Abstract
L-citrulline, a coproduct of nitric oxide synthase (NOS)-catalysed metabolism of L-arginine to nitric oxide (NO), is an important intermediate of the urea cycle and a precursor for L-arginine biosynthesis in vascular cells.
In the present study, we have examined the characteristics of L-citrulline transport, regulation by lipopolysaccharide (LPS) and interferon-γ (IFN-γ) and the ability of L-citrulline to sustain NO synthesis in rat cultured aortic smooth muscle cells.
L-citrulline transport was saturable with an apparent Km=1.6±0.2 mM and Vmax=5.9±0.6 pmol μg−1 protein min−1. Transport was pH-insensitive, partially Na+-dependent and markedly inhibited by substrates selective for amino-acid transport systems L and N but not by L-arginine or substrates for systems A, ASC, xc- or XAG. Moreover, transport was not altered in cells treated with LPS (100 μg ml−1) and IFN-γ (50 U ml−1) for 0–24 h.
Unlike L-arginine, L-citrulline could not sustain maximal NO production in cells expressing iNOS.
Our findings provide the first evidence in vascular smooth muscle cells that L-citrulline transport is mediated via a low-affinity carrier with characteristics resembling systems L and N. Moreover, in L-arginine-deprived rat aortic smooth muscle cells, L-citrulline cannot sustain maximal NO release via iNOS.
Keywords: L-Citrulline, transport, nitric oxide synthesis, inducible nitric oxide synthase, rat aortic smooth muscle cells
Introduction
Nitric oxide is synthesised from the cationic amino acid L-arginine via a series of oxidation reactions which produce L-citrulline as a coproduct. In rat cultured aortic smooth muscle cells (RASMC), the metabolism of L-arginine to NO and L-citrulline is mediated by Ca2+/calmodulin-insensitive NO synthase (iNOS) (Knowles & Moncada, 1994; Alderton et al., 2001), which is induced following exposure of these cells to proinflammatory mediators (Wileman et al., 1995). Once induced, sustained synthesis of NO by iNOS is critically dependent on the availability of extracellular L-arginine (Schott et al., 1993; Escobales et al., 2000) and, as a consequence, supply of L-arginine may become limiting for NO production.
Under physiological conditions, most plasma L-arginine is derived from metabolism of L-citrulline by the kidneys (Windmueller & Spaeth, 1981; Dhanakoti et al., 1990), and is transported into cells via selective cationic amino-acid transporters (CATs; Devés & Boyd, 1998; Palacin et al., 1998; Closs & Mann, 1999; Mann et al., 2003). In vascular smooth muscle, L-arginine can also be generated endogenously from L-citrulline. These cells, although lacking a complete urea cycle, express argininosuccinate synthase (AS) and argininosuccinate lyase (AL) (Hattori et al., 1994), which, respectively, catalyse the synthesis of argininosuccinate from L-citrulline and the cleavage of argininosuccinate to L-arginine and fumarate (Meijer et al., 1990). The capacity of these enzymes to regenerate L-arginine from L-citrulline may represent a rate-limiting mechanism in smooth muscle cells for maintaining substrate supply during sustained NO synthesis. Of direct relevance to this is the fact that AS, the rate-limiting enzyme in the pathway, is markedly enhanced in smooth muscle cells expressing iNOS (Hattori et al., 1994). Moreover, the induction of AS parallels that of iNOS, indicating coordinated regulation of both proteins with perhaps a close functional relationship where enhanced AS expression drives the metabolism of L-citrulline to L-arginine, sustaining substrate supply for iNOS. In agreement with this hypothesis, L-citrulline has been reported to induce NO-dependent vasorelaxation in denuded aortic rings isolated from endotoxin-treated rats (Raghavan & Dikshit, 2001) and to sustain maximal rates of NO production in cultured aortic smooth muscle cells activated with bacterial lipopolysaccharide (LPS) and interferon-γ (IFN-γ) (Hattori et al., 1994). In the latter study, it is worth noting that concentrations of L-citrulline required (EC50=600 μM) for optimal iNOS activity were, however, 20-fold higher than the predicted Km for purified AS (Meijer et al., 1990). This finding, together with the fact that vascular responses to L-citrulline developed slowly, requiring 45 min to reach a maximum (Raghavan & Dikshit, 2001), potentially highlights a limitation in cellular uptake of L-citrulline.
Currently, transport of L-citrulline into vascular cells is poorly understood, with only two reports describing distinct pathways for L-citrulline transport in macrophages (Baydoun et al., 1994) and neural cell cultures (Schmidlin et al., 2000). To our knowledge, the mechanisms mediating L-citrulline transport in vascular smooth muscle cells have not been investigated. In view of the potential role of L-citrulline in regulating NO synthesis and vascular tone under physiological and pathophysiological conditions, we have characterised the system(s) mediating L-citrulline transport into smooth muscle cells cultured from rat aorta and investigated the dependency of iNOS on extracellular L-arginine and L-citrulline. Furthermore, we have examined whether L-citrulline transport is regulated by proinflammatory mediators by examining the effects of LPS in combination with IFN-γ.
Methods
Materials
Tissue culture reagents were purchased from Gibco (Paisley, U.K.). Recombinant murine IFN-γ was from Genzyme (Cambridge, U.K.). Monoclonal anti-α smooth muscle actin, L-citrulline and LPS from Escherichia coli (serotype 0111:B4) were obtained from Sigma (Poole, U.K.). Other chemicals were from Sigma or BDH and of the highest analytical grade obtainable. Radioactive tracers, L-[ureido-14C]citrulline (57.8 mCi mmol−1) and D-[3H]mannitol (49.3 mCi mmol−1), were obtained from New England Nuclear, Dreieich, Germany.
Cell culture
Smooth muscle cells (SMC) were isolated from rat aortic explants as described previously (see Wileman et al., 1995). Briefly, male Sprague–Dawley rats (250–300 g) were stunned and exsanguinated and the thoracic aorta dissected in Dulbecco's modified Eagle's Medium (DMEM) supplemented with 4.4% NaHCO3, penicillin (100 units ml−1) and streptomycin (100 mg ml−1). Following removal of the adventitia and endothelium, each aorta was cut into 2 mm2 segments and placed in a T-25 tissue culture flask containing DMEM, supplemented with 2 mM glutamine and 10% foetal calf serum. Explants were left in culture for 14 days, after which migrating and rapidly dividing cells were harvested with trypsin/EDTA (0.01/0.02%) and cultured to confluence in a T-75 flask. Cells were identified and characterised as SMC by immunostaining of smooth-muscle α-actin, using mouse anti-α-actin antibody and anti-mouse IgG FITC conjugate (Sigma, Poole, Dorset, U.K.), as described by Skalli et al. (1986). All experiments were performed using cells between passages 4 and 7.
Activation of cells with LPS and IFN-γ
Rat aortic SMC were plated into 96-well plates at a seeding density of 8 × 103 cells per well and allowed to grow to confluence. The culture medium was then replaced either with fresh medium or with medium containing LPS (100 μg ml−1) plus IFN-γ (50 U ml−1). Cells were incubated for a further 24 h, after which the supernatant was removed from each well and analysed for nitrite, and transport of L-[14C]citrulline (1 μCi ml−1, 37°C, 0–10 min) determined in cell monolayers incubated with Krebs solution (Wileman et al., 1995).
To investigate the dependency of NO synthesis on extracellular L-arginine or L-citrulline, confluent SMC monolayers in 96-well plates were activated for 24 h with LPS and IFN-γ in the presence of increasing concentrations of either L-arginine (0–1 mM) or in L-arginine-free medium supplemented with increasing concentrations of L-citrulline (0–1 mM). Culture medium was removed after the incubation period and analysed for nitrite. The total protein content of each cell monolayer was determined using Brilliant Blue G reagent (Bradford, 1976).
L-citrulline transport assay
Cell monolayers were rinsed twice with a HEPES-buffered Krebs solution (mM: NaCl: 131; KCl: 5.5; MgCl2: 1; CaCl2: 2.5; NaHCO3: 25; NaH2PO4: 1; D-glucose: 5.5; HEPES: 20; pH 7.4) maintained at 37°C. As described previously (Wileman et al., 1995), transport of L-[14C]citrulline (1 μCi ml−1, 0.1 mM) was measured in confluent cell monolayers in the absence or presence of a 10-fold excess of a given inhibitor (1 mM). Kinetics of L-citrulline transport were assessed over a wide range of substrate concentrations (0.05–5 mM). The effects of pH on L-citrulline transport (0.1 mM) were examined in Krebs solution titrated with 0.1 N HCl or 5 N NaOH to achieve pH values ranging between pH 5 and 8. In sodium-free experiments, Krebs buffer was modified by replacing NaCl, NaHCO3 and NaH2PO4 with choline chloride, choline bicarbonate and KH2PO4, respectively. In some experiments, an extracellular reference tracer, D-[3H]mannitol, was included in the incubation medium and, in these studies, <0.01% of D-mannitol applied was recovered in cell lysates.
Tracer uptake was terminated by placing the 96-well plates on ice and rinsing the cell monolayers three times with 200 μl ice-cold Krebs containing 10 mM unlabelled L-citrulline. Cell protein was determined using Brilliant Blue G and radioactivity (dpm) in formic acid digests of the cells was determined by liquid scintillation counting. Transport was then calculated and expressed as pmol μg protein−1 min−1.
Nitrite analysis
Nitrite accumulation in the culture medium was determined colorimetrically by a diazotisation reaction using the standard Griess reagent (Green et al., 1982; Wileman et al., 1995).
Analysis of intracellular amino-acid concentrations
L-arginine and L-citrulline concentrations in RASMC were determined by reversed-phase hplc. Confluent monolayers from unstimulated or LPS (100 μg ml−1) plus IFN-γ (50 U ml−1)-treated cells were incubated in either L-arginine-containing or L-arginine-deprived medium. Following a 24 h incubation period, the culture medium was removed, and the cell monolayer washed twice with ice-cold PBS, lysed with 0.5 ml ice-cold methanol and stored at −20°C for analysis, as described previously (Baydoun et al., 1990).
Statistics
Data are expressed as means±s.e.m. of measurements in at least three different cell cultures with five to six replicates per experiment. Statistical analyses were performed using either an unpaired Student's t-test, or by analysis of variance when necessary with the overall confidence levels set at 95% (0.05).
Results
Time course and kinetics of L-citrulline transport
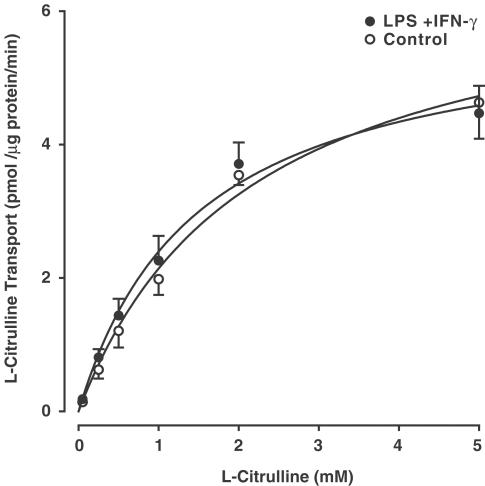
Transport of L-citrulline (0. 1 mM) into RASMC was time-dependent and linear for up to 30 min (see Figure 4b in Wileman et al., 1995). Cells accumulated <0.5 pmol L-citrulline per μg protein in 1 min. In all subsequent experiments, transport was measured over a 10 min incubation period to maximise tracer activity in smooth muscle cell digests. In unstimulated smooth muscle cells, transport of L-citrulline (0.05–5 mM) was fitted best by a single Michaelis–Menten equation, with an apparent Km of 1.6±0.2 mM and Vmax of 5.9±0.6 pmol μg−1 protein min−1 (Figure 1). Pretreatment of smooth muscle cells with LPS (100 μg ml−1) plus IFN-γ (50 U ml−1) for 24 h had no significant effect on either Vmax (6.9±0.9 pmol μg−1 protein min−1) or Km (2.4±0.4 mM).
Figure 1.
Kinetics of L-citrulline transport in RASMC. Kinetics of L-citrulline transport (0.05–5 mM) were examined over a 10 min incubation period in unstimulated aortic smooth muscle cells and following activation of cells with LPS (100 μg ml−1) plus IFN-γ (50 U ml−1) for 24 h. Values denote the means±s.e.m. of experiments in four different cell cultures with five replicate measurements in each experiment.
Characteristics of L-citrulline transport
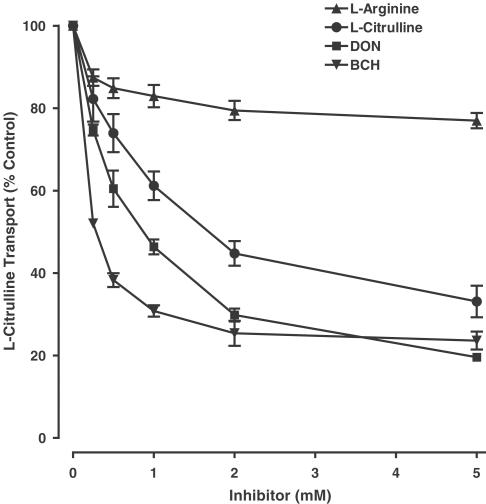
In both unstimulated and LPS/IFN-γ activated cells, transport of L-citrulline was partially dependent on extracellular Na+, but unaffected by changes in extracellular pH ranging from 5 to 8 (Table 1 ). Transport was significantly inhibited by a 10-fold excess of unlabelled L-citrulline (Ki=0.4 mM), 2-aminobicycloheptane-2-carboxylic acid (BCH; model substrate for system L; Ki=0.2 mM) or 6-diazo-5-oxo-L-norleucine (DON; model substrate for system N; Ki=0.8 mM). In contrast, 2-methylaminoisobutyric acid (MeAIB) and L-glutamate (L-Glu), substrates for systems A and xc-/XAG, respectively, were ineffective inhibitors, while L-arginine, a substrate for CATs, caused only marginal (18%) inhibition (Table 2 ).
Table 1.
The effects of extracellular sodium and external pH on L-citrulline transport
| Conditions | L-citrulline transport (% control) |
|---|---|
| Na+-free | 67±5* |
| pH 5 | 112±6 |
| pH 6 | 106±5 |
| pH 8 | 89±1 |
Dependency of L-citrulline transport on extracellular sodium or varying pH was examined using either nominally Na+-free Krebs or buffers with pH values ranging from 5 to 8. Transport of L-citrulline (0.1 mM) was measured in unstimulated cells over a 10 min period. Data are expressed as a percentage of the influx rate obtained in normal Na+-containing Krebs maintained at pH 7.4 (100%=0.31±0.04 pmol μg protein−1 min−1). Values denote the means±s.e.m. of experiments in three different cell cultures with five replicates in each experiment.
P<0.05 compared to control.
Table 2.
Selectivity of L-citrulline transport in rat aortic smooth muscle cells
| Inhibitor (1 mM) | System selectivity | L-citrulline transport (% control) |
|---|---|---|
| L-arginine | y+ | 82±4* |
| L-citrulline | ? | 62±4** |
| BCH | L | 32±2** |
| MeAIB | A | 92±2 |
| DON | N | 46±2** |
| L-glutamate | xc- or XAG | 99±3 |
Transport of L-citrulline (0.1 mM) was measured over 10 min in the absence or presence of a 10-fold excess (1 mM) of various system-selective amino-acid substrates. Data are expressed as a percentage of the influx rate in control cells (100%=0.44±0.05 pmol μg protein−1 min−1). Values denote the means±s.e.m. of experiments in three different cell cultures with five replicates in each experiment.
P<0.05 and P<0.01 compared to control.
Detailed analysis of the inhibition of L-citrulline transport by L-citrulline, BCH or DON revealed that each compound inhibited transport in a concentration-dependent manner, reducing L-citrulline uptake by 67±4, 76±2 and 80±0.4% at 5 mM (Figure 2). In contrast, the marginal inhibition caused by 1 mM L-arginine was not further enhanced by increasing extracellular L-arginine to 5 mM (Figure 2).
Figure 2.
Specificity of L-citrulline transport in RASMC. Inhibition of L-citrulline transport was measured in unstimulated cells incubated with Na+ containing Krebs buffer with increasing concentrations (0.25–5 mM) of either L-arginine, L-citrulline, DON or BCH. Transport of L-citrulline (0.1 mM) was measured over 10 min, and expressed as a percentage of the transport rate determined in the absence of an inhibitor amino acid. Values denote the means±s.e.m. of experiments in three different cell cultures with five replicate measurements in each experiment.
Dependency of nitrite production on extracellular L-citrulline
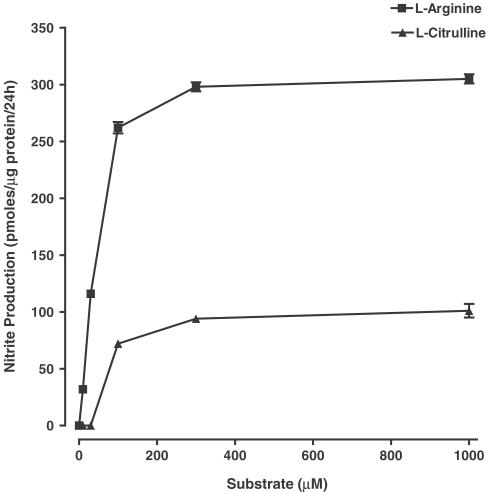
To investigate whether extracellular L-citrulline could sustain NO production by iNOS, RASMCs were activated with LPS (100 μg ml−1) and IFN-γ (50 U ml−1) for 24 h in an L-arginine-free medium containing increasing concentrations of L-citrulline (0–1000 μM). In parallel experiments, the effects of extracellular L-arginine were examined over a similar concentration range. There was no detectable nitrite in the culture medium obtained from LPS/IFN-γ activated smooth muscle cells in the absence of extracellular L-arginine or L-citrulline.
Activation of cells in the presence of exogenous L-arginine caused a concentration-dependent increase in accumulated nitrite levels. This response was near maximal at plasma L-arginine concentrations (∼100 μM; see Baydoun et al., 1990; Hallemeesch et al., 2002; Mann et al., 2003), levelling off at 300 μM nitrite (Figure 3). In contrast to L-arginine, increasing concentrations of L-citrulline failed to sustain maximal NO production. More importantly, there was no detectable nitrite produced at plasma concentrations of L-citrulline (∼30 μM; see Castillo et al., 1993) and at 1 mM, the maximum levels of nitrite detected in the culture medium were approximately one-third those produced in the presence of the same concentration of L-arginine (Figure 3).
Figure 3.
Dependency of LPS plus IFN-γ-stimulated nitrite release from RASMC on extracellular L-arginine or L-citrulline. Confluent smooth muscle cells were activated with LPS (100 μg ml−1) and IFN-γ (50 U ml−1) for 24 h in L-arginine-depleted DMEM supplemented with increasing concentrations of either L-arginine or L-citrulline. Nitrite accumulation in the culture medium was assayed using the Griess reaction. Values denote the means±s.e.m. of experiments in three different cell cultures with five replicate measurements in each experiment.
Chromatographic analyses of cell lysates obtained from smooth muscle cells deprived of L-arginine for 24 h revealed a decrease in intracellular L-arginine levels from 1.43 mM to 260 μM (Table 3 ). In cells activated with LPS and IFN-γ for 24 h, intracellular L-arginine levels decreased to 760 μM, while intracellular L-citrulline levels increased to 3.19 mM, consistent with an enhanced production of NO via iNOS. In the absence of extracellular L-arginine, intracellular L-arginine and L-citrulline levels decreased and were maintained at 300 and 40 μM, respectively, following activation (Table 3).
Table 3.
Intracellular concentrations of L-citrulline and L-arginine in unstimulated and LPS/IFN-γ-stimulated aortic smooth muscle cells
| Amino acid | Control | Control(−L-arginine) | LPS+IFN-γ | LPS+IFN-γ(−L-arginine) |
|---|---|---|---|---|
| L-arginine (mM) | 1.43±0.36 | 0.26±0.01* | 0.76±0.10 | 0.30±0.03⧫ |
| L-citrulline (mM) | 0.16±0.03 | 0.49±0.29 | 3.19±0.11* | 0.04±0.04⧫ |
Unstimulated and activated aortic smooth muscle cells (100 μg ml−1 LPS+50 U ml−1 IFN-γ, 24 h) were extracted and intracellular L-arginine and L-citrulline concentrations determined by reverse-phase hplc. Results are expressed as intracellular amino-acid concentration (mM) assuming an intracellular water space of 1 pl. Data denote the means±s.e.m. of determinations in three different cell cultures.
P<0.05 versus control
P<0.05 versus LPS+IFN-γ.
Discussion
L-citrulline has emerged as an important amino acid both as a product of the urea cycle and as a precursor for L-arginine biosynthesis within the vasculature. In vivo citrulline is synthesised and released from the intestine as an end product of L-glutamine nitrogen metabolism (Windmueller & Spaeth, 1981). Once in the circulation, L-citrulline is rapidly cleared by the kidney and subsequently metabolised and released as L-arginine (Windmueller & Spaeth, 1981; Dhanakoti et al., 1990). In addition to this pathway, various cells within the vasculature possess a truncated urea cycle, expressing both AS and AL, which, respectively, catalyse the metabolism of citrulline to argininosuccinate and then L-arginine. The presence of these enzymes has led to the suggestion that cells may be able to recycle L-citrulline, thereby maintaining cellular concentrations of L-arginine for sustained NO synthesis (Hattori et al., 1994; Raghavan & Dikshit, 2001). Consistent with this hypothesis, conversion of L-citrulline to L-arginine is enhanced in endothelial cells stimulated to release NO (Hecker et al., 1990a,1990b), and in macrophages (Wu & Brosnan, 1992; Baydoun et al., 1994) and smooth muscle cells (Hattori et al., 1994) generating NO, following activation with proinflammatory mediators.
It would appear, however, that the effectiveness of L-citrulline to maintain adequate substrate supply for NOS may be limited by its ability to enter cells. Transport of L-citrulline into RASMC is slow, remaining linear over a period of up to 30 min. At physiological plasma concentrations, initial rates of L-citrulline transport are three-fold lower than rates measured previously for L-arginine (Wileman et al., 1995). Unlike L-arginine, kinetic analysis revealed that transport is mediated via a low-affinity carrier (Km∼1.6 mM), which may limit uptake from plasma in the presence of other circulating competitor amino acids. The lower rate of transport together with the relatively low affinity of the transporter could explain the slow onset of action of L-citrulline in inducing relaxation of preconstricted aortic rings (Raghavan & Dikshit, 2001), and could also account for the significantly high concentrations of exogenous L-citrulline required to sustain maximal iNOS activity in cultured smooth muscle cells (Hattori et al., 1994).
Kinetic cross-inhibition experiments suggest that the transporter for L-citrulline in RASMC is distinct from the CATs mediating L-arginine entry in this cell type. First, transport of L-citrulline was not significantly altered by a 10 to 50-fold excess of L-arginine. Second, transport was also unaffected by LPS and IFN-γ, which are both known to increase L-arginine transport in various cell types including RASMC (Durante et al., 1995; Wileman et al., 1995). The insensitivity of L-citrulline transport to proinflammatory mediators is consistent with our previous findings in J774 macrophages (Baydoun et al., 1994) and with reports in neuronal cell cultures (Schmidlin et al., 2000).
The fact that L-arginine failed to cause a significant modulation of L-citrulline transport in RASMC also rules out a role for the broad-scope amino-acid transporters such as the Na+-dependent system Bo,+ or the Na+-independent system bo,+, capable of transporting both neutral and basic amino acids (Van Winkle et al., 1988;1985). Moreover, the low affinity for L-citrulline transport excludes a significant contribution of the high-affinity system y+L identified in several different cell types (reviewed by Devés & Boyd, 1998;2000; Wagner et al., 2001), including a recent study in human endothelial cells (Sala et al., 2002). Instead, our findings suggest that transport of L-citrulline in RASMC may be mediated predominantly via a carrier with characteristics similar to system L.
The classical Na+-independent transport system L is most reactive with branched chain and aromatic neutral amino acids and is often characterised using the selective nonmetabolisable analogue BCH (Christensen et al., 1969; Shotwell et al., 1983). Transport via system L is trans-stimulated by intracellular substrates of this carrier and in some cases may be increased by lowered extracellular pH. Studies in rat glioma cells, primary astroglial cells and lymphocytes suggested that 4F2hc serve as a necessary component for expression of system L-like transport activity (Bröer et al., 1995). Coexpression of 4F2hc and LAT-1 or LAT-2 (system L transporters) in oocytes induces Na+-independent transport with a broad specificity for small and large zwitterionic amino acids and sensitivity to trans-stimulation (Kanai et al., 1998; Pineda et al., 1999). The marked inhibition of L-citrulline transport by BCH and the trend for increased transport at lowered pH implicates system L as the major transporter of L-citrulline uptake in RASMC. This may not, however, be the only system that is involved. Depletion of Na+ from the transport buffer resulted in a reduction (33%) in L-citrulline influx, indicating a partially Na+-dependent mechanism of entry. This was further investigated by examining the effects of substrates specific for known Na+-dependent transporters including DON (system N; see Goldstein, 1975), MeAIB (system A; Gazzola et al., 1980) and L-glutamate (systems xc−/XAG; see Dall'Asta et al., 1983). L-citrulline transport was virtually unaffected by a 10-fold excess of either MeAIB or L-glutamate, markedly inhibited by DON and not decreased at lowered extracellular pH. It is therefore likely that the Na+-sensitive component of L-citrulline transport was mediated via system N. Inhibition of L-citrulline transport by L-glutamine and inhibitory actions of L-glutamine (and its metabolite glucosamine) on the pentose cycle (Wu et al., 2001) may well account for the reduction of NO synthesis reported in both intact cell systems (Hecker et al., 1990a; Wu & Meininger, 1993) and blood vessels (Swierkosz et al., 1990).
Entry via systems L and N or, an as yet unidentified, carrier with properties similar to system L and/or N, could explain why, in the absence of extracellular L-arginine, L-citrulline was not able to maintain maximal rates of NO production in our studies. Several other amino acids utilise systems L and N for entry into cells. Some of these amino acids, including L-phenylalanine and L-glutamine, are present in high concentrations in various culture media, including DMEM. Thus, under standard experimental conditions, uptake of L-citrulline will be impaired by competing amino acids, with their combined effect causing marked inhibition of L-citrulline transport. Under these conditions, the capacity of cells to recycle L-arginine from L-citrulline would also be limited because other neutral amino acids will inhibit uptake of L-citrulline by competing for the same transporter(s) that facilitate entry of citrulline into cells. As a result, regeneration of L-arginine would not be efficiently maintained to match the consumption by NOS, even if the capacity of AS and AL to generate L-arginine from L-citrulline may be sufficient for maintaining maximal rates of NO synthesis (Hattori et al., 1994). The finding that in LPS- and IFN-γ-activated cells with depleted intracellular L-citrulline and L-arginine levels (Table 3), only limited nitrite is released in the presence of 1 mM extracellular L-citrulline (Figure 3), indicates that L-citrulline transport cannot maintain maximal NO synthesis. It is also possible that L-citrulline available to AS and AL is compartmentalised and not readily exchangeable with transported L-citrulline.
Activation of L-arginine transport in vascular smooth muscle cells by proinflammatory cytokines (Durante et al., 1995; Wileman et al., 1995) provides a mechanism for sustaining L-arginine supply during enhanced utilisation by iNOS. Even though low intracellular L-arginine levels (300 μM) in L-arginine-deprived cells would be expected to sustain NO synthesis, it is possible that as in macrophages (Closs et al., 2000) a nonfreely exchangeable L-arginine pool is not accessible to iNOS. Under these conditions, and as demonstrated in the present study, transport of L-arginine becomes rate-limiting for NO production. This hypothesis is further supported by the finding that iNOS-mediated NO production is reduced significantly in macrophages from CAT2−,− mice (Nicholson et al., 2001). This report and our present findings strongly suggest a functional association between CAT2 and iNOS, with a critical dependency of NO production on L-arginine delivery.
The inability of cytokines to stimulate L-citrulline transport in RASMC suggests that delivery of L-citrulline will not occur at a sufficiently high rate to maintain adequate levels of L-arginine for optimal iNOS activity. L-citrulline would therefore be far less effective than L-arginine in supporting NO synthesis, maintaining, at best, a fraction of the maximal rate of NO synthesis induced by L-arginine, as demonstrated in this and other studies in macrophages (Wu & Brosnan, 1992; Baydoun et al., 1994). The lack of potentiation of L-citrulline transport by proinflammatory cytokines is another limiting factor worth taking into account when evaluating the potential of L-citrulline in maintaining the generation of NO via iNOS.
In summary, our findings establish that uptake of L-citrulline by RASMC is mediated via amino-acid transport systems with properties characteristic of systems L and N. Inhibition by circulating amino acids will restrict the entry of L-citrulline into vascular cells and thereby limit the amount of intracellular L-arginine generated from this source. As a result, L-citrulline has only a limited ability to sustain maximal rates of NO synthesis both in vitro and in vivo.
Acknowledgments
We gratefully acknowledge the support of the British Heart Foundation (PG/91075; FS/94004).
Abbreviations
- AL
argininosuccinate lyase
- AS
argininosuccinate synthase
- BCH
2-aminobicycloheptane-2-carboxylic acid
- CATs
cationic amino-acid transporters
- DMEM
Dulbecco's modified Eagle's medium
- DON
6-diazo-5-oxo-L-norleucine
- IFN-γ
interferon-γ
- iNOS
inducible nitric oxide synthase
- LPS
bacterial lipopolysaccharide
- MeAIB
2-methylaminoisobutyric acid
- RASMC
rat cultured aortic smooth muscle cells
- SMC
smooth muscle cells
References
- ALDERTON W.K., COOPER C.E., KNOWLES R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYDOUN A.R., BOGLE R.G., PEARSON J.D., MANN G.E. Discrimination between citrulline and arginine transport in activated murine macrophages: inefficient synthesis of NO from recycling of citrulline to arginine. Br. J. Pharmacol. 1994;112:487–492. doi: 10.1111/j.1476-5381.1994.tb13099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYDOUN A.R., EMERY P.W., PEARSON J.D., MANN G.E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRÖER S., BRÖER A., HAMPRECHT B. The 4F2hc surface antigen is necessary for expression of system L-like neutral amino acid-transport activity in C6-BU-1 rat glioma cells: evidence from expression studies in Xenopus laevis oocytes. Biochem. J. 1995;312:863–870. doi: 10.1042/bj3120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTILLO L., CHAPMAN T.E., SANCHEZ M., YU Y.M., BURKE J.F., AJAMI A.M., VOGT J., YOUNG V.R. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7749–7753. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN H.N., HANDLOGTEN M.E., LAM I., TAGER H.S., ZAND R. A bicyclic amino acid to improve discriminations among transport systems. J. Biol. Chem. 1969;244:1510–1520. [PubMed] [Google Scholar]
- CLOSS E.I., MANN G.E. Identification of carrier systems in plasma membranes of mammalian cells involved in transport of L-arginine. Methods Enzymol. 1999;301:78–91. doi: 10.1016/s0076-6879(99)01071-x. [DOI] [PubMed] [Google Scholar]
- CLOSS E.I., SCHELD J.S., SHARAFI M., FORSTERMANN U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol. Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- DALL'ASTA V., GAZZOLA G.C., FRANCHI-GAZZOLA R., BUSSOLATI O., LONGO N., GUIDOTTI G.G. Pathways of L-glutamic acid transport in cultured human fibroblasts. J. Biol. Chem. 1983;258:6371–6379. [PubMed] [Google Scholar]
- DEVÉS R., BOYD C.A. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol. Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- DEVÉS R., BOYD C.A. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J. Membr. Biol. 2000;173:165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- DHANAKOTI S.N., BROSNAN J.T., HERZBERG G.R., BROSNAN M.E. Renal arginine synthesis: studies in vitro and in vivo. Am. J. Physiol. 1990;259:E437–E442. doi: 10.1152/ajpendo.1990.259.3.E437. [DOI] [PubMed] [Google Scholar]
- DURANTE W., LIAO L., SCHAFER A.I. Differential regulation of L-arginine transport and inducible NOS in cultured vascular smooth muscle cells. Am. J. Physiol. 1995;268:H1158–H1164. doi: 10.1152/ajpheart.1995.268.3.H1158. [DOI] [PubMed] [Google Scholar]
- ESCOBALES N., RIVERA-CORREA M., ALTIERI P.I., RODRIGUEZ J.F. Relationship between NO synthesis, arginine transport, and intracellular arginine levels in vascular smooth muscle cells. Amino Acids. 2000;19:451–468. doi: 10.1007/s007260070023. [DOI] [PubMed] [Google Scholar]
- GAZZOLA G.C., DALL'ASTA V., GUIDOTTI G.G. The transport of neutral amino acids in cultured human fibroblasts. J. Biol. Chem. 1980;255:929–936. [PubMed] [Google Scholar]
- GOLDSTEIN L. Glutamine transport by mitochondria isolated from normal and acidotic rats. Am. J. Physiol. 1975;229:1027–1033. doi: 10.1152/ajplegacy.1975.229.4.1027. [DOI] [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- HALLEMEESCH M.M., LAMERS W.H., DEUTZ N.E. Reduced arginine availability and nitric oxide production. Clin. Nutr. 2002;21:273–279. doi: 10.1054/clnu.2002.0571. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., CAMPBELL E.B., GROSS S.S. Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle. Role in the regeneration of arginine for nitric oxide synthesis. J. Biol. Chem. 1994;269:9405–9408. [PubMed] [Google Scholar]
- HECKER M., MITCHELL J.A., SWIERKOSZ T.A., SESSA W.C., VANE J.R. Inhibition by L-glutamine of the release of endothelium-derived relaxing factor from cultured endothelial cells. Br. J. Pharmacol. 1990a;101:237–239. doi: 10.1111/j.1476-5381.1990.tb12693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECKER M., SESSA W.C., HARRIS H.J., ANGGARD E.E., VANE J.R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. U.S.A. 1990b;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANAI Y., SEGAWA H., MIYAMOTO K., UCHINO H., TAKEDA E., ENDOU H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J. Biol. Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- KNOWLES R.G., MONCADA S. Nitric oxide synthases in mammals. Biochem. J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN G.E., YUDILEVICH D.L., SOBREVIA L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol. Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- MEIJER A.J., LAMERS W.H., CHAMULEAU R.A. Nitrogen metabolism and ornithine cycle function. Physiol. Rev. 1990;70:701–748. doi: 10.1152/physrev.1990.70.3.701. [DOI] [PubMed] [Google Scholar]
- NICHOLSON B., MANNER C.K., KLEEMAN J., MACLEOD C.L. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J. Biol. Chem. 2001;276:15881–15885. doi: 10.1074/jbc.M010030200. [DOI] [PubMed] [Google Scholar]
- PALACIN M., ESTEVEZ R., BERTRAN J., ZORZANO A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol. Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- PINEDA M., FERNANDEZ E., TORRENTS D., ESTEVEZ R., LOPEZ C., CAMPS M., LLOBERAS J., ZORZANO A., PALACIN M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J. Biol. Chem. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- RAGHAVAN S.A., DIKSHIT M. L-citrulline mediated relaxation in the control and lipopolysaccharide-treated rat aortic rings. Eur. J. Pharmacol. 2001;431:61–69. doi: 10.1016/s0014-2999(01)01407-8. [DOI] [PubMed] [Google Scholar]
- SALA R., ROTOLI B.M., COLLA E., VISIGALLI R., PAROLARI A., BUSSOLATI O., GAZZOLA G.C., DALL'ASTA V.Two-way arginine transport in human endothelial cells: TNF-alpha stimulation is restricted to system y(+) Am. J. Physiol. 2002282C134–C43 [DOI] [PubMed] [Google Scholar]
- SCHMIDLIN A., FISCHER S., WIESINGER H. Transport of L-citrulline in neural cell cultures. Dev. Neurosci. 2000;22:393–398. doi: 10.1159/000017468. [DOI] [PubMed] [Google Scholar]
- SCHOTT C.A., GRAY G.A., STOCLET J.-C. Dependence of endotoxin-induced vascular hyporeactivity on extracellular L-arginine. Br. J. Pharmacol. 1993;108:38–43. doi: 10.1111/j.1476-5381.1993.tb13436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOTWELL M.A., KILBERG M.S., OXENDER D.L. The regulation of neutral amino acid transport in mammalian cells. Biochim. Biophys. Acta. 1983;737:267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- SKALLI O., ROPRAZ P., TRZECIAK A., BENZONANA G., GILLESSEN D., GABBIANI G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J. Cell. Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIERKOSZ T.A., MITCHELL J.A., SESSA W.C., HECKER M., VANE J.R. L-glutamine inhibits the release of endothelium-derived relaxing factor from the rabbit aorta. Biochem. Biophys. Res. Commun. 1990;172:143–148. doi: 10.1016/s0006-291x(05)80184-6. [DOI] [PubMed] [Google Scholar]
- VAN WINKLE L.J., CAMPIONE A.L., GORMAN J.M. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J. Biol. Chem. 1988;263:3150–3163. [PubMed] [Google Scholar]
- VAN WINKLE L.J., CHRISTENSEN H.N., CAMPIONE A.L. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J. Biol. Chem. 1985;260:12118–12123. [PubMed] [Google Scholar]
- WAGNER C.A., LANG F., BROER S. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. 2001;281:C1077–C1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- WILEMAN S.M., MANN G.E., BAYDOUN A.R. Induction of L-arginine transport and nitric oxide synthase in vascular smooth muscle cells: synergistic actions of pro-inflammatory cytokines and bacterial lipopolysaccharide. Br. J. Pharmacol. 1995;116:3243–3250. doi: 10.1111/j.1476-5381.1995.tb15131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDMUELLER H.G., SPAETH A.E. Source and fate of circulating citrulline. Am. J. Physiol. 1981;241:E473–E480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- WU G., HAYES T.E., LI H., YAN W., MEININGER C.J. Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem. J. 2001;353:245–252. doi: 10.1042/0264-6021:3530245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU G., MEININGER C.J. Regulation of L-arginine synthesis from L-citrulline by L-glutamine in endothelial cells. Am. J. Physiol. 1993;265:H1965–H1971. doi: 10.1152/ajpheart.1993.265.6.H1965. [DOI] [PubMed] [Google Scholar]
- WU G.Y., BROSNAN J.T. Macrophages can convert citrulline into arginine. Biochem. J. 1992;281:45–48. doi: 10.1042/bj2810045. [DOI] [PMC free article] [PubMed] [Google Scholar]