Abstract
Magnetic resonance imaging (MRI) was used to study noninvasively the effects of compounds to resolve inflammation induced by ovalbumin (OVA) challenge in the lungs of actively sensitised rats.
Marked oedematous signals were detected between 24 and 96 h following OVA in vehicle-treated animals. When administered 24 h after OVA, budesonide, a glucocorticosteroid, or 4-(8-benzo[1,2,5]oxadiazol-5-yl-[1,7]naphthyridin-6-yl)-benzoic acid (NVP-ABE171), a selective phosphodiesterase 4 inhibitor, increased the rate of resolution of established oedematous signals detected by MRI. The effect was evident 3 h after drug administration and the signals were nearly fully resolved at 96 h postchallenge.
The drug-induced rapid resolution of MRI signals was not accompanied by changes in parameters of inflammation in the bronchoalveolar lavage fluid, but was associated with perivascular oedema detected histologically.
In conclusion, the effects of anti-inflammatory drugs on a component of allergic inflammation can be monitored by following with MRI the rate of resolution of the associated oedematous signals.
Keywords: Allergen, aquaporin, asthma, lung, magnetic resonance imaging (MRI), oedema, oedema clearance, ovalbumin, phosphodiesterase (PDE), phosphodiesterase 4 inhibitor, rat
Introduction
We recently described the noninvasive assessment by magnetic resonance imaging (MRI) of the oedema associated with allergic inflammation induced in the lungs of actively sensitised Brown Norway (BN) rats by challenge with ovalbumin (OVA) (Beckmann et al., 2001). An oedematous signal was detected by MRI in the lung, which correlated with the inflammatory status of the lung determined by bronchoalveolar lavage (BAL) fluid analysis (Beckmann et al., 2001; Tigani et al., 2002). The oedematous signal was not seen if the rats were treated before challenge with budesonide, a glucocorticosteroid used clinically, and this effect was accompanied by a significant decrease in the inflammatory parameters assessed in BAL fluid. MRI analysis also revealed that budesonide could significantly accelerate the resolution of the oedematous signal when administered 24 h after OVA challenge (Beckmann et al., 2001).
In the current study we have investigated in more detail the capacity of budesonide to resolve the oedematous signals induced by OVA challenge in the lungs of actively sensitised BN rats. Comparison has been made with NVP-ABE171 {4-(8-benzo[1,2,5]oxadiazol-5-yl-[1,7]naphthyridin-6-yl)benzoic acid}, a selective inhibitor of phosphodiesterase type 4 (PDE4) which is also a powerful inhibitor of allergic pulmonary inflammation in the rat (Trifilieff et al., 2002). The changes in the MRI signal were compared to changes in the inflammatory parameters in BAL fluid and to tissue oedema quantified by histology.
Methods
Experiments were carried out according to the Swiss federal regulations for animal protection.
Sensitisation
Male BN rats (Iffa-Credo, L'Arbresle, France) weighing approximately 250 g were used in this study. Food and water were available ad libitum. Ovalbumin (OVA; 0.02 mg ml−1; Fluka, Buchs, Switzerland) was mixed (30 min on ice) in a blender (Polytron Kinematica, Brinkmann Instruments Inc., Westbury, NY, U.S.A.) with aluminium hydroxide (20 mg ml−1) and administered subcutaneously (0.5 ml per animal). The sensitisation procedure was repeated 15 and 21 days later.
Antigen exposure
On day 29, animals were anaesthetised with 4% Forene (Abbott, Cham, Switzerland). OVA (0.3 mg kg−1 dissolved in saline, 0.2 ml per animal) was administered intratracheally (i.t.) and the animals were allowed to recover.
Experimental protocol and drug administration
Budesonide and NVP-ABE171 were dissolved in DMSO (4% final concentration) and diluted with saline or Neoral placebo, respectively. A micronised batch of budesonide supplied by Sicor (Milan, Italy) was used in this study. NVP-ABE171 was synthesised in house. Budesonide (1 mg kg−1 i.t.), NVP-ABE171 (1 mg kg−1 p.o. by gavage) or a corresponding vehicle was administered 24 h after OVA challenge. Animals were lightly anaesthetised for administration of budesonide or its vehicle. Budesonide and NVP-ABE171 have ED50 values of 0.2 mg kg−1 (L. Mazzoni, personal communication) and 0.1 mg kg−1 (Trifilieff et al., 2002), respectively, for the OVA-induced eosinophil influx and activation in this rat model of allergic airways inflammation.
All animals (n=72) were imaged by MRI under in vivo conditions. Baseline MR images were acquired 48 h before OVA challenge. Then, rats were imaged at 24 h after OVA, immediately before treatment with either budesonide, NVP-ABE171 or the corresponding vehicle. In all, 18 animals were subsequently measured at 27, 30, 48, 72 and 96 h after OVA. The other 54 animals were measured by MRI at 30 and 72 h following OVA. Out of 54 animals, 36 were submitted to BAL fluid analysis at 30 h (n=18) and 72 h (n=18) following OVA challenge, immediately after an MRI examination. The remaining 18 rats were submitted to histological analysis at 30 h (n=9) and 72 h (n=9) following OVA, immediately after an MRI examination. Histology and BAL fluid analysis were carried out in separate animals to avoid interference between both the procedures.
MRI
Measurements were carried out with a Biospec 47/40 spectrometer (Bruker, Karlsruhe, Germany) operating at 4.7 T. A gradient-echo sequence with repetition time 5.6 ms, echo time 2.7 ms, band width 100 kHz, flip angle of the excitation pulse approximately 15°, field of view 6 × 6 cm2, matrix size 256 × 128 and slice thickness 1.5 mm was used throughout the study. A single slice image was obtained by computing the two-dimensional Fourier transform of the averaged signal from 60 individual image acquisitions and interpolating the data set to 256 × 256 pixels. There was an interval of 530 ms between individual image acquisitions, resulting in a total acquisition time of 75 s for a single slice. The entire lung was covered by 25 consecutive slices. During MRI measurements, rats were anaesthetised with 2% Forene in a mixture of O2/N2O (1 : 2), administered via a face mask. The rats respired spontaneously during image acquisition, and neither respiratory nor cardiac triggering was applied. The body temperature of the animals was maintained at 37±1°C by a flow of warm air. Total examination time per animal, including positioning, was of approximately 25 min. For each animal, a set of baseline images was acquired 48 h before the OVA challenge.
MR image analysis
The volume of the oedematous signal was determined by a person unaware of the treatments using a semiautomatic segmentation procedure described in detail in Beckmann et al. (2001). The segmentation parameters were the same for all the analysed images, chosen to discriminate regions corresponding to high-intensity signals. Since the signals from oedema and vessels were of comparable intensities, the volume corresponding to the vessels was assessed on baseline images and then subtracted from the volumes determined on postchallenge images.
Ex vivo analysis
Immediately after an MRI examination, animals challenged with 0.3 mg kg−1. OVA were killed with an overdose of pentobarbital (250 mg kg−1 i.p.) and either BAL fluid analysis or histology was carried out.
BAL fluid collection and analysis
The following parameters were assessed in the BAL fluid: number of eosinophils, macrophages, lymphocytes, and neutrophils, myeloperoxidase (MPO) and eosinophil peroxidase (EPO) activities and total protein content. The lungs were lavaged using three 4 ml aliquots of solution A (Hank's balanced salt solution (HBSS) × 10, 100 ml; ethylenediaminetetraacetic acid (EDTA) 100 mM, 100 ml; 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) 1 M, 10 ml; distilled water, 790 ml). The recovered solution was pooled (representative mean recovery 11.3±0.1 ml, n=55) and the total volume of recovered fluid adjusted to 12 ml by addition of solution A.
For leukocyte differentiation an automatic cell analysing system was utilised (Cobas Helios 5Diff, Hoffmann-La Roche, Axon Lab, Basel, Switzerland). Both total leukocyte numbers and differential cell counts were obtained directly on 4 ml aliquots of BAL fluid filled into potassium EDTA cups (LIP-VAC, Milian, Switzerland).
The determination of EPO activity was based on the oxidation of o-phenylenediamine (OPD) by EPO in the presence of hydrogen peroxide (H2O2). BAL fluid supernatant (50 μl) obtained by centrifugation (400 × g, 8 min, 4°C; Omnifuge 2.0, Heraeus Sepratech, CH) was mixed with 100 μl of substrate (1 mM OPD, 1 mM H2O2, 0.1% Triton X-100, dissolved in 50 mM Tris-HCl, pH 8.0) in a 96-well flat bottomed microtitre plate and incubated for 30 min at room temperature. The reaction was quenched by adding 50 μl H2SO4 (4 M), and the absorbance measured at 492 nm in a microplate absorbance spectrophotometer (Labsystem Multiskan Plus, Bioconcept, Switzerland). The concentration of EPO was calculated as U ml−1 according to the activity of serial dilutions of a standard horseradish peroxidase.
MPO activity in BAL supernatant was measured using a 96-well plate format colorimetric assay modified according to Lefort et al. (1998). BAL fluid supernatant (50 μl) obtained by centrifugation (400 × g, 8 min, 4°C) was mixed with 100 μl of the substrate buffer (sodium phosphate 50 mM, pH 6.0, containing 0.5% hexadecyltrimethylammonium, 0.167 nM O-dianiside dihydrochloride and 0.01% H2O2) and incubated for 30 min at room temperature. The reaction was stopped by adding 100 μl of 5% sodium azide in distilled water and the absorbance read at 450 nm. Results were expressed as mU ml−1 using a standard curve established with human leukocyte MPO.
The concentration of protein in BAL fluid supernatant was measured by a colorimetric assay (Bio-Rad DC protein assay) as described by the manufacturer (Bio-Rad, Hercules, CA, U.S.A.).
Histological examination
Lungs were fixed by slow in situ inflation with approximately 5 ml of 10% phosphate-buffered neutral formalin, pH 7.2, administered via a trachea cannula. After removal from the thorax, lungs remained in phosphate-buffered neutral formalin for a maximum of 72 h. Following fixation, lungs were trimmed and sections cut longitudinally through the left lobe and transversally through the right caudal lobe so as to include the main bronchi as well as the pulmonary alveoli. After processing to paraffin wax, both sections were embedded in one block. Sections (3 μm) of each block were stained with Verhoeff reaction and the perivascular oedema was quantified on three consecutive sections of the left lobe and two of the right lobe, captured at × 50 magnification. All sections were examined in a blind manner with a light microscope (DMR BE, Leica Microsystems, Glattbrugg, Switzerland) connected to a video camera (ProgRes/3008, Jenoptic LOS, Eching, Germany). Morphometric analyses were performed with the image analysis software ‘Image Access 3.10' (IMAGIC, Glattbrugg, Switzerland).
The external oedema area (exed) and the external lamina elastica area (eel) were manually circumscribed and the perivascular oedema reported as a percentage of the external lamina elastica area (eel):
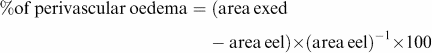 |
The measurements were restricted to vessels with diameters ranging from 80 to 1200 μm.
Statistics
Mean values (±s.e.m.) from n individual animals are presented. Two-way ANOVA multiple comparison tests were carried out at each time point using the Bonferroni method. Significance was assumed at the 5% probability level.
Results
Noninvasive assessment by MRI
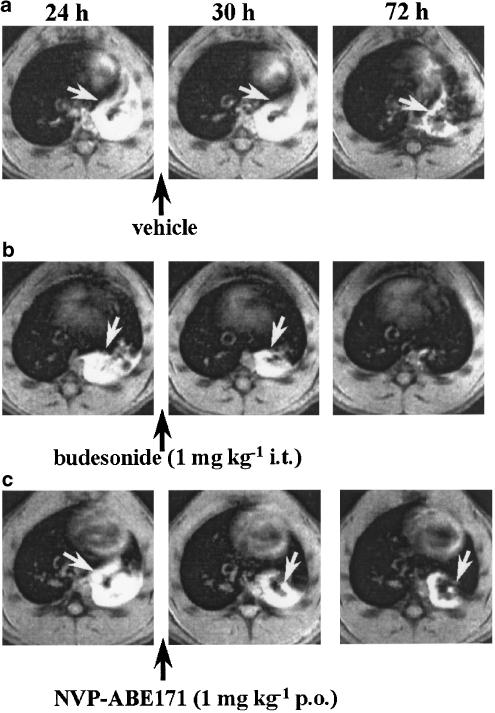
Figure 1 shows representative slices through the chests of three different animals acquired at different time points with respect to OVA challenge. These representative sections show that a marked oedematous signal was detected 24 h following OVA and that this signal was still present 72 h after OVA in vehicle-treated animals (Figure 1a). They further show that treatment with budesonide (Figure 1b) or NVP-ABE171 (Figure 1c) 24 h after OVA challenge increased the rate of resolution of the oedematous signal which was already apparent 6 h after drug administration.
Figure 1.
Transverse sections through the thorax of BN rats acquired at different time points after challenge with OVA. Images correspond to approximately the same anatomical location in each animal. For each animal, the areas corresponding to oedematous signals (indicated by the white arrows) were assessed on 25 transverse sections analogous to those shown here and covering the chest. (a) NVP-ABE171 vehicle (2 ml kg−1 p.o.), (b) budesonide (1 mg kg−1 i.t.), or (c) NVP-ABE171 (1 mg kg−1 p.o.) was administered immediately after the 24 h MRI acquisition (indicated by the black arrows). MRI images were acquired at 24, 30 and 72 h after challenge with OVA (0.3 mg kg−1 i.t., time 0). Neither respiratory nor cardiac triggering was used, and the animals respired spontaneously during image acquisition.
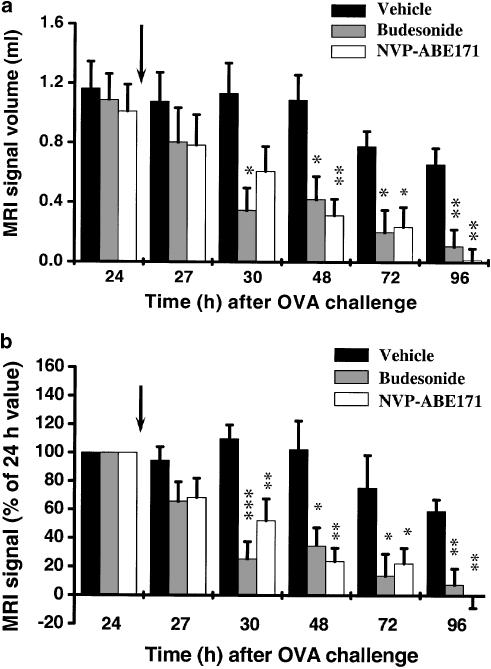
Figure 2a summarises the changes in MRI signal for rats in the different treatment regimens. We have shown previously that for a dose of 0.3 mg kg−1 OVA, oedematous signals are evident as early as 6 h after challenge, their maximum value being attained between 24 and 48 h following allergen administration (Beckmann et al., 2001). Oedematous signals are observed for about 100 h (Beckmann et al., 2001). In confirmation of our recent work (Beckmann et al., 2001), challenge with OVA led to an extensive oedematous signal in the lungs of vehicle-treated animals which persisted up to 96 h after allergen (Figure 2a, b). There were no significant differences in mean MRI signal volumes at 24 h after OVA challenge between the three groups of animals. Treatment with budesonide or NVP-ABE171 accelerated the rate of resolution of the MRI signal (Figure 2a b). For both compounds, a clear trend towards a reduction in the oedematous signal was observed already 3 h after drug administration, and the effect was statistically significant from 6 to 72 h. There was no significant difference between the effects of the compounds at any time point throughout the duration of the experiment.
Figure 2.
Changes in MRI signals in the lungs of BN rats, (a) Absolute volumes of oedematous signals. (b) Values of oedematous signals expressed as percentage, determined by setting the volume at 24 h to 100% for each animal. The data (means±s.e.m.; n=six/group) show the effects of budesonide and NVP-ABE171, administered immediately after the 24 h MRI acquisition (indicated by the black arrows), on the changes in MRI signals in the lungs of actively sensitised BN rats at different time points after OVA challenge. As no significant differences were observed between the treatment with the two different vehicles, data from vehicle-treated animals were pooled. *P<0.05, **P<0.01, ***P<0.001 indicate that the value differs significantly from the equivalent value in the vehicle-treated group.
Comparison between oedematous signals detected by MRI and BAL fluid parameters of inflammation
We next compared the oedematous signals detected by MRI with changes in the BAL fluid parameters carried out on the same animals immediately after the MRI evaluation. In confirmation of our previous observations (Figure 2), administration of budesonide or NVP-ABE171 24 h after OVA challenge led to a rapid decline in oedematous signals, an effect that was not seen following vehicle treatment (Table 1). BAL fluid markers of inflammation were not affected by either compound 6 h after treatment (Table 1). However, 48 h after treatment with budesonide or NVP-ABE171, MPO activity and protein concentrations were significantly reduced and, in NVP-ABE171-treated animals, eosinophil number and EPO activity were significantly diminished as well. A decrease in macrophage numbers was observed between 6 and 48 h after vehicle treatment. Similar, although less pronounced, falls in macrophage numbers were also seen in the drug-treated animals.
Table 1.
Comparison between oedematous signals detected by MRI and BAL fluid parameters of inflammation for post-treatment with either budesonide, NVP-ABE171 or respective vehicle
| Time point for analysis | ||||||
|---|---|---|---|---|---|---|
| Treatment (+24-h/ovalbumin) | ||||||
| 30 h after ovalbumin challenge | 72 h after ovalbumin challenge | |||||
| Vehicle (pooled) | Budesonide (1 mg kg−1 i.t.) | NVP-ABE171 (1 mg kg−1 p.o.) | Vehicle (pooled) | Budesonide (1 mg kg−1 i.t.) | NVP-ABE171 (1 mg kg−1 p.o.) | |
| n | 6 | 6 | 6 | 6 | 6 | 6 |
| MRI signal volumes | 1.4±0.1 | 0.6±0.1*** | 0.9±0.1* | 1.4+0.1 | 0.2±0.1*** | 0.3±0.1*** |
| (114.4±5.5) | (42.1±3.0***) | (71.5±6.0**) | (80.3±6.7) | (20.6±5.3***) | (30.6±8.1***) | |
| Eosinophils | 10.9±1.4 | 11.4±1.1 | 13.5±1.8 | 7.7±0.7 | 8.6±1.0 | 4.0±0.4** |
| Neutrophils | 5.1±1.0 | 6.0±0.3 | 4.7±0.6 | 1.0±0.1 | 1.1±0.1 | 0.7±0.1 |
| Macrophages | 13.4±1.7 | 15.5±0.8 | 15.0±1.8 | 7.1±0.7 | 12.7±1.5* | 11.0±1.4 |
| Lymphocytes | 2.5±0.3 | 2.9±0.2 | 2.3±0.3 | 2.1±0.3 | 2.3±0.2 | 1.6±0.2 |
| EPO | 70.4±2.07 | 68.0±3.06 | 74.89±2.31 | 61.7±4.7 | 48.5±2.01 | 25.01±2.4*** |
| MPO | 637.3±86.3 | 703.8±55.0 | 702.7±77.0 | 245.7±46.7 | 120.0±14.7* | 61.3±6.9*** |
| Protein | 1.11±0.10 | 1.14±0.10 | 1.17±0.09 | 0.75±0.05 | 0.47±0.02*** | 0.38±0.02*** |
Data are expressed as means±s.e.m. for the number of animals (n). MRI signal volumes are expressed in millilitres and in % of 24 h values (in brackets). Eosinophils, neutrophils macrophages and lymphocytes are expressed in 106 cells/12 ml, EPO and MPO in mU ml−1 and protein levels in μg μl−1. As no significant differences were seen between the treatments with the two different vehicles, data from vehicle-treated animals were pooled.
*P<0.05, **P<0.01, ***P<0.001 indicate that the value differs significantly from the equivalent value in the vehicle-treated group.
Histological analysis
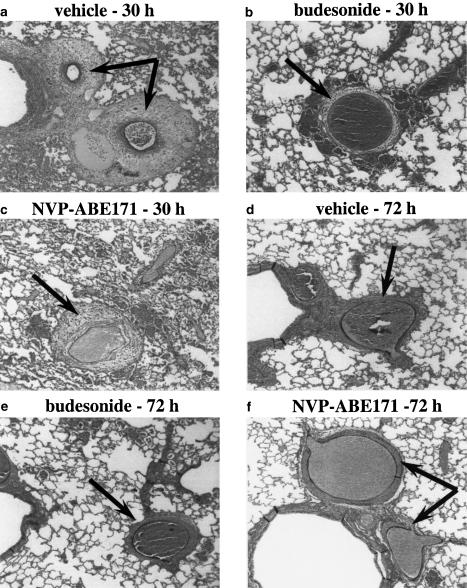
We then compared the oedematous signals detected by MRI and the presence of oedema quantified histologically on tissue from the same animals immediately after the MRI examination. Histological analysis of Verhoeff-reaction-stained lung tissue sections from OVA-challenged animals demonstrated no evidence of pleural fluid in any of the sections thereby excluding blood containing pleural effusion as a contributing factor to the oedematous signals detected by MRI. By contrast, challenge with OVA induced perivascular oedema which was predominantly detected in the left lobe compared to the right lobes (approximate ratio of 3 : 1). Marked perivascular oedema was present in lung sections from vehicle-treated rats 30 h after OVA challenge (Figure 3a). At 72 h, the perivascular oedema was present but less pronounced than at 30 h (Figures 3d and 4). When the rats were given budesonide or NVP-ABE171 24 h after challenge with OVA, significantly less fluid was present in the interstitial space at both time points (Figures 3b,c,e,f and 4). To rule out that the inflation/fixation techniques used could induce the formation of perivascular oedema, additional histological analysis was carried out on lungs removed from nonchallenged animals. No perivascular oedema was observed in these tissue sections (data not illustrated).
Figure 3.
Histological analysis of perivascular oedema. Animals were treated with budesonide, NVP-ABE171 or their respective vehicles 24 h after challenge with OVA. The lungs were removed immediately after corresponding MRI acquisition at 30 (a–c) and 72 h (d–f) after OVA challenge. Tissue sections were stained for Verhoeff reaction, (a) and (d) correspond to the vehicle for budesonide-treated animals. Magnification × 50. Perivascular oedema is indicated by the arrows.
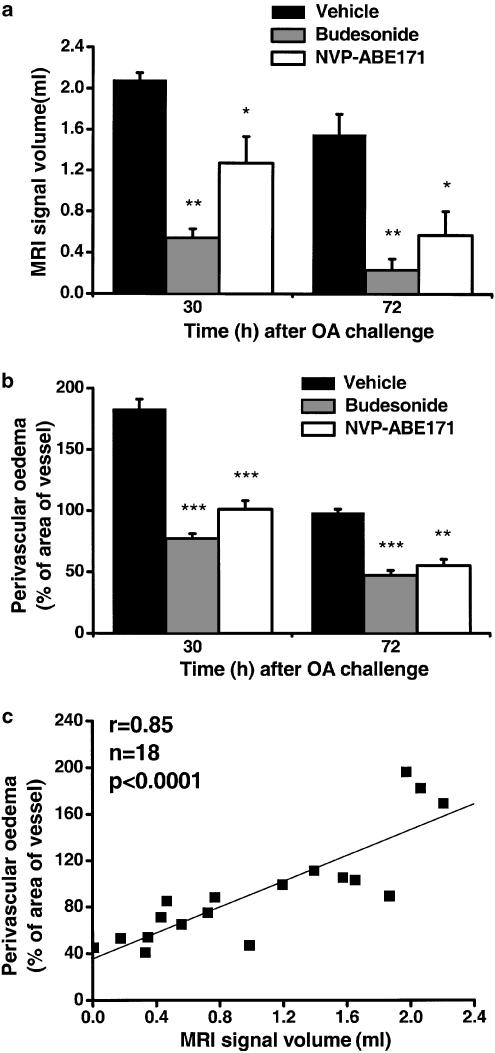
Figure 4.
Comparison between oedematous signals detected by MRI and perivascular oedema quantified by histology. (a) MRI signals and (b) perivascular oedema in the lungs of BN rats induced by ovalbumin challenge, (c) Correlation of oedematous signal volume determined using MRI and perivascular oedema quantified by histology in individual rats. The data (means±s.e.m.; n=3/group) show the effects of budesonide and NVP-ABE171, administered immediately after the 24 h MRI acquisition, on the changes in MRI signals and in the perivascular oedema quantified by histology in the lungs of actively sensitised BN rats 30 and 72 h following OVA challenge. As no significant differences were observed between the treatment with different vehicles, data from vehicle-treated animals were pooled. *P<0.05, **P<0.01, ***P<0.001 indicate that the value differs significantly from the equivalent value in the vehicle-treated rats.
Figure 4 shows that the MRI signal in the lung correlated significantly with the perivascular oedema quantified by histology carried out on the lungs obtained from the same animals killed immediately after the MRI measurement (r=0.85, P<0.0001, n=18).
Discussion
Methods available for the evaluation of the efficacy of potential anti-inflammatory treatments for lung diseases, particularly in small animal models, are limited and generally invasive. They basically rely on the determination of inflammatory parameters in BAL fluid samples (Kirby et al., 1987), in induced sputum (Pavord et al., 1997) or on the analysis of bronchial biopsies (Jeffery et al., 1989). We demonstrated recently that MRI provides a reliable means for assessing a signal associated with the pulmonary inflammation induced in actively sensitised BN rats by challenge with OVA (Beckmann et al., 2001). Since MRI detects mobile species like fluids we refer to the intense and diffuse signal following allergen as an oedematous signal. When the drugs are administered before OVA challenge, the MRI signal correlates highly significantly with BAL fluid parameters of inflammation including protein content (Tigani et al., 2002); it is therefore a component of the inflammatory response elicited by allergen. In the present study, we have applied this method to study the effects of two anti-inflammatory agents on an established inflammation induced by allergen: budesonide, a glucocorticosteroid, and NVP-ABE171, a selective inhibitor of PDE4.
In confirmation of our previous findings (Beckmann et al., 2001), instillation of OVA into the trachea resulted in intense and diffuse signals in the lungs which were present up to 96 h following challenge. Curiously, the oedematous signals were detected primarily on the left side of the animals, despite the fact that the allergen was administered before the bifurcation of the trachea. Histology confirmed that approximately 70% of perivascular oedema was in the left lung. Whereas it cannot be ruled out that the technique of intratracheal allergen administration might have contributed to this observation, we think that the lung anatomy (the right lung being divided into four different lobes) is the primary reason for the imbalance in the response. An important point to take into account is the fact that MRI is not able to discriminate between the individual lung lobes. Thus, a part of the oedematous signals detected on the left anatomical side of the animal could have arisen from the right lung. For this reason, we used the total volume of oedematous signals in our analyses.
The effects of drugs on established inflammation of the airways have been addressed in relatively few studies using post-mortem analyses limited to one or two time points (Underwood et al., 1998; Bundschuh et al., 2001). With MRI, multiple assessments are feasible on the same animal, opening the avenue for a noninvasive evaluation of the efficacy of drugs on established inflammation. Our strategy was to follow the course of MRI signals starting 24 h after challenge with OVA, at which time extensive oedematous signals as well as increases in the number and activation of inflammatory cells and protein concentration are observed (Beckmann et al., 2001). In the event, NVP-ABE171 and budesonide given 24 h after allergen challenge significantly increased the rate of resolution of established oedematous signals detected by MRI in the lungs. This effect appeared promptly (resolution was evident 3 h after drug administration) and was sustained since the oedematous signals were essentially resolved at 96 h post-OVA challenge.
The rapid resolution of the MRI signals was not accompanied by corresponding changes in the BAL fluid parameters, assessed 6 h after drug administration (Table 1), but was clearly associated with perivascular oedema determined histologically. At 48 h following NVP-ABE171 administration, eosinophil numbers, and EPO and MPO levels were significantly decreased, but there was no effect on the neutrophil numbers. At the same time point budesonide inhibited MPO but not neutrophil numbers. These observations suggest that the compounds acted primarily to suppress MPO rather than to decrease the numbers of neutrophils. Curiously, 48 h after compound administration, the number of macrophages detected in the BAL of vehicle-treated animals was smaller than in the drug treatment groups.
To elucidate possible mechanisms underlying the rapid resolution of the MRI signals the effects of drug treatment on the number and activation of cells recovered in BAL fluid were analysed 30 and 72 h after OVA challenge. At 6 h after drug administration, when the MRI signals following budesonide or NVP-ABE171 were reduced compared to vehicle, there were no changes in the inflammatory parameters in the BAL fluid. It seems, therefore, that the rapid resolution of MRI oedematous signals by the anti-inflammatory drugs did not involve general suppression of the inflammatory response. However, the excellent correlation between the oedematous signal and the extent of the perivascular oedema determined histologically suggests that an effect to suppress perivascular oedema underlies the decrease in the MRI signal. In contrast, 48 h after treatment with either budesonide or NVP-ABE171, the BAL protein levels and MPO activity were reduced; additionally, eosinophil numbers and EPO activity were also reduced following NVP-ABE171 administration. These data may provide the mechanistic basis of the sustained resolution of MRI signals.
The question arises as to the mechanism(s) by which budesonide and NVP-ABE171 suppressed perivascular oedema and the oedematous signal in the absence of any effect on BAL fluid parameters of inflammation at 6 h following drug administration. Postulated mechanisms for changes in permeability would be that budesonide suppressed the ongoing protein extravasation by local vasoconstriction (Sommer et al., 1998; Acuna et al., 2002), and that NVP-ABE171 induced cAMP-induced relaxation of tight junctions between epithelial and endothelial cells. Whereas the involvement of such processes cannot be ruled out, other possible mechanisms need to be considered.
In the last two decades, studies of lung liquid transport have provided important new concepts on the cellular mechanisms contributing to lung oedema clearance. Fluid transport is believed to be regulated by ion transport through epithelial sodium channels (ENaC) and Na+–K+–ATPase, with water movement occurring osmotically (for a review see Matthay et al., 2002). Proposed mechanisms for upregulation of sodium transport proteins by cAMP include augmented channel open probability, enhanced regulation of ENaC channels and stimulated Na+–K+–ATPase activity (Blanco et al., 1998; Jiang et al., 1998; Chen et al., 2002). There is evidence that pharmacological treatment with β2 adrenoceptor agonists increases fluid clearance (Berthiaume et al., 1987; Sakuma et al., 1997; Charron et al., 1999) and that cAMP is a second messenger for the effects (Goodman et al., 1989). Another possible mechanism for the prompt resolution of the MRI signals seen at 6 h would be involvement of aquaporins (AQPs), a family of water membrane channel proteins, which has been suggested to have a potential role in water movement between the vascular, interstitial spaces and airway compartments (for a review see Beitz & Schultz, 1999). Towne et al. (2001) showed that the decrease in the expression of AQP5 (expressed in alveolar type I and II cells, tracheal and bronchial epithelium in the lung) in mouse lung epithelial cells induced by tumour necrosis factor-α involved activated nuclear factor κB. The authors concluded that the ability of inflammatory cytokines to decrease aquaporin expression might help to explain the relationship between inflammation and oedema in the lung. Further investigations need to be carried out in order to clarify the mechanisms behind the rapid resolution of oedematous signals reported in our study.
It cannot be ruled out that multiple anaesthetisations could influence the extent of the inflammatory response in the present model. However, we have shown previously that the level of inflammatory cells and their activation in rats subjected to multiple anaesthetisations were not significantly different from those seen in animals following a single anaesthesia (Beckmann et al., 2001; Tigani et al., 2002). Also, repetitive anaesthesia did not affect the condition of the animals reflected in weight gain or general behaviour.
In conclusion, we show that the oedematous signal detected by MRI provides a means for the noninvasive assessment of one component of the inflammatory response to allergen. It allows to determine the anti-inflammatory effects of drugs in this animal model of lung injury. Previously, we had shown that the MRI signals elicited by allergen challenge correlate significantly to the parameters of inflammation determined in the BAL fluid under conditions of no drug intervention, or when the compounds are administered before challenge (Beckmann et al., 2001; Tigani et al., 2002). The significant correlation found here between the MRI signals and the perivascular oedema determined histologically lays the base for the noninvasive assessment of a fundamental component of inflammation in the allergen model, thus enabling rapid effects of drugs on established inflammation to be detected in vivo by monitoring the rate at which oedematous signals resolve. The rapid effects of compounds applied after OVA challenge on oedematous signals were not accompanied by corresponding changes in the BAL fluid parameters of inflammation, suggesting that MRI primarily reflects what happens in the tissues rather than in the lumen. Using MRI we have shown that budesonide and NVP-ABE171 are effective in reducing established inflammation in the BN rat challenge model. Our results imply that oral treatment with selective PDE4 inhibitors may offer an alternative to glucocorticosteroids as an anti-inflammatory therapy in lung diseases.
Abbreviations
- BAL
bronchoalveolar lavage
- BN
Brown Norway
- EDTA
ethylenediaminetetraacetic acid
- ENaC
epithelial sodium channels
- EPO
eosinophil peroxidase
- i.t.
intratracheal
- MPO
myeloperoxidase
- MRI
magnetic resonance imaging
- OVA
ovalbumin
- p.o.
per os
- PDE
phosphodiesterase
- PDE4
phosphodiesterase type 4
References
- ACUNA A.A., GABRIJELCIC J., URIBE E.M., RABINOVICH R., ROCA J., BARBERA J.A., CHUNG K.F., RODRIGUEZ-ROISIN R. Fluticasone propionate attenuates platelet-activating factor-induced gas exchange defects in mild asthma. Eur. Respir. J. 2002;19:872–878. doi: 10.1183/09031936.02.00268802. [DOI] [PubMed] [Google Scholar]
- BECKMANN N., TIGANI B., EKATODRAMIS D., BORER R., MAZZONI L., FOZARD J.R. Pulmonary oedema induced by allergen challenge in the rat: non-invasive assessment by magnetic resonance imaging. Magn. Reson. Med. 2001;45:88–95. doi: 10.1002/1522-2594(200101)45:1<88::aid-mrm1013>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- BEITZ E., SCHULTZ J.E. The mammalian aquaporin water channel family: a promising new drug target. Curr. Med. Chem. 1999;6:457–467. [PubMed] [Google Scholar]
- BERTHIAUME Y., STAUB N.C., MATTHAY M.A. Beta-adrenergic agonists increase lung liquid clearance in anaesthetized sheep. J. Clin. Invest. 1987;79:335–343. doi: 10.1172/JCI112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCO G., SANCHEZ G., MERCER R.W. Differential regulation of Na+–K+–ATPase isozymes by protein kinases and arachidonic acid. Arch. Biochem. Biophys. 1998;359:139–150. doi: 10.1006/abbi.1998.0904. [DOI] [PubMed] [Google Scholar]
- BUNDSCHUH D.S., ELTZE M., BARSIG J., WOLLIN L., HATZELMANN A., BEUME R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J. Pharmacol. Exp. Ther. 2001;297:280–290. [PubMed] [Google Scholar]
- CHARRON P.D., FAWLEY J.P., MARON M.B. Effect of epinephrine on alveolar liquid clearance in the rat. J. Appl. Physiol. 1999;87:611–618. doi: 10.1152/jappl.1999.87.2.611. [DOI] [PubMed] [Google Scholar]
- CHEN X.-J., EATON D.C., JAIN L. Adrenergic regulation of amiloride-sensitive lung sodium channels. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L609–L620. doi: 10.1152/ajplung.00356.2001. [DOI] [PubMed] [Google Scholar]
- GOODMAN B.E., ANDERSON J.L., CLEMENS J.W. Evidence for regulation of sodium transport from airspace to vascular space by cAMP. Am. J. Physiol. Lung Cell. Mol. Physiol. 1989;257:L86–L93. doi: 10.1152/ajplung.1989.257.2.L86. [DOI] [PubMed] [Google Scholar]
- JEFFERY P.K., WARDLAW A.J., NELSON F.C., COLLINS J.V., KAY A.B. Bronchial biopsies in asthma: an ultrastructural quantitative study and correlation with hyperreactivity. Am. Rev. Respir. Dis. 1989;140:1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- JIANG X., INGBAR D.H., O'GRADY S.M. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl− channels. Am. J. Physiol. Cell. Physiol. 1998;275:C1610–C1620. doi: 10.1152/ajpcell.1998.275.6.C1610. [DOI] [PubMed] [Google Scholar]
- KIRBY J.G., HARGREAVE F.E., GLEICH G.J., O'BYRNE P.M. Bronchoalveolar lavage profiles of asthmatic and non-asthmatic subjects. Am. Rev. Respir. Dis. 1987;136:379–383. doi: 10.1164/ajrccm/136.2.379. [DOI] [PubMed] [Google Scholar]
- LEFORT J., SINGER M., LEDUC D., RENESTO P., NAHORI M.A., HUERRE M., CREMINON C., CHIGNARD M., VARGAFTIG B.B. Systemic administration of endotoxin induces bronchopulmonary hyperreactivity dissociated from TNF-alpha formation and neutrophil sequestration into the murine lungs. J. Immunol. 1998;161:474–480. [PubMed] [Google Scholar]
- MATTHAY M.A., FOLKESSON H.G., CLERICI C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- PAVORD I.D., PIZZICHINI M.M., PIZZICHINI E., HARGREAVE F.E. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKUMA T., FOLKESSON H.G., SUZUKI S., OKANIWA G., FUJIMURA S., MATTHAY M.A. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am. J. Respir. Crit. Care Med. 1997;155:506–512. doi: 10.1164/ajrccm.155.2.9032186. [DOI] [PubMed] [Google Scholar]
- SOMMER A., VERAART J., NEUMANN M., KESSELS A. Evaluation of the vasoconstrictive effects of topical steroids by laser-Doppler-perfusion-imaging. Acta Derm. Venereol. 1998;78:15–18. doi: 10.1080/00015559850135751. [DOI] [PubMed] [Google Scholar]
- TIGANI B., SCHAEUBLIN E., SUGAR R., JACKSON A.D., FOZARD J.R., BECKMANN N. Pulmonary inflammation monitored non-invasively by MRI in freely breathing rat. Biochem. Biophys. Res. Commun. 2002;292:216–221. doi: 10.1006/bbrc.2002.6633. [DOI] [PubMed] [Google Scholar]
- TOWNE J.E., KRANE C.M., BACHURSKY C.J., MENON A.G. Tumor necrosis factor-a inhibits aquaporin 5 expression in mouse lung epithelial cells. J. Biol. Chem. 2001;276:18657–18664. doi: 10.1074/jbc.M100322200. [DOI] [PubMed] [Google Scholar]
- TRIFILIEFF A., WYSS D., WALKER C., MAZZONI L., HERSPERGER R. Pharmacological profile of a novel phosphodiesterase 4 inhibitor, 4-(8-benzo[1,2,5]oxadiazol-5-yl-[1,7]naphthyridin-6-yl)-benzoic acid (NVP-ABE171), a 1,7-naphthyridine derivative, with anti-inflammatory activities. J. Pharmacol. Exp. Ther. 2002;301:241–248. doi: 10.1124/jpet.301.1.241. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD D.C., BOCHNOWICZ S., OSBORN R.R., KOTZER C.J., LUTTMANN M.A., HAY D.W.P., GORYCKI P.O., CHRISTENSEN S.B., TORPHY T.J. Antiasthmatic activity of the second-generation phosphodiesterase 4 (PDE4) inhibitor SB 207499 (Ariflo) in the guinea pig. J. Pharmacol. Exp. Ther. 1998;298:988–995. [PubMed] [Google Scholar]