Abstract
We investigated the distribution of morphine and morphine-6β-glucuronide (M6G) in the brain and spinal cord after intracerebroventricular (i.c.v.) injection of each drug in rats.
The cerebrospinal fluid (CSF) concentration of M6G was 5–37 times greater than that of morphine 10, 60 and 120 min after the i.c.v. injection. The apparent elimination clearance of M6G from the CSF was 10 times lower than that of morphine.
The intrathecal CSF concentration of M6G measured by the microdialysis method was 29–79 times greater than that of morphine, and M6G was rapidly distributed into the intrathecal space after the i.c.v. injection.
M6G was detected in the cerebrum, brainstem, cerebellum and spinal cord at concentrations 2–21 times higher than morphine after the i.c.v. injection of each drug. The distribution volume of M6G in rat brain slices was three times lower than that of morphine, and close to the extracellular fluid space in the brain regions corresponding to the vicinity of the opioid receptors.
These brain distribution characteristics of M6G, namely, low clearance from the central nervous system, localization in the extracellular fluid and rapid distribution into the intrathecal space, may contribute to the potent analgesic effect of M6G after i.c.v. injection.
Keywords: Morphine, morphine-6β-glucuronide, brain distribution, microdialysis
Introduction
Morphine-6β-glucuronide (M6G), an active metabolite of morphine in humans, has been shown to be over 100-fold more potent than morphine when administered centrally in rats and mice (Paul et al., 1989; Gong et al., 1991; Frances et al., 1992), although M6G binds preferentially to the μ-opioid receptor with similar affinity to morphine (Abbott & Palmour, 1988; Paul et al., 1989; Frances et al., 1992). It has been hypothesized that the significantly greater analgesic potency of M6G is mediated by distinct receptors that are not activated by morphine, possibly splice variants of the μ-opioid receptor (Pasternak & Standifer, 1995). Osborne et al. (2000) have reported that the relative efficacy of M6G is two to four times higher than that of morphine in the rat locus coeruleus; however, there has been no evidence so far to indicate that a unique receptor for M6G functionally mediates highly potent pharmacological effects in the central nervous system.
Although it has been reported that the blood–brain barrier permeability to M6G is 32–57 times lower than that to morphine (Bickel et al., 1996; Wu et al., 1997), subcutaneously (s.c.) administered M6G has been detected at concentrations two to four times higher than morphine in the brain extracellular fluid space corresponding to the vicinity of the opioid receptors (Aasmundstad et al., 1995; Stain-Texier et al., 1999). Indeed, the analgesic potency of M6G has been shown to be almost equal to that of morphine following s.c. administration (Paul et al., 1989). There might not be a significant difference in the analgesic potency of morphine and M6G based on their concentrations at the target sites in the brain. Payne et al. (1996) have demonstrated in sheep that intracerebroventricular (i.c.v.) and intrathecal administration of lipophilic opioids such as methadone and naloxone produces a different cerebrospinal fluid (CSF) distribution from that of hydrophilic opioids including morphine. The distribution of centrally administered M6G may differ from that of morphine, because the 1-octanol/buffer partition coefficient of M6G is 187 times lower than that of morphine (Wu et al., 1997). The potency of the analgesic effects after the i.c.v. injection of morphine and M6G can be affected by their pharmacokinetics in the brain and spinal cord. We hypothesized that the differences in the analgesic effects after a central administration of morphine and M6G are attributed, at least in part, to different distributions in the central nervous system. To clarify the distribution of morphine and M6G after the i.c.v. injection, we investigated the CSF pharmacokinetics in the ventricle and intrathecal space, as well as the distribution in the brain and spinal cord tissues in rats. We also determined the brain distribution volume of these substances using the brain slice uptake method.
Methods
Animals
Male Sprague–Dawley rats weighing about 250 g were housed three to four per cage with free access to food and water and maintained on a 12 h light/dark cycle in a room with controlled temperature (24±1°C) and humidity (55±5%). This study was conducted according to guidelines approved by the Experimental Animal Ethical Committee of the University of Shizuoka.
Drugs
[3H]sucrose (377.4 GBq mmol−1) and morphine hydrochloride were purchased from Dupont-NEN Co. Ltd (Boston, MA, U.S.A.) and Takeda Chemical Industries, Ltd (Osaka, Japan), respectively. M6G was synthesized from morphine as described previously (Yoshimura et al., 1968; Carrupt et al., 1991). All other chemicals were purchased from commercial sources.
CSF pharmacokinetics of morphine and M6G and their distribution in the brain and spinal cord after the i.c.v. injection
The rats were anesthetized with pentobarbital (50 mg kg−1 i.p.) and their heads were placed in a stereotaxic apparatus (SR-6, Narishige Scientific Instrument Lab., Tokyo, Japan). A guide cannula (AG-8, EICOM Corp., Kyoto, Japan) was inserted into the left lateral ventricle (posterior: 0.9 mm and lateral: 1.5 mm to the bregma as a reference and 3.9 mm ventral to the brain surface). The cannula was then anchored with dental cement to metallic screws placed in the skull. The animals were allowed to recover from the surgery for 2–3 days. Morphine (50 nmol) or M6G (50 nmol) was administered with [3H]sucrose (5 kBq), a CSF bulk flow marker, through the cannula using a stainless steel needle (EICOM Corp., Kyoto, Japan). Then, 10.60 and 120 min later, an aliquot of CSF (approximately 100 μl) was taken by cisternal puncture using a syringe (25G; Terumo, Tokyo, Japan) (Chou & Levy, 1981). Immediately after collecting the CSF, the rats were killed by taking blood from the descending aorta. Then, the brain and spinal cord were removed, and the brain was separated into the cerebrum, brainstem and cerebellum. These brain regions and the spinal cord were weighed and homogenized in a three-fold volume of 0.01 M phosphate buffer (pH 2.0). The homogenate was centrifuged at 4800 × g for 20 min at 4°C. The supernatant and CSF sample were stored at −20°C until the drug concentration was determined. The plasma from the rat blood was separated by centrifugation, and stored at −20°C.
Distribution of morphine and M6G in the intrathecal space after the i.c.v. injection
The intrathecal distribution of i.c.v.-injected morphine and M6G was determined by the microdialysis method (Marsala et al., 1995). The dialysis probe was constructed from Cuprophan hollow fibers (i.d., 0.2 mm; MW cutoff, 12,500; RENAK-E, RE-10M, Kawasumi Chemical Industries Ltd, Tokyo, Japan). The fibers were coated with epoxy glue, except for a 4-cm region in the middle of the fiber. Nichrome wire (o.d., 0.1 mm; Unique Medical Co. Ltd, Tokyo, Japan) was then passed through the fiber and both ends of the fiber were attached to pieces of polyethylene tube (PE-10; Natsume Seisakusho Co. Ltd, Tokyo, Japan). The probe was inserted through an incision in the cisternal membrane of the rats and slowly passed caudally 9 cm into the intrathecal space to leave the uncoated section of the catheter at the Th11–L2 spinal segments. The two PE-10 ends of the dialysis probe were externalized on the top of the head. The rats were allowed to recover from the surgery for 3 days. The dialysis probe was perfused with Ringer's solution (147 mM NaCl, 4 mM KCl, 2.4 mM CaCl2, pH 7.3) at a constant rate of 5 μl min−1. After a stabilization period of 1 h, the in vivo recovery was estimated by retrodialysis (Bouw et al., 2001). The dialysis probes were perfused with Ringer's solution containing morphine (2 μM) or M6G (2 μM) with [3H]sucrose (6.7 kBq ml−1) for 1 h. The in vivo drug recovery was calculated by measuring the loss of drug from the perfusion solution according to the following equation:
where Cin is the drug concentration in the perfusion solution entering the probe and Cout is the drug concentration in the outgoing dialysate. The recoveries for morphine, M6G and [3H]sucrose were 36.4±3.2, 18.7±2.1 and 30.4±3.1%, respectively, and did not change significantly during the 6-h experimental period. The drug concentrations were corrected on the basis of the recovery of the probe to determine the corresponding concentrations in the intrathecal CSF.
After the retrodialysis period, the perfusion solution was changed to Ringer's solution without drugs. A washout period of 1 h before the drug administration was allowed to ensure the complete removal of drugs from the probe. After morphine (50 nmol) or M6G (50 nmol) was administered i.c.v. with [3H]sucrose (5 kBq) through the cannula inserted into the left ventricle as described above, the dialysate samples were collected every 15 min for 1 h and then every 30 min for 2 h during the 3-h experiment. The samples were kept at −20°C until analysis.
Brain slice uptake of morphine and M6G
The distribution volume of morphine, M6G and [3H]sucrose was determined by the in vitro brain slice uptake technique (Ballard et al., 2000). After the rats had been killed, the brain was immediately removed and immersed in a cold Hanks/HEPES buffer (1.3 mM CaCl2, 5 mM KCl, 0.3 mM KH2PO4, 0.8 mM MgCl2, 138 mM NaCl, 0.3 mM Na2HPO4, 5.6 mM D-glucose, 10 mM HEPES, pH 7.4). Brain coronal slices (2 mm thick) were prepared using a rat brain matrix. The brain slices were weighed and preincubated with Hanks/HEPES buffer for 10 min at 37°C, and then transferred into Hanks/HEPES buffer containing morphine (100 μM) or M6G (100 μM) with [3H]sucrose (9.25 kBq ml−1). After incubation at 37°C for 5, 10, 15, 30, 60 and 120 min, the slices were removed and blot dried on filter paper and reweighed. The brain slices were homogenized in a three-fold volume of 0.01 M phosphate buffer (pH 2.0). The homogenate was centrifuged at 4800 × g for 20 min at 4°C and the supernatant was stored at −20°C until the drug concentration was determined.
Determination of morphine and M6G
The concentrations of morphine and M6G were measured by high-performance liquid chromatography (h.p.l.c.) with fluorometric detection (Venn & Michalkiewicz, 1990). Samples of CSF, plasma and tissue homogenates were subjected to solid-phase extraction using an Oasis MCX cartridge (Waters, Milford, MA, U.S.A.) that was first prewetted with methanol (1 ml), followed by water (1 ml). The sample was applied to the cartridge, the cartridge was washed with 1 ml of 0.1 N HCl and 1 ml of methanol, and morphine was then eluted with ethyl acetate with 5% NH4OH. In the case of M6G extraction, the samples were applied and the cartridge was washed with 1 ml of 0.1 N HCl, 1 ml of methanol, ethyl acetate with 5% NH4OH and acetonitrile with 5% NH4OH. Then, M6G was eluted with 5% NH4OH. Both the morphine and M6G elutions were dried under reduced pressure and the residues were reconstituted in the h.p.l.c. mobile phase (6.4% acetonitrile containing 0.1% TFA). The h.p.l.c. system consisted of a pump (880-PU, Japan Spectroscopic Co. (Jasco), Tokyo, Japan), a fluorescence detector (RF-535, Shimadzu, Tokyo, Japan) and an integrator (C-R6A, Shimadzu, Tokyo, Japan). The h.p.l.c. analytical column used was a Nucleosil C18 ODS (4.6 mm × 250 mm, 5 μm particle size, GL Sciences). Gradient elution was carried out at room temperature at a constant flow rate of 1.0 ml min−1. Solvent A was 0.1% TFA in water and solvent B was 0.1% TFA in 40% acetonitrile. The initial concentration of acetonitrile was 6.4%. After injecting the sample, the system was pumped isocratically for 2 min, followed by a gradient from 6.4 to 20% acetonitrile over 10 min, followed by a gradient from 20 to 40% acetonitrile over 2 min to wash the column. The column eluate was monitored fluorometrically at the excitation and emission wavelengths of 280 and 335 nm, respectively. The radioactivity of [3H]sucrose was measured using a liquid scintillation counter.
Data analysis
The kinetic parameters for the elimination of morphine, M6G and [3H]sucrose were determined from the following equation (Ogawa et al., 1994) using the nonlinear regression analysis program:
where CCSF(t), Vd and Kel are the cisternal CSF concentration at time t, the volume of the distribution and the elimination rate constant, respectively. Apparent elimination clearance (CL) was calculated by multiplying Kel by Vd (Kel × Vd). The elimination rate constants of morphine, M6G and [3H]sucrose in the intrathecal space were calculated by linear regression analysis of the last points belonging to the terminal phase. The area under the concentration–time curve from time zero to the last measured concentration at time t (AUC0−t) was calculated by the trapezoidal method from the experimental curve. The extrapolated AUCt−∞ was calculated by dividing the last concentration by Kel.
The amount of uptake in the brain slices (A) was expressed as the ratio to the medium concentration (Cm). The brain distribution volume (Vdbrain) and apparent uptake rate constant (Kapp) were determined from the following equation (Worboys et al., 1997) using nonlinear regression analysis:
Statistical analysis of data was determined by one-way analysis of variance (ANOVA) and the Newman–Keuls test. Statistical significance was accepted at P<0.05.
Results
Elimination of morphine and M6G from the CSF after i.c.v. injection
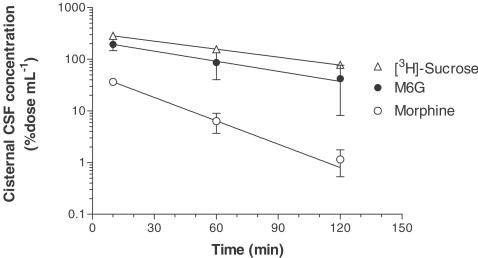
The cisternal CSF concentrations of morphine and M6G were 18 and 97 μM (10 min), 3.1 and 44 μM (60 min), 0.57 and 21 μM (120 min), respectively, after the i.c.v. injection. The cisternal CSF concentration of M6G was 5–37 times greater than that of morphine. Figure 1 shows the time course of the percentage dose of morphine, M6G and [3H]sucrose. The time profile of the CSF concentration of M6G was similar to that of [3H]sucrose. The AUC0−∞ of the cisternal CSF concentration of M6G was 10 times greater than that of morphine (Table 1). The elimination rate constants (Kel) for morphine, M6G and [3H]sucrose were 0.0347, 0.0150 and 0.0119 min−1, and the distribution volumes (Vd) were 1935, 444 and 312 μl per rat, respectively. The CLs from the cisternal CSF of morphine, M6G and [3H]sucrose were estimated to be 67.2, 6.65 and 3.71 μl min−1, respectively.
Figure 1.
Cisternal CSF concentration profiles of morphine, M6G and [3H]sucrose after i.c.v. injection. Morphine (50 nmol) or M6G (50 nmol) was coinjected with [3H]sucrose (5 kBq) into the ventricle. The rats were killed after 10, 60 and 120 min. Each point represents the mean±s.e.m. of three (morphine and M6G) or six ([3H]sucrose) rats. Solid lines were generated from Equation (2) using the parameters listed in Table 1.
Table 1.
Pharmacokinetic parameters calculated from the cisternal CSF concentration of morphine, M6G and [3H]sucrose after i.c.v. injection in rats
| Morphine | M6G | [3H]sucrose | |
|---|---|---|---|
| Kel (min−1) | 0.0347±0.0057† | 0.0150±0.0079* | 0.0119±0.0020 |
| t1/2 (min) | 19.9±3.3 | 46.2±24.4 | 58.4±9.8 |
| Vd (μl) | 1935±160††† | 444±102*** | 312±24 |
| CL (μl min−1) | 67.2±12.3††† | 6.65±3.82*** | 3.71±0.68 |
| AUC0–∞(%dose min μl−1) | 1.49±0.27;† | 15.0±8.6 | 27.0±5.0 |
Each value represents mean±s.e.m. of three (morphine and M6G) or six ([3H]sucrose) rats. Pharmacokinetic parameters were calculated from the data shown in Figure 1. Symbols show a significant difference from the values for morphine (*P<0.05, ***P<0.001) and [3H]sucrose (†P<0.05, †††P<0.001).
Distribution of morphine and M6G in the intrathecal space after the i.c.v. injection
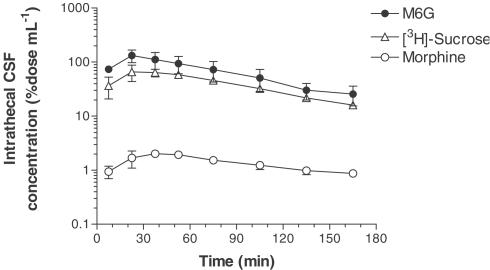
The CSF concentrations of morphine, M6G and [3H]sucrose in the intrathecal space were measured by the microdialysis method after the i.c.v. injection. The intrathecal CSF concentrations of these drugs reached maximum levels 15–30 min (M6G and [3H]sucrose) or 30–45 min (morphine) after the i.c.v. injection, and the level decreased with time (Figure 2). The peak concentrations of morphine and M6G were 1.0 and 66 μM, respectively. The intrathecal CSF concentration of M6G was 29–79 times greater than that of morphine. There was no significant difference in the Kel and elimination half-life (t1/2) among these drugs when calculated from the intrathecal CSF concentration (Table 2). However, the AUC0−∞ of the intrathecal CSF concentration of M6G was 37 times greater than that of morphine. The intrathecal CSF concentration of M6G was at a comparable level to that of M6G in the CSF taken by cisternal puncture and its AUC0−∞ was similar in the cisternal CSF (15.0% dose min μl−1) and intrathecal CSF (13.1% dose min μl−1). On the other hand, the intrathecal CSF concentration of morphine (Figure 2) was much lower than the cisternal CSF concentration (Figure 1) early on, and then the same level of morphine was detected in the cisternal and intrathecal CSF 120 min later. The AUC0−∞ of the intrathecal CSF concentration of morphine was four times lower than that of the cisternal CSF concentration.
Figure 2.
Intrathecal CSF concentration profiles of morphine, M6G and [3H]sucrose after i.c.v. injection. Morphine (50 nmol) or M6G (50 nmol) was coinjected with [3H]sucrose (5 kBq) into the rat ventricle. The intrathecal CSF concentration was measured by the microdialysis method using a probe implanted into the intrathecal space (Th11–L2). Each point represents the mean±s.e.m. of three rats.
Table 2.
Pharmacokinetic parameters calculated from the intrathecal CSF concentration of morphine, M6G and [3H]sucrose after i.c.v. injection in rats
| Morphine | M6G | [3H]sucrose | |
|---|---|---|---|
| Kel (min−1) | 0.00723±0.00118 | 0.0128±0.0013 | 0.0119±0.0020 |
| t1/2 (min) | 102.0±20.0 | 55.4±5.1 | 69.4±15.2 |
| AUC0–∞ (% dose min μl−1) | 0.356±0.060† | 13.1±4.5** | 8.16±0.50 |
Each value represents the mean±s.e.m. of three (morphine, M6G) or six ([3H]sucrose) rats. Pharmacokinetic parameters were calculated from the data shown in Figure 2. Symbols show a significant difference from the values for morphine (**P<0.01) and [3H]sucrose (†P<0.05).
Distribution of morphine and M6G in the brain and spinal cord after the i.c.v. injection
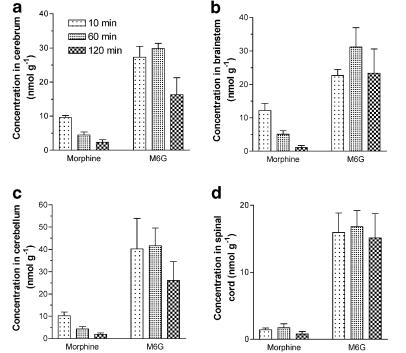
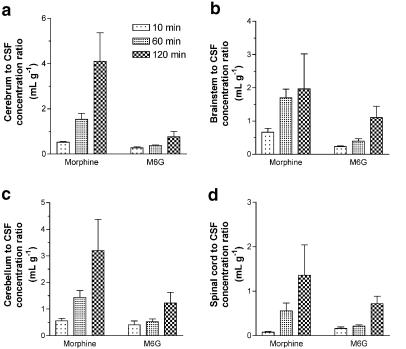
After the i.c.v. injection of morphine or M6G, the drug concentrations in the cerebrum, brainstem, cerebellum and spinal cord were measured 10, 60 and 120 min later. The M6G concentration was 2.9–6.9 times greater than that of morphine in the cerebrum (Figure 3a), 1.9–21 times greater in the brainstem (Figure 3b), 3.9–14 times greater in the cerebellum (Figure 3c) and 9.5–19 times greater in the spinal cord (Figure 3d). The tissue to cisternal CSF concentration ratios of morphine and M6G increased with time (Figure 4). The concentration ratio for M6G was lower than that for morphine except in the spinal cord after 10 min. Morphine was detected in the plasma (0.02% dose ml−1) 10 min after the i.c.v. injection of morphine (data not shown). On the other hand, M6G was not detected in the plasma after the i.c.v. injection of M6G.
Figure 3.
Concentrations of morphine and M6G in the cerebrum (a), brainstem (b), cerebellum (c) and spinal cord (d) after i.c.v. injection in rats. Morphine (50 nmol) or M6G (50 nmol) was injected into the ventricle. The rats were killed after 10, 60 and 120 min. Each column represents the mean±s.e.m. of three rats.
Figure 4.
The ratios of the concentrations in the cerebrum (a), brainstem (b), cerebellum (c) and spinal cord (d) to the CSF concentration of morphine and M6G after i.c.v. injection in rats. Morphine (50 nmol) or M6G (50 nmol) was injected into the ventricle. The rats were killed after 10, 60 and 120 min. Each column represents the mean±s.e.m. of three rats.
Uptake of morphine and M6G in brain slices
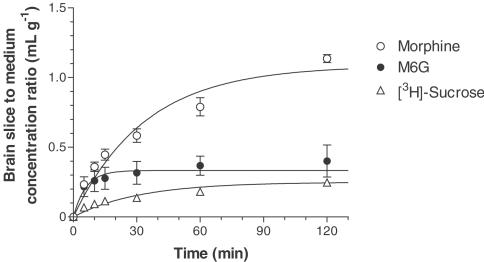
Figure 5 illustrates the time course of the brain slice to medium concentration ratio for morphine, M6G and [3H]sucrose. The slice to medium concentration ratio for morphine increased with time and reached a level of 1.1 ml g−1 after 120 min, whereas that for M6G increased rapidly and reached the equilibrium level within 30 min. The calculated brain distribution volume (Vdbrain) of M6G (0.360 ml g−1) was significantly lower than that of morphine (1.10 ml g−1) (Table 3). There was no significant difference in Vdbrain between M6G and [3H]sucrose, an extracellular marker. The apparent uptake rate constant (Kapp) for M6G was significantly greater than that for morphine or [3H]sucrose.
Figure 5.
Time course of the rat brain to medium concentration ratio of morphine, M6G and [3H]sucrose. Slices were incubated at 37°C in medium containing morphine (100 μM) or M6G (100 μM) in the presence of [3H]sucrose (9.25 kBq ml−1). Each point represents the mean±s.e.m. of three (morphine and M6G) or six ([3H]sucrose) experiments. Solid lines were generated from Equation (3) using the parameters listed in Table 3.
Table 3.
Parameters demonstrating the uptake of morphine, M6G and [3H]sucrose in rat brain slices
| Morphine | M6G | [3H]sucrose | |
|---|---|---|---|
| Kapp (min−1) | 0.0300±0.0059 | 0.138±0.035**†† | 0.040±0.007 |
| Vdbrain (ml g−1) | 1.10±0.06††† | 0.360±0.079*** | 0.235±0.012 |
Each value represents the mean±s.e.m. of three (morphine and M6G) or six ([3H]sucrose) experiments.Kapp and Vdbrain were calculated from the data shown in Figure 5. Symbols show a significant difference from the values for morphine (**P<0.01, ***P<0.001) and [3H]sucrose (††P<0.01, †††P<0.001).
Discussion
In this study, the distribution of i.c.v.-injected morphine and M6G was investigated in the brain and spinal cord of rats. To measure the elimination of morphine and M6G from the CSF, rats were injected i.c.v. with morphine and M6G, and then the drugs remaining in the cisternal CSF were determined. The cisternal CSF concentration of M6G was 5–37 times higher than that of morphine and its AUC0−∞ was 10 times greater than that of morphine. M6G diffused less from the CSF than morphine, since the distribution volume of M6G (0.44 ml) was four times lower than that of morphine (1.9 ml). M6G in the plasma was below the limit of detection after the i.c.v. injection, unlike morphine, indicating that little M6G was transported across the blood–CSF barrier and/or the blood–brain barrier. The apparent clearance of M6G from the CSF was one-tenth that of morphine and comparable to the clearance of [3H]sucrose. Sucrose, like inulin, is a relatively nondiffusible and metabolically stable compound and is used as a marker of CSF bulk flow (Payne et al., 1996). The clearance and distribution volume of [3H]sucrose are comparable to those of [14C]inulin reported by Kitazawa et al. (2000). These findings suggest that the elimination of M6G from the CSF was mainly due to the CSF bulk flow, and that a relatively small amount of M6G was eliminated by transport across the blood–CSF barrier, diffusion into the brain parenchyma and transport across the blood–brain barrier. The CSF half-life of morphine after i.c.v. injection (19.9 min) is shorter than that after s.c. injection (34.1 min), as described by Stain-Texier et al. (1999), whereas those of M6G are similar after i.c.v. (46.2 min) and s.c. (41.9 min) injection. I.c.v.-injected morphine must be transported from the CSF to the blood more rapidly than s.c.-administered morphine, because a steeper CSF–blood concentration gradient exists after i.c.v. injection. The similar half-lives of M6G after i.c.v. and s.c. injection may indicate that M6G is eliminated by a route independent of the CSF–blood concentration gradient, like CSF bulk flow. Another elimination route of i.c.v.-administered drugs is metabolism in the brain. M6G, a metabolic end product, is thought to be metabolically stable, because the hepatic extraction ratio of M6G was lower than that of morphine (Evans & Shanahan, 1995; Stain-Texier et al., 1998).
Ummenhofer et al. (2000) have demonstrated that the spinal cord distribution volume and clearance into the plasma after intrathecal administration of morphine in pigs were lower than those of fentanyl, alfentanil and sufentanil. The intrathecal kinetics of morphine can be explained by its hydrophilicity because morphine is 129–1737 times more hydrophilic than fentanyl, alfentanil or sufentanil. Since M6G is even more hydrophilic than morphine, its hydrophilicity might lead to the small distribution volume and low clearance after i.c.v. injection. In addition, there is a possibility that an active transport system participates between the CSF and blood located at the blood–CSF barrier. King et al. (2001) demonstrated that i.c.v.-administered morphine was transported to the blood by P-glycoprotein. Huwyler et al. (1996) reported that M6G was a substrate for P-glycoprotein in porcine brain capillary endothelial cells, whereas the absence of mdr1a-encoded P-glycoprotein did not enhance the antinociceptive effects of M6G in mdr1a(−/−) knockout mice (Lötsch et al., 2000). In the present study, transport of M6G across the blood–CSF barrier by P-glycoprotein or other transporters was negligible, because the apparent clearance of M6G was extremely low and no M6G was detected in the plasma.
The distribution of morphine and M6G in the intrathecal space after the i.c.v. injection was measured by the microdialysis method. The intrathecal CSF concentration of M6G was 29–79 times greater than that of morphine after the i.c.v. injection and its AUC0−∞ was 37 times greater than that of morphine. The intrathecal CSF concentration of M6G was close to the cisternal CSF concentration. On the other hand, the intrathecal CSF concentration of morphine was much lower than the cisternal CSF concentration early on, and then the same level of morphine was detected in the cisternal and intrathecal CSF 120 min later. The CSF collected by cisternal puncture is thought to correspond to the ventricular CSF (Seki et al., 1994). These data suggest that M6G is distributed more rapidly than morphine from the cerebral ventricle to the intrathecal space after i.c.v. injection. Opioid-induced analgesia is due to actions at several supraspinal and spinal sites (Reisine & Pasternak, 1996). Coadministration of morphine at both spinal (intrathecally) and supraspinal (i.c.v.) sites results in a synergistic analgesic response, with a 10-fold reduction in the total dose of morphine being necessary to produce equivalent analgesia at either site alone (Yeung & Rudy, 1980). Taken together, i.c.v.-administered M6G can access and activate opioid receptors at the dorsal horn of the spinal cord, and the activation of opioid receptors at both the spinal and supraspinal levels may lead to a synergistic increase in analgesia.
Higher concentrations of M6G were detected in the cerebrum, brainstem, cerebellum and spinal cord, compared with morphine after the i.c.v. injection. This result indicated that a relatively high concentration of M6G remained in the central nervous system after the i.c.v. injection. The ratio of brain tissue to the cisternal CSF concentration of M6G 10–120 min after the i.c.v. injection, which may reflect the transfer from the CSF to the brain or spinal cord tissues, was consistently lower than that of morphine in all regions, being consistent with the lower distribution volume of M6G estimated from the cisternal CSF concentration. These data suggest that M6G is distributed in the rat brain in a more limited manner than morphine.
It is important to estimate the brain distribution volume of opioids, because freely diffusible and pharmacologically relevant opioids in the extracellular fluid are expected to decrease as more opioids are distributed or nonspecifically bound to tissue components. From the measurement of brain slice uptake, the distribution volume in the rat brain for M6G (0.36 ml g−1) was one-third of that for morphine (1.1 ml g−1) and close to the value for [3H]sucrose (0.24 ml g−1), reflecting the extracellular space. These data suggest that morphine is distributed in the brain parenchyma cells, whereas M6G is located in the extracellular fluid. Stain-Texier et al. (1999) have reported that AUC ratios of the brain extracellular fluid to the whole brain are 2.4 ml g−1 for morphine and 0.13 ml g−1 for M6G after s.c. injection of each drug in rats. M6G must be located in the extracellular space not only in in vitro conditions but also in in vivo conditions after i.c.v. injection.
We demonstrated that the brain distribution of morphine and M6G differs after i.c.v. injection in rats. Therefore, pharmacokinetic–pharmacodynamic modeling of morphine and M6G based on the drug concentration rather than the drug dosage is important to estimate the pharmacological effect. Meineke et al. (2002) demonstrated that the CSF clearance of M6G was 10 times lower than that of morphine in patients receiving intravenous infusion of 0.5 mg kg−1 morphine. Since there is a good correlation in terms of the lower CSF clearance of M6G in humans and rats, the brain distribution of morphine and M6G may differ in humans as well as rats. The pharmacokinetic–pharmacodynamic modeling of morphine and M6G in rats may provide useful information on the clinical use of these drugs and optical dosage regimen of morphine by considering the contribution of M6G.
In summary, a relatively high concentration of M6G existed in the brain and spinal cord after the i.c.v. injection, and M6G was located in the extracellular fluid in the brain regions corresponding to the vicinity of the opioid receptors. Furthermore, i.c.v.-administered M6G was rapidly distributed into the intrathecal space; therefore, M6G may activate both the spinal and supraspinal opioid receptors and produce synergistic increases in analgesia after i.c.v. injection. These brain distribution characteristics of M6G may contribute to the potent analgesic effect of M6G after i.c.v. injection.
Acknowledgments
We thank Drs O. Sugimoto and K. Umehara (University of Shizuoka, Shizuoka) for synthesis and purification of M6G. This work was supported in part by a Grant-in-Aid for Encouragement of Young Scientists (B) (14771338) provided from the Japan Society for the Promotion of Science.
Abbreviations
- AUC
area under the concentration curve
- CL
apparent elimination clearance
- Kel
elimination rate constant
- M6G
morphine-6β-glucuronide
- Vd
distribution volume
References
- AASMUNDSTAD T.A., MØRLAND J., PAULSEN R.E. Distribution of morphine 6-glucuronide and morphine across the blood–brain barrier in awake, freely moving rats investigated by in vivo microdialysis sampling. J. Pharmacol. Exp. Ther. 1995;275:435–441. [PubMed] [Google Scholar]
- ABBOTT F.V., PALMOUR R.M. Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life Sci. 1988;43:1685–1695. doi: 10.1016/0024-3205(88)90479-1. [DOI] [PubMed] [Google Scholar]
- BALLARD P., LEAHY D.E., ROWLAND M. Prediction of in vivo tissue distribution from in vitro data 1. Experiments with markers of aqueous spaces. Pharm. Res. 2000;17:660–663. doi: 10.1023/a:1007565828856. [DOI] [PubMed] [Google Scholar]
- BICKEL U., SCHUMACHER O.P., KANG Y.-S., VOIGT K. Poor permeability of morphine 3-glucuronide and morphine 6-glucuronide through the blood–brain barrier in the rat. J. Pharmacol. Exp. Ther. 1996;278:107–113. [PubMed] [Google Scholar]
- BOUW M.R., XIE R., TUNBLAD K., HAMMARLUND-UDENAES M. Blood–brain barrier transport and brain distribution of morphine-6-glucuronide in relation to the antinociceptive effect in rats – pharmacokinetic/pharmacodynamic modelling. Br. J. Pharmacol. 2001;134:1796–1804. doi: 10.1038/sj.bjp.0704406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRUPT P.-A., TESTA B., BECHALANY A., TAYAR N.E., DESCAS P., PERRISSOUD D. Morphine 6-glucuronide and morphine 3-glucuronide as molecular chameleons with unexpected lipophilicity. J. Med. Chem. 1991;34:1272–1275. doi: 10.1021/jm00108a005. [DOI] [PubMed] [Google Scholar]
- CHOU R.C., LEVY G. Effect of heparin or salicylate infusion on serum protein binding and on concentrations of phenytoin in serum, brain and cerebrospinal fluid of rats. J. Pharmacol. Exp. Ther. 1981;219:42–48. [PubMed] [Google Scholar]
- EVANS A.M., SHANAHAN K. The disposition of morphine and its metabolites in the in-situ rat isolated perfused liver. J. Pharm. Pharmacol. 1995;47:333–339. doi: 10.1111/j.2042-7158.1995.tb05805.x. [DOI] [PubMed] [Google Scholar]
- FRANCES B., GOUT R., MONSARRAT B., CROS J., ZAJAK J.-M. Further evidence that morphine-6β-glucuronide is a more potent opioid agonist than morphine. J. Pharmacol. Exp. Ther. 1992;262:25–31. [PubMed] [Google Scholar]
- GONG Q.-L., HENDER T., HENDER J., BJÖRKMAN R., NORDBERG G. Antinociceptive and ventilatory effects of the morphine metabolites: morphine-6-glucuronide and morphine-3-glucuronide. Eur. J. Pharmacol. 1991;193:47–56. doi: 10.1016/0014-2999(91)90199-z. [DOI] [PubMed] [Google Scholar]
- HUWYLER J., DREWE J., KLUSEMANN C., FRICKER G. Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. Br. J. Pharmacol. 1996;118:1879–1885. doi: 10.1111/j.1476-5381.1996.tb15619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING M., SU W., CHANG A., ZUCKERMAN A., PASTERNAK G.W. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat. Neurosci. 2001;4:268–274. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- KITAZAWA T., HOSOYA K., TAKAHASHI T., SUGIYAMA Y., TERASAKI T. In-vivo and in-vitro evidence of a carrier-mediated efflux transport system for oestrone-3-sulphate across the blood–cerebrospinal fluid barrier. J. Pharm. Pharmacol. 2000;52:281–288. doi: 10.1211/0022357001773968. [DOI] [PubMed] [Google Scholar]
- LÖTSCH J., TEGEDER I., ANGST M.S., GEISSLINGER G. Antinociceptive effects of morphin-6-glucuronide in homozygous mdr1a P-glycoprotein knockout and in wildtype mice in the hotplate test. Life Sci. 2000;66:2393–2403. doi: 10.1016/s0024-3205(00)00569-5. [DOI] [PubMed] [Google Scholar]
- MARSALA M., MALMBERG A.B., YAKSH T.L. The spinal loop dialysis catheter: characterization of use in the unanesthetized rat. J. Neurosci. Methods. 1995;62:43–53. doi: 10.1016/0165-0270(95)00053-4. [DOI] [PubMed] [Google Scholar]
- MEINEKE I., FREUDENTHALER S., HOFMANN U., SCHAEFFELER E., MIKUS G., SCHWAB M., PRANGE H.W., GLEITER C.H., BROCKMÖLLER J. Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br. J. Clin. Pharmacol. 2002;54:592–603. doi: 10.1046/j.1365-2125.2002.t01-1-01689.x. [DOI] [PubMed] [Google Scholar]
- OGAWA M., SUZUKI H., SAWADA Y., HANANO M., SUGIYAMA Y. Kinetics of active efflux via choroid plexus of β-lactam antibiotics from the CSF into the circulation. Am. J. Physiol. 1994;266:R392–R399. doi: 10.1152/ajpregu.1994.266.2.R392. [DOI] [PubMed] [Google Scholar]
- OSBORNE P.B., CHIENG B., CHRISTIE M.J. Morphine-6β-glucuronide has a higher efficacy than morphine as a mu-opioid receptor agonist in the rat locus coeruleus. Br. J. Pharmacol. 2000;131:1422–1428. doi: 10.1038/sj.bjp.0703697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTERNAK G.W., STANDIFER K.M. Mapping of opioid receptors using antisense oligodeoxynucleotides: correlating their molecular biology and pharmacology. Trends Pharmacol. Sci. 1995;16:344–350. doi: 10.1016/s0165-6147(00)89068-9. [DOI] [PubMed] [Google Scholar]
- PAUL D., STANDIFER K.M., INTURRISI C.E., PASTERNAK G.W. Pharmacological characterization of morphine-6β-glucuronide, a very potent morphine metabolite. J. Pharmacol. Exp. Ther. 1989;251:477–483. [PubMed] [Google Scholar]
- PAYNE R., GRADERT T.L., INTURRISI C.E. Cerebrospinal fluid distribution of opioids after intraventricular & lumbar subarachnoid administration in sheep. Life Sci. 1996;59:1307–1321. doi: 10.1016/0024-3205(96)00456-0. [DOI] [PubMed] [Google Scholar]
- REISINE T., PASTERNAK G.Opioid analgesics and antagonists Goodman and Gillman's The Pharmaceutical Basis of Therapeutics 1996New York: McGraw-Hill; 521–555.9th edn., ed. H ardman , J . G . , Gilman , A . G . & Limbird , L . E . pp [Google Scholar]
- SEKI T., SATO N., HASEGAWA T., KAWAGUCHI T., JUNI K. Nasal absorption of zidovudine and its transport to cerebrospinal fluid in rats. Biol. Pharm. Bull. 1994;17:1135–1137. doi: 10.1248/bpb.17.1135. [DOI] [PubMed] [Google Scholar]
- STAIN-TEXIER F., BOSCHI G., SANDOUK P., SCHERRMANN J.-M. Elevated concentrations of morphine 6-beta-D-glucuronide in brain extracellular fluid despite low blood–brain barrier permeability. Br. J. Pharmacol. 1999;128:917–924. doi: 10.1038/sj.bjp.0702873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAIN-TEXIER F., SANDOUK P., SCHERRMANN J.-M. Intestinal absorption and stability of morphine 6-glucuronide in different physiological compartments of the rat. Drug Metab. Dispos. 1998;26:383–387. [PubMed] [Google Scholar]
- UMMENHOFER W.C., ARENDS R.H., SHEN D.D., BERNARDS C.M. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–753. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- VENN R.F., MICHALKIEWICZ A. Fast reliable assay for morphine and its metabolites using high-performance liquid chromatography and native fluorescence detection. J. Chromatogr. 1990;525:379–388. doi: 10.1016/s0378-4347(00)83414-3. [DOI] [PubMed] [Google Scholar]
- WORBOYS P.D., BRADBURY A., HOUSTON J.B. Kinetics of drug metabolism in rat liver slices III. Relationship between metabolic clearance and slice uptake rate. Drug Metab. Dispos. 1997;25:460–467. [PubMed] [Google Scholar]
- WU D., KANG Y.-S., BICKEL U., PARDRIDGE W.M. Blood–brain barrier permeability to morphine-6-glucuronide is markedly reduced compared with morphine. Drug Metab. Dispos. 1997;25:768–771. [PubMed] [Google Scholar]
- YEUNG J.C., RUDY T.A. Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J. Pharmacol. Exp. Ther. 1980;215:633–642. [PubMed] [Google Scholar]
- YOSHIMURA H., OGURI K., TSUKAMOTO H. Metabolism of drugs. The synthesis of codeine and morphine glucuronides. Chem. Pharm. Bull. 1968;16:2114–2119. doi: 10.1248/cpb.16.2114. [DOI] [PubMed] [Google Scholar]