Abstract
The effect of induction of capacitative Ca2+ entry (CCE) upon tone in small (i.d. 200–500 μm) intrapulmonary (IPA), mesenteric (MA), renal (RA), femoral (FA), and coronary arteries (CA) of the rat was examined.
Following incubation of IPA with 100 nM thapsigargin (Thg) in Ca2+-free physiological salt solution (PSS), a sustained contraction was observed upon reintroduction of 1.8 mM Ca2+, which was unaffected by either diltiazem (10 μM) or the reverse mode Na+/Ca2+ antiport inhibitor KB-R7943 (10 μM). An identical protocol failed to elicit contraction in MA, RA, or CA, while a small transient contraction was sometimes observed in FA.
The effect of this protocol on the intracellular Ca2+ concentration ([Ca2+]i) was assessed using Fura PE3-loaded IPA, MA, and FA. Reintroduction of Ca2+ into the bath solution following Thg treatment in Ca2+-free PSS caused a large, rapid, and sustained increase in [Ca2+]i in all the three types of artery.
100 nM Thg induced a slowly developing noisy inward current in smooth muscle cells (SMC) isolated from IPA, which was due to an increase in the activity of single channels with a conductance of ∼30 pS. The current had a reversal potential near 0 mV in normal PSS, and persisted when Ca2+-dependent K+ and Cl− currents were blocked; it was greatly inhibited by 1 μM La3+, 1 μM Gd3+, and the IP3 receptor antagonist 2-APB (75 μM), and by replacement of extracellular cations by NMDG+.
In conclusion, depletion of intracellular Ca2+ stores with Thg caused capacitative Ca2+ entry in rat small muscular IPA, MA, and FA. However, a corresponding contraction was observed only in IPA. CCE in IPA was associated with the development of a small La3+- and Gd3+-sensitive current, and an increased Mn2+ quench of Fura PE-3 fluorescence. These results suggest that although CCE occurs in a number of types of small arteries, its coupling to contraction appears to be of particular importance in pulmonary arteries.
Keywords: Capacitative calcium entry, intrapulmonary arteries, systemic resistance arteries, intracellular calcium, contraction, nonselective cation current
Introduction
Capacitative Ca2+ entry (CCE) is activated upon depletion of intracellular Ca2+ stores and is demonstrable in a variety of nonexcitable cell types (e.g. Putney, 1990; Berridge, 1995). This pathway is thought to be the key regulatory mechanism in the maintenance of Ca2+ loading of intracellular stores within the sarcoplasmic/endoplasmic reticulum (SR/ER; for a review see Berridge, 1995). However, in recent years it has become apparent that CCE may also be of importance in the regulation of a number of diverse cellular functions such as apoptosis, secretion, and gene transcription (for review see Parekh & Penner, 1997). Although the precise molecular identity of the ion channels involved in CCE is not known, it has been suggested that CCE in vascular smooth muscle is mediated by one or more of a family of seven proteins homologous to the transient receptor potential (TRP) protein of Drosophila (Hardie & Minke, 1993; Birnbaumer et al., 1996; Inoue et al., 2001; Xu & Beech, 2001).
A number of reports have provided evidence for the involvement of CCE in the regulation of smooth muscle tone in a variety of preparations, including mouse anococcygeus (Wallace et al., 1999), guinea-pig and cat gastric fundus (Petkov & Boev, 1996a, 1996b), and rat ileum (Ohta et al., 1995), urinary bladder (Munro & Wendt, 1994), and spleen (Burt et al., 1995; reviewed by McFadzean & Gibson, 2002). Activation of CCE, usually via the application of the selective SR/ER ATPase (SERCA) inhibitors thapsigargin (Thg) or cyclopiazonic acid, has also been shown to modulate tone in vascular smooth muscle preparations. The inhibition of SERCA, and subsequent induction of CCE, has been reported to result in constriction of several conduit arteries, including rat pulmonary (de la Fuente et al., 1995), femoral (Nomura et al., 1997), and carotid arteries (Sekiguchi et al., 1996) and also isolated rat aorta (Kwan et al., 1994; Tepel et al., 1994; Low et al., 1996).
We have previously presented preliminary evidence that activation of CCE in smooth muscle cells (SMC) isolated from the rat intrapulmonary artery (IPA) results in the activation of a Ca2+-permeable cation channel with a conductance of ∼30 pS (Snetkov et al., 2001). Similar results have also been reported by Ng & Gurney (2001), who found that SMC isolated from rat main pulmonary artery possess a small inward current associated with Ca2+ influx that is activated upon inhibition of SERCA. McDaniel et al. (2001) demonstrated a much larger CCE-associated current in cells cultured from the same preparation. These currents appeared to differ not only in amplitude, but also in their sensitivity to La3+, which blocked the current described in the former report with an IC50 of 600 μM, but virtually abolished the current in the cultured PA cells at a concentration of 50 μM.
Little information exists as to the functional effects of CCE in the resistance vasculature, although Flemming et al. (2002) have recently shown that store depletion causes Ca2+ influx, but no constriction in rabbit pial arterioles. Curtis & Scholfield (2001) have also demonstrated the presence of a nifedipine-sensitive CCE-associated current in rabbit choroidal arteriolar myocytes, but did not assess whether this current contributed directly to contraction. In the present study, we have examined the effects of induction of CCE upon tone in small intrapulmonary (IPA), mesenteric (MA), renal (RA), femoral (FA), and coronary (CA) arteries of the rat. We report that although induction of CCE is uniformly associated with a rise in [Ca2+]i, it elicits contraction only in the small pulmonary arteries. CCE in these arteries is associated with the activation of a Na+- and Ca2+-permeable channel with a conductance of ∼30 pS. In contrast to the work of Ng & Gurney (2001), we observe that both the contraction and channel activity associated with CCE are potently blocked by La3+.
Methods
Tension measurement in small muscular arteries
Male Wistar rats (250–350 g) were anaesthetised with sodium pentobarbital (55 mg kg−1 IP) and killed by cervical dislocation as approved by the local Home Office Inspector. The heart, lungs, intestines, kidneys and hind limbs were excised and placed in cold physiological salt solution (PSS, see below). Small (150–600 μm internal diameter (i.d.), median ∼300 μm) IPA, CA, MA, RA, and FA were dissected free of adventitia, mounted in a temperature-controlled myograph at 37°C (Cambustion AM10, Cambustion Ltd, Cambridge, U.K.) or dual channel Mulvany–Halpern myograph, (Danish Myo Technology, Aarhus, Denmark), and gassed continuously with 95% air/5% CO2 (pH 7.35). After 30 min, a length–tension curve was obtained, and the artery stretched to 90% of the diameter that is equivalent to a transmural pressure of 30 mmHg for pulmonary and 100 mmHg for systemic arteries as previously described (Leach et al., 1994). The viability of the preparation was assessed by measuring the response to 80 mM K+ PSS (KPSS; isotonic replacement of NaCl by KCl), and was deemed suitable for use in experiments only when they produced at least 4 and 8 mN mm−1 length for pulmonary and systemic arteries, respectively. Prior to beginning the experiments, arteries were equilibrated with four 2 min exposures to KPSS. Tension recording from small arteries has previously been described in detail (Leach et al., 1994; Robertson et al., 1995).
Measurement of intracellular [Ca2+]i in isolated arteries
In some experiments, [Ca2+]i was measured simultaneously with tension. In this case, following mounting, stretching, and assessment of viability, an artery was incubated with PSS containing 2 μM Fura PE-3/AM for 90 min at room temperature, followed by a further 30 min at 37°C to facilitate intracellular hydrolytic cleavage of the dye. The preparation was then washed several times with fresh PSS and stimulated repeatedly with high KPSS till a stable tension response was achieved. The myograph was mounted on an inverted microscope equipped with spectrofluorimeter (Cairn Research Ltd, Faversham, U.K.) and illuminated alternately with a UV light source at 340 and 380 nm, while measuring the intensities of emitted light using a photomultiplier tube. The ratio of intensities of emitted light (at >510 nm) corresponding to excitation wavelengths of 340 and 380 nm (R340/380) was taken as a measure of [Ca2+]i.
Protocol for induction of CCE with Thg
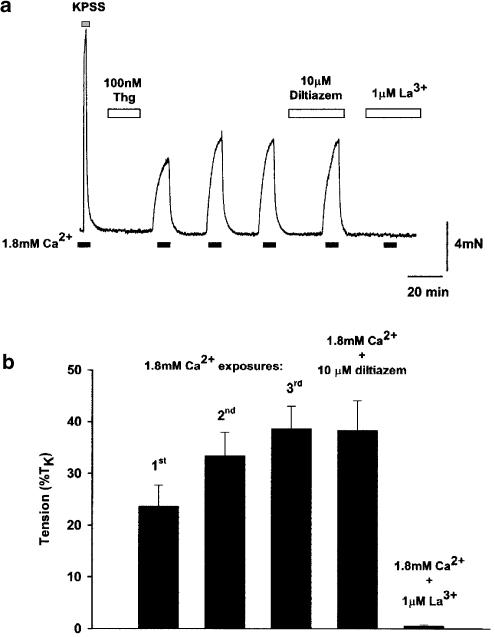
Following the equilibration procedure, the myograph bath solution was exchanged for nominally Ca2+-free PSS for a period of 10 min. Thg (100 nM) was then added to the myograph bath for a period of 10 min, after which 1.8- mM Ca2+ was then reintroduced into the bath solution for 10 min. The myograph bath solution was then replaced again with Ca2+-free PSS. As shown in Figure 1, CCE-related contraction stabilized at the third reintroduction of Ca2+; therefore, in all experiments only the third response was included in the analysis.
Figure 1.
Thg induces sustained constriction in rat small isolated IPA. (a) The trace shows a typical response of rat 232 μm IPA to 100 nM Thg (applied in Ca2+-free PSS). Upon the reintroduction of Ca2+ to the myograph bath, a sustained constriction was observed, which was reversed upon removal of Ca2+ from the myograph bath. The response increased with repeated Ca2+ exposures, yet was reproducible subsequent to the third exposure. This response was unaffected by the voltage-gated channel antagonist diltiazem (10 μM), but was completely abolished by 1 μM La3+. (b) shows the mean response from 10 to 26 IPA.
To prevent precipitation, experiments involving La3+ were performed with HEPES-buffered PSS gassed with air, containing (mM): NaCl 130, MgCl2 1, glucose 5.56, CaCl2 1.8, KCl 4, and HEPES 10, with pH adjusted to 7.4 with NaOH.
Cell isolation for electrophysiology
Dissected small arteries were cut open and placed in 2 ml of Ca2+-free HEPES-buffered PSS, containing (mM): NaCl 135, KCl 5, MgCl2 2, glucose 11, and HEPES 10, pH adjusted to 7.4 with NaOH at 37°C for 20 min. Tissues were then placed in the same solution containing in addition 2 mg ml−1 collagenase (Sigma type XI), 1 mg ml−1 papain, 1 mg ml−1 soyabean trypsin inhibitor, and 1 mM dithiothreitol for 40 min. The tissue was then washed with an enzyme-free dispersion solution four times and left at 37°C for another 20 min. The solution volume was then reduced to ∼0.5 ml and the tissue was gently triturated with a fire-polished Pasteur pipette to release single cells. These were stored in a dispersion solution at 4°C, and then allowed to settle onto a coverslip, which formed the base of the recording chamber, prior to application of the HEPES-buffered solution used for current recording.
Current recording
Single SMC were studied with standard patch-clamp techniques using an Axopatch-1C amplifier (Axon Instruments Inc., Foster City, CA, U.S.A.). The experiments were performed at room temperature. The bath (∼0.2 ml volume) was continuously superfused (1–2 ml min−1) with HEPES-buffered PSS. It should be noted that EGTA was not added to external solutions containing La3+ and Gd3+ in order to avoid chelation. Agonists and drugs were applied via the perfusion system. Whole-cell currents were filtered with −3 dB, low-pass 80 dB dec−1, Bessel-type built-in filter, and digitized using a CED 1401 interface and PATCH v.6 software (Cambridge Electronic Design, Cambridge, U.K.) as appropriate. Whole-cell current–voltage relationships were obtained using a voltage ramp protocol, where the holding potential was maintained at −60 mV, and a 0.5 s ramp from −100 to +100 mV was applied every 5 s. All traces shown are representative of at least four similar experiments.
Measurement of Fura PE-3 quench in SMC isolated from IPA
Single SMC isolated from IPA as above were loaded with Fura-PE3 via incubation of the cells with Fura PE-3/AM (1 μM) for 30 min at room temperature. A drop of cell suspension was placed on the bottom of the chamber mounted on an inverted microscope (Nikon Diaphot, Nikon U.K. Ltd, Kingston, U.K.) equipped with a spectrofluorimeter. SMC were allowed to attach to the bottom of the chamber, washed to remove excess Fura PE-3/AM, and left for another 20 min. Fluorescence recordings were made at room temperature in HEPES-buffered solution with excitation wavelengths of 340, 360, and 380 nm. Changes in [Ca2+]i were assessed by calculating the ratio of the light intensities emitted (>510 nm) when the vessel was illuminated at 340 and 380 nm, respectively. Fluorescence quench was recorded at 360 nm using either 1 mM Mn2+ to study the basal influx or 0.1 mM Mn2+ for Thg-induced influx, where the quench rate was much higher.
Solutions
PSS used for tension recording contained (mM): NaCl 118, NaHCO3 24, KCl 4, CaCl2 1.8, MgSO4 1, NaH2PO4 0.434, and glucose 5.56. HEPES-buffered PSS used for tension recording in the presence of lanthanides contained (mM): NaCl 130, KCl 4, CaCl2 1.8, MgCl2 1, glucose 5.56, and HEPES 10, pH adjusted to 7.4 with NaOH. Low Na+ PSS contained: NMDG 118, choline bicarbonate 24, CaCl2 1.8, MgSO4 1, NaH2PO4 0.434, and glucose 5.56, pH adjusted to 7.4 with HCl.
Electrophysiology
The HEPES-buffered bath solution contained (mM): NaCl 135, KCl 5, CaCl2 2, MgCl2 2, glucose 5, and HEPES 10, pH adjusted to 7.4 with NaOH. In experiments designed to study cation permeability, the latter solution was modified by replacing 140 mM Na+ and 5 mM K+ with 140 mM K+, 140 mM Cs+, or 80 mM Ca2+, respectively. Ca2+-free solution had Ca2+ omitted; no EGTA was used except when 0.1 mM EGTA was added to remove La3+ or Gd3+. Ca2+-, Mg2+-free solution had no Ca2+ or Mg2+ and contained 1 mM EDTA. The pipette solution contained (mM): 140 mM KCl, 2 mM MgCl2, 0.1 mM EGTA, and 10 mM HEPES, pH adjusted to 7.2 with KOH. In experiments designed to minimize K+ and Cl− currents, and with strong intracellular Ca2+ buffering, the pipette solution contained (mM): Cs methanesulphonate 120, BAPTA 10, and HEPES 10, pH adjusted to 7.2 with CsOH. NMDG+, La3+, Gd3+, and Mn2+ were used as chloride salts.
Tension is presented as a percentage of the maximum tension (TK) obtained to the final exposure to KPSS during the equilibration procedure. Mean changes in [Ca2+ ]i are expressed as a percentage of the change in R340/380 induced by KPSS; although not linearly related to [Ca2+]i, this provides a reasonable qualitative index. Results are expressed as mean±s.e.m., and means were compared using paired or unpaired Student's t-test (SigmaStat, SPSS Inc., Chicago, U.S.A.). A difference was deemed significant if P<0.05.
Results
Comparison of the effects of Thg in rat small arteries
Following incubation of rat small IPA with 100 nM Thg in Ca2+-free PSS, reintroduction of Ca2+ resulted in a significant sustained contraction (23.7±4.0% TK, n=26). The size of this response increased with repeated 10 min exposures to Ca2+ (10 min apart), typically stabilizing after the third exposure to Ca2+ (Figure 1; second Ca2+ exposure=33.5±4.5% TK, n=24; third Ca2+ exposure=38.6±4.4% TK, n=18; fourth Ca2+ exposure=38.4±5.7% TK, n=10). In all experiments described subsequently, IPAs were given three 10 min exposures to 1.8 mM Ca2+, 10 min apart, prior to experimental protocols, and time-matched controls were performed in every experiment. In accordance with our previous findings (Robertson et al., 2000), this constrictor response was completely blocked by preincubation with the trivalent cation La3+ at 1 μM (Figure 1b; Thg response to 1.8 mM Ca2+ in the presence of 1 μM La3+=0.6±0.2% TK, n=8, P<0.0001). This response, however, was unaffected by the voltage-gated Ca2+ channel inhibitor diltiazem (10 μM, Figure 1a, n=4).
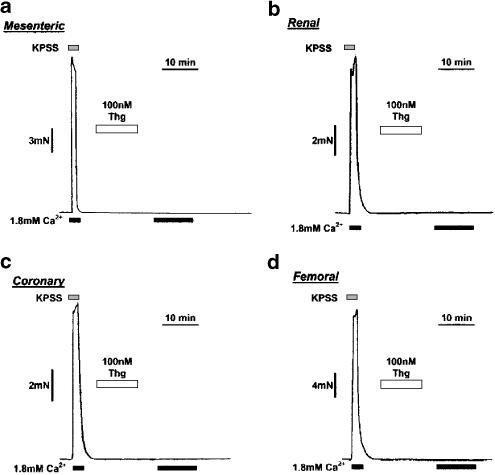
As illustrated in Figure 2, no sustained contractile response could be observed to Thg in any of the ‘systemic' arteries examined in this study. However, in about 1/3 of the FA examined, a small transient contraction that decayed to baseline within 2–3 min was observed (peak response ∼9% TK, see Figure 4 and Figure 5). Removal of the endothelium had no effect on the response of either IPA or MA studied using this protocol (data not shown).
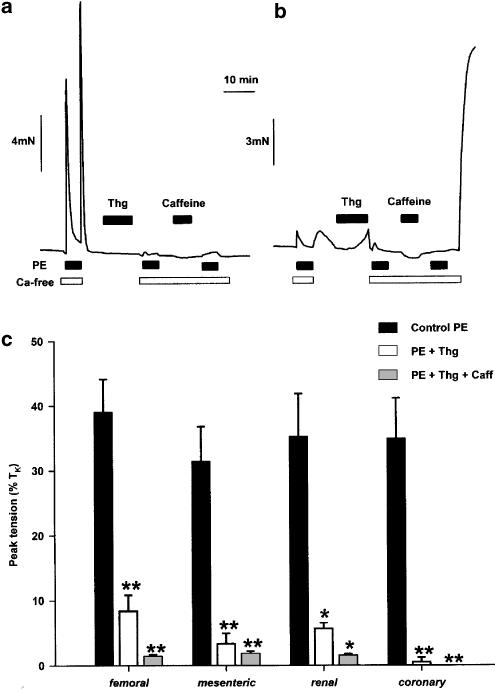
Figure 2.
CCE-induced constriction is not observed in rat small MA, RA, CA, or FA. (a, b, c, d) traces show typical responses to 100- nM Thg and subsequent reintroduction of Ca2+ in rat MA (210 μm i.d.), RA (303 μm i.d.), CA (187 μm i.d.), and FA (324 μm i.d.), respectively, to 100 nM Thg. No constriction was ever observed in MA, RA, or CA upon reintroduction of Ca2+ to the myograph bath (n=8–12). A small transient contraction was observed in four of the 12 FA examined (see Figure 4).
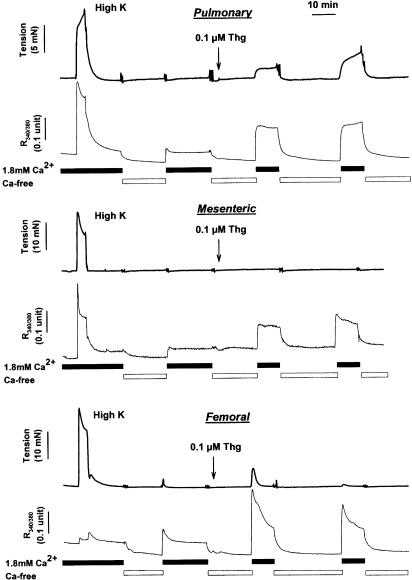
Figure 4.
Simultaneous recording of tension development and [Ca2+]i during application of KPSS and Thg in IPA (upper traces), MA (middle traces), and FA (lower traces). Following addition of KPSS, arteries were exposed to a nominally Ca2+-free solution for 10 min. Subsequent reapplication of Ca2+ caused a small transient increase in [Ca2+]i. Arteries were then exposed to Ca2+-free solution, and 100 nM Thg was applied. Reapplication of Ca2+ in the presence of Thg caused a large and sustained increase in [Ca2+]i, which was stable when the cycle of Ca2+ removal and replacement was repeated. This increases in [Ca2+]i was associated with a sustained contraction in IPA, with no contraction in MA. A transient contraction was sometimes observed in FA, but tended to wane with further cycles of Ca2+ removal and replacement.
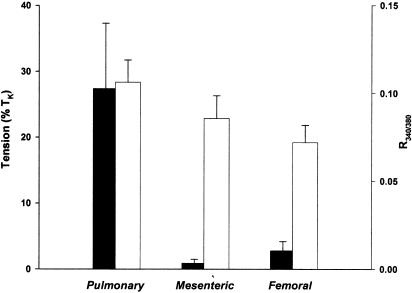
Figure 5.
Summary of CCE-related tension development and [Ca2+]i in IPA, MA, and FA. Effects of reapplication of extracellular Ca2+ following Thg treatment on tension development and [Ca2+]i obtained in individual IPA (n=5), MA (n=6), and FA (n=4) during experiments similar to those shown in Figure 4. The mean±s.e.m. of these responses are shown, with the solid bars representing tension (left axis) and the open bars representing the increase in [Ca2+]i, expressed in terms of the raw R340/380 values (right axis).
The above experiments do not rule out the possibility that the apparent absence of CCE-related contraction in the systemic arteries was due to a less effective Ca2+ store depletion by Thg in these arteries compared to IPA. In order to address this possibility, we used the procedure illustrated in Figure 3a. Arteries were preincubated for 90 s in Ca2+-free solution containing 0.5 mM EGTA, to remove extracellular Ca2+, and then challenged with 10 μM PE (maximally effective concentration). This caused a transient contraction, dependent on Ca2+ release, which provided an index of the amount of releasable Ca2+ in the store. Arteries were then placed in normal PSS for 10 min. This led to a large initial transient contraction, which was presumably caused by the brief simultaneous presence of PE and Ca2+ in the bath, since it was absent if PE was removed 2 min before Ca2+ was reapplied (n=8, data not shown). Thg (100 nM) was then applied for 10 min, after which the arteries were again incubated in Ca2+-free PSS before being exposed to a second PE challenge. The arteries, still in Ca2+-free PSS, were then exposed to 10 mM caffeine for 2 min, before a third and last PE challenge, after which normal Ca2+-containing PSS was reapplied (Figure 3a).
Figure 3.
Contraction after depletion of cellular Ca2+ store with Thg (100 nM), caffeine (10 mM), and phenylephrine (PE, 10 μM). (a) The trace shows three responses to PE, administered 90 s after the removal of Ca2+ from the solution (with addition of 0.5 mM EGTA), in a rat mesenteric resistance artery. After a typical transient control PE contraction (left), stores were refilled by returning Ca2+ to the solution, and Thg was applied. This almost abolished the subsequent response to PE in Ca2+-free solution. After the application and removal of caffeine and then PE in the absence of Ca2+ to ensure further Ca2+ store depletion, readdition of Ca2+ to the solution (at the end of the long open bar) did not cause contraction. (b) A protocol identical to that described above was carried out in a rat IPA. Here, Ca2+ reapplication after store depletion caused a large contraction (right). (c) The mean±s.e.m. amplitude of the three PE contractions were recorded as described above in small FA, MA, RA, and CA. n=4; *P<0.05; **P<0.01.
Thg itself had no effect on tension in MA, RA, FA, or CA, but the tension induced by the subsequent PE challenge was markedly reduced, reflecting a substantially reduced content of the PE-releasable Ca2+ store (Figure 3a, c, solid and open columns). In all types of arteries studied, subsequent treatment with caffeine induced no increase in tension, and the following PE challenge was also without significant effect (Figure 3c). These data strongly suggest that the releasable Ca2+ stores were essentially empty at this point. Nevertheless, in MA, RA, FA, and CA, reapplication of Ca2+-containing PSS caused no increase in tension whatsoever (Figure 3a), whereas in IPA it caused a rapid and powerful contraction (Figure 3b). These experiments show firstly that Thg was effective in depleting intracellular stores of Ca2+ in all the five types of resistance artery, and secondly that the difference between IPA and the systemic arteries persisted even when store depletion was further assured by treating the arteries with PE and caffeine in addition to Thg.
It should be noted that consistent with previous reports (Priest et al., 1997), PE caused only a very small transient contraction in IPA in the absence of extracellular Ca2+, although this was also reduced following application of Thg. Additionally, in IPA, Thg caused a gradually developing contraction in normal PSS (Figure 3b) in contrast to the systemic arteries where it had no effect on tension.
Effect of Thg on [Ca2+]i in isolated arteries
Effects of Thg treatment on [Ca2+]i were investigated in IPA, FA, and MA. Figure 4 illustrates the simultaneous records of tension and [Ca2+]i obtained in an IPA (top), MA (middle), and FA (bottom). In each case, readmission of Ca2+, following treatment of the artery with Thg in Ca2+-free PSS, caused an increase in R340/380, which was comparable to (and in the case of FA, much larger than) the increase observed during application of KPSS. In the FA, the rise in [Ca2+]i in response to KPSS was consistently smaller than in the IPA or MA. Conversely, as described above, tension development upon Ca2+ readmission was negligible in the MA, substantial in the IPA, and small and transient, or absent, in the FA. Pooled measurements from a number of arteries of the CCE-mediated increases in [Ca2+]i and tension are presented in Figure 5, with the bar for tension development in the FA representing the peak of the transient response. These results indicate that CCE occurs in each type of artery, but that it appears to be differentially coupled to tension development.
Effect of Thg upon inward currents in SMC isolated from small IPA
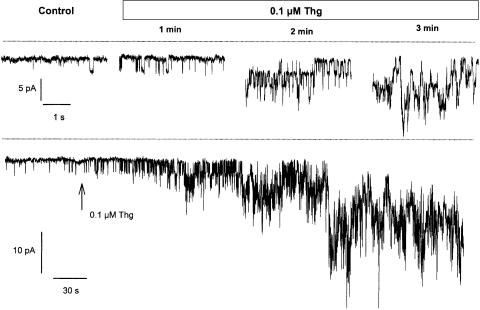
The effect of Thg on the membrane current was studied in cells isolated from the IPA. The high input resistance of these cells meant that the holding current needed to maintain the membrane potential of the whole cell at −80 mV was very small, and occasionally spontaneous single-channel events could be detected in whole-cell mode as inward currents of ∼3 pA (Figure 6). Thg (100 nM) in Ca2+-free solution caused a progressive increase in the frequency of openings, the overlapping of which resulted in a strongly fluctuating inward current with amplitude of 20–30 pA within 3 min (Figure 6). The development of the inward current in the presence of Thg occurred both with standard KCl-based intracellular solution and with a Cs methanesulphonate pipette solution containing 10- mM BAPTA to minimize the contribution of Ca2+-dependent K+ and Cl− currents (not shown).
Figure 6.
Thg causes an inward current in SMC isolated from rat small IPA. Below, the typical trace of the noisy inward current developing in SMC isolated from small IPA in response to 100 nM Thg. Above, the expanded sections of the same trace showing the dramatic increase of single-channel activity. Holding potential −80 mV, intracellular solution – Cs methanesulphonate, 10 -mM BAPTA. Lines indicate zero current level. Traces shown are typical of five to seven experiments.
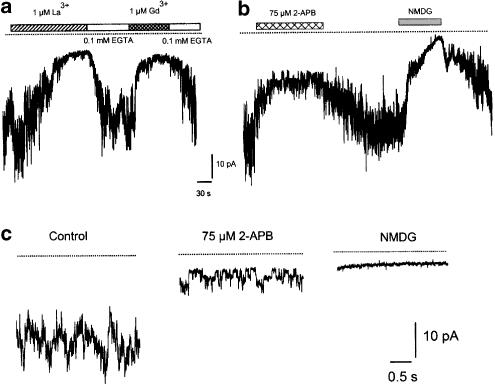
The trivalent cations La3+ or Gd3+ (both 1 μM) were found to block the Thg-induced current rapidly (Figure 7a); for example, in four cells La3+ inhibited the current by 84±4%. Their effect could be reversed by washing with Ca2+-free extracellular solution containing 0.1 mM EGTA, which strongly chelates lanthanides (Halaszovich et al., 2000). The replacement of all extracellular cations by NMDG+ also inhibited the Thg-induced current by more than 90% (Figures 7b, c).
Figure 7.
Block of Thg-induced inward current. (a) Trivalent cations La3+ and Gd3+ block Thg-induced inward current; this block could be reversed by chelating lanthanides with EGTA. (b) 2-APB caused partial and reversible block of the Thg-induced inward current, while replacement of all extracellular permeable cations by NMDG+ abolished the current completely. (c) Single channels underlying Thg-induced whole-cell current were revealed in the presence of 2-APB, which reduced the opening frequency without affecting conductance. No activity could be observed in NMDG+-based solution. Holding potential −80 mV, intracellular solution – Cs methanesulphonate, 10 mM BAPTA. Lines indicate zero current level. Traces shown are typical of six to experiments.
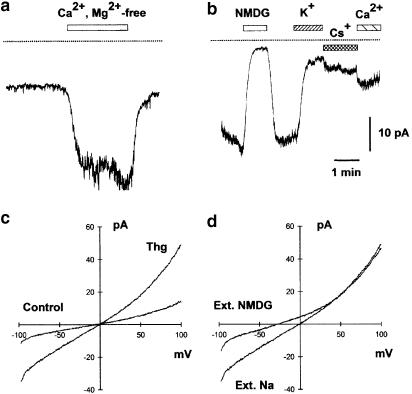
Thg-induced current was not significantly different in Ca2+-free extracellular solution (n=8). However, the current increased considerably when both Ca2+ and Mg2+ were removed using a solution containing 1 mM EDTA to chelate any traces of divalent cations (Figure 8a). Both K+ and Cs+ supported the current to a certain extent and a current of ∼40% of the normal amplitude persisted when 80 mM Ca2+ was the only extracellular cation (Figure 8b). This would be consistent with the channel having a somewhat smaller permeability to Ca2+ than to Na+.
Figure 8.
Ion permeability of Thg-induced inward current. (a) Removal of divalent cations with 1 mM EDTA considerably enhanced Thg-induced current. (b) 140 mM K+, 140 mM Cs+, and 80 mM Ca2+ supported the inward current to some extent, although it was smaller than with 140 mM Na+ (control). The lines indicate zero current level. Traces shown are typical of six to eight experiments. (c) When the membrane potential was ramped from −100 to +100 mV in the absence of both intra- and extracellular K+, Thg treatment (1 μM, 3 min) caused the development of nonrectifying current. (d) After treatment with Thg, the replacement of extracellular Na+ for NMDG+ suppressed the inward current. Holding potential −80 mV, intracellular solution – Cs methanesulphonate, 10 mM BAPTA. Typical traces are from four to eight experiments.
When cells were ramped from –100 to +100 mV in the absence of both intra- and extracellular K+, Thg treatment caused the development of a current which reversed at ∼0 mV (Figure 8c). This current was strongly inhibited at negative potentials by replacement of extracellular Na+ with NMDG+ (Figure 8d). In five cells, the inward current at –80 mV was reduced by 76.5±5.6%, whereas the outward current at +80 mV was reduced by only 19.2±7.1%, when NMDG+ replaced Na+.
Effect of 2-aminoethoxydiphenyl borate (2-APB) and KB-R7943
The putative membrane-permeable IP3 receptor antagonist 2-APB has been reported to block both endogenous store-operated channels and heterologously expressed TRP3 channels in HEK293 cells (Ma et al., 2000). We examined the effect of 2-APB on both IPA constriction and Thg-induced inward currents. 2-APB (75 μM) was found to reduce the inward current (Figure 7b), and in doing so revealed individual channel openings (Figure 7c) similar in amplitude to those recorded spontaneously, and following Thg treatment. 2-APB (75 μM) also completely abolished the Thg-induced contraction in small IPA (n=14), and caused a small but significant inhibition of the KPSS contractile response (KPSS in the presence of 75- μM 2-APB=78.4±11.8% TK, P<0.05, n=5).
Since the current observed after Thg treatment was predominantly due to Na+ influx, it is possible that a subsarcolemmal increase in Na+ could cause an increase in intracellular [Ca2+] and therefore contraction, by activating reverse mode Na+/Ca2+ exchange, as suggested by Lee et al. (2001) for rabbit inferior vena cava. However, the inhibitor of reverse mode Na+/Ca2+ exchange, KB-R7943 (10 μM), did not significantly reduce the Thg-induced contraction in IPA (contraction in the presence of KB-R7943=85±5% of control response, n=5, P>0.05). Moreover, the rise in [Ca2+]i induced by reintroduction of extracellular Ca2+ was unchanged in low Na+ PSS (data not shown; n=4). However, KB-R7943 was found to inhibit the KPSS-induced contraction (contraction in the presence of KB-R7943=31.7±4.6% of control response, n=5, P<0.05), which is mediated by voltage-gated Ca2+ channels. This suggests that this compound is not selective for the reverse mode Na+/Ca2+ exchanger in these arteries.
Basal Mn2+ permeability of SMC from IPA
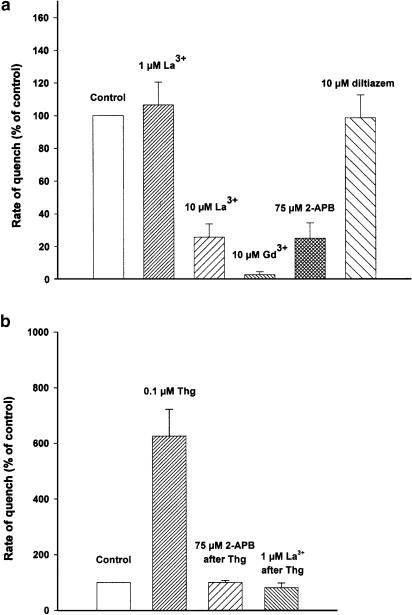
In SMC freshly isolated from IPA and loaded with Fura-PE3, application of extracellular Mn2+ (1 mM) caused a relatively fast quenching of fluorescence under basal conditions. Application of 1 μM La3+ did not affect the rate of quenching (106±8% of control, n=6); however, 10 μM La3+ caused a significant reduction in the rate of quenching (25±4%, n=6), and 10 μM Gd3+ suppressed it entirely (2±1%, n=6). Diltiazem (10 μM) had no effect (98±8%, n=4), while 2-APB (75 μM) markedly blocked basal quenching (25±4%, n=5) (Figure 9a). These results indicate that IPA SMC possess a constitutively active Ca2+ influx pathway permeable to Mn2+. Pretreatment of cells with Thg (100 nM) increased the rate of quenching to 625±96% (n=8, P<0.001) of control, and this increase was abolished by 75 μM 2-APB (n=5) and by 1 μM La3+ (n=4) (Figure 9b).
Figure 9.
SMC isolated from IPA possess a Mn2+-permeable, La3+-, Gd3+-, and 2-APB-sensitive basal and Thg-induced influx pathways. (a) Relative rates of basal Fura-PE3 fluorescence quenching with 1 mM Mn2+ in unstimulated SMC freshly isolated from IPA. La3+ (10 μM, but not 1 μM) caused a significant reduction in the rate of quenching and 10 μM Gd3+ suppressed it entirely. Diltiazem (10 μM) had no effect, while 2-APB (75 μM) attenuated the basal quenching. (b) Thg (100 nM) increased the rate of quenching (measured with 0.1 mM Mn2+) more than six-fold, and this increase was abolished by 75 μM 2-APB or 1 μM La3+. n=10–16, ***P<0.001.
Discussion
CCE subsequent to inhibition of the SERCA has been proposed as a ubiquitous mechanism in smooth muscle controlling not only the Ca2+ load of stores within the ER/SR, but also modulating tone (for review see McFadzean & Gibson, 2002). We have recently reported that the transient portion of the vasoconstrictor response to hypoxia in rat-isolated IPA may be mediated in part via an activation of CCE (Robertson et al., 2000). In the present study, we applied Thg in the absence of Ca2+ to deplete Ca2+ stores, and then restored Ca2+ to the solution to examine CCE. Among the five types of small arteries which we studied, Ca2+ restoration caused a substantial sustained contraction in the IPA, sometimes caused a small transient contraction in the FA, and was not associated with contraction in the MA, RA, or CA. Ca2+ restoration was, however, associated with substantial increases in [Ca2+]i in MA, FA, and IPA, each of the three arteries representing a different pattern of tension development.
CCE and arterial contraction
The majority of investigations into the possible role of CCE in regulating vascular tone have been performed in large conduit arteries. Induction of CCE, via inhibitors of the SERCA or Ca2+-releasing agonists, has consistently been shown to induce a prompt and marked contraction in the rat main pulmonary artery (de la Fuente et al., 1995; McDaniel et al., 2001; Ng & Gurney, 2001), which is associated with a rise in [Ca2+]i (McDaniel et al., 2001; Ng & Gurney, 2001). However, agents that induce CCE tend to cause inconsistent, small, and/or more slowly developing contractions in systemic conduit arteries, including rat aorta (Noguera et al., 1998; Shima & Blaustein, 1992), tail artery (Kwan et al., 1994), carotid artery (Sekiguchi et al., 1996), and FA (Nomura et al., 1997), and mouse aorta (Cohen et al., 1999), in spite of the fact that, when measured, [Ca2+]i has been shown to rise substantially in these arteries (Sekiguchi et al., 1996; Tosun et al., 1998; Cohen et al., 1999). The few analogous studies in smaller systemic arteries suggest that the rise in [Ca2+]i is completely dissociated from contraction. Naganobu & Ito (1994) observed that cyclopiazonic acid caused a sustained rise in [Ca2+]i with no contraction in rat mesenteric resistance arteries, and Flemming et al. (2002) reported a similar effect in rabbit pial arterioles. The present study confirms and extends these findings, showing firstly that CCE is associated with a marked and sustained contraction in smaller pulmonary arteries from rats, and secondly that CCE causes no or a minimal transient contraction in rat small MA, FA, RA, and CA.
The observations of Naganobu & Ito (1994) and Flemming et al. (2002), as well as the results shown in Figure 4 and Figure 5, indicate, however, that Thg-induced store depletion causes a marked and sustained increase in [Ca2+]i in small systemic as well as pulmonary arteries. The basis of the difference between IPA and the other arteries remains obscure. The Thg-induced current appeared to be mainly carried by Na+ (see below), a finding that is consistent with the report of Arnon et al. (2000) that store release greatly elevated [Na+]i in rat cultured mesenteric arterial myocytes. It therefore seemed possible that Na+ influx through store-operated channels could cause an increase in intracellular [Ca2+] and contraction by activating reverse mode Na+/Ca2+ exchange (Lee et al., 2001) in IPA but not the other arteries, especially because our preliminary observations suggest that Na+/Ca2+ exchange is prominent in the IPA, but not in the MA (Moir & Aaronson, unpublished observations). However, we found no evidence for this mechanism, as KB-R7943, an inhibitor of reverse mode Na+/Ca2+ exchange, did not significantly reduce the Thg-induced contraction, and removal of extracellular Na+ did not affect the CCE-associated increase in [Ca2+]i. It should be noted that KB-R7943 inhibited KPSS-induced contractions in IPA, which we have previously found are completely abolished by voltage-gated Ca2+ channel blockers (Robertson et al., 2000). This suggests that KB-R7943 is not selective for reverse-mode Na+/Ca2+ exchange in these arteries, and is consistent with its ability to block a variety of voltage-gated ion channels (Tanaka et al., 2002).
Although IPA responded to CCE with a contraction, it is apparent from Figure 4 that even in these arteries, the CCE-mediated contraction was much smaller than that evoked by high K+, even though both stimuli were associated with comparable increases in [Ca2+]i. This disproportion between increases in [Ca2+]i and tension, an extreme form of which apparently occurs in small systemic arteries and arterioles (Figure 4, see also Naganobu & Ito, 1994; Flemming et al., 2002), was initially highlighted in the rat aorta by Tosun et al. (1998), who proposed that Ca2+ entering during CCE is confined to a compartment that has limited access to the contractile apparatus. The case of the FA is particularly interesting, since here CCE caused an increase in [Ca2+]i that was much larger than that caused by high K+ depolarization, even though it caused a contraction that was negligible compared to the high K+ response. It seems that even in the absence of Ca2+ uptake into the sarcoplasmic reticulum, SMC retain the ability to selectively direct Ca2+ entering the cell via different pathways either towards or away from the contractile apparatus.
Properties of CCE in rat IPAs
Treatment of isolated IPA SMC with Thg caused the development of a noisy inward current in cells held at –80 mV. The current amplitude was ∼30 pA in cells held at, or ramped to, this potential. At negative potentials, the current was not affected when Ca2+ was removed in the presence of a normal [Na+], but was greatly reduced when Na+ was replaced by NMDG+, suggesting that it was mainly carried by Na+ ions. A current of reduced amplitude was, however, observed when Na+ was replaced by 80 mM Ca2+, indicating that the channel is also Ca2+-permeable. The current was also supported by other monovalent cations, and reversed near 0 mV, suggesting that it is mediated by a nonselective cation channel.
During the onset of the current, and following its block by 2-APB, it was possible to observe individual channel openings of ∼3 pA at –80 mV, indicating a channel conductance of ∼30 pS. Although we did not further characterize the properties of these channels, they appear to differ from the store-operated channels in rabbit portal vein, which were recently described in greater detail by Albert & Large (2002), as the latter demonstrated a conductance of ∼2 pS and reversed at a more positive potential under similar conditions.
The current observed in IPA appears to be similar, however, to that previously reported in rat main pulmonary artery by Ng & Gurney (2001), with the difference that the current in IPA is much more potently inhibited by La3+. It seems likely, however, that the relative La3+ insensitivity (IC50∼600 μM) reported by Ng & Gurney (2001) can be explained by their use of a phosphate-containing buffer system. Since lanthanum phosphate is known to be insoluble (Moeller, 1961), the use of phosphate-containing buffers in experiments where La3+ is used would result in the formation of lanthanum phosphate, thereby greatly decreasing the concentration of free La3+ in solution.
It has been reported that La3+ can directly prevent the inhibition of SERCA by Thg (Papp et al., 1991). It is therefore possible that the inhibitory effects of La3+ upon Thg-induced contraction, and channel activation, in pulmonary arteries may be due to stabilization of SERCA by La3+. However, our results suggest that the blockade of the CCE-associated current by La3+ is extracellular since the pipette solution contained EGTA, which would strongly chelate any La3+ entering the cells. The inclusion of EGTA in the pipette solution would also appear to preclude the possibility that La3+ may block CCE in pulmonary arteries via an action on the cytosolic side of the plasma membrane, as reported for the blockade of TRP3 channels by La3+ in Chinese hamster ovary cells (Halaszovich et al., 2000).
In marked contrast to our results, McDaniel et al. (2001) reported a store release-activated current of over 300 pA in pulmonary artery SMC. The most likely explanation for this discrepancy is that McDaniel et al. (2001) utilized cells that had been maintained in primary culture, a procedure that is known to enhance CCE greatly (Golovina, 1999; Dreja et al., 2001).
It is interesting to note that infrequent openings of channels apparently similar to those underlying the Thg-induced current could be recorded prior to Thg exposure (see Figure 6a). It is possible that cell dialysis with high concentrations of Ca2+-chelating agents, such as EGTA or BAPTA, could itself cause depletion of intracellular stores and subsequent activation of CCE (Albert & Large, 2002). However, in nondialysed cells a Mn2+-permeable influx pathway was observed under basal conditions (Figure 9a), which was blocked by 10 μM La3+ but not by 1 μM La3+. Thg preincubation elicited an approximately six-fold increase in Mn2+ quenching of the fluorescence signal, which was abolished by 1 μM La3+ (Figure 9b). There was, therefore, a difference in the La3+ sensitivity of the basal and Thg-associated influx pathways. This suggests the involvement of separate channels, although this requires additional confirmation.
2-APB was effective in abolishing the Thg-induced increases in both inward currents, and constrictor response in rat-isolated small IPA. This agent, which blocks the IP3 receptor (Maruyama et al., 1997), was initially used to assess the possible role that this receptor could play in the regulation of CCE (e.g. Ma et al., 2000). Subsequent observations suggested, however, that 2-APB possesses effects upon CCE that are independent of any action upon the IP3 receptor (Dobrydneva & Blackmore, 2001; Iwasaki et al., 2001; Prakriya & Lewis, 2001; Ma et al., 2002). The present results are consistent with these more recent findings. Irrespective of the mechanism by which 2-APB is acting to inhibit CCE, its effect on the Thg-induced current and contraction in IPA provides additional evidence linking these effects, although the minor inhibition of the contraction to KPSS by 2-APB illustrates its lack of complete selectivity for CCE.
In summary, CCE-induced vasoconstriction appears to be especially prominent in pulmonary arteries, at least at the level of small muscular arteries. The results suggest that the extent to which CCE contributes to agonist-induced vasoconstriction may vary widely between different vascular beds, and underline the importance of this pathway in the pulmonary vasculature. The CCE-associated current in rat IPA involves a nonselective cation channel with a conductance of ∼30 pS, and is sensitive to relatively low concentrations (1 μM) of the trivalent cations La3+ and Gd3+. Further studies are required to determine the basis of the heterogeneity of the contractile response of various arteries to Ca2+ store release.
Acknowledgments
We are grateful to the Wellcome Trust (grant number 062554) for their support.
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- CA
coronary artery
- CCE
capacitative Ca2+ entry
- FA
femoral artery
- IPA
intrapulmonary artery
- MA
mesenteric artery
- PSS
physiological salt solution
- RA
renal artery
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- SMC
smooth muscle cell
- SR/ER
sarco/endoplasmic reticulum
- Thg
thapsigargin
- TRP
transient receptor potential protein
References
- ALBERT A.P., LARGE W.A. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J. Physiol. 2002;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNON A., HAMLYN J.M., BLAUSTEIN M.P. Na+ entry via store-operated channels modulates Ca2+ signalling in arterial myocytes. Am. J. Physiol. 2000;278:C163–C173. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRNBAUMER L., ZHU X., JIANG M., BOULAY G., PEYTON M., VANNIER B., BROWN D., PLATANO D., SADEGHI H., STEFANI E., BIRNBAUMER M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURT R.P., CHAPPLE C.R., MARSHALL I. The role of capacitative Ca2+ influx in the alpha 1B-adrenoceptor-mediated contraction to phenylephrine of the ratspleen. Br. J. Pharmacol. 1995;116:2327–2333. doi: 10.1111/j.1476-5381.1995.tb15073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN R.A., WEISBROD R.M., GERICKE M., YAGHOUBI M., BIERL C., BOLOTINA V.M. Mechanism of nitric-oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ. Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- CURTIS T.M., SCHOLFIELD C.N. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J. Physiol. 2001;532:609–624. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBRYDNEVA Y., BLACKMORE P. 2-Aminoethoxydi-phenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol. Pharmacol. 2001;3:541–552. [PubMed] [Google Scholar]
- DREJA K., BERGDAHL A., HELLSTRAND P. Increased store-operated Ca 2+ entry into contractile vascular smooth muscle following organ culture. J. Vasc. Res. 2001;38:324–331. doi: 10.1159/000051063. [DOI] [PubMed] [Google Scholar]
- FLEMMING R., CHEONG A., DEDMAN A., BEECH D.J. Discrete store-operated calcium influx into an intracellular compartment in rabbit arteriolar smooth muscle. J. Physiol. 2002;542:455–464. doi: 10.1113/jphysiol.2002.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA FUENTE P.G., SAVINEAU J.P., MARTHAN R. Control of pulmonary vascular smooth muscle tone by sarcoplasmic reticulum Ca2+ pump blockers: Thg and cyclopiazonic acid. Pflügers Archiv. Eur. J. Physiol. 1995;429:617–624. doi: 10.1007/BF00373982. [DOI] [PubMed] [Google Scholar]
- GOLOVINA V.A. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am. J. Physiol. 1999;277:C343–C349. doi: 10.1152/ajpcell.1999.277.2.C343. [DOI] [PubMed] [Google Scholar]
- HALASZOVICH C.R., ZITT C., JÜNGLING E., LÜCKHOFF A. Inhibition of TRP3 channels by lanthanides. Block from the cytosolic side of the plasma membrane. J. Biol. Chem. 2000;275:37423–37428. doi: 10.1074/jbc.M007010200. [DOI] [PubMed] [Google Scholar]
- HARDIE R.C., MINKE B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 1993;16:371–376. doi: 10.1016/0166-2236(93)90095-4. [DOI] [PubMed] [Google Scholar]
- INOUE R., OKADA T., ONOUE H., HARA Y., SHIMIZU S., NAITOH S., ITO Y., MORI Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- IWASAKI H., MORI Y., HARA Y., UCHIDA K., ZHOU H., MIKOSHIBA K. 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Receptors Channels. 2001;7:429–439. [PubMed] [Google Scholar]
- KWAN C.Y., CHAUDHARY R., ZHENG X.F., NI J., LEE R.M. Effects of sarcoplasmic reticulum calcium pump inhibitors on vascular smooth muscle. Hypertension. 1994;23:I156–I160. doi: 10.1161/01.hyp.23.1_suppl.i156. [DOI] [PubMed] [Google Scholar]
- LEACH R.M., ROBERTSON T.P., TWORT C.H., WARD J.P.T. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am. J. Physiol. 1994;266:L223–L231. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- LEE C.H., POBURKO D., SAHOTA P., SANDHU J., RUEHLMANN D.O., VAN BREEMEN C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J. Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW A.M., KOTECHA N., NEILD T.O., KWAN C.Y., DANIEL E.E. Relative contributions of extracellular Ca2+ and Ca2+ stores to smooth muscle contraction in arteries and arterioles of rat, guinea-pig, dog and rabbit. Clin. Exp. Pharmacol. Physiol. 1996;23:310–316. doi: 10.1111/j.1440-1681.1996.tb02829.x. [DOI] [PubMed] [Google Scholar]
- MA H.-T., PATTERSON R.L., VAN ROSSUM D.B., BIRNBAUMER L., MIKOSHIBA K., GILL D.L. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- MA H.T., VENKATACHALAM K., PARYS J.B., GILL D.L. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J. Biol. Chem. 2002;277:6915–6922. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- MARUYAMA T., KANAJI T., NAKADE S., KANNO T., MIKOSHIBA K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- MCDANIEL S.S., PLATOSHYN O., WANG J., YU Y., SWEENEY M., KRICK S., RUBIN L.J., YUAN J.X.-J. Capacitative Ca2+ entry in agonist induced pulmonary vasoconstriction. Am. J. Physiol. 2001;280:L870–L880. doi: 10.1152/ajplung.2001.280.5.L870. [DOI] [PubMed] [Google Scholar]
- MCFADZEAN I., GIBSON A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER T.Chemistry of the rare earths The Rare Earths 1961New York: Wiley; 9–20.ed. Spedding, F.H. & Daane, A.H. pp [Google Scholar]
- MUNRO D.D., WENDT I.R. Effects of cyclopiazonic acid on [Ca2+]i and contraction in rat urinary bladder smooth muscle. Cell Calcium. 1994;15:369–380. doi: 10.1016/0143-4160(94)90012-4. [DOI] [PubMed] [Google Scholar]
- NAGANOBU K., ITO K. Handling of cytoplasmic Ca2+ by the sarcoplasmic reticulum during alpha 1-adrenoceptor-mediated contraction of rat mesenteric resistance arteries. Jpn. J. Pharmacol. 1994;64:89–96. doi: 10.1254/jjp.64.89. [DOI] [PubMed] [Google Scholar]
- NG L.C., GURNEY A.M. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ. Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- NOGUERA M.A., MADRERO Y., IVORRA M.D., D'OCON P. Characterization of two different Ca2+ pathways dependent on depletion of internal Ca2+ pools in rat aorta. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:92–99. doi: 10.1007/pl00005156. [DOI] [PubMed] [Google Scholar]
- NOMURA Y., ASANO M., ITO K., UYAMA Y., IMAIZUMI Y., WATANABE M. Potent vasoconstrictor actions of cyclopiazonic acid and Thg on femoral arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 1997;120:65–73. doi: 10.1038/sj.bjp.0700857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHTA T., KAWAI K., ITO S., NAKAZATO Y. Ca2+ entry activated by emptying of intracellular Ca2+ stores in ileal smooth muscle of the rat. Br. J. Pharmacol. 1995;114:1165–1170. doi: 10.1111/j.1476-5381.1995.tb13329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPP B., ENYEDI Á., KOVÁCS T., SARKADI B., WUYTACK F., THASTRUP O., GÁRDOS G., BREDOUX R., LEVY-TOLEDANO S., ENOUF J. Demonstration of two forms of calcium pumps by Thg inhibition and radioimmunoblotting in platelet membrane vesicles. J. Biol. Chem. 1991;266:14593–14596. [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., BOEV K.K. Cyclopiazonic acid-induced changes in contractile activity of smooth muscle strips isolated from cat and guinea-pig stomach. Eur. J. Pharmacol. 1996a;318:109–115. doi: 10.1016/s0014-2999(96)00764-9. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., BOEV K.K. The role of sarcoplasmic reticulum and sarcoplasmic reticulum Ca2+-ATPase in the smooth muscle tone of the cat gastric fundus. Pflügers Archiv. Eur. J. Physiol. 1996b;431:928–935. doi: 10.1007/s004240050087. [DOI] [PubMed] [Google Scholar]
- PRAKRIYA M., LEWIS R.S. Potentiation and inhibition of Ca2+ release activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIEST R.M., HUCKS D., WARD J.P. Noradrenaline, beta-adrenoceptor mediated vasorelaxation and nitric oxide in large and small pulmonary arteries of the rat. Br. J. Pharmacol. 1997;122:1375–1384. doi: 10.1038/sj.bjp.0701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTNEY J.W., JR Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- ROBERTSON T.P., AARONSON P.I., WARD J.P.T. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitisation. Am. J. Physiol. 1995;268:H301–H307. doi: 10.1152/ajpheart.1995.268.1.H301. [DOI] [PubMed] [Google Scholar]
- ROBERTSON T.P., HAGUE D., AARONSON P.I., WARD J.P.T. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J. Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI F., SHIMAMURA K., AKASHI M., SUNANO S. Effects of cyclopiazonic acid and Thg on electromechanical activities and intracellular Ca2+ in smooth muscle of carotid artery of hypertensive rats. Br. J. Pharmacol. 1996;118:857–864. doi: 10.1111/j.1476-5381.1996.tb15478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMA H., BLAUSTEIN M.P. Modulation of evoked contractions in rat arteries by ryanodine, thapsigargin, and cyclopiazonic acid. Circ. Res. 1992;70:968–977. doi: 10.1161/01.res.70.5.968. [DOI] [PubMed] [Google Scholar]
- SNETKOV V.A., ROBERTSON TP AARONSON P.I., WARD J.P.T. Capacitative Ca2+ entry in pulmonary artery smooth muscle cells. Am. J. Resp. Crit. Care Med. 2001;163:A393. [Google Scholar]
- TANAKA H., NISHIMARU K., AIKAWA T., HIRAYAMA W., TANAKA Y., SHGENOBU K. Effect of SEA0400, a novel inhibitor of sodium–calcium exchanger, on myocardial ionic current. Br. J. Pharmacol. 2002;135:1096–1100. doi: 10.1038/sj.bjp.0704574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPEL M., RUESS C., MEHRING N., NEUSSER M., ZIDEK W. Effect of inhibition of sarcoplasmic Ca2+-ATPase on vasoconstriction and cytosolic Ca2+ in aortic smooth muscle from spontaneously hypertensive and normotensive rats. Clin. Exp. Hypertens. 1994;16:493–506. doi: 10.3109/10641969409067958. [DOI] [PubMed] [Google Scholar]
- TOSUN M., PAUL R.J., RAPOPORT R.M. Coupling of store-operated Ca2+ entry to contraction in rat aorta. J. Pharmacol. Exp. Ther. 1998;285:759–766. [PubMed] [Google Scholar]
- WALLACE P., AYMAN S., MCFADZEAN I., GIBSON A. Thg induced tone and capacitative calcium influx in mouse anococcygeus smooth muscle cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:368–375. doi: 10.1007/s002109900100. [DOI] [PubMed] [Google Scholar]
- XU S.Z., BEECH D.J. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ. Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]