 to 8.98 (Table 1). When the pKi values of the five bupranolol analogues that increased HR (DMB, BK-21, BK-22, BK-23 and BK-25) were correlated with their pED50 values, a correlation coefficient r of 0.913 (P<0.05) was obtained.
Figure 4.
Saturation of specific [3H]CGP 12177 binding to rat brain cortex membranes. Membranes were incubated (30°C) for 30 min with [3H]CGP 12177 in the presence of ICI 118,551 (70 nM). Specific binding was defined as that inhibited by propranolol 10 μM. Scatchard analysis of the saturation data is presented in the lower panel. Means from three experiments (in triplicate) are shown (for some data points, s.e.m. is contained within the symbol).
Discussion
The present study was performed to further characterize the atypical cardiostimulant β-adrenoceptor, using the enantiomers and seven analogues of bupranolol. Most of the experiments were carried out on pithed rats in which the atypical β-adrenoceptor has been studied thoroughly (Malinowska & Schlicker, 1996,1997). In the pithed rat, HR is increased by isoprenaline or prenalterol via the β1-adrenoceptor and by CGP 12177 or cyanopindolol via the atypical β-adrenoceptor. To have identical conditions like in our previous two studies, we usually employed the β1-adrenoceptor antagonist CGP 20712 in combination with the β2-adrenoceptor antagonist ICI 118,551 (Malinowska & Schlicker, 1996,1997). Most of the drugs under study showed short-lived depressant effects on diastolic blood pressure and HR, which completely or nearly faded after 5 min, that is, at the time when interacting drugs were given. These effects appear to be of an unspecific nature (e.g. they were not antagonized by CGP 20712 plus ICI 118,551 and/or did not show stereoselectivity and/or were observed at the highest dose only) and will not be further discussed. The affinities of the bupranolol enantiomers and analogues for β1-adrenoceptors were determined in a radioligand binding study on rat brain cortex membranes, using [3H]CGP 12177 (Tadokoro et al., 1997); ICI 118,551 was used to block the β2-adrenoceptors (Alexander et al., 2001).
Our data show that the dose–response curve of CGP 12177 for its positive chronotropic effect, related to the activation of the atypical cardiostimulant β-adrenoceptor, is shifted to the right by a dose of 10 μmol kg−1 of racemic bupranolol and its (−)-enantiomer, but not affected by the same high dose of the (+)-enantiomer. Unfortunately, the amount of stereoselectivity remains uncertain because (+)-bupranolol, due to solubility problems, could not be given at a dose higher than 10 μmol kg−1. The stereoselectivity of bupranolol, previously shown for the classical types of β-adrenoceptors (Lemoine & Kaumann, 1983), is of interest inasmuch as the molecular properties of the atypical cardiostimulant β-adrenoceptor are so far poorly understood and stereoselective effects of ligands are a typical property of receptor-mediated effects.
Next, the question was addressed whether the antagonistic effect of bupranolol at the atypical cardiostimulant β-adrenoceptor is retained by seven analogues. Two compounds, that is, BK-26 and BEV, which, like bupranolol itself, had no or only slight inhibitory effects on basal HR, failed to shift to the right the dose–response curve of CGP 12177 at a dose as high as 10 μmol kg−1, arguing against an antagonistic effect of both drugs at the atypical β-adrenoceptor. On the other hand, a 10-fold lower dose of either drug attenuated the tachycardic effect of the β1-adrenoceptor agonist prenalterol. BEV showed a more marked effect in this respect than BK-26. This is in harmony with our binding data in which the affinity of BEV for β1-adrenoceptor sites markedly exceeded that of BK-26.
The other five bupranolol analogues, that is, DMB, BK-21, BK-22, BK-23 and BK-25, increased HR by themselves. The extent of tachycardia did not exceed 100 beats min−1 at 10 μmol kg−1; this dose caused the maximum effect in the case of DMB and BK-22, whereas for the remaining compounds, the exact maximum could not be determined due to the fact that doses higher than 10 μmol kg−1 could not be dissolved. The extent of tachycardia is much lower than that obtained by CGP 12177 via the atypical cardiostimulant β-adrenoceptor (130 min−1; present study) or by prenalterol or isoprenaline via the β1-adrenoceptor (about 150 min−1; Malinowska & Schlicker, 1996). The β1-adrenoceptor antagonist CGP 20712 (0.1 μmol kg−1) (i.e. at a dose at which the antagonist does not yet block the atypical β-adrenoceptor; Malinowska & Schlicker, 1996) markedly diminished the tachycardia caused by each of the five bupranolol analogues, suggesting that these compounds act by activation of β1-adrenoceptors. The possibility that the five compounds, solely or additionally, act via an indirect sympathomimetic effect (i.e. release catecholamines from the sympathetic nerve endings) seems unlikely, since compounds with a bulky substituent at the nitrogen atom of the side chain are not substrates of the neuronal noradrenaline transporter (Graefe and Bönisch, 1988).
The view that the five compounds increase HR via activation of β1-adrenoceptors is further strengthened by our binding data. Note that the correlation between the potencies from the functional experiments and the binding affinities was reasonably high, although not perfect. This might be related to the fact that the exact pED50 value could not be determined for BK-21, BK-23 and BK-25, and the negative logarithm of the dose causing 50% of the effect obtained at 10 μmol kg−1 was used instead. The inconsistencies might also be caused by pharmacokinetic differences between the compounds.
It was not the scope of the present work to elaborate structure–activity relationships of the bupranolol analogues with respect to the β1-adrenoceptor and few remarks shall suffice. Our study confirms previous data by Lemoine & Kaumann (1982) obtained on isolated cardiac tissues from guinea-pig and kitten in which DMB was virtually equipotent with (−)-bupranolol as a β1-adrenoceptor antagonist, but unlike the parent compound exhibited partial agonism. The synthesis of the four compounds termed here BK-21, BK-22, BK-25 and BK-26 was originally described by Kunz et al. (1967), but in this patent pharmacological data were given for BK-22 only. The latter compound was shown to increase coronary flow in the Langendorff preparation of the guinea-pig heart, possibly by partial agonism at β1-adrenoceptors (which are mainly responsible for coronary dilatation in many mammalian species; see Toda & Okamura, 1990 for references). The structure–activity relationship for bupranolol, DMB and the latter four compounds can easily be reconciled with a frequently described pattern, namely that substitution of the phenoxy moiety with residues like chlorine, methyl and methoxy is more favourable in the ortho or meta than in the para position (see e.g. Hoefle et al., 1975; Phillips, 1980; Louis et al., 1999). Accordingly, compared to BK-25 with unsubstituted phenyl ring, bupranolol, DMB and BK-22 with substitution(s) in ortho and/or meta position had a high affinity, whereas BK-26 with a substituent in meta and para position and, in particular, BK-21 with a substituent in para position had lower affinities. In the structure of BEV, the tert-butyl at the amino group is replaced by a 3,4-dimethoxyphenylethyl moiety conferring an increase in β1-adrenoceptor selectivity (Hoefle et al., 1975). Like in the latter study, in which the potencies of the compounds were determined in the isolated rat atrium, the o-CH3 analogue of BEV, termed here BK-23, had a slightly higher affinity for the β1-adrenoceptor than BEV itself; however, a partial agonistic activity of BK-23 has not been described by Hoefle et al. (1975).
In conclusion, bupranolol is a stereoselective antagonist at the atypical cardiostimulant β-adrenoceptor. An antagonistic effect at the latter receptor is not shown by a high dose of BEV and BK-26. The other five bupranolol analogues, DMB, BK-21, BK-22, BK-23 and BK-25 have a cardiostimulant effect which is, however, at least to the major part, related to the activation of β1-adrenoceptors.
Acknowledgments
This work was supported by the Medical Academy in Białystok (grants 3-13844 and 3-13738) and by the programme BONFOR of the Medical Faculty of the University of Bonn. We are also indebted to the Alexander von Humboldt-Stiftung (Bonn, Germany) for generously providing some of the equipment. We wish to thank Mrs I. Malinowska for her skilled technical assistance and the pharmaceutical company SchwarzPharma AG for gifts of drugs.
Abbreviations
- BEV
bevantolol
- CON
control
- DMB
desmethylbupranolol
- DMSO
dimethyl sulphoxide
- HR
heart rate
- ISA
intrinsic sympathomimetic activity
References
- ALEXANDER S., PETERS J., MATHIE A. TiPS nomenclature supplement. Trends Pharmacol. Sci. (Suppl.) 2001;22:1–146. [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BUNDKIRCHEN A., BRIXIUS K., BOLCK B., SCHWINGER R.H. Bucindolol exerts agonistic activity on the propranolol-insensitive state of β1-adrenoceptors in human myocardium. J. Pharmacol. Exp. Ther. 2002;300:794–801. doi: 10.1124/jpet.300.3.794. [DOI] [PubMed] [Google Scholar]
- COHEN M.L., BLOOMQUIST W., KRIAUCIUNAS A., SHUKER A., CALLIGARO D. Aryl propanolamines: comparison of activity at human β3 receptors, rat β3 receptors and rat atrial receptors mediating tachycardia. Br. J. Pharmacol. 1999;126:1018–1024. doi: 10.1038/sj.bjp.0702364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESTONE N.S., HEUBACH J.F., WETTWER E., RAVENS U., BROWN D., KAUMANN A.J. β4-Adrenoceptors are more effective than β1-adrenoceptors in mediating arrhythmic Ca2+ transients in mouse ventricular myocytes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:445–456. doi: 10.1007/s002109900075. [DOI] [PubMed] [Google Scholar]
- GRAEFE K.H., BÖNISCH H.The transport of amines across the axonal membranes of noradrenergic and dopaminergic neurones Handbook of Experimental Pharmacology 198890/IBerlin: Springer; 193–245.ed. Trendelenburg, U. & Weiner, N. Vol [Google Scholar]
- HOEFLE M.L., HASTINGS S.G., MEYER R.F., COREY R.M., HOLMES A., STRATTON C.D. Cardioselective β-adrenergic blocking agents. 1. 1-[(3,4-dimethoxyphenethyl)amino]-3-aryloxy-2-propanols. J. Med. Chem. 1975;18:148–152. doi: 10.1021/jm00236a007. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. (−)-CGP 12177-induced increase of human atrial contraction through a putative third β1-adrenoceptor. Br. J. Pharmacol. 1996;117:93–98. doi: 10.1111/j.1476-5381.1996.tb15159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., ENGELHARDT S., HEIN L., MOLENAAR P., LOHSE M. Abolition of (−)-CGP 12177-evoked cardiostimulation in double β1-/β2-adrenoceptor knockout mice. Obligatory role of β1-adrenoceptors for putative β4-adrenoceptor pharmacology. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:87–93. doi: 10.1007/s002100000336. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Differences between the third cardiac β-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Modulation of human cardiac function through 4 β-adrenoceptor populations. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:667–681. doi: 10.1007/pl00004999. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., PREITNER F., SARSERO D., MOLENAAR P., REVELLI J.-P., GIACOBINO J.P. CGP 12177 causes cardiostimulation and binds to cardiac putative β4-adrenoceptor in β3-adrenoceptor knockout mice. Mol. Pharmacol. 1998;53:670–675. doi: 10.1124/mol.53.4.670. [DOI] [PubMed] [Google Scholar]
- KOMPA A.R., SUMMERS R.J. Desensitization and resensitization of β1- and putative β4-adrenoceptors mediated responses occur in parallel in a rat model of cardiac failure. Br. J. Pharmacol. 1999;128:1399–1406. doi: 10.1038/sj.bjp.0702920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNZ W., JACOBI H., KOCH K.Verfahren zur Herstellung von basischen Phenyl- und Naphthyläthern und –thioäthern und deren Salzen German Patent, DE 1967. 1236523
- LEMOINE H., KAUMANN A.J. A novel analysis of concentration-dependence of partial agonism. Ring-demethylation of bupranolol results in a high affinity partial agonist (K 105) for myocardial and tracheal β-adrenoceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1982;320:130–144. doi: 10.1007/BF00506313. [DOI] [PubMed] [Google Scholar]
- LEMOINE H., KAUMANN A.J. A model for the interaction of competitive antagonists with two receptor-subtypes characterized by a Schild-plot with apparent slope unity. Agonist-dependent enantiomeric affinity ratios for bupranolol in tracheae but not in right atria of guinea pigs. Naunyn-Schmiedeberg's Arch. Pharmacol. 1983;322:111–120. doi: 10.1007/BF00512383. [DOI] [PubMed] [Google Scholar]
- LOUIS S.N., NERO T.L., IAKOVIDIS D., COLAGRANDE F.M., JACKMAN G.P., LOUIS W.J. β1- and β2-Adrenoceptor antagonist activity of a series of para-substituted N-isopropylphenoxypropanolamines. Eur. J. Med. Chem. 1999;34:919–937. doi: 10.1016/s0223-5234(99)00114-2. [DOI] [PubMed] [Google Scholar]
- LOWE M.D., GRACE A.W., KAUMANN A.J. Blockade of putative β4- and β1-adrenoceptors by carvedilol in ferret myocardium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:400–403. doi: 10.1007/pl00005367. [DOI] [PubMed] [Google Scholar]
- LOWE M.D., GRACE A.W., VANDENBERG J.I., KAUMANN A.J. Action potential shortening through the putative β4-adrenoceptor in ferret ventricle: comparison with β1- and β2-adrenoceptor-mediated effects. Br. J. Pharmacol. 1998;124:1341–1344. doi: 10.1038/sj.bjp.0702013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE M.D., LYNHAM J.A., GRACE A.W., KAUMANN A.J. Comparison of the affinity of β-blockers for two states of the β1-adrenoceptor in ferret ventricular myocardium. Br. J. Pharmacol. 2002;135:451–461. doi: 10.1038/sj.bjp.0704450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Mediation of the positive chronotropic effect of CGP 12177 and cyanopindolol in the pithed rat by atypical β-adrenoceptors, different from β3-adrenoceptors. Br. J. Pharmacol. 1996;117:943–949. doi: 10.1111/j.1476-5381.1996.tb15285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Further evidence for differences between cardiac atypical β-adrenoceptors and brown adipose tissue β3-adrenoceptors in the pithed rat. Br. J. Pharmacol. 1997;122:1307–1314. doi: 10.1038/sj.bjp.0701516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS D.K.Chemistry of alpha- and beta-adrenoceptor agonists and antagonists Handbook of Experimental Pharmacology 198054/IBerlin, Heidelberg, New York: Springer; 3–61.ed. Szekeres, L. Vol [Google Scholar]
- SARSERO D., MOLENAAR P., KAUMANN A.J., FREESTONE N.S. Putative β4-adrenoceptors in rat ventricle mediate increases in contractile force and cell Ca2+: comparison with atrial receptors and relationship to (−)-[3H]-CGP 12177 binding. Br. J. Pharmacol. 1999;128:1445–1460. doi: 10.1038/sj.bjp.0702936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARSERO D., RUSSELL F.D., LYNHAM J.A., RABNOTT G., YANG I., FONG K.M., LI L., KAUMANN A.J., MOLENAAR P. (−)-CGP 12177 increases contractile force and hastens relaxation of human myocardial preparations through a propranolol-resistant state of the β1-adrenoceptor. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:10–21. doi: 10.1007/s00210-002-0652-9. [DOI] [PubMed] [Google Scholar]
- TADOKORO C., KIUCHI Y., YAMAZAKI Y., NARA K., OGUCHI K., KAMIJIAMA K. Behavioral stimulation without alteration of β and 5-HT receptors and adenylate cyclase activity in rat brain after chronic sertraline administration. Psychopharmacology. 1997;130:124–130. doi: 10.1007/s002130050219. [DOI] [PubMed] [Google Scholar]
- TODA N., OKAMURA T. Beta adrenoceptor subtype in isolated human, monkey and dog epicardial coronary arteries. J. Pharmacol. Exp. Ther. 1990;253:518–524. [PubMed] [Google Scholar]