Abstract
The effects of the selective 5-HT7 receptor antagonists SB-269970 (3-300 μg kg−1; n=5–6) and SB-656104 (30 μg kg−1; n=5) administered centrally (i.c.v.) were investigated on the ‘micturition reflex' in the urethane anaesthetized female rat.
In cystometric recordings, SB-269970 caused significant increases in volume of 58±15 and 138±33% and pressure of 140±46 and 149±60% thresholds at 10 and 30 μg kg−1. These changes were associated with significant decreases in distension-induced bladder contraction of 62±14 and 60±11%, respectively. However, there was no change in residual volume. At the higher doses, SB-269970 blocked the micturition reflex. SB-656104 had similar effects to SB-269970 but in addition significantly increased the residual volume.
SB-269970 (10 μg kg−1; n=5) given i.v. had no effect on the micturition reflex.
SB-269970 (30 μg kg−1; n=4) given intrathecally (i.t.) had no effect on micturition reflex, although the selective 5-HT1A receptor antagonist WAY-100635 given i.t. after SB-269970 caused a significant increase in the volume threshold.
Using an isovolumetric method in which urethral changes were measured, SB-269970 (30 μg kg−1; n=4; i.c.v.) failed to have any effect on these urethral-evoked changes although they significantly reduced the amplitude of the bladder contraction.
These data demonstrate that 5-HT7 receptors located supraspinally in the rat are involved in the control of micturition.
Keywords: 5-HT7 receptors, micturition, SB-266970, SB-656104, bladder, rats, WAY-100635, 5-HT1A receptors, blood pressure
Introduction
Central 5-hydroxytryptamine (5-HT) containing neurones, via activation of 5-HT1A receptors, play an important role in the reflex control of parasympathetic outflow to the heart in rats (Bogle et al., 1990), rabbits (Futuro-Neto et al., 1993; Dando et al., 1998; Skinner et al., 2002) and cats (Wang & Ramage, 2001), to the airways in cats (Bootle et al., 1996) and guinea-pigs (Bootle et al., 1998) and to the bladder in rats (Lecci et al., 1992; Testa et al., 1999; Conley et al., 2001; Kakizaki et al., 2001; Pehrson et al., 2002). Even though stimulation of other 5-HT receptors, 5-HT1B/1D (Dando et al., 1998) and 5-HT2C receptors (Steers & De Groat, 1989), can effect reflex activation of parasympathetic outflow, these receptors do not seem to play a physiological role in these reflexes. Moreover, in an examination of the effects of selective antagonists at 5-HT2A, 5-HT2C, 5-HT3, 5-HT4 and 5-HT6 receptors on isovolumetric bladder contractions in the rat, it was concluded that these receptors were not involved in the physiological control of parasympathetic outflow to the bladder (Testa et al., 2001). Interestingly, data from this study indicate that the 5-HT3 receptor antagonist Y 25130 can have an inhibitory action, while zatosetron, also a 5-HT3 receptor antagonist, failed to have any effect. However in the cat, zatosetron given intrathecally (i.t.) was found to decrease volume threshold for micturition (Espey et al., 1998). Further, reflex activation of cardiac vagal outflow in the rabbit has been reported to be attenuated by granisetron, another 5-HT3 receptor antagonist (Dando et al., 1995). Thus, the precise role of 5-HT3 receptors in reflex activation of parasympathetic outflow requires further study. However, there are no reports on the role of 5-HT7 receptors in the reflex control of micturition or other parasympathetic outflows. Interestingly, some of the nonselective 5-HT receptor antagonists, methiothepin, methysergide (To et al., 1995; Gustafson et al., 1996; Wood et al., 2000; Thomas et al., 2002), the 5-HT2C receptor agonist, metachlorophenylpiperazine (mCPP, see Hoyer et al., 2002) and the archetypal 5-HT1A receptor agonist 8-OH-DPAT (To et al., 1995; Wood et al., 2000) bind to 5-HT7 receptors and these ligands have also been shown to interfere with micturition (methysergide, Lecci et al., 1992; Espey et al., 1998; methiothepin, Testa et al., 2001; mCPP, Steers & De Groat, 1989; Guarneri et al., 1996 and 8-OH-DPAT, see above). Further mesulergine, which is considered to be a 5-HT2C receptor antagonist (see Hoyer et al., 2002), can block the effect of the selective 5-HT1A receptor antagonist WAY-100635 on the micturition reflex (Testa et al., 1999) and, at low doses, inhibit isovolumetric bladder contractions in the rat (Testa et al., 2001) and is also a 5-HT7 receptor antagonist (see Hagan et al., 2000). Thus, the present experiments were carried out to investigate the role of 5-HT7 receptors in micturition using the selective 5-HT7 receptor antagonists SB-269970 (Hagan et al., 2000; Lovell et al., 2000) and SB 656104, a 5-HT7 receptor antagonist from the same chemical series but structurally distinct (Thomas et al., 2003), on the ‘micturition reflex' evoked by distension caused by infusing saline into the bladder in anaesthetized rats. Preliminary accounts of the results have been published in abstract form (Read et al., 2002, 2003).
Methods
The experiments were carried out under the Animals (Scientific Procedures) Act, 1986. After completion of experiments, animals were killed by an overdose of pentobarbitone sodium intravenously (i.v.).
Experiments were performed on 67 female Sprague–Dawley rats (200–280 g), initially anaesthetized with isoflurane (4% in 100% oxygen), and maintained with urethane (1.2 g kg−1, i.v.). The depth of anaesthesia was assessed by the stability of blood pressure and heart rate (HR), and by an absence of hind limb withdrawal in response to paw pinch. If needed, supplementary doses of urethane were given (0.1 g kg−1, i.v.). To maintain a patent airway, the trachea was intubated. The left jugular vein was cannulated for anaesthetic and drug administration, and the right common carotid artery was cannulated with a heparinized cannula (20 U ml−1 heparin in 0.9% w v−1 saline) for the measurement of arterial blood pressure and for sampling arterial blood for gas analysis. Blood pressure was measured using a pressure transducer (Gould Statham P23Db), and HR derived electronically from the blood pressure signal using AcqKnowledge version 3.5.3 software (Biopac Systems Inc, U.S.A.). Body temperature was monitored with a rectal temperature probe and maintained between 36°C and 38°C using a homeothermic blanket system (Harvard). All animals were spontaneously breathing oxygen-enriched room air. Blood gases were maintained between 90 and 130 mmHg PO2, 40 and 50 mmHg PCO2 and pH 7.3 and 7.4 with a Corning pH/blood gas analyser (Model 238). The animals were infused (6 ml kg−1 h−1, i.v.) with a solution consisting of 10 ml plasma substitute (Gelofusine), 10 ml distilled water, 0.04 g glucose and 0.168 g sodium bicarbonate to prevent the development of nonrespiratory acidosis and to maintain blood volume. Animals were placed in a stereotaxic frame. The head was tilted so that the animal could lie in the supine position to prevent the weight of the animal affecting the bladder and urethral pressure recordings.
Cannulation of the lateral cerebral ventricle (i.c.v.) and placement of cannula for i.t. administration
A stainless steel guide cannula (22 gauge) was implanted into the right lateral cerebral ventricle. The co-ordinates used from bregma were 3.5 mm ventral, 1.5 mm lateral and 1 mm posterior. Drug and vehicle solutions were administered through an i.c.v. injection cannula (28 gauge) attached by a length of polythene tubing to a 100 μl syringe (Hamilton). For i.t. administration, a polyethylene cannula (PE-10) was inserted through a small puncture in the atlanto-occipital membrane and passed into the subarachnoid space surrounding the spinal cord as described by Yaksh & Rudy (1976). The tubing was measured to the correct length externally prior to insertion and then passed caudally to the appropriate level of the spinal cord for administration. The cannula was clamped in position and drug/vehicle was delivered via a needle inserted into the guide tube, over 20 s. The location of the cannula placement for both injection sites was subsequently confirmed by the administration of 5 μl of 2% pontamine sky blue dye.
Measurement of bladder pressures
Cystometry method
The urinary bladder was exposed by a midline abdominal incision. The proximal ends of each ureter, exiting from the kidney, were exposed by retroperitoneal incisions and cannulated to prevent the bladder filling with urine during experiments. A small incision was made in the bladder dome and a single cuffed cannula (0.86 mm internal and 1.52 external diameter) was inserted into the bladder dome. This was connected via a T-piece to a pressure transducer (Gould Statham P23Db) and a syringe pump for the infusion of saline (0.9% w v−1) at a rate of 0.1 ml min−1, to evoke the micturition reflex. Backflow through this cannula allowed the bladder to be emptied of residual fluid after micturition had occurred.
Measurement of bladder and urethral pressures
Isovolumetric method
The urinary bladder was exposed and the proximal ends of each ureter cannulated, as above. An incision was made in the bladder dome and two cannulae (0.52 mm internal and 1.2 mm external diameter) were inserted into the bladder, one of which was connected to a syringe pump for the infusion of saline to evoke the micturition reflex and the other was connected to a pressure transducer (Gould Statham P23Db). The rate of infusion (0.046 ml min−1) was chosen to simulate the maximal hourly diuresis rate (Klevmark, 1974). Urethral pressure was measured using a method developed by M. Fraser (see Kakizaki et al., 1997). For a detailed methods and diagram of this urethral pressure recording, see Conley et al. (2001).
Experimental protocol
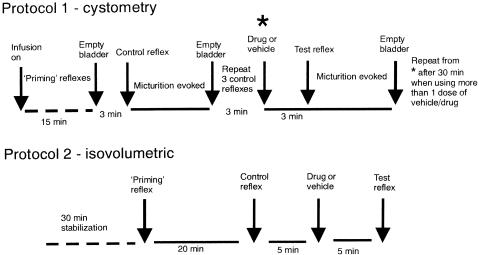
All experiments were left for 30 min after completion of surgery, to stabilize. A diagram for the experimental protocols used is shown in Figure 1.
Figure 1.
Diagram showing the experimental protocols that were used (it should be noted that there is 30 min stabilization after surgery also in Protocol 1).
In Protocol 1 (cystometry), the bladder was infused with saline until threshold was reached and the micturition reflex was evoked. Saline was continuously infused for 15 min to ‘prime' the system and cause a series of infusion-induced micturition reflexes. After 15 min, the infusion was stopped and the bladder emptied; 3 min later, the saline was infused into the bladder until micturition was evoked, the infusion was discontinued, the bladder was emptied and the residual volume was collected and measured. This was repeated three times to ensure the stability of the response. Then, after a period of 3 min, the test drug or vehicle was administered i.c.v., i.v. or i.t. After a further 3 min, the infusion was continued once more to evoke the micturition reflex (a single micturition) in the presence of test drug or vehicle. In some experiments, the test drug caused a continuous rise in bladder pressure with no overt contraction being observed. However, in these cases, after a certain period of time, a few drops of saline leaked from the urethral opening and once this was observed the infusion was switched off. Such responses were taken to indicate that the micturition response had been abolished.
In Protocol 2 (isovolumetric method), saline was infused into the bladder to evoke a ‘priming' reflex. After three consecutive reflex-evoked bladder contractions of similar amplitude, the infusion was discontinued. The infused saline was left in the bladder for 5 min, during which time the reflex was ongoing. The bladder was emptied and after 20 min, was infused and the infusion discontinued as above to acquire a control reflex. The bladder reflex was ongoing for 5 min before emptying the bladder of saline. After 5 min, the test drug or vehicle was administered, and after a further 5 min, the infusion-evoked reflex was repeated.
Data capture and analysis
Arterial blood pressure, bladder and urethral pressures were displayed on a chart recorder (Grass Instruments) and captured (1500 samples s−1) by an MP 100 WSW interface (Biopac systems Inc, U.S.A.) to allow data to be acquired and analysed offline using AcqKnowledge version 3.5.3 software (Biopac Systems Inc, U.S.A.). HRs were derived electronically from the blood pressure signal using the Biopac system.
Analysis of reflex-evoked bladder and urethral responses and baseline values
Saline infusion into the bladder evoked large-amplitude bladder contractions that represent the micturition reflex. The variables mean volume threshold (ml), pressure threshold (mmHg), the evoked bladder contraction (mmHg; maximum intraluminal pressure minus pressure threshold) and residual volume (expressed as percentage of the total volume infused) were measured for the control reflexes in the cystometry method. For details of analysis of bladder and urethral pressures in the isovolumetric method, see Wibberley et al. (2002). Baseline measurements of HR and blood pressure were mean values measured 2 min before reflexes and prior to drug or vehicle administration.
Statistical analysis
Changes in baselines and reflex-evoked effects were compared with vehicle controls by unpaired Students t-test and one-way ANOVA where applicable. Values of P<0.05 were considered statistically significant. All values are expressed as mean±s.e.m.
Drugs and solutions
Drugs and chemicals were obtained from the following sources: SB-269970 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)-ethyl)pyrrolidine-1-sulphonyl)phenolhydrochloride) and SB-656104 (6-((R)-2-{2-[4-(4-chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulphonyl)-1H-in-dole hydrochloride were synthesized at GlaxoSmithKline, Harlow, Essex, U.K.; WAY-100635, urethane and dimethyl sulphoxide (DMSO) from Sigma Aldrich Chemicals, Poole, Dorset, U.K.; pentobarbitone sodium from Rhone Merieux Ltd, Harlow, Essex, U.K.; isoflurane from Baxter Healthcare Ltd, Thetford, Norfolk, U.K.; gelofusine, polyethylene glycol 400 (PEG) from B Braun Medical Ltd, Sheffield, U.K.; sodium chloride, glucose and sodium bicarbonate from Merck/BDH Lab supplies, Poole, Dorset, U.K., heparin from CP Pharmaceuticals Ltd, Wrexham, U.K. SB-269970 was dissolved in 0.9% w v−1 saline, while SB-656104 HCl was dissolved in a mixture of 50%PEG/50%DMSO, which had been diluted 1 in 4 in saline. All i.c.v. and i.t. doses were given in 5 μl volume. All i.v. doses were given in 0.1 ml volume followed by a 0.1 ml flush of saline. All drugs were given as their salts.
Results
Cystometric study
Baseline values
Infusion of saline into the bladder of 59 female rats caused distension of the bladder, and, in turn, evoked the micturition reflex. The reflex was characterized by a rapid increase in bladder pressure (to a maximum of 22.3±0.4 mmHg), the emission of at least three drops of fluid during the detrusor contraction and then a return to baseline, or at least to a pressure lower than that recorded before micturition. The contractions had a mean duration of 21.8±0.7 s and returned to a resting pressure of 1.8±0.3 mmHg after micturition. The mean bladder pressure threshold to evoke the micturition reflex was 6.4±0.2 mmHg, which was reached when 0.55±0.02 ml of saline had been infused. The mean amount of residual volume left in the bladder was 41±2% of the volume infused. The mean baseline bladder pressure, MAP and HR were 1.2±0.1, 135±2 mmHg and 383±5 beats min−1, respectively. The mean baseline data for individual experimental groups are shown in Table 1.
Table 1.
Baseline values of ‘initial' reflex-evoked changes in bladder and urethral pressures caused by intravesical infusion of saline for each experimental group in urethane anaesthetized female rats for the cystometric study
| n | Bladder thresholds | Micturition-evoked bladder contraction | ||||
|---|---|---|---|---|---|---|
| Experimental group | Pressure (mmHg) | Volume (ml) | Amplitude (mmHg) | Duration (s) | Residual volume (%) | |
| Saline i.c.v. | ||||||
| 5 μl | 5 | 7.1±0.6 | 0.42±0.03 | 20.6±1.7 | 19.8±2.2 | 34±5 |
| SB-269970 i.c.v. | ||||||
| 3 μg kg−1 | 6 | 6.9±0.8 | 0.54±0.05 | 13.7±1.2 | 19.9±1.5 | 40±5 |
| 10 μg kg−1 | 5 | 6.7±0.7 | 0.65±0.04 | 19.6±1.0 | 24.5±2.0 | 43±4 |
| 30 μg kg−1 | 6 | 7.2±0.6 | 0.41±0.03 | 19.4±1.7 | 25.6±2.0 | 44±4 |
| 100 μg kg−1 | 5 | 5.4±0.6 | 0.41±0.03 | 17.3±1.3 | 17.9±1.0 | 34±5 |
| 300 μg kg−1 | 5 | 5.6±0.5 | 0.53±0.06 | 16.2±0.6 | 18.4±1.7 | 41±7 |
| 25% PEG/DMSO i.c.v. | ||||||
| 5 μl | 5 | 8.7±0.6 | 0.6±0.1 | 11.3±0.8 | 22±1.2 | 33±3 |
| SB-656104 i.c.v. | ||||||
| 30 μg kg−1 | 5 | 4.8±0.7 | 0.6±0.1 | 12.0±0.03 | 24.3±2.3 | 20±3 |
| Saline i.t. | ||||||
| 5 μl | 4 | 8.7±1.0 | 0.68±0.10 | 11.4±1.1 | 19.4±1.0 | 40±6 |
| SB-269970 i.t. | ||||||
| 30 μg kg−1 | 4 | 4.9±1.0 | 0.40±0.04 | 18.0±1.0 | 17.1±1.2 | 76±22 |
| WAY-100635 i.t. | ||||||
| 10 μg kg−1 | 4* | 4.4±0.9 | 0.43±0.04 | 18.1±2.5 | 19.3±2 | 63±6 |
| Saline i.v. | 4 | 4.2±0.7 | 0.7±0.12 | 13.9±0.7 | 29.1±5.7 | 50±8 |
| SB-269970 i.v. | ||||||
| 10 μg kg−1 | 5 | 6.5±1.1 | 0.76±0.09 | 15.2±0.6 | 22.5±1.1 | 46±5 |
Same animals as for i.t. SB-269970.
Effects of 5-HT7 receptor antagonists, SB-269970 and SB-656104
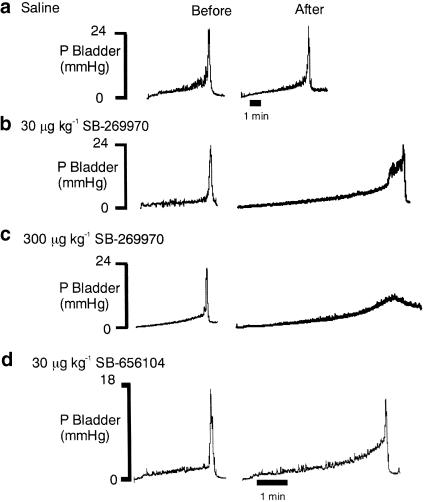
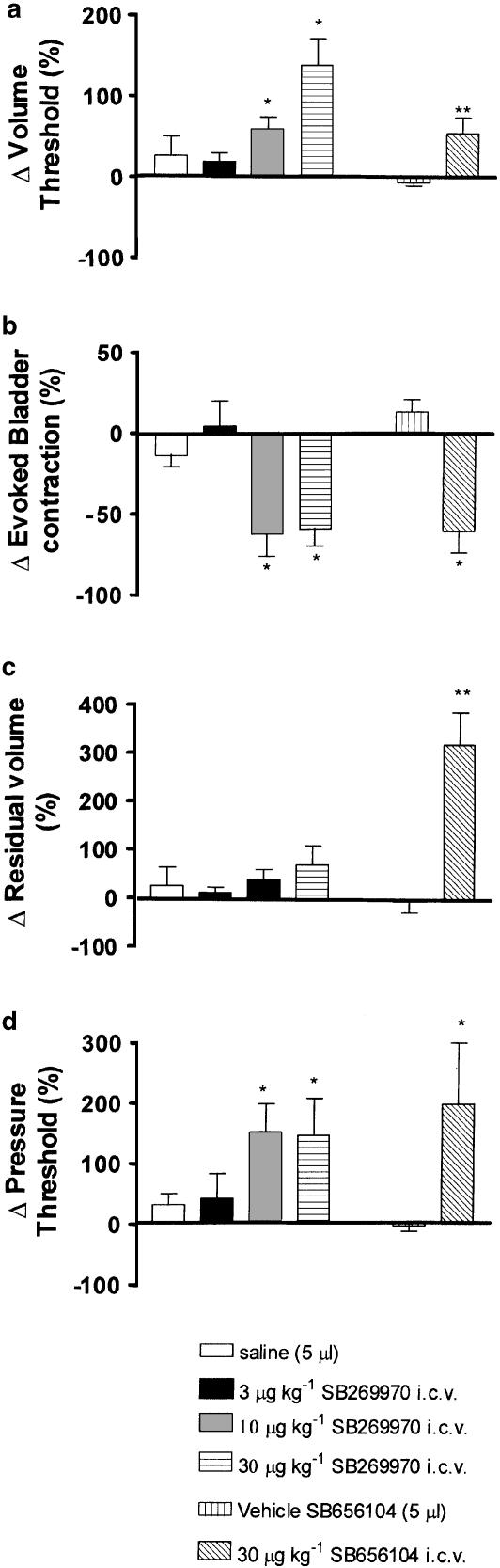
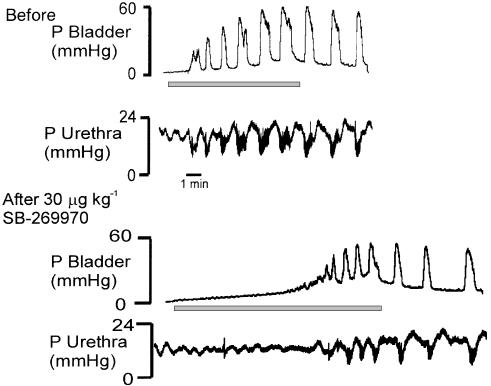
Traces showing the effects of SB-269970 and SB-656104 on distension-evoked changes in bladder pressure are shown in Figure 2. SB-269970 (3, 10, 30 μg kg−1; i.c.v.; n=5–6) caused increases in volume threshold of 18±11, 58±15 and 138±33%, respectively, being statistically significant compared with i.c.v. saline at the doses of 10 and 30 μg kg−1. At these doses, SB-269970 also caused significant reduction in the distension-evoked bladder contraction of −62±14 and −60±11% and a significant increase in pressure threshold of 150±46 and 149±60%, respectively. These effects were not associated with any change in residual volume (see Figure 3). At the doses of 100 and 300 μg kg−1, SB-269970 abolished the reflex (see Figure 2c).
Figure 2.
Urethane anaesthetized female rats: traces comparing the effect of i.c.v. administration of (a) saline, (b) 30 μg kg−1 SB-269970, (c) 300 μg kg−1 SB-269970 and (d) 30 μg kg−1 SB-656104 on the time taken to evoked a distension-induced contraction of the bladder by infusing saline (0.1 ml min−1) into the bladder. The saline infusion began at the beginning of the traces. It should be noted that a different vehicle control was used for SB-656104. The time base in (b, c) is the same as (a).
Figure 3.
Urethane anaesthetized female rats: histograms comparing (Δ) changes caused by i.c.v. injections of saline with SB-269970 (3, 10, 30 μg kg−1) and i.c.v. injections of PEG : DMSO with SB-656104 (30 μg kg−1) on (a) volume threshold, (b) evoked bladder contraction, (c) residual volume, and (d) pressure threshold. Each bar represents the mean value and the error bars show s.e.m. Changes caused by drugs were compared with appropriate vehicle using Student's unpaired t-test. *P<0.05; **P<0.001.
SB-656104 (n=5; Figure 2 and Figure 3) i.c.v. at 30 μg kg−1 had a similar effect to that of SB-269970, causing a significant increase in bladder pressure threshold (196±102%) and volume threshold (54±20%). Further, residual volume was significantly increased by 322±66% when compared to control.
i.t. Administration
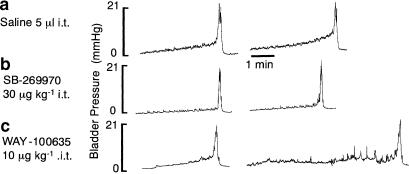
SB-269970 (30 μg kg−1; 5 μl; n=4) given at the L6/S1 level of the spinal cord had no significant effect on any of the variables measured, although WAY-100635 (10 μg kg−1; i.t.; n=4) administered as a positive control 30 min after SB269970 (30 μg kg−1) caused a significant increase in volume threshold of 155±76% (see Figure 4).
Figure 4.
Urethane anaesthetized female rat: traces from the same rat showing (a) the effect of i.t. administration of saline (vehicle) followed by (b) SB-269970 and then (c) WAY-100635 on the time taken to evoke a distension-induced contraction of the bladder by infusing saline (0.1 ml min−1) into the bladder. The saline infusion began at the beginning of the traces.
i.v. Administration
SB-269970 (10 μg kg−1; n=5) had no significant effects on the distension-evoked bladder contraction.
Isovolumetric study
Baseline values
Infusion of saline into the bladder in eight female rats caused distension of the bladder, in turn evoking the micturition reflex. The reflex is characterized by the appearance of rhythmic bladder contractions of 36±2 mmHg. The contractions had a mean duration of 45±3 s. The mean bladder pressure threshold to evoke such contractions (n=8) was 6±1 mmHg, which was reached when 0.17±0.04 ml of saline had been infused into the bladder. Each rhythmic bladder contraction was accompanied by a fall in urethral pressure of 9±1 mmHg that continued for 62±3 s before returning to baseline. High-frequency oscillations occurred in urethral pressure, at the peak of each bladder contraction, that had a mean amplitude of 14±1 mmHg and continued for 35±3 s. Each reflex bladder contraction was accompanied by small increases in MAP and HR. The mean baseline bladder and urethral pressures, MAP and HR were 2±1, 16±1, 136±3 mmHg and 360±13 beats min−1, respectively.
Effect of SB-266970 given i.c.v.
SB-269970 (30 μg kg−1 i.c.v.; 5 μl; n=4) caused a significant increase in volume threshold and pressure threshold and a significant reduction in the amplitude of bladder contractions (219±60, 162±28 and −17±3%, respectively; see Figure 5). However, these changes were associated with no significant effects in urethral relaxations or in urethral pressure high-frequency oscillations.
Figure 5.
Urethane anaesthetized female rats: traces showing changes in bladder and urethral pressures during intravesical infusions of saline before (control) and after i.c.v. injection of SB-2669970 (30 μg kg−1). The length of the line under each trace represents the duration of the intravesical infusions.
Blood pressure and HR
Neither antagonist had any significant effect on baseline values.
Discussion
The present experiments demonstrate that both SB-269970 and SB-656104 given centrally can attenuate distension-evoked bladder contraction. Although these ligands are from the same chemical series, they are sufficiently structurally distinct to minimize the possibility that the effects observed are compound- and not mechanism-specific. For example, by using two different 5-HT7 receptor antagonists, each with different selectivity profiles, the possibility that the effects observed are attributed to a non-5-HT7 receptor-related activity has been minimized. Thus, each compound has high affinity and selectivity for the 5-HT7(a) receptor. SB-269970 has a pKi of 8.9 on both the human recombinant 5-HT7(a) receptor expressed in HEK 293 cells (Lovell et al., 2000) and the human 5-HT7 receptor in cerebral cortex, with an equivalent value of 8.3 being found using guinea-pig cortex (Hagan et al., 2000). SB-656104, has a pKi at the human recombinant receptor was 8.7 (Thomas et al. 2003). The only other receptor that SB-269970 has appreciable affinity for is the 5-HT5A receptor (Lovell et al., 2000; pKi of 7.2), while for SB-656104 a different selectivity profile is apparent, this compound having pKi's of 7.60, 7.20, 7.04 and 7.01 for 5-HT1D, 5-HT2A, 5-HT2B and D2 receptors, respectively (Thomas et al., 2003). Thus, it is concluded that these antagonists, when given centrally, inhibit the micturition reflex by blocking central 5-HT7 receptors. It is known that there are three different splice variants of the 5-HT7 receptor in both the human and rat brain, but as yet there is no evidence for a distinct pharmacology between these variants (see Vanhoenacker et al., 2000); as a result, it remains to be determined as to which variants are involved in the micturition reflex. The present results also indicate that these 5-HT7 receptors are located in the forebrain as the same dose of SB-269970 administered i.c.v. was ineffective when administered i.v. and further no effects of SB-269970 could be observed on the reflex when administered into the sacral portion of the spinal cord. Furthermore, the relatively short half-life of this compound (t1/2<0.5 h when given i.v.; Forbes et al., 2002) also supports the view that it is acting within the CNS; as it redistributes from brain into the circulation, it would rapidly be removed, preventing it from reaching another possible site of action. Nevertheless, further experiments are still required to absolutely rule out an additional peripheral site of action because of this short half-life.
The demonstration that central 5-HT7 receptors along with previous evidence that 5-HT1A receptors are involved in the central control of micturition, especially as both types of antagonist can completely block this reflex (present data and Conley et al., 2001), is intriguing and implies that central 5-HT containing neurones play a very important role in the control of micturition, at least in rats. It is also intriguing that both receptors have a similar overall physiological function in the reflex control of the bladder, although having opposing actions on adenylyl cyclase, 5-HT7 receptors being positively coupled while 5-HT1A receptors are negatively coupled to adenylyl cyclase (see Hoyer et al., 2002). Interestingly, the requirement for both of these 5-HT receptors would seem to only occur at forebrain site/s (Pehrson et al., 2002; Secker et al., 2002) as 5-HT1A receptor-mediated control of the bladder also occurs at the spinal level (Kakizaki et al. (2001) and the present experiments), probably at the level of sacral parasympathetic nucleus, which has been shown by autoradiographic studies to contain 5-HT1A receptors (Thor et al., 1993). Also located in the sacral portion of the spinal cord is Onuf's nucleus, which contains efferent neurones innervating the external urethral sphincter, and in addition contains a high density of 5-HT1A receptors (Thor et al., 1993). However, there is no evidence that blockade of 5-HT1A receptors interferes with the reflex regulation of the urethra (Conley et al., 2001). The present experiments indicate that central 5-HT7 receptors are also not involved in the reflex control of the urethra. However, SB-656104 significantly increased residual volume, which could reflect interference with the urethra, but this was not mirrored by the more selective 5-HT7 receptor antagonist SB-269970. The reason why this increase in bladder residual volume occurs with SB-656104 remains to be determined, but it is probably not related to 5-HT7 receptor blockade.
Central areas in involved in micturition are the hypothalamus, dorsal inferior colliculus, paraquaductal grey, pontine nucleus and nucleus subcoerules (see De Groat et al., 1993) and 5-HT7 receptors have been identified in these regions (To et al., 1995; Gustafson et al., 1996; see Vanhoenacker et al., 2000). These regions also contain 5-HT1A receptors; in fact, 5-HT1A receptors are nearly universally throughout the brain (Verge et al., 1986; see Barnes & Sharp, 1999). Thus, precisely, how and where these two 5-HT receptor systems interact and control micturition remains to be determined. Also, whether 5-HT7 receptors are involved in micturition in a similar way in other species remains to be determined. For instance, in the cat it has been reported that blockade of 5-HT1A receptors with WAY-100635 or LY206130 has no effect on bladder or sphincter activity (Thor et al., 2002), although the other combined data indicate that central 5-HT1A receptors do play a similar role in the control of parasympathetic outflow to the heart in this species as well as in the rabbit, the rat and man (see Ramage, 2001). Further investigations of this discrepancy as well as the role of 5-HT7 receptors are therefore required in the cat.
In conclusion, the present results have identified a major physiological function for central 5-HT7 receptors, that of the control of reflex-induced bladder contraction, at least in the rat. In addition, this study adds to the growing evidence of the importance of central 5-HT containing neurones in the control of parasympathetic outflow.
Acknowledgments
K.R. is in receipt of a BBSRC industrial (with GlaxoSmithkline, Harlow, Essex), PhD scholarship. In addition, we would also like to thank Dr David Thomas of GSK for his support and Mr S. Wilkinson for technical assistance.
Abbreviations
- HR
heart rate
- 5-HT
5-hydroxytryptamine
- i.c.v.
intracerebroventricular
- i.t.
intrathecal
- MAP
mean arterial blood pressure
References
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BOGLE R.G., PIRES J.G.P., RAMAGE A.G. Evidence that central 5-HT1A receptors play a role in the von Bezold–Jarisch reflex in the rat. Br. J. Pharmacol. 1990;100:757–760. doi: 10.1111/j.1476-5381.1990.tb14088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTLE D.J., ADCOCK J.J., RAMAGE A.G. Involvement of central 5-HT1A receptors in the reflex activation of pulmonary vagal motoneurones by inhaled capsaicin in anaesthetized cats. Br. J. Pharmacol. 1996;117:724–728. doi: 10.1111/j.1476-5381.1996.tb15250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTLE D.J., ADCOCK J.J., RAMAGE A.G. The role of central 5-HT receptors in the bronchoconstriction evoked by inhaled capsaicin in anaesthetised guinea-pigs. Neuropharmacology. 1998;37:243–250. doi: 10.1016/s0028-3908(98)00019-7. [DOI] [PubMed] [Google Scholar]
- CONLEY R.K., WILLIAMS T.J., FORD A.P.D.W., RAMAGE A.G. The role of α1-adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. Br. J. Pharmacol. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANDO S.B., JORDAN D., RAMAGE A.G. The role of central 5-HT3 receptors in the modulation of the response to upper airway stimulation in the anaesthetized rabbit. J. Physiol. 1995;489:156. [Google Scholar]
- DANDO S.B., SKINNER M.R., JORDAN D., RAMAGE A.G. Modulation of the vagal bradycardia evoked by stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. Br. J. Pharmacol. 1998;125:409–417. doi: 10.1038/sj.bjp.0702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE GROAT W.C., BOOTH A.M., YOSHIMURA N.Neurophysiology of micturition and its modification in animal models of human disease The Autonomic Nervous System, Vol. 3, Nervous Control of the Urogenital System 1993London: Harwood Academic; 227–290.ed. Maggi, C.A. pp [Google Scholar]
- ESPEY M.J., DU H.J., DOWNIE J.W. Serotonergic modulation of spinal ascending activity and sacral reflex activity evoked by pelvic nerve stimulation in cats. Brain Res. 1998;798:101–108. doi: 10.1016/s0006-8993(98)00401-6. [DOI] [PubMed] [Google Scholar]
- FORBES I.T., DOUGLAS S., GRIBBLE A.D., IFE R.J., LIGHTFOOT A.P., GARNER A.E., RILEY G.J., JEFFREY P., STEVENS A.J., STEAN T.O., THOMAS D.R. SB-656104-A: a novel 5-HT7 receptor antagonist with improved in vivo properties. Bioorg. Med. Chem. Lett. 2002;12:3341–3344. doi: 10.1016/s0960-894x(02)00690-x. [DOI] [PubMed] [Google Scholar]
- FUTURO-NETO H.A., PIRES J.G.P., GILBEY M.P., RAMAGE A.G. Evidence for the ability of central 5-HT1A receptors to modulate the vagal bradycardia induced by stimulating the upper airways in anesthetized rabbits with smoke. Brain Res. 1993;629:349–354. doi: 10.1016/0006-8993(93)91345-s. [DOI] [PubMed] [Google Scholar]
- GUARNERI L., IBBA M., TESTA A., LEONARDI A. The effects of mCPP on bladder voiding contractions in rats are mediated by 5-HT2A/5-HT2C receptors. Neurourol. Urodyn. 1996;15:316–317. [Google Scholar]
- GUSTAFSON E.L., DURKIN M.M., BARD J.A., ZGOMBICK J., BRANCHEK T.A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN J.J., PRICE G.W., JEFFREY P., DEEKS N.J., STEAN T., PIPER D., SMITH M.I., UPTON N., MEDHURST A.D., MIDDLEMISS D.N., RILEY G.J., LOVELL P.J., BROMIDGE S.M., THOMAS D.R. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., HANNON J.P., MARTIN G. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- KAKIZAKI H., FRASER M.O., DP GROAT W.C. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am. J. Physiol. 1997;272:R1647–R1656. doi: 10.1152/ajpregu.1997.272.5.R1647. [DOI] [PubMed] [Google Scholar]
- KAKIZAKI H., YOSHIYAMA M., KOYANAGI T., DE GROAT W.C. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturition-reflex pathway in the rat. Am. J. Physiol. 2001;280:R1407–R1413. doi: 10.1152/ajpregu.2001.280.5.R1407. [DOI] [PubMed] [Google Scholar]
- KLEVMARK B. Motility of the urinary bladder in cats during filling at physiological rates. I. Intravesical pressure patterns studied by a new method of cystometry. Acta Physiol. Scand. 1974;90:565–577. doi: 10.1111/j.1748-1716.1974.tb05621.x. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., SANTICIOLI P., MAGGI C.A. Involvement of 5-hydroxytryptamine1A receptors in the modulation of micturition reflexes in the anesthetized rat. J. Pharmacol. Exp. Ther. 1992;262:181–189. [PubMed] [Google Scholar]
- LOVELL P.J., BROMIDGE S.M., DABBS S., DUCKWORTH D.M., FORBES I.T., JENNINGS A.J., KING F.D., MIDDLEMISS D.N., RAHMAN S.K., SAUNDERS D.V., COLLIN L.L., HAGAN J.J., RILEY G.J., THOMAS D.R. A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl) pyrrolidine-1-sulfonyl) phenol (SB-269970) J. Med. Chem. 2000;43:342–345. doi: 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- PEHRSON R., OJTEG G., ISHIZUKA O., ANDERSSON K.-E. Effects of NAD-299, a new, highly selective 5-HT1A receptor antagonist, on bladder function in rats. Naunyn. Schmiedeberg's Arch. Pharmacol. 2002;366:528–536. doi: 10.1007/s00210-002-0650-y. [DOI] [PubMed] [Google Scholar]
- RAMAGE A.G. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res. Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- READ K.E., SANGER G.J., RAMAGE A.G. Effects of SB-269970, the 5-HT7 receptor antagonist, on micturition. Pharmacologist. 2002;44 suppl. 1:A186. [Google Scholar]
- READ K.E., SANGER G.J., RAMAGE A.G. Evidence that central 5-HT7 receptors are involved in the control of micturition in urethane anaesthetized rats. Br. J. Pharmacol. 2003;138:4p. doi: 10.1038/sj.bjp.0705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SECKER A.G., NAYLOR A.M., RAMAGE A.G. A role for supraspinal 5-HT1A receptors in the control of micturition in female urethane anaesthetized rats. Pharmacologist. 2002;44 suppl. 1:A186. [Google Scholar]
- SKINNER M.R., JORDAN D., RAMAGE A.G. Modulation of reflexly evoked vagal bradycardias by central 5-HT1A receptors in anaesthetized rabbits. Br J. Pharmacol. 2002;137:861–873. doi: 10.1038/sj.bjp.0704941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEERS W.D., DE GROAT W.C. Effects of m-chlorophenylpiperazine on penile and bladder function in rats. Am. J. Physiol. 1989;257:R1441–R1449. doi: 10.1152/ajpregu.1989.257.6.R1441. [DOI] [PubMed] [Google Scholar]
- TESTA R., GUARNERI L., ANGELICO P., VELASCO C., POGGESI E., CILIA A., LEONARDI A. Effect of different 5-hydroxytryptamine receptor subtype antagonists on the micturition reflex in rats. Br. J. Urol. Int. 2001;87:256–264. doi: 10.1046/j.1464-410x.2001.02038.x. [DOI] [PubMed] [Google Scholar]
- TESTA R., GUARNERI L., POGGESI E., ANGELICO P., VELASCO C., IBBA M., CILIA A., MOTTA G., RIVA C, LEONARDI A. Effect of several 5-hydroxytryptamine1A receptor ligands on the micturition reflex in rats: comparison with WAY 100635. J. Pharmacol. Exp. Ther. 1999;290:1258–1269. [PubMed] [Google Scholar]
- THOMAS D.R., ATKINSON P.J., HASTIE P.G., ROBERTS J.C., MIDDLEMISS D.N., PRICE G.W. 3H]-SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacology. 2002;42:74–81. doi: 10.1016/s0028-3908(01)00151-4. [DOI] [PubMed] [Google Scholar]
- THOMAS D.R., MELOTTO S., MASSAGRANDE M., GRIBBLE A.D., JEFFREY P., STEVENS A.J., DEEKS N.J., EDDERSHAW P.J., FENWICK S.H., RILEY G., STEAN T., SCOTT C.M., HILL M.J., MIDDLEMISS D.N., HAGAN J.J., PRICE G.W., FORBES I.T. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br. J. Pharmacol. 2003;139:705–714. doi: 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOR K.B., KATOFIASC M.A., DANUSER H, SPRINGER J., SCHAUS J.M. The role of 5-HT1A receptors in control of lower urinary tract function in cats. Brain Res. 2002;946:290–297. doi: 10.1016/s0006-8993(02)02897-4. [DOI] [PubMed] [Google Scholar]
- THOR K.B., NICKOLAUS S., HELKE C.J. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience. 1993;55:235–252. doi: 10.1016/0306-4522(93)90469-v. [DOI] [PubMed] [Google Scholar]
- TO Z.P., BONHAUS D.W., EGLEN R.M., JAKEMAN L.B. Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHOENACKER P., HAEGEMAN G., LEYSEN J.E. 5-HT7 receptors: current knowledge and future prospects. TiPS. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- VERGE D., DAVAL G., MARCINKIEWICZ M., PATEY A, EL MESTIKAWY S., GOZLAN H., HAMON M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J. Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y, RAMAGE A.G. The role of central 5-HT1A receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J. Physiol. 2001;536:753–767. doi: 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBBERLEY A., NUNN P.A., NAYLOR A.M., RAMAGE A.G. An investigation of the effects of zaprinast, a PDE inhibitor, on the nitrergic control of the urethra in anaesthetized female rats. Br. J. Pharmacol. 2002;136:399–414. doi: 10.1038/sj.bjp.0704735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD M., CHAU BEY M., ATKINSON P., THOMAS D.R. Antagonist activity of meta-chlorophenylpiperazine and partial agonist activity of 8-OH-DPAT at the 5-HT7 receptor. Eur. J. Pharmacol. 2000;396:1–8. doi: 10.1016/s0014-2999(00)00213-2. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., RUDY T.A. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]