Abstract
The nature of the cardiorespiratory effects mediated by cannabinoids in the hindbrain is poorly understood. In the present study we investigated whether cannabinoid receptor activation in the rostral ventrolateral medulla oblongata (RVLM) affects cardiovascular and/or respiratory function.
Initially, we looked for evidence of CB1 receptor gene expression in rostral and caudal sections of the rat ventrolateral medulla (VLM) using reverse transcription–polymerase chain reaction. Second, the potent cannabinoid receptor agonists WIN55,212-2 (0.05, 0.5 or 5 pmol per 50 nl) and HU-210 (0.5 pmol per 50 nl) or the CB1 receptor antagonist/inverse agonist AM281 (1 pmol per 100 nl) were microinjected into the RVLM of urethane-anaesthetised, immobilised and mechanically ventilated male Sprague–Dawley rats (n=22). Changes in splanchnic nerve activity (sSNA), phrenic nerve activity (PNA), mean arterial pressure (MAP) and heart rate (HR) in response to cannabinoid administration were recorded.
The CB1 receptor gene was expressed throughout the VLM. Unilateral microinjection of WIN55,212-2 into the RVLM evoked short-latency, dose-dependent increases in sSNA (0.5 pmol; 175±8%, n=5) and MAP (0.5 pmol; 26±3%, n=8) and abolished PNA (0.5 pmol; duration of apnoea: 5.4±0.4 s, n=8), with little change in HR (P<0.005). HU-210, structurally related to Δ9-tetrahydrocannabinol (THC), evoked similar effects when microinjected into the RVLM (n=4). Surprisingly, prior microinjection of AM281 produced agonist-like effects, as well as significantly attenuated the response to subsequent injection of WIN55,212-2 (0.5 pmol, n=4).
The present study reveals CB1 receptor gene expression in the rat VLM and demonstrates sympathoexcitation, hypertension and respiratory inhibition in response to RVLM-administered cannabinoids. These findings suggest a novel link between CB1 receptors in this region of the hindbrain and the central cardiorespiratory effects of cannabinoids. The extent to which these central effects contribute to the cardiovascular and respiratory outcomes of cannabis use remains to be investigated.
Keywords: Cannabinoid, CB1, cardiorespiratory, sympathoexcitation, apnoea, rostral ventrolateral medulla
Introduction
Δ9-tetrahydrocannabinol (THC), the psychoactive principle of cannabis, profoundly affects neural and physiological functions, including motor, cognitive, nociceptive and thermoregulatory processes, by binding with specific G protein-coupled receptors (Onaivi et al., 2002). The major receptor subtype CB1 is distributed throughout the central and peripheral nervous systems (Herkenham et al., 1991; Tsou et al., 1998) and a second subtype, CB2, is restricted to immune cells (Munro et al., 1993). The absence of many of the neurobehavioural effects of THC in CB1 receptor-knockout mice demonstrates the importance of the neural CB1 receptor (Ledent et al., 1999). Other receptors in the brain and vasculature, such as vanilloid receptors (VR1), may also mediate effects of the endogenous cannabinoid family, including anandamide (Hillard, 2000).
The impact of THC on cardiovascular and respiratory function has long been recognised as both powerful and complex. Cannabis smoking or acute administration of THC in humans induces strong tachycardia with variable effects on blood pressure, while hypotension and bradycardia are prevalent after chronic THC administration (Benowitz & Jones, 1975; Benowitz et al., 1979). Systemic administration of THC and other more potent cannabinoids, such as CP55,940 and WIN55,212-2, generally evoke large falls in blood pressure and heart rate (HR), and respiratory depression, in anaesthetised animals (Vollmer et al., 1974; Doherty et al., 1983; Estrada et al., 1987; Lake et al., 1997a). Cannabinoid-evoked hypotension in vivo is largely caused by CB1 receptor-mediated peripheral sympathoinhibition (Ishac et al., 1996; Niederhoffer & Szabo, 1999); however, direct effects on the vasculature have also been demonstrated (Hillard, 2000). In many animal studies, however, a confounding factor has been the use of anaesthesia, since in conscious animals systemically administered cannabinoids evoke either little or no change in blood pressure, or hypertensive effects (dogs: Jandhyala & Hamed, 1978; rats: Lake et al., 1997b; Kawasaki et al., 1980; Gardiner et al., 2001; 2002; rabbits: Niederhoffer & Szabo, 1999). For example, WIN55,212-2 or HU-210 produced CB1 receptor-mediated regional vasoconstriction and long-lasting pressor effects in conscious rats (Gardiner et al., 2001; 2002). Similarly, in conscious rabbits, intravenously administered WIN55,212-2 augmented sympathetic activity, without eliciting the hypotension and plasma noradrenaline depletion evoked under anaesthesia at similar doses (Niederhoffer & Szabo, 1999).
Owing to the lipophilic nature of cannabinoids, their systemic administration potentially reaches both the central and peripheral nervous systems and vasculature. The cardio-respiratory effects of central cannabinoid administration have not been widely investigated. Most recently, Niederhoffer & Szabo (1999); (2000) found that intracisternal administration of both classical and novel cannabinoids in conscious rabbits evoked sympathoexcitation, an effect that was attenuated by CB1 receptor blockade. Intracisternally administered WIN55,212-2 also reduced respiratory rate (by up to 38%) and minute volume in anaesthetised rats (Szabo et al., 2002). Curiously however, autoradiographical and immunohistochemical studies have demonstrated a paucity of CB1 receptors in the brainstem (Herkenham et al., 1991; Tsou et al., 1998), where potent cardiorespiratory effects can be elicited by other agents. In fact, this absence of receptors is traditionally thought to underlie the cardiorespiratory safety of cannabis.
Several brainstem nuclei are important for the preservation and reflex control of sympathetic activity and hence, blood pressure. In particular, the ventrolateral medulla oblongata (VLM) plays a crucial role in maintaining the integrity of sympathetic vasomotor tone, and contains a network of respiratory neurons that generate and shape the pattern of breathing (Pilowsky, 1995). The rostral region of the VLM (RVLM) contains sympathetic premotor neurons that project directly to the spinal cord (Pilowsky & Goodchild, 2002), as well as inhibitory neurons that are strongly activated during expiration, known as Bötzinger neurons (Pilowsky et al., 1990; Sun et al., 1998). Chemical diversity within these neurons as well as the inputs to this region may underlie the capacity of the RVLM to orchestrate responses to diverse stimuli.
In the present study, we hypothesised that sympathoexcitatory actions of cannabinoids may be mediated by direct effects in the RVLM. Firstly, we tested for the presence of CB1 receptor gene expression throughout the rat VLM. Secondly, we determined the cardiovascular and respiratory responses to microinjection of both novel and classical synthetic CB1/CB2 receptor agonists, R-(+)-WIN55,212-2 and HU-210, and the diarylpyrazole CB1 receptor antagonist/inverse agonist AM281 into the RVLM of urethane-anaesthetised rats.
Methods
All experiments were approved by the joint Animal Care and Ethics Committee of the Royal North Shore Hospital and University of Technology Sydney. Prior to electrophysiological experiments, male Sprague–Dawley rats (400–550 g; n=22) were housed together with access to food and water ad libitum in a 12 h light–dark environment.
Reverse transcription–polymerase chain reaction (RT–PCR)
Sprague–Dawley rats (350–500 g, n=3) were euthanased with an overdose of sodium pentobarbitone (70 mg kg−1 i.p., Nembutal, Merial Australia Pty Ltd) and immediately transcardially perfused with ice-cold sterile saline. Targeted regions of the VLM (rostral C1, caudal C1 (C1/A1) and A1 regions) were removed with a specially designed microcutter, as described by Li et al. (2003). Briefly, parasagittal sections of the medulla were cut caudal to the trapezoid line and 1 mm serial sections were removed from the VLM caudal to the facial nucleus. The remaining tissue was stained with Cresyl Violet to visualise anatomical landmarks and verify dissection of the correct VLM regions. Total RNA was extracted from the dissected brain tissue and treated with DNase I using an SV total RNA isolation system (Promega), following the manufacturers' instructions. Total RNA (150 ng) was reverse transcribed into cDNA in a 40 μl reaction with 15 U AMV reverse transcriptase (Promega), using 500 ng dT7.5 oligonucleotide primers. PCR was performed using template cDNA from each of the three VLM regions and primers complementary to nucleotides 1353–1372 (forward) and 1518–1499 (reverse) of the CB1 receptor gene sequence of the rat (Matsuda et al., 1990) (designed by V. Reja, obtained from SigmaGenosys). Each 20 μl reaction contained 2.5 mM MgCl2, 1 × PCR buffer, 0.1 μM each primer, 0.2 mM dNTPs, 0.625 U Taq polymerase (Promega) and 1 μl template cDNA. A ‘no reverse transcriptase' control (NAC) was run to determine the extent of genomic DNA contamination and a ‘no-template' control (NTC) run to test for contamination of assay reagents. Following an initial denature at 94°C for 180 s, PCR were cycled 30 times at 94°C for 15 s, 57°C for 20 s and 72°C for 25 s (Rotor-Gene 2000, Corbett Research). PCR products were run on 2% Tris borate ethylenediaminetetraacetic acid (TBE) agarose gels with a pGEM® DNA marker (Promega) to confirm the size of the amplicon. The expected CB1 receptor mRNA PCR product size was 166 bp.
Electrophysiological experiments
Anaesthesia
Animals were initially placed in a halothane-filled chamber (3.5% in O2, Fluothane®, Zeneca Limited, U.K.), prior to receiving a bolus injection of urethane (ethyl carbamate) (1.2–1.5 g kg−1 i.p., Sigma®), diluted to 10% (w v−1) to minimise peritoneal irritation. The depth of anaesthesia was assessed by the absence of reflex responses to tactile and nociceptive stimuli, and by carefully monitoring blood pressure and phrenic nerve activity (PNA) after induction of paralysis. Maintenance doses of urethane (0.2 ml i.v.) were administered when required. Body temperature was monitored with a rectal probe and maintained between 37 and 38°C with the aid of an infrared lamp and thermoregulated heating pad (Harvard Apparatus, U.S.A.).
Surgery and electrophysiological recording
The trachea was cannulated and later connected to a respirator (UGO Basile, Biological Research Apparatus, Italy). The right jugular vein and common carotid artery were cannulated for drug administration and arterial blood pressure (AP) measurement, respectively. AP was measured continuously by connecting the cannula to a pressure transducer (Abbott Critical Care Systems, U.S.A.), amplifying the signal (CWE Inc., U.S.A.) and digitally recording real-time changes in pulse pressure using Spike 2 software (Cambridge Electronic Design (CED) Ltd, U.K.). The left greater splanchnic nerve was isolated using a retroperitoneal approach, and was tied and cut to eliminate afferent transmission. The left phrenic nerve was also isolated, tied and cut distally. Nerves rested upon bipolar silver hook recording electrodes and were bathed in paraffin oil. Recording electrodes were connected to a differential preamplifier (CWE Inc., U.S.A.), filtered between 0.03 and 1 kHz and amplified a further 5000-fold through a bioamplifier. The signal was passed through a scaling amplifier and an analogue-to-digital Converter (1401 Plus, CED Ltd, U.K.), sampled at 0.8 kHz and recorded as a waveform in real time using Spike 2 software (CED Ltd, U.K.).
Animals were placed in a stereotaxic frame and heads positioned to yield a horizontal dorsal medullary surface. They were then immobilised using the neuromuscular blocking agent pancuronium bromide (induction: 0.8 mg i.v., maintenance: 0.4 mg h−1, 2 mg ml−1, Astra Pharmaceuticals Pty Ltd, Australia) and artificially ventilated with 100% O2 mixed with room air. End-tidal CO2 was measured continuously using a CO2 analyser (Capstar-100, CWE Inc., U.S.A.) and maintained between 4 and 5% of expired gases.
Intramedullary microinjection
The dorsal surface of the medulla oblongata was approached through the atlanto-occipital membrane and an occipital craniotomy. Single barrel pipettes were used for all experiments, with the exception of eight experiments involving successive injection of AM281 and WIN55,212-2, or sole administration of HU-210. In these cases, triple barrel micropipettes were filled with agonist, antagonist and vehicle, or alternatively with HU-210, L-glutamate and colloidal gold. All drugs were administered as 50 nl microinjections, apart from AM281 where 100 nl injections were used. The RVLM was identified as the site at which microinjection of the excitatory amino acid L-glutamate (50 or 100 mM, Sigma) evoked a pressor and sympathoexcitatory response with short latency and magnitude of at least 20 mmHg and 130% baseline, respectively. At the end of each experiment, microinjection of colloidal gold (100–150 nl) was used to mark injection sites. Marking the dorsal surface of the medulla with the tip of the pipette facilitated accuracy in experiments involving single-barrel micropipettes. All injection sites were verified histologically using the following protocol and only those sites that were anatomically verified and evoked the pressor response defined above were included in the grouped data. Offsite injections were also made in 11 animals into the vicinity of the RVLM, caudal regions of the VLM and/or the caudal midline medulla. In these instances, injections were made at least 8 min apart and were separated by a distance of at least 500 μm.
Histology
Brainstems of rats were harvested following administration of KCl (1–3 M i.v.) and fixed overnight (4% formaldehyde in saline). Brainstems were sectioned at 80 μm using a vibrating microtome (Leica VT1000S, Germany) into four pots containing distilled water. Gold-labelled sections underwent silver enhancement using a Silver Enhancer Kit (Sigma), following the manufacturers' instructions. Sections were mounted onto gelatinised slides in rostrocaudal sequence and, following immersion in a series of alcohols, the sections were counterstained in Cresyl Violet. Counterstained sections were observed using light microscopy (Leica, Wetzlar, Germany), compared to standard sections of Paxinos & Watson (1986), photographed (SPOT2 Digital Camera) and images adjusted for brightness and contrast using SPOT2 software (Diagnostic Instruments Inc.).
Data analysis
Electrophysiological data were analysed off-line using Spike 2 software (CED Ltd, U.K.). Splanchnic and phrenic nerve activities were rectified and smoothed at a time constant of 200 and 20 ms, respectively, for post hoc analysis. Baseline splanchnic nerve activity (sSNA), PNA and MAP were obtained by averaging a 10 s period of activity prior to microinjection. Peak changes in sSNA and MAP following microinjection were measured and expressed as a percentage of baseline activity (% baseline). HR was also derived off-line by continuous integration of systolic intervals. PNA baseline frequency and amplitude were measured and any changes in response to microinjection recorded. Inhibition of PNA was expressed as duration of apnoea, taken as the time (s) from the final preinjection peak until the time activity returned. Grouped data are expressed as mean±standard error of the mean (s.e.m.). Results were analysed by one-way ANOVA, followed by multiple t-tests with Bonferroni's correction. A paired Student's t-test was also performed to compare individual means.
Drugs
The CB1/CB2 receptor agonist WIN55,212-2 and CB1 receptor-selective antagonist AM281 were obtained from Tocris Cookson Ltd (U.K.) and dissolved in dimethylsulphoxide (DMSO)/sodium phosphate buffer (NaPB, 0.1 M) (10% v v−1, pH∼7.4). HU-210 was generously provided by Dr C. Vaughan and dissolved in DMSO. Stock solutions (10 × working concentration WIN55,212-2 and AM281, 1000 × working concentration HU-210) were stored at −20°C for up to 2 weeks. Solutions for microinjection were made up with NaPB (0.1 M) on each experimental day with the final dilution of DMSO always 1% (v v−1). L-Glutamic acid (monosodium salt, Sigma) was diluted in phosphate-buffered saline, at a concentration of either 50 or 100 mM on each experimental day. Albumin–biotin-conjugated colloidal gold (Sigma, 20 nM) was diluted 20–50%.
Results
CB1 receptor gene expression throughout the rat VLM
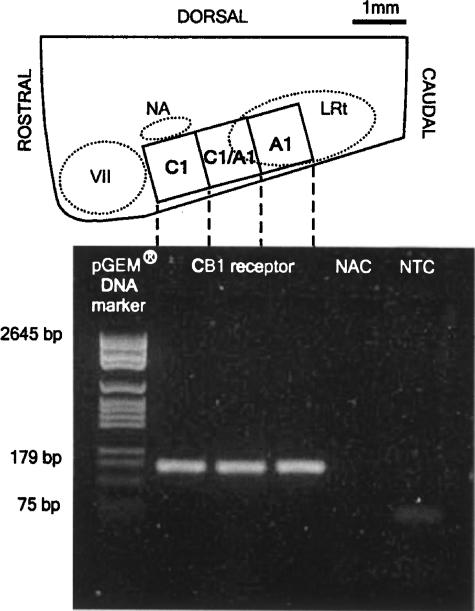
RT–PCR amplification of cDNA from the rostral C1 region (n=3), caudal C1/A1 and A1 regions (n=1) was performed. Figure 1 illustrates PCR products purified by electrophoresis on agarose gel, resulting in the expected amplicon (∼166 bp) in all the three anatomically verified regions. There was no amplification of genomic DNA in NAC, although some primer dimer formation was present in NTC (Figure 1). Although not quantitative, all the three regions show high-intensity bands.
Figure 1.
RT–PCR analysis of CB1 receptor gene expression in the rat ventrolateral medulla. Top: parasagittal section of the medulla showing three regions of the VLM from which RNA was isolated, and below: agarose gel with high-intensity amplicons in all regions confirming the presence of CB1 receptor mRNA (dashed lines indicate the region tested in lanes 2–4). Primers were complementary to bases 1353–1372 (fwd) and 1518–1499 (revs) of the CB1 receptor DNA sequence in the rat (Matsuda et al., 1990). NAC is a ‘no reverse transcriptase' control, and NTC is a ‘no template cDNA' control for assay reagents. NA, nucleus ambiguus; VII, facial nucleus; LRt, lateral reticular nucleus.
Microinjection of WIN55,212-2 or HU-210 into the RVLM of anaesthetised rats evokes cardiorespiratory effects
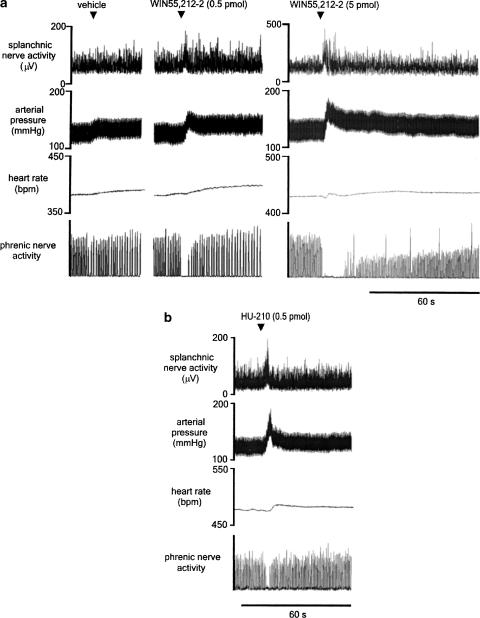
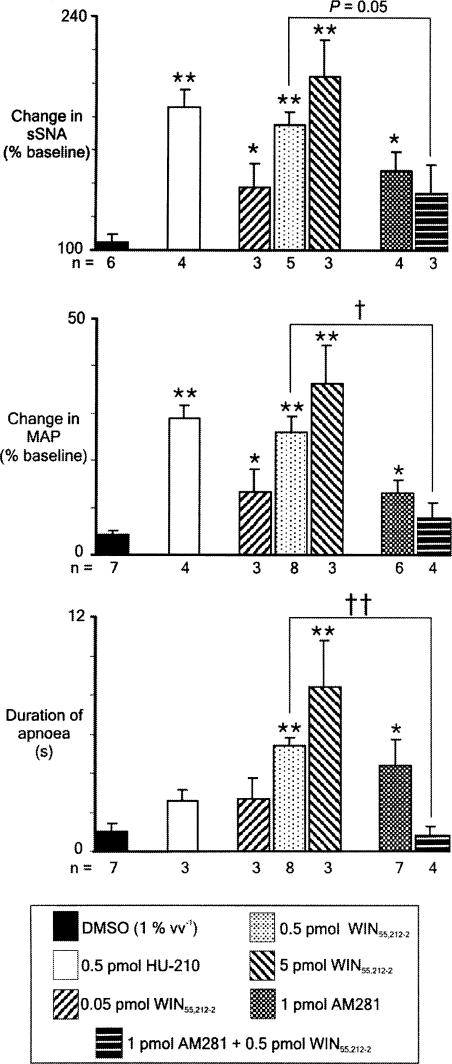
Unilateral microinjection of glutamate was used to identify physiologically bilateral pressor sites of the RVLM in all experiments. All sites were found at the rostral pole of the inferior olive (Figure 2). Vehicle was microinjected at least 5 min prior to WIN55,212-2, or into the contralateral RVLM (n=5), and also in animals never exposed to the cannabinoids (n=2). Microinjection of vehicle alone evokes small increases in sSNA and MAP, and has no effect on HR or PNA (Figure 3a, left). WIN55,212-2 (0.5 pmol, n=8; 5 pmol, n=3) was microinjected unilaterally into the left RVLM. WIN55,212-2 (0.5 pmol) evokes an immediate and short-lasting increase in sSNA and a marked increase in MAP, remaining elevated for several minutes (Figure 3a, middle). PNA is abolished for 5 s, after which time phrenic amplitude returns to baseline. Phrenic frequency was unchanged from baseline after activity returned (Figure 3a, middle). The highest dose of WIN55,212-2 (5 pmol) evokes a similar pattern of effects, but with greater magnitude (Figure 3a, right). Changes in HR were generally absent or small (∼15 beats min−1) and were not further analysed. Grouped data are shown in Figure 6, illustrating the dose–response effect of 0.05, 0.5 and 5 pmol per 50 nl WIN55,212-2 on sSNA, MAP and PNA. In order of increasing dose, WIN55,212-2 evoked highly significant (*P<0.05, **P<0.005) increases in sSNA (138±14.2, 175±7.8, 204±22%) and MAP (13.3±4.9, 26±3.4, 36.3±7.8%) and abolished PNA (duration of apnoea: 2.7±1.1, 5.4±0.4, 8.4±2.4 s). In a separate series of experiments (n=4), HU-210 (0.5 pmol per 50 nl) was microinjected unilaterally into the left RVLM. HU-210 evokes potent and short-lasting increases in sSNA and MAP, but only a small increase in HR (Figure 3b), very similar to the pattern of effects evoked by the same dose of WIN55,212-2 (Figure 3a, middle). Interestingly, compared to WIN55,212-2, HU-210 appeared to have less effect on PNA (Figure 3b). Nevertheless, in every case tested, microinjection of HU-210 into the RVLM resulted in the loss of at least one or two phrenic nerve discharges. Grouped data are shown in Figure 6, illustrating the significant effect (**P<0.005) of HU-210 on sSNA (186±11%) and MAP (29±2.6%), but in contrast the smaller but consistent effect on PNA (duration of apnoea: 2.6±0.6 s). The results suggest that increases in MAP in response to WIN55,212-2 or HU-210 injected into the RVLM are mediated primarily by the increase in sSNA, as the latency of both effects was very short and changes in HR were only minimal.
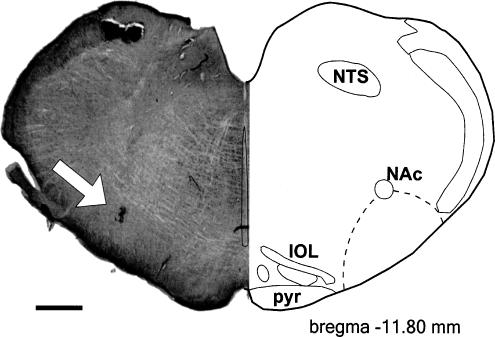
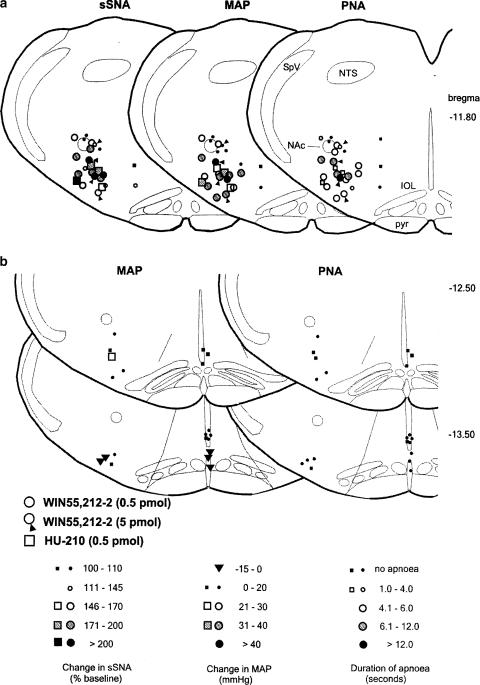
Figure 2.
Microinjection site in the RVLM. The left panel shows a transverse section of the medulla showing silver-enhanced gold particles in the ventral region marking the injection site (arrow). The injection site is located ventral to the NAC within the RVLM (dashed line) at the rostral pole of the inferior olive (approx. bregma −11.80 mm). Scale bar=500 μm. Graphic adapted from Paxinos & Watson (1986). IOL, inferior olivary nucleus; NAc, nucleus ambiguous pars compacta; NTS, nucleus tractus solitarii; pyr, pyramidal tract.
Figure 3.
Effects of unilateral microinjections (50 nl) of vehicle (DMSO 1%, v v−1), WIN55,212-2 (0.5 or 5 pmol per 50nl) or HU-210 (0.5 pmol per 50 nl) into the RVLM of urethane-anaesthetised rats. (a) A small increase in sSNA and arterial pressure is evoked by vehicle alone. In contrast, microinjection of WIN55,212-2 elicits a much larger pressor and sympathoexcitatory response of short latency, as well as a long-lasting apnoea, the higher dose producing larger effects. HR is not significantly affected by treatment with either vehicle or WIN55,212-2. (b) Microinjection of HU-210 into the RVLM also produces sympathoexcitation, hypertension and brief apnoea, as well as a small increase in HR.
Figure 6.
Grouped data demonstrating the change in sSNA, mean arterial pressure (MAP) and duration of apnoea in response to unilateral microinjection of vehicle (DMSO), HU-210 (0.5 pmol per 50 nl), different doses of WIN55,212-2 (0.05, 0.5 or 5 pmol per 50 nl), AM281 (1 pmol) or WIN55,212-2 (0.5 pmol) 5 min after microinjection of AM281 (1 pmol) into the RVLM of urethane-anaesthetised rats. Values are mean±s.e.m., *P<0.05, **P<0.005 (one-way ANOVA with post hoc t-tests and Bonferroni correction), †P<0.01, ††P<0.001 (Student's t-test). Note the similarity in the response to HU-210 and WIN55,212-2, the dose-dependent relationship in the WIN55,212-2-evoked response and that the effects of WIN55,212-2 are significantly attenuated by AM281.
Site-restricted cardiorespiratory effects of WIN55,212-2 in the VLM
All the sites tested, including offsite injections, were plotted onto standard sections to determine the region most sensitive to cannabinoid administration, and whether the effects were anatomically specific (Figure 4). The graded responses in sSNA, MAP and duration of apnoea evoked by WIN55,212-2 (circles, 0.5 pmol, n=8; circles plus arrowhead, 5 pmol, n=3) and HU-210 (squares, 0.5 pmol, n=4) are shown at each site (Figure 4a). Within the RVLM, responses were greatest when located ventrally and slightly medial to the nucleus ambiguus pars compacta (NAc), and were small or absent when dorsal or medial to NAc. Typically, the relative magnitude of the change in sSNA at a given location in the RVLM was reflected in the magnitude of change in MAP and, perhaps surprisingly, duration of apnoea. Microinjection of WIN55,212-2 or HU-210 into more caudal regions of the VLM and into the caudal midline medulla evoked only small changes, if any, in MAP, and had no effect on PNA (Figure 4b). The sympathoexcitatory and apneustic response to WIN55,212-2 and HU-210 appears to be well restricted to the RVLM (Figure 4).
Figure 4.
(a) Microinjection sites and responses evoked by unilateral microinjection of 0.5 (circles, n=8) or 5 pmol (circles plus arrowhead, n=3) WIN55,212-2 or 0.5 pmol HU-210 (squares, n=4) into the RVLM in urethane-anaesthetised rats. The magnitude of the change in sSNA, MAP and duration of apnoea in response to WIN55,212-2 or HU-210 is shown in identical transverse sections of the medulla oblongata at the level of the RVLM (∼−11.80 mm bregma). Responses were greatest when the site of injection was ventral and slightly medial to NAc in the RVLM. (b) Control microinjections of 0.5 pmol WIN55,212-2 and HU-210 into more caudal regions of the VLM and into the midline evoked small increases or decreases in MAP, but did not elicit apnoea. Each level is representative of injections made within 250 μm of that level. Graphics adapted from Paxinos & Watson (1986). IOL, inferior olivary nucleus; NAc, nucleus ambiguous pars compacta; NTS, nucleus tractus solitarii; pyr, pyramidal tract; SpV, spinal trigeminal tract.
Cardiorespiratory effects of WIN55,212-2 in the RVLM attenuated by AM281
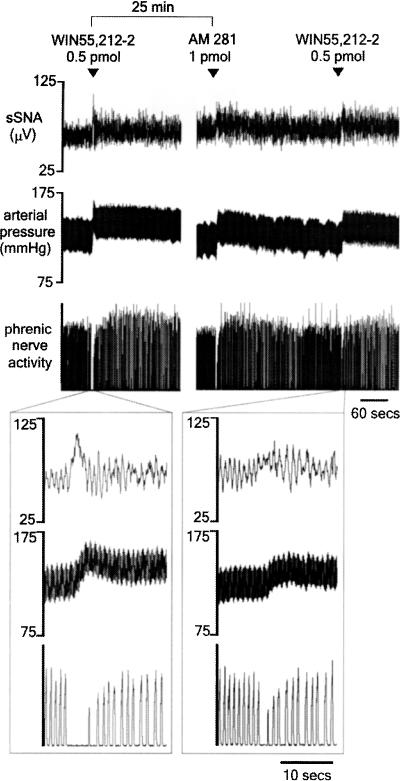
In seven animals, the CB1 receptor-selective antagonist/inverse agonist AM281 (1 pmol) was microinjected unilaterally into the RVLM to determine whether or not CB1 receptors were tonically activated in this region. Figure 5 shows the effects evoked by AM281 in an original recording in one animal. AM281 itself evokes an increase in sSNA, MAP and briefly inhibits PNA, a response very similar to that evoked by the agonists. Grouped data show that the AM281-evoked response was comparable to the lowest dose of WIN55,212-2 (0.05 pmol) (Figure 6). Figure 5 shows that a repeat injection of WIN55,212-2 (0.5 pmol), 5 min after injection of the antagonist, resulted in a marked attenuation of the response to WIN55,212-2. In one animal, WIN55,212-2-evoked effects were completely abolished, although an attenuated response was more typical (Figure 6, †P<0.01, ††P<0.001). In contrast, injection of vehicle alone 5 min prior to WIN55,212-2 did not affect the response to WIN55,21-2. Repeat injections of WIN55,212-2 in antagonist experiments were separated by at least 30 min. This protocol was followed to prevent desensitisation to agonist exposure, as the response to WIN55,212-2 (0.5 pmol) was found to be attenuated by ∼30% when injected into the RVLM within 15 min of a previous injection of WIN55,212-2 (data not shown).
Figure 5.
Response to unilateral microinjection of WIN55,212-2 (0.5 pmol) into the RVLM before and after injection of AM281 (1 pmol) into the same site in urethane-anaesthetised rats. Initially, WIN55,212-2 evokes a sympathoexcitatory and pressor response as well as persistent apnoea (exploded trace – left). AM281 evokes a similar pattern of effects, albeit a smaller response. The effects of WIN55,212-2 were markedly attenuated by AM281 (exploded trace – right).
Discussion
The major finding of the present study is that the cannabinoids WIN55,212-2 and HU-210 evoke sympathoexcitation and hypertension and abolish central respiratory drive when injected into the RVLM of urethane-anaesthetised rats. It is likely that activation of CB1 receptors underlies their effects, as these agents differ structurally but have strong affinity for CB1/CB2 receptors (Howlett et al., 2002), and pretreatment with the CB1 receptor antagonist/inverse agonist AM281 attenuated the response to WIN55,212-2. We confirmed that the CB1 receptor gene was expressed in the RVLM, and also in more caudal regions of the rat VLM, areas of the hindbrain that are also critically involved in the control of cardiorespiratory function (Pilowsky & Goodchild, 2002).
Previously, WIN55,212-2 (novel CB1/CB2 receptor agonist) and CP55,940 (potent THC analogue) were shown to evoke sympathoexcitatory and pressor responses when administered into the cisterna magna of conscious rabbits, effects that were attenuated by intravenously administered SR141716-A (CB1 receptor antagonist) (Niederhoffer & Szabo, 1999; 2000). Increases in plasma noradrenaline and blood pressure in response to intracisternally administered WIN55,212-2 have also been reported in anaesthetised rats (Szabo et al., 2002). The present findings indicate that the RVLM is one site at which cannabinoid receptor agonists evoke sympathetically mediated increases in blood pressure. The RVLM contains constitutively active spinally projecting sympathoexcitatory neurons (Pilowsky, 1995; Miyawaki et al., 2003) that receive a variety of tonic excitatory and inhibitory inputs (Pilowsky & Goodchild, 2002). It is possible that cannabinoids act to disinhibit these neurons in the RVLM, a characteristic mechanism of CB1 receptor-mediated neuromodulation throughout the CNS (Howlett et al., 2002). In essence, CB1 receptors are likely to be located presynaptically in the RVLM, although postsynaptic and non-neuronal CB1 receptors have been found in some regions of the CNS (Salio et al., 2001).
AM281 was developed as a CB1 receptor-selective compound that is structurally related to SR141716A and suitable for radiolabelling CB1 receptors in vivo (Gatley et al., 1998). AM281 is known to block or attenuate responses to established cannabinoid agonists in vitro and in vivo (Howlett et al., 2002). There are also reports that both AM281 and SR141716A share inverse agonist properties (Cosenza et al., 2000). Surprisingly, AM281 elicited similar effects to the agonists used in this study when injected into the RVLM, raising the possibility that it also acts as a partial agonist. However, although there are no previous reports that AM281 acts in this way, Martin et al. (1998) reported that SR141716A has a small, albeit not significant, agonist-like effect on the tail-flick reflex when microinjected into the rostral ventromedial medulla in conscious rats. It is possible that the physiological effects of some antagonists depend on the resting activity state of the G-protein-coupled receptor (GPCR) population (Milligan & Bond, 1997). In GPCR populations with higher basal activity, independent of the presence of endogenous ligand, ‘antagonists' may be shown to have a weak partial agonist or inverse agonist function by virtue of the proportions of active and ‘ground' state GPCRs (Milligan & Bond, 1997).
We found that WIN55,212-2-evoked sympathoexcitation and hypertension were dose-dependent, consistent with the afore-mentioned studies administering this agent intracisternally (Niederhoffer & Szabo, 1999; 2000). Furthermore, the cardiorespiratory effects evoked by WIN55,212-2 and HU-210 are likely to reflect activation of CB1 receptors, as the doses used were derived from the EC50 of WIN55,212-2 at CB1 receptors in medullary preparations in vitro (Vaughan et al., 1999). However, at a dose at least 2000-fold greater than the doses of WIN55,212-2 administered in the present study, injection of WIN55,212-2 (5 μg) into the RVLM produced a small depressor response in anaesthetised rats (Niederhoffer et al., 2001). Although this appears incongruous, dose-related biphasic effects of cannabinoids have been observed in other brain regions; for example, cannabinoids stimulate hippocampal and cortical acetylcholine release at low doses but inhibit release at high doses (Howlett et al., 2002). Dose-related variations in the cardiovascular responses to systemically administered cannabinoids have also been observed in both humans (Benowitz & Jones, 1975; Benowitz et al., 1979) and animals (Lake et al., 1997a; Niederhoffer & Szabo, 1999). In conscious rabbits, for instance, low-to-moderate systemic doses of WIN55,212-2 evoke sympathoexcitation, whereas high doses (0.5 mg kg−1) evoke sympathoinhibition and hypotension (Niederhoffer & Szabo, 1999).
The extent to which RVLM CB1 receptors contribute to the cardiovascular effects of systemically administered cannabinoids is not known. Taking dose into consideration, we noted that pressor responses to RVLM-administered WIN55,212-2 were much greater than responses to intracisternal administration (Niederhoffer & Szabo, 1999; 2000), suggesting that cannabinoids influence blood pressure at more than one site in the hindbrain. It is likely that one site may be the caudal VLM, as we found CB1 receptor mRNA in this region and observed small changes in AP in response to microinjection of WIN55,212-2 and HU-210. Also of note are the cardiovascular effects of cannabinoids at sites rostral of the brainstem, one example of which is the bradycardia evoked by lateral cerebroventricular injection of THC in anaesthetised cats (Vollmer et al., 1974). Overall cardiovascular responses to cannabinoids are therefore particularly complex, as there are multiple sites of action of both peripheral (Niederhoffer & Szabo, 1999) and central origin (Vollmer et al., 1974; Niederhoffer & Szabo, 2000), some of which do not involve CB1 receptors (Gardiner et al., 2002). However, in conscious rats, the prominent cardiovascular effects of intravenously administered WIN55,212-2 and HU-210 are CB1 receptor-mediated vasoconstriction and long-lasting pressor responses, effects that are prevented by ganglionic blockade (Gardiner et al., 2001; 2002). This response is altered dramatically by the presence of anaesthesia (Lake et al., 1997a). The underlying reasons for this are unknown, but may involve a balance of central sympathoexcitatory and peripheral hypotensive effects of cannabinoids that is altered by the animal's state of consciousness (Niederhoffer & Szabo, 2000).
In addition to cardiovascular effects, a dose-dependent and persistent inhibition of PNA was evoked by microinjection of WIN55,212-2 into the RVLM. HU-210 also consistently evoked at least single- or two-breath apnoea. There are previous reports of apnoea in response to systemic administration of THC (Graham & Li, 1973) and anandamide (Lake et al., 1997b), although anandamide may evoke these effects through CB1 receptor-independent actions in the lung (Smith & McQueen, 2001). In the present study, an interaction with Bötzinger neurons, respiratory-related neurons that are intermingled with RVLM sympathoexcitatory neurons and together occupy the same rostrocaudal extent of the VLM (Kanjhan et al., 1995; Sun et al., 1998), is likely to account for the cannabinoid-evoked inhibition of PNA. Bötzinger neurons possess a glycinergic phenotype (Schreihofer et al., 1999), make widespread inhibitory connections with medullary respiratory neurons (Ezure, 1990), and when chemically stimulated produce apnoea (Chitravanshi & Sapru, 1999). The neuronal mechanism/s of cannabinoid-evoked respiratory inhibition in the RVLM are unknown. CB1 receptors interact with a diversity of neurotransmitter systems, and often the release of different neurotransmitters within the same brain region can be modulated via CB1 receptor activation (Vaughan et al., 2000; Howlett et al., 2002).
The most profound respiratory outcome of cannabinoid administration is respiratory depression (Doherty et al., 1983; Estrada et al., 1987), an effect that is mimicked by intracisternally administered WIN55,212-2 (Szabo et al., 2002). Although cannabis-related fatality is extremely rare in humans, there are reports of gradual respiratory failure following administration of nabilone, N-methylevonantradol and THC in anaesthetised cats (Doherty et al., 1983). It is not clear where cannabinoids evoke these effects, although the medulla, which comprises vital respiratory networks (Bianchi et al., 1995), has been implicated previously (Doherty et al., 1983). The present findings support this view, indicated by the presence of CB1 receptor mRNA throughout the VLM and involvement of CB1 receptors in cannabinoid-evoked respiratory inhibition in the RVLM.
The presence of CB1 receptor gene expression throughout the VLM is consistent with earlier in situ hybridisation studies that showed expression localised in nuclei specifically associated with the functionally described RVLM (Matsuda et al., 1993). Others report generally low levels of expression in the hindbrain of rats (Matsuda et al., 1993), and even less in humans (Mailleux et al., 1992). However, an anatomical foundation for cardiorespiratory effects of cannabinoids mediated in the ventral medulla is lacking, as both radio-ligand binding (Herkenham et al., 1991) and immunohistochemical studies (Tsou et al., 1998) demonstrate low CB1 receptor levels throughout much of the brainstem. Conversely, CB1 receptors in medullary regions play major roles in some physiological processes, as illustrated by the antinociceptive properties of cannabinoids in the rostral ventromedial medulla (Vaughan et al., 1999). The divergence of anatomical and functional evidence may be explained by the relative abundance of CB1 receptors throughout the brain, compared to peptide receptors, for example (Herkenham et al., 1991; Breivogel et al., 1997), the large receptor reserve (Gifford et al., 1999), and a higher efficiency of receptor-G protein coupling in the brainstem compared to regions of greater CB1 receptor density, for example, hippocampus (Breivogel et al., 1997). Studies in CB1 receptor knockout mice also demonstrate that hippocampal and cortical slices retain some sensitivity to anandamide and WIN55,212-2 (Breivogel et al., 2001), suggesting the potential for novel cannabinoid receptors in the brain.
The physiological role of endogenous cannabinoid systems in the medulla oblongata is not well understood. The nucleus tractus solitarii (NTS), densely populated by CB1 receptors (Herkenham et al., 1991), is one site at which endogenous cannabinoids have been shown to be involved in vivo in baroreflex control via CB1 receptors (Rademacher et al., 2003) and putative vanilloid receptor-mediated effects on respiration (Geraghty & Mazzone, 2002). A role for endogenous cannabinoids in brainstem cardiovascular and respiratory control may be uncovered by future studies.
Conclusions
In conclusion, the present findings demonstrate that CB1 receptor activation in the RVLM evokes cardiorespiratory effects, and support the notion that cannabinoids interact with central pathways controlling sympathetic activity and respiration. The degree to which these centrally mediated effects contribute to cardiorespiratory responses to cannabinoids introduced into the systemic circulation remains undetermined. Further investigation of endogenous cannabinoids in the ventral medulla may also lead to new insights into the central control of cardiorespiratory function.
Note added in proof: During revision, a publication by Niederhoffer et al. (Niederhoffer, N., Schmid, K., Szabo, B. (2003). Nauynyn-Schmiederberg's Arch. Pharmacol., 367, 434–443) appeared describing the AP and HR response to microinjection of WIN55,212-2 into the RVLM and NTS of anaesthetised rats. At doses used by this group (1 or 5 μg per 500 nl; at least 2000-fold greater than doses used in this study), with injections of successive doses separated by 17 min, WIN55,212-2 microinjected into the RVLM had no effect on AP nor HR, except for a small drop in AP (∼20%) at the highest dose.
Acknowledgments
Work in the authors' laboratory is supported by grants from the National Health and Medical Research Council (211023, 211196), National Heart Foundation of Australia (G00S0716), Garnett Passe and Rodney Williams Memorial Foundation, the North Shore Heart Research Foundation (14-00/01, 17-00/01), and Northern Sydney Health. The authors would like to thank Valin Reja and Dr Chris Vaughan for their technical advice and generosity during the course of this study.
Abbreviations
- PNA
phrenic nerve activity
- RVLM
rostral ventrolateral medulla
- sSNA
splanchnic sympathetic nerve activity
References
- BENOWITZ N.L., JONES R.T. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin. Pharmacol. Ther. 1975;18:287–297. doi: 10.1002/cpt1975183287. [DOI] [PubMed] [Google Scholar]
- BENOWITZ N.L., ROSENBERG J., ROGERS W., BACHMAN J., JONES R.T. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin. Pharmacol. Ther. 1979;25:440–446. doi: 10.1002/cpt1979254440. [DOI] [PubMed] [Google Scholar]
- BIANCHI A.L., DENAVIT-SAUBIE M., CHAMPAGNAT J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol. Rev. 1995;75:2–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., GRIFFIN G., DI MARZO V., MARTIN B.R. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- BREIVOGEL C.S., SIM L.J., CHILDERS S.R. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J. Pharmacol. Exp. Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- CHITRAVANSHI V.C., SAPRU H.N. Phrenic nerve responses to chemical stimulation of the subregions of ventral medullary respiratory neuronal group in the rat. Brain Res. 1999;821:443–460. doi: 10.1016/s0006-8993(99)01139-7. [DOI] [PubMed] [Google Scholar]
- COSENZA M., GIFFORD A.N., GATLEY J., PYATT B., LIU Q., MAKRIYANNIS A., VOLKOW N.D. Locomotor activity and occupancy of brain cannabinoid CB1 receptors by the antagonist/inverse agonist AM281. Synapse. 2000;38:477–482. doi: 10.1002/1098-2396(20001215)38:4<477::AID-SYN13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- DOHERTY P.A., McCARTHY L.E., BORISON H.L. Respiratory and cardiovascular depressant effects of nabilone, N-methyllevonantradol and delta-9-tetrahydrocannabinol in anaesthetised cats. J. Pharmacol. Exp. Ther. 1983;227:508–516. [PubMed] [Google Scholar]
- ESTRADA U., BRASE D.A., MARTIN B.R., DEWEY W.L. Cardiovascular effects of delta-9- and delta-9(11)-tetrahydrocannabinol and their interaction with epinephrine. Life Sci. 1987;41:79–87. doi: 10.1016/0024-3205(87)90559-5. [DOI] [PubMed] [Google Scholar]
- EZURE K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog. Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. Regional haemodynamic responses to the cannabinoid agonist, WIN55,212-2, in conscious, normotensive rats, and in hypertensive, transgenic rats. Br. J. Pharmacol. 2001;133:445–453. doi: 10.1038/sj.bjp.0704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. Influence of the CB1 receptor antagonist, AM251, on the regional haemodynamic effects of WIN55,212-2 or HU-210 in conscious rats. Br. J. Pharmacol. 2002;136:581–587. doi: 10.1038/sj.bjp.0704750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GATLEY S.J., LAN R., VOLKOW N.D., PAPPAS N., KING P., WONG C.T., GIFFORD A.N., PYATT B., DEWEY S.L., MAKRIYANNIS A. Imaging the brain marijuana receptor: development of a radioligand that binds to cannabinoids CB1 receptors in vivo. J. Neurochem. 1998;70:417–423. doi: 10.1046/j.1471-4159.1998.70010417.x. [DOI] [PubMed] [Google Scholar]
- GERAGHTY D.P., MAZZONE S.B. Respiratory actions of vanilloid receptor agonists in the nucleus of the solitary tract: comparison of resiniferotoxin with non-pungent agents and anandamide. Br. J. Pharmacol. 2002;137:919–927. doi: 10.1038/sj.bjp.0704931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIFFORD A.N., BRUNEUS M., GATLEY S.J., LAN R.X., MAKRIYANNIS A., VOLKOW N.D. Large receptor reserve for cannabinoid actions in the central nervous system. J. Pharmacol. Exp. Ther. 1999;288:478–483. [PubMed] [Google Scholar]
- GRAHAM J.D., LI D.M. Cardiovascular and respiratory effects of cannabis in cat and rat. Br. J. Pharmacol. 1973;49:1–10. [PMC free article] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., JOHNSON M.R., MELVIN L.S., DE COSTA B.R., RICE K.C. Characterisation and localisation of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARD C.J. Endocannabinoids and vascular function. J. Pharmacol. Exp. Ther. 2000;294:27–32. [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- ISHAC E.J.N., JIANG L., LAKE K.D., VARGA K., ABOOD M.E., KUNOS G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDHYALA B.S., HAMED A.T. Pulmonary and systemic hemodynamic effects of delta9-tetrahydrocannabinol in conscious and morphine-chloralose-anaesthetised dogs: anesthetic influence on drug action. Eur. J. Pharmacol. 1978;53:63–68. doi: 10.1016/0014-2999(78)90268-6. [DOI] [PubMed] [Google Scholar]
- KANJHAN R., LIPSKI J., KRUSZEWSKA B., RONG W. A comparative study of pre-sympathetic and Botzinger neurons in the rostral ventrolateral medulla (RVLM) of the rat. Brain Res. 1995;699:19–32. doi: 10.1016/0006-8993(95)00814-7. [DOI] [PubMed] [Google Scholar]
- KAWASAKI H., WATANABE S., OISHI R., UEKI S. Effects of delta-9-tetrahydrocannabinol on the cardiovascular system, and pressor and behavioral responses to brain stimulation in rats. Jpn. J. Pharmacol. 1980;30:493–502. doi: 10.1254/jjp.30.493. [DOI] [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997a;281:1030–1037. [PubMed] [Google Scholar]
- LAKE K.D., MARTIN B.R., KUNOS G., VARGA K. Cardiovascular effects of anandamide in anesthetised and conscious normotensive and hypertensive rats. Hypertension. 1997b;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETITET F., AUBERT J.F., BESLOT F., BOHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- LI Q., GOODCHILD A.M., PILOWSKY P.M. The effect of haemorrhage on neurotransmitter-related genes in rat ventrolateral medulla: a quantitative real-time-RT–PCR study. Mol. Brain Res. 2003;114:46–54. doi: 10.1016/s0169-328x(03)00131-1. [DOI] [PubMed] [Google Scholar]
- MAILLEUX P., PARMENTIER M., VANDERHAGEN J.J. Distribution of cannabinoid receptor messenger RNA in the human brain: an in situ hybridization histochemistry with oligonucleotides. Neurosci. Lett. 1992;143:200–204. doi: 10.1016/0304-3940(92)90265-9. [DOI] [PubMed] [Google Scholar]
- MARTIN W.J., TSOU K., WALKER J.M. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci. Lett. 1998;242:33–36. doi: 10.1016/s0304-3940(98)00044-5. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., BONNER T.I., LOLAIT S.J. Localisation of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTEIN M.J., YOUNG A.C., BONNER T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MILLIGAN G., BOND R.A. Inverse agonism and the regulation of receptor number. Trends Pharmacol. Sci. 1997;18:468–474. doi: 10.1016/s0165-6147(97)01139-5. [DOI] [PubMed] [Google Scholar]
- MIYAWAKI T., GOODCHILD A.K., PILOWSKY P.M. Maintenance of sympathetic tone by a nickel chloride sensitive mechanism in the rostral ventrolateral medulla of the adult rat. Neuroscience. 2003;116:455–464. doi: 10.1016/s0306-4522(02)00705-4. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterisation of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SCHMID K., SZABO B. Analysis of the cardiovascular depressive effects of cannabinoids in anaesthetised rats (abstract) Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:R27. [Google Scholar]
- NIEDERHOFFER N., SZABO B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- ONAIVI E.S., LEONARD C.M., ISHIGURO H., ZANG P.W., LIN Z., AKINSHOLA B.E., UHL G.R. Endocannabinoid and receptor genetics. Prog. Neurobiol. 2002;567:1–38. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1986London: Academic Press; 2nd edn [Google Scholar]
- PILOWSKY P.M. Good vibrations? Respiratory rhythms in the central control of blood pressure. Clin. Exp. Pharmacol. Physiol. 1995;22:594–604. doi: 10.1111/j.1440-1681.1995.tb02072.x. [DOI] [PubMed] [Google Scholar]
- PILOWSKY P.M., GOODCHILD A.K. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J. Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- PILOWSKY P.M., JIANG C., LIPSKI J. An intracellular study of respiratory neurons in the rostral ventrolateral medulla of the rat and their relationship to catecholamine-containing neurons. J. Comp. Neurol. 1990;301:604–617. doi: 10.1002/cne.903010409. [DOI] [PubMed] [Google Scholar]
- RADEMACHER D.J., PATEL S., HOPP F.A., DEAN C., HILLARD C.J., SEAGARD J.L. Microinjection of a cannabinoid receptor antagonist into the NTS increases baroreflex duration in dogs. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1570–H1576. doi: 10.1152/ajpheart.00772.2002. [DOI] [PubMed] [Google Scholar]
- SALIO C., FISCHER J., FOSCA FRANZONI M., MACKIE K., KANEKO T., CONRATH M. CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12:3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- SCHREIHOFER A.M., STORNETTA R.L., GUYENET P.G. Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J. Comp. Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- SMITH P.J.W., McQUEEN D.S. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetised rat. Br. J. Pharmacol. 2001;134:655–663. doi: 10.1038/sj.bjp.0704296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN Q.-J., GOODCHILD A.K., CHALMERS J., PILOWSKY P.M. The pre-Botzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- SZABO B., PFITZER T., NIEDERHOFFER N. Central respiratory effects of cannabinoids in rats. Conference Proceedings, 12th Annual Symposium on the Cannabinoids, California, U.S.A. 2002.
- TSOU K., BROWN S., SANUDO-PENA M.C., MACKIE K., WALKER J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- VAUGHAN C.W., CONNOR M., BAGLEY E., CHRISTIE M.J. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal grey neurons in vitro. Mol. Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- VAUGHAN C.W., McGREGOR I.S., CHRISTIE M.J. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventrolateral medulla neurons in vitro. Br. J. Pharmacol. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLLMER R.R., CAVERO I., ERTEL R.J., SOLOMON T., BUCKLEY J.P. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (−)-delta-9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 1974;26:186–192. doi: 10.1111/j.2042-7158.1974.tb09252.x. [DOI] [PubMed] [Google Scholar]