Abstract
The extent to which impulse-independent release of noradrenaline and/or inhibition of its reuptake contribute to the response to d-amphetamine in vivo is unclear. Here, dual-probe microdialysis was used to investigate this question in the rat frontal cortex and hypothalamus.
After systemic administration of d-amphetamine (10 mg kg−1), or its local infusion (10 μM), the increase in noradrenaline efflux in the hypothalamus was greater than in the frontal cortex.
In contrast, during local infusion of the noradrenaline reuptake inhibitor, BTS 54 354 (50 μM), the noradrenaline response was similar in the frontal cortex and hypothalamus, even after systemic administration of the α2-antagonist, atipamezole, to block presynaptic inhibition of transmitter release and neuronal firing.
In the frontal cortex, but not the hypothalamus, the noradrenaline response to 10 μM d-amphetamine was constrained by activation of α2-adrenoceptors. This suggests that, at this concentration, inhibition of reuptake of noradrenaline, following its impulse-dependent release, is evident in the frontal cortex, but that the noradrenaline response in the hypothalamus derives mostly from impulse-independent release (retrotransport).
Atipamezole did not affect the noradrenaline response to 100 μM d-amphetamine in either brain region possibly because, at this higher concentration, retrotransport of noradrenaline masks any compensatory reduction in impulse-evoked release.
It is concluded that inhibition of reuptake and retrotransport make different contributions to the noradrenaline response to d-amphetamine in the frontal cortex and hypothalamus and that retrotransport increases with the concentration of d-amphetamine.
Keywords: α2-Adrenoceptor, d-amphetamine, frontal cortex, hypothalamus, microdialysis, noradrenaline
Introduction
The mechanisms by which d-amphetamine increases the concentration of extracellular noradrenaline in the brain are not at all clear. Early studies of cortical and hypothalamic synaptosomes in vitro led to the conclusion that the main action of this drug is to inhibit neuronal reuptake of noradrenaline (Heikkila et al., 1975; Raiteri et al., 1975). More recent findings, based on cloned noradrenaline transporters in a COS–7 expression system in vitro, suggested that d-amphetamine inhibits noradrenaline uptake at high micromolar concentrations but that, at lower concentrations, impulse-independent release of noradrenaline (‘retrotransport') predominates (Pifl et al., 1999). However, microdialysis studies of the hippocampus in vivo, in which there would be tonic release of noradrenaline from intact neurones, suggest the opposite: uptake inhibition is evident only at low (0.5 mg kg−1) doses of d-amphetamine, whereas retrotransport occurs at higher (1.75 and 5 mg kg−1) doses (Florin et al., 1994).
So far, there have been no microdialysis studies to test whether these actions of d-amphetamine in the hippocampus generalise to other brain regions. However, there are reasons to predict that the relative contributions of mechanisms by which d-amphetamine increases the concentration of extracellular noradrenaline might not be the same throughout the brain. This is because noradrenergic neurones projecting to different forebrain areas often derive from different brainstem nuclei (Moore & Bloom, 1979; Waterhouse et al., 1983) and so could express different neurochemical and topographical characteristics that affect their response to a pharmacological challenge.
One key variable is likely to be the influence of α2-adrenoceptors on noradrenergic cell bodies and their terminals which blunt the neuronal firing rate and impulse-evoked release of noradrenaline, respectively. d-Amphetamine reduces both the spontaneous firing rate of noradrenergic neurones projecting from the locus coeruleus (Grahame & Aghajanian, 1971; Engberg & Svensson, 1979; Ryan et al., 1985) and the excitable state of their nerve terminals (Ryan et al., 1985). This is thought to be due to activation of α2-adrenoceptors following an increase in the concentration of extracellular noradrenaline. Indeed, we have already shown that systemic administration, or local infusion, of an α2-adrenoceptor antagonist augments the cortical noradrenaline response to d-amphetamine in anaesthetised rats (Wortley et al., 1999a). As a consequence, the net increase in noradrenaline efflux caused by d-amphetamine would depend on the extent to which its effects on impulse-independent release and inhibition of reuptake of noradrenaline are offset by a reduction in neuronal firing rate and impulse-evoked release of this transmitter.
In order to investigate this point in more detail, we used dual-probe microdialysis in freely moving rats to measure noradrenaline efflux (i.e., the migration of noradrenaline into the probe) simultaneously in the frontal cortex and hypo-thalamus. This provides an index of changes in the concentration of extracellular noradrenaline in these two brain regions, after treatment with d-amphetamine. We studied these two brain regions because their noradrenergic innervation derives from different sources: whereas the frontal cortex is innervated exclusively by neurones projecting from the locus coeruleus, the hypothalamus is innervated mainly by neurones with cell bodies in the lateral tegmental nuclei (Moore & Bloom, 1979; Waterhouse et al., 1983).
Since there was a clear regional difference in the noradrenaline response to systemic (intraperitoneal (i.p.)) administration of d-amphetamine, we went on to investigate mechanisms that could contribute to this variation. As a first step, we used local infusion of d-amphetamine in order to monitor its actions at noradrenergic nerve terminals. Our main aim was to establish whether inhibition of noradrenaline reuptake and impulse-independent release make different contributions to noradrenaline efflux in the frontal cortex and hypothalamus. To achieve this, we first compared the extent to which the noradrenaline response to local infusion of the noradrenaline reuptake inhibitor, BTS 54 354 (an active metabolite of sibutramine which, like d-amphetamine, is an antiobesity agent, Luscombe et al., 1989), resembles that of d-amphetamine. We then investigated whether the noradrenaline response to d-amphetamine was offset by indirect activation of presynaptic α2-adrenoceptors on noradrenergic nerve terminals and/or the cell bodies and whether any such effect differed across brain regions.
Methods
Subjects
All procedures complied with the U.K. Animals (Scientific Procedures) Act 1986. Experiments were carried out on male Sprague – Dawley rats (230–360 g) obtained from the colony at University College London. These were housed at 21°C and 55% relative humidity with a light – dark cycle of 12 h (lights on at 0800) and were given unlimited access to food and water at all stages of the experiment.
Surgical procedures
Microdialysis probes were constructed as described in McQuade and Stanford (2000). They were equipped with cuprophan membrane (inside diameter 180 μm, outside diameter 250 μm, molecular weight cutoff 10 kDa; Medicell International Ltd, England) forming an active dialysis window of 2 mm. Anaesthesia of the rats was induced in a closed chamber delivering 5% halothane combined with 95% O2/5% CO2 at 2 l min−1. The rats were then placed in a Kopf stereotaxic frame and anaesthesia maintained by the delivery (via a face mask) of 1.5 – 2% halothane combined with 95% O2/5% CO2 (1 l min−1). Rats' core body temperature was maintained at 37°C using a homeothermic heating blanket and rectal probe (Harvard Instruments). A small incision was made in the scalp to expose the skull and reveal bregma. Then, following craniotomy, microdialysis probes, primed with modified Ringer's solution (mM: NaCl 145, KCl 4, CaCl2 1.3), were implanted into the frontal cortex (mm: AP +3.5, ML +1.5, DV −4.0) and the hypothalamus (mm: AP −1.8, ML −0.4, DV –9.2) (Paxinos & Watson, 1986). A small screw was inserted into the skull in order to anchor the dental cement that was used to secure the probes. At the end of the surgery, the rats were allowed to recover from anaesthesia in an incubation chamber and then transferred to individual plastic cages overnight. All experiments were carried out on the day following probe implantation.
Microdialysis
At 0800 on the day after surgery, rats were transferred, in their home cages, to the laboratory. There, the dialysis probes were perfused with the modified Ringer's solution at a rate of 2 μl min−1 and dialysis samples collected at 20 min intervals. In all experiments, drug challenges started after a minimum of three consecutive basal samples indicated a stable baseline for spontaneous noradrenaline efflux.
Experimental protocol
The first series of experiments compared the effects of systemic injection of d-amphetamine on noradrenaline efflux in the frontal cortex and hypothalamus. Rats were divided into three treatment groups of five to nine rats per group and were given an i.p. injection of either d-amphetamine (3 or 10 mg kg−1) or saline (1 ml kg−1). Dialysis samples were collected from both brain regions for 240 min.
In the second set of experiments, the rats were divided into three time-matched treatment groups of 7–11 per group. In one group, basal samples were collected for 60 min and then the noradrenaline reuptake inhibitor, BTS 54 354 (50 μM) was infused locally, via both probes, and dialysis samples collected for the following 240 min. The second group of rats was injected with the α2-antagonist, atipamezole (1 mg kg−1, i.p.). After 60 min, to allow noradrenaline efflux to stabilise after the stress of the injection, dialysis samples were collected for the following 300 min. The third group was also injected with the α2-adrenoceptor antagonist, atipamezole (1 mg kg−1). Then, after 60 min, three basal samples (60 min) were collected before infusion of BTS 54 354 (50 μM) for 240 min.
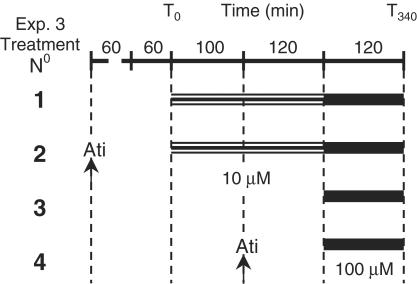
In the third set of experiments, there were four groups of rats of 8 – 11 per group. Each rat group was assigned one of the following time-matched treatments (see also, Figure 1): (1) 10 μM d-amphetamine was infused, via the microdialysis probes, for 220 min. The concentration of d-amphetamine was then increased to 100 μM for 120 min. (2) The α2-adrenoceptor antagonist atipamezole (1 mg kg−1, i.p.) was administered 60 min before collection of three basal samples. Then, d-amphetamine was infused locally (10 μM followed by 100 μM) as in group 1. (3) Ringer's solution was infused for 220 min before infusing d-amphetamine (100 μM) for 120 min. (4) Ringer's solution was infused for 100 min before administration of atipamezole. After a further 120 min, d-amphetamine (100 μM) was infused for 120 min.
Figure 1.
Protocol for Experiment 3. Animals were assigned to one of four treatment groups. They received either a single (100 μM, for 120 min) or sequential (10 μM, for 220 min and 100 μM, for 120 min) infusion of d-amphetamine. Two groups of rats also received an injection of atipamezole, as indicated by the arrows.
HPLC and electrochemical detection
The concentration of noradrenaline in the dialysis samples was determined by high-pressure liquid chromatography coupled to electrochemical detection. Solutes were separated by reversed-phase ion-pair chromatography on a Hypersil ODS 5 μm column (250 × 4.6 mm) maintained at 43°C and protected by an Aquapore guard column (30 × 4.6 mm). The mobile phase contained (mM): octane sulphonic acid, 2; sodium dihydrogen orthophosphate, 100; EDTA, 0.67 and methanol, 12%, adjusted to pH 3.75. This was pumped through the system at 1.3 ml min−1. Noradrenaline was detected using a high-performance analytical cell comprising two electrodes in series (ESA, model 5014A), controlled by a Coulochem detector (ESA, model 5100A): the conditioning electrode was set at −280 mV and the measuring electrode was set at +180 mV. The mobile phase was conditioned by a guard cell (ESA, model 5020) set at +350 mV. The electrochemical signal was integrated by either a Spectra-Physics Data Jet integrator or TurboChrom package. The noradrenaline content of the samples was calculated from the peak height of the chromatogram with reference to an external standard. Their content was not corrected for probe recovery.
Drugs and reagents
d-Amphetamine sulphate was obtained from Sigma, Poole, U.K. The α2-adrenoceptor antagonist, atipamezole (Newman-Tancredi et al., 1998), and halothane were purchased commercially from Pfizer, Sandwich, U.K. and Rhodia, Bristol, U.K., respectively. BTS 54 354 was kindly supplied by Knoll Pharmaceuticals Research. Drugs to be administered i.p. were dissolved in 0.9% saline and given at a volume of 1 ml kg−1. All buffer reagents were either AnalaR or HPLC grade.
Data analysis
Statistical analysis was carried out routinely on the raw data. When the increase in noradrenaline efflux was to be compared in different groups of rats or across brain regions, the net change in efflux, after administration of test drug, was analysed, also. This was calculated by subtracting the mean efflux of the three consecutive basal samples before administration of the drug from all experimental samples. The significance of any differences in noradrenaline efflux was assessed using repeated measures three-way, two-way or one-way ANOVA (SPSS PC+), as appropriate. In all cases, a significant effect on the main factor(s), or interactions between them, was taken as the criterion for progressing to (post hoc) two-way or one-way ANOVA. In all cases, ‘time' and ‘brain region' were treated as ‘within subjects' factors and ‘dose' was treated as a ‘between' subjects factor; ‘concentration' was a ‘between' subjects factor except when infusions were consecutive; in this case it was treated as a ‘within' subjects factor. Comparisons of changes in different groups of rats were carried out on time-matched samples. Drug-induced changes in noradrenaline efflux within each treatment group were compared with the three consecutive basal samples, thereby enabling the animals to serve as their own controls. In these comparisons, statistical analyses were carried out on bins of three consecutive samples so as to balance the ANOVA matrix. When appropriate, the Greenhouse – Geisser ‘ɛ' correction was applied to correct for any violation of sphericity of the variance – covariance matrix. The criterion for statistical significance was set at P⩽0.05. Finally, Mead's ‘Resource Equation' (1988) was used to confirm that sample sizes were sufficient to detect a statistically significant difference, should one exist.
Results
Systemic administration of d-amphetamine
In animals that received systemic administration of d-amphetamine, there was no difference in basal efflux of noradrenaline in the frontal cortex and the hypothalamus. Basal concentrations obtained from the pooled data were 9±1 and 12±2 fmol 20 min−1, in rats destined for 3 and 10 mg kg−1 d-amphetamine, respectively.
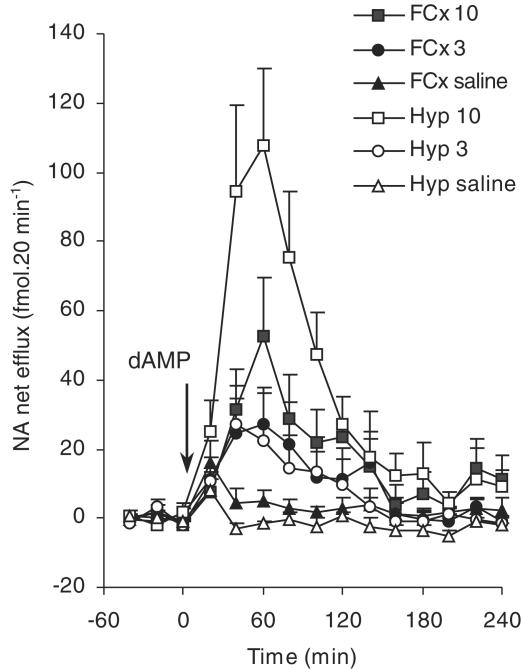
In the frontal cortex, saline injection did not affect noradrenaline efflux, which remained stable throughout the experiment. In the hypothalamus, injection of saline increased noradrenaline efflux during the 20 min following injection (T0–T20, F(1,4)=7.66; P=0.05). Thereafter, efflux returned to basal and remained stable for the remaining 220 min of the experiment (Figure 2).
Figure 2.
The effects of i.p. administration of d-amphetamine (dAMP, 3 or 10 mg kg−1) or saline (1 ml kg−1) administered at time ‘0' (T0, indicated by arrow) on noradrenaline (‘NA') efflux in the frontal cortex (FCx) or hypothalamus (Hyp) of freely moving rats. Noradrenaline efflux is expressed as fmol 20 min−1. Points show mean±s.e.m. noradrenaline efflux. N=5 – 9 in each group. For results of statistical analysis, see text.
In the frontal cortex, there was no difference in the increase in noradrenaline efflux following injection of 3 or 10 mg kg−1 d-amphetamine (Figure 2). In contrast, the noradrenaline response in the hypothalamus was dose-dependent. Specifically, the response at 10 mg kg−1 was greater than that seen at 3 mg kg−1 between T20 and T240 (F(1,14)=4.985; P<0.05) (Figure 2). Thus, after the 3 mg kg−1 dose, there was no difference in the increase in noradrenaline efflux in the frontal cortex and hypothalamus. However, in the frontal cortex, the noradrenaline response to 10 mg kg−1 d-amphetamine was considerably smaller than that in the hypothalamus for up to 100 min postinjection (Figure 2; F(1,7)=6.21; P<0.05).
Effects of α2-adrenoceptor antagonist, atipamezole (1 mg kg−1 i.p), on noradrenaline response to local infusion of BTS 54 354 (50 μM)
In none of the three groups of experimental animals was there a difference in basal efflux of noradrenaline in the frontal cortex and the hypothalamus. Basal levels obtained from the pooled data were 8±1, 14±1 and 13±1 fmol 20 min−1, in animals destined for BTS 54 354 alone, atipamezole alone and BTS 54 354, following injection of atipamezole, respectively.
In animals given atipamezole alone, there was no change in noradrenaline efflux in either brain region (i.e. on the main factor, ‘time') for at least 4 h after its administration (Figure 3b).
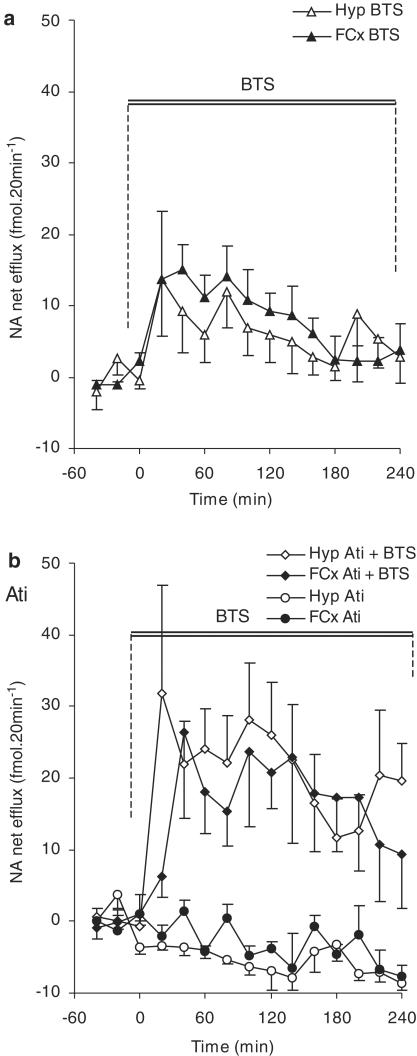
Figure 3.
The effects of local infusion of the noradrenaline reuptake inhibitor BTS 54 354 (‘BTS', 50 μM) on noradrenaline (‘NA') efflux in the frontal cortex (FCx) or hypothalamus (Hyp) of freely moving rats. (a) Shows the data from animals given an infusion of BTS 54 354, alone. (b) All animals received a systemic injection of the α2-adrenoceptor antagonist, atipamezole (‘Ati', 1 mg kg−1; i.p.). After 2 h, half the group was infused with BTS 54 354 (50 μM). In both groups of rats treated with BTS 54 354, the infusion started at T0 and was maintained for the following 240 min (i.e., until T240). Points show mean±s.e.m. noradrenaline efflux. N=8–9 in each group. For results of statistical analysis, see text.
In both brain areas, local infusion of the noradrenaline reuptake inhibitor, BTS 54 354 (50 μM) alone, caused an immediate increase in the efflux of noradrenaline which remained greater than basals for at least 1 h (frontal cortex: F(1,18)=17.40; P=0.001 and hypothalamus: F(1,18)=4.45; P=0.05) (Figure 3a). However, there was no overall difference in the noradrenaline response in these two brain regions over the 4 h of infusion of BTS 54 354 and no interaction between the factors ‘time' and ‘brain region'.
During infusion of BTS 54 354 in atipamezole-pretreated rats, there was no difference in the noradrenaline response in the frontal cortex and hypothalamus (Figure 3b). However, in both brain regions, noradrenaline efflux was greater than in rats given BTS 54 354 alone: in the frontal cortex, this increase was evident over the period T160 – T200 (F(1,15)=4.68; P<0.05) and, in the hypothalamus, between T20 – T240 (F(1,13)=6.11; P<0.05) (cf. Figure 3a and b).
Local infusion of d-amphetamine
There was no difference in basal efflux of noradrenaline in the frontal cortex and hypothalamus of animals destined for local infusion of d-amphetamine, alone. Basal levels obtained from the pooled data were 7±1 and 2±1 fmol 20 min−1, in animals destined for sequential infusion of d-amphetamine (10 μM followed by 100 μM), or a single concentration (100 μM) of d-amphetamine, respectively.
In both the frontal cortex and hypothalamus, the noradrenaline response to infusion of 10 μM d-amphetamine or 100 μM d-amphetamine, alone, increased with the drug concentration (Figure 4a). However, at 10 μM d-amphetamine, the increase in efflux in the hypothalamus was greater than that in the frontal cortex between T0 – T80 (F(1,6)=7.53; P<0.05). In contrast, there was no regional difference in the noradrenaline response to 100 μM d-amphetamine (Figure 4a).
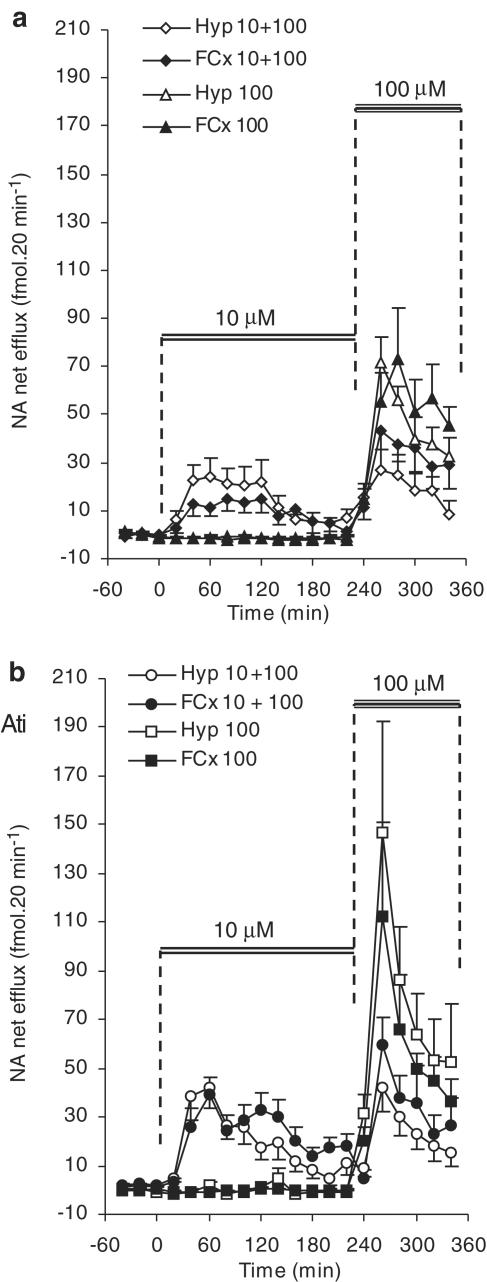
Figure 4.
The effects of infusion of d-amphetamine (cumulative: 10 and 100 μM, ‘10+100'; or 100 μM alone ‘100') on noradrenaline (‘NA') efflux in the frontal cortex (FCx) or hypothalamus (Hyp) of freely moving rats. (a) Shows the results from animals that were given d-amphetamine, only. (b) All animals were given a systemic injection of the α2-adrenoceptor antagonist, atipamezole (‘Ati', 1 mg kg−1; i.p.), 2 h before infusion of d-amphetamine. The duration of infusions of d-amphetamine are indicated by the solid bars. Noradrenaline efflux is expressed as fmol 20 min−1 and points show mean±s.e.m. N=9 – 11 in each group. For results of statistical analysis, see text.
Remarkably, the noradrenaline response to 10 μM d-amphetamine declined abruptly in both brain regions after 2 h, despite continued infusion of this drug. However, when the two concentrations of d-amphetamine were infused sequentially, substitution of the higher dose (100 μM) restored the noradrenaline response in both brain regions. This reached a peak within 40 min, but declined rapidly thereafter (Figure 4a). In the frontal cortex, noradrenaline efflux during the 120 min of infusion of this higher dose was greater than that during the first 120 min of infusion of the 10 μM dose (F(1,18)=6.49; P<0.05). However, in the hypothalamus, there was no such dose-related difference (Figure 4a).
Since there was no regional difference in the noradrenaline response after substitution of the higher concentration of d-amphetamine, we then compared the noradrenaline response to this higher concentration with that in time-matched samples from animals infused with 100 μM d-amphetamine, alone (i.e. with no preinfusion of 10 μM d-amphetamine). In the frontal cortex, the apparent increase in noradrenaline efflux in these two groups did not reach the criterion for significance (Figure 4a). In contrast, in the hypothalamus, the noradrenaline response to 100 μM d-amphetamine was diminished over the period T240 – T340 by the 10 μM preinfusion (F(1,16)=15.79; P=0.01) (Figure 4a).
Effects of α2-adrenoceptor antagonist, atipamezole (1 mg kg−1 i.p. ), on noradrenaline response to local infusion of d-amphetamine
There was no difference in basal efflux of noradrenaline in the frontal cortex and the hypothalamus of animals destined for local infusion of d-amphetamine after administration of atipamezole. Basal efflux, obtained from the pooled data was 5±1 and 3±1 fmol 20 min−1, in animals given consecutive infusions of 10 μM and then 100 μM d-amphetamine, or a single concentration (100 μM) of d-amphetamine, respectively.
Between T40 – T120, the increase in noradrenaline efflux in the frontal cortex induced by d-amphetamine (10 μM) in atipamezole-pretreated rats was greater than that in time-matched samples from rats which did not receive atipamezole (F(1,19)=4.27, P=0.05) (cf. Figure 4a and b). In contrast, in the hypothalamus, there was no such difference (cf. Figure 4a and b). Furthermore atipamezole abolished the regional difference in noradrenaline efflux seen during infusion of 10 μM d-amphetamine (Figure 4b). In neither brain area did atipamezole prevent the abrupt dissipation of the noradrenaline response to 10 μM d-amphetamine, despite continued infusion of this drug.
The apparent increase in the noradrenaline response to 100 μM d-amphetamine alone, caused by atipamezole, did not reach criterion for statistical significance. Atipamezole also did not affect the noradrenaline response to 100 μM d-amphetamine when this followed a preinfusion at the lower concentration (cf. Figure 4a and b). Finally, in neither of these groups of rats was there any difference in the noradrenaline response to 100 μM d-amphetamine in the frontal cortex and hypo-thalamus.
Discussion
In this study, we have compared the actions of d-amphetamine in the rat frontal cortex and the hypothalamus. Our main aim was to investigate the contribution of retrotransport of noradrenaline and the inhibition of its reuptake to the overall increase in noradrenaline efflux induced by this drug. As in the past, we have studied these two brain regions because they are innervated by noradrenergic neurones projecting from different brainstem nuclei. To the best of our knowledge, there have been no previous studies of the noradrenaline response to systemic d-amphetamine in the hypothalamus and none has compared simultaneously the effects of either systemic administration or local infusion of this drug on noradrenaline efflux across different brain regions.
The first experiments compared the effects of systemic administration (i.p.) of d-amphetamine on the noradrenaline response in the frontal cortex and hypothalamus. There was no regional difference in the increase in noradrenaline efflux induced by 3 mg kg−1 d-amphetamine (i.p.). However, when challenged with a higher drug dose (10 mg kg−1), the increase in the hypothalamus was considerably greater than that in the frontal cortex. Moreover, in the hypothalamus, but not the frontal cortex, the increase seen at 10 mg kg−1 was greater than that at 3 mg kg−1. It seems that different factors determine the noradrenaline response to d-amphetamine in these two brain regions.
As a first step in explaining this regional disparity, the next experiments went on to explore differences in the effects of d-amphetamine on neuronal reuptake and impulse-independent release of noradrenaline at the level of the nerve terminals. d-Amphetamine was infused, via the probe, simultaneously into the frontal cortex and hypothalamus. The lower concentration of 10 μM d-amphetamine was chosen because this increases noradrenaline efflux when infused in the medial prefrontal cortex (Finlay et al., 1997). However, it must be borne in mind that the drug concentration in the extracellular fluid surrounding the probe will be less than this and will decline with increasing distance from the probe.
The first finding was that the initial increase in noradrenaline efflux, induced by local infusion of 10 μM d-amphetamine, was greater in the hypothalamus than in the frontal cortex. There are several possible explanations for this. One is that d-amphetamine inhibits noradrenaline reuptake (Florin et al., 1994) and that this process is less effective in the frontal cortex than the hypothalamus. This could be because there are differences in the populations of noradrenaline transporters or that differences in the neuronal cytoarchitecture affect the migration of noradrenaline towards the probe. Alternatively, there could be less tonic release of noradrenaline in the frontal cortex, compared with the hypothalamus, and so inhibition of reuptake would have less impact on its extraneuronal concentration in the former brain region.
Despite published evidence that d-amphetamine blocks uptake of noradrenaline, neither of these explanations alone seems likely. This is because, when the reuptake inhibitor, BTS 54 354, was infused simultaneously into the frontal cortex and hypothalamus, there was no difference in the ensuing increase in noradrenaline efflux. This similarity is obviously upheld, regardless of any difference in the activity of neurones projecting to these two brain areas, and/or their regional topography (such as the density of innervation or dimensions of the extraneuronal space). Consequently, the finding that the noradrenaline response to 10 μM d-amphetamine was greater in the hypothalamus than the frontal cortex suggests that different mechanisms contribute to the overall response to d-amphetamine in these two brain regions.
Another factor contributing to the regional differences in noradrenaline response to d-amphetamine could be activation of α2–autoreceptors (following accumulation of extracellular noradrenaline) and attenuation of impulse-evoked release of noradrenaline and/or neuronal firing rate (see: Grahame & Aghajanian, 1971; Engberg & Svensson, 1979; Ryan et al., 1985). Either of these processes could offset any impulse-independent increase in efflux caused by d-amphetamine.
So far, the effects of d-amphetamine on neuronal firing rate have been studied only in the locus coeruleus and there is clearly scope for investigating this further. Nevertheless, we tested whether there are regional differences in the extent to which α2-adrenoceptors influence the noradrenaline response to local infusion of d-amphetamine. This involved comparing the effects of the selective α2-adrenoceptor antagonist, atipamezole, on the increase in noradrenaline efflux caused by d-amphetamine in the frontal cortex and hypothalamus. Atipamezole was given systemically so as to ensure that it was distributed throughout the brain and so would prevent α2-adrenoceptor–mediated inhibition of noradrenaline release and/or neuronal firing rate. The latter could conceivably be modified through polysynaptic pathway(s), following increased release of noradrenaline in the terminal field. The influence of such a process could well differ in the frontal cortex and hypothalamus, but systemic administration of atipamezole would eliminate this as a confounding factor. Systemic administration would also help to avoid other complications that could arise from giving both drugs via the probe. These could include the impact of differences in the pharmacokinetics of d-amphetamine and atipamezole and, possibly, mutual interference of two solutes which could disrupt their flux (or that of noradrenaline) to and from the probe. Our previous work has confirmed that, at the dose used here, atipamezole blocks α2-adrenoceptors for at least 5.3 h in the rat (Wortley et al., 1999a;1999b), a period which is more than adequate for the present experiments.
Atipamezole alone had no effect on noradrenaline efflux during the experiment. However, it caused a long-lasting increase in the noradrenaline response to d-amphetamine (10 μM) in the frontal cortex. In contrast, there was no such increase in the hypothalamus of the same animals. In fact, pretreatment with this antagonist abolished the regional difference in the response to d-amphetamine (10 μM). It follows that, at this concentration of d-amphetamine, there is a compensatory reduction in impulse-evoked noradrenaline release in the frontal cortex but not the hypothalamus. Such an effect could contribute to the blunted response to systemic d-amphetamine seen in the frontal cortex at the doses used here. It remains to be seen whether this attenuation of noradrenaline release depends on activation of α2-adrenoceptors on the noradrenergic nerve terminals, their cell bodies, or both.
A further point is that, in atipamezole-pretreated rats, BTS 54 354 increased noradrenaline efflux to the same extent in the two brain regions. Systemic administration of atipamezole also increased the noradrenaline response to its parent compound, sibutramine, in both the frontal cortex and the hypothalamus (Wortley et al., 1999b) and so it is clear that α2-adrenoceptors normally modulate noradrenaline release in both these brain areas. Against this background, the lack of any influence on the hypothalamic noradrenaline response to 10 μM d-amphetamine suggests that, at this concentration, the increase in efflux derives mostly from impulse-independent transmitter release (retrotransport).
We were also interested in discovering why the noradrenaline response to 10 μM d-amphetamine dissipated in both brain regions after approximately 2 h, despite continued drug infusion. In the frontal cortex, the increase in noradrenaline efflux induced by 10 μM d-amphetamine was augmented by pretreatment with atipamezole, suggesting that there is a large reserve of releasable transmitter. Thus, in this brain region at least, the decline in the noradrenaline response is unlikely to be due to exhaustion of releasable transmitter. To test this further, we investigated whether the noradrenaline response could be restored by infusion of a higher concentration of d-amphetamine. At the same time, we could investigate whether impulse-independent release of noradrenaline in the frontal cortex increases at higher concentrations of d-amphetamine, as has been suggested for the hippocampus (Florin et al., 1994).
In both brain regions, there was a resurgence of the noradrenaline response when the concentration of d-amphetamine was increased to 100 μM. This makes it unlikely that the dissipation of the noradrenaline response at the lower concentration is due to inhibition of retrotransport by d-amphetamine, following its accumulation inside the neurones (see: Pifl et al., 1999). If this were the case, increasing the concentration of d-amphetamine would have increased the inhibition of retrotransport rather than reduced it. It also rules out a reduction in the number of noradrenaline transporter sites on exposure to d-amphetamine, as a possible explanation (Saunders et al., 2000; Carvelli et al., 2002). Finally, the poor penetration of brain tissue by d-amphetamine (Westerink & De Vries, 2001) argues against the higher drug concentration simply recruiting a noradrenaline response from more distant neurones with intact transmitter stores.
The most likely explanation for the increased efflux at the higher dose of d-amphetamine is that it increases impulse-independent release of noradrenaline, as has been found in the hippocampus (Florin et al., 1994). Such a proposal is supported by the finding that atipamezole had no appreciable effect on the noradrenaline response to 100 μM d-amphetamine (note that the Resource Equation was used to confirm that the sample size was large enough to detect a significant change, should one exist). It seems that even if α2-adrenoceptors are activated indirectly during infusion of 100 μM d-amphetamine, they have little/no impact on transmitter efflux in either brain region. Together with the results from our study with BTS 54 354, it follows that the increase in noradrenaline efflux at this concentration of d-amphetamine is unlikely to be due to inhibition of reuptake following its impulse-dependent release.
A final finding was that, in the frontal cortex, the noradrenaline response to sequential infusion of 100 μM d-amphetamine was greater than that during the 10 μM preinfusion. This supports our suggestion that the pool of releasable noradrenaline in the frontal cortex was not exhausted by the preinfusion. In contrast, in the same animals, there was no increase in the hypothalamic noradrenaline response when the concentration of d-amphetamine was increased from 10 to 100 μM. Possibly, the pool of releasable noradrenaline in the hypothalamus is too small, and/or not replenished rapidly enough, to rally a greater response to 100 μM d-amphetamine. Alternatively, the noradrenaline response to d-amphetamine in the hypothalamus could be limited by saturation of the (retro)transporters. However, this latter case seems unlikely because noradrenaline efflux in the hypothalamus was greater after 100 μM than at 10 μM d-amphetamine if there was no preinfusion.
In conclusion, the results of this dual-probe microdialysis study suggest that the noradrenaline response to d-amphetamine depends not only on drug dose, but also on brain region. In the frontal cortex, the noradrenaline response to local infusion of a low concentration of d-amphetamine seems to be constrained through activation of α2-adrenoceptors that blunt impulse-evoked transmitter release. No such compensatory inhibition of impulse-evoked release is evident in the hypothalamus. At higher probe concentrations (100 μM) of d-amphetamine, there is no significant influence of presynaptic α2-adrenoceptors in either brain region. Under these conditions, noradrenaline efflux in both the frontal cortex and hypothalamus seems mainly to derive from its impulse-independent release. Finally, in the frontal cortex, the decline of noradrenaline efflux, despite continued infusion of 10 μM d-amphetamine, cannot be explained by depletion of releasable transmitter. However, in the hypothalamus, limitations on the rate at which the pool of releasable noradrenaline is replenished could limit the response to this drug.
The extent to which noradrenaline responses to local infusion of d-amphetamine help explain its actions when given systemically needs further investigation. Nevertheless, regional differences in the noradrenaline response to d-amphetamine and its derivatives could well influence their psychotropic effects (e.g. arousal and mood) versus their disruption of homeostasis (e.g. autonomic function and food intake).
Acknowledgments
This work was funded by Knoll Ltd Research and Development, Nottingham.
References
- CARVELLI L., MORON J.A., KAHLIG K.M., FERRER J.V., SEN N., LECHLEITER J.D., LEEB-LUNDBERG L.M., MERRILL G., LAFER E.M., BALLOU L.M., SHIPPENBERG T.S., JAVITCH J.A., LIN R.Z., GALLI A. PI 3-kinase regulation of dopamine uptake. J. Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- ENGBERG G., SVENSSON T.H. Amphetamine-induced inhibition of central noradrenergic neurons: a pharmacological analysis. Life Sci. 1979;24:2245–2254. doi: 10.1016/0024-3205(79)90101-2. [DOI] [PubMed] [Google Scholar]
- FINLAY J.M., JEDEMA H.P., RABINOVIC A.D., MANA M.J., ZIGMOND M.J., SVED A.F. Impact of corticotropin-releasing hormone on extracellular norepinephrine in prefrontal cortex after chronic cold stress. J. Neurochem. 1997;69:144–150. doi: 10.1046/j.1471-4159.1997.69010144.x. [DOI] [PubMed] [Google Scholar]
- FLORIN S.M., KUCZENSKI R., SEGAL D.S. Regional extracellular norepinephrine responses to amphetamine and cocaine and effects of clonidine pretreatment. Brain Res. 1994;654:53–62. doi: 10.1016/0006-8993(94)91570-9. [DOI] [PubMed] [Google Scholar]
- GRAHAME D.D., AGHAJANIAN G.K. Effects of amphetamine on single cell activity in a catecholamine nucleus, the locus coeruleus. Nature. 1971;234:100–102. doi: 10.1038/234100b0. [DOI] [PubMed] [Google Scholar]
- HEIKKILA R.E., ORLANSKY H., MYTILINEOU C., COHEN G. Amphetamine evaluation of d- and l-isomers as releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. J. Pharmacol. Exp. Ther. 1975;194:47–56. [PubMed] [Google Scholar]
- LUSCOMBE G.P., HOPCROFT R.H., THOMAS P.C., BUCKET W.R. The contribution of metabolites to the rapid and potent down-regulation of rat cortical β-adrenoceptors by the putative antidepressant sibutramine hydrochloride. Neuropharmacology. 1989;28:129–134. doi: 10.1016/0028-3908(89)90048-8. [DOI] [PubMed] [Google Scholar]
- MEAD R. The Design of Experiments. Cambridge, NY: Cambridge University Press; 1988. [Google Scholar]
- McQUADE R., STANFORD S.C. A microdialysis study of the noradrenergic response in rat frontal cortex and hypothalamus to a conditioned cue for aversive, naturalistic environmental stimuli. Psychopharmacology. 2000;148:201–208. doi: 10.1007/s002130050043. [DOI] [PubMed] [Google Scholar]
- MOORE R.Y., BLOOM F.E. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Ann. Rev. Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., NICHOLAS J.P., AUDINOT V., GAVAUDAN S., VERRIELE L., TOUZARD M., CHAPUT C., RICHARD N., MILLAN M.J. Actions of α2-adrenoceptor ligands at α2A and 5-HT1A receptors: the antagonist atipamezole, and the agonist, medetomidine, are highly selective for α2A-adrenoceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. Australia: Academic Press; 1986. [Google Scholar]
- PIFL C., AGNETER E., DROBNY H., SITTE H.H., SINGER E.A. Amphetamine reverses or blocks the operation of the noradrenaline transporter depending on its concentration: superfusion studies on transfected cells. Neuropharmacology. 1999;38:157–165. doi: 10.1016/s0028-3908(98)00155-5. [DOI] [PubMed] [Google Scholar]
- RAITERI M., BERTOLLINI A., ANGELINI F., LEVI G. d-Amphetamine as a releaser or reuptake inhibitor of biogenic amines in synaptosomes. Eur. J. Pharmacol. 1975;34:189–195. doi: 10.1016/0014-2999(75)90239-3. [DOI] [PubMed] [Google Scholar]
- RYAN L.J., TEPPER J.M., YOUNG S.J., GROVES P.M. Amphetamine's effects on terminal excitability of noradrenergic locus coeruleus neurons are impulse-dependent at low but not high doses. Brain Res. 1985;341:155–163. doi: 10.1016/0006-8993(85)91483-0. [DOI] [PubMed] [Google Scholar]
- SAUNDERS C., FERRER J.V., SHI L., CHEN J., MERRILL G., LAMB M.E., LEEB-LUNDBERG L.M., CARVELLI L., JAVITCH J.A., GALLI A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERHOUSE B.D., LIN C.S., BURNE R.A., WOODWARD D.J. The distribution of neocortical projection neurons in the locus coeruleus. J. Comp. Neurol. 1983;21:418–431. doi: 10.1002/cne.902170406. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H.C., DE VRIES J.B. A method to evaluate the diffusion rate of drugs from a microdialysis probe through brain tissue. J. Neurosci. Methods. 2001;109:53–58. doi: 10.1016/s0165-0270(01)00401-0. [DOI] [PubMed] [Google Scholar]
- WORTLEY K.E., HUGHES Z.A., HEAL D.J., STANFORD S.C. Comparison of changes in the extracellular concentration of noradrenaline in rat frontal cortex induced by sibutramine or d-amphetamine: modulation by α2–adrenoceptors. Br. J. Pharmacol. 1999a;127:1860–1866. doi: 10.1038/sj.bjp.0702720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORTLEY K.E., HEAL D.J., STANFORD S.C. Modulation of sibutramine-induced increases in extracellular noradrenaline concentration in rat frontal cortex and hypothalamus by α2–adrenoceptors. Br. J. Pharmacol. 1999b;128:659–666. doi: 10.1038/sj.bjp.0702859. [DOI] [PMC free article] [PubMed] [Google Scholar]