Abstract
We investigated the effect of the cannabinoid CB1 receptor antagonist, SR 141716, on indomethacin-induced small intestine inflammation and Escherichia coli lipopolysaccharide (LPS)-induced plasma TNF-α (TNF) release in comparison to the cannabinoid CB2 receptor antagonist, SR 144528, in rodents.
In rats, indomethacin induced significant ulcer formation in the small intestine; this was accompanied by an increase in tissue TNF levels and myeloperoxidase (MPO) activity. SR 141716 prevented the ulcers and the rise in TNF levels (ID50 3.3, 0.4 mg kg−1, respectively) and MPO activity. SR 144528 prevented intestinal ulcers only.
The effect of SR 141716 against indomethacin-induced ulcers and increase of plasma TNF levels after LPS was also studied in wild-type and CB1 receptor knockout mice. Indomethacin induced intestinal ulcers in mice, but not tissue TNF production and MPO activity. SR 141716 reduced the ulcers to a similar extent in wild-type and CB1 receptor knockout mice. In rats and wild-type mice, but not in CB1 receptor knockout mice, SR 141716 inhibited the LPS-induced increase in plasma TNF levels.
These findings provide evidence that the indomethacin model of intestinal lesions differs in rat and mouse and support the existence of several mechanisms for the antiulcer activity of SR141716, the most important involving the inhibition of TNF production. The potent anti-inflammatory activity of SR141716 in rodents indicated its potential therapeutic interest in chronic immune-inflammatory diseases.
Keywords: Cannabinoids, CB1 receptor, TNF-α, SR141716, Rimonabant, gut, ulcers, indomethacin, cytokines, inflammation
Introduction
Cannabinoid agonists and antagonists display several pharmacological effects through interactions with at least two receptor subtypes, the cannabinoid CB1 and CB2 receptors (Howlett, 1995; Pertwee, 1997). The cannabinoid CB1 receptor subtype accounts for most of the central effects of marijuana and synthetic cannabinoid agonists. It is not only abundant in the central nervous system (Devane et al., 1988; Wilson & Nicoll, 2002), but is also present in several peripheral organs, including the gut, where it appears to modulate gastroprotection, gastrointestinal motility and secretion (Croci et al., 1998; Izzo et al., 2001; Mancinelli et al., 2001; Pertwee, 2001; Landi et al., 2002; Pinto et al., 2002).
In vitro functional evidence of prejunctional neuronal cannabinoid CB1 receptors in the human ileum longitudinal smooth muscle and colon has been provided (Croci et al., 1998; Manara et al., 2002). Cannabinoid CB1-binding sites (Pertwee, 1997) and immunoreactivity (Kulkarni-Narla & Brown, 2000) and cannabinoid CB1 transcripts have also been reported in peripheral tissues (Bouaboula et al., 1993; Galiegue et al., 1995). The cannabinoid CB2 receptor subtype is virtually absent from the brain, but largely distributed in the periphery and on the surface of immune cells, where it exerts important modulatory functions (Munro et al., 1993; Galiegue et al., 1995; Klein et al., 2000). There is substantial evidence that in different in vitro and in vivo inflammatory conditions, both endogenous and synthetic cannabinoid receptor agonists downregulate mast cells and granulocytes, and reduce cytokine release by acting mainly at cannabinoid CB2 receptors (Aloe et al., 1993; Mazzari et al., 1996; Richardson et al., 1998; Parolaro, 1999; Klein et al., 2000; Massi et al., 2000; Sacerdote et al., 2000; Smith et al., 2000,2001a; Conti et al., 2002). The importance of the cannabinoid CB1 receptor in immunological and inflammatory events is less clearly established than the cannabinoid CB2 receptor, in spite of the expression and presence of CB1 receptors on the surface of immune cells (Kaminski et al., 1992; Galiegue et al., 1995). SR 141716 (Rimonabant) is a potent and highly selective cannabinoid CB1 receptor antagonist with potential therapeutic properties in obesity (Le Fur et al., 2001); it is now widely used as a pharmacological tool to block cannabinoid CB1 receptors selectively in vitro and in vivo (Rinaldi-Carmona et al., 1994; Bouaboula et al., 1997).
The aim of this study is to investigate the role of cannabinoid CB1 receptors in inflammation and to characterize the actions of SR 141716 further in the indomethacin toxicity model in the gut. Indomethacin-induced small intestinal injury in the rat is a simple inflammatory model in which local tumor necrosis factor-α (TNF-α) tissue production is clearly involved in the early stage of the mechanism leading to the lesions (Bertrand et al., 1998; Konaka et al., 1999). We compared the effect of SR 141716 with that of the selective cannabinoid CB2 receptor antagonist, SR 144528 (Rinaldi-Carmona et al., 1998; Bouaboula et al., 1999) on indomethacin-induced intestinal ulcers, TNF-α production and myeloperoxidase (MPO) tissue activity in rats and mice. We also investigated the action of SR 141716 on plasma TNF-α levels after systemic administration of Escherichia coli lipopolysaccaride (LPS): (a) in rats, to see whether the inhibitory effect of SR141716 on local intestinal TNF-α production is not just a consequence, but rather one of the major steps in ulcer healing; (b) in wild-type and CB1-receptor knockout mice, to understand the role of cannabinoid CB1 receptors in the anti-inflammatory activity of SR141716.
A preliminary account of this study was presented at the 2002 Symposium on the cannabinoids (Croci et al., 2002).
Methods
Animals
Male Crl : CD BR rats weighing 210±30 g were purchased from Charles River, Italy. Male C57BL/6N,Crl BR mice (wild type) and cannabinoid CB1 receptor knockout mice, weighing 28±3 g, were bred at the Sanofi-Synthelabo animal care facility (Montpellier, France), according to the method of Robbe et al. (2002). All the animals were treated according to internationally accepted principles for care of laboratory animals (E.E.C. Council Directive 86/609, OJ L358, 1, December 12, 1987). The experimental protocol was approved by the local Animal Care and Use Committee of Sanofi-Synthelabo Recherche. Animals were housed under controlled environmental conditions (22±1°C, 55±15% relative humidity, 12 h light from 630 to 1830 h) for at least 7 days before the experiment and had free access to filtered tap water and pellet diet (4RF 21, Mucedola, Italy).
Indomethacin-induced small intestine ulcers in rats and mice
Small intestine ulcers were induced by indomethacin according to the method of Bertrand et al. (1998) in rats and of Ettarh & Carr (1993) in mice, with minor modifications.
Overnight fasted rats, with water ad libitum, were treated subcutaneously with 20 mg kg−1 of indomethacin or vehicle (3% NaHCO3 in distilled water) and euthanized by cervical dislocation 24 h later. Food was made available only 6 h after indomethacin treatment, just after oral treatment with SR141716 (0.3–30 mg kg−1), SR144528 (0.3–30 mg kg−1) or the vehicle (2 ml kg−1; 9% Tween 80 in ultrapure water).
To study compound interaction, SR 144528 (1, 3, 10 mg kg−1) and SR141716 (3 mg kg−1) were given orally, either alone or together, 6 h after indomethacin (20 mg kg−1 s.c.), and rats were killed 24 h later.
Mice, with water ad libitum, were treated subcutaneously once a day for two consecutive days, with 100 mg kg−1 of indomethacin or vehicle (10 ml kg−1, body weight). Oral SR141716 (10 mg kg−1 day−1) was given 6 h after each dose of indomethacin. Food was withdrawn after the last dose of SR141716. At 24 h after the last indomethacin injection, the animals were euthanized by cervical dislocation, the small intestine was excised, from the ileo-cecal valve to the pylorus, rinsed with saline, opened along its length and stretched over a filter paper for identifying and counting ulcers.
A piece of small intestine in the area reflecting the severity of ulceration of each rat or mouse was dissected (about 300 mg) and immediately cooled on dry ice for MPO and TNF-α assays.
Intestinal transit in mice
Small intestine transit was assessed 20 min after a standard charcoal meal (distilled water suspension containing 10% gum arabic, 10% vegetable charcoal and 20% starch) given by gavage (0.1 ml per 10 g body weight). Mice were euthanized by cervical dislocation and the percentage of small intestine traversed by the front of the test meal was measured. (+)-WIN 55212-2, dissolved in 10% propylene glycol acid solution, was injected i.p. in a volume of 10 ml kg−1 immediately before the test meal. SR141716, dissolved in 9% Tween 80 in ultrapure water, was given orally 60 min before the agonist, in a volume of 10 ml kg−1.
LPS treatment and TNF-α assay in the small intestine or plasma of rats and mice
A submaximal effective dose of lipopolysaccharide from E. coli (dissolved in sterile water) was given either i.v. in rats (1 mg kg−1 ml−1, body weight) or i.p. in mice (10 mg kg−1 10 ml−1) 120 min after oral SR141716, SR144528 or vehicle (9% Tween 80 in ultrapure water). Blood was drawn by cardiac puncture 90 min after LPS injection in animals anesthetized with 60 mg kg−1, i.p. of pentobarbital. Blood was collected in tubes containing approximately 1/10 (v v−1) of 0.106 M sodium citrate. Plasma was obtained by centrifugating blood at 1400 × g for 20 min, and was then stored at −20°C until TNF-α assay.
The frozen intestinal samples were first homogenized in 20 vol of 0.05 M phosphate buffer (pH 6) in a Polytron homogenizer (24,000 r.p.m. for 40 s) and centrifuged at 10,000 × g. The supernatants were kept frozen (−20°C) until TNF-α assay. TNF-α protein in the plasma and small intestine of rats and mice was measured with ELISA kits specific for rat or mouse TNF-α (BIOTRAK, Amersham Pharmacia Biotech).
MPO activity in small intestine samples
MPO activity, as an index of leukocyte infiltration into the intestinal mucosa, was measured following the method of Bradley et al. (1982), with minor modifications. The frozen intestinal sample was first homogenized in 20 vol of 0.05 M phosphate buffer (pH 6) in a Polytron homogenizer (24,000 r.p.m. for 40 s). After taking two 0.5 ml aliquots for the protein and TNF-α assays, the homogenate was centrifuged at 40,000 × g for 20 min at 4°C in an L-70 Beckman ultracentrifuge. The pellet was suspended in hexadecyl-trimethylammonium (0.5% in 0.05 M phosphate buffer) and again homogenized (24,000 r.p.m. for 20 s), and then sonicated for 20 s. An aliquot of this homogenate was frozen at −80°C, and then subjected to three alternating freezing–thawing cycles and finally centrifuged at 18,000 × g for 16 min. The supernatant was assayed spectrophotometrically for MPO activity.
The assay was carried out on a 96-well microplate at 4°C. A volume of 50 μl of each sample, or an equivalent volume of standard solution of horseradish peroxidase, was combined with 200 μl solution containing 26.32 μg of tetramethylbenzene and 22.5 nmol of H2O2 in 0.1 M acetate buffer and added to each well. The kinetics of absorbance at λ=630 nm was followed from 5 to 50 min in a spectrophotometer at 4°C. The reaction was stopped with 50 μl of 1 M H2SO4 at 50 min, and 10 min later a further reading was taken at λ=450 nm. For each sample, the change in absorbance (MPO activity) was expressed in microunits (μU) mg−1 of total protein, measured according to the method of Bradford (1976). The calculation of microunits was based on a standard curve of horseradish peroxidase in the same experimental conditions. One international unit (IU) of MPO activity degrades 1 μmol of peroxide per min.
Calculation and statistical analysis
Results are expressed as means±s.e.m. The ID50 with their 95% confidence limits were obtained using the four-parameter logistic model according to Ratkowsky & Reedy (1986). Adjustment was carried out by nonlinear regression analysis using the Levenberg–Marquandt algorithm in RS/1 software (B.B.N., Cambridge, MA, U.S.A.). The means were compared by a completely randomized one-way analysis of variance (ANOVA), followed by Duncan's test for multiple comparisons using RS/1 software. A probability of less than 0.05 was considered significant.
Chemicals
N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR 141716) and N-(1S)-[1,3,3-trimethylbicyclo(2.2.1)-hept-2-endoyl)-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3-carboxamide (SR 144528) were synthesized at Sanofi-Synthelabo Recherche, Montpellier, France. The following chemicals were purchased from the commercial sources indicated: Sigma-Aldrich S.r.l. (Milan, Italy): indomethacin, (+)-WIN 55,212-2 (R(+)-[2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate, E. coli LPS (lot.51K4115); Calbiochem-Novabiochem GmbH (Schwalbach, Germany): horseradish peroxidase, 230 U mg−1.
Results
Effects of SR 141716 and SR 144528 on intestinal ulcers, TNF-α production and MPO activity induced by indomethacin in the rat ileum
Indomethacin (20 mg kg−1 s.c.) induced the formation of 107±5 (mean±s.e.m., n=32) ulcers along the rat small intestine 24 h after the treatment. The lesions appeared as rounded or slightly elongated hemorrhagic lesions covering the entire small intestine. These were accompanied by an increase of tissue TNF-α levels and MPO activity; the basal values were: MPO 0.20±0.05 μU mg−1 protein, TNF-α 25±4 pg mg−1 protein, in the vehicle-treated rats; MPO 0.68±0.06 μU mg−1 protein, TNF-α 142±13 pg mg−1 protein, in indomethacin-treated rats.
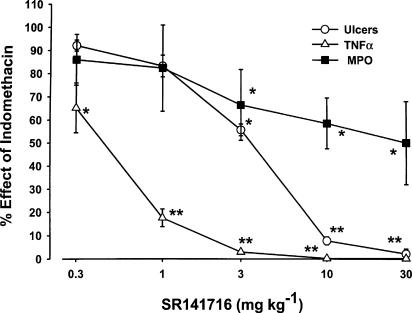
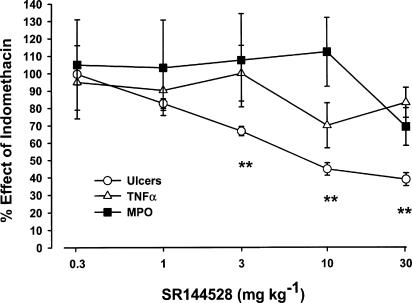
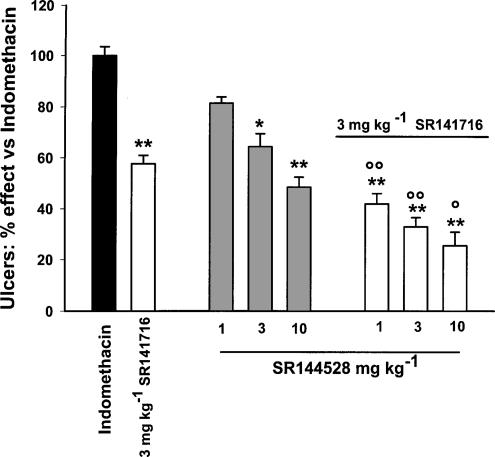
The effects of the cannabinoid CB1 and CB2 receptor antagonists SR 141716 and SR 144528 on indomethacin-induced ulcers, TNF-α levels and MPO activity in the rat small intestine are shown in Figure 1 and 2. The ID50 on the above parameters are reported in Table 1. Oral SR 141716 (0.3–30 mg kg−1 given 6 h after indomethacin) dose-dependently prevented the ulcers (ID50, 3.32 mg kg−1 with maximal effect at 10 mg kg−1) and produced a significant reduction of TNF-α levels (ID50 0.43 mg kg−1 with maximal effect at 3 mg kg−1). SR141716 also reduced intestinal MPO activity (50% inhibition at 30 mg kg−1). As shown in Figure 2 and Table 1, SR 144528 was less potent (ID50∼7 mg kg−1) than SR 141716 in preventing the ulcerogenic action of indomethacin (maximal inhibition: ∼60% at 30 mg kg−1). At doses up to 30 mg kg−1, it also failed to inhibit TNF-α and MPO activity. As shown in Figure 3, the antiulcer effect of a submaximal dose of SR 141716 was additive with that of submaximal doses of SR 144528.
Figure 1.
Effect of oral SR 141716 on ulcers, TNF-α production and MPO activity induced by indomethacin in the rat small intestine. SR 141716 (0.3–30 mg kg−1) was given 6 h after indomethacin (20 mg kg−1 s.c.) and rats were killed 24 h later. Results are expressed as mean %±s.e.m. of indomethacin effect (100%), from five independent experiments, each including at least five animals. The basal values were: number of ulcers 0, MPO 0.19±0.05 μU mg−1 protein, TNF-α 25±4 pg mg−1 protein, in the vehicle-treated rats; number of ulcers 104±4, MPO 0.70±0.06 μU mg−1 protein, TNF-α 142±13 pg mg−1 protein, in indomethacin-treated rats. *P<0.01, **P<0.05 vs control vehicle (Duncans's test).
Figure 2.
Effect of oral SR 144528 on ulcers, TNF-α production and MPO activity induced by indomethacin in the rat ileum. SR 144528 (0.3–30 mg kg−1) was given 6 h after indomethacin (20 mg kg−1 s.c.) and rats were killed 24 h later. Results are expressed as mean %±s.e.m. of indomethacin effect (100%) from at least 12 animals per dose. Basal values were: number of ulcers 0, MPO 0.21±0.06 μU mg−1 protein, TNF-α 25±4 pg mg−1 protein, in the vehicle-treated rats; number of ulcers 111±4, MPO 0.65±0.05 μU mg−1 protein, TNF-α 142±13 pg mg−1 protein, in indomethacin-treated rats. **P<0.01 vs control vehicle (Duncans's test).
Table 1.
Inhibition (ID50 mg kg−1, p.o.) of IND-induced ulcers, TNF-α production and myeloperoxidase (MPO) activity in the rat small intestine by SR 141716 and SR 144528
| Ulcers | TNF-α | MPO | |
|---|---|---|---|
| SR 141716 | 3.32 (2.84–3.95) | 0.43 (0.17–0.62) | ∼30a |
| SR 144528 | ∼7b; | >30 | >30 |
In parentheses, 95% confidence limits.
Maximal inhibition 50%.
Maximal inhibition 60%.
Figure 3.
Additive effects of SR 141716 and SR 144528 on indomethacin ulcer prevention in rats. SR 144528 (1, 3, 10 mg kg−1) and SR 141716 (3 mg kg−1) were given orally, either alone or together, 6 h after indomethacin (20 mg kg−1 s.c.) and rats were killed 24 h later. Results are expressed as mean %±s.e.m. of indomethacin effect (100%), from three independent experiments, each including at least five animals. *P<0.05, **P<0.01 vs indomethacin; ○P<0.05, ○○P<0.01 vs SR 144528 alone (Duncan's test).
Effects of SR 141716 and SR 144528 on E. coli LPS-induced increased levels of TNF-α in rat plasma
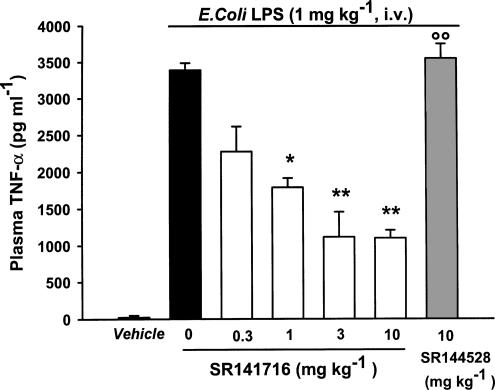
As shown in Figure 4, oral SR 141716 (0.3–10 mg kg−1) dose-dependently prevented the increase of TNF-α levels in rat plasma induced by LPS (1 mg kg−1 i.v.) with an ID50 value of 0.38 mg kg−1 (95% confidence limits 0.2–0.7). A peak effect was seen at a dose of 3 mg kg−1: (mean±s.e.m.) percentage inhibition 68±9. In contrast, oral SR 144528 (10 mg kg−1) had no effect on the increase in plasma TNF-α levels induced by the endotoxin (mean pg ml−1±s.e.m.): control vehicle 23±16; LPS 3388±97; LPS plus SR144528, 3350±201.
Figure 4.
Effect of oral SR 141716 and SR 144528 on LPS-induced increase of plasma TNF-α in rats. SR 141716 (0.3–10 mg kg−1) and SR 144528 (10 mg kg−1) were given orally 120 min before LPS or vehicle. Plasma was collected 90 min later. Results are mean±s.e.m. of at least six rats. ○○P<0.01 vs vehicle; *P<0.05, **P<0.01 vs LPS (Duncan's test).
Effects of SR 141716 on ulcers, TNF-α production and MPO activity induced by indomethacin in the small intestine of wild-type (+/+) and cannabinoid CB1 receptor knockout (−/−) mice
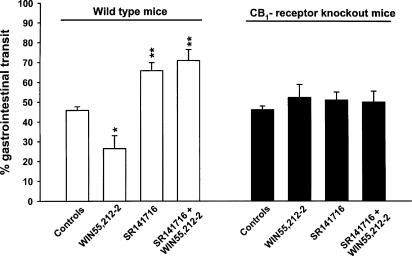
In a preliminary experiment, we verified whether CB1 receptor knockout mice totally lacked CB1 receptor-mediated functional responses. As shown in Figure 5, the knockout mice, unlike the wild type, were fully resistant to the inhibitory effect on gastrointestinal transit of the cannabinoid agonist (+)-WIN 55,212-2, as well as to SR141716's intrinsic effect, for example, stimulation of gastrointestinal transit.
Figure 5.
Effect of (+)-WIN 55,512-2 and SR 141716 on the intestinal propulsion of a charcoal meal in wild-type and CB1 receptor knockout mice. The values on the ordinate are the percentages of small intestine traversed by the charcoal meal during 20 min. SR 141716 (10 mg kg−1) was given orally, 60 min before (+)-WIN 55,512-2, which was injected (0.5 mg kg−1 i.p.) immediately before the charcoal meal. Results are mean±s.e.m. of at least six mice. *P<0.05, **P<0.01 vs vehicle (Duncan's test).
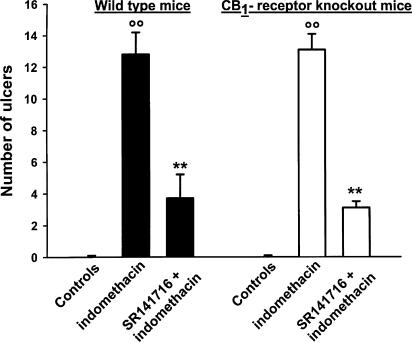
Mice were much less sensitive than rats to indomethacin as they had fewer, almost punctiform small intestinal ulcers more concentrated in the ileum, near the cecum. As shown in Figure 6, indomethacin (100 mg kg−1 s.c., once a day for two consecutive days) induced small intestinal ulcers to a similar extent in wild-type and CB1 receptor knockout mice; (mean number±s.e.m.): 12.8±1.5; 13.1±1.0. Oral SR 141716 (10 mg kg−1 day−1 for 2 days), given 6 h after the indomethacin dose, produced a similar inhibitory effect on the lesions in wild-type and knockout mice, mean % inhibition±s.e.m. being 71±4 and 76±6, respectively (Figure 6). However, indomethacin did not increase intestinal MPO activity and TNF-α production in both wild-type and CB1 receptor knockout mice; they were both below the limit of detection (10 pg mg−1 protein and 0.05 μU mg−1 protein) in vehicle- and indomethacin-injected mice.
Figure 6.
Prevention of indomethacin-induced intestinal ulcers by SR 141716 in wild-type and CB1 receptor knockout mice. Mice were given vehicle or indomethacin 100 mg kg−1 s.c. once a day for two consecutive days. SR 141716 (10 mg kg−1 day−1) was administered orally 6 h after each indomethacin injection. The ulcers were counted 24 h after the second dose of indomethacin. Results are mean±s.e.m. of at least 10 mice. **P<0.01 vs indomethacin (Duncan's test).
Effect of SR 141716 on LPS-induced increase of plasma TNF-α levels in wild-type (+/+) and CB1 receptor knockout (−/−) mice
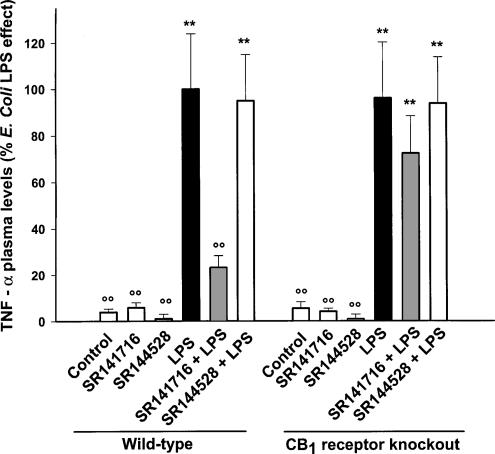
As shown in Figure 7, LPS (10 mg kg−1 i.p.) substantially raised plasma TNF-α levels similarly in wild-type and CB1 receptor knockout mice (mean pg ml−1±s.e.m.): wild-type mice, vehicle 18±7, LPS 483±99; CB1 receptor knockout mice, vehicle 23±9, LPS 465±100. Oral SR141716 (10 mg kg−1) significantly (P<0.01) antagonized the increase of plasma TNF-α levels induced by LPS in wild-type mice, but not in CB1 receptor knockout mice (mean % inhibition±s.e.m. 77±6 and 22±18). SR144528 (10 mg kg−1) had no effect in both wild-type and CB1 receptor knockout mice.
Figure 7.
Effect of oral SR 141716 and SR 144528 on TNF-α plasma levels increased by LPS in wild-type and CB1 receptor knockout mice. SR 141716 (10 mg kg−1) or SR 144528 (10 mg kg−1) was given orally 120 min before LPS (10 mg kg−1, i.p.). Mice were anesthetized and euthanized 90 min after LPS. Results are mean±s.e.m. of 8–11 mice from two independent experiments, calculated as a percentage of the mean wild-type value (100%). **P<0.01 vs vehicle, ○P<0.01 vs LPS (Duncan's test).
Discussion
We found that the highly potent and selective cannabinoid CB1 receptor antagonist SR 141716 dose-dependently prevented indomethacin-induced small intestinal ulcers in the rat; this effect was associated with a marked dose-related inhibition of tissue TNF-α levels and MPO activity. In previous in vivo and in vitro studies, SR 141716 has been used to demonstrate the role of peripheral cannabinoid CB1 receptors in gastrointestinal motility and propulsion (Croci et al., 1998; Izzo et al., 1999; Pertwee, 2001; Landi et al., 2002).
The prevention of indomethacin-induced intestinal injury in rats by relatively low oral doses indicates that this compound may interfere peripherally with the immune-inflammatory pathogenic mechanisms underlying indomethacin's ulcerogenic action. There is, in fact, substantial evidence that indomethacin and other nonsteroidal anti-inflammatory agents may paradoxically cause intestinal damage in rats by local tissue production and release of inflammatory cytokines and other components of the inflammation cascade, such as prostaglandins and nitric oxide (Bertrand et al., 1998; Konaka et al., 1999). Low doses of indomethacin are able to enhance TNF-α production in rat paw inflammed tissue and pleural exudate (Pinheiro & Calixto, 2002). Exogenous TNF-α injection has been reported to induce ileum inflammation with necrosis of villi and endothelial cell damage (Tracey et al., 1986; Remick et al., 1987). It is also worth noting that the systemic, but not central, administration of SR 141716 has been reported to improve rat survival and hypotension induced by LPS, through macrophage and platelet-derived endogenous cannabinoids acting on CB1 receptors (Varga et al., 1998). Indomethacin-induced small intestinal ulcers in rats may therefore serve as a good model to test the anti-inflammatory action of drugs with potential inhibitory action on TNF-α production and/or release.
In our experiments, the ulcerogenic action of indomethacin was accompanied by a six-fold increase of tissue levels of TNF-α and a three-fold increase of MPO activity, indicating an inflammatory reaction with recruitment and local participation of immune cells. SR 141716 was more potent in preventing TNF-α production (ID50, 0.4 mg kg−1) than ulcers (ID50, 3.3 mg kg−1), indicating that TNF-α synthesis and release, as suggested by Bertrand et al. (1998), may be key events in ulcer formation.
In the same experimental model, the selective cannabinoid CB2 antagonist, SR 144528 (Rinaldi-Carmona et al., 1998; Bouaboula et al., 1999) also prevented indomethacin lesions, but was less potent than SR141716. However, unlike SR 141716, it did not affect intestinal TNF-α and MPO activity, suggesting that these two antagonists have protective effects against indomethacin toxicity through different mechanisms. This conclusion is also supported by the fact that SR 141716 and SR 144528 have additive effects on ulcer healing. It is likely that SR 141716, unlike SR 144528, prevented indomethacin lesions mainly through its inhibition of tissue TNF-α production. In the indomethacin model of small intestinal lesions, the cannabinoid agonists, (+)-WIN 55, 212-2 and CP 55940, had no effects; they neither increased ulcers nor counteracted the curative action of SR141716 (data not shown). This result apparently seems to exclude the involvement of cannabinoid CB1 receptors on indomethacin-induced intestinal lesions. However, the nonselective agonist activity on cannabinoid receptors (CB1 vs CB2) and most probably their less-persistent effect relative to SR 141716 prevent us from drawing any conclusion.
In our study, SR 141716 not only prevented the intestinal TNF-α formation associated with indomethacin ulcers in rats, but also significantly reduced the plasma TNF-α increase in rats challenged with LPS, an established model for testing drugs active on the cytokines involved in inflammation and immunity. The cannabinoid CB2 antagonist SR 144528, unlike SR 141716, did not prevent the increase in TNF-α induced by the endotoxin. This further supports the above findings on indomethacin toxicity, where cannabinoid CB1, but not cannabinoid CB2 receptors, are involved in the effect on TNF-α levels. Smith et al. (2000) found that two synthetic nonselective cannabinoid agonists, (+)-WIN 55, 212-2 and HU-210, prevented the increases in serum TNF-α and IL-12 levels in mice challenged with an LPS injection, and that both agonists modulated LPS responses through the cannabinoid CB1 receptors. The selective cannabinoid CB1 receptor antagonist SR 141716 not only inhibited the agonist's action but also, surprisingly, at high doses, had an intrinsic activity partially modulating cytokine responses similar to the agonists; the author suggested that SR 141716 partial agonism at the cannabinoid CB1 receptor may account for such effects. However, the real role of the cannabinoid CB1 receptor in inflammatory conditions is still far from clear.
Therefore, in order to characterize the potential anti-inflammatory properties of SR 141716 better and to confirm the involvement of cannabinoid CB1 receptors, we compared its effects in indomethacin- or LPS-treated CB1-receptor knockout mice, in which the function of the gene encoding for the CB1 receptor is suppressed.
Cannabinoid CB1 receptor knockout mice show poor responses in a number of behavioral and physiological tests (Ledent et al., 1999; Zimmer et al., 1999), but little is known about their response in immune-inflammatory tests (Smith et al., 2001a,2001b). Mice are less sensitive than rats to the ulcerogenic action of indomethacin. In mice, 100 mg kg−1 indomethacin for two consecutive days was necessary to induce a minimal ulcer response compared to rats in which a significant response was obtained with a single dose of 20 mg kg−1 (mean number of ulcers: 107 in rats, 13 in mice). However, indomethacin lesions were similar in wild-type and CB1 receptor knockout mice. SR 141716 significantly reduced the number of lesions, to a similar extent in wild-type and CB1 knockout mice, suggesting that its antiulcerogenic action does not depend on its antagonism at cannabinoid CB1 receptors. However, indomethacin did not significantly increase intestinal TNF-α levels in mice, suggesting that their lower sensitivity than rats to indomethacin toxicity is linked to its reduced ability to increase local tissue TNF-α levels.
By contrast, LPS increased plasma TNF-α to similar measurable levels in wild-type and CB1 receptor knockout mice. As in rats, SR144528 had no effect on plasma TNF-α in either type of mice, while SR 141716 completely prevented the endotoxin-induced increase of plasma TNF-α levels in wild-type mice, but not in the knockout mice; these results indicate that the immune-inflammatory response to LPS challenge involves the cannabinoid CB1 receptor.
In conclusion, our study not only shows that the indomethacin model of intestinal lesions substantially differs in rats and mice, but also strongly supports the existence of two different mechanisms to explain the SR 141716 ulcer-preventing effect. The first is based on TNF-α inhibition, whereas the second is totally independent and may be similar to that activated by the CB2 receptor antagonist SR 144528 in the rat, only partially preventing indomethacin toxicity. SR 144528 has been reported to have anti-inflammatory properties in a mouse peritonitis model, apparently not through the cannabinoid CB1/CB2 receptors (Smith et al., 2001a,2001b). The existence of a new cannabinoid receptor subtype has been recently postulated in order to explain several non-CB1 – non-CB2 – receptor responses elicited by endogenous and synthetic cannabinoids (Howlett et al., 2002). The effects of SR 141716 on the immune system should be further investigated, using these and other established in vivo and in vitro models of inflammation and immunity in which the cannabinoid system and TNF-α are involved (Mazzari et al., 1996; Berdyshev et al., 1998; Ross et al., 2000; Sacerdote et al., 2000). Thus, SR 141716 and generally cannabinoid CB1 receptor antagonists could be potential new anti-inflammatory therapeutic agents to be used in acute and chronic diseases such as arthritis, pulmonary inflammation and Crohn's disease, as well as other pathologies in which inflammation and cytokines play an important role. SR 141716 (Rimonabant) has also been shown to be effective in human obesity (Le Fur et al., 2001) and it is now under investigation on a large number of patients. The inhibition of TNF-α production by SR 141716 through the cannabinoid CB1 receptor is of particular interest, since plasma levels of this cytokine are generally high in obese subjects, and it is apparently involved in the regulation of glucose transport and insulin sensitivity (Hube & Hauner, 1999).
Acknowledgments
We thank Mrs Antonella Larovere, Mrs Cristina Cattaneo and Mrs Maria-Giovanna Marongiu for their technical support; Dr Robert Matyas for knockout mice preparation and Dr Alberto Bianchetti for his help in manuscript preparation.
Abbreviations
- LPS
Escherichia coli lipopolysaccharide
- MPO
myeloperoxidase
- TNF-α
tumor necrosis factor α
References
- ALOE L., LEON A., LEVI-MONTALCINI R. A proposed autacoid mechanism controlling mastocyte behavior. Agents Actions. 1993;39:C145–C147. doi: 10.1007/BF01972748. [DOI] [PubMed] [Google Scholar]
- BERDYSHEV E., BOICHOT E., CORBEL M., GERMAIN N., LAGENTE V. Effects of cannabinoid receptor ligands on LPS-induced pulmonary inflammation in mice. Life Sci. 1998;63:PL125–PL129. doi: 10.1016/s0024-3205(98)00324-5. [DOI] [PubMed] [Google Scholar]
- BERTRAND V., GUIMBAUD R., TULLIEZ M., MAUPRIVEZ C., SOGNI P., COUTURIER D., GIROUD J.P., CHAUSSADE S., CHAUVELOT-MOACHON L. Increase in tumor necrosis factor-α production linked to the toxicity of indomethacin for the rat small intestine. Br. J. Pharmacol. 1998;124:1385–1394. doi: 10.1038/sj.bjp.0701968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUABOULA M., DESNOYER N., CARAYON P., COMBES T., CASELLAS P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol. Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- BOUABOULA M., PERRACHON S., MILLIGAN L., CANAT X., RINALDI-CARMONA M., PORTIER M., BARTH F., CALANDRA B., PECCEU F., LUPKER J., MAFFRAND J.P., LE FUR G., CASELLAS P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor–ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- BOUABOULA M., RINALDI-CARMONA M., CARAYON P., CARILLON C., DELPECH B., SHIRE D., LE FUR G., CASELLAS P. Cannabinoid-receptor expression in human leukocytes. Eur. J. Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRADLEY P.P., CHRISTENSEN R.D., ROTHSTEIN G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–622. [PubMed] [Google Scholar]
- CONTI S., COSTA B., COLLEONI M., PAROLARO D., GIAGNONI G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002;135:181–187. doi: 10.1038/sj.bjp.0704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCI T., LANDI M., MARINI P., RINALDI-CARMONA M., MANARA L.Inhibition of TNF-α production by the cannabinoid CB1 receptor antagonist SR141716 may account for its prevention of indomethacin intestinal injury in rats 20021202002 Symposium on the Cannabinoids, Burlington Vermont, International Cannabinoid Research Society. p
- CROCI T., MANARA L., AUREGGI G., GUAGNINI F., RINALDI-CARMONA M., MAFFRAND J.P., LE FUR G., MUKENGE S., FERLA G. In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br. J. Pharmacol. 1998;125:1393–1395. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANE W.A., DYSARZ F.A., JOHNSON M.R., MELVIN L.S., HOWLETT A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- ETTARH R.R., CARR K.E. Structural and morphometric analysis of murine small intestine after indomethacin administration. Scand. J. Gastroenterol. 1993;28:795–802. doi: 10.3109/00365529309104012. [DOI] [PubMed] [Google Scholar]
- GALIEGUE S., MARY S., MARCHAND J., DUSSOSSOY D., CARRIERE D., CARAYON P., BOUABOULA M., SHIRE M., LE FUR G., CASELLAS P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C. Pharmacology of cannabinoid receptors. Annu. Rev. Pharmacol. Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.C., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- HUBE F., HAUNER H. The role of TNFalfa in human adipose tissue: prevention of weight gain at the expense of insulin resistance. Hormone Metab. Res. 1999;31:626–631. doi: 10.1055/s-2007-978810. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., FEZZA F., CAPASSO R., BISOGNO T., PINTO L., IUVONE T., ESPOSITO G., MASCOLO N., DI MARZO V., CAPASSO F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., BORRELLI F., CAPASSO F. Defaecation, intestinal fluid accumulation and motility in rodents: implications of cannabinoid CB1 receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1999;359:65–70. doi: 10.1007/pl00005325. [DOI] [PubMed] [Google Scholar]
- KAMINSKI N.E., ABOOD M.E., KESSLER F.K., MARTIN B.R., SCHATZ A.R. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol. Pharmacol. 1992;42:736–742. [PMC free article] [PubMed] [Google Scholar]
- KLEIN T.W., LANE B., NEWTON C.A., FRIEDMAN H. The cannabinoid system and cytokine network. Proc. Soc. Exp. Biol. Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- KONAKA A., NISHIJIMA M., TANAKA A., KUNIKATA T., KATO S., TAKEUCHI K. Nitric oxide, superoxide radicals and mast cells in pathogenesis of indomethacin-induced small intestinal lesions in rats. J. Physiol. Pharmacol. 1999;50:25–38. [PubMed] [Google Scholar]
- KULKARNI-NARLA A., BROWN D.R. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000;302:73–80. doi: 10.1007/s004410000261. [DOI] [PubMed] [Google Scholar]
- LANDI M., CROCI T., RINALDI-CARMONA M., MAFFRAND J.P., LE FUR G., MANARA L. Modulation of gastric emptying and gastrointestinal transit in rats through intestinal cannabinoid CB1 receptors. Eur. J. Pharmacol. 2002;450:77–83. doi: 10.1016/s0014-2999(02)02053-8. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETITET F., AUBERT J.F., BESLOT F., BOHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- LE FUR G., ARNONE M., RINALDI-CARMONA M., BARTH F., HESHMATI H.SR141716, a selective antagonist of CB1 receptors and obesity 2001101Symposium on the Cannabinoids, Burlington Vermont, International Cannabinoid Research Society. p
- MANARA L., CROCI T., GUAGNINI F., RINALDI-CARMONA M., MAFFRAND J.P., LE FUR G., MUKENGE S., FERLA G. Functional assessment of neuronal cannabinoid receptors in the muscular layers of human ileum and colon. Digest. Liver Dis. 2002;34:262–269. doi: 10.1016/s1590-8658(02)80146-3. [DOI] [PubMed] [Google Scholar]
- MANCINELLI R., FABRIZI A., DEL MONACO S., AZZENA G.B., VARGIU R., COLOMBO G.C., GESSA G.L. Inhibition of peristaltic activity by cannabinoids in the isolated distal colon of mouse. Life Sci. 2001;69:101–111. doi: 10.1016/s0024-3205(01)01110-9. [DOI] [PubMed] [Google Scholar]
- MASSI P., FUZIO D., VIGANO D., SACERDOTE P., PAROLARO D. Relative involvement of cannabinoid CB1 and CB2 receptors in the Ä9-tetrahydrocannabinol-induced inhibition of natural killer activity. Eur. J. Pharmacol. 2000;387:343–347. doi: 10.1016/s0014-2999(99)00860-2. [DOI] [PubMed] [Google Scholar]
- MAZZARI S., CANELLA R., PETRELLI L., MARCOLONGO G., LEON A. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- PAROLARO D. Presence and functional regulation of cannabinoid receptors in immune cells. Life Sci. 1999;65:637–644. doi: 10.1016/s0024-3205(99)00286-6. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINHEIRO R.M., CALIXTO J.B. Effect of the selective COX-2 inhibitors, celecoxib and rofecoxib in rat acute models of inflammation. Inflamm. Res. 2002;51:603–610. doi: 10.1007/pl00012435. [DOI] [PubMed] [Google Scholar]
- PINTO L., IZZO A.A., CASCIO M.G., BISOGNO T., HOSPODAR-SCOTT K., BROWN D.R., MASCOLO N., DI MARZO V., CAPASSO F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- RATKOWSKY D.A., REEDY T.J. Choosing near-linear parameters in the four-parameter logistic model for radioligand and related assays. Biometrics. 1986;42:575–582. [PubMed] [Google Scholar]
- REMICK D.G., KUNKEL R.G., LARRICK J.W., KUNKEL S.L. Acute in vivo effects of human recombinant tumor necrosis factor. Lab. Invest. 1987;56:583–590. [PubMed] [Google Scholar]
- RICHARDSON J.D., KILO S., HARGREAVES K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIERE J.C., LE FUR G. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.C., LE FUR G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- ROBBE D., KOPF M., REMAURY A., BOCKAERT J., MANZONI O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., PERTWEE R.G. Inhibition of nitric oxide production in RAW 264.7 macrophages by cannabinoids and palmitoylethanolamide. Eur. J. Pharmacol. 2000;401:121–130. doi: 10.1016/s0014-2999(00)00437-4. [DOI] [PubMed] [Google Scholar]
- SACERDOTE P., MASSI P., PANERAI A.E., PAROLARO D. In vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J. Neuroimmunol. 2000;109:155–163. doi: 10.1016/s0165-5728(00)00307-6. [DOI] [PubMed] [Google Scholar]
- SMITH S.R., DENHARDT G., TERMINELLI C. The anti-inflammatory activities of cannabinoid receptor ligands in mouse peritonitis models. Eur. J. Pharmacol. 2001a;432:107–119. doi: 10.1016/s0014-2999(01)01477-7. [DOI] [PubMed] [Google Scholar]
- SMITH S.R., TERMINELLI C., DENHARDT G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharmacol. Exp. Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- SMITH S.R., TERMINELLI C., DENHARDT G. Modulation of cytokine responses in Corynebacterium parvum-primed endotoxemic mice by centrally administered cannabinoid ligands. Eur. J. Pharmacol. 2001b;425:73–83. doi: 10.1016/s0014-2999(01)01142-6. [DOI] [PubMed] [Google Scholar]
- TRACEY K.J., BEUTLER B., LOWRY S.F., MERRYWEATHER J., WOLPE S., MILSARK I.W., HARIRI R.J., FAHEY T.J., III, ZENTELLA A., ALBERT J.D., SHIRE G.T., CERAMI A. Shock and tissue injury induced by the recombinant human cachetin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- VARGA K., WAGNER J.A., BRIDGEN D.T., KUNOS G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- WILSON R.I., NICOLL R.A. Endocannabinoid signaling in the brain. Science. 2002;296:668–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- ZIMMER A., ZIMMER A.M., HOHMANN A.G., HERKENHAM M., BONNER T.I. Increased mortality, hypoactivity and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]