Abstract
A new group IIa sPLA2 inhibitor was compared with selective inhibitors of COX-1, COX-2 and an LTC4 antagonist for effects on local and remote tissue injuries following ischaemia and reperfusion (I/R) of the small intestine in rats.
In an acute model of ischaemia (30 min) and reperfusion (150 min) injury in the absence of inhibitors, there was significant intestinal haemorrhage, oedema and mucosal damage, neutropenia, elevated serum levels of aspartate aminotransferase (AST) and hypotension.
Preischaemic treatment with the inhibitor of sPLA2 (Group IIa), at 5 mg kg−1 i.v. or 10 mg kg−1 p.o. significantly inhibited I/R-induced neutropenia, the elevation of serum levels of AST, intestinal oedema and hypotension.
Pretreatment with the COX-2 inhibitor celebrex (10 mg kg−1 i.v.) and the LTC4 antagonist zafirlukast (1 mg kg−1 i.v.) also showed marked improvement with I/R-induced AST, oedema and neutropenia. Hypotension was only reduced by the LTC4 antagonist. The COX-1 inhibitor flunixin (1 mg kg−1 i.v.) did not effect improvement in the markers of tissue injury.
Histological examination of rat I/R injury showed that all of the drugs offered some protection to the mucosal layer damage compared to no drug treatment. Given i.v., the sPLA2 inhibitor was more effective than either the COX-1 or COX-2 inhibitors in preventing rat I/R injury.
These results indicate that a potent new inhibitor of sPLA2 (group IIa) protects the rat small intestine from I/R injury after oral or intravenous administration. COX-2 and LTC4 inhibitors also showed some beneficial effects against intestinal I/R injury. Our study suggests that sPLA2 (Group IIa) may have a pathogenic role in intestinal I/R in rats.
Keywords: Gut ischaemia–reperfusion, sPLA2, COX-1, COX-2, LTC4, neutropenia, inflammation, histopathology, pharmacokinetics
Introduction
Intestinal ischaemia occurs as a result of inadequate systemic blood flow or local vascular abnormalities, and the metabolic demand of the tissue exceeds the delivery of oxygen. Bowel obstruction, abdominal aortic aneurism, haemorrhagic shock, sepsis and traumatic injury can all induce intestinal ischaemia (Fink, 1991; Christenson et al., 1996; Tadros et al., 2000; Wattanasirichaigoon et al., 2000). Diseases such as necrotising enterocolitis, mesenteric insufficiency in the elderly and intestinal dysfunction following bowel transplantation are thought to have a component of ischaemia–reperfusion (I/R) in their pathogenesis (Haglund et al., 1987; Schoenberg & Beger, 1993). Reperfusion of blood to an ischaemic tissue further increases acute ischaemic injury (Granger et al., 1981). In addition to damaging the bowel, intestinal I/R injury can induce pathology at sites remote from the initial injury (Chiu et al., 1970; Koike et al., 1992b; Poggetti et al., 1992; Sun et al., 1999). Intestinal I/R can lead to adult respiratory distress syndrome and multiple organ dysfunction syndrome (MODS) (Sheng et al., 1991).
Reperfusion injury is caused by the release of a variety of endogenous agents including oxygen radicals (Granger et al., 1986; Arumugam et al., 2002a), polymorphonuclear leucocytes (PMNs) (Grisham et al., 1986), tumour necrosis factor-alpha (TNF-α) (Caty et al., 1990), leukotrienes (Karasawa et al., 1991), platelet activating factor (PAF) (Kim et al., 1995) and complement products (Wada et al., 2001; Arumugam et al., 2002b). Phospholipases A2 (PLA2) are also important components of the inflammatory response in intestinal I/R injury, although it is not known precisely which specific subtype(s) of this enzyme family are involved.
PLA2-mediated tissue injury results through either a direct action of the enzyme(s) or through subsequent actions of its products, which include PAF, leukotrienes, lipoxins, prostaglandins and thromboxanes (Chang et al., 1987). Evidence in support of the role of PLA2 in intestinal I/R has been shown in several studies using the nonspecific PLA2 inhibitor quinacrine (Otamiri et al., 1987, 1988; Otamiri & Tagesson, 1989; Koike et al., 1992a), which reduced manifestations of gut I/R injury. The PLA2 inhibitor used in the present study is an orally active, potent inhibitor of group IIa secretory PLA2 (sPLA2) (Hansford et al., 2003). Group IIa sPLA2 is a human enzyme reported to induce lung injury after intestinal I/R (Koike et al., 2000). Although numerous agents are reported to inhibit ‘PLA2' activity via different mechanisms, there are actually only a handful of bona fide inhibitors (Balsinde et al., 1999) of this specific isoform found in human platelets and synoviocytes. We have shown that this particular sPLA2 (group IIa) inhibitor is highly selective for the group IIa enzyme and has potent anti-inflammatory activity in rats (Hansford et al., 2003; unpublished observations).
PLA2 hydrolyses membrane phosphoglycerides to liberate free fatty acids (arachidonic acid) and lysophospholipids (Scheuer, 1989). Cyclooxygenases are involved in the biosynthesis of prostaglandins from arachidonic acid. Two isoforms of the enzyme have been described: cyclooxygenase-1 (COX-1), which is constitutively expressed in most cells and required for physiological functions, and cyclooxygenase-2 (COX-2), which is an inducible form arising in response to inflammatory stimuli (Feng et al., 1993). sPLA2 regulates the release of arachidonic acid (AA) from membrane phospholipids, while COX converts AA to prostaglandins. Accumulating evidence suggests that sPLA2-IIa and sPLA2-V are functionally coupled with COX-1 and COX-2 pathways for prostaglandin biosynthesis (Murakami et al., 1999).
To determine the roles of COX-1 and COX-2 in intestinal I/R injury, the present study used flunixin meglumine (Flunixin, Mavlab P/L, Brisbane, Australia) and celecoxib (Celebrex, Pfizer, Australia). Flunixin is a relatively selective COX-1 inhibitor (Brideau et al., 2001) commonly used for the management of intestinal ischaemia, colic and endotoxemia in equids (Jochle et al., 1989; Semrad et al., 1993), while celebrex is a relatively selective inhibitor of COX-2 approved for the treatment of rheumatism and osteoarthritis (Davies et al., 2000). The other major metabolites of the arachidonate pathway are the leukotrienes, which are generated by the action of lipoxygenases (Bingham & Austen, 1999). Lipoxygenase inhibitors and leukotriene B4 receptor antagonists have frequently been investigated in animal models of intestinal I/R (Karasawa, et al., 1991; Goldman et al., 1992; Mangino et al., 1994; Kirschner et al., 1995). The cysteinyl leukotrienes are also elevated in the bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome (Stephenson et al., 1988), a common consequence of intestinal I/R. Zafirlukast (Accolate, Zeneca) is a potent and selective cysteinyl leukotriene receptor antagonist (Calhoun, 1998) and was also used in the present study as a comparator drug.
This study sought to test the effectiveness of a new sPLA2 (group IIa) inhibitor in alleviating intestinal I/R-induced injury. As a comparison to this sPLA2 blockade, this study also investigated the relative contribution of a number of inflammatory mediators in intestinal I/R by selectively blocking different stages of the eicosanoid inflammatory cascade. This was achieved with a relatively selective COX-2 inhibitor, a predominantly COX-1 inhibitor and a cysteinyl leukotriene receptor LTC4 antagonist.
Methods
sPLA2 inhibitor preparation
The sPLA2 inhibitor (5-(4-benzyloxyphenyl)-4S-(7-phenylheptanoylamino)-pentanoic acid) was synthesised, purified by reversed phase HPLC, and fully characterised by mass spectrometry and proton NMR spectroscopy as described (Hansford et al., 2003). The sPLA2 inhibitor is active in vitro using a standard enzyme assay (Reynolds et al., 1992) as an inhibitor of the action of human recombinant nonpancreatic sPLA2 (group IIa) (IC50=0.029 μM, 0.000019 mole fraction, compound 2b in Hansford et al., 2003).
Pharmacokinetics of sPLA2 inhibitor
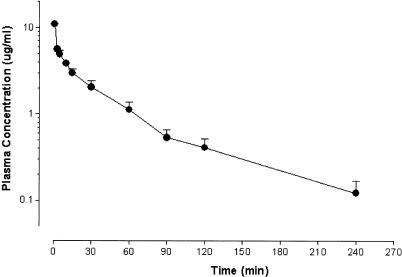
Female Wistar rats were used to monitor clearance of the sPLA2 inhibitor from serum after i.v. administration. Anaesthetised rats were injected with 5 mg kg−1 sPLA2 inhibitor in 70% dimethyl formamide (DMF; Merck, U.K.). Blood samples were collected from the tail at regular intervals over a period of 4 h (Figure 1). Blood samples were then centrifuged to remove red blood cells and an aliquot of plasma (50 μl) transferred to a clean tube and stored at −20°C until sample analysis.
Figure 1.
Pharmacokinetics of the sPLA2 inhibitor. Rats were injected with a single dose of sPLA2 inhibitor (5 mg kg−1 i.v.) and plasma collected over 4 h (n=4). Plasma levels of sPLA2 inhibitor were determined by LC-MS. Data are expressed as mean concentration of the sPLA2 inhibitor±s.e.m.
To determine the concentration of sPLA2 inhibitor in the plasma samples, liquid chromatography mass spectrometry (LC-MS) analysis was employed. An internal standard (50 μl containing 5 μg ml−1 of an inhibitor analogue) was added to each sample. The tubes were acidified with a solution of 5% w v−1 citric acid in water (400 μl) and extracted with HPLC-grade dichloromethane (500 μl) by vortexing at full speed for 20 s. The tubes were centrifuged to facilitate separation of the layers, the bottom layer was removed and transferred to a new tube. The dichloromethane was removed using a centrifugal evaporator (Genevac) and the residue was dissolved in the mobile phase (50 μl) by vortexing for 20 s and then transferred to an autoinjector vial.
A set of standard solutions for the generation of a calibration curve was prepared by adding a stock solution of inhibitor (in 80% acetonitrile/20% water) and internal standard containing 5 μg ml−1 of an inhibitor analogue in 50 μl rat plasma, vortexed briefly then extracted with dichloromethane/citric acid as described above. Samples were analysed on a PE-Sciex API-3000 triple quadruple mass spectrometer equipped with an Agilent 1100 HPLC system under isocratic conditions using a mobile phase consisting of 72% acetonitrile, 27.9% water and 0.1% formic acid. The column was a Phenomenex Luna C18, 5 μm, 100 Å, 50 × 2 mm with flow rate 200 μl min−1, retention times: internal standard 2.4 min, sPLA2 inhibitor 2.8 min. The parent ions for the sPLA2 inhibitor MH+ 488 and internal standard MH+ 474 were fragmented producing ions both at m/e 282 that were focused into Q3. Data were smoothed (Kalman and moving average) prior to integration and the area ratio of drug to internal standard was used for quantitation from a standard curve using the commercial software MacQuan 1.6 (PE-Sciex).
Model of intestinal I/R injury
Adult female Wistar rats weighing 200–250 g were fasted for 12–14 h before experimentation, but were allowed free access to water. Rats were anaesthetised by the intraperitoneal injection of 10 mg kg−1 of a mixture of zolazepam and tiletamine (Zoletil 100, Virbac, Australia) and 10 mg kg−1 xylazine (Xylazil-20, Ilium, Australia) and normal body temperature was maintained by placing rats on a heating pad.
The abdomen was opened by a midline incision to expose the superior mesenteric artery (SMA), the main supply of blood to the small intestine. Intestinal I/R was achieved by placing a nontraumatic occlusive device on the artery for a 30 min ischaemic phase, then removing the clamp to allow reperfusion of blood for 150 min (Arumugam et al., 2002b). At 15 min prior to occlusion, the right femoral vein was isolated and an injection of either 5 mg kg−1 PLA2 inhibitor in 75%, DMF 1 mg kg−1 flunixin in 15% ethanol, 1 mg kg−1 zafirlukast in saline, or 10 mg kg−1 celebrex in saline to drug-treated rats, or 75% DMF, 15% ethanol or sterile, pyrogen-free saline for I/R injury control rats, in 0.2 ml volume, was administered. Saline, 75% DMF or 15% ethanol was also infused into weight-matched rats undergoing sham operation, in which the SMA was exposed, but not occluded. Infusions were made over 2 min. Oral dosing of 10 mg kg−1 sPLA2 inhibitor in 75% DMF in 0.2 ml volume was achieved by gavage 60 min prior to SMA occlusion.
Blood samples (50 μl) were collected into heparinised tubes at regular intervals over the 180 min duration of the experiments for the estimation of leucocyte numbers. In a separate series of identical experiments, whole blood was collected at regular intervals over the 180 min and allowed to clot on ice, and serum samples collected and stored at −20°C for later measurement of aspartate aminotransferase (AST). At the end of the reperfusion period, the animals were euthanised by cervical dislocation.
Neutropenia assay
Blood (50 μl) for PMN counts was placed into heparinised tubes and then layered over an equal volume of Histopaque 1083 (Sigma, U.S.A.). PMNs were isolated as previously described (Short et al., 1999), and cell number counted on a haemocytometer. Concentrations of PMNs were presented as mean percentage±s.e.m. of the values obtained immediately prior to SMA occlusion.
Intestinal oedema measurement
After 180 min of I/R, a section of the occluded ileum was removed. The lumen was rinsed with saline, the intestine blotted dry and then weighed. Specimens were dried in an oven for 24 h at 80°C and weighed again, to obtain the tissue dry weight. Intestinal oedema was determined by assessing the wet and dry tissue weight ratio.
Aspartate aminotransferase assay
Plasma AST (AST/GOT; Sigma, USA) concentrations were measured according to manufacturer's instructions within 48 h of collecting plasma. Plasma AST concentrations were derived from a calibration curve. Results are expressed in Sigma-Franke (SF) units ml−1.
Blood pressure measurement
In a separate set of experiments (30 min of ischaemia and 120 min of reperfusion) rats were anaesthetised and placed on a heat pad for 30 min or until the heart rate and blood pressure stabilised. Drug or solvents were administered intravenously into the right femoral vein 15 min prior to inducing ischaemia. Systolic blood pressure was recorded using a pressure transducer (AD Instruments, Sydney, Australia) and an accompanying tail cuff. An inflatable cuff was placed on the tail above the pulse transducer, connected to a pressure transducer and amplifier. The electrical signal was recorded with a computerised chart recording system (MacLab/8). The tail cuff was inflated, inhibiting pulse signal, and systolic blood pressure was recorded as the point when the tail blood pressure exceeded cuff pressure. This was repeated ⩾3 times for each time point, and the mean value recorded.
Histopathology analysis
After I/R for 180 min, segments of ischaemic and normal intestine (ileum) were harvested and rinsed with saline and immediately fixed in 10% buffered formaldehyde–saline solution for histological studies. Fixed specimens were embedded in paraffin wax, sectioned serially, and stained with haematoxylin and eosin. Histological assessment was carried out in a blinded fashion by an independent investigator, with the mean of the observations being used for analysis. The grading scheme for intestinal injury was adapted from modified scoring of Chiu et al., 1970 (Park et al., 1990). Thus, injury was classified using a semiquantitative grading system ranging from 0 to 4, where a numerical score was assigned based on the degree of mucosal and submucosal damage. Normal mucosa was scored as grade 0. Epithelial cell damage, seen as loss of cells and separation of the epithelial cells from the underlying villus was scored between grades 1–3, while loss of villous tissue was scored as grade 4.
Statistical analysis
All experiment results are expressed as mean±s.e.m. Analyses were performed using GraphPad Prism 3.0 software (GraphPad Software, Inc., U.S.A.). Statistical analysis for Figures 2 and 5 was performed using one-way repeated-measures ANOVA. Figures 3 and 4 were analysed by one-way ANOVA followed by Newman–Keuls comparison test analysis; P<0.05 was considered significant.
Figure 2.
Neutropenia induced by gut ischaemia–reperfusion. Gut I/R caused significant reduction in circulating PMN levels compared with sham-operated animals (a–c). Pretreatment of rats with (a) sPLA2 inhibitor (5 mg kg−1 i.v. or 10 mg kg−1 p.o.) significantly inhibited I/R-induced neutropenia; (b) celebrex (10 mg kg−1 i.v.) or flunixin (1 mg kg−1 i.v.) had negligible effect for up to 2 h postischaemic phase; (c) zafirlukast (1 mg kg−1 i.v.) resulted in significant improvement in I/R-induced neutropenia. Both flunixin and zafirlukast caused neutrophilia at 2–3 h postischaemic phase. Data are shown as means±s.e.m. (n=6–10 in each group). *, P<0.05 vs I/R animals between groups. Bar shows 30 min period of ischaemia.
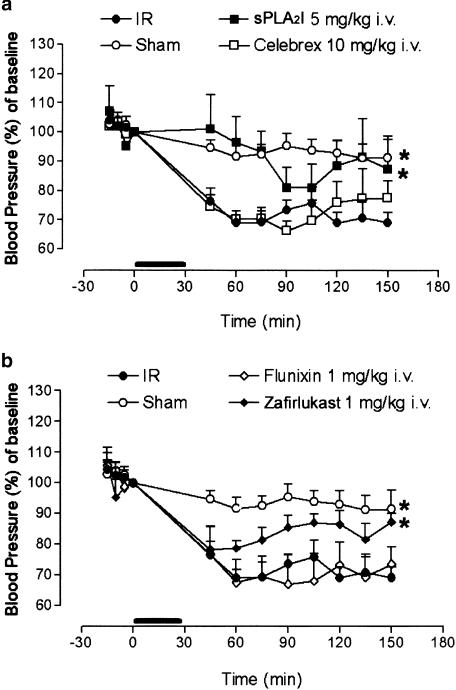
Figure 5.
Hypotension induced by gut ischaemia–reperfusion. Intestinal I/R resulted in a significant decrease in blood pressure compared to sham-operated animals. Pretreatment with: (a) the sPLA2 inhibitor (5 mg kg−1 i.v.) offered some protection against this hypotension, whereas there was no effect with the COX-2 inhibitor celebrex (10 mg kg−1 i.v.); (b) zafirlukast (1 mg kg−1 i.v.) also offered some protection, but the COX-1 inhibitor flunixin (1 mg kg−1 i.v.) did not prevent I/R-induced hypotension. Data are shown as means±s.e.m. (n=6–10 in each group). *, P<0.05 vs I/R control animals between groups. The horizontal bar represents ischaemic phase.
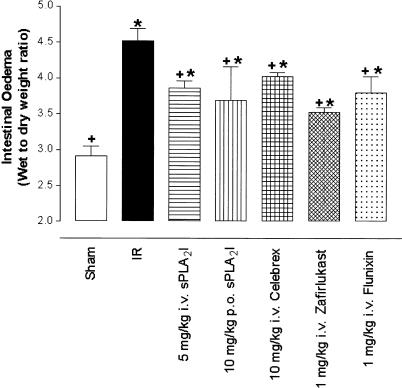
Figure 3.
Intestinal oedema induced by ischaemia–reperfusion. Wet-to-dry weight ratio of the small intestine is significantly elevated after I/R compared to sham-operated animals. Pretreatment with all the drug groups resulted in significant increase in tissue oedema from sham-operated animals as well as significant drop from I/R injury animals. Data are shown as means±s.e.m. (n=6–10 in each group). *, P<0.05 vs sham-operated animals.+, P<0.05 vs I/R animals.
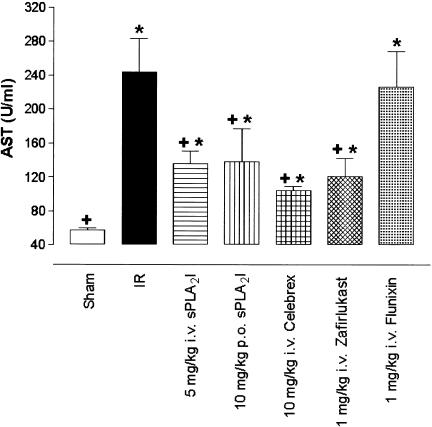
Figure 4.
ASA induction by gut ischaemia–reperfusion. Gut I/R resulted in a significant increase in plasma AST levels compared to sham-operated animals. Pretreatment with the sPLA2 inhibitor (5 mg kg−1 i.v., 10 mg kg−1 p.o.) or zafirlukast (1 mg kg−1 i.v.) or celebrex (10 mg kg−1 i.v.), but not flunixin (1 mg kg−1 i.v.), significantly reduced gut I/R-induced AST levels compared to I/R animals. Drug-treated groups also showed significant increase against sham-operated animals level of AST. Data are shown as means±s.e.m. (n=6–10 in each group). *, P<0.05 vs sham-operated animals. +, P<0.05 vs I/R animals.
Results
Pharmacokinetics of sPLA2 inhibitor
Intravenous administration of 5 mg kg−1 sPLA2 inhibitor resulted in peak plasma levels of ∼10 μg ml−1 which declined to ∼0. 5 μg ml−1 at 2 h (Figure 1). Thus, significant levels of the sPLA2 inhibitor remained throughout the experimental period. Oral administration of this sPL2 inhibitor at 5 mg kg−1 resulted in plasma levels of ∼0.1–0.2 μg ml−1 within 15 min and this level remained constant for at least 6 h (unpublished data). To optimise the plasma levels of sPLA2 inhibitor for the present study after single-dose oral administration, we used 10 mg kg−1 p.o.
Effect of drugs on I/R-induced neutropenia
Intestinal I/R caused a marked (∼30% of baseline) reduction in circulating PMN levels compared with sham-operated animals. Circulating levels of PMNs also declined progressively in the sham-operated animals to ∼70–80% of baseline (Figure 2). Administration of the sPLA2 inhibitor (5 or 10 mg kg−1 p.o.) 15 or 60 min prior to SMA occlusion significantly inhibited the neutropenia caused by I/R (Figure 2a). The solvent vehicle DMF used for the sPLA2 inhibitor did not affect the I/R-induced neutropenia when administered. Intravenous administration of zafirlukast (1 mg kg−1) given 15 min prior to SMA occlusion also inhibited the I/R-induced neutropenia, and at later time points (150–180 min) there was a marked neutrophilia (Figure 2c), which was also apparent in sham-operated animals treated with the drug (data not shown). Neither celebrex (10 mg kg−1 i.v.) nor flunixin (1 mg kg−1 i.v.) 15 min prior to SMA occlusion reduced the I/R-induced neutropenia in the first 120 min after ischaemia (Figure 2b).
Effect of drugs on I/R-induced intestinal oedema
The I/R animals exhibited marked intestinal oedema (wet/dry weight=4.5±0.2) compared to the sham-operated animals (2.9±0.1, P<0.05). All the drugs used in the present study significantly (P<0.05) reduced intestinal oedema compared to I/R control animals, but mean values were also significantly increased (P<0.05) from sham-operated animals (Figure 3).
Effect of drugs on serum AST following I/R
After the reperfusion phase, serum concentrations of AST at 180 min increased by approximately five-fold, from 57±2 U ml−1 in sham-operated animals, to 244±39 U ml−1 in I/R animals (P<0.05). Intravenous (5 mg kg−1) and oral administration (10 mg kg−1) of the PLA2 inhibitor significantly (P<0.05) inhibited the rise in serum AST at 180 min (Figure 4). Zafirlukast (1 mg kg−1 i.v., P<0.05) and celebrex (10 mg kg−1 i.v., P<0.05) also caused reductions in I/R-induced AST. However, there was no reduction in AST levels after administration of flunixin (1 mg kg−1 i.v., P>0.05) after intestinal I/R (Figure 4).
Effect of drugs on I/R-induced hypotension
Blood pressure was measured over the 150 min of the experiment. For animals receiving solvent only, no difference was observed between the sham-operated and the I/R injury rats, and so the data were combined. In sham-operated animals, blood pressure was maintained consistently above 90% of preocclusion levels during the 150 min experiment (Figure 5). In contrast, there is a significant (P<0.05) decrease in blood pressure in I/R injury animals, the greatest decrease to 69.0±3% observed at 60 min. The blood pressure in rats treated with either (5 mg kg−1 i.v.) sPLA2 inhibitor or (1 mg kg−1 i.v.) zafirlukast also decreased after reperfusion; however, these drugs significantly (P<0.05) prevented I/R-induced hypotension. The intravenous administration of either flunixin (1 mg kg−1) or celebrex (10 mg kg−1) did not prevent I/R-induced hypotension (Figure 5a, b).
Histopathology
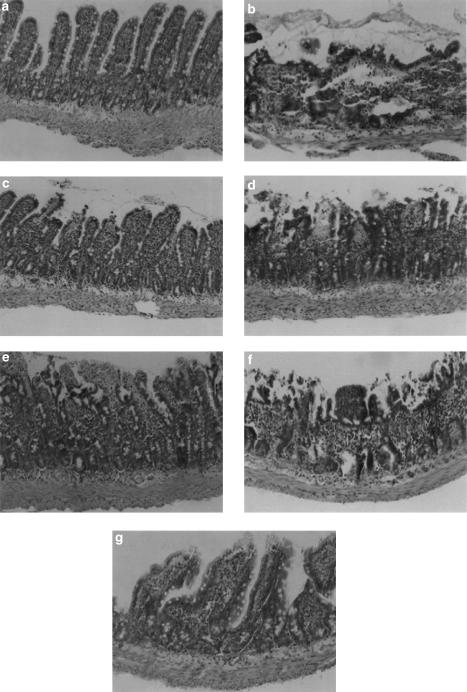
The intestines from rats, which were subject to occlusion of the mesenteric artery followed by reperfusion, showed significant structural changes with loss of epithelial cells from the villi and damage to those villi, but without infarction of the crypt layer or the mucosal layer (Figure 7b). The different solvents used to solubilise drugs did not affect the histopathological appearance of sham-operated animals or I/R injury animals. There were obvious differences between the capacities of the drugs to affect the histopathology during I/R injury (Figure 6). The greatest degree of tissue protection was afforded by the sPLA2 inhibitor given either i.v. (Figure 7c), p.o. (Figure 7d) or by zafirlukast (Figure 7g). In animals treated with 10 mg kg−1 p.o. sPLA2 inhibitor, greater damage was observed, with some villi showing loss of epithelium and haemorrhage at the villous tips (Figure 7d). Intravenous dosing of Celebrex (10 mg kg−1) also gave some detectable protection–most villi had haemorrhage and loss of the epithelial cell layer at the tip of the villi (Figure 7e). Flunixin administration (1 mg kg−1) demonstrated little or no preservation of normal mucosal structure compared to I/R injury animals (Figure 7f).
Figure 7.
I/R-induced mucosal injury in the rat small intestine. Microscopic image of small intestinal tissue section from (a) Sham (score 0); (b) I/R injury (score 4); and I/R injury following pretreatment with: (c) 5 mg kg−1 i.v. sPLA2 inhibitor (score 1); (d) 10 mg kg−1 p.o. sPLA2 inhibitor (score 2); (e) 10 mg kg−1 i.v. celebrex (score 2); (f) 1 mg kg−1 i.v. flunixin (score 3); and (g) 1 mg kg−1 i.v. zafirlukast (score 1). Images are typical and representative of each treatment group, and the score indicated is for the section shown. Original magnification × 200.
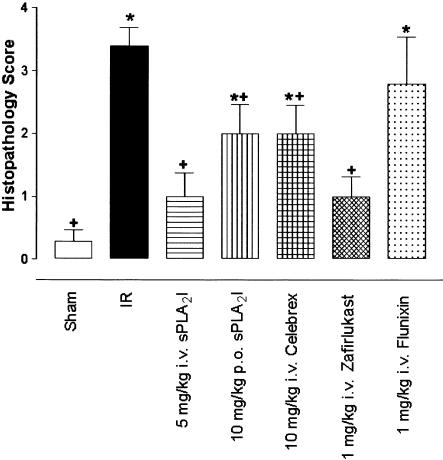
Figure 6.
Histopathology score for gut I/R reflects damage to the intestine (3+) compared to sham-operated (0.2+) animals. Pretreatment with either sPLA2 inhibitor (5 mg kg−1 i.v.) or zafirlukast (1 mg kg−1 i.v.) was most effective in reducing gut I/R-induced tissue damage. Data are shown as means±s.e.m. (n=6–10 in each group). *, P<0.05 vs sham-operated animals. +, P<0.05 vs I/R animals.
Discussion
I/R injury causes changes in the affected tissues as well as damage in organs remote from the initial injury site. This paper describes a rat model of intestinal I/R and the protective effects of a new and isoform-selective inhibitor of secretory phospholipase A2 (group IIa). Neutropenia, serum aspartate aminotransferase, intestinal oedema, blood pressure and histopathology were measured to assess changes associated with both local and remote tissue injury. Four drug treatments (zafirlukast, a cysteinyl leukotriene receptor antagonist; flunixin, a relatively selective COX-1 inhibitor; celebrex, a relatively selective COX-2 inhibitor; and the sPLA2 (IIa) inhibitor) were compared for efficacy.
In this study, initial experiments measuring numbers of PMNs established that intestinal I/R caused a significant neutropenia. The loss of PMNs from the circulation observed after intestinal I/R is explained by the attraction, activation, adherence and transendothelial migration of PMNs to the intestinal tissue and into remote organs (Botha et al., 1995; Partrick et al., 1996). The administration of the sPLA2 inhibitor and zafirlukast prevented this loss of PMNs from the circulation. In contrast, there was no reduction in neutropenia seen after administration of the COX inhibitors, celebrex and flunixin, suggesting that blocking the synthesis of leukotrienes and/or PAF has a greater role in protecting against PMN infiltration than inhibiting prostanoid synthesis alone. The lack of protection by conventional nonsteroidal anti-inflammatory drugs may also be due to the inhibition of prostacyclin, a prostaglandin produced by endothelial cells.
The first detectable sign of intestinal mucosal injury in ischaemia is increased capillary permeability, resulting in an intestinal oedema (Haglund, 1994). In this study, there was a clear change in wet to dry weight ratio of I/R intestinal tissue compared to sham-operated animals, demonstrating the formation of oedema. Inhibition of eicosanoids reduced, but did not abolish intestinal oedema, suggesting that factors other than eicosanoids or PAF are probably also involved in the process of vascular leakage.
Aspartate aminotransferase is a reliable marker released from liver parenchymal cells and kidney cells under stress. It has been reported that liver I/R injury and kidney I/R injury both significantly increase serum AST levels (Vajdova et al., 2000; Chatterjee et al., 2001). It is shown here that intestinal I/R also increased serum AST levels. This increase may be subsequent to local intestinal I/R injury, presumably caused by circulating mediators such as proinflammatory cytokines and PMN damage to liver and kidney cells. The elevation of plasma AST levels with I/R injury was inhibited by the sPLA2 inhibitor, zafirlukast, celebrex, but not flunixin. PLA2 and prostanoids have been implicated in remote organ damage in models of intestinal I/R (Koike et al., 1992a). Inhibition of ‘PLA2' was shown to decouple lung injury from intestinal I/R (Koike et al., 1992a), and inhibition of thromboxane-A2 also prevented lung injury (Turnage et al., 1995, 1997).
Blood pressure was measured to determine the haemodynamic effects of intestinal I/R, because the release of PMNs, bacterial products across the compromised intestinal barrier (translocation), and other inflammatory mediators lead to significant distant pathophysiological effects, including systemic hypotension (Khanna et al., 2001). Previous studies measuring blood pressure have shown a steep rise in pressure immediately upon the induction of intestinal ischaemia that gradually diminished to preocclusion levels, and then dropped dramatically upon reperfusion (Hayward & Lefer, 1998; Khanna et al., 2001). This immediate rise was also seen in our experiments which was not affected by any of the drugs used in this study (data not shown). In I/R injury animals, reperfusion after 30 min of ischaemia caused an abrupt and sustained decrease in systemic blood pressure, indicating circulatory shock. This precipitous decrease in blood pressure has been proposed to be primarily mediated by the release of PAF from the postischaemic intestine (Filep et al., 1991; Hayward & Lefer, 1998). It was observed that the sPLA2 inhibitor displayed some protection against intestinal I/R-induced hypotension, consistent with the assumption that PAF plays an important role in mediating this response. In contrast, the COX inhibitors celebrex and flunixin did not prevent intestinal I/R-induced hypotension. Interestingly, the leukotriene receptor antagonist zafirlukast also gave some protection, indicating some involvement for leukotrienes in intestinal I/R-induced hypotension.
Histopathological examination clearly demonstrated the tissue-protective effects of the various drugs in the pretreatment of intestinal I/R. The mucosa is the most metabolically active layer in the gut wall and so it is the first tissue layer to demonstrate signs of ischaemia. The earliest changes seen in intestinal ischaemia are at the tip of the intestinal villi. Within 10 min of ischaemia, ultrastructural changes become detectable, and cellular damage is extensive within 30 min. Sloughing of the villi tips in the small bowel and the superficial mucosal layer of the intestine is followed by oedema, submucosal haemorrhage and eventual transmural necrosis (Chiu et al., 1970). In this study, sham-operated animals showed little or no histological changes in the small intestine, but I/R caused extensive damage of the villi of the small intestine. The preadministration i.v. of either the sPLA2 inhibitor or zafirlukast strongly protected the intestine. Conversely, pretreatment i.v. with celebrex or flunixin intravenously provided less protection against intestinal I/R-induced histopathological changes, suggesting that prostaglandins may play a protective role in the gut mucosa after I/R. Pajdo et al. (2001), using a model of rat ischaemic preconditioning to determine the role of prostaglandins, found that gastric ischaemic preconditioning stimulates a protective effect against prolonged I/R injury, which involves prostaglandins derived from COX-1 and COX-2 enzymes. Moreover, it has been found that endogenous prostaglandin derived from the COX enzymes are involved in the mechanism of mucosal recovery from I/R-induced acute gastric erosions (Brzozowski et al., 1999).
Oral administration of the sPLA2 inhibitor provided less protection from histopathological changes than intravenous administration, suggesting that our method of oral administration or the intrinsic oral bioavailability of the inhibitor did not provide sufficient blood concentrations of the drug as the intravenous route for protection against intestinal injury. On the other hand, we did show that intravenous administration provided a blood concentration of inhibitor that was many times higher than following oral administration of the same dosage (unpublished observations). Greater protection against intestinal tissue injury may have been found with higher oral doses.
Clearly, there are many inflammatory mediators and mechanisms of injury in intestinal I/R causing pathology that is the result of a combination of factors including mucosal oedema, degradation of mucosal structure, loss of haemodynamic homeostasis and remote organ injury. The present study measured some of the features of intestinal I/R injury, not merely focusing on measuring one injury in a variety of different ways. It was therefore expected that the drugs in this study might protect against some measures of injury, while showing no protection against other features of intestinal I/R injury.
The finding that the NSAIDs flunixin and celebrex provided limited protection against intestinal I/R tends to support clinical impressions that treating horses with colic with flunixin may actually be detrimental (Campbell & Blikslager, 2000). The success of the leukotriene receptor antagonist in this study was also surprising because blocking one peptido leukotriene receptor of the AA cascade still leaves many other mediators free to cause damage. It is interesting, however, to compare this to a study by Sare et al. (1996), who measured leukotriene C4 and prostaglandin E2 production in intestinal I/R. They found that what should have been a substantial protection from intestinal I/R provided by blocking cysteinyl leukotriene production was limited as a result of the overflow effect of 5-lipoxygenase inhibition causing an increase in production of proinflammatory prostanoids such as prostaglandin E2. It is assumed that zafirlukast does not cause this overflow effect, as it is a receptor antagonist and thus the cysteinyl leukotrienes are still produced, but their actions are blocked. This may explain why zafirlukast has provided greater protection in this model than previously seen using lipoxygenase inhibitors (Sare et al., 1996).
SPLA2 (IIa) has been implicated as a candidate for I/R-induced membrane phospholipids degradation because of its Ca2+ dependency and nonspecific hydrolytic action toward the acylglycerol bonds of phospholipids (Van Bilsen & Van der Vusse, 1995; Murakami et al., 1998). One study clearly indicated that postischaemic cardiac accumulation of total unesterified fatty acids in general, and AA in particular, did not differ between the two substrains, which does not support a crucial role for group IIa sPLA2 in I/R-induced myocardial cell damage (De Windt et al., 2001). That study also suggested that PLA2 enzymes other than group IIa sPLA2 may be responsible for the enhanced phospholipid degradation in the transiently ischaemic heart (De Windt et al., 2001). Another study indicated that pretreatment with a nonspecific PLA2 inhibitor (methylprednisolone, dexamethasone or quinacrine) was ineffective in diminishing the reperfusion injury in either case (Boros et al., 1993). However, this contrasts with other studies which used the nonspecific PLA2 inhibitor quinacrine (Otamiri et al., 1987, 1988; Otamiri & Tagesson, 1989; Koike et al., 1992a), which reduced manifestations of gut I/R injury.
A potent group IIa inhibitor (LY315920) has been described (Snyder et al., 1999) and has been tested in an intestinal I/R model (Koike et al., 2000). This and close analogues (Hansford et al., 2003) are reported to be nonselective for group IIa over group V human recombinant enzymes (Chen & Dennis, 1998; Singer et al., 2002). Our sPLA2 inhibitor is ∼two-fold more potent against the group IIa enzyme than LY311299 (Hansford et al., 2003), but 170-fold more selective for the group IIa isoform over the group V isoform enzyme (Reid, unpublished). This motivated us to test only the current sPLA2-IIa inhibitor in this model, it being the only compound that we are aware of which is selective for IIa over V forms of sPLA2. We are therefore confident that the effects reported in the present study are most likely due to inhibition of the group IIa enzyme.
In summary, this study has demonstrated very promising tissue protection by an inhibitor of sPLA2 (IIa) and an LTC4 antagonist in a rat model of intestinal I/R injury. Since these compounds directly inhibit human receptors, they may also be effective in human I/R injury, reducing the degree of the damage of the gastrointestinal mucosa in occlusive conditions. However, the effectiveness of these compounds in clinically relevant (i.e. systemic low-flow-induced intestinal ischaemia) situations is uncertain and further studies are warranted.
Acknowledgments
We thank Mr Paul Addison for excellent technical assistance. All animal experimentation conducted in this study was performed in accordance with National Health & Medical Research Council (NHMRC) guidelines. This work was supported by a grant from the NHMRC of Australia.
Abbreviations
- AST
aspartate aminotransferase
- celebrex
celecoxib
- COX
cyclooxygenases
- flunixin
flunixin meglumine
- I/R
ischaemia–reperfusion
- LT
leukotrienes
- PAF
platelet activating factor
- PLA2
phospholipase A2
- PMNs
polymorphonuclear leucocytes
- SMA
superior mesenteric artery
- sPLA2I
sPLA2 inhibitor
- TNF
tumour necrosis factor
References
- ARUMUGAM T.V., SHIELS I.A., MARGOLIN S.B., TAYLOR S.M., BROWN L. Pirfenidone attenuates ischaemia–reperfusion injury in the rat small intestine. Clin. Exp. Pharmacol. Physiol. 2002a;29:996–1000. doi: 10.1046/j.1440-1681.2002.03766.x. [DOI] [PubMed] [Google Scholar]
- ARUMUGAM T.V., SHIELS I.A., WOODRUFF T.M., REID R.C., FAIRLIE D.P., TAYLOR S.M. Protective effect of a new C5a receptor antagonist against ischemia–reperfusion injury in the rat small intestine. J. Surg. Res. 2002b;103:260–267. doi: 10.1006/jsre.2002.6369. [DOI] [PubMed] [Google Scholar]
- BALSINDE J., BALBOA M.A., INSEL P.A., DENNIS E.A. Regulation and inhibition of phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- BINGHAM C.O., AUSTEN K.F. Phospholipase A2 enzymes in eicosanoid generation. Proc. Assoc. Am. Physicians. 1999;111:516–524. doi: 10.1046/j.1525-1381.1999.99321.x. [DOI] [PubMed] [Google Scholar]
- BOROS M., KARACSONY G., KASZAKI J., NAGY S. Reperfusion mucosal damage after complete intestinal ischemia in the dog: the effects of antioxidant and phospholipase A2 inhibitor therapy. Surgery. 1993;113:184–191. [PubMed] [Google Scholar]
- BOTHA A.J., MOORE F.A., MOORE E.E., SAUAIA A., BANERJEE A., PETERSON V.M. Early neutrophil sequestration after injury: a pathogenic mechanism for multiple organ failure. J. Trauma. 1995;39:411–417. doi: 10.1097/00005373-199509000-00003. [DOI] [PubMed] [Google Scholar]
- BRIDEAU C., VAN STADEN C., CHAN C.C. In vitro effects of cyclooxygenase inhibitors in whole blood of horses, dogs, and cats. Am. J. Vet. Res. 2001;62:1755–1760. doi: 10.2460/ajvr.2001.62.1755. [DOI] [PubMed] [Google Scholar]
- BRZOZOWSKI T., KONTUREK P.C., KONTUREK S.J., SLIWOWSKI Z., DROZDOWICZ D., STACHURA J., PAJDO R., HAHN E.G. Role of prostaglandins generated by cyclooxygenase-1 and cyclooxygenase-2 in healing of ischemia–reperfusion-induced gastric lesions. Eur. J. Pharmacol. 1999;385:47–61. doi: 10.1016/s0014-2999(99)00681-0. [DOI] [PubMed] [Google Scholar]
- CALHOUN W.J. Summary of clinical trials with zafirlukast. Am. J. Respir. Crit. Care. Med. 1998;157:S238–S245. [PubMed] [Google Scholar]
- CAMPBELL N.B., BLIKSLAGER A.T. The role of cyclo-oxygenase inhibitors in repair of ischaemic-injured jejunal mucosa in the horse. Equine Vet. J. Suppl. 2000;32:59–64. doi: 10.1111/j.2042-3306.2000.tb05335.x. [DOI] [PubMed] [Google Scholar]
- CATY M.G., GUICE K.S., OLDHAM K.T., REMICK D.G., KUNKEL S.I. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia–reperfusion injury. Ann. Surg. 1990;212:694–700. doi: 10.1097/00000658-199012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG J., MUSSER J.H., MCGREGOR H. Phospholipase A2: function and pharmacological regulation. Biochem. Pharmacol. 1987;36:2429–2436. doi: 10.1016/0006-2952(87)90512-0. [DOI] [PubMed] [Google Scholar]
- CHATTERJEE P.K., BROWN P.A., CUZZOCREA S., ZACHAROWSKI K., STEWART K.N., MOTA-FILIPE H., MCDONALD M.C., THIEMERMANN C. Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int. 2001;59:2073–2083. doi: 10.1046/j.1523-1755.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- CHEN Y., DENNIS E.A. Expression and characterisation of human group V phospholipase A2. Biochim. Biophys. Acta. 1998;1394:57–64. doi: 10.1016/s0005-2760(98)00098-8. [DOI] [PubMed] [Google Scholar]
- CHIU C.J., MCARDLE A., BROWN R., SCOTT H.J., GURD F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- CHRISTENSON J.T., AEBERHARD J.M., BADEL P., PEPCAK F., MAURICE J., SIMONET F., VELEBIT V., SCHMUZIGER M. Adult respiratory distress syndrome after cardiac surgery. Cardiovasc. Surg. 1996;4:15–21. doi: 10.1016/0967-2109(96)83778-1. [DOI] [PubMed] [Google Scholar]
- DAVIES N.M., MCLACHLAN A.J., DAY R.O., WILLIAMS K.M. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin. Pharmacokinet. 2000;38:225–242. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]
- DE WINDT L.J., WILLEMS J., ROEMEN T.H., COUMANS W.A., RENEMAN R.S., VAN DER VUSSE G.J., VAN BILSEN M. Ischemic–reperfused isolated working mouse hearts: membrane damage and type IIA phospholipase A2. Am. J. Physiol. 2001;280:H2572–H2580. doi: 10.1152/ajpheart.2001.280.6.H2572. [DOI] [PubMed] [Google Scholar]
- FENG L., SUN W., XIA Y., TANG W.W., CHANMUGAM P., SOYOOLA E., WILSON C.B., HWANG D. Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch. Biochem. Biophys. 1993;307:361–368. doi: 10.1006/abbi.1993.1601. [DOI] [PubMed] [Google Scholar]
- FILEP J., BRAQUET P., MOZES T. Significance of platelet-activating factor in mesenteric ischemia–reperfusion. Lipids. 1991;26:1336–1339. doi: 10.1007/BF02536561. [DOI] [PubMed] [Google Scholar]
- FINK M.P. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit. Care Med. 1991;19:627–641. doi: 10.1097/00003246-199105000-00009. [DOI] [PubMed] [Google Scholar]
- GOLDMAN G., WELBOURN R., KLAUSNER J.M., VALERI C.R., SHEPRO D., HECHTMAN H.B. Oxygen free radicals are required for ischemia-induced leukotriene B4 synthesis and diapedesis. Surgery. 1992;111:287–293. [PubMed] [Google Scholar]
- GRANGER D.N., MCCORD J.M., PARKS D.A., HOLLWARTH M.E. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986;90:80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- GRANGER D.N., RUTILI G., MCCORD J.M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- GRISHAM M.B., HERNANDEZ L.A., GRANGER D.N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am. J. Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- HAGLUND U. Gut ischaemia. Gut. 1994;35:S73–S76. doi: 10.1136/gut.35.1_suppl.s73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGLUND U., BULKLEY G.B., GRANGER D.N. On the pathophysiology of intestinal ischemic injury. Acta. Chir. Scand. 1987;153:321–324. [PubMed] [Google Scholar]
- HANSFORD K.A., REID R.C., CLARK C.I., TYNDALL J.D.A., WHITEHOUSE M.W., GUTHRIE T., MCGEARY R.P., SCHAFER K., MARTIN J.L., FAIRLIE D.P. D-Tyrosine as a chiral precursor to potent inhibitors of human non-pancreatic secretory phospholipase A2 (IIa) with anti-inflammatory activity. Chem. Bio. Chem. 2003;4:101–105. doi: 10.1002/cbic.200390029. [DOI] [PubMed] [Google Scholar]
- HAYWARD R., LEFER A.M. Time course of endothelial–neutrophil interaction in splanchnic artery ischemia–reperfusion. Am. J. Physiol. 1998;275:H2080–H2086. doi: 10.1152/ajpheart.1998.275.6.H2080. [DOI] [PubMed] [Google Scholar]
- JOCHLE W., MOORE J.N., BROWN J., BAKER G.J., LOWE J.E., FUBINI S., REEVES M.J., WATKINS J.P., WHITE N.A. Comparison of detomidine, butorphanol, flunixin meglumine and xylazine in clinical cases of equine colic. Equine Vet. J. 1989;7:111–116. doi: 10.1111/j.2042-3306.1989.tb05668.x. [DOI] [PubMed] [Google Scholar]
- KARASAWA A., GUO J.P., MA X.L., TSAO P.S., LEFER A.M. Protective actions of a leukotriene B4 antagonist in splanchnic ischemia and reperfusion in rats. Am. J. Physiol. 1991;261:G191–G198. doi: 10.1152/ajpgi.1991.261.2.G191. [DOI] [PubMed] [Google Scholar]
- KHANNA A., ROSSMAN J.E., FUNG H.L., CATY M.G. Intestinal and hemodynamic impairment following mesenteric ischemia/reperfusion. J. Surg. Res. 2001;99:114–119. doi: 10.1006/jsre.2001.6103. [DOI] [PubMed] [Google Scholar]
- KIM F.J., MOORE E.E., MOORE F.A., BIFFL W.L., FONTES B., BANERJEE A. Reperfused gut elaborates PAF that chemoattracts and primes neutrophils. J. Surg. Res. 1995;58:636–640. doi: 10.1006/jsre.1995.1100. [DOI] [PubMed] [Google Scholar]
- KIRSCHNER R.E., CHIAO J.J., FYFE B.S., HOFFMAN L.A., DAVIS J.M., FANTINI G.A. Neutrophil lipoxygenase activation and leukosequestration in postischemic myocutaneous flaps: role of LTB4. Am. J. Physiol. 1995;268:H2167–H2174. doi: 10.1152/ajpheart.1995.268.6.H2167. [DOI] [PubMed] [Google Scholar]
- KOIKE K., MOORE E.E., MOORE F.A., CARL V.S., PITMAN J.M., BANERJEE A. Phospholipase A2 inhibition decouples lung injury from gut ischemia–reperfusion. Surgery. 1992a;112:173–180. [PubMed] [Google Scholar]
- KOIKE K., MOORE F.A., MOORE E.E., POGGETTI R.S., TUDER R.M., BANERJEE A. Endotoxin after gut ischemia/reperfusion causes irreversible lung injury. J. Surg. Res. 1992b;52:656–662. doi: 10.1016/0022-4804(92)90145-p. [DOI] [PubMed] [Google Scholar]
- KOIKE K., YAMAMOTO Y., HORI Y., ONO T. Group IIA phospholipase A2 mediates lung injury in intestinal ischemia–reperfusion. Ann. Surg. 2000;232:90–97. doi: 10.1097/00000658-200007000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANGINO M.J., MURPHY M.K., ANDERSON C.B. Effects of the arachidonate 5-lipoxygenase synthesis inhibitor A-64077 in intestinal ischemia–reperfusion injury. J. Pharmacol. Exp. Ther. 1994;269:75–81. [PubMed] [Google Scholar]
- MURAKAMI M., KAMBE T., SHIMBARA S., KUDO I. Functional coupling between various phospholipase A2 s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., SHIMBARA S., KAMBE T., KUWATA H., WINSTEAD M.V., TISCHFIELD J.A., KUDO I. The function of five distinct mammalian phospholipase A2 s in regulating arachidonic acid release. J. Biol. Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- OTAMIRI T., FRANZEN L., LINDMARK D., TAGESSON C. Increased phospholipase A2 and decreased lysophospho-lipase activity in the small intestinal mucosa after ischaemia and revascularisation. Gut. 1987;28:1445–1453. doi: 10.1136/gut.28.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTAMIRI T., LINDAHL M., TAGESSON C. Phospholipase A2 inhibition prevents mucosal damage associated with small intestinal ischaemia in rats. Gut. 1988;29:489–494. doi: 10.1136/gut.29.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTAMIRI T., TAGESSON C. Role of phospholipase A2 and oxygenated free radicals in mucosal damage after small intestinal ischemia and reperfusion. Am. J. Surg. 1989;157:562–566. doi: 10.1016/0002-9610(89)90699-5. [DOI] [PubMed] [Google Scholar]
- PAJDO R., BRZOZOWSKI T., KONTUREK P.C., KWIECIEN S., KONTUREK S.J., SLIWOWSKI Z., PAWLIK M., PTAK A., DROZDOWICZ D., HAHN E.G. Ischemic preconditioning, the most effective gastroprotective intervention: involvement of prostaglandins, nitric oxide, adenosine and sensory nerves. Eur. J. Pharmacol. 2001;427:263–276. doi: 10.1016/s0014-2999(01)01246-8. [DOI] [PubMed] [Google Scholar]
- PARK P.O., HAGLUND U., BULKLEY G.B., FALT K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574–580. [PubMed] [Google Scholar]
- PARTRICK D.A., MOORE F.A., MOORE E.E., BARNETT C.C., JR, SILLIMAN C.C. Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New Horiz. 1996;4:194–210. [PubMed] [Google Scholar]
- POGGETTI R.S., MOORE F.A., MOORE E.E., BENSARD D.D., ANDERSON B.O., BANERJEE A. Liver injury is a reversible nuetrophil-mediated event following gut ischaemia. Arch. Surg. 1992;127:175–179. doi: 10.1001/archsurg.1992.01420020057009. [DOI] [PubMed] [Google Scholar]
- REYNOLDS L.J., HUGHES L.L., DENNIS E.A. Analysis of human synovial fluid phospholipase A2 on short chain phosphatidylcholine-mixed micelles: development of a spectrophotometric assay suitable for a microtiterplate reader. Anal. Biochem. 1992;204:190–197. doi: 10.1016/0003-2697(92)90160-9. [DOI] [PubMed] [Google Scholar]
- SARE M., BOZKURT S., ONUK E., OGUZ M., GUREL M., ERCAN S. The effects of indomethacin, NDGA, allopurinol and superoxide dismutase on prostaglandin E2 and leukotriene C4 levels after mesenteric ischemia–reperfusion injury. Prostaglandins Leukot. Essent. Fatty Acids. 1996;55:379–383. doi: 10.1016/s0952-3278(96)90120-9. [DOI] [PubMed] [Google Scholar]
- SCHEUER W. Phospholipase A2–regulation and inhibition. Klin. Wochenschr. 1989;67:153–159. doi: 10.1007/BF01711343. [DOI] [PubMed] [Google Scholar]
- SCHOENBERG M.H., BEGER H.G. Reperfusion injury after intestinal ischemia. Crit. Care Med. 1993;21:1376–1386. doi: 10.1097/00003246-199309000-00023. [DOI] [PubMed] [Google Scholar]
- SEMRAD S.D., SAMS R.A., HARRIS O.N., ASHCRAFT S.M. Effects of concurrent administration of phenylbutazone and flunixin meglumine on pharmacokinetic variables and in vitro generation of thromboxane B2 in mares. Am. J. Vet. Res. 1993;54:1901–1905. [PubMed] [Google Scholar]
- SHENG Z.Y., DONG Y.L., WANG X.H. Bacterial translocation and multiple system organ failure in bowel ischemia and reperfusion. Chin. Med. J. 1991;104:897–903. [PubMed] [Google Scholar]
- SHORT A.J., PACZKOWSKI N.J., VOGEN S.M., SANDERSON S.D., TAYLOR S.M. Response-selective C5a agonists: differential effects on neutropenia and hypotension in the rat. Br. J. Pharmacol. 1999;128:511–514. doi: 10.1038/sj.bjp.0702847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER A.G., GHOMASHCHI F., LE CALVEZ C BOLLINGER J., BEZZINE S., ROUAULT M., SADILEK M., NGUYEN E., LAZDUNSKIL M., LAMBEAU G., GELB M.H. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- SNYDER D.W., BACH N.J., DILLARD R.D., DRAHEIM S.E., CARLSON D.G., FOX N., ROEHM N.W., ARMSTRONG C.T., CHANG C.H., HARTLEY L.W., JOHNSON L.M., ROMAN C.R., SMITH A.C., SONG M., FLEISCH J.H. Pharmacology of LY315920/S-5920, [[3-(aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy] acetate, a potent and selective secretory phospholipase A2 inhibitor: a new class of anti-inflammatory drugs, SPI. J. Pharmacol. Exp. Ther. 1999;288:1117–1124. [PubMed] [Google Scholar]
- STEPHENSON A.H., LONIGRO A.J., HYERS T.M., WEBSTER R.O., FOWLER A.A. Increased concentrations of leukotrienes in bronchoalveolar lavage fluid of patients with ARDS or at risk for ARDS. Am. Rev. Respir. Dis. 1988;138:714–719. doi: 10.1164/ajrccm/138.3.714. [DOI] [PubMed] [Google Scholar]
- SUN Z., WANG X., LASSON A., BORJESSON A., LEVEAU P., HARALDSEN P., ANDERSSON R. Roles of platelet-activating factor, interleukin-1beta and interleukin-6 in intestinal barrier dysfunction induced by mesenteric arterial ischemia and reperfusion. J. Surg. Res. 1999;87:90–100. doi: 10.1006/jsre.1999.5746. [DOI] [PubMed] [Google Scholar]
- TADROS T., TRABER D.L., HEGGERS J.P., HERNDON D.N. Angiotensin II inhibitor DuP753 attenuates burn- and endotoxin-induced gut ischemia, lipid peroxidation, mucosal permeability, and bacterial translocation. Ann. Surg. 2000;231:566–576. doi: 10.1097/00000658-200004000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNAGE R.H., KADESKY K.M., BARTULA L., MYERS S.I. Pulmonary thromboxane release following intestinal reperfusion. J. Surg. Res. 1995;58:552–557. doi: 10.1006/jsre.1995.1087. [DOI] [PubMed] [Google Scholar]
- TURNAGE R.H., LANOUE J.L., KADESKY K.M., MENG Y., MYERS S.I. Thromboxane A2 mediates increased pulmonary microvascular permeability after intestinal reperfusion. J. Appl. Physiol. 1997;82:592–598. doi: 10.1152/jappl.1997.82.2.592. [DOI] [PubMed] [Google Scholar]
- VAJDOVA K., SMREKOVA R., KUKAN M., LUTTEROVA M., WSOLOVA L. Bile analysis as a tool for assessing integrity of biliary epithelial cells after cold ischemia–reperfusion of rat livers. Cryobiology. 2000;41:145–152. doi: 10.1006/cryo.2000.2276. [DOI] [PubMed] [Google Scholar]
- VAN BILSEN M., VAN DER VUSSE G.J. Phospholipase A2-dependent signaling in the heart. Cardiovasc. Res. 1995;30:518–529. doi: 10.1016/0008-6363(95)00098-4. [DOI] [PubMed] [Google Scholar]
- WADA K., MONTALTO M.C., STAHL G.L. Inhibition of complement C5 reduces local and remote organ injury after intestinal ischemia/reperfusion in the rat. Gastroenterology. 2001;120:126–133. doi: 10.1053/gast.2001.20873. [DOI] [PubMed] [Google Scholar]
- WATTANASIRICHAIGOON S., MENCONI M.J., FINK M. Lisofylline ameliorates intestinal and hepatic injury induced by hemorrhage and resuscitation in rats. Crit. Care Med. 2000;28:1540–1549. doi: 10.1097/00003246-200005000-00047. [DOI] [PubMed] [Google Scholar]