Abstract
L-selectin, constitutively expressed by leukocytes, is involved in the initial binding of leukocytes to activated endothelium. Anti-inflammatory drugs like glucocorticoids can induce shedding of L-selectin, but the mechanism is still unknown. Annexin 1, a protein whose synthesis and externalization/secretion are induced during the inflammatory response, has been proposed as a mediator of the anti-inflammatory actions of glucocorticoids.
The monocytic cell line U-937 strongly expresses Annexin 1 after 24 h of phorbol 12-myristate 13-acetate (PMA, 1 nM) treatment and externalizes/releases the protein after additional 16 h of dexamethasone (1 μM) treatment.
This study investigated the possible regulation of cell surface L-selectin shedding by endogenous Annexin 1, and its role in glucocorticoid-induced L-selectin shedding in the U-937 cell line.
PMA- and dexamethasone treatment-induced L-selectin shedding was potentially mediated by Annexin 1, since neutralizing antibodies against Annexin 1 reduced dexamethasone- and Annexin 1-induced shedding.
Immunoprecipitation and binding assays provided support for the suggestion that this effect could be mediated by an interaction between externalized Annexin 1 and L-selectin. Such interaction involved the N-terminal domain of Annexin 1 and was calcium-dependent. Confocal microscopy studies demonstrated increased colocalization of Annexin 1 and L-selectin on the cell surface.
Overall, our study provides new insights into the potential role of endogenous ANXA1 as a mediator of dexamethasone-induced L-selectin shedding, which may contribute to the anti-inflammatory activity of glucocorticoids.
Keywords: Annexin 1, L-selectin, shedding, monocytes, inflammation
Introduction
Leukocyte recruitment to inflammatory sites is essential for generation of appropriate host defense responses. This is achieved through a sequence of interactions between selectins on leukocytes and activated endothelial cells, which are controlled by cytokines and chemoattractants, secreted from activated endothelial cells, connective tissue and invading microorganisms. This initial step rapidly activates cells with a concomitant shedding of L-selectin and an upregulation of Mac-1 (CD11b/CD18), which leads cells to adhere more firmly to the endothelial cell surface (Ebnet & Vestweber, 1999).
L-selectin, which mediates reversible adhesive interactions between endothelium, leukocytes and platelets, is a highly glycosylated leukocyte protein that is constitutively expressed on all classes of leukocytes, except a substantial population of memory T cells (Ebnet & Vestweber, 1999). This protein is concentrated at the tips of leukocyte microvilli, which has been suggested to facilitate leukocyte tethering by concentrating the receptor and increasing its avidity for endothelial ligands (Bruehl et al., 1996). Expression of L-selectin is regulated on the surface of leukocytes. In vitro stimulation with chemotactic factors, or other activating agents, leads to L-selectin shedding from neutrophils and monocytes (Kishimoto et al., 1989; 1990; Palecanda et al., 1992). This shedding is thought to be mediated by a metalloprotease on the leukocyte surface, named sheddase (Preece et al., 1996; Peschon et al., 1998). Inhibition of leukocyte accumulation in inflamed tissues is a well-known effect of anti-inflammatory corticosteroids in humans. Several studies have demonstrated that in vivo administration of dexamethasone caused nearly complete downregulation of L-selectin on blood neutrophils (Burton et al., 1995; O'Leary et al., 1996; Jilma et al., 1997) or human monocytes (Waisman et al., 1998).
Annexin 1 (ANXA1), a calcium-binding protein characterized by a C-terminal core structure, which binds calcium and phospholipids and an N-terminus domain (Raynal & Pollard, 1994), is particularly abundant in granulocytes and monocytes (Ernst et al., 1986; Perretti et al., 1996; 1999). ANXA1 protein is strongly upregulated by glucocorticoids, and mediates some of their anti-inflammatory properties (Blackwell et al., 1980; 1982; Solito et al., 1991; Goulding & Guyre, 1993a). Although glucocorticoids are widely used for the control of inflammatory diseases, the mechanisms by which the glucocorticoid-inducible protein ANXA1 may reduce leukocyte influx and inflammation are not completely understood. In experimental inflammation, neutrophil migration and accumulation are markedly reduced by dexamethasone-induced translocation of ANXA1 (Perretti & Flower, 1993; Perretti, 1998; Perretti et al., 1999) or by administration of the N-terminal peptide (Perretti et al., 1993a). Externalization of endogenous ANXA1 following neutrophil adhesion to endothelial monolayers in vitro or to venular endothelium in vivo, downregulates leukocyte transmigration (Perretti et al., 1996). Moreover, in vivo studies reported that mice treated with human recombinant ANXA1 or its mimetic peptide Ac (2–26) attenuate the degree of neutrophil adhesion and emigration, suggesting that ANXA1 may interfere during the steps occurring at the leukocyte–endothelium interface on the inflamed post capillary venule (Lim et al., 1998). Recently, we showed in vitro that ANXA1 may play a role as a feedback mechanism in this process in modulating monocyte firm adhesion to the endothelium through a direct interaction between ANXA1 and α4 integrin in the U-937 cell line (Solito et al., 2000).
In the present study, we have addressed the potential modulating role of endogenous ANXA1 on monocyte L-selectin cell-surface expression, by examining the effects of externalization of this molecule in U-937 cells. This immortalized cell line has been used in many studies in which the results are generally reported as being applicable to monocytes or macrophages (Hass et al., 1989). In addition to this, our choice was supported by three other specific reasons: first, U-937 cell line expresses increased amount of endogenous ANXA1 during cell differentiation by phorbol ester (24 h treatment), and subsequent glucocorticoid treatment for 16 h produced translocation of ANXA1 to the external cell surface and facilitated its release in the medium (Solito et al., 1991; Parente et al., 1992; De Caterina et al., 1993; Solito et al., 1993; 1994); second, we have demonstrated that ANXA1 modulates U-937 cell adhesive properties by interfering with α4β1 (CD49d/CD29) (Solito et al., 2000) for the same time course of PMA and dexamethasone treatment we used in this present study; and, third, we have recently shown U-937 cell migration in vivo in SCID mice (Perretti et al., 2002).
Data obtained in this study show that endogenous ANXA1 translocated on the membrane following dexamethasone treatment, colocalized with L-selectin and possibly contributed to its shedding through a protein/protein interaction.
Methods
Cell line
The monomyelocytic U-937 cell line was maintained in culture in RPMI 1640 supplemented with 10% FCS, 1% L-glutamine and 1% penicillin–streptomycin at 37°C with 5% CO2 atmosphere. U-937 cells resuspended at 1 × 106 cells ml−1 were cultured for 24 h in low concentration of serum (RPMI 1640 supplemented with 5% of FCS) before treatment.
Chymotrypsin experiments were performed on 1 × 106 U-937 cells/100 μl of RPMI 1640. Cells were treated with 1–200 U of chymotrypsin for 15 min at 37°C, and then used for immunoprecipitation or stained for flow cytometry analysis.
Flow cytometry analysis
Briefly, 1 × 106 cells were washed in cold-HEPES buffer (25 mM HEPES supplemented with 1% BSA, 1 mM CaCl2, 1 mM MgCl2) and incubated for 30 min on ice with specific primary antibodies for ANXA1 (rabbit polyclonal antibody, 1 : 1000), Dreg-56 FITC-conjugated antibody (1 : 100) or with isotype-matched control antibody. Cells were resuspended in 1 ml of cold-HEPES buffer, and cell viability was assessed by exclusion of propidium iodide (1 μg ml−1). Log fluorescence histograms were obtained from 10,000 viable cells for each sample, using a dual-laser FACStar+ cell sorter (Becton Dickinson, San Jose, CA, U.S.A.). Data acquisition and analysis were accomplished with CellQuest software, version 3.1 (Becton Dickinson) and expressed as relative fluorescence intensity (RFI).
Immunoprecipitation and Western blot analysis
Cells (107) were collected and lysed in 500 μl of a lysis buffer (10 mM Tris, pH 7.2, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2 and 1% Nonidet P-40) for 30 min on ice in the presence of protease inhibitors (100 mM PMSF, 1 μg ml−1 leupeptin, 1 μg ml−1 aprotinin, 1 μg ml−1 pepstatin). After centrifugation, supernatants were collected and cell lysates (500 μg) were preincubated with protein-A sepharose and rabbit preimmune serum for 1 h at 4°C. The recovered supernatants were incubated at 4°C overnight with protein-A sepharose and a specific polyclonal antibody directed against ANXA1 (1 : 200). After two washes in low salt (150 mM NaCl) and three washes in high salt (500 mM NaCl) buffers, IX Laemmli sample buffer was added to the pellets and boiled for 5 min. Immunoprecipitated proteins were separated on 12% polyacrylamide gels according to the method of Laemmli (1970), and electroblotted onto nitrocellulose membranes (Biorad, Hercules, CA, U.S.A.). Immunostaining was performed using a polyclonal ANXA1 antibody (1 : 1000) (Solito et al., 1998a), the monoclonal DREG-56 antibody (1 : 500), or their respective control antibody. The immunoreactive bands were detected with an anti-rabbit or mouse IgG-HRP secondary antibody (1 : 10,000; Sigma), and peroxidase reaction was developed using enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech, Buckinghamshire, U.K.).
ELISA for membrane-associated ANXA1 and free released ANXA1
Cell viability of nonstimulated, PMA or PMA+dexamethasone-treated cells was checked by the trypan blue exclusion test (found to be >95%). Supernatants were collected and cellular debris was removed by centrifugation at 2000 rpm for 15 min, followed by syringe filtration through a 0.2 μm filter. Media were three-fold concentrated through Amicon-Millipore concentrators (Amicon Inc, Beverly, MA, U.S.A). ANXA1 is a calcium-dependent phospholipid-binding protein; therefore, to take into account all the fractions of externalized ANXA1 present on the outer membrane, we used a calcium chelator to extract the protein. U-937 cells were washed for 10 min at room temperature in PBS supplemented with 1 mM EDTA and a cocktail of protease inhibitors (100 mM PMSF, 1 μg ml−1 leupeptin, 1 μg ml−1 aprotinin, 1 μg ml−1 pepstatin) and cell-free supernatants were collected. Both the extracellular media and the EDTA supernatants were then processed for ELISA. 96-well plates (Immulon 2HB from Dynex, Chantilly, VA, U.S.A.) were coated overnight at 4°C with 100 μl of human ANXA1 polyclonal antibody, at a concentration of 3 μg ml−1, and blocked in PBS–3% BSA for 2 h at room temperature with washing steps between each reaction with PBS–0.1% Tween 20. Concentrated supernatants were applied in 100 μl aliquots to the wells, and the presence of a soluble form of ANXA1 was detected using a monoclonal ANXA1 antibody (1 : 1000), followed by a goat anti-mouse biotinylated antibody (1 : 10,000). Staining was performed using SAP antibody (1 : 500) for 45 min and addition of 100 μl per well of a freshly prepared diethanolamine buffer (1% diethanolamine, 0.5 mM MgCl2, 0.02% NaN3, pH 9) with PNP-PO4 for detection. The optical density (OD) of the reaction mixture was read at 405 nm within an hour, using a plate spectrophotometer (Biorad, model 3550, Hercules, CA, U.S.A.). A standard curve was constructed using recombinant human ANXA1 over a range of 150–9400 ng ml−1.
Measurement of soluble L-selectin by ELISA
Plates (96-well) were coated overnight with 3 μg ml−1 of L-selectin polyclonal antibody, blocked for 2 h with PBS–3% BSA, and washed three times in PBS containing 0.1%. Tween 20. Supernatants from U-937 cells were collected and cellular debris was removed as previously described. 1 × supernatant (100 μl) was applied per well for 1 h at room temperature. The captured L-selectin was detected using the monoclonal DREG-56 antibody (1 : 500), followed by a goat anti-mouse biotinylated antibody (4 μg ml−1, Sigma). Staining was performed using streptavidin–alkaline phosphatase-conjugated antibody, as previously described.
Measurement of L-selectin binding to immobilized annexin proteins
The sandwich ELISA used to determine L-selectin binding to ANXA1 was performed in 96-well microtiter plates with extra-high binding capacity for proteins (Immulon 4HBX, Dynex, Chantilly, VA, U.S.A.). The ability of ANXA1 to bind directly to the plastic was assessed by plotting a standard curve with ANXA1 coated on the plastic, compared to the coated monoclonal antibody capturing increasing concentrations of ANXA1; in both cases, a specific polyclonal antibody detected the amount of ANXA1. Human recombinant proteins for ANXA1, ANXA5 and ANXA1–5 (N-terminal of ANXA1 (2–26) and core domain of ANXA5) were serially diluted in coating buffer (PBS, 1 mM Ca2+, 1 mM Mg2+) and immobilized overnight at 4°C. After the blocking step, concentrated murine Lec-IgM chimera was incubated at the indicated concentrations during 1 h at room temperature. Detection was performed using a goat anti-mouse IgM-biotinylated antibody incubated for 1 h at room temperature (1 : 500), and the detection procedure was performed as previously described.
Immunofluorescence and confocal imaging
U-937 cells were cultured for 24 h in low serum concentration (RPMI 1640-5% FBS) before stimulation. Cells were washed three times in ice-cold PBS buffer (1% albumin, 1 mM CaCl2, 1 mM MgCl2, 0.1% sodium azide) and labeled for 45 min on ice with either a polyclonal ANXA1 antibody (1 : 1000), a monoclonal DREG-56 antibody (1 : 500) or both. For negative controls, respective isotype-matched control antibody was used as primary antibody. An anti-rabbit phycoerythrine-labeled secondary antibody (1 : 100), an anti-mouse fluoresceinated secondary antibody (1 : 100) or the combination were applied for 30 min on ice. Samples were washed and fixed for 10 min with 2% paraformaldehyde at room temperature. Cells were finally mounted in Vectashield® and observed using a Bio-Rad MRC 1000 laser-scanning confocal microscope, a Zeiss Axiovert × 100 microscope equipped with a Zeiss × 100 Plan-Apochromat oil immersion objective for clearer localization. Data shown are representative of the average staining observed over 80–100 cells obtained from five independent experiments.
Reagents and antibodies
PMA, dexamethasone-21 phosphate (disodium salt, MW 516.4), chymotrypsin (EC 3.4.21.1, type VII) and propidium iodide were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). The recombinant human L-selectin/human IgM chimeric protein was provided by Dr S. Rosen (University of California at San Francisco), and was used as crude tissue culture supernatant of transiently transfected COS-7 cells (Bruehl et al., 2000). Recombinant protein ANXA1, ANXA5 or chimeric ANXA1-5 were produced and purified by FPLC, as reported previously (Lim et al., 1998). P-nitrophenyl phosphate, disodium salt (PNP-PO4) was obtained from Pierce (Rockford, IL, U.S.A.) and the Vectashield® mounting medium, from Vector Laboratories (Burligame, CA, U.S.A.).
A neutralizing selective monoclonal antibody (mAB1-A), a mouse IgG2a raised against human ANXA1, was obtained as previously described (Pepinsky et al., 1990). Its isotype-matched control antibody (mouse IgG2a), monoclonal L-selectin antibody (Dreg-56 antibody or FITC-conjugated), goat anti-mouse biotinylated antibody and streptavidin-alkaline phosphatase-conjugated (SAP) antibody were all purchased from Caltag (Burligame, CA, U.S.A.). Monoclonal ANXA1 antibody was obtained from Biogenesis (Brentwood, NH, U.S.A.), L-selectin polyclonal antibody (N-18) from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.) and rabbit or mouse secondary conjugated antibodies (PE or FITC) from Pierce.
Statistical analysis
Data are shown as mean±s.e.m. of n distinct observations. Statistical comparisons were analyzed with ANOVA followed by Tukey's post hoc test. In all cases, a threshold value of P⩽0.05 was considered as the minimum level of significance.
Results
PMA and dexamethasone treatment induces differential modulation of the cell-surface level of ANXA1 and externalization of the protein. These effects are concomitant with a decrease in L-selectin cell-surface expression
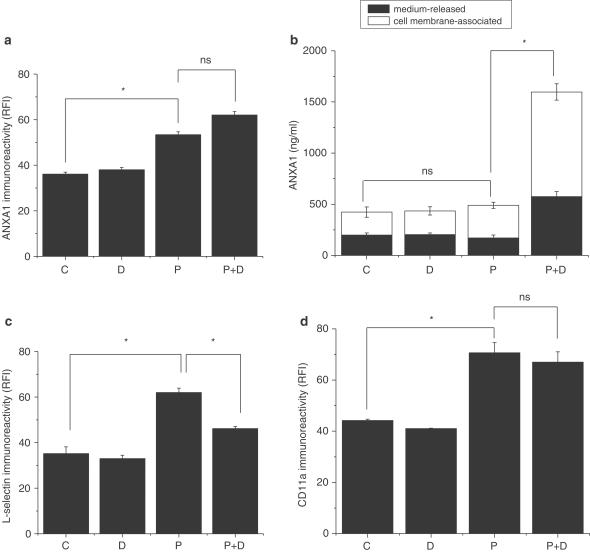
Incubation of U-937 cells with 1 nM PMA for 24 h and with 1 μM dexamethasone for an additional 16 h was used as a standard procedure to increase ANXA1 expression and externalization on the outer surface of the membrane, as previously reported (Solito et al., 1991; 1993; 1994; 2000; Parente et al., 1992; De Caterina et al., 1993). Figure 1a showed 50% increase of ANXA1 surface level above untreated control cells after PMA treatment, while dexamethasone alone (16 h incubation) failed to induce the protein. The combined treatment, PMA+dexamethasone, slightly increased the cell-surface expression of ANXA1, but not significantly compared to PMA-treated cells. These results are in line with previous findings (Solito et al., 1991; 1994; Kang et al., 1996; Willmott et al., 1997). Since PMA+dexamethasone treatment stimulates translocation/release of ANXA1 in differentiated U-937 cells, it was thus important to take into account the total amount of protein (membrane-associated and released) to correctly understand the importance of the phenomenon. ELISA assays were therefore performed on concentrated U-937 supernatants, as well as on EDTA wash for the indicated treatments (Figure 1b). The extracted levels of membrane-associated ANXA1 in the supernatant of untreated U-937 was equivalent to 200 ng ml−1, and was not significantly different from the amount detected in dexamethasone or PMA-treated cells. PMA+dexamethasone treatment increased the amount of ANXA1 released from differentiated U-937. The amount of cell membrane-associated ANXA1 that could be removed from the extracellular surface by EDTA wash was strongly increased after PMA+dexamethasone treatment, compared to unstimulated cells or cells stimulated with PMA or dexamethasone alone (Figure 1b, white bars). After PMA+dexamethasone treatment, ANXA1 level was equivalent to a concentration of 1.6 μg ml−1, as measured by ELISA. Figure 1a showed that PMA increased cell-surface ANXA1 expression, while the sum of the medium-released ANXA1 and the cell membrane-associated ANXA1 in PMA-treated cells was not different from control (Figure 1b). This apparent inconsistency could be explained by a calcium-independent interaction of ANXA1 with phospholipid monolayers that has already been reported (Sheets et al., 1987; Rosengarth et al., 1998). These data showed that a fraction of ANXA1 still remained present on the cell surface even after EDTA wash. Figure 1c represents flow cytometry analysis and showed that long-term incubation with PMA upregulated L-selectin expression on the cell surface (+77% increase compared to untreated control or dexamethasone-treated cells), while dexamethasone by itself did not modify L-selectin expression. On the contrary, PMA+dexamethasone treatment resulted in 27% decrease in the cell-surface L-selectin expression, as demonstrated by a reduction in the relative fluorescence intensity. In parallel, we examined the cell-surface expression of CD11a adhesion molecule. CD11a expression increased after PMA treatment, as L-selectin did, but it did not change after PMA+dexamethasone (Figure 1d), supporting the specificity of the relationship between ANXA1 and L-selectin. These findings showed a potential association between the effect of dexamethasone on PMA-enhanced cell-surface expression of ANXA1 and decreased expression of L-selectin.
Figure 1.
Effect of dexamethasone-induced ANXA1 expression and externalization/release on L-selectin cell-surface expression in differentiated U-937 cells. Nonstimulated U-937 cells (C) were treated with 1 nM PMA for 24 h (P) or with 1 μM dexamethasone for 16 h (D) or with the combination PMA (24 h)+dexamethasone (additional 16 h) (P+D). Control samples were incubated in RPMI+5% serum medium for the same period (24 h+16 h). Flow cytometry histograms show ANXA1 (a), L-selectin (c) and CD11a (d) immunoreactivities on U-937 cell surface after the indicated treatments. Data are shown as relative fluorescence intensity (RFI) and represent mean±s.e.m. of five independent experiments done in duplicate, *P⩽0.05. FITC-conjugated antibody alone was used as an internal control for background fluorescence, (b) Analysis of the amount of cell membrane-associated ANXA1 that was EDTA-extracted (white bars) or released in U-937 supernatants (black bars) from nonstimulated (C), PMA 24 h (P), dexamethasone 16 h (D) or PMA and dexamethasone (P+D)-treated cells. Cell-free supernatants were harvested from U-937 cells and concentrated three times before ELISA; EDTA-wash was used for the assay. Soluble and membrane-associated ANXA1 were captured with a polyclonal antibody anti-ANXA1, and detected with a monoclonal antibody as described in Methods. Soluble ANXA1 levels were quantitated by constructing a standard curve using human recombinant ANXA1 protein. Data represent mean±s.e.m. of three independent experiments done in quadruplicate, *P≤0.05.
Glucocorticoids-induced L-selectin shedding is mediated by ANXA1
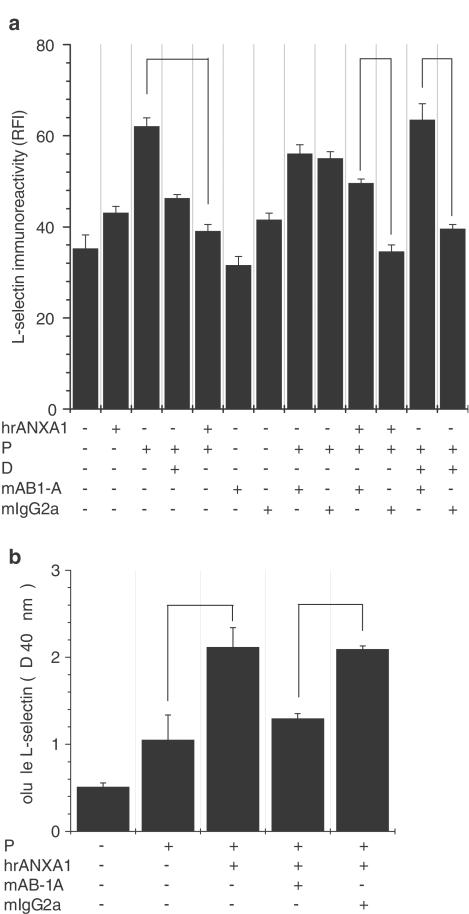
Since externalized/released ANXA1 after dexamethasone treatment was measured around 1.6 μg ml−1, U-937 cells were treated with a saturating concentration of recombinant human ANXA1 (5 μg ml−1) previously determined by Solito et al. (2000). Exogenous ANXA1 mimicked the effect of dexamethasone-induced ANXA1 on L-selectin cell-surface expression after PMA treatment (25.5% decrease in L-selectin shedding with dexamethasone and 37.1% decrease with hrANXA1, Figure 2a). ANXA1 and dexamethasone treatments were strongly reversed by the ANXA1-neutralizing monoclonal antibody (mAB1-A), whereas control mouse IgG (mIg2a) was without significant effect, suggesting a role for ANXA1 as a mediator of dexamethasone-induced shedding. mAB1-A incubation showed little effect on L-selectin cell-surface expression on control or PMA-treated cells. A similar observation was made for the U-937 cells treated with hrANXA1, mAB1-A or mIgG2a alone. To further confirm that the loss of fluorescence detected by flow cytometry resulted from shedding of L-selectin rather than internalization or capping of L-selectin by externalized ANXA1, we measured the amount of soluble L-selectin present in the medium by ELISA. L-selectin detected in the cell-free supernatant of U-937 cells treated with PMA was not significantly different from unstimulated cells (Figure 2b). In contrast, a higher amount of soluble L-selectin was found in the supernatant of U-937 cells treated with PMA+hrANXA1. As expected, ANXA1-neutralizing antibody prevented the ANXA1-induced L-selectin shedding.
Figure 2.
Exogenous human recombinant ANXA1 mimicked the endogenous protein by inducing L-selectin shedding. Both effects were reversed by a neutralizing antibody. Nonstimulated U-937 cells (C) were treated with 1 nM PMA for 24 h (P) or with 1 μM dexamethasone for 16 h (D) or with the combination PMA (24 h)+dexamethasone (additional 16 h) (P+D). Control samples were incubated in RPMI+5% serum medium for the same period (24 h+16 h), (a) The contribution of ANXA1 to the downregulation of L-selectin expression was confirmed by PMA treatment of U-937 cells for 24 h, followed by 30 min incubation at 37°C with 5 μg ml−1 of human recombinant ANXA1 (PMA+hrANXA1). Cells were treated with PMA for 24 h, then incubated with a specific anti-ANXA1 mAb at a concentration of 20 μg ml−1 (+mAB1-A) for 10 min, and finally incubated with either hrANXA1 for 30 min or dexamethasone for 16 h. A control antibody (+mIgG2a) was used at the same concentration and incubated for the same period of time. Data represent mean±s.e.m. of three independent experiments done in duplicate, *P⩽0.05. (b) ELISA analysis for soluble L-selectin in U-937 cell-free supernatants. Neutralizing anti-ANXA1 mAb (20 μg ml−1) or its isotype-matched control antibody (+mIgG2a) was incubated with PMA-treated cells 10 min before adding hrANXA1 for 30 min. Soluble L-selectin was trapped with a polyclonal L-selectin antibody and detected with a monoclonal L-selectin antibody (Dreg-56), as described in Methods. Data represent mean±s.e.m. of three independent experiments done in triplicate, P≤0.05.
Direct interaction between ANXA1 and L-selectin
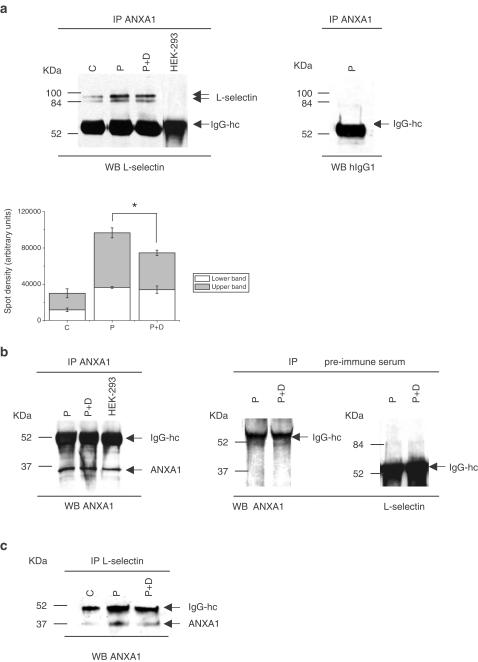
Crosslinking of the surface antigen has been reported to result in L-selectin shedding in human leukocytes (Palecanda et al., 1992). Therefore, we tested whether externalized ANXA1 would interact with the extracellular domain of L-selectin and triggers specific signaling events that lead to cleavage of the adhesion molecule. To test this hypothesis, U-937 lysates were incubated with specific antibodies directed against ANXA1. Immunoprecipitated proteins were transferred on nitrocellulose membrane and probed with L-selectin antibodies or with the isotype-matched control IgG1. Two different forms of L-selectin (around 84 kDa) were present and immunoprecipitated with ANXA1 (Figure 3a, left panel). Their intensity is enhanced after PMA treatment, while reduced after PMA+dexamethasone treatment. The densitometric analysis of the L-selectin immunoreactivity for each band detected by Western blot is illustrated in Figure 3a lower panel. The results showed that the level of L-selectin immunoreactivity in PMA-treated U-937 cells was strongly increased (by 230% compared to control cells). Dexamethasone treatment reduced the amount of L-selectin immunoreactivity by 33% compared to PMA-treated cells. The relative proportion between the upper and the lower bands in nontreated cells remains unchanged after PMA treatment (2 : 1), suggesting that the interaction, which is barely detectable in nontreated cells, is enhanced after stimulation. Control experiments performed on L-selectin-deficient cell line HEK-293 and on PMA-treated U-937 cells did not show either of these high molecular weight bands. ANXA1 immunoreactivity was similar after PMA or PMA+dexamethasone treatments, confirming the presence of equal amounts of ANXA1 in the immunoprecipitates. HEK-293 cells also expressed a high level of ANXA1 (Figure 3b, left panel). In the same experiment, we performed internal controls including immunoprecipitation with a rabbit preimmune serum followed by ANXA1 or L-selectin antibodies blotting (Figure 3b, right panel). Specificity of the interaction was confirmed by precipitating L-selectin from cell lysates and ANXA1 antibody used as affinity probe (IP: L-selectin antibody followed by WB: ANXA1 antibody, Figure 3c). A similar observation showed L-selectin and ANXA1 in the same complex.
Figure 3.
Coimmunoprecipitation experiments suggest an interaction between ANXA1 and L-selectin. (a) Immunoprecipitation analysis of ANXA1. Upper panel: total protein lysates from HEK-293 and nontreated (C), PMA (P) or PMA+dexamethasone (P+D)-treated U-937 cells were extracted, immunoprecipitated with specific antibodies for ANXA1 (IP) and immunoblotted for L-selectin or for its isotype-matched antibody, hIgG1 (WB). Lower panel: relative intensities of L-selectin immunolabeling in the samples. Bars indicate mean±s.e.m. of three independent experiments, *P⩽0.05. (b) Presence of ANXA1 protein in the immunoprecipitation was confirmed by reprobing the membrane with specific ANXA1 antibody. Internal control for the immunoprecipitation was demonstrated by using nonspecific control antibody (rabbit IgG), and blotting with ANXA1 and L-selectin-specific antibodies, (c) Specificity of the interaction is shown by reversion of the immunoprecipitation–immunoblot experiment in U-937-treated cells. All data shown are representative of three independent experiments giving similar results.
Chymotrypsin treatment decreases the interaction between Annexin 1 and L-selectin
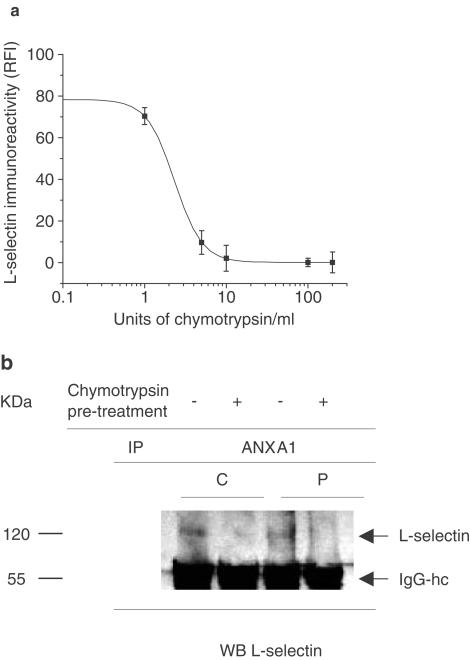
To confirm the identity of the immunoprecipitated bands (shown in Figure 3a) as L-selectin protein, we tested if chymotrypsin-induced loss of L-selectin would interfere in the interaction of L-selectin with ANXA1. Cells were treated with 1–200 U (1 × 106)−1 cells of chymotrypsin in 100 μl for 15 min at 37°C and then analyzed by flow cytometry for L-selectin immunoreactivity. A dose of 10 U (1 × 106)−1 cells caused an 80% reduction in L-selectin expression (Figure 4a). Pretreatment of 1 × 106 U-937 cells with 10 U of chymotrypsin reduced the interaction between L-selectin and ANXA1 in resting or PMA-treated cells as illustrated in Figure 4b.
Figure 4.
Chymotrypsin-induced loss of L-selectin reduced ANXA1 interaction with L-selectin. (a) Effect of different concentrations of chymotrypsin on U-937 expression of L-selectin. Cells were incubated with different doses of chymotrypsin (U (1 × 106)−1 cells) for 15 min at 37°C. The expression of L-selectin antigen was analyzed by flow cytometry using the FITC-DREG-56 antibody. Chymotrypsin induced a dose-dependent decrease in L-selectin expression with a maximal effect achieved at 10 U per 1 × 106 cells (−80%). Bars indicate mean±s.e.m. of three independent experiments, (b) Loss of L-selectin antigen by chymotrypsin is associated with a decrease in the interaction between ANXA1 and L-selectin. U-937 cells (C) were stimulated with 1 nM PMA (P) for 24 h and then with 10 U per 1 × 106 cells of chymotrypsin for 15 min at 37°C, before performing the immunoprecipitation with a specific ANXA1 antibody, as described above. L-selectin immunoreactivity was detected with DREG-56 (WB). Data shown are representative of three independent experiments giving similar results.
ANXA1 N-terminal domain interacts with the extracellular domain of L-selectin in a calcium-dependent manner
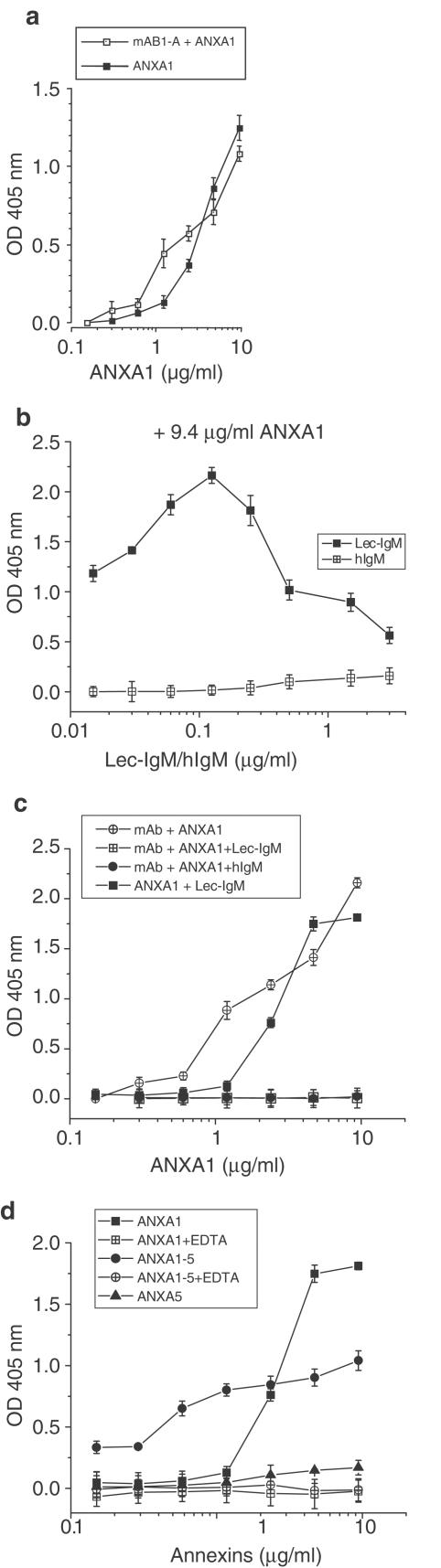
To further characterize the interaction between ANXA1 and L-selectin, binding assays were performed with the recombinant L-selectin/IgM chimera (Lec-IgM), used as a probe for L-selectin, and the human recombinant protein ANXA1 expressed in a prokaryotic system. In initial experiments, we validated the model of ANXA1 binding to plastic, showing that the amount of ANXA1 coated on an extra-high binding capacity plastic was similar to the amount of ANXA1 presented by a specific monoclonal antibody, both revealed with a polyclonal antibody (Figure 5a).
Figure 5.
L-selectin binding to ANXA1 is calcium-dependent and involves the N-terminal domain of ANXA1. (a) Comparison of the amounts of ANXA1 detected after presentation of the protein by a specific mAb or after direct binding to plastic. A monoclonal ANXA1 antibody was coated onto microtiter plates (3 μg ml−1) to capture varying concentrations of ANXA1 (mAB1-A+ANXA1). This binding was compared to the binding for the same range of ANXA1 recombinant protein concentrations directly coated on extra-high binding capacity plastic (ANXA1). In both cases, ANXA1 was detected with a polyclonal ANXA1 antibody and showed no major difference between the two methods, (b–d) Direct binding between annexins and L-selectin chimeric protein (Lec-IgM) in the presence or absence of calcium, (b) Increasing concentrations of Lec-IgM (black square) were immobilized on plastic and then incubated with 9.4 μg ml−1 of ANXA1. Internal control for nonspecific binding was determined in the presence of human IgM (hIgM) (open square), (c) Lec-IgM or human IgM were added to increasing concentrations of ANXA1, either directly coated on the plastic (ANXA1) or presented by the monoclonal antibody mAB1-A (mAB-1A+ANXA1). Presence of the ANXA1 in the assay was detected with a polyclonal antibody (mAB1-A+ANXA1) as in (a), (d) Effect of the presence of calcium of the N-terminal domain of ANXA1 on the interaction between ANXA1 and Lec-IgM. Binding of Lec-IgM (0.125 μg ml−1) to increasing concentrations of ANXA1 was compared to the binding to the same concentration range of the chimeric protein ANXA1-5 (N-terminal domain of ANXA1 (1–26) and core domain of ANXA5) or to an unrelated protein, ANXA5. Experiments were done in PBS+1 mM Ca2++1 mM Mg2+ with or without 10 mM EDTA (+EDTA). Each point represents the mean±s.e.m. of three independent experiments done in duplicates.
The results shown in Figures 5b–d investigated the binding/interaction of the extracellular domain of L-selectin (Lec-IgM) with ANXA1. Optimal binding was observed with a concentration of ANXA1 around 5 μg ml−1, and a concentration of Lec-IgM of 0.125 μg ml−1; no binding was detected in the presence of human IgM (Figures 5b and c). The bell-shaped pattern of the dose–response curve observed in Figure 5b may relate to additional characteristics of ANXA1-binding sites in the presence of L-selectin, like dimerization (Solito et al., 2000). Half-saturable binding ranged from 0.15 to 2.8 μg ml−1 (4–74.5 nM) of ANXA1. Presentation of ANXA1 by a monoclonal antibody (mAb) directed against the N-terminal portion of the ANXA1 protein (amino acids 1–346) prevented its binding to Lec-IgM (Figure 5c, open square). Several studies have indicated that ANXA1 N-terminal peptide is almost as potent as the full-length protein (Perretti et al., 1993a; 1995; Flower & Rothwell, 1994). Taking this into consideration, we investigated the role of the N-terminal domain of ANXA1 (amino acids 1–33) in the interaction using a chimeric ANXA1-5 protein constituted of the N-terminus domain of ANXA1 (1–26) and the core domain of ANXA5, a closely related protein displaying a core domain similar to ANXA1 (44% identity and 63% homology), but a completely unrelated N-terminal which is only six amino acids long. The chimeric protein ANXA1–5 was also able to bind to Lec-IgM, but achieved only 43% as much binding as did the wild-type protein (Figure 5d). While ANXA5 showed no binding, low concentrations of ANXA1–5 induced a significant higher binding to Lec-IgM (1 μg ml−1) than ANXA1 by itself, suggesting a role for ANXA1 N-terminus and ANXA5 core domains in the interaction with L-selectin. Since ANXA1 requires calcium for its biological activity, we examined the importance of this ion for the interaction. Figure 5d showed that the presence of 10 mM EDTA completely prevented the interaction of Lec-IgM either with ANXA1 or with ANXA1–5 to a level similar to ANXA5.
ANXA1 colocalizes with L-selectin on U-937 cell surface
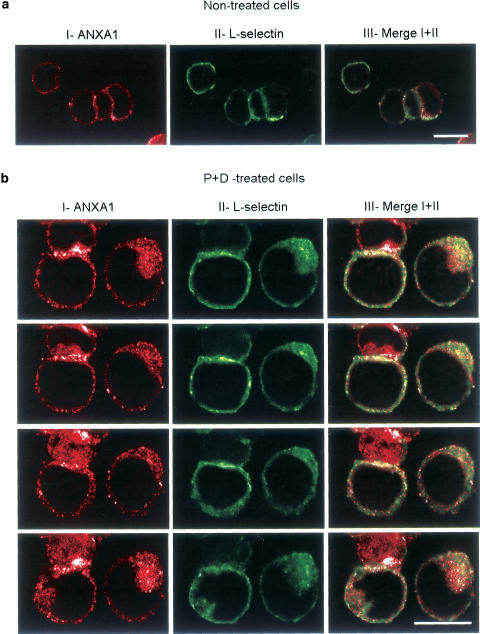
As the results described above provided evidence that ANXA1 could interact with L-selectin on the cell surface, we next characterized their cellular codistribution by confocal microscopy. Figure 6 represents Z-sections through the same cell and showed one or two representative cells for each staining. ANXA1 was not or only slightly detectable on the cell surface of nontreated U-937 cells (Figure 6aI, red), while L-selectin distributed over the cell membrane (Figure 6aII, green). A very light colocalization between ANXA1 and L-selectin could be observed (Figure 6a, right panel, yellow). Endogenous ANXA1, externalized after PMA+dexamethasone treatment, was mostly concentrated to one pole of the cell and showed a subtle punctate appearance (Figure 6bI, red). L-selectin staining exhibits uniform distribution on the surface of U-937 cells, with some concentration at one pole of the cell (Figure 6bII, green). This staining was observed even in the presence of azide, which is known to prevent cap formation (Rosenman et al., 1993). After PMA+dexamethasone treatment, ANXA1 partially colocalized with L-selectin, as shown in Figure 6aIII and bIII (yellow). All the cells examined exhibit the same pattern of expression and colocalization. Qualitatively, we did not find any difference in ANXA1 or L-selectin cell-surface staining between PMA- and PMA+dexamethasone-treated cells (data not shown), but we did not perform additional experiments to estimate the number of ANXA1 and L-selectin molecules present on the cell surface.
Figure 6.
Colocalization of ANXA1 and L-selectin on U-937 cell surface by confocal microscopy. ANXA1 (panels I in red) and L-selectin (panels II in green) cell-surface immunostaining were performed on nonstimulated U-937 cells (a) or after PMA+dexamethasone treatment (b). Column III (a, b) shows the distribution of fluorescence signal in merged images. To show the distribution of the colocalization, successive Z-sections through the same cell are illustrated in (b). A monoclonal anti-α-tubulin antibody was used to confirm that the plasma membrane was not permeabilized during immunofluorescence analysis and showed no staining (data not shown). Pictures show representative pattern of polarity and colocalization observed in four different high-power fields (× 100 lens) per condition. Results were obtained from five separate experiments. Scale bars, 20 μm.
Discussion
It has been shown that blockade of L-selectin in vivo does not impair neutrophil emigration (Johnston et al., 1997) or that prevention of L-selectin proteolytic cleavage does not enhance or inhibit neutrophil–endothelial cell adhesive interactions (Allport et al., 1997). On the contrary, several studies investigating the role of L-selectin shedding in leukocyte migration demonstrated an important role for L-selectin in enabling leukocytes to respond and migrate effectively to chemotactic stimuli (Jung et al., 1998; Jung & Ley, 1999; Hickey et al., 2000). Blockade of L-selectin shedding decreased leukocyte rolling in vivo (reviewed in Ebnet & Vestweber, 1999) and diminished neutrophil adhesion (Kuijper et al., 1997). While these latter studies appear paradoxical, it is possible that: first, L-selectin is not critical for leukocyte migration while it may still have a functional role in capturing leukocytes; second, studies have shown that selectins have overlapping functions (Jung & Ley, 1999; Steeber et al., 1999).
Exogenous recombinant ANXA1 protein has been recently reported to induce, in a dose- and time-dependent manner, L-selectin shedding from peripheral blood neutrophils (Strausbaugh & Rosen, 2001). However, mechanisms by which ANXA1 regulate L-selectin cell-surface expression remains unclear. Since, much evidence now exist that ANXA1 has the highest binding to the external cell surface of monocytes with a lower binding to circulating neutrophils and not with lymphocytes (Perretti et al., 1996; Euzger et al., 1999), we decided to take advantage of the U-937 cell line as a surrogate of human monocytes expressing L-selectin, to investigate for the first time, the role of externalized ANXA1 protein (monitored by steroids) on L-selectin shedding and to depict the mechanism.
We and previous investigators have reported that in U-937 cells, endogenous ANXA1 could be upregulated and membrane translocated by dexamethasone after PMA treatment (24 h) (Solito et al., 1991; 1993; 1994; Parente et al., 1992; De Caterina et al., 1993; Parente & Solito, 1994; Comera & Russo, 1995). Data indicate that there is no significant difference in the cell-surface expression of ANXA1 in cells treated with PMA or PMA+dex. However, ANXA1 has been shown to translocate on the outer membrane and to be released/secreted upon dexamethasone stimulation. Therefore, we performed an ELISA on the stimulated U-937 cell supernatant to take into account the total amount of protein (membrane-associated and free-released ANXA1). The results showed an increase of ANXA1 in the medium, suggesting that dexamethasone increased the amount of ANXA1 on the cell surface, that is, fraction of ANXA1 detectable only by ELISA. U-937 cells expressed a basal level of L-selectin. Its expression was enhanced by PMA treatment, while PMA+dexamethasone-induced ANXA1 externalization was correlated with increased shedding of cell-surface L-selectin. Treatment of U-937 cells with PMA markedly increases cell-surface expression of CD11a, the αL subunit of the β2 integrin known as lymphocyte function-associated antigen (LFA-1) (Pedrinaci et al., 1990), but remained unchanged after PMA+dexamethasone. Translocation of ANXA1 on the cell surface has been related in other models like epithelial cells (Solito et al., 1998b), human leukocytes (Perretti et al., 1996), or C6 glioma cells (McLeod et al., 1995); however, the mechanism by which ANXA1 is translocated still remains unclear. A secretion of ANXA1 from human neutrophils has been reported via a degranulation event involving gelatinase granules (Perretti et al., 2000). We cannot exclude that a fraction of ANXA1 is released from damaged cells in our assays, which is inevitable during handling. However, our flow cytometry experiments were performed on viable cells, that is, in the presence of propidium iodide to specifically exclude from the analysis dying or dead cells (less than 1% of the cells, including apoptotic bodies).
We also demonstrated that ANXA1-neutralizing antibody counteracted dexamethasone as well as exogenous ANXA1 effects on L-selectin cell-surface expression, but had little effect on nonactivated cells. The amount of soluble L-selectin shed was slightly increased following PMA treatment, suggesting that phorbol esters may increase the activity of a cell-surface metalloproteinase responsible for L-selectin shedding (named L-selectin sheddase) (Spertini et al., 1991; Preece et al., 1996) or alter the conformation of L-selectin, increasing its susceptibility to enzymatic cleavage. However, we found here that low concentration of PMA (1 nM) induced L-selectin shedding, but in a lesser extent than it increased L-selectin density on U-937 cell surface. According to Kaldjian & Stoolman (1995), low phorbol ester concentration (0.5 nM) induced rapid L-selectin shedding, followed by progressive increases in L-selectin density and mRNA levels in Jurkat cells (Kaldjian & Stoolman, 1995).
Previously, we showed by binding studies of hrANXA1 to U-937 that 5 μg ml−1 of this protein saturated all the binding sites present on U-937 cell surface (Solito et al., 2000). This concentration mimicked dexamethasone treatment by markedly increasing L-selectin shedding. We are aware that the amount of human recombinant protein (5 μg ml−1) we used to mimic the actual effect of dexamethasone was slightly higher than the regular amount of ANXA1 detected as a soluble form or as calcium membrane-associated protein (1.6 μg ml−1). However, in vivo studies showed that ANXA1 can reach a concentration as high as 100 μg ml−1 in epithelial (Fava et al., 1989) and endothelial (Romisch et al., 1992) environments or in prostatic secretions (Christmas et al., 1991), suggesting that in some conditions the ANXA1 concentration we used is found.
Previous observations demonstrating that crosslinking of leukocyte L-selectin results in shedding activation (Palecanda et al., 1992), prompted us to investigate whether ANXA1 would interact with L-selectin in U-937 cells. Using immunoprecipitation studies, we showed that ANXA1 co-precipitated with L-selectin and that this interaction was enhanced after PMA treatment. While we detected two forms of L-selectin, since the experiments were performed in the presence of protease inhibitors, they may correspond to different glycosylated forms of the protein as reported in other studies. Indeed, L-selectin was immunodetected around 65 and 70 kDa in human plasma (Palecanda et al., 1992), around 50 and 74 kDa in transfected CHO cells (Arribas et al., 1996) or around 68 kDa in transfected COS cells, where two bands could be clearly observed on the blot (Migaki et al., 1995). Another study showed that expression of wild-type L-selectin gene transfected in two different cell lines (BHK and K562) resulted in expression of two differently glycosylated recombinant L-selectin, which were detected by Western blot as broad bands of 60 and 65 kDa. Despite different molecular weights, these two proteins presented no change in their ligand affinity (Fieger et al., 2000). Intact neutrophil L-selectin is larger than the one detected on lymphocytes (Zhao et al., 2001); no data have been demonstrated so far for the U-937 cell line, and a different pattern of L-selectin glycosylation could possibly justify molecular weights of 80–85 kDa. ANXA1 and L-selectin interaction increased after externalization of ANXA1 (upon PMA), but decreased after release/secretion of ANXA1 by dexamethasone. Densitometric analysis of the L-selectin immunodetected bands revealed that the interaction was barely detectable by Western blot in nontreated cells, unless stimulated by PMA where more proteins were involved. Despite the direct interaction of ANXA1 with L-selectin, we cannot rule out that binding of ANXA1 to another molecule may also initiate the L-selectin downregulation. Furthermore, ANXA1 is not to be expected to only interact with L-selectin. ANXA1 has been shown to bind to the N-formyl-methionyl-leucyl-phenylalanine (fMLP) receptor, leading to a specific inhibition of the transendothelial migration of neutrophils in vitro (Walther et al., 2000) and in vivo (Perretti et al., 2001). Furthermore, previously we demonstrated that ANXA1 could interact with the integrin VLA4, which suggests that ANXA1 interaction with a selectin or an integrin may occur at different points in time or during distinct steps in the process of cell recruitment.
In the last part of the study, we conducted ELISA assays with the recombinant protein ANXA1 that was not glycosylated (Goulet et al., 1992) or did not undergo any post-translational modification because it was expressed in a prokaryotic system (Houdebine, 1993). Binding assays confirmed and extended these findings, showing a dose-dependent reciprocal binding between ANXA1 and the extracellular domain of L-selectin. A parallel in the pattern of binding between Lec-IgM and L-selectin-expressing Jurkat T cells to the L-selectin ligands strongly validates the use of the chimera as a probe for L-selectin binding (Bruehl et al., 2000). In this study, we found that half-saturable binding of ANXA1 to L-selectin ranged from 5 to 74.5 nM, which is consistent with the characteristics of ANXA1 binding to human monocytes (half-saturable binding between 13.3 and 80 nM) (Goulding et al., 1996). The N-terminus domain of ANXA1 mimics the potent anti-inflammatory effect of the full-length ANXA1 protein (Cirino et al., 1993; Perretti et al., 1993a; 1995; Getting et al., 1997; Croxtall et al., 1998; Lim et al., 1998; Perretti, 1998). The predominant role of this N-terminal domain in the interaction with L-selectin was also observed in our model. Indeed, the monoclonal antibody directed against the first 346 amino acids of ANXA1 totally prevented the interaction, and a comparative binding study showed that both ANXA1 and ANXA1–5 bind Lec-IgM whereas ANXA5 did not. The spatial conformation of the chimeric protein ANXA1–5 is different from that of ANXA1, and may account for its reduced binding at high concentrations.
This interaction required calcium that may involve either the annexin's calcium-binding sites or the C-type lectin domain of L-selectin, which is important in cell–cell adhesion or attachment to extracellular matrix (Drickamer, 1999). At low concentration, ANXA1–5 showed a higher affinity for Lec-IgM than ANXA1, suggesting that the fourth calcium-binding site that does not exist in the ANXA1 core domain did participate in the binding and may help stabilize the complex or anchor ANXA1 on the cell membrane.
Finally, evidence for an association between ANXA1 and L-selectin was extended with immunolocalization studies. After PMA+dexamethasone treatment, externalized ANXA1 showed a punctate distribution on the plasma membrane, which is consistent with the idea of specific binding protein(s) for ANXA1 present on leukocyte cell surface (Goulding & Guyre, 1992; 1993b; Perretti et al., 1993b; Goulding et al., 1996; Euzger et al., 1999).
In this study, we identified a potential cellular mechanism responsible for the L-selectin shedding during inflammation. We propose that endogenous ANXA1 externalized on the monocyte cell surface by glucocorticoids could facilitate L-selectin shedding through a calcium-dependent interaction with the selectin. Our findings may provide the basis for further understanding of physiological actions of externalized ANXA1 for modulation of inflammatory reactions.
Acknowledgments
This work was supported by a grant from the National Institute of Health AM 32634 and by the Wellcome Trust (grant number 051887/Z/97A) for financial support (E.S.). We thank Dr Paul Dazin for his assistance with flow cytometry, Dr Fabien Shmidlin for his help with confocal analysis and Mark Singer for useful advice. We are also grateful to Dr Holly J. Strausbaugh and Dr Steven D. Rosen for their critical review of the data and comments on the manuscript.
Abbreviations
- ANXA1
Annexin 1
- ANXA5
Annexin 5
- ANXA1–5
chimeric protein with the N-terminal domain of Annexin 1 and the core domain of Annexin 5
- FBS
fetal bovine serum
- HEK-293
human embryonal kidney cells 293
- IP
immunoprecipitation
- PMA
phorbol 12-myristate 13-acetate
References
- ALLPORT J.R., DING H.T., AGER A., STEEBER D.A., TEDDER T.F., LUSCINSKAS F.W. L-selectin shedding does not regulate human neutrophil attachment, rolling, or transmigration across human vascular endothelium in vitro. J. Immunol. 1997;158:4365–4372. [PubMed] [Google Scholar]
- ARRIBAS J., COODLY L., VOLLMER P., KISHMOTO T.K., ROSE-JOHN S., MASSAGUE J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J. Biol. Chem. 1996;271:11376–11382. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]
- BLACKWELL G.J., CARNUCCIO R., DI R.M., FLOWER R.J., LANGHAM C.S., PARENTE L., PERSICO P., RUSSEL S.N., STONE D. Glucocorticoids induce the formation and release of anti-inflammatory and anti-phospholipase proteins into the peritoneal cavity of the rat. Br. J. Pharmacol. 1982;76:185–194. doi: 10.1111/j.1476-5381.1982.tb09205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKWELL G.J., CARNUCCIO R., DI ROSA M., FLOWER R.J., PARENTE L., PERSICO P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980;287:147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- BRUEHL R.E., BERTOZZI C.R., ROSEN S.D. Minimal sulfated carbohydrates for recognition by L-selectin and the MECA-79 antibody. J. Biol. Chem. 2000;275:32642–32648. doi: 10.1074/jbc.M001703200. [DOI] [PubMed] [Google Scholar]
- BRUEHL R.E., SPRINGER T.A., BAINTON D.F. Quantitation of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J. Histochem. Cytochem. 1996;44:835–844. doi: 10.1177/44.8.8756756. [DOI] [PubMed] [Google Scholar]
- BURTON J.L., KEHRLI M.E., JR, KAPIL S., HORST R.L. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 1995;57:317–325. doi: 10.1002/jlb.57.2.317. [DOI] [PubMed] [Google Scholar]
- CHRISTMAS P., CALLAWAY J., FALLON J., JONES J., HAIGLER H.T. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. J. Biol. Chem. 1991;266:2499–2507. [PubMed] [Google Scholar]
- CIRINO G., CICALA C., SORRENTINO L., CILIBERTO G., ARPAIA G., PERRETTI M., FLOWER R.J. Anti-inflammatory actions of an N-terminal peptide from human lipocortin 1. Br. J. Pharmacol. 1993;108:573–574. doi: 10.1111/j.1476-5381.1993.tb12843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMERA C., RUSSO M.F. Glucocorticoid-induced annexin 1 secretion by monocytes and peritoneal leukocytes. Br. J. Pharmacol. 1995;115:1043–1047. doi: 10.1111/j.1476-5381.1995.tb15916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROXTALL J.D., CHOUDHURY Q., FLOWER R.J. Inhibitory effect of peptides derived from the N-terminus of lipocortin 1 on arachidonic acid release and proliferation in the A549 cell line: identification of E-Q-E-Y-V as a crucial component. Br. J. Pharmacol. 1998;123:975–983. doi: 10.1038/sj.bjp.0701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CATERINA R., SICARI R., GIANNESSI D., PAGGIARO P.L., PAOLETTI P., LAZZERINI G., BERNINI W., SOLITO E., PARENTE L. Macrophage-specific eicosanoid synthesis inhibition and lipocortin-1 induction by glucocorticoids. J. Appl. Physiol. 1993;75:2368–2375. doi: 10.1152/jappl.1993.75.6.2368. [DOI] [PubMed] [Google Scholar]
- DRICKAMER K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999;9:585–590. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- EBNET K., VESTWEBER D. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines. Histochem. Cell. Biol. 1999;112:1–23. doi: 10.1007/s004180050387. [DOI] [PubMed] [Google Scholar]
- ERNST J.D., MEERS P., HONG K., DUZGUNES N., PAPAHADJOPOULOS D., GOLDSTEIN I.M. Human polymorphonuclear leukocytes contain synexin, a calcium-binding protein that mediates membrane fusion. Trans. Assoc. Am. Phys. 1986;99:58–66. [PubMed] [Google Scholar]
- EUZGER H.S., FLOWER R.J., GOULDING N.J., PERRETTI M. Differential modulation of annexin I binding sites on monocytes and neutrophils. Mediators Inflamm. 1999;8:53–62. doi: 10.1080/09629359990720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVA R.A., MCKANNA J., COHEN S. Lipocortin I (p35) is abundant in a restricted number of differentiated cell types in adult organs. J. Cell. Physiol. 1989;141:284–293. doi: 10.1002/jcp.1041410209. [DOI] [PubMed] [Google Scholar]
- FIEGER C.B., EMIG-VOLLMER S., PETRI T., GRAFE M., GOHLKE M., DEBUS N., SEMMLER W., TAUBER R., VOLZ B. The adhesive properties of recombinant soluble L-selectin are modulated by its glycosylation. Biochim. Biophys. Acta. 2000;1524:75–85. doi: 10.1016/s0304-4165(00)00143-4. [DOI] [PubMed] [Google Scholar]
- FLOWER R.J., ROTHWELL N.J. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 1994;15:71–76. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- GETTING S.J., FLOWER R.J., PERRETTI M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br. J. Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULDING N., GUYRE P. Glucocorticoids, lipocortins and the immune response. Curr. Opin. Immunol. 1993a;5:108–113. doi: 10.1016/0952-7915(93)90089-b. [DOI] [PubMed] [Google Scholar]
- GOULDING N.J., GUYRE P.M. Regulation of inflammation by lipocortin 1. Immunol. Today. 1992;13:295–297. doi: 10.1016/0167-5699(92)90040-E. [DOI] [PubMed] [Google Scholar]
- GOULDING N.J., GUYRE P.M. Lipocortin 1 binding to human leukocytes correlates with its ability to inhibit IgG interactions with Fc gamma receptors. Biochem. Biophys. Res. Commun. 1993b;192:351–358. doi: 10.1006/bbrc.1993.1422. [DOI] [PubMed] [Google Scholar]
- GOULDING N.J., PAN L., WARDWELL K., GUYRE V.C., GUYRE P.M. Evidence for specific annexin I-binding proteins on human monocytes. Biochem. J. 1996;316:593–597. doi: 10.1042/bj3160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULET F., MOORE K.G., SARTORELLI A.C. Glycosylation of annexin I and annexin II. Biochem. Biophys. Res. Commun. 1992;188:554–558. doi: 10.1016/0006-291x(92)91091-4. [DOI] [PubMed] [Google Scholar]
- HASS R., BARTELS H., TOPLEY N., HADAM M., KOHLER L., GOPPELT-STRUBE M., RESCH K. TPA-induced differentiation and adhesion of U937 cells: changes in ultrastrucrure, cytoskeletal organization and expression of cell surface antigens. Eur. J. Cell. Biol. 1989;48:282–293. [PubMed] [Google Scholar]
- HICKEY M.J., FORSTER M., MITCHELL D., KAUR J., DE CAIGNY C., KUBES P. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J. Immunol. 2000;165:7164–7170. doi: 10.4049/jimmunol.165.12.7164. [DOI] [PubMed] [Google Scholar]
- HOUDEBINE L.M. Expression of recombinant proteins in the milk of transgenic animals. Rev. Fr. Transfus. Hemobiol. 1993;36:49–72. doi: 10.1016/s1140-4639(05)80168-6. [DOI] [PubMed] [Google Scholar]
- JILMA B., VOLTMANN J., ALBINNI S., STOHLAWETZ P., SCHWARZINGER I., GLEITER C.H., RAUCH A., EICHLER H.G., WAGNER O.F. Dexamethasone down-regulates the expression of L-selectin on the surface of neutrophils and lymphocytes in humans. Clin. Pharmacol. Ther. 1997;62:562–568. doi: 10.1016/S0009-9236(97)90052-7. [DOI] [PubMed] [Google Scholar]
- JOHNSTON B., WALTER U.M., ISSEKUTZ A.C., ISSEKUTZ T.B., ANDERSON D.C., KUBES P. Differential roles of selectins and the alpha4-integrin in acute, subacute, and chronic leukocyte recruitment in vivo. J. Immunol. 1997;159:4514–4523. [PubMed] [Google Scholar]
- JUNG U., LEY K. Mice lacking two or all three selectins demonstrate overlapping and distinct functions for each selectin. J. Immunol. 1999;162:6755–6762. [PubMed] [Google Scholar]
- JUNG U., RAMOS C.L., BULLARD D.C., LEY K. Gene-targeted mice reveal importance of L-selectin-dependent rolling for neutrophil adhesion. Am. J. Physiol. 1998;274:H1785–H1791. doi: 10.1152/ajpheart.1998.274.5.H1785. [DOI] [PubMed] [Google Scholar]
- KALDJIAN E.P., STOOLMAN L.M. Regulation of L-selectin mRNA in Jurkat cells. Opposing influences of calcium- and protein kinase C-dependent signaling pathways. J. Immunol. 1995;154:4351–4362. [PubMed] [Google Scholar]
- KANG S.A., CHO Y.J., MOON H.B., NA D.S. Translocation of lipocortin (annexin) 1 to the membrane of U937 cells induced by phorbol ester, but not by dexamethasone. Br. J. Pharmacol. 1996;117:1780–1784. doi: 10.1111/j.1476-5381.1996.tb15354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISHIMOTO T.K., JUTILA M.A., BERG E.L., BUTCHER E.C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- KISHIMOTO T.K., JUTILA M.A., BUTCHER E.C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUIJPER P.H., GALLARDO TORRES H.I., VAN DER LINDEN J.A., LAMMERS J.W., SIXMA J.J., ZWAGINGA J.J., KOENDERMAN L. Neutrophil adhesion to fibrinogen and fibrin under flow conditions is diminished by activation and L-selectin shedding. Blood. 1997;89:2131–2138. [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LIM L.H., SOLITO E., RUSSO-MARIE F., FLOWER R.J., PERRETTI M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEOD J.D., GOODALL A., JELIC P., BOLTON C. Changes in the cellular distribution of lipocortin-1 (Annexin-1) in C6 glioma cells after exposure to dexamethasone. Biochem. Pharmacol. 1995;50:1103–1107. doi: 10.1016/0006-2952(95)00234-q. [DOI] [PubMed] [Google Scholar]
- MIGAKI G.I., KAHN J., KISSHMOTO T.K. Mutational analysis of the membrane-proximal cleavage site of L-selectin: relaxed sequence specificity surrounding the cleavage site. J. Exp. Med. 1995;182:549–557. doi: 10.1084/jem.182.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'LEARY E.C., MARDER P., ZUCKERMAN S.H. Glucocorticoid effects in an endotoxin-induced rat pulmonary inflammation model: differential effects on neutrophil influx, integrin expression, and inflammatory mediators. Am. J. Respir. Cell. Mol. Biol. 1996;15:97–106. doi: 10.1165/ajrcmb.15.1.8679228. [DOI] [PubMed] [Google Scholar]
- PALECANDA A., WALCHECK B., BISHOP D.K., JUTILA M.A. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur. J. Immunol. 1992;22:1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- PARENTE L., SOLITO E. Association between glucocorticosteroids and lipocortin 1 (letter; comment) Trends Pharmacol. Sci. 1994;15:362. doi: 10.1016/0165-6147(94)90154-6. [DOI] [PubMed] [Google Scholar]
- PARENTE L., SOLITO E., GIANNESSI P., PAGGIARI P., SICARI R., DE C.R. Inhibition of eicosanoid formation and lipocortin induction by glucocorticoids in human cell lines and in patients with inflammatory lung disease. Pharmacol. Res. 1992;2:44–45. doi: 10.1016/1043-6618(92)90591-x. [DOI] [PubMed] [Google Scholar]
- PEDRINACI S., RUIZ-CABELLO F., GOMEZ O., COLLADO A., GARRIDO F. Protein kinase C-mediated regulation of the expression of CD14 and GD11/CD18 in U937 cells. Int. J. Cancer. 1990;45:294–298. doi: 10.1002/ijc.2910450215. [DOI] [PubMed] [Google Scholar]
- PEPINSKY R.B., SINCLAIR L.K., DOUGAS I., LIANG C.M., LAWTON P., BROWNING J.L. Monoclonal antibodies to lipocortin-1 as probes for biological function. FEBS Lett. 1990;261:247–252. doi: 10.1016/0014-5793(90)80564-y. [DOI] [PubMed] [Google Scholar]
- PERRETTI M. Lipocortin 1 and chemokine modulation of granulocyte and monocyte accumulation in experimental inflammation. Gen. Pharmacol. 1998;31:545–552. doi: 10.1016/s0306-3623(98)00039-1. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., AHLUWALIA A., HARRIS J.G., GOULDING N.J., FLOWER R.J. Lipocortin-1 fragments inhibit neutrophil accumulation and neutrophil-dependent edema in the mouse. A qualitative comparison with an anti-CD11b monoclonal antibody. J. Immunol. 1993a;151:4306–4314. [PubMed] [Google Scholar]
- PERRETTI M., CHRISTIAN H., WHELLER S.K., AIELLO I., MUGRIDGE K.G., MORRIS J.F., FLOWER R.J., GOULDING N.J. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol. Int. 2000;24:163–174. doi: 10.1006/cbir.1999.0468. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., CROXTALL J.D., WHELLER S.K., GOULDING N.J., HANNON R., FLOWER R.J. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat. Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., FLOWER R.J. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J. Immunol. 1993;150:992–999. [PubMed] [Google Scholar]
- PERRETTI M., FLOWER R.J., GOULDING N.J. The ability of murine leukocytes to bind lipocortin 1 is lost during acute inflammation. Biochem. Biophys. Res. Commun. 1993b;192:345–350. doi: 10.1006/bbrc.1993.1421. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., GETTING S.J., SOLITO E., MURPHY P.M., GAO J.L. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 2001;158:1969–1973. doi: 10.1016/S0002-9440(10)64667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRETTI M., INGEGNOLI F., WHELLER S.K., BLADES M.C., SOLITO E., PITZALIS C. Annexin 1 modulates monocyte–endothelial cell interaction in vitro and cell migration in vivo in the human SCID mouse transplantation model. J. Immunol. 2002;169:2085–2092. doi: 10.4049/jimmunol.169.4.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRETTI M., WHELLER S.K., CHOUDHURY Q., CROXTALL J.D., FLOWER R.J. Selective inhibition of neutrophil function by a peptide derived from lipocortin 1 N-terminus. Biochem. Pharmacol. 1995;50:1037–1042. doi: 10.1016/0006-2952(95)00238-u. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., WHELLER S.K., FLOWER R.J., WAHID S., PITZALIS C. Modulation of cellular annexin I in human leukocytes infiltrating DTH skin reactions. J. Leukoc. Biol. 1999;65:583–589. doi: 10.1002/jlb.65.5.583. [DOI] [PubMed] [Google Scholar]
- PESCHON J.J., SLACK J.L., REDDY P., STOCKING K.L., SUNNARBORG S.W., LEE D.C., RUSSELL W.E., CASTNER B.J., JOHNSON R.S., FITZNER J.N., BOYCE R.W., NELSON N., KOZLOSKY C.J., WOLFSON M.F., RAUCH C.T., CERRETTI D.P., PAXTON R.J., MARCH C.J., BLACK R.A. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- PREECE G., MURPHY G., AGER A. Metalloproteinase-mediated regulation of L-selectin levels on leucocytes. J. Biol. Chem. 1996;271:11634–11640. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- RAYNAL P., POLLARD H.B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- ROMISCH J., SCHULER E., BASTIAN B., BURGER T., DUNKEL F.G., SCHWINN A., HARTMANN A.A., PAQUES E.P. Annexins I to VI: quantitative determination in different human cell types and in plasma after myocardial infarction. Blood Coagul. Fibrinolysis. 1992;3:11–17. [PubMed] [Google Scholar]
- ROSENGARTH A., WINTERGALEN A., GALLA H.J., HINZ H.J., GERKE V. Ca2+-independent interaction of annexin I with phospholipid monolayers. FEBS Lett. 1998;438:279–284. doi: 10.1016/s0014-5793(98)01318-0. [DOI] [PubMed] [Google Scholar]
- ROSENMAN S.J., GANJI A.A., TEDDER T.F., GALLATIN W.M. Syn-capping of human T lymphocyte adhesion/activation molecules and their redistribution during interaction with endothelial cells. J. Leukoc. Biol. 1993;53:1–10. doi: 10.1002/jlb.53.1.1. [DOI] [PubMed] [Google Scholar]
- SHEETS E.E., GIUGNI T.D., COATES G.G., SCHLAEPFER D.D., HAIGLER H.T. Epidermal growth factor dependent phosphorylation of a 35-kilodalton protein in placental membranes. Biochemistry. 1987;26:1164–1172. doi: 10.1021/bi00378a026. [DOI] [PubMed] [Google Scholar]
- SOLITO E., DE CATERINA R., GIANNESSI D., PAGGIARO P.L., SICARI R., PARENTE L. Studies on the induction of lipocortin-1 by glucocorticoids. Ann. 1st Super Sanita. 1993;29:391–394. [PubMed] [Google Scholar]
- SOLITO E., DE COUPADE C., PARENTE L., FLOWER R.J., RUSSO-MARIE F. Human annexin 1 is highly expressed during the differentiation of the human epithelial cell line A 549. Involvement of NF-IL6 in phorbol ester induction of annexin 1. Cell Growth Differ. 1998a;9:327–336. [PubMed] [Google Scholar]
- SOLITO E., DE COUPADE C., PARENTE L., FLOWER R.J., RUSSO-MARIE F. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 1998b;10:514–521. doi: 10.1006/cyto.1997.0325. [DOI] [PubMed] [Google Scholar]
- SOLITO E., NUTI S., PARENTE L. Dexamethasone-induced translocation of lipocortin (annexin) 1 to the cell membrane of U-937 cells. Br. J. Pharmacol. 1994;112:347–348. doi: 10.1111/j.1476-5381.1994.tb13075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLITO E., RAUGEI G., MELLI M., PARENTE L. Dexamethasone induces the expression of the mRNA of lipocortin 1 and 2 and the release of lipocortin 1 and 5 in differentiated, but not undifferentiated U-937 cells. FEBS Lett. 1991;291:238–244. doi: 10.1016/0014-5793(91)81293-h. [DOI] [PubMed] [Google Scholar]
- SOLITO E., ROMERO I.A., MARULLO S., RUSSO-MARIE F., WEKSLER B.B. Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the alpha 4 beta 1 integrin. J. Immunol. 2000;165:1573–1581. doi: 10.4049/jimmunol.165.3.1573. [DOI] [PubMed] [Google Scholar]
- SPERTINI O., FREEDMAN A.S., BELVIN M.P., PENTA A.C., GRIFFIN J.D., TEDDER T.F. Regulation of leukocyte adhesion molecule-1 (TQ1, Leu-8) expression and shedding by normal and malignant cells. Leukemia. 1991;5:300–308. [PubMed] [Google Scholar]
- STEEBER D.A., TANG M.L., GREEN N.E., ZHANG X.Q., SLOANE J.E., TEDDER T.F. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and ICAM-1 pathways. J. Immunol. 1999;163:2176–2186. [PubMed] [Google Scholar]
- STRAUSBAUGH H.J., ROSEN S.D. A potential role for annexin 1 as a physiologic mediator of glucocorticoid-induced L-selectin shedding from myeloid cells. J. Immunol. 2001;166:6294–6300. doi: 10.4049/jimmunol.166.10.6294. [DOI] [PubMed] [Google Scholar]
- WAISMAN D., VAN EEDEN S.F., HOGG J.C., SOLIMANO A., MASSING B., BONDY G.P. L-selectin expression on polymorphonuclear leukocytes and monocytes in premature infants: reduced expression after dexamethasone treatment for bronchopulmonary dysplasia. J. Pediatr. 1998;132:53–56. doi: 10.1016/s0022-3476(98)70484-6. [DOI] [PubMed] [Google Scholar]
- WALTHER A., RIEHEMANN K., GERKE V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol. Cell. 2000;5:831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- WILLMOTT N.J., CHOUDHURY Q., FLOWER R.J. Effects of dexamethasone and phorbol ester on P2 receptor-coupled a2+ signalling and lipocortin 1 presentation in U937 cells. Br. J. Pharmacol. 1997;122:1055–1060. doi: 10.1038/sj.bjp.0701490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO L.C., EDGAR J.B., DAILEY M.O. Characterization of the rapid proteolytic shedding of murine L-selectin. Dev. Immunol. 2001;8:267–277. doi: 10.1155/2001/91831. [DOI] [PMC free article] [PubMed] [Google Scholar]