Abstract
The electrophysiological properties of detrusor smooth muscles have been studied almost exclusively in small mammals and the relevance of the information to the human bladder has been questioned. In the present study, electrical properties of detrusor smooth muscles of the pig and human were investigated using intracellular recording techniques.
Bladder smooth muscles of the pig and human exhibited nifedipine (10 μM)-sensitive spontaneous action potentials, and their frequency was highly sensitive to membrane polarization.
During bursts of action potentials, each action potential was followed by a fast after-hyperpolarization (fast AHP). Charybdotoxin (CTX, 50 nM) increased the amplitude and duration of action potentials but failed to inhibit the fast AHPs, while apamin (0.1 μM) blocked the fast AHPs and induced action potential complexes, which were followed by slow AHPs. 4-Aminopyridine (4-AP, 1 mM) suppressed the slow AHP and increased action potential frequency.
In the human bladder, transmural stimuli initiated inhibitory junction potential-like hyperpolarizations, which were followed by action potential discharges. The hyperpolarizations were blocked by atropine (1 μM) and by apamin (0.1 μM) but not by CTX (50 nM). In the pig bladder, transmural stimuli evoked excitatory junction potentials (EJPs), which triggered action potentials. After desensitizing P2x receptors with α,β methylene-ATP (10 μM), nerve-evoked responses were similar to those of human bladder.
These results indicate that detrusor smooth muscles of the pig share many features of electrical properties with those of the human. In addition to large conductance (BK) and small conductance (SK) Ca2+-activated K+ channels, voltage-dependent K+ (VK) channels may play an important role in the regulation of electrical activity of detrusor smooth muscles.
Keywords: Action potential, neuromuscular transmission and urinary bladder
Introduction
Bladder smooth muscle strips exhibit phasic contractions either spontaneously or in response to transmural nerve stimuli. Since spontaneous contractions occur locally and do not readily spread throughout the tissue (Hashitani et al., 2000), probably due to the relative poor coupling between smooth muscle cells (Bramich & Brading, 1996; Hashitani et al., 2001), they do not increase the intravesical pressure. In contrast, the excitation of nerves initiates synchronized contractions of the tissue, and thus causes a rise in the intravesical pressure to void urine.
In the guinea-pig, spontaneous action potentials and associated calcium transients have been shown to underlie spontaneous contractions (Hashitani et al., 2001). Action potentials result from the opening of voltage-dependent L-type Ca2+ channels and their frequency is highly sensitive to membrane polarization (Brading & Mostwin, 1989). A double sucrose gap study in the guinea-pig bladder showed that increasing action potential frequency by current injection facilitated phasic contractions (Mostwin, 1986). Several pharmacological agents, such as ATP-sensitive K+ channel openers and β-adrenoceptor agonists, which hyperpolarize the membrane of bladder smooth muscles, inhibit action potentials and also suppress spontaneous contractions (Seki et al., 1992; Hashitani et al., 1996; Nakahira et al., 2001).
In response to transmural stimulation, acetylcholine (ACh) and ATP are released from nerves to contract bladder smooth muscles (Fujji, 1988; Brading & Mostwin, 1989; Hashitani et al., 2000). Neurally released ATP initiates excitatory junction potentials (EJPs), which trigger action potentials (Bramich & Brading, 1996; Hashitani et al., 2000). ATP has been suggested to contract bladder smooth muscles mainly by opening nonselective cation channels causing depolarization and thus Ca2+ entry through L-type Ca2+ channels and subsequent Ca2+-induced Ca2+ release (CICR) from internal stores (Schneider et al., 1991; Ganitkevich & Isenberg, 1992; Hashitani et al., 2000). The activation of muscarinic receptors by ACh is thought to increase the production of InsP3 which releases Ca2+ from internal stores to contract bladder smooth muscles (Iacovou et al., 1990; Andersson et al., 1991; Wu et al., 1999). It has also been demonstrated that neurally released ACh caused slow depolarizations, which trigger action potentials and oscillatory increases in [Ca2+]i (Hashitani et al., 2000). Therefore, regardless of the mode of excitation, the discharge of action potentials seems to be a key mechanism for contraction of bladder smooth muscles, and thus understanding the regulation of electrical activity is essential for understanding the mechanisms of bladder contractions.
Most of our knowledge about the electrical properties of bladder smooth muscles relies on work carried out on small laboratory animals, for example, guinea-pig, rat and rabbit (Creed et al., 1983; Brading & Mostwin, 1989; Hashitani et al., 2001). However, contractile studies reveal considerable species differences in bladder smooth muscles (Sibley, 1984). Furthermore, different experimental conditions have been applied for contractile studies and electrophysiological investigations; organ bath studies of bladder smooth muscles are carried out under reasonably physiological conditions, while electrical properties are studied either in isolated cells (Heppner et al., 1997; Sui et al., 2001) or in tissue preparations in which spontaneous muscle contractions have been diminished by increasing extracellular osmorality (Brading & Mostwin, 1989; Heppner et al., 1997). Microelectrode studies on bladders from larger mammals have rarely been successfully undertaken, therefore, we have very limited information about the electrical properties of detrusor smooth muscles in either pig or human bladder, particularly about the neuromuscular transmission (however, see Visser et al., 2000a, 2000b).
In the present study, the electrical properties of detrusor smooth muscles of the pig and human urinary bladder were investigated using intracellular recording techniques in normal physiological saline. Limitations in the availability of normal human bladder have meant that more complete results are presented for pig detrusor. First, passive properties of the detrusor smooth muscle were examined by measuring membrane potential changes induced by intracellular current injection. Second, spontaneous action potentials were characterized, and their modulation by either membrane polarization or several pharmacological agents was investigated. Finally, the pharmacological profile of nerve-evoked changes in the membrane potentials was studied. The results of the present study were compared to findings in guinea-pig bladder in which the electrical properties have been most widely studied.
Methods
General
Pig bladder samples were obtained from female pigs freshly killed in the local abattoir by exsanguination and human bladder samples were obtained from either transplant donors or patients undergoing surgery for bladder cancer. This study was approved by the hospital ethical committee, and was carried out as a project of the Oxford Continence Group. Samples were transported to the laboratory in cold physiological saline, and dissection was then undertaken immediately. From human and pig bladders, thin single smooth muscle bundles, 2–5 mm long and 0.3–1 mm width, were dissected. Smooth muscle bundles were pinned out on a Sylgard plate (silicone elastomer, Dow Corning Corporation, Midland, Michigan, U.S.A.) at the bottom of a recording chamber, and the connective tissue, which covered the surface of the muscle bundle, was carefully removed using a fine tungsten wire held by forceps.
Male guinea-pigs, weighing 200–400 g, were killed by a blow to the head followed by a cervical dislocation. The procedures described have been approved by the animal experimentation ethics committee at the University of Oxford. The urinary bladder was removed and its ventral wall was opened longitudinally from the top of the dome to the bladder neck. The mucosal layer, connective tissues and several smooth muscle layers were then removed leaving an underlying single layer of smooth muscle bundles attached to the serosal layer. A serosal sheet that contains a single bundle of smooth muscle 2–3 mm long and 0.3–0.7 mm wide was then prepared as described previously (Hashitani et al., 2001).
Intracellular recordings
Preparations were pinned out on a Sylgard plate at the bottom of a recording chamber (volume, approximately 1 ml), which was mounted on the stage of an inverted microscope. The preparations were superfused with warmed (36°C) physiological saline at a constant flow rate (2 ml min−1). Individual bladder smooth muscle cells were impaled with glass capillary microelectrodes, filled with 0.5 M KCl (tip resistance, 150–250 MΩ). Membrane potential changes were recorded using a high-input impedance amplifier (Axoclamp-2A, Axon Instruments, Inc., Foster City, California, U.S.A.), and displayed on a cathode-ray oscilloscope (Data SYS 740, Gould Nicolet Technologies, Ilford, Essex, U.K.). After low-pass filtering (cutoff frequency, 1 kHz), membrane potential changes were digitized using PowerLab/4SP (ADInstruments Ltd, Grove House, Hastings, U.K.) and stored on a personal computer for later analysis.
The passive electrical properties of detrusor smooth muscles from human and pig bladder were determined; following neutralization of the tip resistance, both inward and outward current was passed through the recording electrode and resultant voltage changes were recorded. In some experiments on the pig bladder, preparations were impaled with two independent microelectrodes and the distance and location of each electrode determined with the inverted microscope. To study electrical coupling between cells, one electrode was used to inject current and the other was used to record resultant electrotonic potentials.
Intramural nerves were stimulated selectively by passing brief square-stimulating pulses (duration 50–100 μs) between a pair of electrodes (platinum plate and wire) placed above and below the bladder preparation. The neural selectivity of the stimulating pulses was confirmed by abolishing the evoked responses with tetrodotoxin (1 μM).
Solution
The composition of physiological saline was (in mM): NaCl, 120; KCl, 5.9; MgCl2, 1.2; CaCl2, 2.5; NaHCO3, 15.5; NaH2PO4, 1.2 and glucose, 11.5. The solution was bubbled with 95% O2 and 5% CO2.
Drugs used were 4-aminopyridine (4-AP) (from ICN Biomedicals Ltd, Aurora, OH, U.S.A.), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (BAPTA-AM) (from Calbiochem-Novabiochem Ltd, San Diego, CA, U.S.A.), α,β methylene-ATP (Me-ATP), apamin, atropine sulphate, carbamylcholine chloride (carbachol), charybdotoxin (CTX), nifedipine (from Sigma, St Louis, MO, U.S.A.), cyclopiazonic acid (CPA) (from Tocris Cookson Ltd, Bristol, U.K.), levcromakalim (SmithKline-Beecham, Harlow, U.K.) and tetrodotoxin (from Alomone Lab., Jerusalem, Israel). 4-AP, Me-ATP, apamin, atropine, carbachol, CTX and tetrodotoxin were dissolved in distilled water. Nifedipine were dissolved in 100% ethanol, and BAPTA-AM, CPA and levcromakalim were dissolved in dimethyl sulphoxide (DMSO). The final concentration of these solvents in the physiological saline did not exceed 1 : 1000.
Calculations and statistic
The following parameters were measured: peak amplitude, measured as the value from the resting membrane potential to the peak of the action potential (which was defined as an average of 0.1 ms on either side of the maximum point); leading dV/dt (dV/dtL), measured as the slope between 20 and 80% of the peak amplitude of the events on the raising phase; half width, measured as the time between 50% peak amplitude on the rising and falling phases; trailing dV/dt (dV/dtT), measured as the slope between 20 and 80% of the peak amplitude of the events on the falling phase. The amplitude of after-hyperpolarizations was measured as the value from the resting membrane potential to the peak of the after-hyperpolarization.
Measured values were expressed as the mean±standard deviation (s.d.). Statistical significance was tested using Student's t-test, and probabilities of less than 5% different from the control were considered significant.
Results
General observations
In 31 out of 35 detrusor smooth muscle preparations obtained from the pig bladder, smooth muscle cells exhibited spontaneous action potentials (Figure 1A). The remaining four preparations were electrically quiescent, but were capable of generating action potentials in response to intracellular current injection. Resting membrane potentials, determined at the most negative, stable potential between each action potential, ranged between −50 and −39 mV (mean −43.1±2.9 mV, n=35). Two types of spontaneous action potential discharges were observed; in about 60% of preparations, prolonged bursts of action potentials were generated with irregular intervals between the bursts (see Figures 1Aa, 4Aa and Ba) and during the bursts each action potential was followed by an after-hyperpolarization (AHP), which had a relatively fast time course (the fast AHP; Figure 1Ab, Da). In the remaining 40% of preparations, either single or clustered action potentials which were followed by slow AHPs (Figure 1Da), were generated periodically (see Figure 5B, C). The fast AHPs had amplitudes of 7.3±2.2 mV and half-widths of 661.4±96.9 ms (n=18, Figure 1Da), while the slow AHPs had amplitudes of 6.9±1.9 mV and half-widths of 1889.8±552.9 ms (n=13, Figure 1Da). When bursts of action potentials were generated, they occurred at a frequency of 1.4±0.39 min−1 (range, 0.8 and 2 min−1, n=18) and each burst consisted of three to 20 action potentials. For the entire experiments, action potentials occurred with a frequency ranging between 4 and 18.5 min−1 (mean 10.1±5.2 min−1, n=31). Action potentials had peak amplitudes ranging between 34.6 and 49 mV (mean 40.6±3.9 mV, n=31), leading dV/dt (dV/dtL) ranging between 0.5 and 2.1 mV ms−1 (mean 0.95±0.42 mV ms−1, n=31), half-widths ranging between 8.5 and 72.1 ms (mean 23.9±3.7 ms, n=31), and trailing dV/dt (dV/dtT) ranging between −7.5 and −0.25 mV ms−1 (mean −2.4±1.8 mV ms−1, n=31).
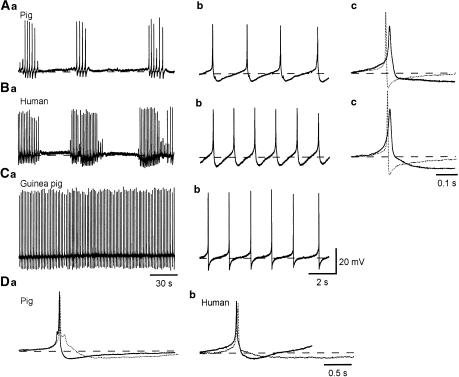
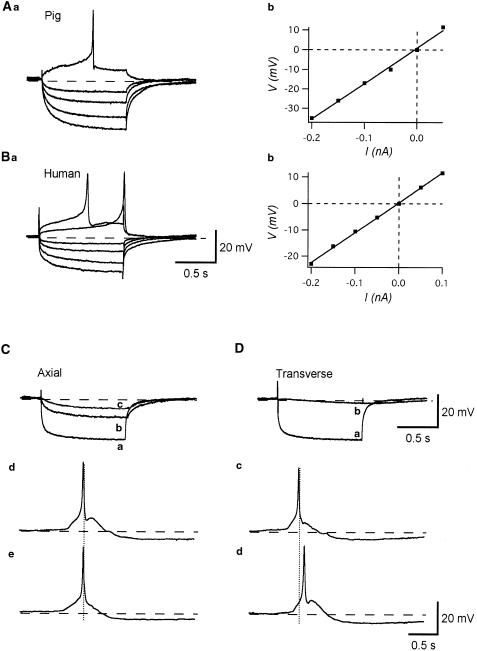
Figure 1.
Comparison of action potentials in the human, pig and guinea-pig bladder. Detrusor smooth muscle cells of the pig and human bladder exhibited bursts of action potentials (Aa, Ba), which had an amplitude of some 40 mV and consisted of a slow depolarizing phase and a regenerative depolarization which lasted for some 20 ms (Ab, Bb). The regenerative depolarization of the inter-burst action potentials was followed by a repolarizing phase which continued to a fast AHP (Ab, Bb). Overlaid traces show action potentials recorded from pig or human (full lines) and guinea-pig (dotted line) bladders (Ac, Bc). Note that the AHP of the guinea-pig bladder is sharper and of shorter duration than the fast AHP in the other species. Continuous action potentials recorded from the guinea-pig bladder (Ca) had an amplitude of some 50 mV, and consisted of a slow depolarizing phase and a rapid regenerative depolarizations which lasted less than 10 ms. The depolarizations were followed by a rapid repolarizing phase and an AHP (Cb). The types of AHP in pig and human bladder are shown in Da and Db. Full lines show action potentials followed by a fast AHP characteristic of the inter-burst action potentials and dotted lines show action potentials followed by a slow AHP, characteristic of preparations showing sporadic or complex action potentials. Resting membrane potentials were −43 mV for A, −41 mV for B and −42 mV for C.
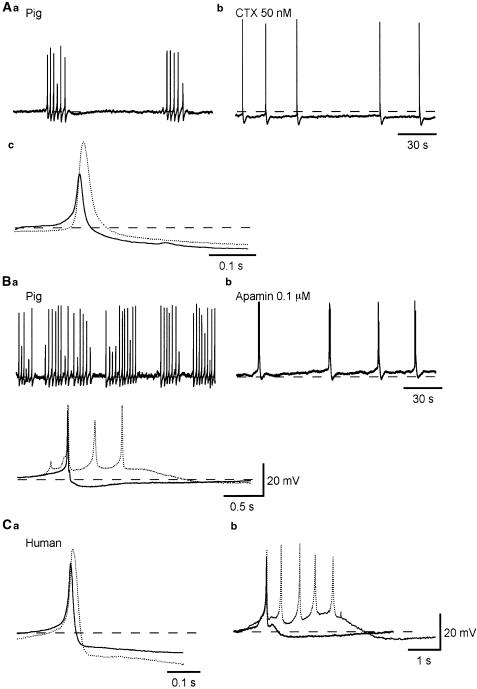
Figure 4.
Effects of CTX and apamin on spontaneous action potentials recorded from pig and human bladder smooth muscles. Bladder smooth muscle cells of the pig exhibiting bursts of action potentials (A, B) in control conditions, had action potentials with an amplitude of about 35 mV, which were followed by a fast AHP (Aa,c). After some 10 min exposure to CTX (50 nM), the membrane was hyperpolarized by some 5 mV and action potentials occurred individually with a reduced frequency (Ab). In the presence of CTX, action potentials had an amplitude of some 60 mV and were followed by a fast AHP (dotted line, Ac). In another preparation exhibiting bursts of action potentials (B), action potentials had an amplitude of about 40 mV, and were followed by a fast AHP in control conditions (Bb,c). After some 10 min exposure to apamin (0.1 μM), the membrane was depolarized by a few mV and complex action potentials were generated (Bb,c). In the presence of apamin, each action potential complex consisted of three to six action potentials and was followed by a slow AHP (dotted line, Bc). In a preparation of human detrusor, similar effects of CTX (dotted line, Ca) and of apamin (dotted line, Cb) were seen. In (Ca) and (Cb), full lines represent control action potentials. Resting membrane potentials were −39 mV in (A), and −43 mV in (B) and −41 mV in (C).
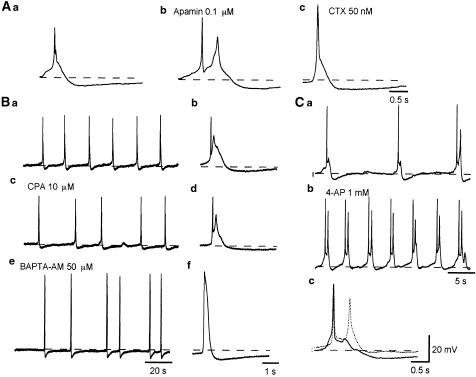
Figure 5.
Pharmacological profile of the slow AHPs recorded from pig-bladder smooth muscle. Action potentials followed by slow AHPs were recorded from detrusor smooth muscle of the pig bladder (Aa). Apamin (0.1 μM) did not inhibit the slow AHPs (Ab). CTX (50 nM) increased the amplitude and duration of action potentials, but failed to block the slow AHPs (Ac). In bladder smooth muscle cells of the pig exhibiting action potential complexes that were followed by slow AHPs (Ba, b), after some 20 min exposure to CPA (10 μM), action potential complexes, which had a similar morphology to those in control condition, were generated, and were still followed by slow AHPs (Bc, d). In the same preparation which had been exposed to BAPTA-AM (50 μM) for some 20 min, action potentials had an increased amplitude and duration, and again were still followed by slow AHPs (Be, f). Traces (Bb, d and f) are expanded action potentials from traces (Ba, c and d). Resting membrane potentials were −42 mV. In another preparation in which bladder smooth muscle cells exhibited action potential complexes (Ca), 4-AP (1 mM) depolarized the membrane by about 3 mV and increased action potential frequency (Cb). Note that the slow depolarizations between action potentials were greatly shortened in the presence of 4-AP. In the presence of 4-AP, action potentials had a similar morphology to those in the control condition but the slow AHPs had reduced amplitude shown by dotted line in (Cc). Resting membrane potentials were −42 mV.
In 12 out of 15 preparations examined, human bladder smooth muscle cells exhibited spontaneous action potentials that occurred with a frequency ranging between 4 and 17.5 min−1 (mean 7.9±4.2 min−1). The remaining three preparations were electrically quiescent, although action potentials could be evoked either by injecting depolarizing current or by transmural stimuli. In 10 preparations, bursts of action potentials were generated (Figure 1Ba) and during the burst each action potential was followed by fast AHPs (Figure 1, Db). In the remaining two preparations, action potential complexes, which were followed by slow AHPs (Figure 1, Db), were exhibited periodically. Resting membrane potentials, determined at the most negative, stable potential between each action potential, ranged between −45 mV and −38 mV (mean −40.5 ± 1.7 mV, n=15). Each action potential consisted of an initial slow depolarizing phase and a subsequent regenerative depolarization (Figure 1Bb,c). The regenerative depolarization was followed by a repolarizing phase. Action potentials had peak amplitudes ranging between 33.7 and 46.5 mV (mean 38.5±3.7 mV, n=12), leading dV/dt (dV/dtL) ranging between 0.28 and 5.4 mV ms−1 (mean 0.54±0.26 mV ms−1, n=12), half-widths ranging between 15.4 and 28.5 ms (mean 21.9±4.5 ms, n=12) and trailing dV/dt (dV/dtT) ranging between −3.2 and −1 mV ms−1 (mean −1.8±0.68 mV ms−1, n=12). Amplitudes of AHPs (both types) ranged between 5.3 and 11.5 mV (mean 8.2±2.7 mV, n=12).
In human and pig bladders, the action potential frequency was lower than that of the guinea-pig bladder (31.6±21.3 min−1, n=53) in which action potentials were mostly continuously discharged (Figure 1Ca,b). Guinea-pig action potentials had a larger amplitude (52.3±5.1 mV), faster dV/dtL (2.9±0.9 mV ms−1), faster dV/dtT (mean −15.1±2.6 mV ms−1) and a shorter half-width (mean 6.2±1.3 ms) than those of the pig and human bladders, and fast AHPs in the guinea-pig had a much faster time course and larger amplitude than those of human and pig bladders (12.1±2.7 mV, Figure 1Ac, Bc).
In both human and pig bladders, spontaneous action potentials were abolished by nifedipine (10 μM, n=3 each, Figure 2B), indicating that they result from the opening of L-type calcium channels as has been reported for action potentials in the guinea-pig bladder (Mostwin, 1986).
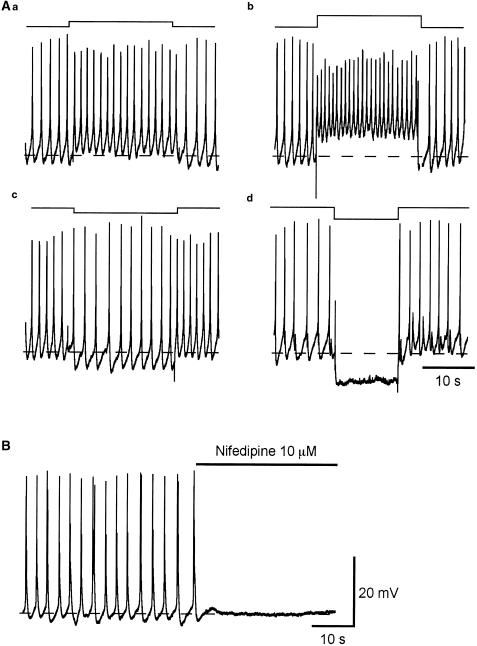
Figure 2.
Effects of the membrane polarization on spontaneous action potentials recorded from pig-bladder smooth muscle. Pig-bladder smooth muscle cells generated spontaneous action potentials with a frequency of about 40 min−1. Outward current with an amplitude of 0.05 nA injected through the recording electrode depolarized the membrane by about 5 mV and increased action potential frequency to about 60 min−1 (Aa). A larger outward current (0.1 nA) depolarized the membrane by about 10 mV and increased the frequency of action potentials to some 80 min−1 (Ab). During the depolarization, the amplitude of action potentials was reduced. In the same preparation, inward current with an amplitude of 0.05 nA hyperpolarized the membrane by about 5 mV and reduced the frequency of action potentials to 30 min−1 (Ac). During the hyperpolarization, the amplitude of action potentials was increased. A larger outward current (−0.1 nA) hyperpolarized the membrane by about 10 mV and prevented the generation of action potentials (Ad). Resting membrane potential was −41 mV. Nifedipine (10 μM) abolished the action potentials (B).
Effect of changes in the membrane potential on action potential frequency
To examine the effect of membrane polarization on the frequency of spontaneous action potentials in pig bladders, prolonged outward or inward currents were injected through the recording electrode. Outward currents induced depolarizations of the membrane and increased the action potential frequency in a voltage-dependent manner (Figure 2Aa,b). During depolarization, the amplitude of action potentials was reduced (Figure 2Aa,b). Inward currents injected into a cell caused hyperpolarizations in a voltage-dependent manner, and either reduced the frequency of spontaneous action potentials (Figure 2Ac) or prevented their generation (Figure 2Ad). During hyperpolarization, the action potential amplitude was increased (Figure 2Ac). These results demonstrate a high voltage dependency of the action potential frequency in the pig bladder, and are consistent with findings obtained from guinea-pig bladders (Mostwin, 1986).
Passive properties of pig and human bladder smooth muscles
In pig and human bladders, passive electrical properties of detrusor smooth muscles were examined by brief intracellular current injections. In the pig bladder, the relation between the amplitude of injected current and resultant membrane potential changes was linear (Figure 3Ab), and apparent input resistances calculated from a slope of the I–V relation ranged between 90 and 390 MΩ (mean 221.9±95.2 MΩ, n=10, Figure 3Ab). The decay of electronic potentials could be fitted by a single exponential curve, and the time constants of decay ranged between 37.5 and 222 ms (mean 114.6±57.6 ms, n=10). Similar results were obtained from human bladders (Figure 3Ba). The relation between the amplitude of injected currents and resultant membrane potential changes were again linear (Figure 3Bb), and apparent input resistances ranged between 78 and 200 MΩ (mean 125.3±48.9 MΩ, n=8). The decay of the resultant electrotonic potentials could be fitted by a single exponential curve, and had time constants ranging from 33 to 142 ms (mean 74±39.1 ms, n=8).
Figure 3.
Passive membrane properties of detrusor smooth muscle of the human and pig bladder. Overlaid traces show the membrane potential changes in the pig (Aa) and human (Ba) bladder induced by intracellular current injection of +0.1, +0.05, −0.05, −0.1, −0.15 and −0.2 nA for 1 s. The relation between the amplitude of injected currents and resultant membrane potential changes was linear in both cases. The input resistance calculated from the slope of I–V relation for pig detrusor was 160 MΩ (Ab), and for human detrusor, 110 MΩ (Bb). Resting membrane potentials were −40 mV in (A) and −39 mV in (B). Intracellular injection of inward current of −0.2 nA for 2 s induced a hyperpolarization with an amplitude of some 25 mV (Ca). The electrotonic potential recorded from a cell located about 200 μM apart in the axial direction had an amplitude of some 13 mV (Cb) and that in a cell located 400 μM apart axially had an amplitude of some 7 mV (Cc). In the same preparation, an injection of inward current of −0.2 nA for 2 s caused a hyperpolarization with an amplitude of some 25 mV (Da). The resultant membrane potential changes in a cell located 50 μM away in the transverse direction had an amplitude of some 4 mV (Db). In a different preparation, when two electrodes were placed 400 μM apart axially, action potentials recorded from both electrodes occurred almost simultaneously (Cd, e). When electrodes were placed 100 μM apart transversely, some 100 ms of delay between the peaks of paired action potentials could be detected (Dc, d). Resting membrane potential was −40 mV in (C) and (D) and −43 mV in (E) and (F).
Electrical coupling between detrusor smooth muscle cells in the pig bladder
To further examine the passive electrical properties of detrusor smooth muscles of the pig bladder, electrical coupling between cells was studied using the two-electrode technique. Inward currents were injected into cells through the first electrode and resultant membrane potential changes were recorded with the second electrode.
When two electrodes were placed in the axial direction, injected current induced a hyperpolarization, which had a slower time course in the second cell (Figure 3Cb). By increasing the separation between two electrodes, the amplitude of electrotonic potentials detected with the second electrode became small (Figure 3Cc). When two electrodes were placed axially, synchronized action potentials were recorded from both electrodes with little (<10 μs) or no measurable delay between the peaks of paired action potentials (Figure 3Cd,e). When two electrodes were placed in the transverse direction, only a very small electrotonic potential, which had a very slow time course, was detected from the second cell (Figure 3Db). When the second electrode was placed over some 70–80 μM apart transversely, no detectable membrane potential changes were recorded from the second electrode. When two electrodes were placed transversely, action potentials recorded from both electrodes were still synchronized. However, an obvious delay between the peaks of paired action potentials was invariably detected (Figure 4Dc,d), and sometimes action potentials failed to propagate.
Effects of blockers for BK and SK channels on action potentials recorded from human and pig bladders
To identify ion channels that are involved in the repolarizing phase of action potentials and fast AHPs, the effects of some K+ channel blockers were examined. In pig-bladder smooth muscle preparations, CTX (50 nM), a blocker for large-conductance Ca2+-activated K+ channels (BK channels), increased the amplitude and duration of action potentials but did not inhibit fast AHPs (Figure 4Ab,c). In five preparations which had been exposed to CTX for some 10 min, action potentials had a mean amplitude of 70.1±7.4 mV, dV/dtL of 2.9±1.2 mV ms−1, half-widths of 53.1±23.9 ms and dV/dtT of −1±0.57 mV ms−1. The amplitudes of fast AHPs were 8.4±2.6 mV. Apamin (0.1 μM), a blocker for small-conductance Ca2+-activated K+ channels (SK channels), inhibited the fast AHPs, and the slow depolarizing phase between action potentials was greatly shortened so that action potential complexes with several spikes occurred followed by slow AHPs (Figure 4Bb,c). The amplitude and time course of the individual action potentials were, however, not much affected by apamin (Figure 4Bc).
In detrusor smooth muscles of the human bladder, CTX (50 nM) also increased the amplitude and duration of action potentials but did not inhibit fast AHPs (Figure 4Ca). Apamin (0.1 μM), as in the pig, inhibited the fast AHPs without affecting either the amplitude or time course of action potentials (Figure 4Cb). In the presence of apamin, action potential complexes with several spikes were again generated, and were followed by slow AHPs.
Pharmacological profile of slow AHPs recorded from pig bladders
To investigate ion channels that contribute to slow AHPs and the role of Ca2+ release from intracellular stores in their generation, the effects of CTX, apamin, 4-AP, CPA and BAPTA-AM were studied on pig detrusor smooth muscles.
When action potential complexes, which were followed by slow AHPs, were spontaneously generated, neither apamin (0.1 μM) nor CTX (50 nM) inhibited the slow AHPs (Figure 5Ab, c).
CPA (10 μM) initially increased and then reduced action potential frequency, but did not change the shape of action potentials and failed to inhibit the slow AHPs (Figure 5Bc, d). In four preparations that had been treated with CPA for some 20 min, action potentials had an amplitude of 40.1±1.4 mV, dV/dtL of 0.98±0.13 mV ms−1, half-width of 27.7±3 ms and dV/dtT of −1.9±0.27 mV ms−1. The slow AHPs had an amplitude of 7.2±1.6 mV and half-widths of 1924.5±227.2 ms.
BAPTA-AM (50 μM), a chelator of intracellular calcium ions, increased the amplitude and duration of action potentials, but did not inhibit the slow AHPs (Figure 5Be, f). In four preparations that had been exposed to BAPTA-AM for some 20 min, action potentials had an amplitude of 69.8±5.4 mV, dV/dtL of 2.3±0.68 mV ms−1, half-width of 429.5±88.2 ms and dV/dtT of −0.15±0.57 mV ms−1. The slow AHPs had an amplitude of 7.5±1.7 mV and half-width of 2073.3±482.5 ms.
To examine a possible involvement of voltage-dependent K+ (VK) channels in slow AHPs, the effect of 4-AP on action potentials of the pig bladders was studied. 4-AP (1 mM) depolarized the membrane by about 3 mV (−38.8±2.5 mV, n=4) and increased the frequency of action potentials (13.3±2.1 min−1) without significantly changing their shape (Figure 5Cb, c). In the presence of 4-AP, action potentials had an amplitude of 42.1±2.2 mV (n=4), dV/dtL of 1.3±0.22 mV ms−1 (n=4), half-width of 29±2.6 ms (n=4) and dV/dtT of −1.9±0.31 mV ms−1 (n=4). 4-AP also diminished slow AHPs (Figure 5Cc) and greatly reduced the quiescent periods between action potentials (Figure 5Cb).
Effects of carbachol, α,β-methylene ATP and levcromakalim on spontaneous action potentials in pig bladders
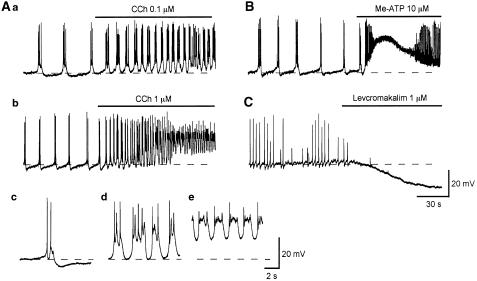
To investigate the pharmacological profile of spontaneous action potentials of the pig bladder, the effects of bath-applied excitatory neuromuscular transmitters and an ATP-sensitive K+ channel opener on electrical activity were examined. Carbachol (CCh, 10 nM) increased the frequency of spontaneous action potentials without changing the membrane potential (n=3), while CCh (0.1 μM) depolarized the membrane (−38.2±1.8 mV in 0.1 μM, n=4) and increased the action potential frequency (Figure 6Aa). During the application of CCh (0.1 μM), slow AHPs were suppressed (Figure 6Aa). A higher concentration of CCh (1 μM) caused larger depolarizations (−31±2 mV in 1 μM, n=3) and greatly increased the action potential frequency (Figure 6Ab). During the application of CCh (1 μM), slow AHPs were initially suppressed (Figure 6Ad) and then oscillatory deprolarizations, which had plateau phases, were generated (Figure 6Ae). α,β-methylene ATP (Me-ATP, 10 μM) caused large depolarizations which returned during continuous application to the original level within some 2 min (−41±1.4 mV in control, −12.1±2.2 mV in Me-ATP, n=4; Figure 6B). During application of Me-ATP, the frequency of action potentials was initially increased and their amplitude reduced and then the generation of action potentials ceased.
Figure 6.
Effects of CCh, Me-ATP and levcromakalim on spontaneous action potentials recorded from pig-bladder smooth muscle. In a control solution, bladder smooth muscle of pig exhibited spontaneous action potentials (Aa). CCh (0.1 μM) depolarized the membrane by about 3 mV, and increased the action potential frequency (Aa). In another preparation, a higher concentration of CCh (1 μM) depolarized the membrane by some 20 mV and greatly increased action potential frequency (Ab). Me-ATP (10 μM) caused a transient depolarization with an amplitude of some 25 mV which returned to the original level within some 2 min (B). On the rising phase of the depolarization, action potential frequency was dramatically increased and their amplitude was suppressed (B). In a different preparation, levcromakalim (1 μM) hyperpolarized the membrane by about 20 mV and prevented the generation of action potentials (C). Resting membrane potential was −40 mV in (Aa), −41 mV in (Ab and B) and −40 mV in (C).
The effects of levcromakalim, an ATP-sensitive K+ channel opener, which has been widely investigated in the bladders of laboratory animals, on spontaneous action potentials in the pig bladder were also examined. Levcromakalim (1 μM) hyperpolarized the membrane by about 25 mV (−41.3±3.2 mV in control, −64.7±2.3 mV in levcromakalim, n=4) and prevented the generation of action potentials (Figure 6C).
Nerve-evoked changes in the membrane potential of pig and human bladders
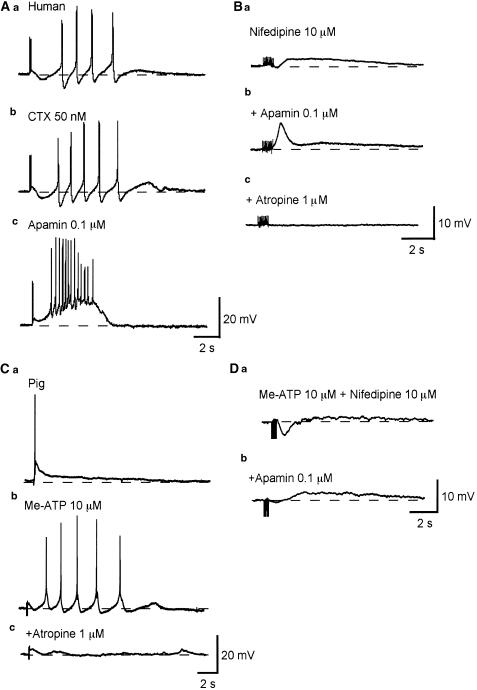
In 10 preparations of the human bladder, transmural nerve stimulation with a single impulse did not cause any detectable membrane potential changes. In six of these preparations, trains of impulses (three to 10 impulses delivered at 20 Hz) initiated an IJP-like hyperpolarization and a slow depolarization that triggered several action potentials (Figure 7Aa). All these responses were blocked by atropine (1 μM). In the remaining four preparations, no detectable membrane potential change was evoked by nerve stimulation. IJP-like hyperpolarizations were blocked by apamin (0.1 μM, n=6 Figure 7Ac) but not CTX (50 nM, n=4, Figure 7Ab). After apamin treatment, nerve stimulation initiated a large depolarization that triggered multiple action potentials (Figure 7Ac). In the presence of nifedipine (10 μM), nerve stimulation initiated a small IJP-like hyperpolarization and slow depolarization (n=4, Figure 7Ba). Apamin (0.1 μM) blocked the hyperpolarization and unmasked a fast depolarization, which was followed by a slow depolarization (Figure 7Bb). All these responses were again blocked by atropine (1 μM, n=4, Figure 7Bc).
Figure 7.
Nerve-evoked changes in the membrane potential recorded from human and pig-bladder smooth muscle. In a human detrusor smooth muscle preparation, three transmural stimuli initiated an IJP-like hyperpolarization which was followed by a discharge of several action potentials (Aa). CTX (50 nM) did not inhibit the hyperpolarization, but increased the amplitude of the action potentials (Ab). Apamin (0.1 μM) abolished the hyperpolarization and unmasked a depolarization which triggered multiple action potentials (Ac). In the presence of nifedipine (10 μM), 10 impulses initiated a small IJP-like hyperpolarization which was followed by a slow depolarization (Ba). Apamin (0.1 μM) abolished the hyperpolarization and unmasked a fast depolarization which was followed by a slow depolarization (Bb). Atropine (1 μM) abolished the biphasic depolarization (Bc). In a pig detrusor smooth muscle preparation, three impulses initiated an EJP which triggered an action potential (Ca). After desensitizing P2x receptors with Me-ATP (10 μM), the same stimulus failed to initiate an EJP but generated several action potentials (Cb). These responses were blocked by atropine (1 μM, Cc). In another preparation, which had been exposed to Me-ATP (10 μM) and nifedipine (10 μM), five impulses initiated an IJP-like hyperpolarization, followed by a small slow depolarization (Da). Apamin (0.1 μM) reduced the amplitude of the hyperpolarization and enhanced the slow depolarization (Db). All transmural stimuli were delivered at 20 Hz and had a duration of 50 μs. Resting membrane potential was −41 mV in (A) −40 mV in (B) −43 mV in (C) and −39 mV in (D).
In all 15 preparations of the pig bladder studied, single impulses failed to induce any detectable membrane potential changes. In eight out of 15 preparations, trains of impulses (three to 10 impulses) delivered at 20 Hz initiated a fast depolarization EJP) which triggered an action potential (Figure 7Ca). The remaining seven preparations did not respond to nerve stimulation.
In preparations in which the P2x receptor had been desensitized with Me-ATP (10 μM), nerve stimulation failed to cause an EJP but triggered delayed discharges of action potentials (Figure 7Cb). These responses were blocked by atropine (1 μM, Figure 7Ac). In desensitized preparations, in the presence of nifedipine (10 μM), nerve stimulation initiated a transient hyperpolarization similar to an inhibitory junction potential (IJP) which was followed by a slow depolarization (n=4, Figure 7Da). Apamin (0.1 μM) blocked the IJP-like hyperpolarizations and increased the amplitude of the slow depolarizations (Figure 7Db).
Discussion
In the present study, the electrical properties of detrusor smooth muscles of the pig and human were investigated using intracellular recording techniques. Detrusor smooth muscle cells of the pig and human bladders exhibit nifedipine-sensitive spontaneous action potentials. The repolarizing phase of action potentials results from the opening of BK channels and Ca2+-mediated inactivation of L-type Ca2+ channels. SK channels and VK channels contribute to fast and slow AHPs, respectively. In the pig, nerve stimulation initiates an EJP mediated by P2x receptors, but when these receptors are desensitized the responses to nerve stimulation are similar to the human tissue, and in both species these nerve-mediated changes in the membrane potential are characterized by cholinergic, apamin-sensitive IJP-like hyperpolarizations and a following slow depolarization which triggers action potentials.
The passive properties of detrusor smooth muscle of the pig and human bladder were examined by intracellular current injections. The input resistance and time constant of detrusor smooth muscles of the pig were approximately 222 mΩ and 115 ms, respectively. Corresponding values of the human bladder were 125 mΩ and 74 ms, respectively. These values were larger than those obtained in the guinea-pig bladder using same technique (65 mΩ and 12 ms, Bramich & Brading, 1996; 79 mΩ and 56 ms, Hashitani et al., 2001), suggesting that electrical coupling between detrusor smooth muscle cells of the pig and human is less extensive than that of the guinea-pig bladder. Electrical coupling between cells was further investigated in the pig bladder by impaling the tissues with two independent electrodes. The obtained results are in good agreement with the findings in the guinea-pig bladder (Bramich & Brading, 1996; Hashitani et al., 2001), indicating that detrusor smooth muscle cells of the pig bladder coupled electrically more readily with cells located axially than those located transversely. In the guinea-pig bladder, regardless of the short length constant, successive action potentials were propagated to cells within a muscle bundle – particularly in the transverse direction, presumably due to the regenerative nature of action potentials (Hashitani et al., 2001). However, in the pig bladder, action potentials sometimes failed to propagate to neighboring cells located transversely, suggesting that the spread of action potentials in the pig bladder may not be as good as that of the guinea-pig. Consistently, it was reported that spontaneous action potentials could not be recorded in the pig bladder smooth muscles using sucrose-gap recording (Fujji, 1988).
Action potentials in detrusor smooth muscles of the pig and human were blocked by nifedipine, suggesting that they result from the opening of L-type Ca2+ channels as in the guinea-pig (Mostwin, 1985). Since neither CPA nor BAPTA-AM prevented action potential generation in the pig bladder, Ca2+ release from internal stores is not essential for their generation as is also the case for the guinea-pig bladder (Hashitani et al., 2000). The frequency of action potentials in the pig bladder was highly sensitive to membrane polarization. Depolarizations of the membrane induced by either intracellular current injection or bath-applied agonists, for example, CCh and Me-ATP, increased the frequency of action potentials, while hyperpolarizations induced by current injection or levcromakalim inhibited the generation of action potentials. These results are again consistent with findings in detrusor smooth muscle of the guinea-pig (Fujii et al., 1990; Hashitani et al., 1996), indicating that the nature of action potentials of the pig bladder is qualitatively similar to that of the guinea-pig bladder. However, action potentials in the human and pig bladder had a smaller amplitude and a slower time course that those of the guinea-pig bladder as it has been reported in a previous study on isolated detrusor muscle cells (Sui et al., 2001). More recently, it has been reported that the current density of voltage-sensitive Ca2+ channels of the guinea-pig detrusor smooth muscle is larger than that of the human and pig (Kajioka et al., 2002). Furthermore, in the human and pig bladder, bursts of action potentials were dominantly generated and their frequency was much lower than that of the guinea-pig bladder in which action potentials were mostly generated continuously. There are, therefore, several qualitative differences between action potentials of the pig and human bladders and those of the guinea-pig.
An unexpected finding of the present study was the failure of blocking AHPs by CTX in both pig and human bladders. In the guinea-pig bladder, the opening of BK channels contributes to both the repolarizing phase of action potential and the AHP (Hashitani & Brading, paired submission). Findings from the guinea-pig bladder also suggested that Ca2+-induced Ca2+ release (CICR) from internal stores is required for the activation of BK channels to initiate AHPs (Herrera et al., 2002; Hashitani & Brading, paired submission). Both fast and slow AHPs in the pig and human bladders lacked the rapid phase which may be attributable to the activation of BK channels by CICR in the guinea-pig bladder. Although CTX failed to block AHPs, it increased the amplitude and duration of action potentials in the pig and human bladder, indicating that BK channels are activated during action potentials probably by depolarization and entered Ca2+ through L-type channels. In the guinea-pig bladder, both iberiotoxin and ryanodine are reported to augment phasic contractions (Herrera et al., 2000), while in the pig bladder, the amplitude of phasic contractions was increased by iberiotoxin but was reduced by ryanodine (Buckner et al., 2002). This discrepancy may be explained by the different relation between BK channels and internal stores in the pig and guinea-pig bladder. The colocalization of BK channels and internal stores that is seen in the guinea-pig, which may allow a preferential access to BK channels by released Ca2+, may not be present in the pig and human bladders (Ohi et al., 2001).
The blockade of fast AHPs by apamin shortened the duration of bursts and produced complexes of multiple spikes that were followed by slow AHPs. Consistently, apamin increased the amplitude of phasic contractions and reduced their frequency and duration in the pig bladder (Buckner et al., 2002). Slow AHPs were not blocked by apamin, CTX, CPA or BAPTA-AM, suggesting that neither Ca2+-activated K+ channels nor increases in [Ca2+]c contribute to the AHPs. 4-AP suppressed slow AHPs and shortened the slow depolarizations between action potentials to increase action potential frequency. VK channels are activated by membrane depolarization and are expected to contribute to the repolarizing phase of action potentials in detrusor smooth muscle cells (Klöckner and Isenberg, 1985). In guinea-pig ureters, 4-AP indeed dramatically increased the action potential duration and also inhibited AHPs and refractory periods (Exintaris and Lang, 1999). However, in the pig bladder, the repolarizing phase of action potentials seems to result from the opening of BK channels and Ca2+-mediated inactivation of L-type channels, and the contribution of VK channels may be relatively small. Since the inactivation of these channels occurs slowly, these channels may contribute to both slow AHPs and the slow depolarizations in the pig bladder. One might expect 4-AP to inhibit the slow AHPs indirectly by releasing ACh from nerves rather than by blocking VK channels on smooth muscle membrane. Indeed, the effects of 4-AP on action potentials resembled those of bath-applied CCh. Although we could not exclude this possibility, our studies on guinea-pig bladders showed that effects of K+ channel blockers, including 4-AP, on spontaneous action potentials were not greatly changed by a cocktail of neurotransmitter blockers (Hashitani & Brading, paired submission). Furthermore, 4-AP simply increased action potential frequency without changing either action potential shape or AHPs in the guinea-pig bladder, while it apparently suppressed slow AHPs. Alternatively, muscarinic stimulation on detrusor smooth muscles may inhibit a particular type of K+ channel which contributes to the slow AHPs.
A striking finding of the present study is apamin-sensitive IJP-like hyperpolarizations, which were observed in both human and pig bladder. Apamin-sensitive IJPs mediated by nonadrenergic, noncholinergic nerves have been reported in several smooth muscles (Seki & Suzuki, 1989; Vogalis & Sanders, 1990; Rae & Muir, 1996) and are associated with relaxations of the muscle. In bladder smooth muscles, IJP-like hyperpolarizations are mediated by neurally released ACh, and are probably associated with muscle contractions. After inhibiting these hyperpolarizations, transmural stimuli initiated larger depolarization and triggered bursts of action potentials, indicating that the hyperpolarizations may work as a negative feedback to prevent excessive calcium entry through L-type calcium channels. Muscarinic stimulation in the bladder smooth muscle is thought to increase [Ca2+]i initially by the Ca2+ release from InsP3 receptors on internal store membranes (Iacovou et al., 1990; Andersson et al., 1991; Wu et al., 1999), and then additionally through an increase in action potential frequency, by Ca2+ influx through L-type Ca2+ channels. Therefore, IJP-like hyperpolarizations presumably result form the activation of SK channels by Ca2+ release through InsP3 receptors.
In conclusion, detrusor smooth muscles of the pig bladder share several characteristic electrical properties with those of the human, and thus pig bladders provide a suitable model to investigate human bladder function. In both human and pig, BK channels are activated by depolarizations and Ca2+ influx through L-type Ca2+ channels and contribute to the repolarizing phase of action potentials. SK channels cause a ‘negative feedback' on L-type calcium channels by producing fast AHPs and IJP-like hyperpolarizations, and would be a novel target for pharmacological treatment of the unstable bladder. In addition, VK channels may contribute to the slow AHPs and play a ‘negative feedback' role by determining action potential frequency.
Acknowledgments
This work was supported by grants from Action Research, Japan Society for the Promotion of Science and Uehara Memorial Foundation. We thank Wyeth Research for the gift of some of the compounds used.
Abbreviations
- AHP
after-hyperpolarization
- 4-AP
4-aminopyridine
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester)
- BK channel
large conductance Ca2+-activated K+ channel
- CICR
calcium-induced calcium release
- CPA
cyclopiazonic acid
- CTX
charybdotoxin
- [Ca2+]c
cytosolic concentration of free calcium ions
- DMSO
dimethyl sulphoxide
- dV/dtL
leading slope
- dV/dtT
trailing slope
- EJP
excitatory junction potential
- IJP
inhibitory junction potential
- SK channel
small conductance Ca2+-activated K+ channel
- VK channel
voltage-sensitive K+ channels
References
- ANDERSSON K.-E., HOLMQUIST F., FOVAEUS M., HEDLUND H., SUNDLER R. Muscarinic receptor stimulation of phosphoinositide hydrolysis in the human isolated urinary bladder. J. Urol. 1991;146:1156–1159. doi: 10.1016/s0022-5347(17)38030-8. [DOI] [PubMed] [Google Scholar]
- BRADING A.F., MOSTWIN J.L. Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. Br. J. Pharmacol. 1989;98:1083–1090. doi: 10.1111/j.1476-5381.1989.tb12651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAMICH N.J., BRADING A.F. Electrical properties of smooth muscle in the guinea-pig urinary bladder. J. Physiol. 1996;492:185–198. doi: 10.1113/jphysiol.1996.sp021300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKNER S.A., MILICIC I., DAZA A.V., COGHLAN M.J., GOPALAKRISHNAN M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br. J. Pharmacol. 2002;135:639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREED K.E., ISHIKAWA S., ITO Y. Electrical and mechanical activity of recorded from rabbit urinary bladder in response to nerve stimulation. J. Physiol. 1983;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EXINTARIS B., LANG R.J. Effects of nerve stimulation on spontaneously active preparations of the guinea pig ureter. Urol. Res. 1999;27:328–335. doi: 10.1007/s002400050159. [DOI] [PubMed] [Google Scholar]
- FUJJI K. Evidence for adenosine triphosphate as an excitatory transmitter in the guinea-pig, rabbit and pig urinary bladder. J. Physiol. 1988;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJII K., FOSTER C.D., BRADING A.F., PAREKH A.B. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br. J. Pharmacol. 1990;99:779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANITKEVICH V.Y., ISENBERG G. Contribution of Ca2+-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. J. Physiol. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., SUZUKI H., KUMAZAWA J. Effects of Y-26763, a novel K-channel opener, on electrical responses of smooth muscles in the guinea pig bladder. J. Urol. 1996;155:1454–1458. [PubMed] [Google Scholar]
- HASHITANI H, BRAMICH N.J., HIRST G.D.S. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J. Physiol. 2000;524:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., FUKUTA H., TAKANO H., KLEMM M., SUZUKI H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J. Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPNER T.J., BONEV A.D., NELSON M.T. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am. J. Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- HERRERA G.M., HEPPNER T.J., NELSON M.T. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am. J. Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- HERRERA G.M., NELSON M.T. Differential regulation of SK and BK channels by Ca(2+) signals from Ca(2+) channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J. Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IACOVOU J.W., HILL S.J., BIRMINGHAM A.T. Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by differential mechanisms. J. Urol. 1990;144:775–779. doi: 10.1016/s0022-5347(17)39590-3. [DOI] [PubMed] [Google Scholar]
- KAJIOKA S., NAKAYAMA S., McMURRAY G., ABE K., BRADING A.F. Ca2+ channel properties in smooth muscle cells of the urinary bladder from pig and human. Eur. J. Pharmacol. 2002;443:19–29. doi: 10.1016/s0014-2999(02)01593-5. [DOI] [PubMed] [Google Scholar]
- KLOCKNER U., ISENBERG G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflugers Arch. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- MOSTWIN J.L. Receptor operated intracellular calcium stores in the smooth muscle of the guinea pig bladder. J. Urol. 1985;133:900–905. doi: 10.1016/s0022-5347(17)49277-9. [DOI] [PubMed] [Google Scholar]
- MOSTWIN J.L. The action potential of guinea pig bladder smooth muscle. J. Urol. 1986;135:1299–1303. doi: 10.1016/s0022-5347(17)46079-4. [DOI] [PubMed] [Google Scholar]
- NAKAHIRA Y., HASHITANI H., FUKUTA H., SASAKI S., KOHRI K., SUZUKI H. Effects of isoproterenol on spontaneous excitations in detrusor smooth muscle cells of the guinea pig. J. Urol. 2001;166:335–340. [PubMed] [Google Scholar]
- OHI Y., YAMAMURA H., NAGANO N., OHYA S., MURAKI K., WATANABE M., IMAIZUMI Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J. Physiol. 2001;534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAE M.G., MUIR T.C. Neuronal mediators of inhibitory junction potentials and relaxation in the guinea-pig internal anal sphincter. J. Physiol. 1996;493:517–527. doi: 10.1113/jphysiol.1996.sp021400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIBLEY G.N.A. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J. Physiol. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER P., HOPP H.H., ISENBERG G. Ca2+ influx through ATP-gated channels increments [Ca2+]i and inactivates ICa in myocytes from guinea-pig urinary bladder. J. Physiol. 1991;440:479–496. doi: 10.1113/jphysiol.1991.sp018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKI N., SUZUKI H. Electrical and mechanical activity of rabbit prostate smooth muscles in response to nerve stimulation. J. Physiol. 1989;419:651–663. doi: 10.1113/jphysiol.1989.sp017891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKI N., KARIM O.M., MOSTWIN J.L. Effect of pinacidil on the membrane electrical activity of guinea pig detrusor muscle. J. Pharmacol. Exp. Ther. 1992;263:816–822. [PubMed] [Google Scholar]
- SUI G.-P., WU C., FRY C. The electrophysiological properties of cultured and freshly isolated detrusor smooth muscle cells. J. Urol. 2001;165:627–632. doi: 10.1097/00005392-200102000-00085. [DOI] [PubMed] [Google Scholar]
- VISSER A.J., VAN MASTRIGT R. Simultaneous recording of mechanical and intracellular electrical activity in guinea-pig urinary bladder smooth muscle: a comparison with human detrusor contraction. Urology. 2000a;56:696–701. doi: 10.1016/s0090-4295(00)00679-8. [DOI] [PubMed] [Google Scholar]
- VISSER A.J., VAN MASTRIGT R. Simultaneous recording of mechanical and intracellular electrical activity in human urinary bladder smooth muscle. Br. J. Urol. Int. 2000b;86:113–120. doi: 10.1046/j.1464-410x.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- VOGALIS F., SANDERS K.M. Excitatory and inhibitory neural regulation of canine pyloric smooth muscle. Am. J. Physiol. 1990;259:G125–G133. doi: 10.1152/ajpgi.1990.259.1.G125. [DOI] [PubMed] [Google Scholar]
- WU C., BAYLISS M., NEWGREEN D., MUNDY A.R., FRY C.H. A comparison of the mode of action of ATP and carbachol on isolated human detrusor smooth muscle. J. Urol. 1999;162:1840–1847. [PubMed] [Google Scholar]