Abstract
Liraglutide is a long-acting GLP-1 derivative, designed for once daily administration in type II diabetic patients. To investigate the effects of liraglutide on glycemic control and β-cell mass in rat models of β-cell deficiencies, studies were performed in male Zucker diabetic fatty (ZDF) rats and in 60% pancreatectomized rats.
When liraglutide was dosed s.c. at 150 μg kg−1 b.i.d. for 6 weeks in ZDF rats 6–8 weeks of age at study start, diabetes development was markedly attenuated. Blood glucose was approximately 12 mM lower compared to vehicle (P<0.0002), and plasma insulin was 2–3-fold higher during a normal 24-h feeding period (P<0.001). Judged by pair feeding, approximately 53% of the antihyperglycemic effect observed on 24-h glucose profiles was mediated by a reduction in food intake, which persisted throughout the study and averaged 16% (P<0.02).
Histological analyses revealed that β-cell mass and proliferation were significantly lower in prediabetic animals still normoglycemic after 2 weeks treatment compared to vehicle-treated animals that had begun to develop diabetes. When the treatment period was 6 weeks, the liraglutide-treated animals were no longer completely normoglycemic and the β-cell mass was significantly increased compared to overtly diabetic vehicle-treated animals, while β-cell proliferation was unaffected.
In the experiments with 60% pancreatectomized rats, 8 days treatment with liraglutide resulted in a significantly lower glucose excursion in response to oral glucose compared to vehicle treatment. Again, part of the antihyperglycemic effect was due to reduced food intake. No effect of liraglutide on β-cell mass was observed in these virtually normoglycemic animals.
In conclusion, treatment with liraglutide has marked antihyperglycemic effects in rodent models of β-cell deficiencies, and the in vivo effect of liraglutide on β-cell mass may in part depend on the metabolic state of the animals.
Keywords: Glucagon-like peptide-1, type II diabetes, Zucker diabetic fatty, 60% pancreatectomy
Introduction
Glucagon-like peptide-1 (GLP-1) is secreted from the intestinal L-cells in response to ingested carbohydrates and fat (Kreymann et al., 1987; Ørskov, 1992; Holst, 1997). The molecule has a spectrum of physiological effects, including glucose-dependent stimulation of insulin secretion and inhibition of glucagon secretion (Gromada et al., 1998), inhibition of small bowel motility (Tolessa et al., 1998) and gastric emptying (Nauck et al., 1997), and reduction of appetite (Flint et al., 1998; Toft-Nielsen et al., 1999). Moreover, recent evidence suggests that GLP-1 and analogs act as trophic agents in the pancreas, causing β-cell proliferation and neogenesis (Buteau et al., 1999; Edvell & Lindström, 1999; Xu et al., 1999; Perfetti et al., 2000; Moldrup et al., 2001; Farilla et al., 2002; Rolin et al., 2002), and some studies indicate that it also inhibits β-cell apoptosis (Bregenholt et al., 2001; Farilla et al., 2002; Li et al., 2003). Thus, GLP-1 may be an important physiological regulator of β-cell mass. Taken as a whole, these properties make GLP-1 ideally suited to be developed as an antidiabetic agent, particularly since many of its effects are glucose-dependent and therefore treatment would likely be associated with a very low risk of hypoglycemia. Consequently, many studies have been undertaken and shown GLP-1 to be highly effective in reducing blood glucose levels in patients with type II diabetes (Nauck et al., 1993; Gutniak et al., 1994; Rachman et al., 1997; Larsen et al., 2001a,2001b). Native GLP-1 is, however, rapidly degraded by dipeptidyl peptidase IV (DPPIV; Mentlein et al., 1993; Deacon et al., 1995) and cleared by the kidneys (Deacon et al., 1996), giving the biologically active peptide a half-life of less than 2 min after i.v. administration (Deacon et al., 1996) and 1–2 h after s.c. administration (Knudsen et al., 2000). Since the presence of an elevated GLP-1 concentration is necessary in type II diabetic patients in order to obtain a continuous effect, the pharmacokinetic properties of the native peptide make it less than ideal for therapeutic use. Liraglutide (Arg34Lys26-(N-ɛ-(γ-Glu(N-α-hexadecanoyl)))-GLP-1(7-37)), also known as NN2211, is a novel long-acting analog, obtained by derivatizing GLP-1 with a fatty acid side chain, which promotes albumin binding and prevents degradation by DPPIV (unpublished observations). Liraglutide and GLP-1 are equipotent in vitro (Knudsen et al., 2000). When injected s.c., these features, combined with a slow release from the injection site, result in a compound with a prolonged plasma half-life of 14 h in pigs (Knudsen et al., 2000) and 10–12 h in man (Agersø et al., 2002; Juhl et al., 2002), which makes it suitable for once daily administration (Knudsen et al., 2000).
The present study was undertaken to investigate the effects of liraglutide on glycemia and β-cell mass in two β-cell-deficient rat models: male Zucker diabetic fatty (ZDF) rats, a model of type II diabetes in which insulin resistance and β-cell defects are prominent features (Clark et al., 1983; Etgen & Oldham, 2000), and 60% pancreatectomized Sprague–Dawley rats, a model of β-cell deficiency (Liu et al., 2000).
Methods
Animals
All studies were carried out with permits from the Animal Experiments Inspectorate, Ministry of Justice, Denmark. Male ZDF (fa/fa) rats (ZDF) and lean male ZDF (fa/+ or +/+) rats (lean) were purchased from Genetic Models Inc. (Indianapolis, IN, U.S.A.) and housed two per cage (study 1a and 1c) or individually (study 1b). Male Sprague–Dawley rats were obtained from M&B, Lille Skensved, Denmark, and were housed individually. Unless otherwise stated, animals had free access to food (Purina 5008) and drinking water.
Experimental procedures
Dosing
In all studies, the animals were dosed with subcutaneous injections (1 ml kg−1) of vehicle or liraglutide at the various doses indicated at approximately 0715–0800 and 1400–1415 h. Twice daily dosing was used because the pharmacokinetic half-life of liraglutide is only approximately 4 h in rats (Novo Nordisk, data on file).
Blood sampling
Blood for the determination of whole-blood glucose concentration and HbA1c was collected into heparinized 10 and 5 μl glass tubes, respectively, by puncture of the capillary vessels in the tail tip, and diluted in analysis buffer. Samples for insulin measurements were also collected from the tail into 100-μl heparin-coated glass tubes and were centrifuged at 4°C for 10 min and plasma was separated and stored at −20°C until assayed.
Oral glucose tolerance test
Oral glucose tolerance tests (OGTTs) were performed in fasted animals. For ZDF rats, 1 g kg−1 glucose solution and for 60% pancreatectomized rats, 2 g kg−1 glucose solution was administered by gavage. Compound or vehicle was administered at the regular time points. Samples for the measurement of blood glucose and, in some cases, plasma insulin were drawn immediately prior to glucose administration and at intervals thereafter.
Studies in ZDF rats
These studies were performed to investigate the ability of liraglutide to influence diabetes development and β-cell proliferation and mass in the ZDF rat model.
Study 1a
Three groups of animals (n=6; age 7–8 weeks at the start of the study) were housed two per cage and treated b.i.d. with either vehicle or liraglutide (low dose, 30 μg kg−1; high dose, 150 μg kg−1) for 6 weeks. Initial random glucose concentrations were not significantly different between the three groups (vehicle: 6.55±0.33; low dose: 6.40±0.22; high dose: 6.42±0.20 mM). Blood samples for 24-h profiles of glucose were taken approximately every 4 h on day 9, OGTTs were performed on days 21 and 36, and a final 24-h profile of glucose and insulin was carried out on day 41. Food and water intake were monitored daily (at approx. 0800 h), and body weight was measured regularly.
Study 1b
Pair feeding was employed in order to investigate to what extent the blood glucose-lowering effect observed with 150 μg kg−1 liraglutide in study 1a could be ascribed to the reduction in food intake. Four groups of animals (age 6 weeks at the start of the study) were individually housed and treated b.i.d. with either vehicle (lean, n=9; ZDF vehicle, n=7; ZDF pair-fed, n=8) or liraglutide (150 μg kg−1, n=8) for 6 weeks. Pair-matching was based on the animals' initial body weights. Daily food consumption was measured for each liraglutide-treated animal and this amount of food was then made available to the pair-matched vehicle-treated animal on the following day. Glucose and insulin profiles (24 h) were assessed on day 38. Food and water intake were measured daily and body weight was recorded twice weekly.
Study 1c
Two groups of animals (n=9–10; age 8 weeks at the start of the study) received either vehicle or liraglutide (200 μg kg−1) b.i.d. for 2 weeks. OGTTs were performed after the first dose and again after 13 days. Food and water consumption were recorded throughout the study period. Body weight and fructosamine were measured before and at the end of the study.
Studies in 60% pancreatectomized rats
These studies were performed to test the effect of short-term liraglutide treatment on glucose tolerance and β-cell mass in 60% pancreatectomized rats, a nongenetic model of β-cell deficiency.
Study 2a
In all, 12 male Sprague–Dawley rats of 80–100 g received presurgical streptocillin treatment (50 μl (100 g−1) i.m.) prior to isofluoran anesthesia and were subjected to 60% pancreatectomy using the method previously described (Liu et al., 2000). Briefly, the portion of the pancreas bordered by the spleen and ventriculus extending to the duodenum was carefully removed by gentle abrasion with cotton applicators, leaving the ligament with arteries and veins intact. The remnant pancreatic tissue was anatomically defined as the duodenal segment. All animals received postsurgical pain relief Finadyne (20 μl (100 g−1) i.m.) and were allowed to recuperate for 4 days postoperatively. Animals were allocated into two groups (n=6), based on an OGTT on day 4, after which they received either vehicle or liraglutide (100 μg kg−1) b.i.d. for 4 days. A second OGTT was carried out on day 8.
Study 2b
In all, 24 male Sprague–Dawley rats were subjected to 60% pancreatectomy and allocated into three groups (n=8) after an OGTT on day 4. One group was thereafter treated with vehicle, a second vehicle-treated group was pair-fed relative to the liraglutide-treated group, and the third group received liraglutide (150 μg kg−1) b.i.d., all for 4 days. A second OGTT was carried out on day 8.
Histology
In all studies, 4 h prior to killing, animals were injected with bromodeoxyuridine (BrdU, 100 mg kg−1 i.p.). The animals were anesthetized with CO2, decapitated, and approximately 5 ml whole blood for determination of fructosamine (study 1a) and liraglutide concentrations (study 1b) was collected from the trunk into 10 ml tubes containing 0.12 ml 15% K3 ETDA. The pancreata were immediately isolated for histological analysis, weighed, fixed in 4% paraformaldehyde overnight, cut in small pieces, fractionated according to the smooth fractionator principle (Bock et al., 1999), dehydrated, and embedded in paraffin. Paraffin sections (3 μm) were cut, deparaffinized, rehydrated, the endogenous peroxidase blocked by H2O2 in 96% ethanol, and blocked with avidin and then biotin, before use for immunohistochemical staining of islet cell markers, which was carried out with slides from all pancreata in parallel. For antigen retrieval, the sections in 0.01 M citrate buffer pH 6.0, preheated to 90°C, were heated 3 × 5 min in a microwave oven, before blocking with avidin and biotin blocking solutions (DAKO) and 10% normal goat serum. BrdU was stained by monoclonal mouse anti-BrdU (M0744, DAKO) 1 : 50 in 7% goat serum 3% rat serum in TBS, biotinylated goat anti-mouse Ig (E0433, DAKO) 1 : 300, and streptavidin peroxidase (Vectastain, Vector). The peroxidase activity was developed 5 min with 0.066% diaminobenzidine (DAB)+0.01% H2O2+2.5% NiSO4 to render BrdU containing nuclei black. Insulin was stained with guinea-pig antiinsulin (#651041, ICN) 1 : 400 in 7% rabbit serum 3% rat serum in TBS-T, peroxidase-coupled rabbit anti-guinea-pig Ig (P141, DAKO) 1 : 100, and developed with DAB for brown cytoplasm or with Vector Nova Red (Vector) according to the manufacturer, to stain the β-cell cytoplasm reddish brown. Finally, the slides were lightly counterstained with Mayer's hematoxylin. Neighboring sections were stained for non-β-cells with a mixture of monoclonal mouse antiglucagon (GLU-001, Novo Nordisk) 1 : 800+rabbit antisomatostatin (A566, DAKO) 1 : 600+rabbit antipancreatic polypeptide (A619, DAKO) 1 : 1000 in 4% swine serum+4% goat serum+3% rat serum in TBS-T, detected using biotinylated goat anti-mouse IgG (E0433, DAKO) 1 : 400+biotinylated swine anti-rabbit (E353, DAKO) 1 : 400, streptavidin peroxidase, and developed with DAB and NiSO4, as above. In ZDF studies 1a and 1b, β-cells were subsequently stained for insulin as described above. Reagents were obtained from DAKO, Copenhagen, Denmark, if not otherwise stated. BrdU staining of cell nuclei was examined in 1000–1500 β-cells per section in an Olympus BX-50 microscope with a video camera and monitor (Olympus, Copenhagen, Denmark), at a total n-screen magnification of × 1920. The sections were systematically scanned using a PC-controlled motorized stage and CAST-GRID software (Olympus). The volume fractions of β- and non-β-cells were estimated by point-counting stereological techniques, at a total on-screen magnification of × 960, a grid of 6 × 64 points, and step lengths of 1200 × 1000 mm2. The sections were examined with their origin blinded to the observer. Two sections cut 250 μm apart were stained and analyzed for each set of estimations.
Assays
Glucose concentrations were measured by the immobilized glucose oxidase method using an EBIO Plus autoanalyser (Eppendorf, Germany). Plasma insulin concentration was measured with an in-house ELISA method (Johansen et al., 1999) using 15 μl samples. Rat insulin was used as standard. Per cent HbA1c (Roche A/S) was measured by the enzymatic calorimetric method (COBAS MIRA Plus Autoanalyser, Roche Diagnostic Systems, Basel, Switzerland). The concentration of liraglutide was measured by sandwich ELISA (Wilken et al., 2000).
Statistics
Paired and unpaired t-tests and one-way analysis of variance (ANOVA) with Duncan's and Tukey's post hoc tests for pairwise group comparisons were employed. Area under the curve (AUC) was calculated with the trapezoidal rule. Data are expressed as mean±s.e.m. Differences with P<0.05 were considered significant.
Results
ZDF studies: metabolic parameters
Glucose tolerance tests
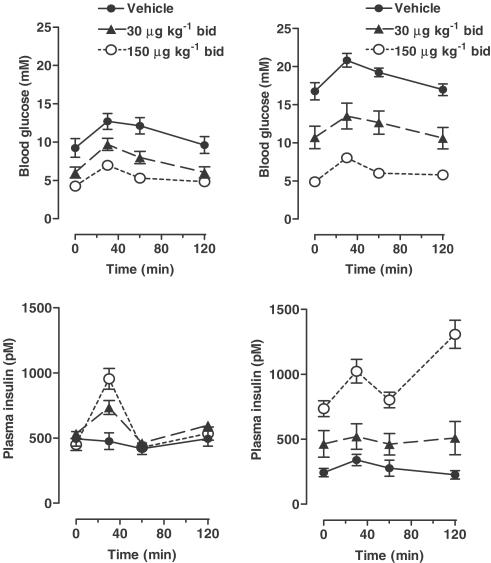
Study 1a: Both the low and high doses of liraglutide significantly lowered glucose excursions (AUC) compared to vehicle treatment. Differences were seen both during the first OGTT (Figure 1; day 21; P<0.0005, ANOVA), and during the second OGTT (Figure 1; day 36; AUCs: vehicle 2250±90 mM min vs low dose 1450±180 mM min vs high dose 760±40 mM min, P<0.0002, ANOVA, all groups different by post hoc testing), demonstrating a continuing antihyperglycemic effect at both dose levels. Plasma insulin concentrations were also increased in a dose-dependent manner (AUCs: vehicle 33±5 nM min and low dose 59±12 nM min vs high dose 117±8 nM min, P<0.0002, ANOVA, high dose different from low dose and vehicle) and the effect of the drug increased with time. Thus, in the high-dose group, the AUC of insulin was increased between the first and second OGTT (P<0.005, paired t-test), the increase averaging 66%. In contrast, in the vehicle group, the AUC of insulin was decreased between the first and second OGTT (P<0.0005, paired t-test), the decrease averaging 40%. In the second OGTT, the effect on the second phase of insulin secretion appeared particularly pronounced in the high-dose group.
Figure 1.
ZDF study 1a. OGTT after 21 days (left) and 36 days liraglutide treatment (right). Glucose (1 g kg−1) was administered by gavage at time 0 to overnight fasted animals and BG and plasma insulin were measured basally and after the glucose challenge. All the three groups differed significantly with respect to total AUC after the glucose challenge (P<0.0005 by ANOVA, day 21, P<0.0002 by ANOVA, day 36).
Study 1c: In the shorter-term study, no effect of the first dose was observed on glucose or insulin excursion during the OGTT, while significant effects on both parameters were observed on day 13 (data not shown).
Profiles (24 h), fructosamine and HbA1c
Study 1a: After 41 days treatment, the final 24-h glucose profile (Figure 2) was significantly lower in the high-dose liraglutide group, the difference to the vehicle-treated group averaged ∼12 mM (glucose AUCs: vehicle 31±1 M min and low dose 26±3 M min vs high dose 14±2 M min, P<0.0002, ANOVA, high dose different from low dose and vehicle). In contrast, the 24-h insulin profile demonstrated increased insulin levels in the high-dose group (insulin AUCs: vehicle 0.74±0.12 μM min and low dose 0.92±0.30 μM min vs high dose 2.35+0.34 μM min, P<0.002, ANOVA, high dose different from low dose and vehicle). Fructosamine was significantly reduced in the high-dose group (vehicle 229±7 μM and low dose 219±11 μM vs high dose 174±8 μM, P<0.002, ANOVA).
Figure 2.
ZDF study 1a. Glucose and insulin profiles (24 h) after 41 days liraglutide treatment. Animals had free access to food throughout. Judged by 24-h AUCs, BG was significantly decreased (P<0.0002 by ANOVA) and plasma insulin was significantly increased (P<0.002 by ANOVA) in the group receiving high-dose liraglutide compared to low dose and vehicle.
Study 1b: After treatment for 38 days, the AUCs of the 24-h glucose profiles were significantly different between all four groups (Figure 3). Lean animals had the lowest and ZDF vehicle animals the highest glucose AUC. Both liraglutide and pair feeding reduced the glucose AUC compared to the ZDF vehicle group. The reduction achieved by pair feeding alone was 7.5 M min, that is, approximately 53% of the 14.2 M min reduction observed with liraglutide treatment (Table 1). Similarly, HbA1c was significantly reduced in both ZDF liraglutide and ZDF pair-fed animals compared to vehicle, but was not normal compared with lean vehicle (Table 1). AUC for plasma insulin did not differ between ZDF liraglutide and ZDF pair-fed, but both were higher than lean vehicle and ZDF vehicle, which were similar to each other (Table 1). The reproducibility of the glucose and insulin results compared with study 1a strongly suggests that any stress caused to the animals by the individual housing did not influence the development of diabetes.
Figure 3.
ZDF study 1b. Glucose and insulin profiles (24 h) after 38 days liraglutide treatment. Animals had free access to food throughout. Judged by 24-h AUCs, BG was significantly decreased (P<0.0001 by ANOVA) and plasma insulin was significantly increased (P<0.0001 by ANOVA) in animals receiving liraglutide compared to vehicle (see Table 1 for detailed analysis).
Table 1.
ZDF, study 1b: AUC of glucose and insulin during 24-h profile and HbA1c after 38 days treatment
| AUC glucose (M min) | AUC insulin (μM min) | HbA1c (%) | |
|---|---|---|---|
| Lean vehicle | 8.3±0.1 | 0.6±0.02 | 3.9±0.04 |
| ZDF vehicle | 30.4±0.6* | 0.8±0.1 | 8.6±0.2* |
| ZDF pair-fed | 22.9±0.2*† | 2.0±0.3*† | 6.3±0.4*‡ |
| ZDF liraglutide | 16.2±0.2‡§∥ | 2.5±0.4*‡ | 5.5±0.4‡§ |
| ANOVA | P<0.0001 | P<0.0001 | P<0.0001 |
P<0.001 vs lean vehicle;
P<0.01 vs ZDF vehicle;
P<0.001 vs ZDF vehicle;
P<0.01 vs lean vehicle;
P<0.05 vs ZDF pair-fed, Tukey's post hoc test. Data are mean±s.e.m.
Study 1c: Fructosamine levels were decreased in the liraglutide group compared to vehicle.
Food and water consumption, body weight
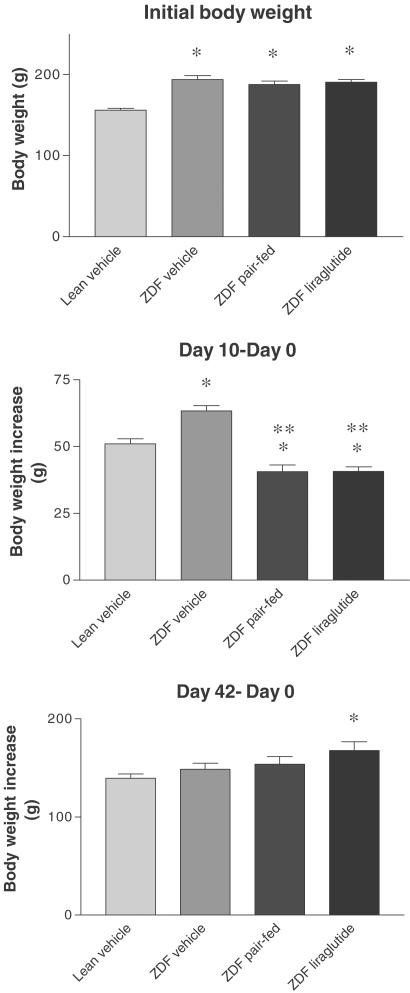
In all the three ZDF studies, liraglutide treatment had an immediate strong effect on lowering both food and water intake immediately after dosing was initiated. With continued dosing, a less pronounced, but significant effect on food and water consumption was observed. At the end of the study, the overtly hyperglycemic vehicle-treated ZDF rats consumed significantly more water than the remaining groups. The data from study 1b are representative and are summarized in Table 2. Hematocrit measured at the end of study 1a was not reduced by liraglutide treatment (data not shown), indicating that the reduced water consumption was not associated with dehydration. Within each of the three ZDF studies, initial body weight did not differ between ZDF groups and in the early treatment period, the reduced food intake in liraglutide-treated animals resulted in an attenuated weight gain. However, as the treatment duration increased, the difference in weight disappeared, reflecting a treatment-related reduced loss of calories due to glucosuria. Data on body weight from study 1b are representative and are illustrated in Figure 4.
Table 2.
ZDF, study 1b: food and water intake
| Average daily food intake (g) | Average daily food intake, final week (g) | Average daily water intake, final week (g) | |
|---|---|---|---|
| Lean vehicle | 20.2±0.4 | 20.0±0.6 | 29±1# |
| ZDF vehicle | 30.4±0.7* | 30.8±0.9* | 94±11 |
| ZDF pair-fed | 23.2±0.3*# | 23.5±0.5§# | 56±4§# |
| ZDF liraglutide | 23.8±0.4*# | 25.1±0.6*# | 38±3# |
| ANOVA | P<0.0001 | P<0.0001 | P<0.0001 |
P<0.001 vs lean vehicle;
P<0.001 vs ZDF vehicle;
P<0.01 vs lean vehicle. Data are mean±s.e.m.
Figure 4.
ZDF study 1b. Initial body weight and body weight increase after 10 and 42 days liraglutide treatment. *P<0.01 vs lean vehicle; **P<0.001 vs ZDF vehicle.
Plasma concentration liraglutide
In study 1b, the total plasma concentration (albumin bound+free) of liraglutide was 160±12 nM (approximately 5–7 h after the final dose). The high total concentration reflects the albumin-binding properties of liraglutide.
ZDF studies: histology
Proliferation of β-cells was demonstrated by the incorporation of BrdU into β-cell nuclei (Figure 5). It should be noted that the number of non-β-cells in islets, duct cells, and exocrine cells that incorporated BrdU exceeded that of β-cells. The β-cell mass was estimated by point-counting morphometry. In study 1a, after 6 weeks treatment in the study, β-cell mass (volume fraction) was increased in both the low- and high-dose liraglutide groups, although only statistically significant for the low-dose group (Figure 6a), and there was a weak trend towards increased β-cell proliferation as estimated by the BrdU indices. In general, the islet morphology in all groups of ZDF rats was quite irregular and the β-cell insulin-staining intensity variable with no treatment-related differences in staining intensity (not shown). In study 1b, β-cell volume fractions in liraglutide-treated and pair-fed groups were higher than in the lean control group, but not higher than in the vehicle-treated ZDF rats (Figure 6b). When expressed in terms of total β-cell mass, the liraglutide-treated group of ZDF rats had a higher β-cell mass than the ZDF vehicle animals (20.9±2.6 vs 12.4±1.8 mg, P<0.03). There was no difference between the β-cell proliferation rates of the three groups of ZDF rats and no obvious differences between insulin-staining intensities.
Figure 5.
BrdU incorporation in β- and non-β-cells of the pancreas of a 13-week-old vehicle-treated diabetic ZDF rat from ZDF study 1a. Black nuclei=BrdU incorporation, brown cytoplasm=insulin in β-cells. Filled arrows show BrdU-positive β-cells, empty arrow shows a BrdU-positive non-β-cell (exocrine or centro-acinar duct cell?). Horizontal bar indicates 50 μm.
Figure 6.
Quantitation of β-cell proliferation and mass (volume fraction) in ZDF experiments. Study 1a: after 6 weeks treatment, β-cell volume fraction was increased in both the low- and high-dose liraglutide groups, though only statistically significant for the low-dose group (P<0.05) There was a weak trend towards increased β-cell proliferation as estimated by the BrdU indices. Study 1b: β-cell volume fraction in liraglutide-treated and pair-fed groups were higher than in the lean control group, but not significantly higher than in the vehicle-treated ZDF rats. There was no difference between the β-cell proliferation rates of the three groups of ZDF rats. Study 1c: β-cell volume fraction was significantly lower in the animals treated with liraglutide (P<0.002), and this relative decrease occurred concomitantly with a markedly reduced β-cell proliferation (P<0.006).
After only 2 weeks treatment in study 1c, β-cell mass (volume fraction) was significantly lower in the animals treated with liraglutide (0.66±0.04 vs 0.90±0.06%, P<0.002), and this relative decrease occurred concomitantly with a markedly reduced β-cell proliferation (0.13±0.04 vs 0.46±0.07%, P<0.006, Figure 6c). In contrast, the insulin-staining intensity of β-cells in the liraglutide-treated rats was markedly higher than in the vehicle group (Figure 7).
Figure 7.
Increased insulin staining intensity after 2 weeks liraglutide treatment. Representative sections from pancreata of ZDF rats show higher and more regular insulin-staining intensity in the liraglutide-treated rat than in the vehicle-treated rat. Note the better staining pattern and intensity of this 10-week-old newly diabetic vehicle-treated rat than that of the 13-week-old diabetic rat from the experiment shown in Figure 5.
Non-β-cells were demonstrated by a cocktail of antibodies against glucagon, somatostatin, and pancreatic polypeptide. Stereological measurements of the volume fractions of non-β-cells in the pancreata from these experiments showed no treatment-related differences between the liraglutide-treated groups of ZDF rats and the vehicle- (study 1a and 1b) or vehicle plus pair feeding (study 1b)-treated groups (not shown).
Studies in 60% pancreatectomized rats: metabolic parameters
In study 2a, liraglutide (100 μg kg−1 b.i.d.) treatment resulted in a tendency towards lower blood glucose during the OGTT compared to vehicle at day 8, but the difference in AUC for glucose was not statistically significant (Figure 8, top). However, in study 2b, the higher dose of liraglutide (150 μg kg−1 b.i.d.) resulted in significantly lower AUC for blood glucose at day 8 compared with both the vehicle and pair-fed groups. Furthermore, pair feeding itself resulted in slightly, but significantly, reduced delta AUC during the OGTT (Figure 8, bottom).
Figure 8.
OGTTs in experiments performed in 60% pancreatectomized rats. After an overnight, fast glucose (2 g kg−1) was administered by gavage at time 0 to overnight fasted animals and BG was measured basally and after the glucose challenge. Top: study 2a. After dosing vehicle or 100 μg kg−1 liraglutide b.i.d. for 4 days, a nonsignificant trend towards improved glucose tolerance was observed in the treated animals. Bottom: study 2b. After dosing vehicle or 150 μg kg−1 liraglutide b.i.d. for 4 days, significantly lower AUC for BG was observed in both the vehicle and pair-fed groups. Furthermore, pair feeding itself resulted in slightly, but significantly, reduced delta AUC during the OGTT.
Studies in 60% pancreatectomized rats: histology
In both studies 2a and 2b, the distribution of islet sizes showed a trend towards an increase in the liraglutide-treated animals. However, point-counting morphometry at sections through the remnant pancreas from study 2b revealed no significant differences in the total β-cell volume fractions (1.27±0.16% in the liraglutide group, 1.15±0.15% in the vehicle group, and 0.95±0.14% in the pair-fed group).
There was no visible difference between the vehicle- and liraglutide-treated groups in the size or number of islets located in the regenerated area. Immunostaining for BrdU revealed a marked proliferation mainly in exocrine cells located at the border of the regeneration zones in both vehicle- and liraglutide-treated animals. At this time point, there was no effect of liraglutide treatment on the proliferative index in islets or in any other areas of remnant or regenerating pancreas.
Discussion and conclusions
We have demonstrated in two different rat models of β-cell deficiency that liraglutide has marked antihyperglycemic effects. In male ZDF rats, treatment with liraglutide strongly attenuated diabetes development in prediabetic animals, while treatment in 60% pancreatectomized rats reduced glucose excursions after an OGTT. In both models, the effects were partly, but not completely, mediated by a reduction in food intake. Furthermore, we have demonstrated that increases in β-cell proliferation and mass did not occur in situations when treatment was able to maintain normoglycemia.
The male ZDF rat is a commonly used model of type II diabetes (Clark et al., 1983; Friedman et al., 1991; Sturis et al., 1995; Sreenan et al., 1996; Etgen & Oldham, 2000; Farilla et al., 2002). Homozygous males are severely insulin resistant compared to lean controls and have compensatory hyperinsulinemia at a young age (Etgen & Oldham, 2000). These features are caused by a mutation in the leptin receptor (Phillips et al., 1996) and, in this regard, the ZDF rat is like the Zucker fa/fa rat. Unlike the Zucker rat, however, the male ZDF rat develops frank diabetes, beginning between weeks 6 and 10 of age, when fed a diet containing 6.5% (weight%) fat (Purina 5008), and the animals simultaneously display a progressive deterioration of β-cell function (Etgen & Oldham, 2000). A genetic β-cell defect that segregates independently from the fa locus has recently been demonstrated in the ZDF rat (Griffen et al., 2001). Thus, it appears that two distinct genetic defects are responsible for diabetes in this model, one causing insulin resistance, and the other causing a progressive deterioration of β-cell function in the presence of insulin resistance, with the severity of disease influenced by dietary fat intake.
Exogenously administered GLP-1 stimulates insulin secretion and inhibits glucagon secretion, with both actions being glucose-dependent (Willms et al., 1996). Thus, at fasting glucose concentrations, GLP-1 causes little stimulation of insulin secretion, while at higher glucose concentrations, a much larger stimulation occurs. The signal transduction mechanism of GLP-1 on insulin secretion is not fully understood, but most likely involves cAMP generation and thus PKa activation, thereby potentiating the normal metabolic stimulus secretion mechanisms that regulate insulin secretion via the KATP channel (MacDonald et al., 2002). Liraglutide stimulates insulin secretion glucose-dependently in a pig model of glucose intolerance (Ribel et al., 2002), in keeping with the compound's mechanism of action as a full agonist at the GLP-1 receptor with an affinity equal to that of the native peptide (Knudsen et al., 2000). In addition to these pancreatic effects, exogenous GLP-1 inhibits gastric emptying dramatically (Wettergren et al., 1993; Willms et al., 1996; Nauck et al., 1997). The lack of an acute effect of liraglutide on insulin secretion in the present study is, thus, compatible with the animals' relatively low blood glucose levels, combined with a delay in gastric emptying. Indeed, it has been suggested that GLP-1's effect on gastric emptying is the major determinant of its glucose-lowering effects in healthy humans (Nauck et al., 1997). However, glucose tolerance tests in the ZDF animals showed that liraglutide treatment had a marked long-term effect, both on glucose tolerance and insulin secretion. Whereas vehicle-treated ZDF rats, as expected, started to become less hyperinsulinemic with the progressing disease, the liraglutide-treated animals exhibited an increase in insulin secretion over time. Since GLP-1 does not have a major direct insulin-sensitizing effect, at least acutely (Vella et al., 2000), the data suggest that liraglutide treatment leads to an improved ability of the β-cells to compensate for the insulin resistance in these animals. Recent evidence does suggest, however, that chronic (6 weeks) treatment with GLP-1 in type II diabetic patients can actually improve insulin sensitivity (Zander et al., 2002) and GLP-1 extrapancreatic effects at the liver have been demonstrated (Burcelin et al., 2001), raising the possibility that some of the effects of liraglutide on insulin secretory function are mediated via extrapancreatic effects.
GLP-1 has been demonstrated to reduce food intake (Flint et al., 1998) by mechanisms involving both reduced gastric emptying (Willms et al., 1996) as well as effects on satiety and appetite (Flint et al., 1998). In accordance with its mechanism of action as a GLP-1 receptor agonist (Knudsen et al., 2000), liraglutide reduced food intake in the present study. Liraglutide also reduces food and water intake and body weight in normal rats (Larsen et al., 2001a,2001b). In the present study, the reduced food intake was initially accompanied by a reduction in body weight, relative to the vehicle-treated control animals. However, as the vehicle-treated rats became more severely diabetic, glucosuria and the resultant calorie loss meant that they stopped gaining any weight, in contrast to the less diabetic, liraglutide-treated animals. In this regard, it is worth noting that in diabetes-prevention studies with troglitazone and pioglitazone in ZDF rats, much greater weight gains compared to the liraglutide-treated animals in the present study have been observed (Sturis et al., 1995; Sreenan et al., 1996). This suggests that, despite the trend to gain weight caused by the improving diabetes and resultant reduction in calorie loss, liraglutide actually has a long-term weight-reducing effect in the ZDF rat. Similarly, studies with exendin-4 have demonstrated sustained reductions in weight (Greig et al., 1999; Szayna et al., 2000), while a study in man suggests that native GLP-1 given continuously over 6 weeks also causes weight loss (Zander et al., 2002). Reduced food intake per se has been demonstrated to affect diabetes development in the ZDF rat (Ohneda et al., 1995), leading us to carry out a second study utilizing pair feeding. We found that approximately half of the beneficial effect on glycemia associated with liraglutide treatment could be ascribed to a reduced food intake, confirming that the antihyperglycemic effects of liraglutide are likely to be mediated via more than one mechanism. These findings were corroborated by the results obtained in the 60% pancreatectomized rats in that liraglutide also reduced glucose AUC after an OGTT in that model, with pair-fed animals showing an intermediate response.
Several studies have demonstrated that agonists of the GLP-1 receptor, including native GLP-1, exendin-4, and liraglutide, are capable of enhancing β-cell mass, both in vitro and in vivo. The signal transduction mechanisms mediating these effects are incompletely understood, but involve upregulation of PDX-1 (MacDonald et al., 2002). Increased proliferation rate of existing β-cells, stimulation of neogenesis and inhibition of apoptosis have all been implicated in the mechanism by which the mass increases (Buteau et al., 1999; Edvell & Lindström, 1999; Xu et al., 1999; Perfetti et al., 2000; Bregenholt et al., 2001; Moldrup et al., 2001; Farilla et al., 2002; Rolin et al., 2002; Li et al., 2003). The majority of in vivo studies have thus demonstrated an upregulation of β-cell mass. In the present study, the difference in islet histology between ZDF rats treated for 2 and 6 weeks is striking, with an expected increased β-cell mass in animals treated for 6 weeks, but a surprising decrease in β-cell proliferation and mass when the treatment period was only 2 weeks. Whereas animals treated for 6 weeks were somewhat hyperglycemic, animals treated for only 2 weeks were still normoglycemic. Also, in normoglycemic, 60% pancreatectomized rats, we observed no effect on β-cell proliferation or mass after 4 days of treatment. It thus appears that the presence of normoglycemia may decrease the ability of liraglutide to increase β-cell mass. In support of our findings, the mass of native β-cells was not found to differ between exendin-4 and control treatment in a study in normoglycemic athymic nude mice into which human fetal islets were transplanted, whereas increased mass and improved function of the β-cells in the graft of transplanted fetal islets were found (Movassat et al., 2002). However, other studies have indicated that exogenously administered GLP-1 (Perfetti et al., 2000) and exendin-4 (Xu et al., 1999) are able to increase β-cell mass in only slightly glucose intolerant (Perfetti et al., 2000) or in normoglycemic (Xu et al., 1999) rats. In those studies, drug delivery was discontinued several days prior to the assessment of β-cell mass, while our results were obtained during drug exposure. It is possible that a change in effect on β-cell mass occurs once compound exposure disappears, particularly since GLP-1 is known to stimulate somatostatin (d'Alessio et al., 1989), which in turn, has been shown to inhibit proliferation of RINm5F insulinoma cells (Stark & Mentlein, 2002). The differences between our results with liraglutide and those observed with GLP-1 and exendin-4 are unlikely to be due to a reduced ability of liraglutide to stimulate β-cell mass compared to GLP-1 and Exendin-4, since a recent study in db/db mice demonstrated liraglutide to be at least as efficacious as Exendin-4 on β-cell mass in that model (Rolin et al., 2002).
In conclusion, treatment with the long-acting GLP-1 derivative liraglutide attenuates diabetes development in prediabetic ZDF rats and has antihyperglycemic effects in 60% pancreatectomized rats. The effect is partly mediated by a reduction in food intake, but other features of liraglutide as a GLP-1 agonist are involved. In situations where normoglycemia is maintained, liraglutide does not cause expansion of β-cell mass, while on the longer term, liraglutide treatment confers protection of β-cell mass and function in ZDF rats, suggesting that the influence of GLP-1 agonism on β-cell mass dynamics in vivo may, directly or indirectly, depend on the glycemic state.
Acknowledgments
We thank Marianne Jappe, Susanne Primdahl, Anne-Mette Gjedsted, Helle Nygaard, Anne-Grethe Juul, Else Bentsen, Jesper Damgaard, Birte Jørgensen, Kent E. Pedersen, Pia Rothe, and Steen Kryger for technical assistance and the staff at the animal unit for taking care of the animals.
Abbreviations
- AUC
area under the curve
- b.i.d.
twice a day
- BrdU
bromodeoxyuridine
- DPPIV
dipeptidyl-peptidase IV
- GLP-1
glucagon-like peptide-1
- OGTT
oral glucose tolerance test
- ZDF
Zucker diabetic fatty
References
- AGERSØ H., JENSEN L.B., ELBRØND B., ROLAN P., ZDRAVKOVIC M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- BOCK T., SVENSTRUP K., PAKKENBERG B., BUSCHARD K. Unbiased estimation of total beta-cell number and mean beta-cell volume in rodent pancreas. APMIS. 1999;107:791–799. doi: 10.1111/j.1699-0463.1999.tb01474.x. [DOI] [PubMed] [Google Scholar]
- BREGENHOLT S., MOLDRUP A., KNUDSEN L.B., PETERSEN J.S. The GLP-1 derivative NN2211 inhibits cytokine-induced apoptosis in primary rat β-cells. Diabetes. 2001;50 Suppl 2:A31. [Google Scholar]
- BURCELIN R., DA COSTA A., DRUCKER D., THORENS B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720–1728. doi: 10.2337/diabetes.50.8.1720. [DOI] [PubMed] [Google Scholar]
- BUTEAU J., RODUIT R., SUSINI S., PRENTKI M. Glp-1 promotes DNA synthesis, activates phosphatidylinositol-3-kinase and increases transcription factor pancreatic and duodenal homebox gene 1 (PDX-1) DNA binding activity in beta (INS-1) cells. Diabetologia. 1999;42:856–864. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- CLARK J.B., PALMER C.J., SHAW W.N. The diabetic Zucker Fatty rat. Proc. Soc. Exp. Biol. Med. 1983;173:68–75. doi: 10.3181/00379727-173-41611. [DOI] [PubMed] [Google Scholar]
- D'ALESSIO D.A., FUJIMOTO W.Y., ENSINCK J.W. Effects of glucacon-like peptide I-(7–36) on release of insulin, glucagon, and somatostatin by rat pancreatic islet cell monolayer cultures. Diabetes. 1989;38:1534–1538. doi: 10.2337/diab.38.12.1534. [DOI] [PubMed] [Google Scholar]
- DEACON C.F., JOHNSEN A.H., HOLST J.J. Degradation of glucagons-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide which is a major endogenous metabolite in vivo. J. Clin. Endocrinol. Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- DEACON C.F., PRIMDAL L., KLARSKOV L., OLESEN M., HOLST J.J. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in anesthetized pig. Am. J. Physiol. 1996;271:E458–E464. doi: 10.1152/ajpendo.1996.271.3.E458. [DOI] [PubMed] [Google Scholar]
- EDVELL A., LINDSTRÖM P. Initiation of increased pancreatic islet growth in young normoglycaemic mice (Umeå +/?) Endocrinology. 1999;140:778–783. doi: 10.1210/endo.140.2.6514. [DOI] [PubMed] [Google Scholar]
- ETGEN G.J., OLDHAM B.A. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000;49:684–688. doi: 10.1016/s0026-0495(00)80049-9. [DOI] [PubMed] [Google Scholar]
- FARILLA L., HUI H., BERTOLOTTO C., KANG E., BULOTTA A., DI MARIO U., PERFETTI R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143:4397–4408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- FLINT A., RABEN A., ASTRUP A., HOLST J.J. GLP-1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN J.E., DE VENTE J.E., PETERSON R.G., DOHM G.L. Altered expression of muscle glucose transporter GLUT-4 in diabetic fatty Zucker rats (ZDF/Drt-fa) Am. J. Physiol. 1991;261:E782–E788. doi: 10.1152/ajpendo.1991.261.6.E782. [DOI] [PubMed] [Google Scholar]
- GREIG N.H., HOLLOWAY H.N., DEORE K.A., JANI D., WANG Y., ZHOU J., GARANT M.J., EGAN J.M. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia. 1999;42:45–50. doi: 10.1007/s001250051111. [DOI] [PubMed] [Google Scholar]
- GRIFFEN S.C., WANG J., GERMAN M.S. A genetic defect in β-cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes. 2001;50:63–68. doi: 10.2337/diabetes.50.1.63. [DOI] [PubMed] [Google Scholar]
- GROMADA J., HOLST J.J., RORSMAN P. Cellular regulation of islet hormone secretion by the incretin hormone GLP-1. Pflugers Arch. Eur. J. Phys. 1998;435:583–594. doi: 10.1007/s004240050558. [DOI] [PubMed] [Google Scholar]
- GUTNIAK M.K., LINDE B., HOLST J.J., EFENDIC S. Subcutaneous injection of the incretin hormone GLP-1 abolishes postprandial glycemia in NIDDM. Diabetes Care. 1994;17:1039–1044. doi: 10.2337/diacare.17.9.1039. [DOI] [PubMed] [Google Scholar]
- HOLST J.J. Enteroglucagon. Annu. Rev. Physiol. 1997;59:257–271. doi: 10.1146/annurev.physiol.59.1.257. [DOI] [PubMed] [Google Scholar]
- JOHANSEN T., DECKERT M., MANDRUP-POULSEN T., MALMLÖF K. The role of growth hormone and glucocorticoid in glucose handling in vivo. J. Endocrinol. 1999;162:87–93. doi: 10.1677/joe.0.1620087. [DOI] [PubMed] [Google Scholar]
- JUHL C.B., HOLLINGDAL M., PORKSEN N., STURIS J., JAKOBSEN G., SCHMITZ O. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in Type 2 diabetes. Diabetes. 2002;51:484–492. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- KNUDSEN L.B., NIELSEN P.F., HUUSFELDT P.O., JOHANSEN N.L., MADSEN K., PEDERSEN F.Z., THØGERSEN H., WILKEN M., AGERSØ H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J. Med. Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- KREYMANN B., GHATEI M.A., WILLIAMS G., BLOOM S.R. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;II:1300–1303. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- LARSEN J., HYLLEBERG G., NG K., DAMSBO P. Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care. 2001a;24:1416–1421. doi: 10.2337/diacare.24.8.1416. [DOI] [PubMed] [Google Scholar]
- LARSEN P.J., FLEDELIUS C., KNUDSEN L.B., TANG-CHRISTENSEN M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001b;50:2530–2539. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- LI Y., HANSOTIA T., YUSTA B., RIS F., HALBAN P.A., DRUCKER D.J. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J. Biol. Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- LIU Y.Q., NEVIN P.W., LEAHY J.L. Beta-cell adaptation in 60% pancreatectomy rats that preserves normoinsulinemia and normoglycemia. Am. J. Physiol. 2000;278:E68–E73. doi: 10.1152/ajpendo.2000.279.1.E68. [DOI] [PubMed] [Google Scholar]
- MACDONALD P.E., EL-KHOLY W., RIEDEL M.J., SALAPATEK A.M.F., LIGHT P.E., WHEELER M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51 Suppl 3:S434–S442. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- MENTLEIN R., GALLWITZ B., SCHMIDT W.E. Dipeptidyl peptidase IV hydrolyses gastric inhibitory polypeptide, glucagons-like peptide-1 (7-36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- MOLDRUP A., GRAM V.K., FRIEDRICHSEN B.N., NIELSEN J.H., PETERSEN J.S. Glucagon-like peptide-1 (GLP-1) and the long-acting GLP-1 derivative, NN2211, stimulate expansion of primary rat beta-cells in a camp/protein kinase A (PKA)-dependent manner. Diabetes. 2001;50 Suppl 2:A336. [Google Scholar]
- MOVASSAT J., BEATTIE G.M., LOPEZ A.D., HAYEK A. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J. Clin. Endocrinol. Metab. 2002;87:4775–4781. doi: 10.1210/jc.2002-020137. [DOI] [PubMed] [Google Scholar]
- NAUCK M.A., KLEINE N., ORSKOV C., HOLST J.J., WILLMS B., CREUTZFELDT W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- NAUCK M.A., NIEDEREREICHHOLZ U., ETTLER R., HOLST J.J., ØRSKOV C., RITZEL R., SCHMIGEL W.H. Glucagon-like peptide-1 inhibition of gastric-emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 1997;273:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- OHNEDA M., INMAN L.R., UNGER R.H. Caloric restriction in obese pre-diabetic rats prevents beta-cell depletion, loss of beta-cell GLUT 2 and glucose incompetence. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- ØRSKOV C. Glucagon-like peptide-1, a new hormone of the enteroinsular axis. Diabetologia. 1992;35:701–711. [PubMed] [Google Scholar]
- PERFETTI R., ZHOU J., DOYLE M.E., EGAN J.M. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- PHILLIPS M.S., LIU Q., HAMMOND H.A., DUGAN V., HEY P.J., CASKEY C.J., HESS J.F. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- RACHMAN J., BARROW B.A., LEVY J.C., TURNER R.C. Near normalization of diurnal glucose concentrations by continuous administration of GLP-1 in subjects with NIDDM. Diabetologia. 1997;40:205–211. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- RIBEL U., LARSEN M.O., ROLIN B., CARR R.D., WILKEN M., STURIS J., WESTERGAARD L., DEACON C.F., KNUDSEN L.B. NN2211: a long-acting glucagon-like peptide-1 derivative with anti-diabetic effects in glucose-intolerant pigs, Eur. J. Pharmacol. 2002;451:217–225. doi: 10.1016/s0014-2999(02)02189-1. [DOI] [PubMed] [Google Scholar]
- ROLIN B., LARSEN M.O., GOTFREDSEN C.F., DEACON C.F., CARR R.D., WILKEN M., KNUDSEN L.B. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases β-cell mass in diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2002;283:E745–E752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- SREENAN S., STURIS J., PUGH W.L., BURANT C.F., POLONSKY K.S. Prevention of hyperglycemia in the Zucker diabetic fatty rat by treatment with metformin or troglitazone. Am. J. Physiol. 1996;271:E742–E747. doi: 10.1152/ajpendo.1996.271.4.E742. [DOI] [PubMed] [Google Scholar]
- STARK A., MENTLEIN R. Somatostatin inhibits glucagon-like peptide-1-induced insulin secretion and proliferation of RINm5F insulinoma cells. Regul. Pept. 2002;108:97–102. doi: 10.1016/s0167-0115(02)00152-0. [DOI] [PubMed] [Google Scholar]
- STURIS J., PUGH W.L., TANG J., POLONSKY K.S. Prevention of diabetes does not completely prevent insulin secretory defects in the ZDF Rat. Am. J. Physiol. 1995;269:E786–E792. doi: 10.1152/ajpendo.1995.269.4.E786. [DOI] [PubMed] [Google Scholar]
- SZAYNA M., DOYLE M.E., BETKEY J.A., HOLLOWAY H.W., SPENCER R.G., GREIG N.H., EGAN J.M. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141:1936–1941. doi: 10.1210/endo.141.6.7490. [DOI] [PubMed] [Google Scholar]
- TOFT-NIELSEN M.B., MADSBAD S., HOLST J.J. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22:1137–1143. doi: 10.2337/diacare.22.7.1137. [DOI] [PubMed] [Google Scholar]
- TOLESSA T., GUTNIAK M., HOLST J.J., EFENDIC S., HELLSTRÖM P.M. Inhibitory effect of glucagon-like peptide-1 on small bowel motility. J. Clin. Invest. 1998;102:764–774. doi: 10.1172/JCI942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELLA A., SHAH P., BASU R., BASU A., HOLST J.J., RIZZA R.A. Effect of glucagon-like peptide 1(7–36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes. 2000;49:611–617. doi: 10.2337/diabetes.49.4.611. [DOI] [PubMed] [Google Scholar]
- WETTERGREN A., SCHJOLDAGER B., MORTENSEN P.E., MYHRE J., CHRISTIANSEN J., HOLST J.J. Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig. Dis. Sci. 1993;38:665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- WILKEN M., LARSEN F.S., JUUL A.-G., JENSEN L.B., RIBEL U. An immunoassay for the GLP-1 derivative NN2211. Diabetologia. 2000;43 Suppl. 1:A148. [Google Scholar]
- WILLMS B., WERNER J., HOLST J.J., ØRSKOV C., CREUTZFELDT W., NAUCK M. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous GLP-1 (7–36)amide in type 2 (noninsulin-dependent) diabetic patients. J. Clin. Endocrinol. Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- XU G., STOFFERS D.A., HABENER J.F., BONNER-WEIR S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- ZANDER M., MADSBAD S., HOLST J.J. GLP-1 treatment for 6 weeks improves glycaemic control, insulin sensitivity and β-cell function in type 2 diabetic patients. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]