Abstract
Prenatal patency of the ductus arteriosus is maintained by prostaglandin (PG) E2, conceivably in concert with nitric oxide (NO). Local PGE2 formation is sustained by cyclooxygenase-1 (COX1) and cyclooxygenase-2 (COX2), a possible exception being the mouse in which COX1, or both COXs, are reportedly absent. Here, we have examined the occurrence of functional COX isoforms in the near-term mouse ductus and the possibility of COX deletion causing NO upregulation.
COX1 and COX2 were detected in smooth muscle cells by immunogold electronmicroscopy, both being located primarily in the perinuclear region. Cytosolic and microsomal PGE synthases (cPGES and mPGES) were also found, but they occurred diffusely across the cytosol. COX1 and, far more frequently, COX2 were colocalised with mPGES, while neither COX appeared to be colocalized with cPGES.
The isolated ductus from wild-type and COX1−/− mice contracted promptly to indomethacin (2.8 μM). Conversely, the contraction of COX2−/− ductus to the same inhibitor started only after a delay and was slower.
NG-nitro-L-arginine methyl ester (L-NAME, 100 μM) weakly contracted the isolated wild-type ductus. Its effect, however, increased three- to four-fold after deleting either COX, hence equalling that of indomethacin.
In vivo, the ductus was patent in all mice foetuses, whether wild-type or COX-deleted. Likewise, no genotype-related difference was noted in its postnatal closure.
We conclude that the mouse ductus has a complete system for PGE2 synthesis comprising both COX1 and COX2. The two enzymes respond differently to indomethacin but, nevertheless, deletion of either one results in NO upregulation. PGE2 and NO can function synergistically in keeping the ductus patent.
Keywords: Ductus arteriosus, cyclooxygenase, prostaglandin E synthase, nitric oxide synthase, prostaglandin E2, nitric oxide, indomethacin, L-NAME, oxygen, foetal and neonatal physiology
Introduction
It is known that prenatal patency and postnatal closure of the ductus arteriosus are actively induced (Smith, 1998). Prostaglandin (PG) E2 plays a major role in both the conditions, namely, through foetal life by exerting a potent relaxant effect (Clyman, 1987; Coceani & Olley, 1988), possibly in conjunction with nitric oxide (NO) (Coceani et al., 1994; Momma & Toyono, 1999; Seidner et al., 2001; Takizawa et al., 2001), and after birth by abruptly withdrawing its action. Two synergistic events occur during the immediate neonatal period that account for the lesser efficacy of PGE2, viz, the fall in blood levels of the compound (Challis et al., 1976; Clyman et al., 1980; Jones et al., 1993) and the waning expression within the ductus of PGE2 receptor subtypes subserving relaxation (Bouayad et al., 2001). Based on this premise, much interest has focused on the ontogeny of cyclooxygenase (COX), a key enzyme in the PG synthetic pathway existing in two isoforms (i.e. COX1 and COX2) (Smith et al., 1996). Studies, carried out in the lamb and the pig, have shown that COXs develop unevenly in the ductus, with COX1 preceding COX2 in the course of gestation (Guerguerian et al., 1998; Takahashi et al., 2000; Coceani et al., 2001). Hence, both COX1 and COX2 have been implicated in PGE2 formation at term, while COX1 has been assigned a predominant role in the premature (Coceani et al., 2001). In contradiction with this scheme, however, it has recently been reported that the mouse ductus lacks COX1 at term gestation and may, at least in vivo, fail to constrict to the dual COX1/COX2 inhibitor, indomethacin, once its COX2 had been deleted (Loftin et al., 2001). Furthermore, another investigation could not detect either COX in the same vessel or, at most, could demonstrate a minimal, and reportedly insignificant, expression of the two enzymes (Reese et al., 2000). The latter finding is in clear contrast with our observation that indomethacin exerts a potent constrictor effect on the isolated mouse ductus (Coceani et al., 1999).
The present investigation was carried out to resolve these apparent inconsistencies and gain, at the same time, a better insight into the functional organisation of the PG synthetic system in the ductus. This was achieved by examining the occurrence in the term mouse ductus of the two COX isoforms and the terminal enzymes for PGE2 synthesis (i.e. microsomal and cytosolic PGE synthase; mPGES and cPGES) (Murakami et al., 2000; Tanioka et al., 2000). Concomitantly, the effect of indomethacin was assessed on the isolated vessel from wild-type vs COX(1 or 2)-deleted animals. In addition, the possible upregulation of the NO system upon COX deletion was verified by testing on the same preparations the NO synthase (NOS) inhibitor, NG-nitro-L-arginine methyl ester (L-NAME). Experiments in vitro were complemented by a study in vivo in which, using a whole-body freezing technique (Hörnblad & Larsson, 1967; Coceani et al., 1999), the calibre of the ductus was monitored from the term foetal through the early neonatal periods before and after deleting either COX isoform.
A preliminary account of this work has been reported (Coceani et al., 2003).
Methods
Experiments were carried out in 129/B6 mice with COX1−/− and COX2−/− genotypes (Morham et al., 1995) (litter size, 2–12; mean, 6), and wild-type C57/B6 mice (litter size 4–12; mean, 7) provided a control after having confirmed in preliminary experiments with the inhibitors (see below) that they behave as the wild-type 129/B6 mice. COX2+/− mice were also used in selected experiments. As expected, animals from the various groups did not differ in general appearance and, consequently, their genotype was verified on tail specimens by PCR (Ballou et al., 2000). Surgical procedures and experimental protocols were approved by the Animal Care Committee of the Ministry of Health.
In vitro studies
Term foetal mice, both wild-type and gene-deleted (gestational age, 19 days, body wt. 0.9–1.4 g, mean 1.1), were delivered by Caesarean section under halothane anaesthesia and were killed by cervical dislocation. The procedure for dissection of the ductus arteriosus, normalisation of internal circumference and mechanical record has been described previously (Coceani et al., 1999). In brief, the animal was secured with its left side up in a dissection chamber containing ice-cold Krebs solution gassed with 5% CO2 in N2. Through a thoracotomy, the ductus was exposed, separated from the adjoining large blood vessels and then suspended onto 25-μm tungsten wires (Cooner wire, Chatsworth, U.S.A.) inside an organ bath. The fluid of the bath was gassed with gas mixtures containing 2.5 or 12.5% O2 to mimic, respectively, the foetal and the neonatal condition. Preparations were then equilibrated (about 60 min at 37°C) with a minimal stretch being applied (all values in mN mm−1) (COX1−/−, 0.04±0.001 (n=8); COX2−/−, 0.05±0.001 (n=16); COX2+/−, 0.05±0.001 (n=11); wild-type, 0.04±0.0009 (n=24)) and the attendant internal circumference (C0) with the related resting dimensions (Table 1) served as a reference for choosing the appropriate load. Afterwards, tension was applied to attain an operating circumference (i.e. C50) coinciding with the condition in vivo (COX1−/−, 0.49±0.008 (n=8); COX2−/−, 0.47±0.01 (n=16); COX2+/−, 0.46±0.007 (n=11); wild-type, 0.52±0.006 (n=24); all values in mN mm−1), and the actual experiment was started following a second, 60–80-min period of equilibration.
Table 1.
Resting dimension of isolated ductus arteriosus from wild-type and COX-deficient term foetal mice
| Dimension (μm) | Wild-type (n=25) | COX1−/− (n=8) | COX2−/− (n=16) | COX2+/− (n=11) |
|---|---|---|---|---|
| Internal diameter | 177±3 | 162±4† | 155±4†† | 157±4†† |
| Vessel length* | ||||
| Short side | 522±8 | 530±19 | 466±13†† | 475±17†† |
| Long side | 542±8 | 548±19 | 483±13†† | 488±17†† |
| Wall thickness | 18±0.6 | 21±0.7 | 20±0.6 | 19±0.7 |
Values are mean±s.e.m.
Vessel length of the ductus arteriosus is uneven because of its insertion into aorta at an angle. Short side was taken for normalisation of internal circumference and calculation of tension output (see In vitro studies).
vs wild-type, P<0.05 (Student's t-test);
vs. wild-type, P<0.01 (Student's t-test). Note that, in spite of its overall smaller size, the COX2−/− ductus was outwardly normal on light microscopy and had, specifically, a well-developed muscle layer (C. Ackerley and F. Coceani, unpublished data).
The study comprised two main protocols depending on whether COX or NOS function was investigated. In protocol 1 (n=33), indomethacin (2.8 μM) was added to the perfusion fluid, and its effect was ascertained on basal tone at either 2.5 or 12.5% O2. Preliminary experiments with the wild-type confirmed the occurrence of a concentration-dependent contraction to oxygen, mimicking in magnitude (0.12±0.02, 0.22±0.03 and 0.39±0.04 mN mm−1 with 12.5, 30 and 95% O2, respectively; n=3) and pattern that of 129/SvEv mice (see Coceani et al., 1999). In certain cases, the contraction to indomethacin did not subside, or subsided only partially, after the tissue had been returned to the control medium (see Results). The tissue was then treated (5–7-min application) with the relaxant sodium nitroprusside (SNP) (100 μM) in the attempt of reversing this persistent response. The same procedure was used with protocol 2 (n=39), except for the fact that L-NAME (100 μM) was tested. In either protocol, concentrations of the inhibitors were selected on the basis of previous reports (Coceani et al., 1994,1999), and treatment lasted 60 min or as long as required to achieve a maximal effect. The contraction to the TXA2 analogue (0.1 μM) provided in both cases a reference. In the majority of experiments, only one protocol was used with each vessel. However, when the two treatments were performed in succession, L-NAME was tested after indomethacin (interval, 2.5–3.5 h) and on the condition that the baseline had returned to control values.
In vivo studies
Experiments were carried out in foetuses (gestational age, 19 days) or newborns (max. 12 h) depending on the protocol. Animals (wild-type and COX-deleted) were delivered by Caesarean section under halothane anaesthesia in the former case, while in the latter they were used at different intervals after a vaginal delivery. Time zero (i.e. the time at which delivery was completed) was assessed for each animal in the litter. Throughout the survival period, newborns were placed on a warm metal plate (∼37°C) and were also heated with a lamp. All animals were killed by cervical dislocation.
Changes in the ductus calibre through the transition from the pre- to the postnatal condition were assessed by fixing the vessel in situ with the whole-body freezing technique (Hörnblad & Larsson, 1967; Coceani et al., 1999). Briefly, animals were placed with their right side up in a Petri dish and were covered with an embedding medium (Tissue-Tek optimum cutting temperature compound; Sakura Finetek, Torrance, U.S.A.). The dish with the animal was then wrapped with aluminium foil and immersed in liquid N2. Once frozen, specimens were stored at −20°C for further work-up. To prepare a block of tissue with the ductus, the carcass was freed of its embedding in the frozen state. With a razor blade, soft tissues covering the dorsum were removed, making certain that the exposed surface would be parallel to the underlying descending aorta and hence perpendicular to the ductus. Serial, transversal sections (5-μm thick), progressing from this cut through the whole length of the vessel, were obtained on a Zeiss freezing microtome (model HM500 OM, Walldorf, Germany) and were stained with 1% methylene blue. Care was taken to collect a complete series of ductus sections. Afterwards, each section was photographed with a CCD solid-state camera (COHU San Diego, U.S.A.) and lumen area of the ductus was measured with a MCID-M4 program (Brock University, St Catharines, Canada). Both maximal and minimal values of this area were retrieved from each series for computation.
Preparation of total RNA and RT–PCR
Total RNA was isolated from the foetal ductus (gestational age, 19 days; wild-type and COX1−/−) with the TriPure Isolation Reagent (Roche Diagnostic Corporation, Indianapolis, U.S.A.), as recommended by the manufacturer, following a modification of the method of Chomczynsky & Sacchi (1987). Briefly, 30–40 specimens of ductus were pooled, homogenised in TriPure solution (1 ml) and then 0.2 ml of chloroform was added. The resulting mixture was centrifuged (12,000 × g; 15 min at 4°C), the aqueous phase was separated and transferred to a tube where RNA was precipitated with 0.5 ml of isopropanol. The precipitate was pelleted by centrifugation (12,000 × g; 10 min at 4°C), and the pellet was washed with ethanol–water (7.5 : 2.5, by volume) before being dissolved into sterile Rnase-free water. Any genomic DNA contamination was subsequently removed by treatment with Rnase-free Dnase I (Roche). RNA (1 μg) was reverse transcribed with 1 U of ThermoScript RT (Invitrogen Corporation, Carlsbad, U.S.A.) in the presence of random hexanucleotide primers according to the manufacturer's instructions. The cDNA product was used for the amplification of COX1 (32 cycles), COX2 (35 cycles), eNOS (36 cycles), iNOS (38 cycles) and nNOS (38 cycles). Forward/reverse primers and general procedure were the same of early reports for both COX (Zhang et al., 2002) and NOS (Kabayashi et al., 2001; Winkler et al., 2001) isoforms. Cyclophilin (forward primer: 5′-AGTTCCATCGTGTCATCAAGG-3′; reverse primer: 5′-GTTTCTCCACTTCGATCTTGC-3′; primers based on the reported gene sequence, GenBank accession no. NM-022563) was used as internal reference (see Haendler et al., 1987) after confirming its steady expression through the perinatal period (S. Barogi, unpublished data), and separate samples being processed without cDNA ruled out any contamination. Agarose gels (1.5% w v−1) were stained with ethidium bromide and the bands were quantified by NIH 1.60 software (National Institute of Health, Bethesda, U.S.A.). Integrated densities were normalised to cyclophilin values for a semiquantitative assessment of individual transcript levels. Preliminary experiments confirmed that PCR conditions and image analysis were in the linear range of detection.
Electron microscopy immunogold histochemistry
Wild-type foetal ductus specimens, which had been prepared in the usual manner and pinned to a wax board, were fixed in 2 or 4% paraformaldehyde/0.1% glutaraldehyde in 0.1 M phosphate buffer (PBS; pH 7.4). They were then rinsed thoroughly with PBS before being stored at 4°C in the same buffer containing sodium azide (20 mM). Specimens were subsequently infused with 2.3 M sucrose and, after being trimmed to a trapezoidal shape, were mounted on aluminium pins and plunge frozen in liquid N2. Cryosections (−90°C) of approximately 100-nm thickness were prepared with a diamond knife in a Reichert Jung Ultracut S microtome equipped with a cryochamber. These ultrathin sections were transferred to glow-discharged carbon-coated nickel grids by use of a loop of molten sucrose. After any residual aldehyde had been neutralised by treatment with 0.15% glycine–0.5% bovine serum albumen (BSA) in PBS, sections were washed repeatedly with BSA-supplemented PBS (0.5%) and were then incubated with antiserum against COX1 (dilution, 1 : 50), COX2 (dilution, 1 : 200), mPGES (dilution, 1 : 10) or cPGES (dilution, 1 : 150). The antiserum was subsequently removed by repeated rinsing with BSA-supplemented PBS, and specimens were treated for 60 min with gold-labelled goat anti-mouse immunoglobulin (particle diameter, 5 or 10 nm) (dilution, 1 : 20 for both). After another thorough wash with PBS/BSA and distilled water in succession, sections were embedded in a thin film of methylcellulose containing 0.2% of uranyl acetate and then were viewed and photographed in a JEOL model 1230 (Peabody, U.S.A.) transmission electron microscope. Specificity of binding was verified by omitting the primary antiserum or by neutralising the primary antiserum with excess enzyme protein (10 : 1 ratio). Gold particles were counted in smooth muscle cells either at random within putative immunoreactive regions (i.e., perinuclear and peripheral cytoplasm; see Coceani et al., 2001) (5 fields cell−1 at × 25,000 magnification) or in the whole cytoplasm (20 fields cell−1 at × 50,000 magnification). The size of gold particles in the two analyses was, respectively, 10 and 5 nm. Number of cells examined in each specimen (n=4) varied from a minimum of 25, when the plan of section crossed the perinuclear cytoplasm, to a maximum of 43. Possible colocalisation of COX and PGES enzymes was also verified in a separate analysis of the perinuclear and peripheral regions of the cell (three fields at × 50,000 magnification for each region; min. 50 cells per specimen) through dual tagging with gold particles of different size (COX isozymes, 10 nm, PGES isozymes, 5 nm). Quantification of particles in the entire cytoplasm as well as in discrete regions of the cell (expressed in both cases as particles μm−2) was done with the Image ProPlus morphometric programme (Media Cybernetics, Des Moines, U.S.A.) on images being acquired via a digital camera (AMT, Danver, U.S.A.) from the transmission electron microscope. COX/PGES colocalisation was quantified by measuring the ratio (in percentage) between COX-linked and free PGES.
Solutions and drugs
The Krebs medium had the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1, MgSO4 0.9, dextrose 11.1 and NaHCO3 25. Depending on the protocol, the solution was bubbled with gas mixtures containing either no O2 or O2 in one of two concentrations (2.5 and 12.5%) plus 5% CO2 and balance N2. PO2 was measured with a Chiron gas analyser (mod. 248, Halstead, U.K.) and was 1.09±0.03, 2.11±0.01 and 7±0.02 kPa (pH 7.42±0.002) when gas mixtures were used with 0, 2.5 and 12.5% O2, respectively.
Purified seminal vesicle COX1, placental COX2 (both from sheep), mPGES and cPGES (both human type) were supplied by Cayman (Ann Arbor, U.S.A.). COX1 and COX2 antisera (courtesy of Merck Frosst, Montreal, Canada) and cPGES antiserum (obtained from Cayman) were all polyclonal and had been generated in the rabbit. mPGES antiserum (courtesy of NIH Scor Program, Cell and Tissue Banking Resource, Michigan State University, Ann Arbor, U.S.A.) was instead monoclonal and was derived from a murine lymphoblast cell line. Colloidal gold-labelled (particle diameter, 5 or 10 nm) goat anti-rabbit or anti-mouse immunoglobulins were purchased from Amersham (Piscatoway, U.S.A.).
The thromboxane A2 (TXA2) analogue 9,11-epithio-11,12-methano-TXA2 (ONO-11113, courtesy of ONO Pharmaceutical, Osaka, Japan) was dissolved in distilled ethanol (5 mg ml−1), and aliquots (stored at −70°C) were diluted with Tris buffer (pH 7.4). Indomethacin, a dual COX1/COX2 inhibitor (Sigma, St Louis, U.S.A), was also dissolved in distilled ethanol (10 mg ml−1) before preparation of the final solution in the Krebs medium. Ethanol in the fluid bathing isolated ductus preparations did not exceed 0.01% (indomethacin) or 0.001% (ONO-11113). The NOS inhibitor L-NAME and SNP (both from Sigma) were dissolved directly in saline. Precautions were taken to protect the SNP solution from light. Doses of all compounds are given in molar concentrations and refer to their final concentration in the bath. Vehicle alone, without or with ethanol (see above), had no effect on the vessel tone.
Analysis of data
Baseline contractile tension, which varied with the preparation (see Results), refers to the net active tension (i.e., total tension minus applied tension) developed by the preparation prior to any treatment. Responses to test agents are given as the fractional change from baseline.
Data are expressed as the mean±s.e.m. Comparisons were made using a Student's t-test or ANOVA, followed when required by Duncan's multiple-range test. Differences are considered significant for P<0.05.
Results
mRNA analysis
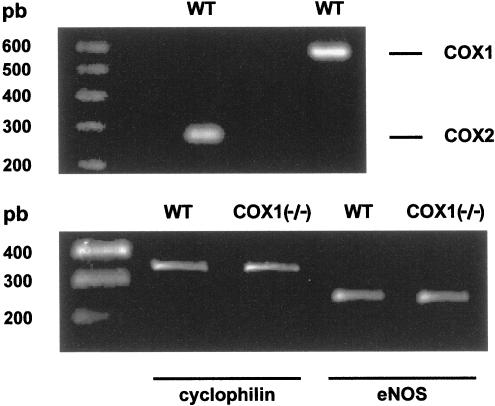
The ductus arteriosus from the wild-type foetal mouse expressed mRNA for both COX1 and COX2 at term gestation (Figure 1, upper panel). Conversely, only one of the NOS isoforms, eNOS mRNA, was detected in the same preparation and its expression did not change appreciably upon deleting COX1 (Figure 1, lower panel). Furthermore, iNOS mRNA and nNOS mRNA remained undetectable in the COX1−/− mutant.
Figure 1.
Gel electrophoresis of RT–PCR-amplified COX (upper panel) and eNOS (lower panel) isoforms of mRNA in the foetal ductus arteriosus from wild-type (WT) mice. Note that the level of eNOS transcript was normalised to that of cyclophilin and did not change upon COX1 deletion (lower panel). COX2−/− animals were not used since they behave as the COX1−/− animals in showing an enhanced NOS-based relaxing activity (see text and Figure 8).
Localisation of cyclooxygenase and PGE synthase isoforms
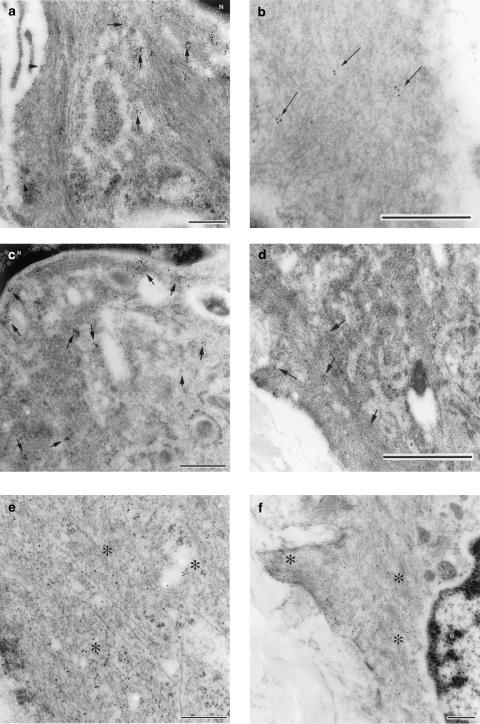
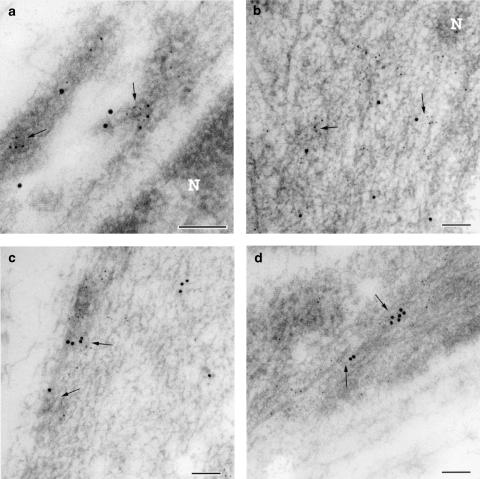
As previously reported in the lamb (Coceani et al., 2001), muscle cells from the wild-type mouse ductus showed greater immunogold reactivity for COX1 than COX2 (Figure 2a). Likewise, both enzymes were preferentially located in the perinuclear rather than the peripheral region of the cytoplasm, often in association with vesicular structures (Figure 2a). In contrast, cPEGS and mPGES were similarly expressed and were also distributed diffusely across the cytosol (Figure 2b). Figure 3 provides representative examples of COX (a–d) and PGES (e, f) antigenicity in ductus muscle cells. In those instances in which the two groups of enzymes had concomitantly been tagged, COX1- and COX2-linked gold particles were found to be colocalised with mPGES-linked particles in both the inner (Figure 4a and b) and the outer (Figure 4c and d) regions of the cytoplasm. However, at either site, the incidence of COX2/mPGES complexes (6.5±1.1 and 7.3±2.6%, respectively, in the perinuclear and peripheral cytoplasm) exceeded greatly that of the COX1/mPGES complexes (0.25±0.1 and 0.3±0.15%, respectively, in the perinuclear and peripheral cytoplasm). No obvious evidence, on the other hand, was found of either COX being linked with cPGES.
Figure 2.
Transmission immunoelectron microscopy from the muscle layer of the foetal mouse ductus arteriosus at term (n=4). (a) COX1 and COX2 immunogold labelling of the whole cytoplasm (cyt) and its perinuclear and peripheral components. Note that gold particle size was different in the two analyses (whole cytoplasm: 5 nm; perinuclear/peripheral cytoplasm: 10 nm). (b) Immunogold labelling for microsomal and cytosolic PGE synthase (mPGES and cPGES). *vs cytoplasm perinuclear, P<0.05 (Student's t-test); †vs COX1, whole cytoplasm, P<0.01 (Student's t-test).
Figure 3.
Transmission electron micrograph of ultrathin cryosections from the muscle layer of the foetal mouse ductus arteriosus. (a) Immunogold labelling for COX1; note the gold particles being clustered in the cytoplasm (indicated with arrows) which surrounds the nucleus (N) and in the plasma membrane (arrowheads). (b) Immunogold labelling for COX1 in the peripheral cytoplasm; label (arrows) is not as dense as that seen in the perinuclear cytoplasm. (c) Immunogold labelling for COX2 in the perinuclear cytoplasm; note clusters of label in proximity of the nucleus (N). (d) Immunogold labelling for COX2; note few clusters of label (arrows) in the peripheral cytoplasm and along the plasma membrane. (e) Immunogold labelling for cytosolic PGE synthase; label was scattered throughout the cytoplasm (asterisks). (f) Immunogold labelling for microsomal PGE synthase; label was found diffusely throughout the perinuclear (N) and peripheral cytoplasm (asterisks). Bar represents 0.25 μm (panels a, c, e, f) or 0.5 μm (panels b and d).
Figure 4.
Transmission electron micrograph of ultrathin cryosections from the muscle layer of the foetal mouse ductus arteriosus. (a) Dual immunogold labelling for COX1 (particle, 10 nm) and microsomal PGE synthase (particle, 5 nm); note colocalisation of the two labels (arrows) in vesicle-like structures close to the nucleus (N). (b) Dual immunogold labelling for COX2 (particle, 10 nm) and microsomal PGE synthase (particle, 5 nm); colocalisation of the two labels (arrows) in proximity of the nucleus (N). (c) Dual immunogold labelling for COX1 (particle, 10 nm) and microsomal PGE synthase (particle, 5 nm) in the peripheral cytoplasm; colocalisation of the two labels in vesicle-like structures (arrows). (d) Dual immunogold labelling for COX2 (particle, 10 nm) and microsomal PGE synthase (particle, 5 nm) in the peripheral cytoplasm; colocalisation of the two labels (arrows). Note that in both perinuclear and peripheral cytoplasm, COX2 colocalised more frequently than COX1 with microsomal PGE synthase. Bar represents 0.1 μm.
In vitro studies
The isolated ductus arteriosus was relatively small in the COX mutant compared to the wild-type control, the lesser size being particularly evident in the case of COX2 deletion (Table 1). In agreement with a previous study (Coceani et al., 1999), the wild-type vessel contracted variably during the initial period of equilibration, and the steady tension being attained in 40–60 min was, as expected (see Methods), moderately greater at 12.5 than 2.5% O2 (0.36±0.07 mN mm−1, n=10 vs 0.20±0.03 mN mm−1, n=16; P<0.05). Comparable values of tension were observed with COX-deleted animals. However, more often than in controls (80 vs 30% of experiments), their baseline tone had transient contractions of uneven amplitude (0.1–0.47 mN mm−1) and/or phasic discharges superimposed. Whatever the genotype, the ductus contracted promptly to the TXA2 analogue ONO-11113 (0.1 μM) (Figure 5), but the magnitude of the contraction (at 2.5% O2) was slightly smaller with COX2−/− (0.83±0.19 mN mm−1, n=5; P<0.05) than with either COX1−/− (1.52±0.08 mN mm−1, n=4) or wild-type (1.23±0.08 mN mm−1, n=10) preparations.
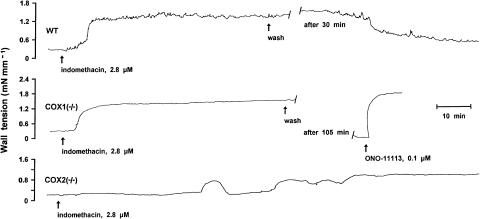
Figure 5.
Representative responses to indomethacin in the wild-type (WT) vs COX-deleted foetal mouse ductus arteriosus. Contraction to the TXA2 analogue, ONO-11113, given as a reference. Length of the vessel is 540, 444 and 481 μm (short side, see Methods) for wild-type, COX1−/− and COX2−/−, respectively.
Indomethacin (2.8 μM) had a contractile effect on the vessel and, in the case of the wild-type and the COX1−/− mutant, this response developed after short latency (2–7 min) and proceeded rapidly to a peak. As shown in Figure 5, the progression of the contraction was often biphasic in the wild-type, while in the COX1−/− it was linear most of the times. In contrast, the contractile response of the COX2−/− ductus to indomethacin was delayed in onset (32–67 min) and slow in development, with transient contractions often superimposed on the rising slope (Figure 5). The peak contraction, once attained (70–140 min, mean 104), was sustained and showed no significant difference in magnitude with the genotype (Figure 6a and b). However, reversal of the contraction upon washing out the inhibitor was complete, or nearly complete, with the COX1−/− ductus, while with the COX2−/− vessel it was partial (avg. 43%) or absent altogether (three of eight exps.). Significantly, in the latter case, the vessel regained its elevated tone even after being relaxed with SNP (100 μM; see Methods). The wild-type preparation, on the other hand, behaved most variably after stopping the treatment with indomethacin and showed complete reversal of the contraction (five of 12 exps), partial reversal (avg. 58%) with or without the SNP priming, or total lack of reversibility (four of 12 exps.). No obvious difference from the wild-type was noted instead in the COX2+/− preparation with regard to both magnitude (0.96 and 0.57±0.10 mN mm−1 at 2.5 and 12.5% O2, respectively; n=2 and 3) and pattern of the indomethacin contraction.
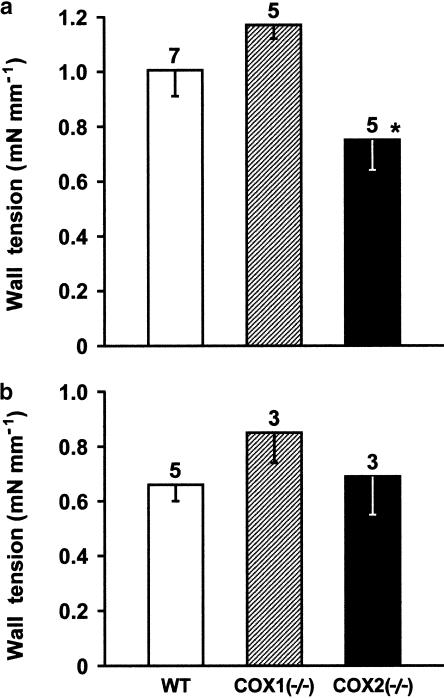
Figure 6.
Comparison of contractile responses to indomethacin (2.8 μM) in the isolated ductus arteriosus from wild-type (WT) vs COX-deleted mouse foetus. (a) 2.5% O2; (b) 12.5% O2. Wall tension (mN mm−1) prior to treatment, respectively, at 2.5 and 12.5% O2, was as follows: WT, 0.36±0.04 and 0.36±0.15; COX1−/−, 0.30±0.03 and 0.29±0.13; COX2−/−, 0.25±0.08 and 0.36±0.03. For each group, the number of experiments is given above each column, and no significant difference between wild-type and COX-deleted preparations is reached at either O2 tension. A significant difference in the panel (a) relative to COX1−/− is indicated with *P<0.05 (ANOVA). Note that in the case of wild-type and COX1−/− preparations, responses are slightly lower at 12.5 than 2.5% O2 (P<0.05).
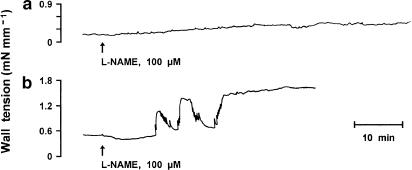
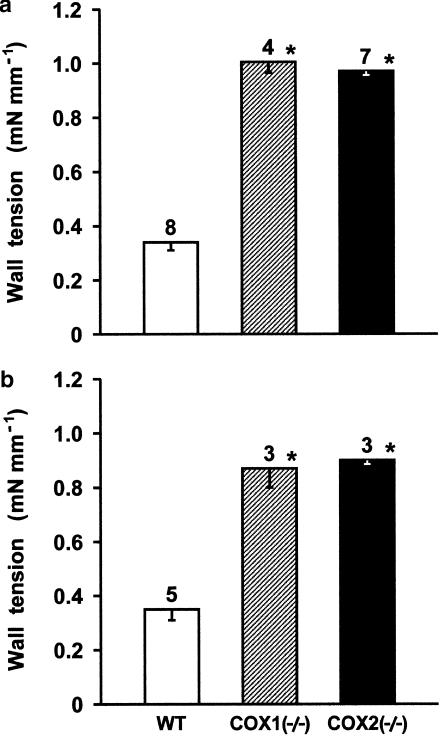
Contrary to indomethacin, L-NAME (100 μM) caused a small and gradually developing contraction in the wild-type preparation (Figure 7a). Only occasionally did the rising tone have transient contractions and/or phasic discharges superimposed. The resulting peak response (in 50–150 min, mean 97), as shown in Figure 8, was about one-third of that to indomethacin (compare with Figure 6) and did not change with the oxygen tension of the medium. However, upon deleting either COX, the contraction to L-NAME became greater, hence equalling in magnitude the indomethacin contraction (Figure 8). Its shape was similar in the two null mutants and consisted of an initial gradual rise in tone, often with transient contractions superimposed, which after variable delay (25–90 min, mean 56) changed into an abrupt, secondary contraction (Figure 7b). Exceptionally did the fast contraction occur immediately after the start of the L-NAME treatment. Whatever the pattern, the response of COX-deleted preparations to L-NAME was characterised by frequent bursts of phasic activity. An enhanced effect of L-NAME was seen even with preparations heterozygous for the COX2 deletion, their contraction being 0.71±0.12 mN mm−1 (n=6) and 0.76±0.12 mN mm−1 (n=3), respectively, at 2.5 and 12.5% O2.
Figure 7.
Representative response to L-NAME in the wild-type vs COX-deleted foetal mouse ductus arteriosus. (a) wild-type; (b) COX1−/− mutant. Note that response pattern is similar in COX1−/− and COX2−/−. Length of the vessel is 540 and 548 μm (short side, see Methods) for wild-type and COX1−/−, respectively.
Figure 8.
Comparison of contractile responses to L-NAME (100 μM) in the isolated ductus arteriosus from wild-type (WT) vs COX-deleted mouse foetus. (a) 2.5% O2; (b) 12.5% O2. Wall tension (mN mm−1) prior to treatment, respectively, at 2.5 and 12.5% O2, was as follows: WT, 0.07±0.03 and 0.37±0.04; COX1−/−, 0.24±0.1 and 0.14±0.08; COX2−/−, 0.08±0.05 and 0.2±0.12. For each group, the number of experiments is given above the columns, and a significant difference (P<0.01) between wild-type and COX-deleted preparations is indicated with an asterisk.
In vivo studies
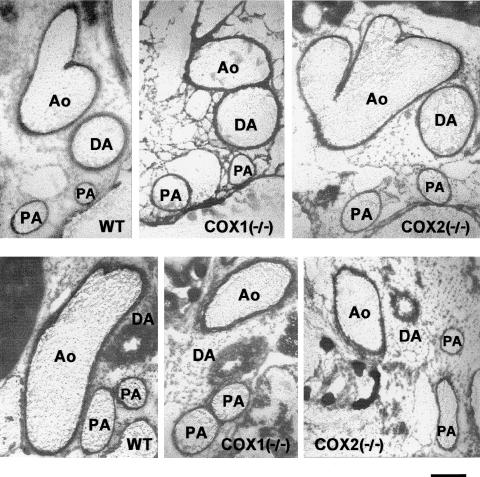
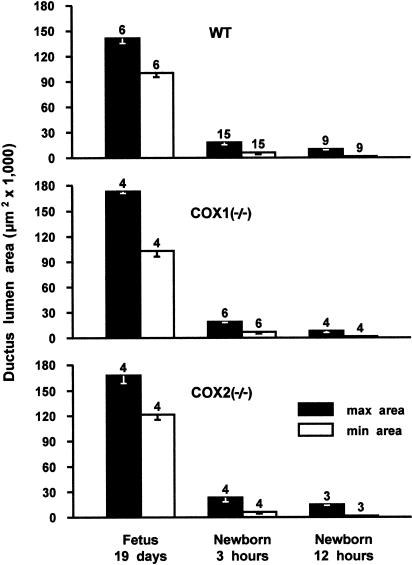
The ductus was patent in all foetuses (Figure 9, upper panel) and no difference in either maximal or minimal lumen area was noted depending on the genotype (Figure 10). Likewise, in both wild-type and COX-deleted animals, the vessel constricted rapidly during the first 3 h after vaginal delivery (Figure 9, lower panel) and closure, or virtual closure, was attained by 12 h (Figure 10). Within this period, maximal narrowing occurred in either, or both, the central and aortic portions of the ductus, thus leaving the pulmonary end wider throughout the closing process.
Figure 9.
Cross-section of the ductus arteriosus and the large blood vessels in the wild-type and COX-deleted frozen mice. (upper panel) Foetus at 19 days gestation; (lower panel) newborn at 3 h. WT, wild-type; DA, ductus arteriosus; Ao, aorta; PA, pulmonary artery. Scale bar, 250 μm.
Figure 10.
Time course of ductus closure in wild-type (WT) and COX-deleted mice. For each group, the number of animals is given above the columns.
Discussion
In contrast to previous reports (Reese et al., 2000; Loftin et al., 2001), this study demonstrates the presence of both COX enzymes, COX1 and COX2, in the foetal mouse ductus. The same vessel is also endowed with the two isoforms of PGES. Hence, the mouse shares with other species, specifically the lamb (Coceani et al., 2001; C. Ackerley and F. Coceani, unpublished data), a complete system for PGE2 synthesis with its two sets of enzymes being unevenly distributed within the cell. In fact, in both species COX enzymes have primarily a perinuclear location, while PGES enzymes are scattered diffusely across the cytosol. Accordingly, the isolated mouse ductus, as an equivalent preparation from the lamb (Smith, 1998), contracts forcefully to indomethacin. Significant in this context is the fact that the guinea-pig ductus, which is peculiarly unresponsive to indomethacin and other nonsteroidal anti-inflammatory drugs (Coceani et al., 1978), has exceedingly low levels of either COX (avg. <4 particles μm−2), lacking in addition any particular regional localisation, while coincidentally it presents a full complement of PGES enzymes (C. Ackerley and F. Coceani, unpublished data). From these data, one may conclude that an appropriately high and region-selective expression of COX enzymes is required for attaining values of PGE2 synthesis effective on ductal muscle. COX1 and COX2, however, differ in their susceptibility to indomethacin, and this raises intriguing questions on their individual operation. Leaving aside the latter point, our study confirms that NO is naturally formed in the ductus and also shows that the compound, despite its seemingly weak action under normal conditions, may become important for ductus patency should the efficacy of PGE2 abate for any reason. Indeed, the marked enhancement in the L-NAME contraction upon deletion of either COX implies a complementary role for the two agents, whereby interference with the PGE2 mechanism generates a signal for NO upregulation conceivably at the post-transcriptional level (see Results). eNOS appears to be the only NO-forming enzyme in the ductus and, in that respect, the mouse differs from the lamb where both iNOS (Clyman et al., 1998) and nNOS (Mason et al., 1999; Rairigh et al., 2000) have also been detected. Based on this premise, the discussion will address two issues: the reason for the differential response of COX1 vs COX2 to indomethacin, and the implications, conceptual and practical, from NO upregulation subsequent to COX removal.
The uneven development of the indomethacin contraction in COX1−/− (rapid) vs COX2−/− (slow) preparations is unexpected and reveals a new aspect in the operation of individual COX isoforms. Its knowledge is important particularly when dealing with structures, as the one being considered here, where both isoforms are constitutively expressed. Our finding is unlikely linked to methodological factors such as the accessibility of the target to the inhibitor or the effect of any NO upregulation on the capability of the ductus to contract. The wall of the vessel is exceedingly thin in all animals, whether wild-type or COX-deficient (see Table 1), and should allow a facile diffusion of indomethacin across the tissue. Furthermore, there are no obvious differences in the subcellular distribution of the two COX enzymes that could account for their uneven susceptibility to the inhibitor. An activated NO system, on the other hand, may well curtail the contractile drive of the vessel but its influence, if potent enough, should be equally evident with the COX1−/− and COX2−/− genotypes. Therefore, it is safe to conclude that differences in the response to indomethacin reside in the actual operation of the enzymes. One possibility is that the COX/indomethacin interaction varies with the isoform. Indeed, it has been shown that, despite the lack of any isoform-linked diversity in the occupancy of the catalytic site by indomethacin, inhibition is slower and not complete with COX1 compared to COX2 (Laneuville et al., 1994). Accordingly, the indomethacin effect may need some time to reach the point at which PGE2 formation falls below a critical level for the maintenance of ductus relaxation. Alternatively, the observed differences could be related not to the COX enzymes themselves, but rather to the way such enzymes are operationally coupled with the PGES enzymes. Of relevance in this connection is our finding (see Results) that COX1 is colocalised minimally with mPGES and seemingly not at all with cPGES. Conversely, there is the frequent coexistence of COX2 with mPGES, hence implying preferential formation of PGE2 through a functional coupling of these enzymes. In addition, it must be pointed out that a second mPGES (i.e. mPGES2), to be distinguished from the conventional synthase studied by us and now renamed mPGES1, has been characterised in both foetal and adult tissues (Tanikawa et al., 2002). The presence of mPGES2, should it be ascertained in the ductus, may provide a new route, perhaps isoform-specific, for PGE2 synthesis. Clearly, all these possibilities are not mutually exclusive and their verification warrants further investigation.
Whatever the reason for the differential behaviour of the COX enzymes, our results may explain the reported inability to observe a COX1-based contraction of the ductus in vivo when indomethacin (Loftin et al., 2001) or a selective inhibitor (Loftin et al., 2002) is given to the mother. Possibly, a delayed responsiveness coupled with the inevitable fall of drug levels with time might have hindered the detection of the enzyme.
While certain facts concerning COX function remain to be elucidated, our findings validate the role of NO as an alternative agent to PGE2 in maintaining ductus patency. In fact, it would appear that the two agents are intertwined operationally to the point that even a fractional suppression of COX function, as it happens in the COX2+/− genotype, promotes the activation of the NO mechanism. The existence of this efficient synergism raises several questions. For example, it has been shown by us (see Results) and others (Loftin et al., 2001) that the ductus remains patent in utero after removal of either COX and, intuitively, the absence of any change has been ascribed to compensation by the residual enzyme. However, the rebound upregulation of NO could also account, at least in part, for this finding. Clearly, in view of our data, the persistent ductus patency in foetuses missing both COXs (Reese et al., 2000; Loftin et al., 2001) is expected to rely on an efficient NO mechanism. It remains to be ascertained whether the indomethacin-resistant patency seen in animals without the EP4 receptor subtype for PGE2 (Nguyen et al., 1997; Kobayashi & Narumiya, 2002) is also linked to the same process of compensation. An additional outstanding question is whether inhibition of COX by pharmacological means may mimic the gene deletion in promoting NO. Findings in another vascular district of the perinatal animal support this possibility (Zhang & Leffler, 2001). Any such occurrence in the ductus would have important implications for the interpretation of experimental and clinical data. It could provide an explanation for the apparent loss of efficacy of a COX inhibitor as ductus constrictor when the administration is chronic rather than acute (Loftin et al., 2002). In addition, the observation of a higher incidence of persistent ductus in infants from mothers treated antenatally with indomethacin (Norton et al., 1993) could find a reason in the overriding action of NO. In fact, if one extends this reasoning further, a pharmacologically driven rebound of NO could become an important causative factor with any failure of the indomethacin therapy in prematures with persistent ductus.
In conclusion, this study demonstrates the presence in the mouse ductus of a complete enzyme system for the synthesis of PGE2 including both COX1 and COX2. COX enzymes differ in their susceptibility to the inhibitor action of indomethacin and this finding may denote distinct functional properties. However, despite this difference, deletion of either COX results in the acceleration of NO synthesis. A cooperative interaction between PGE2 and NO is expectedly important for ductus patency in the foetus and may be a significant factor for persistence of the vessel in the sick infant.
Acknowledgments
This work was supported by the Heart and Stroke Foundation of Ontario (Grant T-3329). F.B. is the recipient of a graduate studentship from the Scuola Superiore S. Anna. The technical assistance of V. Gattai is gratefully acknowledged.
Abbreviations
- BSA
bovine serum albumin
- COX
cyclooxygenase
- L-NAME
NG-nitro-L-arginine methyl ester hydrochloride
- NO
nitric oxide
- NOS
nitric oxide synthase
- OCT
optimum cutting temperature compound
- PBS
phosphate buffer solution
- PG
prostaglandin
- PGES
prostaglandin E synthase
- TX
thromboxane
References
- BALLOU L.R., BOTTING R.M., GOORHA S., ZHANG J., VANE J.R. Nociception in cyclooxygenase isozyme-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10272–10276. doi: 10.1073/pnas.180319297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUAYAD A., KAJINO H., WALEH N., FOURON J.-C., ANDELFINGER G., VARMA D.R., SKOLL A., VAZQUEZ A., GOBEIL F., JR, CLYMAN R.I., CHEMTOB S. Characterisation of PGE receptors in fetal and newborn lamb ductus arteriosus. Am. J. Physiol. 2001;280:H2342–H2349. doi: 10.1152/ajpheart.2001.280.5.H2342. [DOI] [PubMed] [Google Scholar]
- CHALLIS J.R.G., DILLEY S.R., ROBINSON J.S., THORBURN G.D. Prostaglandins in the circulation of the fetal lamb. Prostaglandins. 1976;11:1041–1052. doi: 10.1016/0090-6980(76)90011-3. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKY P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thyocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CLYMAN R.I. Ductus arteriosus: current theories of prenatal and postnatal regulation. Semin. Perinatol. 1987;11:64–71. [PubMed] [Google Scholar]
- CLYMAN R.I., MAURAY F., RUDOLPH A.M., HEYMANN M.A. Circulating prostaglandin E2 concentrations and patent ductus arteriosus in fetal and neonatal lambs. J. Pediatr. 1980;97:455–461. doi: 10.1016/s0022-3476(80)80205-8. [DOI] [PubMed] [Google Scholar]
- CLYMAN R.I., WALEH N., BLACK S.M., RIEMER R.K., MAURAY F., CHEN Y.-Q. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, and vasa vasorum. Pediatr. Res. 1998;43:633–644. doi: 10.1203/00006450-199805000-00012. [DOI] [PubMed] [Google Scholar]
- COCEANI F., ACKERLEY C., SEIDLITZ E., KELSEY L. Function of cyclooxygenase-1 and cyclooxygenase-2 in the ductus arteriosus from foetal lamb: differential development and change by oxygen and endotoxin. Br. J. Pharmacol. 2001;132:241–251. doi: 10.1038/sj.bjp.0703779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCEANI F., BARAGATTI B., BRIZZI F., BAROGI S., ACKERLEY C.Cyclooxygenase (COX) function in the ductus arteriosus: another look Advances in Prostaglandin, Leukotriene and Other Bioactive Lipid Research: Basic Science and Clinical Applications. 2003Dordrecht. THE Netherlands: Kluwer Academics Publishers; ed. Yazici, Z., Folco, G., Drazen, J., Nigam, S. & Shimizu, T(in press) [Google Scholar]
- COCEANI F., KELSEY L., SEIDLITZ E. Occurrence of endothelium-derived relaxing factor-nitric oxide in the lamb ductus arteriosus. Can. J. Physiol. Pharmacol. 1994;72:82–88. doi: 10.1139/y94-013. [DOI] [PubMed] [Google Scholar]
- COCEANI F., LIU Y.-A., SEIDLITZ E., KELSEY L., KUWAKI T., ACKERLEY C., YANAGISAWA M. Endothelin A receptor is necessary for O2 constriction but not closure of ductus arteriosus. Am. J. Physiol. 1999;277:H1521–H1531. doi: 10.1152/ajpheart.1999.277.4.H1521. [DOI] [PubMed] [Google Scholar]
- COCEANI F., OLLEY P.M. The control of cardiovascular shunts in the fetal and perinatal period. Can. J. Physiol. Pharmacol. 1988;66:1129–1134. doi: 10.1139/y88-185. [DOI] [PubMed] [Google Scholar]
- COCEANI F., OLLEY P.M., BISHAI I., BODACH E., WHITE E.P.Significance of the prostaglandin system to the control of muscle tone of the ductus arteriosus Advances in Prostaglandin and Thromboxane Research. 19784New York: Raven Press; 325–333.ed. Coceani, F. & Olley, P.M. Vol [PubMed] [Google Scholar]
- GUERGUERIAN A.-M., HARDY P., BHATTACHARYA M., OLLEY P., CLYMAN R.I., FOURON J.-C., CHEMTOB S. Expression of cyclooxygenases in ductus arteriosus of fetal and newborn pigs. Am. J. Obstet. Gynecol. 1998;179:1618–1626. doi: 10.1016/s0002-9378(98)70035-3. [DOI] [PubMed] [Google Scholar]
- HAENDLER B., HOFER-WARBINEK R., HOFER E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987;6:947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HÖRNBLAD P.Y., LARSSON K.S. Studies on closure of the ductus arteriosus, I. Whole-body freezing as improvement of fixation procedures. Cardiologia. 1967;51:231–241. [PubMed] [Google Scholar]
- JONES S.A., ADAMSON S.L., BISHAI I., LEES J., ENGELBERTS D., COCEANI F. Eicosanoids in third ventricular cerebrospinal fluid of fetal and newborn sheep. Am. J. Physiol. 1993;264:R135–R142. doi: 10.1152/ajpregu.1993.264.1.R135. [DOI] [PubMed] [Google Scholar]
- KABAYASHI H., HATAISHI R., MITSUFUJI H., TANAKA M., JACOBSON M., TOMITA T., ZAPOL W.M., JONES R.C. Antiinflammatory properties of inducible nitric oxide synthase in acute hyperoxic lung injury. Am. J. Resp. Cell Biol. 2001;24:390–397. doi: 10.1165/ajrcmb.24.4.4218. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., NARUMIYA S. Function of prostanoid receptors: studies on knockout mice. Prostagland. Other Lipid Mediat. 2002;68–69:557–573. doi: 10.1016/s0090-6980(02)00055-2. [DOI] [PubMed] [Google Scholar]
- LANEUVILLE O., BREUER D.K., DEWITT D.L., HLA T., FUNK C.D., SMITH W.L. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 1994;271:927–934. [PubMed] [Google Scholar]
- LOFTIN C.D., TRIVEDI D.B., LANGEBACH R. Cyclooxygenase-1-selective inhibition prolongs gestation in mice without adverse effects on the ductus arteriosus. J. Clin. Invest. 2002;110:549–557. doi: 10.1172/JCI14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOFTIN C.D., TRIVEDI D.B., TIANO H.F., CLARK J.A., LEE C.A., EPSTEIN J.A., MORHAM S.G., BREYER M.D., NGUYEN M.T., HAWKINS B.M., GOULET J.L., SMITHIES O., KOLLER B.H., LANGENBACH R. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1059–1064. doi: 10.1073/pnas.031573498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON C.A.E., CHANG P., FALLERY C., RABINOVITCH M. Nitric oxide mediates LC-3-dependent regulation of fibronectin in ductus arteriosus intimal cushion formation. FASEB J. 1999;13:1423–1434. doi: 10.1096/fasebj.13.11.1423. [DOI] [PubMed] [Google Scholar]
- MOMMA K., TOYONO M. The role of nitric oxide in dilating the fetal ductus arteriosus in rats. Pediatr. Res. 1999;46:311–315. doi: 10.1203/00006450-199909000-00010. [DOI] [PubMed] [Google Scholar]
- MORHAM S.G., LANGENBACH R., LOFTIN C.D., TIANO H.F., VOULOUMANOS N., JENNETTE J.C., MAHLER J.F., KLUCKMAN K.D., LEDFORD A., LEE C.A., SMITHIES O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., NARABA H., TANIOKA T., SEMMYO N., NAKATANI Y., KOJIMA F., IKEDA T., FUEKI M., UENO A., OHISHI S., KUDO I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- NGUYEN M.T., CAMENISCH T., SNOUWAERT J.N., HICKS E., COFFMAN T.M., ANDERSON P.A.W., MALOUF N.N., KOLLER B.H. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- NORTON M.E., MERRILL J., COOPER B.A.B., KULLER J.A., CLYMAN R.I. Neonatal complications after the administration of indomethacin for preterm labor. N. Engl. J. Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- RAIRIGH R.L., STORME L., PARKER T.A., LE CRAS T.D., MARKHAM N., JAKKULA M., ABMAN S.H. Role of neuronal nitric oxide synthase in regulation of vascular and ductus arteriosus tone in the ovine fetus. Am. J. Physiol. 2000;278:L105–L110. doi: 10.1152/ajplung.2000.278.1.L105. [DOI] [PubMed] [Google Scholar]
- REESE J., PARIA B.C., BROWN N., ZHAO X., MORROW J.D., DEY S.K. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9759–9764. doi: 10.1073/pnas.97.17.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDNER S.R., CHEN Y.Q., OPRYSKO P.R., MAURAY F., TSE M.M., LIN E., KOCH C., CLYMAN R.I. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr. Res. 2001;50:365–373. doi: 10.1203/00006450-200109000-00012. [DOI] [PubMed] [Google Scholar]
- SMITH G.C.S. The pharmacology of the ductus arteriosus. Pharmacol. Rev. 1998;50:35–58. [PubMed] [Google Scholar]
- SMITH W.L., GARAVITO R.M., DEWITT D.L. Prostaglandin endoperoxide H synthases (cyclooxygenase)-1 and -2. J. Biol. Chem. 1996;271:35157–35160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI Y., ROMAN C., CHEMTOB S., TSE M.M., LIN E., HEYMANN M.A., CLYMAN R.I. Cyclooxygenase-2 inhibitors constrict the fetal lamb ductus arteriosus both in vitro and vivo. Am. J. Physiol. 2000;278:R1496–R1505. doi: 10.1152/ajpregu.2000.278.6.R1496. [DOI] [PubMed] [Google Scholar]
- TAKIZAWA T., KIHARA T., KAMATA A. Increased constriction of the ductus arteriosus with combined administration of indomethacin and L-NAME in fetal rats. Biol. Neonate. 2001;80:64–67. doi: 10.1159/000047122. [DOI] [PubMed] [Google Scholar]
- TANIKAWA N., OHMIYA Y., OHKUBO H., HASHIMOTO K., KANGAWA K., KOJIMA M., ITO S., WATANABE K. Identification and characterisation of a novel type of membrane-associated prostaglandin E synthase. Biochem. Biophys. Res. Commun. 2002;291:884–889. doi: 10.1006/bbrc.2002.6531. [DOI] [PubMed] [Google Scholar]
- TANIOKA T., NAKATANI Y., SEMMYO N., MURAKAMI M., KUDO I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- WINKLER F., KODEL U., KASTENBAUER S., PFISTER W. Differential expression of nitric oxide synthase in bacterial meningitis: role of the inducible isoform for blood–brain barrier breakdown. J. Infect. Dis. 2001;183:1749–1759. doi: 10.1086/320730. [DOI] [PubMed] [Google Scholar]
- ZHANG J., GOORHA S., RAGHOW R., BALLOU L.R. The tissue-specific, compensatory expression of cyclooxygenase-1 and -2 in transgenic mice. Prostagland. Other Lipid Mediat. 2002;67:121–135. doi: 10.1016/s0090-6980(01)00177-0. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., LEFFLER C.W. Compensatory role of NO in cerebral circulation of piglets chronically treated with indomethacin. Am. J. Physiol. 2001;282:R400–R410. doi: 10.1152/ajpregu.00256.2001. [DOI] [PubMed] [Google Scholar]