Abstract
Little is known about the interaction of 17β-estradiol (E2) and the vasoactive endothelin system in the heart. Endothelin signaling is activated in a failing heart and may contribute to myocardial dysfunction and remodeling. Therefore, we investigated the regulation of proteins of the endothelin system (ppET-1, ECE and ETA-R and ETB-R) in the hearts of female spontaneously hypertensive rats (SHR) with respect to E2.
Relative expression levels of the respective cardiac mRNA obtained from sham-operated, ovariectomized and ovariectomized E2-substituted SHR were quantified by real-time PCR. Ovariectomy led to a significant upregulation of the ETB-R mRNA (2.6±0.8-fold) in the left ventricular myocardium, which was not attendant with an alteration of ETA-R, ECE and ppET-1 mRNA expression.
An upregulation of the relative expression level of ETB-R protein due to ovariectomy was also demonstrated by radioligand binding assay.
Upregulation of both ETB-R mRNA and ETB-R protein expression was completely inhibited by E2 replacement.
To confirm these results in in vitro experiments, we quantified the mRNA of ET-R subtypes from isolated cardiomyocytes in the presence and absence of E2 (10−8 M, 24 h). Our data showed a markedly downregulated level of ETB-R mRNA in cardiomyocytes stimulated with E2. ETB-R downregulation was not attendant with the alteration of ETA-R, ECE and ppET-1 mRNA expression.
Taken together, these data demonstrate that estrogen regulates the expression of ETB-R in rat ventricular myocardium in vivo and in vitro. These observations may help to understand gender-based differences found in cardiovascular disease.
Keywords: Endothelin, endothelin receptor A and B, estrogen, cardiomyocytes, real-time PCR
Introduction
Endothelin-1 (ET-1), a 21-amino-acid peptide, was originally isolated from porcine aortic endothelial cells in 1988 and is the most potent vasoconstrictive peptide identified to date (Yanagisawa et al., 1988). Three structurally and pharmacologically distinct endothelin isopeptides are known, ET-1, ET-2 and ET-3. Each is encoded by a different gene (Inoue et al., 1989). ET-1 appears to be the predominant and most relevant isoform in humans (Saetrum Opgaard et al., 2000). It became clear that ET-1 also exerts important cardiac effects. These include positive inotropic action (Ishikawa et al., 1988) and growth-promoting properties inducing hypertrophy (Ito et al., 1991). Thus, ET-1 might contribute considerably to the development of cardiac hypertrophy and cardiac disease.
Mammalian ET-1 gene contains five exons that encode prepro-endothelin (ppET-1). ppET-1 is cleaved to form big ET-1, which is further cleaved by the endothelin-converting enzyme (ECE), resulting in the 21-amino-acid active peptide (Xu et al., 1994). Local ET-1 synthesis in the heart has been demonstrated (Suzuki et al., 1993). ET-1 is secreted by myo- and endocardium, thereby acting in a paracrine and autocrine fashion on cardiomyocytes (Suzuki et al., 1993). The physiological effects of ET-1 are mediated by at least two subtypes of ET receptors (ET-R), designated as the ETA (ETA-R) and ETB (ETB-R) receptors. Both ETA-R and ETB-R coexist in human and rat hearts (Molenaar et al., 1993). The different regulation of these proteins in myocardium under physiological and pathophysiological conditions is poorly understood. Recent observations suggest that there is a gender-based difference in ET-R-mediated changes of cardiovascular hemodynamics (Tatchum-Talom et al., 2000). However, it remains to be evaluated whether estrogen affects the progression of hypertensive heart disease via the endothelin system.
In the present study, we examined the status of ET-1, ppET-1, ECE and ET-R in the cardiac tissue of SHR, a well-established animal model for hypertension, and in isolated cardiomyocytes, and the influence of E2 on the endothelin system in both models.
Methods
Animals
Female spontaneously hypertensive rats (SHR, Harlan Winkelmann, Borchen, Germany) were ovariectomized or sham operated 13 weeks after birth. All treatment groups consisted of 10 animals each. Ovariectomized animals were E2 supplemented by subcutaneous pellets inserted into the dorsal neck region under local anesthesia. To achieve typical physiological E2 levels found in midcycle, a 60-day release pellet containing 1.7 mg E2 (Innovative Research, Sarasota, FL, U.S.A.) was used. All animal experiments were performed in accordance with the German animal protection law and after approval by the Animal Welfare Committee at the University of Maastricht, The Netherlands.
Blood pressure measurement and quantitative determination of E2 plasma level
At 5 weeks after surgery, rats were anesthetized (ketamine 100 mg kg−1 body weight i.p. and xylazine 5 mg kg−1 body weight i.p.) and a stretched PE catheter was inserted into the femoral artery and exteriorized at the neck. Animals were allowed to recover for 48 h before blood pressure measurements were performed in conscious animals. Thereafter, animals were anesthetized as described above, killed by decapitation and the hearts were excised. Blood samples were withdrawn from the vena cava inferior and the concentration of E2 was determined using a radioimmunoassay kit (DPC Biermann, Bad Nauheim, Germany). The left ventricular tissue was rapidly frozen in liquid nitrogen and stored at −80°C.
Isolation, culture and treatment of neonatal cardiomyocytes
The hearts of 1–2-day-old rats (Wistar-Kyoto) were isolated and digested with 10 ml of Spinner solution (116- mM NaCl, 5.3 -mM KCl, 8 -mM NaH2PO4, 22.6 -mM NaHCO3, 10 -mM HEPES, 5 -mM D-Glucose, pH 7.4) containing 0.1% (w v−1) collagenase (Cytogen; Berlin, Germany) for 10 min at 37°C in eight consecutive steps as previously described (Nuedling et al., 2001). Cell suspension was centrifuged at 400 × g for 5 min and the cell pellet was resuspended in 20 ml of Hams' F10 supplemented with 10% horse serum (Biochrom; Berlin, Germany) and 10% estrogen-free FCS (Biochrom KG, Berlin, Germany) and plated on culture dishes. After 75 min, the medium that contained the cardiomyocyte fraction of the digested tissue was removed. Cardiomyocytes were counted in a Fuchs–Rosenthal chamber and seeded on culture dishes at a density of 2 × 104 cells cm−2 in phenol red-free DMEM. Serum-starved cells were treated with 10−8 M E2 for 24 h. Control cells were incubated with 0.1% (v v−1) ethanol, the solvent of E2.
Real-time PCR
Total RNA was isolated from 10 left ventricles of each treatment group using the guanidine thiocyanate and phenol/chloroform extraction method (Trizol reagent, Life Technologies, Karlsruhe, Germany). RNA was reconstituted in 20 μl diethylcarbonate-treated water and then stored at −80°C. The quality of the RNA was verified by agarose gel electrophoresis and quantified spectrophotometrically. Total isolated RNA (2 μg) was reverse transcribed to first-strand cDNA using random primers of the Superscript™ preamplication system (Life Technologies).
Primers and probes were chosen with the assistance of the computer software Primer Express (PE Applied Biosystems, Weiterstadt, Germany), as shown in Table 1 . Both were synthesized by PE Applied Biosystems. Probes were covalently labeled with a reporter dye (6-carboxy-fluoresin) at the 5′ end and a quencher dye (5-carboxytetramethylrhodamine) at the 3′ end. To prevent probe extension, phosphate groups were attached to the 3′ ends of all probes.
Table 1.
Oligonucleotide sequences used for real-time PCR amplification
| Gene | Oligonucleotide | Sequence | PCR product size (bp) |
|---|---|---|---|
| 18S RNA | Forward primer | ACGGCTACCACATCCAAGGA | |
| Reverse primer | AAGGATTTAAAGTGGACTCATTCCA | 100 | |
| Probe | CGCGCAAATTACCCACTCCCGA | ||
| ETA-R | Forward primer | CAGCCACAAGGACAGCATGA | |
| Reverse primer | TGCTGCGTGACCGTTTCA | 86 | |
| Probe | CCGAGCAGTGTGCCTTCGAGTCC | ||
| ETB-R | Forward primer | CCTGCGGAGGTGACCAAA | |
| Reverse primer | GGCATGATACAATCGTGTTGATG | 124 | |
| Probe | AGTCCCGCCAAGATCCTTCCCTCC | ||
| ECE | Forward primer | GAGAAGCGCCGGGATGA | |
| Reverse primer | GGCATTCAGAAAGGGTAACCAG | 99 | |
| Probe | AGCTCATCTACCACAAAGTCACGGCTGC | ||
| ppET-1 | Forward primer | TTGATGGACAAGGAGTGTGTCTACTT | |
| Reverse primer | TTCCTAGTCCATACGGGACGAC | 88 | |
| Probe | CCACCTGGACATCATCTGGGTCAACA |
Real-time PCR was performed according to the TaqMan™ protocol using the ABI Prism 7700 Sequence Detector (PE Applied Biosystems). The PCR reaction mixture (50 μl) for single measurements contained 25 μl of 2 × TaqMan Universal PCR Master Mix, 50–900 nM of each primer (each primer concentration was optimized giving a maximum normalized reporter signal), 200 nM of probe and 5 μl of sample cDNA. The thermal cycling conditions comprised two initial steps at 50°C for 2 min required for optimal AmpErase uracil-N-glycosylase enzyme activity and 95°C for 10 min required to activate AmpliTaq Gold DNA polymerase, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min.
The threshold cycle or CT value at which a statistically significant increase in signal, associated with an exponential growth of PCR product, is first detected, provided the quantitative values that represent the mRNA of the genes of interest (using PE Applied Biosystems analysis software Sequence Detector version 1.7). Standard curves for all target genes and the endogenous reference gene (18S-rRNA) to be amplified were made by using six four-fold dilutions of cDNA. Relative amounts of transcripts were calculated by comparison with standard curves. Experiments were performed in triplicate for each data point. Data were normalized on the basis of its 18S-RNA content. Final results, expressed as N-fold differences in target gene expression relative to reference (18S-rRNA), and calibrator, termed NX, were determined as follows: NX=2(ΔCTtarget−ΔCTcalibrator).
Radioligand binding
Left ventricular tissue was placed in 5 ml ice-cold assay buffer (Tris/HCl 50 mM, Tris 0.01 mM, NaCl 137 mM, CaCl2 1.8 mM, MgCl 1.1 mM, N-acetyl-methionin 1 mM, aprotinine 1 μM, pH 7.4) containing 250- mM sucrose and homogenized. The homogenates were centrifuged at 1000 × g for 10 min at 4°C. The supernatants were recentrifuged at 50,000 × g for 20 min. They were discarded and the pellets were resuspended in 3 ml assay buffer. The protein concentration was determined by Bio-Rad Bradford reagent (Bio-Rad Laboratories, Munich, Germany). The volume was adjusted to yield a protein concentration of 100 μg/400 μl and BSA was added to reach a final concentration of 0.3% BSA.
To assess the relative amount of ETA-R and ETB-R, displacement experiments were performed with the selective ETA-R antagonist BQ123 (10−7 M; Bachem Biochemica, Heidelberg, Germany), which was preincubated with the membrane fraction for 30 min at 21°C. 125J-ET binding was performed in polypropylene tubes in a total assay volume of 500 μl consisting of 400 μl membrane fraction (100 μg protein), 50 μl radioligand (2000 Ci mmol−1, Amersham Pharmacia, Braunschweig, Germany) and 50 μl unlabeled ET-1 (Bachem Biochemica) in different concentrations ranging from 10−11 to 10−9 M. Tubes were incubated for 3 h at 21°C in a shaking water bath. The bound ET-1 was separated by vacuum filtration over GF/C filters (Whatman, Maidstone, U.K.) that had been presoaked in 0.33% polyethyleneimine for 20 min. The filters were washed three times with 2 ml ice-cold NaCl (0.9%), and membrane-bound radioactivity was measured by a gamma counter (Gamma C12, Berthold, Bad Wilberg, Germany). To define the level of nonspecific binding, 10−7 M unlabeled ET-1 was used for maximal displacement of the radioligand. Specific binding was determined as the difference between the amount of total and nonspecific 125J-ET binding. Binding data were analyzed by a Kell program (Biosoft, Cambridge, U.K.).
Histology
In the hearts of five animals of each treatment group, collagen content was determined. Hearts of anesthetized rats were arrested in diastole by injection of 1 ml 100- mM CdCl2 into the vena cava. Then, the hearts were retrogradely perfused in situ with PBS at a pressure of 90 mmHg to remove blood components. Afterwards, the hearts were fixed by perfusion with 10% formalin in PBS for 10 min. The hearts were excised and kept for an additional 50 min in fixation solution. They were further processed for paraffin embedding. Cross-sections, 6 μm thick, were prepared from representative regions. The collagen fibers in the sections were stained with Sirius Red (0.1%; Sigma, Taufkirchen, Germany) for 90 min (Junqueira et al., 1979). The sections were visualized by a Leitz Ortholux Microscope with PL Fluotar × 16 and × 40 nosepieces (Leitz, Wetzlar, Germany). The collagen content was calculated as a fraction of the total area determined by means of a Quantimed 570 Image Analyzer (Leica Ltd, Cambridge, U.K.).
Results
Cardiac and circulatory parameters
E2 serum levels were lower in ovariectomized rats (5.87±1.69 pM) compared to sham-operated rats (130.7±46.6 pM). Ovariectomized E2-substituted rats were adequately replaced with E2, as serum levels were comparable to those in animals with intact ovaries (199.3±64.2 pM). The mean arterial pressure in sham-operated SHR amounted to 160±4 mmHg and ventricles weighed 882±27 mg. Both the parameters were not significantly different among the treatment groups.
Detections of protein expression may be influenced by a shift in cellular composition and/or an increase of the extracellular matrix of the tissue investigated. As a control, the collagen content in Sirius Red-stained sections was determined in the right, left ventricular myocardia and in the septum. The relative contribution of collagen in % was not significantly different between sham-operated (right ventricle: 8.0±2.2; left ventricle: 4.4±1.3; septum: 3.4±0.7), ovariectomized (right ventricle: 7.7±1.4; left ventricle: 4.5±0.7; septum: 3.4±0.7) and ovariectomized E2-replaced animals (right ventricle: 8.6±2.2; left ventricle: 4.4±2.3; septum: 3.1±0.4).
Effect of estrogen deficiency on ET-receptor mRNA expression
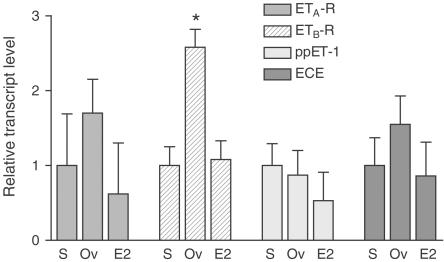
The relative expression levels of ET receptors, ppET-1 and ECE, were quantified in left ventricular tissues from 10 animals of each treatment group. After optimization of probe and primer concentration, we obtained a highly specific Real-time PCR assay for detection of the respective RNA target genes. The calibration curves for all transcripts showed linearity over the entire quantification range with correlation coefficients >0.98, indicating a precise log-linear relationship. The relative expression level was determined from the CT values obtained as ratios between the target gene and the reference gene to correct for variations in the amount of cDNA. Individual results for each target were standardized for each treatment group. The data are illustrated in Figure 1 and summarized in Table 2 .
Figure 1.
Relative expression of ETA-R, ETB-R, ppET-1 and ECE in the left ventricular tissue obtained from the three groups (S=sham, Ov=ovariectomized, E2=E2 substituted-ovariectomized). Expression levels for each target gene have been normalized to the mean expression of sham-operated rats taken as a value of 1. Expression level of ETB-R mRNA was significantly upregulated in estrogen-deficient rats, whereas there was no significant difference between the three groups concerning expression levels of ETA-R, ECE and ppET-1 (*P<0.005 vs S and vs E2, n=10 for each treatment group).
Table 2.
The relative expression of target genes in the left ventricle was quantified by real-time PCR and determined as the ratio of the target gene CT and the reference gene CT for each individual
| ETA-R | ETB-R | ppET-1 | ECE | |
|---|---|---|---|---|
| Sham | 8.01±0.48 | 9.95±0.44 | 12.07±1.29 | 6.97±0.99 |
| Ovariectomized | 7.25±1.42 | 8.58±0.88 | 12.26±0.42 | 6.34±0.94 |
| E2-substituted | 8.69±0.63 | 9.83±0.55 | 12.99±0.42 | 7.19±0.43 |
The mean CT-values±s.d. for each treatment group (n=10 for each group) are given.
The ETA-R appeared to be the predominant ET-R; the calculated average ratio of ETA-R and ETB-R mRNA was 3.8 : 1 in the left ventricular myocardium obtained from sham-operated rats. There was no significant difference in expression levels of ppET-1, ECE and ETA-R in the left ventricular tissue of the three treatment groups (Figure 1). In contrast, there was a highly significant upregulation (2.6-fold) of ETB-R in the left ventricular tissue of E2-deficient rats compared to sham-operated or E2-substituted animals.
Effect of estrogen deficiency on ET-R protein density
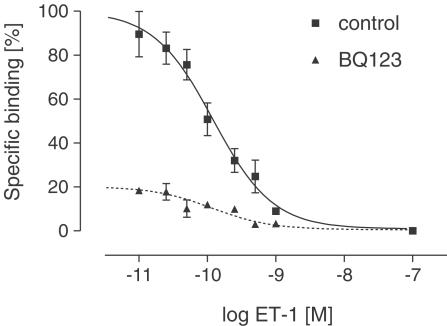
To confirm the estrogen-dependent regulation of gene expression studied by real-time PCR, we performed a receptor-binding assay. Addition of increasing concentrations of 125J-ET-1 to membrane preparations from the left ventricular myocardium resulted in specific, saturable high-affinity binding. Under our assay conditions, we obtained a specific binding that comprised 70–80% of the total signal. 125J-ET binding was displaced by ET-1 in a monophasic manner as shown in Figure 2. The mean ET-R density (Bmax) was 54±25 fmol mg−1 protein in sham-operated rats and revealed comparable values in E2-deficient and E2-substituted/ovariectomized rats. Relatively high s.e.m. resulted from individual differences between the animals within each treatment group and were <15% for each individual. The estimated Kd values were 47.33±18.85 pM (sham-operated) vs 70.40±23.94 pM (ovariectomized); however, these differences did not reach significance.
Figure 2.
Representative competition curves of binding of 125J-ET-1 to membrane preparations from the left ventricular tissue of SHR (here E2-substituted-ovariectomized SHR). Membranes were preincubated with the ETA-R antagonist BQ123 (10−7 M, dotted line) or vehicle (control) at 21°C for 30 min. 125J-ET-1 was competitively displaced by unlabeled ET-1. Each point represents the mean±s.e.m. from three experiments performed in triplicate.
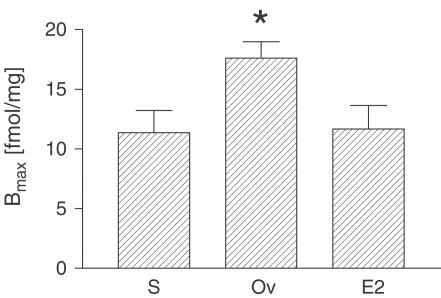
To discriminate between ETA-R and ETB-R, BQ123, a specific ETA antagonist was applied. Figure 2 shows the competition curves of membrane preparations preincubated with BQ123 (dotted line) compared to membrane preparations preincubated with vehicle. The maximum number of binding sites (mean Bmax value) after inhibition of ETA-R was 11.35±1.87 fmol mg−1 in sham-operated rats, suggesting that 21% of the receptor population is ETB-R protein. This receptor ratio was consistent with the results obtained from membrane preparations of ovariectomized E2-substituted rats (Bmax=11.67±1.97). In ovariectomized rats, Bmax was calculated as 17.61±1.37 fmol mg−1. These data indicate that ETB-R density was significantly increased in estrogen-deficient rats compared to sham-operated and estrogen-substituted animals (Figure 3).
Figure 3.
Density of ETB-R in the left ventricle of SHR. Bmax values derived from computer-assisted nonlinear regression of specific bound vs free data in the presence of ETA-R antagonist BQ123. Each column bar represents mean±s.e.m. of 10 animals per group (S=Sham, Ov=Ovariectomized, E2=E2 substituted-ovariectomized, *P<0.001).
Taken together, these results from radioligand binding and real-time PCR strongly suggest a regulation of ET-R subtypes in the myocardium by estrogen. Both mRNA level and protein level of the ETB-R in the left ventricle were significantly higher in rats with an estrogen deficiency.
Effect of estrogen on ET-R subtypes in isolated cardiomyocytes
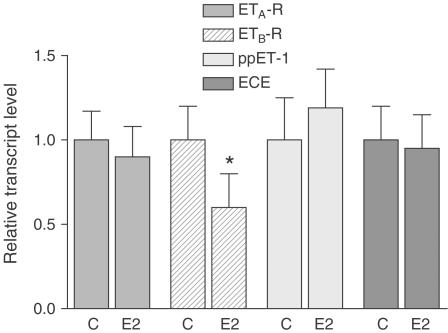
To further confirm the data revealed from the in vivo model, we investigated the direct effects of estrogen on ET-R subtypes, ppET-1 and ECE in cultured neonatal cardiomyocytes by real-time PCR. Expression of ET-R subtypes in cardiomyocytes from neonatal rats was quantified after incubation in the presence and absence of E2 (10−8 M, 24 h). CT values obtained as ratios between the target gene and the reference gene are shown in Table 3 . The ETA-R appeared to be the dominant ET-R in membrane preparations of neonatal cardiomyocytes and the ratio of ETA-R mRNA to ETB-R mRNA (29 : 1) was much higher than in membrane preparations of the left ventricular tissue. The relative expression of the RNA target genes obtained from three independent experiments is summarized in Figure 4. Our data show a downregulated level of ETB-R mRNA after incubation with E2 (40%), which was not attendant with the alteration of ETA-R, ECE and ppET-1 mRNA expression. Taken together, these data demonstrate that E2 downregulates the expression of ETB-R in rat ventricular myocardium in vivo and in vitro.
Table 3.
The relative expression of target genes in neonatal cardiomyocytes stimulated for 24 h with E2 (10−8 M) or vehicle (control) obtained by real-time PCR determined as ΔCT
| ETA-R | ETB-R | ppET-1 | ECE | |
|---|---|---|---|---|
| Control | 9.25±0.23 | 14.11±0.23 | 10.76±0.24 | 7.58±0.25 |
| E2-stimulated | 9.41±0.23 | 14.84±0.27 | 10.51±0.54 | 7.66±0.27 |
ΔCT were first obtained as ratios between the target gene CT and the reference gene 18S rRNA CT for each experiment to correct for variations in amount of cDNA. Thereafter, ΔCT were averaged for each treatment group (mean±s.d.).
Figure 4.
Relative expression of ETA-R, ETB-R, ppET-1 and ECE in neonatal cardiomyocytes in response to E2. Cells were stimulated for 24 h with E2 (10−8 M) or vehicle alone (C=control). PCR results were normalized for each target gene to control cells in such a manner that the mean expression of control equals a value of 1. The expression level of ETB-R mRNA was significantly downregulated due to E2 treatment, whereas there was no significant difference concerning expression levels of ETA-R, ECE and ppET-1. Results are mean±s.d. from three experiments (n=3, *P<0.005 vs control).
Discussion
In the present study, we show that the myocardium of SHR contains an intact armamentarium of all relevant proteins of the ET system including ppET-1, ECE, ETA-R and ETB-R. In the left ventricular tissue of SHR, ETA-R are prevalent compared to ETB-R, a finding consistent with studies in WKY rats (Kobayashi et al., 1999), SHR (Thibault et al., 1995) and human myocardium (Molenaar et al., 1993). E2 was found to decrease ETB-R density and mRNA abundance in the left ventricular myocardium of SHR. Incubation of neonatal cardiomyocytes by E2 for 24 h also reduced ETB-R mRNA expression compared to E2-deprived cells. To our knowledge, this is the first demonstration of modulation of the expression of ETB-R by E2 in cardiomyocytes in an in vivo model of arterial hypertension and in cultured cardiomyocytes.
In principle, regulation of the ET system is possible through different mechanisms (1) via transcriptional and/or translational regulation of ppET-1, (2) via change of the converting rate of ppET-1 into the physiological active form of ET-1, which is mediated by the endopeptidase ECE and (3) via transcriptional and/or translational regulation of ET-R. Results of this study suggest that E2 may not regulate the production of ET-1 in the myocardium at the level of gene expression, as no differences were observed in mRNA for ppET-1. There was no apparent regulation of the converting rate by change of ECE production, as mRNA levels of the ECE were not different between intact and sham-operated SHR. In accordance with this finding, E2 had no effect on ppET-1 and ECE expression in isolated cardiomyocytes. Interestingly, earlier studies demonstrate that E2 attenuates ET-1 expression and release in vascular endothelial cells (Akishita et al., 1998; Bilsel et al., 2000), the main source of circulating ET-1. Gender-related differences of the circulating concentration of ET-1 may therefore relate to inhibitory effects of E2 on the release of ET-1 from endothelial cells and smooth muscle cells, but not from cardiomyocytes.
Regulation of ETB-R has also been reported by angiotensin II, which is probably mediated by stimulation of angiotensin receptor type 1 (Kanno et al., 1993). E2-dependent regulation of ETB-R might be mediated by the estrogen receptor. Interestingly, the known structure of the estrogen response element is found in the 5′ upstream region of ETB-R gene (Cheng et al., 1993). Thus, it seems possible that regulation of ETB-R in cardiac tissue may be influenced by an estrogen receptor-dependent mechanism. This is consistent with the finding of Smith et al. (2000), who showed that in ovariectomized rats myocardial ischemia induced an increase in cardiac ETB-R mRNA, which was downregulated by E2-substitution.
To avoid interference with the pathological events present in SHR, additional experiments were carried out in isolated cells of WKY rats. Neonatal myocytes in culture are a valid model for the evaluation of regulatory effects of estrogen independent of any hemodynamic modifications (Nuedling et al., 1999a). In these cells, ETB-R was found to be expressed in low amounts. Despite the low basic expression level of ETB-R mRNA, the presence of E2 caused a further decrease, while ETA-R mRNA remained unchanged.
It has been shown that E2 regulates the expression of various proteins, which are critical for the development of cardiovascular diseases. Regulatory mechanisms in cardiomyocytes between E2 and NO synthases (Nuedling et al., 1999b), MAPK (Nuedling et al., 1999a), the angiotensin type I receptor (Nickenig et al., 1998) and the IGF-1 signalling pathway (Kahlert et al., 2000) have been described. Also, an attenuation of ET-1 synthesis by E2 in endothelial cells has been reported, as mentioned above (Akishita et al., 1998; Bilsel et al., 2000). Although the physiological role of ET-1 has not been fully established, there is increasing evidence of its involvement in pathological situations such as hypertension, myocardial infarction and congestive heart failure (Smith et al., 2000). Regulation of ETB by E2, which has been shown in the case of ischemia by Smith et al. (2000) and in the case of hypertension and in vitro here, represents another pathway by which E2 can influence cardiovascular diseases.
In isolated human cardiac muscle, ET-1 exerts positive inotropic action via both ETA-R and ETB-R (Saetrum Opgaard et al., 2000). However, the potential role of either ETA-R or ETB-R subtype in the development of cardiac hypertrophy and heart disease remains poorly understood. The growth-promoting effect of ET-1 in rat cardiomyocytes seems to be exclusively mediated by ETA-R stimulation (Hilal-Dandan et al., 1994). Several experimental studies have raised the possibility that ETA-R mediate myocardial and coronary activities, whereas ETB-R act in the local clearance of ET-1 (Fukuroda et al., 1994; Modesti et al., 1999). Recent studies in ETB-R knockout mice suggest that the ETA-R system plays an aggravating role in vascular remodeling after injury, whereas the ETB-R system plays a favorable inhibitory role, at least partly through NO release (Murakoshi et al., 2002). Asano et al. (2002) demonstrated an altered expression of endothelin receptors in failing human ventricles. Chronic left ventricular failure from either idiopathically dilated or ischemic cardiomyopathy was accompanied by a significant increase in ETA-R density and mRNA abundance, and a significant decrease in ETB-R density and mRNA abundance (Asano et al., 2002). However, the lowering of the ETB-R level could be either a mechanism to compensate ventricular failure or a mechanism contributing to the development of heart failure. As long as we do not understand the physiological consequences of ETB-R depression, it is impossible to validate the interaction of E2 with the endothelin signaling.
Taken together, the present study demonstrates a decrease of ETB-R density and mRNA by E2 in cardiomyocytes in vivo and in vitro, which may have important implications for the pathophysiology of the heart. Further studies are required to determine the physiological and clinical significance of an E2-depending alteration in ET-R expression in the heart.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft Gr729/8-1, Faculty Grant BONFOR O-708 and Dutch Heart Foundation.
Abbreviations
- E2
17β-estradiol
- ECE
endothelin-converting enzyme
- ET
endothelin
- ET-R
endothelin receptor
- ETA-R
endothelin receptor type A
- ETB-R
endothelin receptor type B
- FCS
fetal calf serum
- PCR
polymerase chain reaction
- ppET-1
prepro-endothelin 1
- SHR
spontaneously hypertensive rats
References
- AKISHITA M., KOZAKI K., ETO M., YOSHIZUMI M., ISCHIKAWA M., TOBA K., ORIMO H., OUCHI Y. Estrogen attenuates endothelin-1 production by bovine endothelial cells via estrogen receptor. Biochem. Biophys. Res. Commun. 1998;251:17–21. doi: 10.1006/bbrc.1998.9409. [DOI] [PubMed] [Google Scholar]
- ASANO K., BOHLMEYER T.J., WESTCOTT J.Y., ZISMAN L., KINUGAWA K., GOOD M., MINOBE W.A., RODEN R., WOLFEL E.E., LINDENFELD J., DAVID PORT J., PERRYMAN M.B., ClLEVEL J., LOWSS B.D., BRISTOW M.R. Altered expression of endothelin receptors in failing human left ventricles. J. Mol. Cell. Cardiol. 2002;34:833–846. doi: 10.1006/jmcc.2002.2022. [DOI] [PubMed] [Google Scholar]
- BILSEL A.S., MOINI H., TETIK E., AKSUNGAR F., KAYNAK B., ÖZER A. 17β-Estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc. Res. 2000;46:579–584. doi: 10.1016/s0008-6363(00)00046-8. [DOI] [PubMed] [Google Scholar]
- CHENG H.F., SU Y.M., YEH J.R., CHANG K.J. Alternative transcript of the nonselective-type endothelin receptor from rat brain. Mol. Pharmacol. 1993;44:533–538. [PubMed] [Google Scholar]
- FUKURODA T., FUJIKAWA T., OZAKI S., ISHIKAW K., YANO M., NISHIKIBE M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem. Biophys. Res. Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- HILAL-DANDAN R., MERCK S.T., LUJAN J.P., BRUNTON L.L. Coupling of the type A endothelin receptor to multiple responses in adult rat cardiomyocytes. Mol. Pharmacol. 1994;45:1183–1190. [PubMed] [Google Scholar]
- INOUE A., YANAGISAWA M., KIMURA S., KASUYA Y., MIYAUCHI T., GOTO K., MASAKI T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three different genes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKAWA T., YANAGISAWA M., KIMURA S., GOTO K., MASAKI T. Positive inotropic action of a novel vasoconstrictor peptide endothelin on guinea pig atria. Am. J. Physiol. 1988;255:H970–H973. doi: 10.1152/ajpheart.1988.255.4.H970. [DOI] [PubMed] [Google Scholar]
- ITO H., HIRATA Y., HIROE M., TSUJINO M., ADACHI S., TAKAMOTO T., NITTA M., TANIGUCHI K., MARUMO F. Endothelin-1 induces hypertrophy with enhanced expression of muscle-specific genes in cultured neonatal rat cardiomyocytes. Circ. Res. 1991;69:209–215. doi: 10.1161/01.res.69.1.209. [DOI] [PubMed] [Google Scholar]
- JUNQUEIRA L., BIGNOLAS G., BRENTANI R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- KAHLERT S., NUEDLING S., VAN EICKELS M., VETTER H., MEYER R., GROHÈ C. Estrogen receptor a rapidly activates the IGF-1 receptor pathway. J. Biol. Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- KANNO K., HIRATA Y., TSUJINO M., IMAI T., SHIRICHI M., ITO H., MARUMO F. Upregulation of ETB receptor subtype mRNA by angiotensin II in rat cardiomyocytes. Biochem. Biophys. Res. Commun. 1993;194:1282–1287. doi: 10.1006/bbrc.1993.1962. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., MIYAUCHI T., SAKAI S., KOBAYASHI M., YAMAGUCHI I., GOTO K., SUGISHITA Y. Expression of endothelin-1, ETA and ETB receptors, and ECE and distribution of endothelin-1 in failing rat heart. Am. J. Physiol. 1999;276:H1197–H1206. doi: 10.1152/ajpheart.1999.276.4.H1197. [DOI] [PubMed] [Google Scholar]
- MODESTI P.A., VANNI S., PANICCIA R., BANDINELLI B., BERTOLOZZI I., POLIDORI G., SANI G., SERNERI G.G.N. Characterization of endothelin-1 receptor subtypes in isolated human cardiomyocytes. J. Cardiovasc. Pharmacol. 1999;34:333–339. doi: 10.1097/00005344-199909000-00003. [DOI] [PubMed] [Google Scholar]
- MOLENAAR P., ÓREILLY G., SHARKEY A., KUC R.E., HARDING D.P., PLUMPTON C., GRESHAM G.A., DAVENPORT A.P. Characterization and localization of endothelin receptor subtypes in the human atrioventricular conducting system and myocardium. Circ. Res. 1993;72:526–538. doi: 10.1161/01.res.72.3.526. [DOI] [PubMed] [Google Scholar]
- MURAKOSHI N., MIYAUCHI T., KAKINUMA Y., OHUCHI T., GOTO K., YANAGISAWA M., YAMAGUCHI I. Vascular endothelin-B receptor system in vivo plays a favorable inhibitory role in vascular remodeling after injury revealed by endothelin-B receptor-knockout mice. Circulation. 2002;106:1991–1998. doi: 10.1161/01.cir.0000032004.56585.2a. [DOI] [PubMed] [Google Scholar]
- NICKENIG G., BAUMER A.T., GROHÈ C., KAHLERT S., STREHLOW K., ROSENKRANZ S., STABLEIN A., BECKERS F., SMITS J.F.M., DAEMEN M.J.A.P., VETTER H., BÖHM M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- NUEDLING S., KAHLERT S., LÖBBERT K., DOEVENDANS P.A.F.M., MEYER R., VETTER H., GROHÈ C. 17β-Estradiol stimulates expression of inducible and endothelial NO synthase in rat myocardium in-vivo and in-vitro. Cardiovasc. Res. 1999b;43:666–674. doi: 10.1016/s0008-6363(99)00093-0. [DOI] [PubMed] [Google Scholar]
- NUEDLING S., KAHLERT S., LÖBBERT K., MEYER R., VETTER H., GROHÈ C. Differential effects of 17β-estradiol on mitogen-activated protein kinase pathways in rat cardiomyocytes. FEBS Lett. 1999a;454:271–276. doi: 10.1016/s0014-5793(99)00816-9. [DOI] [PubMed] [Google Scholar]
- NUEDLING S., KARAS R.H., MENDELSOHN M.E., KATZENELLENBOGEN J.A., KATZENELLENBOGEN B.S., MEYER R., VETTER H., GROHÉ C. Activation of estrogen receptor β is a prerequisite for estrogen-dependent upregulation of NO synthases in cardiomyocytes. FEBS Lett. 2001;502:103–108. doi: 10.1016/s0014-5793(01)02675-8. [DOI] [PubMed] [Google Scholar]
- SAETRUM OPGAARD O., MÖLLER S., DE VRIES R., EDVINSSON L., SAXENA P.R. Positive inotropic responses mediated by endothelin ET (A) and ET (B) receptors in human myocardial trabeculae. Clin. Sci. 2000;99:161–168. doi: 10.1042/cs19990302. [DOI] [PubMed] [Google Scholar]
- SUZUKI T., KUMAZAKI T., MITSUI Y. Endothelin is produced and secreted by neonatal rat cardiac myocytes in vitro. Biochem. Biophys. Res. Commun. 1993;191:823–830. doi: 10.1006/bbrc.1993.1291. [DOI] [PubMed] [Google Scholar]
- SMITH P.J.W., ORNATZKY O., STEWART D.J., PICARD P., DAWOOD F., WEN W.-H., LIU P.P., WEBB D.J., MONGE J.C. Effects of estrogen replacement on infarct size, cardiac remodeling, and the endothelin system after myocardial infarction in ovariectomized rats. Circulation. 2000;102:2983–2989. doi: 10.1161/01.cir.102.24.2983. [DOI] [PubMed] [Google Scholar]
- TATCHUM-TALOM T., MARTEL M., LABRIE C., LABRIE F., MARETTE A. Gender differences in hemodynamic responses to endothelin-1. J. Cardiovasc. Pharmacol. 2000;36:102–104. doi: 10.1097/00005344-200036051-00033. [DOI] [PubMed] [Google Scholar]
- THIBAULT G., ARGUIN C., GARCIA R. Cardiac endothelin-1 content and receptor subtype in spontaneously hypertensive rats. J. Mol. Cell. Cardiol. 1995;27:2327–2336. doi: 10.1016/s0022-2828(95)91911-2. [DOI] [PubMed] [Google Scholar]
- XU D., EMOTO N., GIAID A. A membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKIi Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;3322:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]