Abstract
Long-term treatment of rats with Nω-nitro-L-arginine methyl ester (L-NAME) induces hypertension associated with inflammatory and vascular changes. Leukotrienes are proinflammatory vasoactive products that are suspected to be involved in the pathogenesis of hypertension. We investigated, in rats chronically treated with L-NAME, the involvement of leukotrienes in the in vivo regulation of blood pressure and the in vitro contraction elicited by noradrenaline in isolated aorta.
Rats were randomly assigned to four groups and orally treated for 3 weeks with L-NAME (1 mg ml−1), L-NAME (1 mg ml−1) plus the leukotriene biosynthesis inhibitor MK-886 (0.1 mg ml−1), MK-886 (0.1 mg ml−1) alone or vehicle (Methocel, 0.1%). All the drugs were added to the drinking fluid.
The mean arterial blood pressure (MABP) increased significantly in L-NAME-treated rats (173.3±9.4 mmHg (n=25)) vs Methocel-treated rats (110.7±4.8 mmHg (n=11), P<0.001). Chronic treatment with MK-886 prevented this rise in MABP.
Aortic rings with or without endothelium were suspended in organ baths for recording isometric changes in response to noradrenaline. Pretreatment with either MK-886 (10 μM), the CysLT1 receptor antagonist MK571 (1 μM) or the dual CysLT1/CysLT2 receptor antagonist BAY-u9773 (0.1 μM) reduced (P<0.05) noradrenaline-induced contractions in intact aortic rings from L-NAME-treated rats only.
Noradrenaline (0.3 μM) induced a two-fold increase in cysteinyl leukotriene (CysLT) release (measured by enzyme immunoassay) in intact aortic rings from L-NAME-treated rats only.
These data suggested (1) a role for the 5-lipoxygenase pathway in the regulation of blood pressure in L-NAME-treated rats and (2) the involvement of endothelial CysLTs in noradrenaline-induced contraction in aorta from L-NAME-treated rats.
Keywords: Cysteinyl leukotrienes, endothelium, 5-lipoxygenase, nitric oxide inhibition, noradrenaline, hypertension, rat aorta
Introduction
Nitric oxide (NO) is an important modulator of vascular function and blood pressure and chronic inhibition of NO synthesis by Nω-nitro-L-arginine methyl ester (L-NAME) in normotensive rats induces sustained hypertension. This experimental model of hypertension is of particular interest because it is associated with alteration of vascular reactivity (Henrion et al., 1996) and inflammatory changes (Tomita et al., 1998).
Leukotrienes are vasoactive and proinflammatory metabolites of arachidonic acid. Their synthesis is initiated by the calcium-dependent activation of 5-lipoxygenase (5-LOX) (Rouzer & Kargman, 1988) and its further interaction with the nuclear membrane-bound 5-LOX activating protein (FLAP) (Dixon et al., 1990; Peters-Golden & Brock, 2001). Compelling evidence suggests that cysteinyl leukotrienes (CysLTs) may play a major role in the pathogenesis of cardiovascular diseases (Folco et al., 2000), particularly hypertension (Stanke-Labesque et al., 2001). In addition, the aorta from hypertensive rats displays an increased sensitivity to CysLTs (Stanke-Labesque et al., 2001) and CysLTs enhance the contractile response to noradrenaline in normotensive rats (Lawson et al., 1988).
In L-NAME-treated hypertensive rats, adrenergic tone plays an important role in the rise in blood pressure, since this is prevented by chronic treatment with the alpha1 adrenoceptor antagonist prazosin (Wangensteen et al., 2002). Moreover, noradrenaline can modulate leukotriene release (Kerttula et al., 1995). This action is primarily a consequence of phospholipase activation, resulting in the formation of free arachidonic acid, which is then available for metabolism by oxygenases (LaBelle & Polyak, 1998).
The aim of the present study was therefore to investigate the effect of inhibition of leukotriene biosynthesis with the FLAP inhibitor MK-886 on blood pressure elevation and on the contractile response to noradrenaline in L-NAME-treated rats.
Methods
The care and use of animals in this work were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1985).
Experimental groups
Experiments were conducted on 63 adult male Wistar rats (weight range 220–240 g) from IFFA CREDO (France). Rats were separated randomly into four groups. The first group (Methocel rats) received Methocel (methylcellulose, 0.1%) in their drinking water, since 0.1% Methocel was recommended by Merck Frosst for the solubilisation of MK-886. The second group (L-NAME rats) received the NO synthase inhibitor L-NAME (1 mg ml−1) and 0.1% Methocel (Tomita et al., 1998; Usui et al., 2000) in their drinking water. At this concentration, the daily intake of L-NAME was 30–40 mg day−1. This dose of L-NAME has been chosen, since chronic treatment with L-NAME (1 mg ml−1) induced inflammatory changes in the vessels (Katoh et al., 1998; Usui et al., 2000). The third group (L-NAME-MK-886 rats) received the leukotriene synthesis inhibitor MK-886 (0.1 mg ml−1) (Salas et al., 1998), L-NAME (1 mg ml−1) and 0.1% Methocel in their drinking water. This dose of MK-886 has been reported to effectively block leukotriene release in rats (Raychaudhuri et al., 1993). The fourth group (MK-886 rats) received MK-886 (0.1 mg ml−1) and 0.1% Methocel in their drinking water. We measured the actual volume of water drunk by each rat on a daily basis and confirmed that all animals drank 30–40 ml of the water containing the drugs. The treatments were carried out for 3 weeks.
The rats were anaesthetised by intraperitoneal injection of sodium pentobarbital (50 mg kg−1), and mean arterial blood pressure (MABP) was measured as previously described (Stanke-Labesque et al., 2001; 2002). The right carotid artery of the rats was cannulated with polyethylene tubing (PE-50) connected to a pressure transducer (Statham, U.S.A.) for recording arterial blood pressure on a polygraph (Windograph, Gould Instruments, U.S.A.).
Vessel preparation
After measurement of MABP, the thoracic aorta was excised, transferred to a dish filled with Krebs bicarbonate buffer, cleared of periadventitial tissue and cut into ring segments (3.0 mm in length). In some rings, the endothelium was removed by gentle rubbing of the intimal surface with small forceps; in the remaining rings, care was taken not to touch the inner surface of the blood vessels.
Measurement of isometric tension in rings of aorta
Studies of tension development were performed on aortic ring segments using a previously reported method (Stanke-Labesque et al., 2001; 2002). The aortic rings were initially stretched to a given preload of 1.5 g. After the 60-min equilibration period, experiments were initiated by inducing a reference contraction in response to KCl (90 mM) in each ring. In intact aortic rings, the contractions elicited by 90 mM KCl were, for each group of rats, 3578±120 mg (Methocel rats, n=71), 3561±98 mg (L-NAME rats, n=135), 4094±111 mg (L-NAME-MK-886 rats, n=73) and 4090±170 mg (MK-886 rats, n=28). In endothelium-denuded aortic rings, the contractions elicited by 90 mM KCl were: 2904±134 mg (Methocel rats, n=57), 2699±155 mg (L-NAME rats, n=57), 2779±118 mg (L-NAME-MK-886 rats, n=32) and 2510±150 mg (MK-886 rats, n=21).
Endothelial function was assessed by testing the relaxant effect of acetylcholine (10 nM–0.1 mM) on aortic rings precontracted with phenylephrine (30 nM–0.1 μM). The failure of acetylcholine to elicit relaxation of aortic rings previously subjected to rubbing of the intimal surface was taken as a proof of endothelium removal. Subsequently, the rings were allowed to equilibrate for another hour, during which the Krebs solution was changed every 15 min. Only one cumulative concentration–response curve was established for noradrenaline (0.5 log increments, 0.1 nM–3 μM) in each ring.
The contribution of CysLTs to the contractile response to noradrenaline was assessed by prior incubation of the preparations for 30 min with the specific FLAP inhibitor MK-886 (10 μM) (Rouzer et al., 1990), the specific CysLT1 receptor antagonist MK571 (1 μM) (Lynch et al., 1999) or the dual CysLT1/CysLT2 receptor antagonist BAY-u9773 (0.1 μM) (Heise et al., 2000; Nothacker et al., 2000). For all experiments, appropriate controls (incubation with vehicle) were run under similar experimental conditions in rings obtained from the same aorta.
The contractile effects of the CysLTs were also assessed in intact aortic rings from both Methocel and L-NAME rats. Cumulative concentration–response curves were constructed for LTC4 and LTD4 (1 log increment, 1 pM–0.1 μM) vs basal tone. In addition, cumulative concentration–response curves for LTD4 were performed on aortic rings precontracted with phenylephrine (30 nM–0.1 μM) in the presence of MK571 (1 μM, 30 min) or vehicle. Lastly, since LTD4 has been shown to enhance noradrenaline-induced contraction in rat aorta (Lawson et al., 1988), the effect of LTD4 on the contractile response to noradrenaline was studied on intact aortic rings from Methocel rats. Aortic rings were pretreated with LTD4 (1, 10 nM or 0.1 μM) for 3 min (Lawson et al., 1988) before the cumulative addition of noradrenaline.
Measurement of CysLT release in rings of rat aorta
The levels of total CysLTs (LTC4, LTD4 and LTE4) released by aortic rings in response to noradrenaline were measured as previously described (Stanke-Labesque et al., 2001; 2002). Briefly, intact and endothelium-denuded aortic rings from all rat groups were incubated for 30 min with either noradrenaline (0.3 μM, 30 min) or vehicle (control experiments). The intact aortic rings from L-NAME rats were also challenged with noradrenaline (3 μM) in order to check whether noradrenaline induced a concentration-dependent release of CysLTs. The Krebs solution was collected and samples were frozen at −80°C for later measurement of CysLT levels. The rings were dried in an oven for measurement of dry weight tissue. CysLTs were measured by enzyme immunoassay according to the manufacturer's instructions using reagents purchased from Cayman (Ann Arbor, U.S.A.). The detection limit of the assay was 3.2 pg ml−1, the EC50 value (50% B B0−1) was 36.6 pg ml−1 and the intra and interassay coefficients of variation were <10%.
Drugs
KCl (Prolabo Normapur grade), acetylcholine, noradrenaline, AA861 (2,3,5-trimethyl-6-(12-hydroxy-5, 10-dodecadynyl)-1, 4-benzoquinone), L-NAME and Methocel were from Sigma (L'isle d'Abeau, France). Leukotriene C4, leukotriene D4, leukotriene E4 and the CysLT1 receptor antagonist MK571 (propanoic acid, 3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl][3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]-(E)-, sodium salt) were purchased from Cayman (Ann. Arbor, U.S.A.) 3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2-dimethylpropanoic acid (MK-886) was kindly provided by Merck Frosst, Zuebee, Canada. BAY-u9773 was from Biomol. Plymouth, U.S.A. Drugs were kept at –20°C or, for leukotrienes −80°C, and freshly dissolved in distilled water to the appropriate concentration expressed as the final molar concentration in the organ bath.
Statistical analysis
Contractile responses were expressed as a percentage of the contraction induced by 90 mM KCl. The maximal effect (Emax) was the greatest response obtained with the agonist. The concentration of agonist producing 50% of the maximal effect (EC50) was determined from each curve using a logistic curve-fitting equation. The pD2 value is the negative logarithm of the EC50. The data for CysLTs were expressed as percentages of the control values. The data for the control values were given as pg mg−1 dry weight tissue. Results are expressed as means±standard error of the mean (s.e.m.) for the specified number (n) of preparations tested. Statistical analyses were performed using analysis of variance (ANOVA) for repeated measures, followed by the Bonferroni corrected t-test. Individual comparisons were made by Student's t-test for unpaired data. P-values <0.05 were considered significant.
Results
Body weight and MABP
After the 3-week treatment period, body weight was significantly less in L-NAME rats than in Methocel rats, whereas it was similar in Methocel, MK-886 and L-NAME-MK-886 rats (Table 1).
Table 1.
Body weight (expressed as g) and MABP (expressed as mmHg) of Methocel, L-NAME, L-NAME-MK-886 and MK-886 rats
| Parameters | Methocel | L-NAME | L-NAME-MK-886 | MK-886 |
|---|---|---|---|---|
| Body weight | 409.5±21.1 | 370.0±9.5* | 395.6±16.9 | 440.0±6.7 |
| MABP | 110.7±4.8 | 173.3±9.4* | 128.4±8.2 | 127.5±3.8 |
| Numbers of rats | 11 | 25 | 17 | 10 |
Results are expressed as means±s.e.m. for the number of animals indicated.
P<0.001 vs Methocel, MK-886 and L-NAME-MK-886 rats.
Mean arterial blood pressure was markedly increased in the L-NAME group compared with the Methocel group (Table 1). Chronic treatment with MK-886 prevented this elevation of MABP (Table 1). In contrast, MABP was similar in Methocel and MK-886 rats (Table 1).
Organ chamber experiments
Noradrenaline induced concentration-dependent contractions of isolated aortic rings from all rat groups (Figure 1). In terms of potency (pD2), the contractions elicited by noradrenaline in aortic rings with the endothelium present were greater in L-NAME rats than in Methocel rats (Table 2). In terms of efficacy, the response to noradrenaline (expressed either as a percentage of the contraction elicited by KCl (90 mM) or as milligrams of isometric force) was not significantly different in rings with or without endothelium from Methocel and L-NAME rats (Table 2). On the other hand, the amplitude of the contraction elicited by noradrenaline was significantly reduced in intact aortic rings from L-NAME-MK-886 rats compared with those in intact aortic rings from Methocel, L-NAME and MK-886 rats (Table 2).
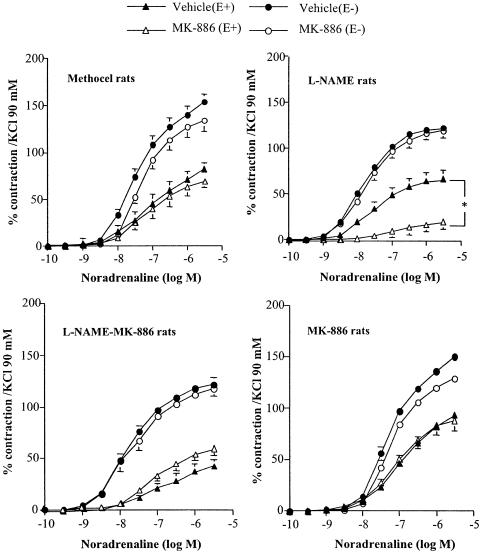
Figure 1.
Effect of MK-886 (10 μM) on concentration–response curves for noradrenaline in aortic rings with (E+) or without (E−) endothelium from Methocel, L-NAME, L-NAME-MK-886 and MK-886 rats. Results are expressed as a percentage of the contraction elicited by 90 mM KCl and are presented as means±s.e.m. of experiments performed on n aortic rings as indicated in Table 2. *P<0.05 vs respective vehicle.
Table 2.
Effects of MK-886 (10 μM), AA861 (10 μM), MK571 (1 μM) and BAY-u9773 (0.1 μM) or vehicle on noradrenaline-induced contraction in aorta from all rat groups
| Methocel | L-NAME | L-NAME MK-886 | MK-886 | ||
|---|---|---|---|---|---|
| With endothelium | |||||
| Vehicle | pD2 | 6.9±0.2 (19) | 7.3±0.1* (22) | 6.9±0.1 (17) | 6.9±0.1 (8) |
| Emax (%) | 90.0±9.2 (19) | 74.7±7.7 (22) | 31.1±4.6 (17) | 95.8±4.5 (8) | |
| Emax (mg) | 2834±260 | 2281±186 | 1348±205 | 3370±312 | |
| MK-886 | pD2 | 6.7±0.2 (6) | 7.0±0.1** (8) | 6.8±0.1 (10) | 7.1±0.1 (6) |
| Emax | 79.0±4.2 (6) | 18.8±3.6** (8) | 24.7±4.0 (10) | 87.8±9.7 (6) | |
| AA861 | pD2 | 7.2±0.1 (5) | 7.2±0.1 (10) | 7.2±0.1 (10) | 6.9±0.1 (6) |
| Emax | 94.7±10.2 (5) | 47.0±8.4** (10) | 29.8±6.4 (6) | 102.9±16.9(6) | |
| MK571 | pD2 | 7.1±0.3 (6) | 7.0±0.1** (7) | 7.0±0.1 (7) | 6.9±0.1 (6) |
| Emax | 73.0±11.1 (6) | 43.4±9.4** (7) | 48.8±3.4 (7) | 93.3±7.8 (6) | |
| BAY-u9773 | pD2 | ND | 7.0±0.1* (9) | ND | ND |
| Emax | ND | 27.6±3.2** (9) | ND | ND | |
| Without endothelium | |||||
| Vehicle | pD2 | 7.4±0.1 (18) | 7.7±0.1 (17) | 7.7±0.1 (15) | 7.3±0.1 (8) |
| Emax (%) | 155.7±9.1 (18) | 122.6±5.6 (17) | 113.0±5.1 (15) | 150.6±4.1 (8) | |
| Emax (mg) | 3032±222 | 2322±207 | 2765±0.365 | 3195±234 | |
| MK-886 | pD2 | 7.5±0.1 (6) | 7.6±0.1 (7) | 7.6±0.2 (9) | 7.3±0.1 (6) |
| Emax | 134.2±8.0 (6) | 119.4±7.6 (13) | 95.9±7.2 (9) | 134.0±6.6 (6) | |
| MK571 | pD2 | 7.5±0.1 (4) | 7.8±0.1 (6) | 7.6±0.2 (6) | 7.3±0.1 (6) |
| Emax | 127.2±5.4 (4) | 132.1±7.0 (6) | 117.4±5.7 (6) | 146.7±9.8 (6) | |
| BAY-u9773 | pD2 | ND | 7.6±0.1 (6) | ND | ND |
| Emax | ND | 140.7±20.8 (6) | ND | ND | |
The Emax values of noradrenaline in the presence of vehicle are expressed as a percentage of the contraction elicited by 90 mM KCl and as mg of isometric force. All results are expressed as mean±s.e.m. of n aortic rings as indicated in parentheses.
P<0.05 vs Methocel, L-NAME-MK-886 and MK-886 rats; P<0.001 vs Methocel, MK-886 and L-NAME rats;
P<0.05 vs respective vehicle; ND: not determined.
In intact aorta from L-NAME rats, the FLAP inhibitor MK-886 (10 μM) reduced the resting tone by 36.7±20.6 mg (n=8) and significantly reduced the amplitude of the contraction elicited by noradrenaline by 68% (Figure 1, Table 2). In the same preparation, pretreatment with the 5-LOX inhibitor AA861 (10 μM) also decreased the resting tone, by 26.0±10.0 mg (n=10), and reduced the contractile response to noradrenaline by 31.7% (Table 2). In contrast, in endothelium-denuded aortic rings from L-NAME rats, MK-886 did not affect the noradrenaline-induced contractions (Figure 1, Table 2). In aortic rings from Methocel, L-NAME-MK-886 and MK-886 rats, with or without endothelium, MK-886 (10 μM) did not affect the resting tone and did not change the noradrenaline-induced contractions (Figure 1, Table 2).
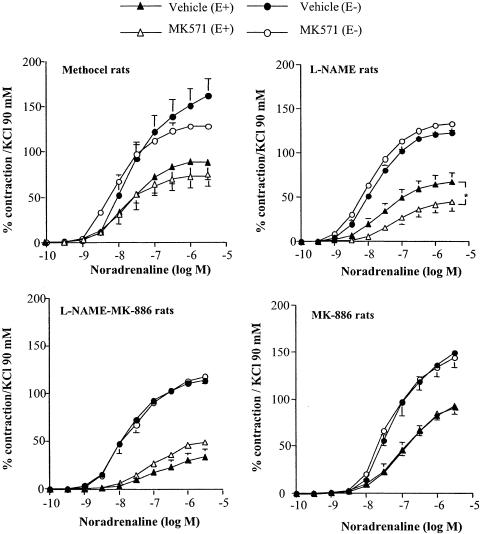
The CysLT1 receptor antagonist MK571 (1 μM) decreased the resting tone in intact aortic rings from L-NAME rats by 21.9±8.3 mg (n=13) and significantly reduced, by 47.8%, the contraction elicited by noradrenaline (Figure 2, Table 2). In the same preparation, the CysLT1/CysLT2 receptor antagonist BAY-u9773 (0.1 μM) also decreased the resting tone by 26.6±13.6 mg (n=9) and significantly reduced the amplitude of the noradrenaline-induced contraction by 59.7%. Pretreatment with MK571 or BAY-u9773, on the other hand, did not change the contraction elicited by noradrenaline in endothelium-denuded aortic rings from L-NAME rats. Similarly, in aortic rings with or without endothelium from Methocel, L-NAME-MK-886 and MK-886 rats, MK571 did not significantly change the noradrenaline-evoked contraction (Figure 2).
Figure 2.
Effect of MK571 (1 μM) on concentration–response curves for noradrenaline in aortic rings with (E+) or without (E−) endothelium from Methocel, L-NAME, L-NAME-MK-886 and MK-886 rats. Results are expressed as a percentage of the contraction elicited by 90 mM KCl and are presented as means±s.e.m. of experiments performed on n aortic rings as indicated in Table 2. *P<0.05 vs respective vehicle.
LTC4, LTD4 and LTE4 induced no contraction of intact aortic rings from Methocel rats (n=3 for each leukotriene, data not shown). In aortic rings from L-NAME rats, LTC4 and LTE4 had no effect on the basal tone (n=3 for LTC4 and LTE4, data not shown), whereas LTD4 at a concentration of 0.1 μM elicited weak but stable contractions (78.7±35.6 mg, n=3). In phenylephrine-precontracted aortic rings, LTD4 induced a biphasic effect. At concentrations between 10 pM and 10 nM, LTD4 induced a concentration-dependent relaxation of the aortic rings that was much weaker (P<0.001) in L-NAME than Methocel rats (Figure 3). At a concentration of 0.1 μM, LTD4 induced a contraction that was greater (P<0.001) in aortic rings from L-NAME rats than in rings from Methocel rats (Figure 3). Pretreatment with MK571 (1 μM) abolished the LTD4-induced contraction (Figure 3). Furthermore, when aortic rings from Methocel rats were pretreated with subthreshold concentrations of LTD4, the contractile responses to noradrenaline were not significantly changed: the pD2 values were 7.1±0.1 (n=6), 7.1±0.1 (n=6), 7.0±0.1 (n=6) and 7.0±0.2 (n=6), and the Emax values were 110.6±9.2 (n=6), 113.9±4.3 (n=6), 117.9±7.4 (n=6) and 99.8±9.4 (n=6), in the presence of 1, 10 nM and 0.1 μM LTD4 and vehicle, respectively.
Figure 3.
(a) Concentration–response curves for LTD4 in aortic rings with endothelium from Methocel and L-NAME rats. (b) Effect of MK571 (1 μM) on LTD4-evoked vascular effects in aortic rings with endothelium from L-NAME rats. Results are expressed as a percentage of relaxation/phenylephrine-induced tone and are presented as means±s.e.m. of experiments performed on four aortic rings from four Methocel and four L-NAME rats. *P<0.001 vs Methocel rats, **P<0.05 vs vehicle.
Release of CysLTs in rings of rat aorta
Noradrenaline induced a significant two-fold increase over the control values in CysLT release in intact aortic rings from L-NAME rats (Table 3). This increase in CysLTs in response to noradrenaline was concentration-dependent, since 3 μM noradrenaline induced a greater increase in CysLT release (percentage of control values: 320.7±79.2 (n=4), P<0.001 vs control values, P<0.01 vs 0.3 μM noradrenaline). In contrast, in endothelium-denuded aortic rings from L-NAME rats and in aortic rings from Methocel, L-NAME-MK-886 and MK-886 rats, with or without endothelium, noradrenaline failed to modify CysLT release (Table 3).
Table 3.
Release of CysLTs in response to noradrenaline (0.3 μM) or vehicle (control values) in aortic rings from Methocel, L-NAME, L-NAME-MK-886 and MK-886-treated rats
| Methocel | L-NAME | L-NAME-MK-886 | MK-886 | |
|---|---|---|---|---|
| With endothelium | ||||
| Noradrenaline | 120.0±22.1 | 199.4±26.8* | 102.1±11.3 | 121.6±13.7 |
| n | 6 | 6 | 6 | 6 |
| Without endothelium | ||||
| Noradrenaline | 98.49±31.5 | 116.8±15.7 | 110.1±25.8 | 93.9±17.0 |
| n | 6 | 6 | 6 | 6 |
Results are expressed as per cent over the respective control values and are presented as mean±s.e.m. In rings with endothelium, the control values were: (pg mg−1dry weight tissue) 32.6±3.9 (n=7), 24.7±1.8 (n=11), 18.8±5.1 (n=6) and 20.9±1.5 (n=6) for Methocel-, L-NAME-, L-NAME-MK-886- and MK-886-treated rats, respectively. In rings without endothelium, the control values were: 37.7±5.4 (n=6), 31.0±4.5 (n=7), 19.4±2.4 (n=6) and 35.8±5.8 (n=6) for Methocel-, L-NAME-, L-NAME-MK-886- and MK-886-treated rats, respectively. n refers to the number of aortic rings.
*P<0.05 vs respective control values.
Discussion
Long-term inhibition of endothelial NO synthesis by administration of L-NAME to rats induces experimental hypertension. The present study demonstrated for the first time that inhibition of leukotriene biosynthesis prevents L-NAME-induced hypertension, suggesting the involvement of 5-LOX-derived products in the regulation of MABP in L-NAME-treated rats. Consistent with this finding, previous studies have reported that nonspecific lipoxygenase inhibitors decreased arterial blood pressure in spontaneously hypertensive rats (Stern et al., 1993), aortic coarctation-induced hypertensive rats (DelliPizzi et al., 2000) and renovascular hypertensive rats (Nozawa et al., 1990). In the present study, MK-866 was used to investigate the involvement of leukotrienes in MABP regulation, since MK-866 blocks the binding of 5-LOX to the membrane by specifically interacting with the membrane-bound activating protein FLAP (Rouzer et al., 1990), which is necessary for cellular leukotriene biosynthesis (Dixon et al., 1990). Since in our experiments MABP was similar in Methocel- and MK-886-treated rats, a nonspecific effect of MK-886 appeared unlikely. In agreement with this result, intravenous administration of MK-886 did not change MABP in pigs (Provost et al., 1998).
In addition to increasing MABP, chronic oral treatment with L-NAME induced marked functional alteration of large conduit arteries such as the aorta (Henrion et al., 1996). The present study demonstrated that CysLTs are involved in the contractile effect of noradrenaline in aortic rings from L-NAME- but not Methocel-treated rats. Indeed, inhibition of leukotriene biosynthesis with either the FLAP inhibitor MK-886 (Rouzer et al., 1990) or the redox 5-LOX inhibitor AA861 (Steinhilber, 1999) significantly reduced the noradrenaline-induced contractions in intact aorta from L-NAME- but not Methocel-treated rats. In contrast, in our endothelium-denuded aortic rings from L-NAME- and Methocel-treated rats, inhibition of leukotriene biosynthesis did not affect the contractions elicited by noradrenaline. Collectively, these data suggested that part of the noradrenaline-induced contraction is supported by endothelial contractile 5-LOX-derived products in intact aorta from L-NAME- but not Methocel-treated rats. Consistent with these data, a recent study reported that CysLTs are involved in the endothelium-dependent contraction in response to acetylcholine in rat aorta (Mazzetti et al., 2003). However, there was no significant difference in the amplitude of the contraction evoked by noradrenaline between L-NAME- and Methocel-treated rats, suggesting that the signaling pathways involved in the contractile response to noradrenaline are different, with contractile 5-LOX-derived products being recruited in L-NAME- but not in Methocel-treated rats. Among the 5-LOX-derived products, CysLTs modulate the vascular tone in aorta from hypertensive rats (Stanke-Labesque et al., 2001; 2002). Therefore, the contribution of the CysLT receptors to noradrenaline-induced contraction was studied next using the selective CysLT1 receptor antagonist MK571 and the dual CysLT1/CysLT2 receptor antagonist BAY-u9773. In L-NAME-treated rats, the contraction elicited by noradrenaline was significantly reduced in the presence of MK571 in aortic rings with endothelium, but not in endothelium-denuded aortic rings. These data are consistent with the inhibitory effect of 5-LOX inhibitors on noradrenaline-induced contraction in intact aortic rings from L-NAME-treated rats and with the endothelial location of CysLT1 receptors (Walch et al., 2000), and suggest that stimulation of CysLT1 receptors could be involved in the noradrenaline-evoked contractile response in aorta from L-NAME-treated rats. However, since pretreatment with the dual CysLT1/CysLT2 receptor antagonist BAY-u9773 produced a greater reduction in the amplitude of the contraction elicited by noradrenaline, the activation of a contractile CysLT2 receptor (Walch et al., 2000) cannot be excluded.
The contribution of CysLTs to noradrenaline-induced contractions was confirmed by the finding that noradrenaline induced the release of CysLTs in intact aortic rings from L-NAME-treated rats, but failed to stimulate the release of CysLTs in either endothelium-denuded aortic rings from L-NAME-treated rats or aortic rings with or without endothelium from Methocel-treated rats. It is now well established that angiotensin II-dependent hypertension is associated with alteration of eicosanoid metabolism (Nasjletti, 1998). In particular, previous studies have demonstrated an increased release of CysLTs in response to angiotensin II in aortic rings from hypertensive rats (Stanke-Labesque et al., 2001; 2002). The present study demonstrated that noradrenaline can also promote the release of CysLTs in aortic rings from hypertensive rats; however, the presence of the endothelium was required to stimulate CysLT synthesis in response to noradrenaline. These data suggest that noradrenaline induces CysLT release through the stimulation of an endothelial adrenoceptor. Furthermore, since CysLTs contribute to the contraction elicited by noradrenaline, the adrenoceptors involved in their release are likely to be receptors that mediate the contractile response, that is, alpha receptors. Further experiments are, however, required to identify the adrenoceptor subtype involved in noradrenaline-induced CysLT release. In addition, it is now well established that CysLTs can be produced in the vessel wall by endothelial or vascular smooth muscle cells from neutrophil-derived leukotrienes (Sala & Folco, 2001). An increase in the adhesion of polymorphonuclear cells to the endothelium has been found in rat aorta following chronic treatment with L-NAME (Tomita et al., 1998) and inhibition of NO synthesis has been reported to enhance transcellular metabolism-derived CysLT synthesis in the rabbit coronary artery (Buccellati et al., 1997). All these data are consistent with our findings that noradrenaline enhances CysLT release in aortic rings with intact endothelium from L-NAME-treated rats.
We studied the vascular effects of LTC4 and LTD4 in intact aortic rings from all the rat groups. In aortic rings from Methocel-treated rats, LTD4 induced either no change to the basal tone or, at concentrations up to 10 nM, a concentration-dependent relaxation in phenylephrine-induced tone. This relaxation has been reported to be NO-dependent (Pawloski & Chapnick, 1993). Our results support these findings, showing that the relaxation was inhibited in aortic rings from L-NAME rats. In contrast, at a concentration of 0.1 μM, LTD4 elicited weak contractions in aortic rings with intact endothelium from L-NAME rats, whether the rings were under basal tone or precontracted with phenylephrine. In line with these findings, inhibition of NO synthase with Nω-nitro-L-arginine enhanced LTD4-evoked contraction in aorta from spontaneously hypertensive rats (Stanke-Labesque et al., 2001). This weak contraction was inhibited by MK571, suggesting that LTD4 induces contraction through stimulation of CysLT1 receptors. These data are in agreement with previous reports of an increased sensitivity to CysLTs of aortic rings from hypertensive rats (Stanke-Labesque et al., 2001; 2002) and suggest that CysLTs may contribute to the contractile effect of noradrenaline in aorta from L-NAME-treated rats at least in part through stimulation of CysLT1 receptors. However, in contrast to previous data obtained in aorta from Sprague–Dawley rats (Lawson et al., 1988), the addition of exogenous LTD4 in our experiments did not modify the contractile response to noradrenaline in aorta from Methocel-treated rats. This discrepancy could be explained by the different rat species used in the studies. However, the present findings are consistent with the hypothesis that different signaling pathways are involved in the contraction elicited by noradrenaline in normotensive and hypertensive rats, with the recruitment of a CysLT-mediated constrictor response in hypertensive rats but not in control normotensive rats. Moreover, the observation that the contractile response to noradrenaline was weaker (in terms of efficacy and potency) in L-NAME-MK-886- than L-NAME-treated rats provides additional evidence for the involvement of constrictor CysLTs in the noradrenaline-induced contraction in aorta from L-NAME-treated rats. Again, a nonspecific effect of MK-886 can be excluded since the contraction elicited by noradrenaline was similar in Methocel- and MK-886-treated rats. Indeed, in rat pulmonary arterial myocytes, MK-886, at the same concentration used in the present study for in vitro experiments (10 μM), activated the delayed rectifier K+ current IKCa (Smirnov et al., 1998). Since the activation of IKCa leads to an increase in intracellular Ca2+ concentration, a greater contraction in response to noradrenaline would be expected in aorta from MK-886-treated rats. Furthermore, this effect of MK-886 occurred independently on any leukotriene biosynthesis. In contrast, the present study clearly demonstrates that MK-886 reduces noradrenaline-induced contraction in L-NAME-treated rats through inhibition of leukotriene synthesis. We have previously reported that CysLTs modulate the contraction elicited by angiotensin II in hypertensive rats (Stanke-Labesque et al., 2001; 2002). The present study extends these findings and demonstrates a modulatory effect of CysLTs on noradrenaline-induced contraction in hypertensive states.
In conclusion, the present study demonstrates that inhibition of leukotriene biosynthesis by the FLAP inhibitor MK-886 prevents in vivo the L-NAME-induced increase in blood pressure and reduces in vitro the contraction elicited by noradrenaline. This reduction in the contractile response to noradrenaline could account, at least in part, for the prevention of L-NAME-induced hypertension by MK-886. In addition to increasing the evidence for the involvement of lipoxygenase-derived products in the pathogenesis of hypertension (Gonzalez-Nunez et al., 2001), these data are of potential clinical interest, suggesting a role for inhibition of the 5-LOX pathway in the treatment of hypertension. Additional experiments on resistance arteries from L-NAME-treated rats will further address the role of 5-LOX-derived products in the regulation of blood pressure in experimental hypertensive states.
Abbreviations
- CysLTs
cysteinyl leukotrienes
- FLAP
5-lipoxygenase activating protein
- L-NAME
Nω-nitro-L-arginine methyl ester
- 5-LOX
5-lipoxygenase
- MABP
mean arterial blood pressure
- MK-886
3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2-dimethylpropanoic acid
References
- BUCCELLATI C., ROSSONI G., BONAZZI A., BERTI F., MACLOUF J., FOLCO G., SALA A. Nitric oxide modulation of transcellular biosynthesis of cys-leukotrienes in rabbit leukocyte-perfused heart. Br. J. Pharmacol. 1997;120:1128–1134. doi: 10.1038/sj.bjp.0700994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLIPIZZI A., GUAN H., TONG X., TAKIZAWA H., NASJLETTI A. Lipoxygenase-dependent mechanisms in hypertension. Clin. Exp. Hypertens. 2000;22:181–192. doi: 10.1081/ceh-100100071. [DOI] [PubMed] [Google Scholar]
- DIXON R.A., DIEHL R.E., OPAS E., RANDS E., VICKERS P.J., EVANS J.F., GILLARD J.W., MILLER D.K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- FOLCO G., ROSSONI G., BUCCELLATI C., BERTI F., MACLOUF J., SALA A. Leukotrienes in cardiovascular diseases. Am. J. Respir. Crit. Care Med. 2000;161:S112–S116. doi: 10.1164/ajrccm.161.supplement_1.ltta-22. [DOI] [PubMed] [Google Scholar]
- GONZALEZ-NUNEZ D., CLARIA J., RIVERA F., POCH E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 2001;37:334–338. doi: 10.1161/01.hyp.37.2.334. [DOI] [PubMed] [Google Scholar]
- HEISE C.E., O'DOWD B.F., FIGUEROA D.J., SAWYER N., NGUYEN T., IM D.S., STOCCO R., BELLEFEUILLE J.N., ABRAMOVITZ M., CHENG R., WILLIAMS D.L., JR, ZENG Z., LIU Q., MA L., CLEMENTS M.K., COULOMBE N., LIU Y., AUSTIN C.P., GEORGE S.R., O'NEILL G.P., METTERS K.M., LYNCH K.R., EVANS J.F. Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- HENRION D., DOWELL F.J., LEVY B.I., MICHEL J.B. In vitro alteration of aortic vascular reactivity in hypertension induced by chronic NG-nitro-L-arginine methyl ester. Hypertension. 1996;28:361–366. doi: 10.1161/01.hyp.28.3.361. [DOI] [PubMed] [Google Scholar]
- KATOH M., EGASHIRA K., USUI M., ICHIKI T., TOMITA H., SHIMOKAWA H., RAKUGI H., TAKESHITA A. Cardiac angiotensin II receptors are upregulated by long-term inhibition of nitric oxide synthesis in rats. Circ. Res. 1998;83:743–751. doi: 10.1161/01.res.83.7.743. [DOI] [PubMed] [Google Scholar]
- KERTTULA T., RIUTTA A., KAUKINEN S., METSA-KETELA T., SEPPALA E., VAPAATALO H., ALANKO J. Noradrenaline and dopamine infusions modulate arachidonic acid cyclooxygenase and 5-lipoxygenase pathways ex vivo in man. Prostagland. Leukotr. Essent. Fatty Acids. 1995;53:47–52. doi: 10.1016/0952-3278(95)90082-9. [DOI] [PubMed] [Google Scholar]
- LABELLE E.F., POLYAK E. Norepinephrine stimulates arachidonic acid release from vascular smooth muscle via activation of cPLA2. Am. J. Physiol. 1998;274:C1129–C1137. doi: 10.1152/ajpcell.1998.274.4.C1129. [DOI] [PubMed] [Google Scholar]
- LAWSON D.L., SMITH C., MEHTA J.L., MEHTA P., NICHOLS W.W. Leukotriene D4 potentiates the contractile effects of epinephrine and norepinephrine on rat aortic rings. J. Pharmacol. Exp. Ther. 1988;247:953–957. [PubMed] [Google Scholar]
- LYNCH K.R., O'NEILL G.P., LIU Q., IM D.S., SAWYER N., METTERS K.M., COULOMBE N., ABRAMOVITZ M., FIGUEROA D.J., ZENG Z., CONNOLLY B.M., BAI C., AUSTIN C.P., CHATEAUNEUF A., STOCCO R., GREIG G.M., KARGMAN S., HOOKS S.B., HOSFIELD E., WILLIAMS D.L., JR, FORD-HUTCHINSON A.W., CASKEY C.T., EVANS J.F. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- MAZZETTI L., FRANCHI-MICHELI S., NISTRI S., QUATTRONE S., SIMONE R., CIUFFI M., ZILLETTI L., FAILLI P. The ACh-induced contraction in rat aortas is mediated by the Cys Lt(1) receptor via intracellular calcium mobilization in smooth muscle cells. Br. J. Pharmacol. 2003;138:707–715. doi: 10.1038/sj.bjp.0705087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASJLETTI A. Arthur C. Corcoran Memorial Lecture. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension. 1998;31:194–200. doi: 10.1161/01.hyp.31.1.194. [DOI] [PubMed] [Google Scholar]
- NOTHACKER H.P., WANG Z., ZHU Y., REINSCHEID R.K., LIN S.H., CIVELLI O. Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol. Pharmacol. 2000;58:1601–1608. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- NOZAWA K., TUCK M.L., GOLUB M., EGGENA P., NADLER J.L., STERN N. Inhibition of lipoxygenase pathway reduces blood pressure in renovascular hypertensive rats. Am. J. Physiol. 1990;259:H1774–H1780. doi: 10.1152/ajpheart.1990.259.6.H1774. [DOI] [PubMed] [Google Scholar]
- PAWLOSKI J.R., CHAPNICK B.M. Leukotrienes C4 and D4 are potent endothelium-dependent relaxing agents in canine splanchnic venous capacitance vessels. Circ. Res. 1993;73:395–404. doi: 10.1161/01.res.73.2.395. [DOI] [PubMed] [Google Scholar]
- PETERS-GOLDEN M., BROCK T.G. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Lett. 2001;487:323–326. doi: 10.1016/s0014-5793(00)02374-7. [DOI] [PubMed] [Google Scholar]
- PROVOST P., BORGEAT P., MERHI Y. Platelets, neutrophils, and vasoconstriction after arterial injury by angioplasty in pigs: effects of MK-886, a leukotriene biosynthesis inhibitor. Br. J. Pharmacol. 1998;123:251–258. doi: 10.1038/sj.bjp.0701611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYCHAUDHURI A., CHERTOCK H., PEPPARD J., WHITE W.D., KOELER J., DIPASQUALE G. Effect of 5-lipoxygenase inhibitors on in situ LTB4 biosynthesis following calcium ionophore stimulation in the rat pleural cavity. Agents Actions. 1993;39:C43–C45. doi: 10.1007/BF01972715. [DOI] [PubMed] [Google Scholar]
- ROUZER C.A., FORD-HUTCHINSON A.W., MORTON H.E., GILLARD J.W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J. Biol. Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- ROUZER C.A., KARGMAN S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J. Biol. Chem. 1988;263:10980–10988. [PubMed] [Google Scholar]
- SALA A., FOLCO G. Neutrophils, endothelial cells, and cysteinyl leukotrienes: a new approach to neutrophil-dependent inflammation. Biochem. Biophys. Res. Commun. 2001;283:1003–1006. doi: 10.1006/bbrc.2001.4865. [DOI] [PubMed] [Google Scholar]
- SALAS A., PANES J., ELIZALDE J.I., CASADEVALL M., ANDERSON D.C., GRANGER D.N., PIQUE J.M. Mechanisms responsible for enhanced inflammatory response to ischemia-reperfusion in diabetes. Am. J. Physiol. 1998;275:H1773–H1781. doi: 10.1152/ajpheart.1998.275.5.H1773. [DOI] [PubMed] [Google Scholar]
- SMIRNOV S.V., KNOCK G.A., AARONSON P.I. Effects of the 5-lipoxygenase activating protein inhibitor MK886 on voltage-gated and Ca2+-activated K+ currents in rat arterial myocytes. Br. J. Pharmacol. 1998;124:572–578. doi: 10.1038/sj.bjp.0701855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANKE-LABESQUE F., DEVILLIER P., VEITL S., CARON F., CRACOWSKI J.L., BESSARD G. Cysteinyl leukotrienes are involved in angiotensin II-induced contraction of aorta from spontaneously hypertensive rats. Cardiovasc. Res. 2001;49:152–160. doi: 10.1016/s0008-6363(00)00238-8. [DOI] [PubMed] [Google Scholar]
- STANKE-LABESQUE F., HARDY G., VERGNAUD S., DEVILLIER P., PEOC'H M., RANDON J., BRICCA G., CARON F., CRACOWSKI J.L., BESSARD G. Involvement of cysteinyl leukotrienes in angiotensin II-induced contraction in isolated aortas from transgenic (mRen-2)27 rats. J. Hypertens. 2002;20:263–272. doi: 10.1097/00004872-200202000-00016. [DOI] [PubMed] [Google Scholar]
- STEINHILBER D. 5-Lipoxygenase: a target for antiinflammatory drugs revisited. Curr. Med. Chem. 1999;6:71–85. [PubMed] [Google Scholar]
- STERN N., NOZAWA K., GOLUB M., EGGENA P., KNOLL E., TUCK M.L. The lipoxygenase inhibitor phenidone is a potent hypotensive agent in the spontaneously hypertensive rat. Am. J. Hypertens. 1993;6:52–58. doi: 10.1093/ajh/6.1.52. [DOI] [PubMed] [Google Scholar]
- TOMITA H., EGASHIRA K., KUBO-INOUE M., USUI M., KOYANAGI M., SHIMOKAWA H., TAKEYA M., YOSHIMURA T., TAKESHITA A. Inhibition of NO synthesis induces inflammatory changes and monocyte chemoattractant protein-1 expression in rat hearts and vessels. Arterioscler. Thromb. Vasc. Biol. 1998;18:1456–1464. doi: 10.1161/01.atv.18.9.1456. [DOI] [PubMed] [Google Scholar]
- USUI M., EGASHIRA K., TOMITA H., KOYANAGI M., KATOH M., SHIMOKAWA H., TAKEYA M., YOSHIMURA T., MATSUSHIMA K., TAKESHITA A. Important role of local angiotensin II activity mediated via type 1 receptor in the pathogenesis of cardiovascular inflammatory changes induced by chronic blockade of nitric oxide synthesis in rats. Circulation. 2000;101:305–310. doi: 10.1161/01.cir.101.3.305. [DOI] [PubMed] [Google Scholar]
- WALCH L., NOREL X., GASCARD J.P., BRINK C. Functional studies of leukotriene receptors in vascular tissues. Am. J. Respir. Crit. Care Med. 2000;161:S107–S111. doi: 10.1164/ajrccm.161.supplement_1.ltta-21. [DOI] [PubMed] [Google Scholar]
- WANGENSTEEN R., O'VALLE F., DEL MORAL R., VARGAS F., OSUNA A. Chronic alpha1-adrenergic blockade improves hypertension and renal injury in L-NAME and low-renin L-NAME-DOCA hypertensive rats. Med. Sci. Monit. 2002;8:BR378–BR384. [PubMed] [Google Scholar]