Abstract
Blockers of the rapid component of the delayed rectifier potassium current (IKr) prolong cardiac action potential duration (APD) and effective refractory period (ERP) in a reverse rate-dependent manner. Since activation of β-adrenoceptors attenuates prolongation of APD evoked by IKr blockers, rate-dependent neuronal noradrenaline liberation in the myocardium may contribute to the reverse rate-dependent nature of the effects of IKr blockers. In order to test this hypothesis, we studied the effects of dofetilide, a pure IKr blocker, on ERP after activation or blockade of β-adrenoceptors and after catecholamine depletion in guinea-pig left atrial myocardium paced at 3, 2 and 1 Hz, in vitro.
Dofetilide (100 nM) lengthened ERP in a reverse rate-dependent manner in the left atrial myocardium of guinea-pigs. Strong activation of β-adrenoceptors using 10 nM isoproterenol abolished the dofetilide-induced lengthening of ERP at all pacing rates.
Blockade of the β-adrenoceptors with metoprolol (1 μM), atenolol (3 μM) or propranolol (300 nM) increased the dofetilide-evoked prolongation of ERP at 3 and 2 Hz, but not at 1 Hz. As a consequence, metoprolol attenuated while propranolol and atenolol fully eliminated the reverse rate-dependent nature of the dofetilide-induced ERP lengthening. In catecholamine-depleted atrial preparations of the guinea-pig (24 h pretreatment with 5 mg kg−1 reserpine i.p.), the effect of dofetilide on ERP was not frequency dependent, and propranolol did not alter the effects of dofetilide.
In contrast to results obtained in guinea-pig atrial preparations, propranolol failed to change the reverse rate-dependent effect of dofetilide on ERP in the right ventricular papillary muscles of rabbits and guinea-pigs.
As an indication of the functional consequences of rate-dependent noradrenaline liberation, propranolol decreased twitch tension at 3 and 2 Hz but not at 1 Hz in the atrial myocardium of control guinea-pigs, whereas no such effect was detected in catecholamine-depleted atrial preparations. Propranolol failed to change contractility of ventricular myocardium in guinea-pigs and rabbits.
It is concluded that rate-dependent noradrenaline release and the ensuing β-adrenoceptor activation contributed to the reverse rate-dependent nature of ERP prolongation caused by IKr blockers in isolated guinea-pig atrial myocardium.
Keywords: Guinea-pig, atrium, dofetilide, metoprolol, propranolol, atenolol, catecholamine depletion, reserpine, rate-dependence, refractoriness
Introduction
The rapid and slow components of the delayed rectifier potassium currents (IKr and IKs, respectively) play an important role in the initiation of repolarisation and in determining the duration of action potential (APD), and effective refractory period (ERP) in mammalian myocardial cells (Noble & Tsien, 1969; Sanguinetti & Jurkiewicz, 1990; Varró et al., 1993; Iost et al., 1998). The majority of class III antiarrhythmic drugs inhibit IKr. It has been shown that the lengthening effect of IKr blockers on APD and ERP is more pronounced at slow than at rapid driving rates (Hondeghem et al., 1990). This phenomenon, known as reverse frequency-dependence, is undesirable since antiarrhythmic effect is most needed during tachyarrhythmias. According to the classic hypothesis, reverse rate-dependency is due to differences in the kinetics of IKr and IKs channels (Jurkiewicz & Sanguinetti, 1993). However, some recent experimental findings contradicted this theory (Groh et al., 1997; Gintant, 1998; Lengyel et al., 2001; Rocchetti et al., 2001). Thus, the precise mechanism of the reverse rate-dependent effect of IKr blockers has not yet been explained despite the great practical significance of understanding this phenomenon.
β-Adrenoceptor activation can counteract the electrophysiological effects of various antiarrhythmic drugs (Jazayeri et al., 1989; Sanguinetti et al., 1991). High doses of isoproterenol, a nonselective β-adrenoceptor agonist, fully reverted the effects of IKr blockers on APD in vitro (Newman et al., 1993, Martin et al., 1996; Marschang et al., 2000), and abolished the sematilide-induced prolongation of APD and myocardial refractoriness in humans in vivo (Sager et al., 1994). Thus, gradual increases in the stimulation frequency and the concomitant β-adrenoceptor activation due to neuronal noradrenaline release in the myocardium can proportionally shorten the prolongation of APD and ERP caused by IKr blockers. However, D,L-sotalol, a mixed β-adrenoceptor antagonist and IKr blocker caused a reverse frequency-dependent prolongation of ERP in right ventricular papillary muscle of rabbits and guinea-pigs in vitro (Berman & Dorian, 1993, Kovács et al., 2001). These results exclude the application of this hypothesis to the ventricular myocardium, but there are no data available on such effects of β-adrenoceptors in the atrial myocardium. Our aim was to study the role of β-adrenoceptor activation in the reverse frequency-dependency of ERP prolongation elicited by IKr blockers. Therefore, the effects of dofetilide on ERP were studied either in the absence and presence of β-adrenoceptor blockade or after catecholamine depletion in guinea-pig left atrial tissues stimulated at 3, 2 and 1 Hz, in vitro.
Methods
The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and was approved by the local Animal Ethics Committee.
Preparations
Male Dunkin–Hurtley guinea-pigs (400–600 g) and male New Zealand white rabbits (2.5–3.0 kg) were killed by cervical dislocation in pentobarbitone sodium anaesthesia and the heart was rapidly excised. The left atrium of guinea-pigs was dissected and mounted vertically in a 20 ml organ chamber filled with Krebs solution, containing (in mM) NaCl 118, KCl 4.6, NaHCO3 27.2, glucose 11.1, MgSO4 1.2, KH2PO4 1.0, CaCl2 2.6, pH 7.4 (Schuler organ bath, Hugo-Sachs, Hugstetten, Germany). The tissue was connected to a force transducer coupled to a recorder through a DC-bridge amplifier allowing the measurement of isometric tension. Immediately after the application of preload (500 mg), the preparations were stimulated (field stimulation) using rectangular current pulses of 1 ms duration and 1.3 times of diastolic threshold amplitude at the rate of 2 Hz. Resting tension was maintained during the 90 min equilibration period and the preparations were rinsed with fresh bathing solution every 20 min. The bathing solution was bubbled with carbogene (95% O2 plus 5% CO2) and the temperature was set to 35°C.
ERP was measured using a standard extrastimulus technique. Extrastimuli (S2) were interposed between normally paced stimuli (S1) in such a way that the S1–S2 interval was initially below the refractory period of the tissue. The S1–S2 interval was then lengthened gradually by 1 ms increment after every 10th normal stimulus until postextrasystolic potentiation (120% of the control twitch tension) was observed. ERP was defined as the smallest S1–S2 interval, which produced postextrasystolic potentiation.
The concentration- and frequency-dependent effects of dofetilide were studied at concentrations of 10, 30 and 100 nM. As the effect of dofetilide on ERP only slightly increased from 30 to 100 nM, the latter concentration was selected for further experiments. The concentration-dependent effects of β-adrenoceptor antagonists on the increase in twitch tension induced by isoproterenol (30 nM) were tested in separate experiments. The concentration of β-adrenoceptor antagonists selected for further studies inhibited the positive inotropic effect of isoproterenol by 84–92% in the atrial myocardium of guinea-pigs.
Experimental protocol
Control (baseline) ERP values were measured at 3, 2 and 1 Hz stimulation frequencies. The test compounds were applied and incubated for 30 min at 2 Hz. At the end of the incubation period, ERP values were measured at 3, 2 and 1 Hz stimulation rates. Measurement of ERP always commenced 3 min after changing stimulation frequency. When the effect of β-adrenoceptor blockade was examined, four groups of atrial tissues were studied (control, β-adrenoceptor antagonist, dofetilide and β-adrenoceptor antagonist+dofetilide). The effects of isoproterenol (10 nM) on the dofetilide-induced ERP prolongation were studied at three pacing rates. Isoproterenol was applied 3 min after changing stimulation frequency. ERP was measured close to the time of peak effect of isoproterenol (3 min) and then a 15-min interval was allowed for recovery. Catecholamine depletion was performed by pretreatment of the guinea-pigs with reserpine (5 mg kg−1 i.p.) 24 h before the experiment. The compound was dissolved in 10% ascorbic acid solution and injected in a volume of 1 ml kg−1 b.w.
Similar experimental techniques were applied when measuring ERP in the right ventricular papillary muscles of guinea-pigs or rabbits. The rabbit was also chosen for measuring the frequency-dependent effect of dofetilide on ERP in the ventricular tissue, since both IKr density and the ERP lengthening effect of IKr blockade were shown to be small in guinea-pig ventricular myocardium (Ohmoto-Sekine et al., 1999). The right ventricle was opened and the papillary muscles were dissected and mounted in the experimental chamber. Following a 120-min period of incubation, the experiments were performed in the same way as described for the guinea-pig atrial myocardium.
Drugs
Reserpine, metoprolol and dofetilide were synthesised at the Division of Chemical Research of EGIS Pharmaceuticals Ltd (Budapest, Hungary). Propranolol and atenolol were purchased from Research Biochemicals International (Natick, MA, U.S.A.) and Sigma Chemical Co. (St Louis, MO, U.S.A.), respectively, and isoproterenol was obtained from Fluka Chemie AG. (Buchs, Switzerland).
Statistics
Data are presented as mean±s.e.m. values. Statistical analysis of the results involved Newman–Keuls post hoc test after checking the homogeneity of variances with two-way ANOVA for repeated measures (Statistica Software Package, Statsoft, Tulsa, U.S.A.).
Results
All baseline ERP values obtained in the guinea-pig atrial myocardium in the control, propranolol-, dofetilide- and dofetilide+propranolol-treated groups were pooled for untreated and reserpinised animals. In untreated tissues, ERP was short and showed reverse frequency-dependence (Table 1). The frequency-dependent changes in ERP were small but statistically significant. In contrast to these untreated preparations, ERP values were not frequency-dependent in atrial tissues obtained from reserpine-treated animals. Thus, reverse frequency-dependence of the baseline ERP was eliminated by catecholamine depletion.
Table 1.
Effect of stimulation frequency on the effective refractory period in left atrial myocardium of untreated and reserpinised guinea-pigs
| Treatment | ERP (ms) | ||
|---|---|---|---|
| 3Hz | 2Hz | 1Hz | |
| Untreated (n=39) | 67.4±0.7 | 70.7±0.8*** | 72.0±1.0*** |
| Reserpinised (n=35) | 73.2±1.5°°° | 72.9±1.6° | 72.9±1.7 |
All ERP values, measured before drug treatment in the control-, propranolol-, dofetilide- and dofetilide+propranolol-treated groups, were combined for both untreated and reserpinised guinea-pigs. Values are means±s.e.m., n indicates the number of experiments. Data were evaluated using two-way ANOVA for repeated measures (pacing rate), followed by Newman–Keuls post hoc test (***P<0.001 vs 3 Hz in the same group, °P<0.01 °°° P<0.001 vs untreated tissues at the same frequency).
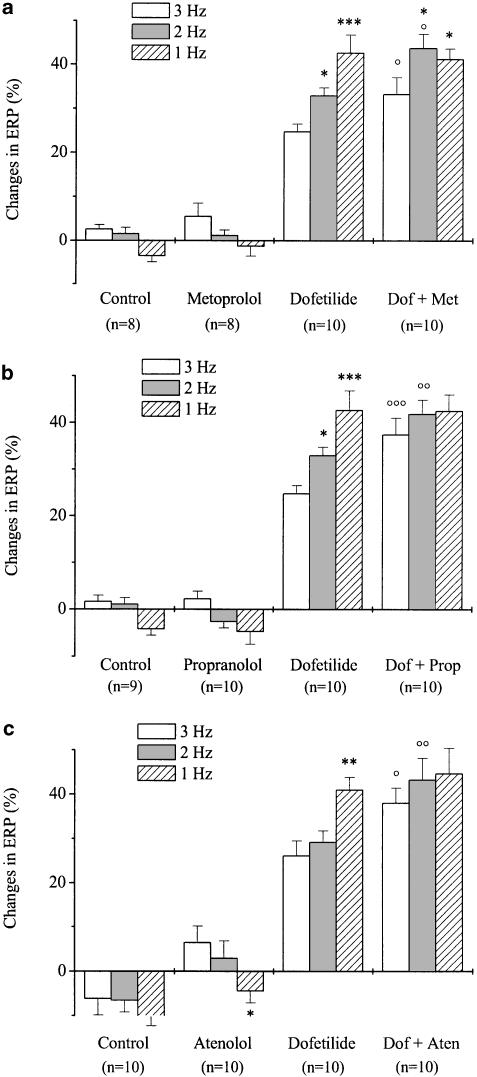
The effects of dofetilide on ERP showed reverse frequency-dependent properties (Figure 1a–c). Metoprolol, atenolol and propranolol did not influence ERP at any driving rate (even though ERP values were different between 1 and 3 Hz in the group treated with atenolol). Metoprolol (1 μM) increased the dofetilide-induced prolongation of ERP at high (3 and 2 Hz), but not at low (1 Hz) stimulation frequency (Figure 1a), that is, the reverse frequency-dependent character of the dofetilide-induced ERP prolongation was attenuated by metoprolol. Similar results were obtained when other β-receptor blockers, like propranolol (300 nM, Figure 1b) or atenolol (3 μM, Figure 1c), were applied. In these experiments, pretreatment with either propranolol or atenolol completely abolished the reverse frequency-dependent nature of the dofetilide-induced lengthening of ERP, while this effect was only partial with metoprolol.
Figure 1.
Frequency-dependent effects of dofetilide in the presence of metoprolol (a), propranolol (b) or atenolol (c) on the effective refractory period in guinea-pig left atrial myocardium. After measuring baseline values, separate groups of preparations were treated with either metoprolol (1 μM), dofetilide (100 nM), dofetilide+metoprolol (Dof+Met) or were used as control in (a). In panels (b and c) propranolol (300 nM) or atenolol (3 μM) was used, respectively. The incubation period lasted for 30 min. Values are means±s.e.m., n indicates the number of experiments. Data are expressed as percentage changes in comparison with the baseline values (*P<0.05, ***P<0.001 vs 3 Hz in the same group; °P<0.05, °°P<0.01, °°°P<0.001 vs dofetilide at the same frequency).
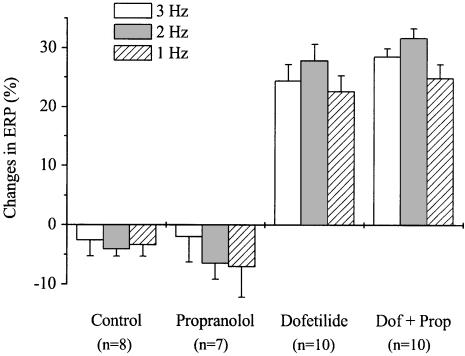
The frequency-dependent effects of dofetilide, with or without pretreatment with propranolol, were also studied in guinea-pig atrial preparations obtained from animals, having their noradrenaline pools depleted by pretreatment with reserpine. In these reserpinised tissues, dofetilide (100 nM) caused 24.4, 27.9 and 22.7% increases in ERP at 3, 2 and 1 Hz, respectively, which were statistically not different from each other (Figure 2). Thus, the dofetilide-induced ERP prolongation was independent of stimulation frequency, and propranolol (300 nM) failed to alter the dofetilide-induced changes in ERP in reserpinised atria. The effects of dofetilide on ERP were directly compared in untreated and reserpinised tissues in Table 2. The ERP lengthening effect of dofetilide was not different in the two groups of preparations at 3 Hz, it was moderately reduced at 2 Hz in the reserpine-treated hearts, while at 1 Hz the prolongation of ERP was smaller in atria of reserpinised animals than in untreated ones.
Figure 2.
Frequency-dependent effects of dofetilide and propranolol on the effective refractory period in atria obtained from reserpinised guinea-pigs. Reserpine (5 mg kg−1 i.p.) was administered 24 h before the experiments. After measuring baseline values, separate groups of preparations were treated with propranolol (300 nM), dofetilide (100 nM), dofetilide+propranolol (Dof+Prop) or were used as control. Each drug was applied for 30 min. Values are means±s.e.m., n indicates the number of experiments. Data are expressed as percentage changes in comparison with the baseline values. No significant changes were observed between the dofetilide- and the dofetilide+propranolol-treated groups.
Table 2.
Effects of catecholamine depletion and isoproterenol on the dofetilide-induced lengthening of ERP in guinea-pig left atrial tissues
| Treatment | ERP prolongation (%) | ||
|---|---|---|---|
| 3Hz | 2Hz | 1Hz | |
| Untreated (n=10) | 24.7±1.8 | 32.9±1.8* | 42.6±4.2*** |
| Reserpinised (n=10) | 24.4±2.8 | 27.9±2.8 | 22.7±2.6°°° |
| Isoproterenol (n=10) | 3.3±4.3°°° | 3.8±5.9°°° | 3.8±6.6°°° |
Reserpine (5 mg kg−1 i.p.) was administered 24 h before the experiments. Isoproterenol (10 nM) was repeatedly applied with a 15-min interval between subsequent measurements in left atrial myocardium of untreated guinea-pigs. Values are means±s.e.m., n indicates the number of experiments. Data were evaluated with two-way ANOVA for repeated measures (pacing rate), followed by Newman–Keuls post hoc test (*P<0.05, ***P<0.001 vs 3 Hz in the same group, P<0.001 vs untreated tissues at the same frequency).
Application of isoproterenol (10 nM), a full activator of β-adrenoceptors, eliminated the dofetilide-evoked prolongation of ERP at all pacing rates (Table 2), demonstrating that the effect of an IKr blocker on ERP is critically dependent on the intensity of β-adrenoceptor activation.
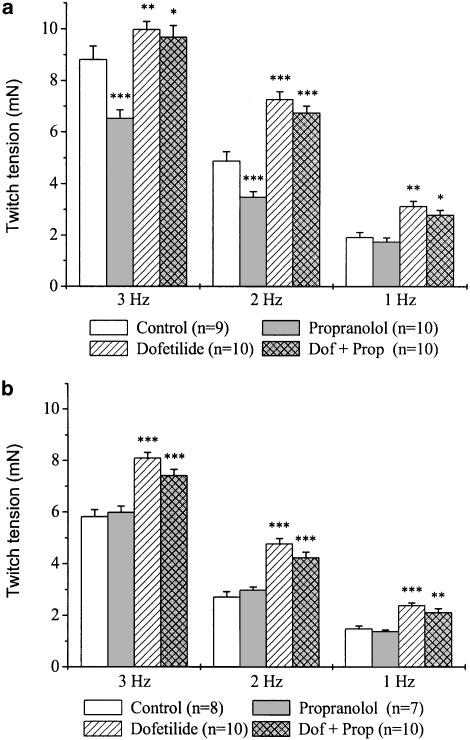
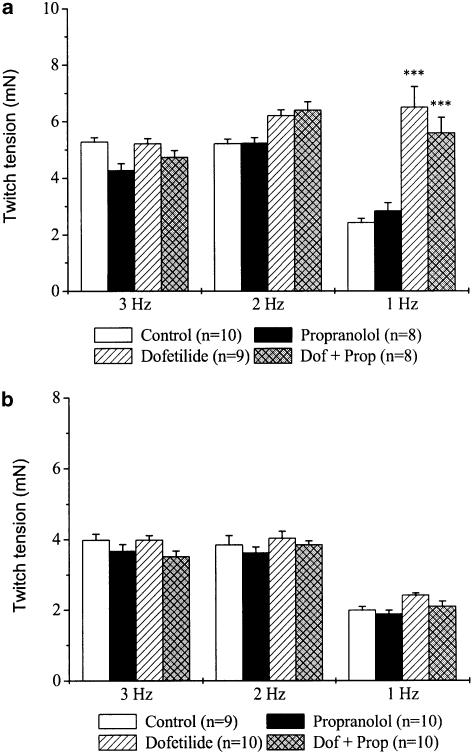
In order to study the β-adrenoceptor-mediated functional effects of rate-dependent catecholamine liberation in the atrial myocardium, the effects of propranolol on contractility in untreated and reserpinised tissues were also measured (Figure 3). In untreated preparations, propranolol induced a marked reduction in twitch tension at 3 Hz, it produced a moderate decrease at 2 Hz, but failed to alter contractility at 1 Hz pacing rate compared with values measured in the control group at the same frequencies (Figure 3a). On the other hand, in atria with depleted noradrenaline pools, propranolol did not change twitch tension at either stimulation frequency (Figure 3b). Compared with values measured in control tissues, dofetilide increased twitch tension at all three pacing rates in both untreated and reserpinised atria. Interestingly, this effect was not much affected by the presence of propranolol.
Figure 3.
Frequency-dependent effects of dofetilide and propranolol on twitch tension in left atria obtained from untreated (a) and reserpinised (b) guinea-pigs. Reserpine (5 mg kg−1 i.p.) was administered 24 h before the experiments. After measuring baseline values, separate groups of preparations were treated with propranolol (300 nM), dofetilide (100 nM), dofetilide+propranolol (Dof+Prop) or were used as control. Each incubation period lasted for 30 min. Values are means±s.e.m., n indicates the number of experiments. (*P<0.05, **P<0.01, ***P<0.001 vs control at the same frequency).
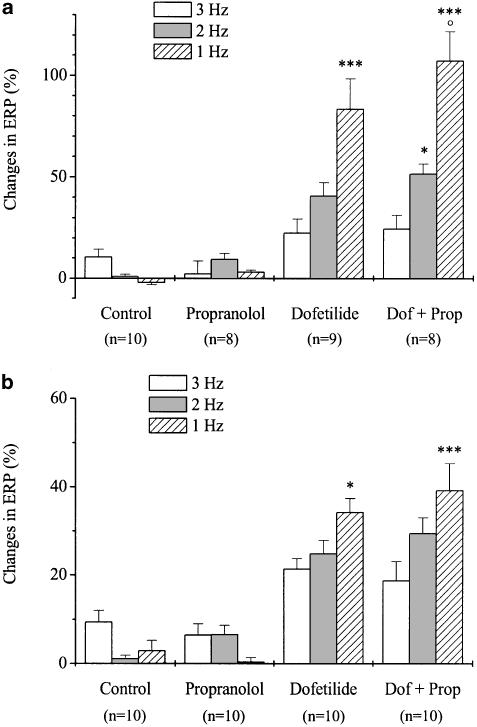
All experiments presented above were performed in the left atrial myocardium of the guinea-pig. For studying the role of β-adrenoceptor activation in the reverse rate dependency of ERP prolongation elicited by dofetilide in ventricular myocardium, the frequency-dependent effects of dofetilide on ERP were also evaluated in the right ventricular papillary muscles of guinea-pigs and rabbits. Dofetilide (100 nM) prolonged ERP in a reverse rate-dependent manner in papillary muscle preparations obtained from both rabbits and guinea-pigs; however, its effect on ERP was much larger in the former than in the latter species (Figure 4a–b). The frequency-dependence of the dofetilide-induced ERP response was not influenced by propranolol in either species. In contrast to results obtained in guinea-pig atrial myocardium, propranolol increased the effect of dofetilide on ERP at 1 Hz but not at other stimulation rates in rabbit papillary muscles, while it did not change the effect of dofetilide in guinea-pig papillary muscle prepa-rations.
Figure 4.
Frequency-dependent effects of dofetilide and propranolol on the effective refractory period in the right ventricular papillary muscles of rabbits (a) and guinea-pigs (b). After measuring baseline values, separate groups of preparations were treated with pro-pranolol (300 nM), dofetilide (100 nM), dofetilide+propranolol (Dof+Prop) or were used as control. Each incubation period lasted for 30 min. Values are means±s.e.m., n indicates the number of experiments. Data are expressed as percentage changes in comparison with the baseline values (*P<0.05, ***P<0.001 vs 3 Hz in the same group. °P<0.05 vs dofetilide at the same frequency).
The level of the rate-dependent β-adrenoceptor activation was smaller in ventricular myocardium than in atrial tissues, as propranolol failed to decrease twitch tension in papillary muscles of either species at any stimulation rate (Figure 5a–b). Dofetilide and dofetilide+propranolol augmented twitch tension in the rabbit ventricle, at 1 Hz only. The inotropic effect of dofetilide was much larger in the ventricular myocardium of rabbits than in guinea-pigs, similarly to its effects on ERP between the two species.
Figure 5.
Frequency-dependent effects of dofetilide and propranolol on twitch tension in right ventricular papillary muscles of rabbits (a) and guinea-pigs (b). After measuring baseline values, separate groups of tissues were treated with propranolol (300 nM), dofetilide (100 nM), dofetilide+propranolol (Dof+Prop) or were used as control. Each incubation period lasted for 30 min. Values are means±s.e.m., n indicates the number of experiments. Asterisks (***P<0.001) denote significant differences vs control at the same frequency.
Discussion
In this study, dofetilide, one of the most selective IKr blockers known, was chosen to examine a feasible mechanism of the phenomenon called reverse frequency-dependence. Dofetilide evoked a reverse frequency-dependent prolongation of ERP in the guinea-pig left atrial myocardium. These results are in accordance with earlier results obtained in atrial and ventricular preparations of various species (Martin et al., 1996; Lee et al., 1998; Marschang et al., 1998; Li et al., 2001). The dofetilide-induced lengthening of ERP was augmented by β-adrenoceptor blockade (metoprolol, atenolol and propranolol) in case of high-frequency stimulation, whereas application of β-receptor blockers alone failed to modify ERP substantially. The modulatory effects of β-adrenoceptor blockers on the dofetilide-induced lengthening of ERP were not observed in catecholamine-depleted animals.
The most important finding in this study was that full activation of the β-adrenergic pathway (by isoproterenol) and its suppression (by β-adrenoceptor blockers or reser-pine pretreatment) abolished the dofetilide-induced ERP prolongation or eliminated its reverse rate-dependent character in the guinea-pig atrial myocardium. Therefore, our observations suggest that the rate-dependent release of endogenous catecholamines and the concomitant β-adrenoceptor activation play an important role in the development of reverse rate-dependency of the dofetilide-induced prolongation of ERP in this preparation. In contrast, the modulatory action of β-adrenoceptor stimulation was not observed in the ventricular myocardium of rabbits and guinea-pigs.
It is obvious and well documented that noradrenaline release increased proportionally with the rate of electrical stimulation in various cardiac preparations (Junstad & Wennmalm, 1973; Johnston & Majewski, 1986; Foucart & Majewski, 1989; Lamontagne et al., 1990; Murphy et al., 1992; Molderings et al., 2000). Under the conditions of the present experiments, a functional consequence of rate-dependent noradrenaline liberation was that propranolol decreased twitch tension at 3 and 2 Hz but not at 1 Hz in atrial myocardium of untreated animals. As it was expected, propranolol did not alter contractility in catecholamine-depleted preparations. These observations clearly show that the level of β-adrenoceptor activation was high enough to increase contractility and to reduce the dofetilide-induced ERP lengthening at high rates of electrical stimulation in atrial myocardium. On the other hand, propranolol did not change contractility in the papillary muscles of either species indicating that the level of noradrenaline release was insufficient to induce detectable functional changes up to 3 Hz in ventricular myocardium. Thus, in various parts of the heart reverse rate-dependency of APD and ERP prolongation by IKr blockers may be the consequence of diverse mechanisms. The reason for this inconsistency is unclear at present; however, it can likely be attributed to differences in the composition, kinetics and regulation of ionic currents governing repolarisation in atrial and ventricular myocardium (Hume & Uehara, 1985; Hafner et al., 1988; Baskin & Lynch, 1998).
β-Adrenoceptor stimulation may enhance a great variety of ion currents and pumps in cardiac cells, including the inward sodium current (Matsuda et al., 1992), the delayed rectifier potassium current (Hoffmann & Singer, 1967), the Na–K pump current (Boyett & Fedida, 1984), the transient outward potassium current (Nakayama & Fozzard, 1988), the chloride current (Harvey & Hume, 1989), the slow calcium current (Reuter, 1983) and the pacemaker current (Di Francesco, 1985). It has been clearly demonstrated that IKr current was also increased by β-adrenocep-tor stimulation in guinea-pig ventricular myocytes, an effect mediated both by protein kinase A and protein kinase C and involved calcium entry into the cells (Heath & Terrar, 2000). IKr current was less sensitive to blockade by E-4031, a pure IKr blocker, in myocytes treated with isoproterenol than in control myocytes. It was also demonstrated that IKr currents in both the humans and guinea-pigs were suppressed (having their activation curve shifted towards positive voltages) by a cAMP- and protein kinase A-dependent pathway that was due to phosphorylation of the channel protein (Kiehn et al., 1998; Thomas et al., 1999). The reduced IKr current and decreased sensitivity of IKr current to blockade by drugs after increasing intracellular cAMP levels, demonstrated in these studies, are compatible with our findings. Since all previous results on this issue were obtained in ventricular myocytes and the dofetilide-induced reverse frequency-dependent ERP prolongation was diminished by β-adrenoceptor blockade only in atrial but not ventricular myocardium in our study, to explain the precise mechanisms by which β-adrenoceptor activation attenuated the effects of dofetilide on ERP at high pacing rate in the atrium remains to be elucidated.
Dofetilide induced smaller changes in ERP at all stimulation rates in reserpinised preparations than in untreated ones exposed or not to β-adrenoceptor blockers. The effects of noradrenaline on myocardial α-adrenoceptors may be an important factor in these seemingly contradictory findings. Activation of α-adrenoceptors prolonged APD in rabbit and human atrium by inhibiting Ito (Fedida et al., 1989; 2000), reducing Ito and IK1 in canine ventricular myocytes (Wang et al., 2001), decreasing IKur, in canine and human atrial myocardium (Li et al., 1996; Yue et al., 1999) and also by suppressing a repolarising chloride current (Oleksa et al., 1996; Hool & Harvey, 1997). When any repolarising current is reduced, the relative contribution of other currents to repolarisation may increase (Biliczki et al., 2002). Therefore, the effect of dofetilide on ERP can be more pronounced after β-adrenoceptor blockade than after catecholamine depletion. However, it is not known which currents were involved in such an effect in the guinea-pig atrial myocardium in our study. The release of acetylcholine from the cholinergic nerve terminals can be inhibited by noradrenaline via activation of prejunctional α2-adrenoceptors in guinea-pig atria (Loiacono et al., 1986). Acetylcholine can reduce the effects of selective IKr blockers on APD and ERP, and this action is more pronounced in noradrenaline-depleted preparations than in control ones (Zaza et al., 1995). In addition, α1-adrenoceptor activation is known to desensitise acetylcholine-activated potassium currents (IK,Ach) that cannot occur in preparations obtained from reserpine-pretreated guinea-pigs (Cho et al., 2001). Another important factor involved could be cardiac remodelling due to exposition of the heart to high catecholamine concentrations after reserpine treatment (Ramirez et al., 2000; Wichter et al., 2000). The combination of some or all of the above effects may have brought about adjustments in several ion currents that resulted in a diminished lengthening of ERP by dofeti-lide in catecholamine-depleted atrial myocardium of the guinea-pig.
ERP is a more reliable parameter for the assessment of therapeutic utility of antiarrhythmic agents than APD, because it reflects functional alterations of electrical excitability of the myocardium. Although ERP and APD can change disproportionally (Pankucsi et al., 1997; Watanabe et al., 2001), changes in ERP and APD are strictly proportional for atrial and ventricular myocardium in the case of selective IKr blockers (Yang et al., 1992a, 1992b; Ishii et al., 1995; Marschang et al., 1998). Our results were based on measurements of ERP detected as changes in myocardial contractility, which is a proper method that has been used previously by several investigators (Gwilt et al., 1991; Lynch et al., 1995; Baskin & Lynch, 1998; Kovács et al., 2001; Li et al., 2001; 2002). Baseline ERP values were within the range obtained in electrophysiological measurements in the guinea-pig atrial myocardium (Ishii et al., 1995; Raatikainen et al., 2000; Li et al., 2001).
We have shown that in the presence of propranolol and atenolol or after catecholamine depletion, the dofetilide-evoked ERP prolongation turned to be frequency independent in guinea-pig left atrial preparations. Our results are in agreement with the findings of some previous studies in which the authors did not aim to reveal the role of β-adrenoceptors in the development of reverse frequency-dependent effects of IKr blockers. In anaesthetised and vagotomised newborn dogs pretreated with propranolol, both dofetilide and ibutilide evoked reverse rate-dependent monophasic APD prolongation in the ventricle, but their effect was independent of the stimulation rate in the atrium (Pickoff & Stolfi, 2001). Ishii et al. (1995) found that in isolated left atrial preparations obtained from reserpinised guinea-pigs, the effect of sematilide on APD90 was independent of the pacing frequency. Nifecalant, a pure IKr blocker, prolonged atrial monophasic APD and ERP in a reverse rate-dependent manner, whereas absolute changes in ERP did not depend on the pacing rate after treatment with D,L-sotalol in a human electrophysio-logical study in vivo (Watanabe et al., 2001). These results together strongly suggest that the interaction between β-adrenoceptor stimulation and IKr blockade is likely a general regulatory mechanism in the mammalian atrium, and it is not a specific feature of the guinea-pig. However, according to our knowledge, we were the first to demonstrate that both β-adrenoceptor antagonists and catecholamine depletion attenuated the reverse frequency-dependent character of the dofetilide-evoked ERP prolongation in a mammalian atrial tissue.
In summary, β-adrenoceptor inhibition or catecholamine depletion attenuated or fully eliminated the reverse frequency-dependency of the dofetilide-induced prolongation of the effective refractory period in the left atrial myocardium of guinea-pigs. These results suggest that rate-dependent noradrenaline release and ensuing β-adrenoceptor activation can explain, at least partly, the development of the reverse frequency-dependent nature of the effects of IKr blockers in the mammalian atrium. However, no such mechanism was detected in the ventricular tissue of guinea-pigs and rabbits.
Acknowledgments
We thank Ms Éva Árvai for her valuable technical assistance.
Abbreviations
- APD
action potential duration
- ERP
effective refractory period
- IKr
rapid component of the delayed rectifier potassium current
- IKs
slow component of the delayed rectifier potassium current
- IKur
ultrarapid component of the delayed rectifier potassium current
- Ito
transient outward potassium current
- IK1
inward rectifier potassium current
- If
hyperpolarization-activated current
References
- BASKIN E.P., LYNCH J.J. Differential atrial versus ventricular activities of class III potassium channel blockers. J. Pharmacol. Exp. Ther. 1998;285:135–142. [PubMed] [Google Scholar]
- BERMAN N.D., DORIAN P. Cellular mechanism underlying the efficacy of the sotalol-quinidine combination. J. Cardiovasc. Pharmacol. 1993;21:609–614. doi: 10.1097/00005344-199304000-00015. [DOI] [PubMed] [Google Scholar]
- BILICZKI P., VIRAG L., IOST N., PAPP J.G., VARRO A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br. J. Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYETT N.R., FEDIDA D. Changes in the electrical activity of the dog Purkinje fibers at high heart rates. J. Physiol. (London) 1984;350:361–391. doi: 10.1113/jphysiol.1984.sp015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO H., NAM G.B., LEE S.H., EARM Y.E., HO W.K. Phosphatidylinositol 4,5-bisphosphate is acting as a signal molecule in α1-adrenergic pathway via the modulation of acetylcholine-activated K+ channels in mouse atrial myocytes. J. Biol. Chem. 2001;276:159–164. doi: 10.1074/jbc.M004826200. [DOI] [PubMed] [Google Scholar]
- DIFRANCESCO D. The cardiac hyperpolarizing-activated current, If: origins and developments. Prog. Biophys. Mol. Biol. 1985;46:163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- FEDIDA D., SHIMONI Y., GILES W.R. Alpha-adrenergic modulation of the transient outward current in rabbit atrial myocytes. J Physiol. (London) 2000;423:257–277. doi: 10.1113/jphysiol.1990.sp018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEDIDA D., SHIMONI Y., GILES W.R. A novel effect of noradrenaline on cardiac cells is mediated by alpha 1-adrenoceptors. Am. J. Physiol. 1989;256:H1500–H1504. doi: 10.1152/ajpheart.1989.256.5.H1500. [DOI] [PubMed] [Google Scholar]
- FOUCART S., MAJEWSKI H. Inhibition of noradrenaline release by neuropeptide Y in mouse atria does not involve inhibition of adenylate cyclase or a pertussis toxin-susceptible G protein. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;340:658–665. doi: 10.1007/BF00717741. [DOI] [PubMed] [Google Scholar]
- GINTANT G.A. Azimilide causes reverse rate-dependent block while reducing both components of cardiac delayed rectifier current in canine ventricular myocytes. J. Cardiovasc. Pharmacol. 1998;31:945–953. doi: 10.1097/00005344-199806000-00020. [DOI] [PubMed] [Google Scholar]
- GROH W.J., GIBSON K.J., MAYLIE J.G. Comparison of the rate-dependent properties of the class III antiarrhythmic agents azimilide (NE-10064) and E-4031: considerations on the mechanism of reverse rate-dependent action potential prolongation. J. Cardiovasc. Electrophysiol. 1997;8:529–536. doi: 10.1111/j.1540-8167.1997.tb00821.x. [DOI] [PubMed] [Google Scholar]
- GWILT M., WILLIAMS R.C., HIGGINS A.J. Effects of action potential prolongation via different cellular mechanisms on the electrophysiological properties of rat and guinea-pig ventricular myocardium. Arch. Int. Pharmacodyn. Ther. 1991;312:66–78. [PubMed] [Google Scholar]
- HAFNER D., BERGER F., BORCHARD A., KULLMANN A., SCHERLITZ A. Electrophysiological characterisation of the class III activity of sotalol and its enantiomers. New interpretation of use-dependent effects. Arzneim.-Forsch. Drug Res. 1988;38:231–237. [PubMed] [Google Scholar]
- HARVEY R.D., HUME J.R. Isoproterenol activates a chloride current, not the transient outward current, in rabbit ventricular myocytes. Am. J. Physiol. 1989;253:C1177–C1181. doi: 10.1152/ajpcell.1989.257.6.C1177. [DOI] [PubMed] [Google Scholar]
- HEATH B.M., TERRAR D.A. Protein kinase C enhances rapidly activating delayed rectifier potassium current IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J. Physiol. (London) 2000;522:391–402. doi: 10.1111/j.1469-7793.2000.t01-2-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMANN B.F., SINGER D. Appraisal the effects of catecholamines on cardiac electrical activity. Ann. NY Acad. Sci. 1967;193:914–924. doi: 10.1111/j.1749-6632.1967.tb41261.x. [DOI] [PubMed] [Google Scholar]
- HONDEGHEM L.M., SNYDERS D.J. Class III antiarrhythmic agents have a lot of potential but a long way to go: reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- HOOL L.C., HARVEY R.D. Role of beta1- and beta2-adrenergic receptors in regulation of Cl− and Ca2+ channels in guinea pig ventricular myocytes. Am. J. Physiol. 1997;273:H1669–H1676. doi: 10.1152/ajpheart.1997.273.4.H1669. [DOI] [PubMed] [Google Scholar]
- HUME J.R., UEHARA A. Ionic basis of the different action potential configurations of single guinea pig atrial and ventricular myocytes. J. Physiol. (London) 1985;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOST N., VIRÁG L., OPANCARIU M., VARRÓ A., PAPP J.GY. Delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc. Res. 1998;40:508–515. doi: 10.1016/s0008-6363(98)00204-1. [DOI] [PubMed] [Google Scholar]
- ISHII Y., MURAKI K., KURIHARA A., IMAIZUMI Y., WATANABE M. Effects of sematilide, a novel class III antiarrhythmic agent, on action potential in guinea pig atrium. Jpn. J. Pharmacol. 1995;68:175–182. doi: 10.1254/jjp.68.175. [DOI] [PubMed] [Google Scholar]
- JAZAYERI M.R., VAN WYHE G., AVITALL B., MCKINNIE J., TCHOU P., AKHTAR M. Isoproterenol reversal of antiarrhythmic effects in patients with inducible sustained ventricular tachyarrhythmias. J. Am. Coll. Cardiol. 1989;14:705–711. doi: 10.1016/0735-1097(89)90114-9. [DOI] [PubMed] [Google Scholar]
- JOHNSTON H., MAJEWSKI H. Prejunctional beta-adrenoceptors in rabbit pulmonary artery and mouse atria: effect of alpha-adrenoceptor blockade and phosphodiesterase inhibition. Br. J. Pharmacol. 1986;87:553–562. doi: 10.1111/j.1476-5381.1986.tb10197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNSTAD M., WENNMALM A. Prostaglandin mediated inhibition of noradrenaline release at different nerve impulses. Acta Physiol. Scand. 1973;89:544–549. doi: 10.1111/j.1748-1716.1973.tb05548.x. [DOI] [PubMed] [Google Scholar]
- JURKIEWICZ N.K., SANGUINETTI M.C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating cardiac delayed rectifier K+ current by dofetilide. Circ. Res. 1993;71:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- KIEHN J., KARLE C., THOMAS D., YAO X., BRACHMANN J., KUBLER W. HERG potassium channel activation is shifted by phorbol esters via protein kinase A-dependent pathways. J. Biol. Chem. 1998;273:25285–25291. doi: 10.1074/jbc.273.39.25285. [DOI] [PubMed] [Google Scholar]
- KOVÁCS A., GYÖNÖS I., MAGYAR J., BÁNYÁSZ T., NÁNÁSI P.P., SPEDDING M., SZÉNÁSI G. Effects of EGIS-7229 (S 21407), a novel class III antiarrhythmic drug, on myocardial refractoriness to electrical stimulation in vivo and in vitro. J. Cardiovasc. Pharmacol. 2001;37:78–88. doi: 10.1097/00005344-200101000-00009. [DOI] [PubMed] [Google Scholar]
- LAMONTAGNE D., YAMAGUCHI N., LAMBERT C., DE CHAMPLAIN J., NADEAU R. Absence of prejunctional sympathetic effect of amiodarone in hearts of open-chest anesthetized dogs. Can. J. Physiol. Pharmacol. 1990;68:144–149. doi: 10.1139/y90-023. [DOI] [PubMed] [Google Scholar]
- LEE K., LEE J.Y., KIM H.Y., KWON L.S., SHIN H.S., TANABE S., KOZONO T., PARK S.D., CHUNG Y.S. KCB-328: a novel class III antiarrhythmic agent with little reverse frequency dependence in isolated guinea pig myocardium. J. Cardiovasc. Pharmacol. 1998;31:609–617. doi: 10.1097/00005344-199804000-00021. [DOI] [PubMed] [Google Scholar]
- LENGYEL C., IOST N., VIRÁG L., VARRÓ A., LATHROP D.A., PAPP J.G.Y. Pharmacological block of the slow component of the outward delayed rectifier current (IKr) fails to lengthen rabbit ventricular muscle QTc and action potential duration. Br. J. Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G.R., FENG J., WANG Z., FERMINI B. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ. Res. 1996;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- LI H.T., WANG Y.Q., DAI D.Z., KONG R.Z. Frequency dependent prolongation of effective refractory period by a complex class III antiarrhythmic agent CPU-86017. Acta Pharmacol. Sin. 2001;22:32–36. [PubMed] [Google Scholar]
- LIU H., JI M., LUO X., SHEN J., HUANG X., HUA W., JIANG H., CHEN K. New p-methylsulfonamido phenylethylamine analogues as class III antiarrhythmic agents: design, synthesis, biological assay, and 3D-QSAR analysis. J. Med. Chem. 2002;45:2953–2969. doi: 10.1021/jm010574u. [DOI] [PubMed] [Google Scholar]
- LOIACONO R.E., STORY D.F. Effect of alpha-adrenoceptor agonists and antagonists on cholinergic transmission in guinea-pig isolated atria. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;334:40–47. doi: 10.1007/BF00498738. [DOI] [PubMed] [Google Scholar]
- LYNCH JR J.J., BASKIN E.P., NUTT E.M., GUINOSSO JR P.J., HAMILL T., SALATA J.J., WOODS C.M. Comparison of binding to rapidly activating delayed rectifier K+ channel, IKr, and effects on myocardial refractoriness for class III antiarrhythmic agents. J. Cardiovasc. Pharmacol. 1995;25:336–340. doi: 10.1097/00005344-199502000-00021. [DOI] [PubMed] [Google Scholar]
- MARSCHANG H., BRACHMANN J., KAROLYI L., KÜBLER W., SCHÖLS W. Isoproterenol specifically modulates reverse rate-dependent effects of D,L-sotalol, D-sotalol, and dofetilide. J. Cardiovasc. Pharmacol. 2000;35:443–450. doi: 10.1097/00005344-200003000-00015. [DOI] [PubMed] [Google Scholar]
- MARSCHANG H., BEYER T., KAROLYI L., KÜBLER W., BRACHMANN J. Differential rate and potassium-dependent effects of the class III agents D-sotalol and dofetilide on guinea pig papillary muscle. Cardiovasc. Drugs Ther. 1998;12:573–583. doi: 10.1023/a:1007743521932. [DOI] [PubMed] [Google Scholar]
- MARTIN C.L., PALOMO M.A., MCMAHON E.G. Comparison of bidisomide, flecainide and dofetilide on action potential duration in isolated canine atria: effect of isoproterenol. J. Pharmacol. Exp. Ther. 1996;278:154–162. [PubMed] [Google Scholar]
- MATSUDA J.J., LEE H., SHIBATA E.F. Enhancement of rabbit cardiac sodium channels by β-adrenergic stimulation. Circ. Res. 1992;70:199–207. doi: 10.1161/01.res.70.1.199. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., LIKUNGU J., GOTHERT M. N-type calcium channels control sympathetic neurotransmission in human heart atrium. Circulation. 2000;101:403–407. doi: 10.1161/01.cir.101.4.403. [DOI] [PubMed] [Google Scholar]
- MURPHY T.V., FOUCART S., MAJEWSKI H. Prejunctional alpha 2-adrenoceptors in mouse atria function through G-proteins which are sensitive to N-ethylmaleimide, but not pertussis toxin. Br. J. Pharmacol. 1992;106:871–876. doi: 10.1111/j.1476-5381.1992.tb14427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAYAMA T., FOZZARD H.A. Adrenergic modulation of the transient outward current, in rabbit ventricular myocytes. Circ. Res. 1988;62:162–172. doi: 10.1161/01.res.62.1.162. [DOI] [PubMed] [Google Scholar]
- NEWMAN D., DORIAN P., FEDER-ELITUV R. Isoproterenol antagonises drug-induced prolongation of action potential duration in humans. Can. J. Physiol. Pharmacol. 1993;71:755–760. doi: 10.1139/y93-113. [DOI] [PubMed] [Google Scholar]
- NOBLE D., TSIEN R.W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J. Physiol. (London) 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMOTO-SEKINE Y., UEMURA H., TAMAGAWA M., NAKAYA H. Inhibitory effects of aprindine on the delayed rectifier K+ current and the muscarinic acetylcholine receptor-operated K+ current in guinea-pig atrial cells. Br. J. Pharmacol. 1999;126:751–761. doi: 10.1038/sj.bjp.0702334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLEKSA L.M., HOOL L.C., HARVEY R.D. Alpha 1-adrenergic inhibition of the beta-adrenergically activated Cl− current in guinea pig ventricular myocytes. Circ. Res. 1996;78:1090–1099. doi: 10.1161/01.res.78.6.1090. [DOI] [PubMed] [Google Scholar]
- PANKUCSI C., BÁNYÁSZ T., MAGYAR J., GYÖNÖS I., KOVÁCS A., SZÉNÁSI G., VARRÓ A., NÁNÁSI P.P. Electrophysiological effects of EGIS-7229, a new antiarrhythmic agent, in isolated guinea pig papillary muscle. Gen. Pharmacol. 1997;29:275–280. doi: 10.1016/s0306-3623(96)00461-2. [DOI] [PubMed] [Google Scholar]
- PICKOFF A.S., STOLFI A. Comparison of the rate dependent effects of dofetilide and ibutilide in the newborn heart. Pacing Clin. Electrophysiol. 2001;24:816–823. doi: 10.1046/j.1460-9592.2001.00816.x. [DOI] [PubMed] [Google Scholar]
- RAATIKAINEN M.J., MOREY T.E., DRUZGALA P., MILNER P., GONZALEZ M.D., DENNIS D.M. Potent and reversible effects of ATI-2001 on atrial and atrioventricular nodal electrophysiological properties in guinea pig isolated perfused heart. J. Pharmacol. Exp. Ther. 2000;295:779–785. [PubMed] [Google Scholar]
- RAMIREZ R.J., NATTEL S., COURTEMANCHE M. Mathematical analysis of canine atrial action potentials: rate, regional factors, and electrical remodelling. Am. J. Physiol. 2000;279:H1767–H1785. doi: 10.1152/ajpheart.2000.279.4.H1767. [DOI] [PubMed] [Google Scholar]
- REUTER H. Calcium channel modulation by neurotransmitter, enzymes and drugs. Nature. 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- ROCCHETTI M., BESANA L., GURROLA G.B., POSSANI L.D., ZAZA A. Rate dependency of delayed rectifier currents during the guinea pig ventricular action potential. J. Physiol. (London) 2001;534:721–732. doi: 10.1111/j.1469-7793.2001.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER P.T., FOLLMER C., UPPAL P., PRUITT C., GODFREY R. The effects of β-adrenergic stimulation on the frequency-dependent electrophysiologic actions of amiodarone and sematilide in humans. Circulation. 1994;90:1811–1819. doi: 10.1161/01.cir.90.4.1811. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWITZ N.K., SCOTT A., SIEGL P.K.S. Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circ. Res. 1991;68:77–84. doi: 10.1161/01.res.68.1.77. [DOI] [PubMed] [Google Scholar]
- THOMAS D., ZHANG W., KARLE C.A., KATHOFER S., SCHOLS W., KUBLER W., KIEHN J. Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J. Biol. Chem. 1999;274:27457–27462. doi: 10.1074/jbc.274.39.27457. [DOI] [PubMed] [Google Scholar]
- VARRÓ A., LATHROP D.A., HESTER S.B., NÁNÁSI P.P., PAPP J.G.Y. Ionic currents and action potentials in rabbit, rat, and guinea-pig ventricular myocytes. Basic Res. Cardiol. 1993;88:93–102. doi: 10.1007/BF00798257. [DOI] [PubMed] [Google Scholar]
- WANG H., YANG B., ZHANG Y., HAN H., WANG J., SHI H., WANG Z. Different subtypes of alpha 1-adrenoceptor modulate different K+ currents via different signaling pathways in canine ventricular myocytes. J. Biol. Chem. 2001;276:40811–40816. doi: 10.1074/jbc.M105572200. [DOI] [PubMed] [Google Scholar]
- WATANABE H., WATANABE I., NAKAI T., OSHIKAWA N., KUNIMOTO S., MASAKI R., KOJIMA T., SAITO S., OZAWA Y., KANMATUSE K. Frequency-dependent electrophysiological effects of flecainide, nifekalant and D,L-sotalol on the human atrium. Jpn. Circ. J. 2001;65:1–6. doi: 10.1253/jcj.65.1. [DOI] [PubMed] [Google Scholar]
- WICHTER T., SCHAFERS M., RHODES C.G., BORGGREFE M., LERCH H., LAMMERTSMA A.A., HERMANSEN F., SCHOBER O., BREITHARDT G., CAMICI P.G. Abnormalities of cardiac sympathetic innervation in arrhythmogenic right ventricular cardiomyopathy: quantitative assessment of presynaptic noradrenaline reuptake and postsynaptic beta-adrenergic receptor density with positron emission tomography. Circulation. 2000;101:1552–1558. doi: 10.1161/01.cir.101.13.1552. [DOI] [PubMed] [Google Scholar]
- YANG T.T., TANDE P.M., LATHROP D.A., REFSUM H. Effects of altered extracellular potassium and pacing cycle length on the class III antiarrhythmic actions of dofetilide (UK-68,798) in guinea pig papillary muscle. Cardiovasc. Drugs Ther. 1992a;6:429–436. doi: 10.1007/BF00054193. [DOI] [PubMed] [Google Scholar]
- YANG T.T., TANDE P.M., REFSUM H. Electromechanical action of dofetilide and D-sotalol during simulated metabolic acidosis in isolated guinea pig ventricular muscle. Cardiovasc. Pharmacol. 1992b;20:889–894. doi: 10.1097/00005344-199212000-00007. [DOI] [PubMed] [Google Scholar]
- YUE L., FENG J., WANG Z., NATTEL S. Adrenergic control of the ultrarapid delayed rectifier current in canine atrial myocytes. J. Physiol. 1999;516:385–398. doi: 10.1111/j.1469-7793.1999.0385v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAZA A., MALFATTO G., SCHWARTZ P.J. Effects on atrial repolarization of the interaction between K+ channel blockers and muscarinic receptor stimulation. J. Pharmacol. Exp. Ther. 1995;273:1095–1104. [PubMed] [Google Scholar]