Abstract
The neurotransmitters involved in NANC relaxation and their possible interactions were investigated in mouse isolated stomach, recording the motor responses as changes of endoluminal pressure from whole organ.
Field stimulation produced tetrodotoxin-sensitive, frequency-dependent, biphasic responses: rapid transient relaxation followed by a delayed inhibitory component.
The inhibitor of the synthesis of nitric oxide (NO), L-NAME, abolished the rapid relaxation and significantly reduced the slow relaxation. Apamin, blocker of Ca2+-dependent K+ channels, or ADPβS, which desensitises P2y purinoceptors, reduced the slow relaxation to 2–8 Hz, without affecting that to 16–32 Hz or the fast relaxation. α-Chymotrypsin or vasoactive intestinal polypeptide 6–28 (VIP6–28), antagonist of VIP receptors, failed to affect the fast component or the delayed relaxation to 2–4 Hz, but antagonised the slow component to 8–32 Hz.
Relaxation to sodium nitroprusside was not affected by L-NAME, apamin or ADPβS, but was reduced by α-chymotrypsin or VIP6–28. Relaxation to VIP was abolished by α-chymotrypsin, antagonised by VIP6–28, but was not affected by L-NAME, apamin or ADPβS. Relaxation to ATP was abolished by apamin, antagonised by ADPβS, but was not affected by L-NAME or α-chymotrypsin.
The present results suggest that NO is responsible for the rapid relaxation and partly for the slow relaxation. ATP is involved in the slow relaxation evoked by low frequencies of stimulation. VIP is responsible for the slow relaxation evoked by high frequencies of stimulation. The different neurotransmitters appear to work in parallel, although NO could serve also as a neuromodulator that facilitates release of VIP.
Keywords: NANC inhibitory neurotransmitters, mouse stomach, nitric oxide, VIP, ATP, gastric relaxation
Introduction
In the stomach, nonadrenergic, noncholinergic (NANC) inhibitory neurones are responsible for receptive relaxation, a response through which the organ adapts to receive a large volume with only a minimal increase in pressure during food intake (Abrahamsson, 1973; Desai et al., 1991). Nitric oxide (NO) has been shown to be a critical mediator of relaxation (Desai et al., 1991), but other inhibitory transmitters, such as vasoactive intestinal polypeptide (VIP) and adenosine triphosphate (ATP) may also contribute to the gastric inhibitory neurotransmission (Grider et al., 1985; Jenkinson & Reid, 2000).
There is a lot of controversy about the interaction between NO and VIP in mediating relaxation of the gastric smooth muscle. Some studies on guinea-pig and rat have indicated that NO influences the release of VIP and vice versa (Grider et al., 1992; Makhlouf & Grider, 1993; Jin et al., 1996), whereas other studies in the rat, guinea-pig, cat, pig and dog have suggested that NO and VIP act in parallel (Lefebvre et al., 1992; Barbier & Lefebvre, 1993; Desai et al., 1994; Bayguinov et al., 1999; Dick & Lefebvre, 2000; Ergün et al., 2001). Moreover, NOS may colocalise with ATP or VIP in the myenteric plexus (Belai & Burnstock, 1994), but the nature of the interaction between NO and purinergic neurotransmitters remains unclear.
In the stomach of the mouse, which is being used increasingly as experimental model because of the advent of gene-targeting technology, the interaction between VIP and NO as NANC neurotransmitters has not been fully clarified. In mouse, NO acts as a NANC inhibitory neurotransmitter in the gut including gastric fundus (Yano et al., 1995; Mashimo et al., 1996; Pfeifer et al., 1998; Baccari et al., 2000; Ny et al., 2000; Selemidis & Cocks, 2000; Ergün & Öğülener, 2001; Mulè & Serio, 2002). Some reports also suggest the participation of a peptide, such as VIP, in NANC transmission in the mouse stomach (Mashimo et al., 1996; Baccari et al., 2000; Ergün & Öǧülener, 2001). However, it is still unsettled if VIP and NO are sequentially linked or act in parallel to induce relaxation. Furthermore, none of these studies investigated whether the relaxation to NO depends on VIP release from the enteric nerves. In addition, although recent data indicate that ATP acting on P2y receptors causes relaxation of the murine gastric fundus (Giaroni et al., 2002), little is known about the contribution of ATP in the neurogenic mechanical relaxation of the gastric smooth muscle of the mouse and its possible interplay with the other neurotransmitters.
Therefore, the purpose of the present study was to investigate the contribution of NO, VIP and ATP to NANC responses, and the possible interactions between the different neurotransmitters in mouse isolated stomach. It has been reported that it is important to consider the experimental method when studying the influence of NOS inhibitors on the relaxation induced by VIP in gastric preparations (Dick & Lefebvre, 2000; Dick et al., 2000). In fact, many of the studies upon which the serial cascade model is based were performed on isolated gastric smooth muscle cells, whereas the majority of studies on gastric muscular strips have not supported the serial cascade model of enteric inhibitory neurotransmission. So, we carried out experiments using the isolated whole organ, in order to study the muscle function under conditions where the influence of external factors is removed, but the muscle itself performs in a manner analogous to its in vivo capacity.
Methods
Experiments were authorised by the Ministero della Sanità (Rome, Italy). Mice (male, C57BL/10SnJ, 20–35 g) were killed by cervical dislocation. The abdomen was immediately opened, the oesophagus was tied proximal to the lower oesophageal sphincter, and the entire stomach was excised. Preparations were mounted in a custom-designed organ bath continuously perfused with oxygenated (95% O2 and 5% CO2) and heated (37°C) Krebs solution with the following composition (mM): NaCl 119; KCl 4.5; MgSO4 2.5; NaHCO3 25; KH2PO4 1.2; CaCl2 2.5; glucose 11.1. The Krebs solution always contained atropine (1 μM) and guanethidine (1 μM) to block cholinergic and adrenergic responses, and thus to impose NANC recording conditions. The pyloric end was tied around the mouth of a J-tube, which was connected via a T catheter to a standard pressure transducer (Statham Mod. P23XL). The mechanical activity was recorded on an ink-writer polygraph (Grass model 7D). To provide electrical field stimulation (EFS), a pair of platinum plates was placed in parallel on either side of the entire stomach. EFS was applied by an S88 square-wave pulse generator (Grass Medical Instruments, Quincy, MA, U.S.A.) coupled via a stimulus isolation unit (Grass SIU5) to the electrodes. Preparations were allowed to equilibrate for about 60 min before starting the experiment.
Experimental protocol
After the equilibration time, EFS was performed or relaxant agents were administered. EFS (0.5 ms duration, supramaximal voltage, in trains of 5 s, 2–32 Hz) was applied to the tissue at 10 min intervals. After the relaxant responses to NANC nerve stimulation had been obtained, the Krebs solution was changed with one containing one or more antagonists of the putative mediators or of mechanisms of NANC relaxation and the tissue was incubated for at least 30 min before recording a second series of responses to EFS. The antagonists tested were: Nω-nitro-L-arginine methyl ester (L-NAME, 300 μM), apamin (0.1 μM), α-chymotrypsin (10 U ml−1), VIP6–28 (10 μM) and adenosine 5′-O-(2-thiodiphosphate) (ADPβS) (10 μM). The efficacy and the specificity of these blocking drugs at the concentrations used were verified by testing their effects on the relaxant responses induced by exogenous agonists (data not shown). Then, in separate experimental gastric preparations, the responses to noncumulative concentrations of sodium nitroprusside (SNP), VIP and ATP were examined in the absence and presence of the different inhibitors/antagonists. The agonists were added to the bath after switching off the perfusion.
Data analysis and statistical tests
The amplitude values of the relaxant responses to EFS refer to the maximal peak obtained during the stimulation period. Responses to SNP, VIP and ATP have been expressed as a percentage of the maximum response obtained in the same tissue to 10 μM SNP, 0.3 μM VIP and 1 mM ATP, respectively. All data are expressed as mean values±s.e.m. The letter n indicates the number of experiments, and it is equivalent to the number of experimental animals. Statistical analysis was performed by means of paired Student's t-test or analysis of variance, followed by Bonferroni's t-test, when appropriate. A probability value of less than 0.05 was regarded as significant.
Drugs
The following drugs were used: ATP, atropine sulphate, ADPβS, guanethidine monosulphate, L-NAME, tetrodotoxin (TTX), apamin, α-chymotrypsin, VIP, VIP6–28, SNP (all purchased from Sigma Chemical Corp., St Louis, MO, U.S.A.). The stock solutions were prepared by dissolving all drugs in distilled water and kept frozen. The working solutions were prepared fresh on the day of the experiment by diluting the stock solutions with Krebs solution.
Results
Responses to NANC nerve stimulation
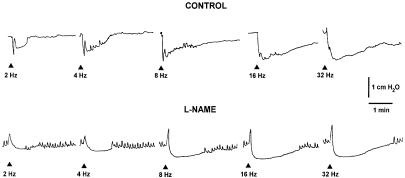
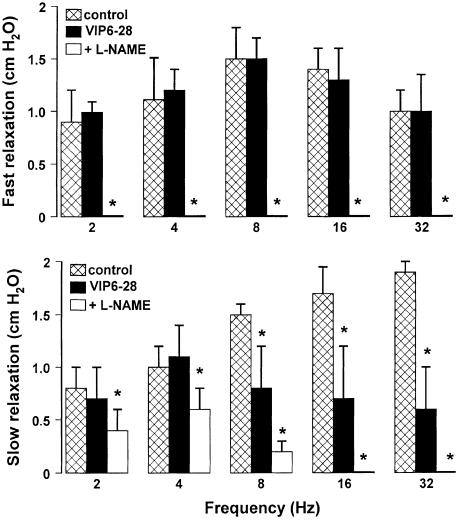
EFS (2–32 Hz) induced TTX-sensitive, frequency-dependent relaxations. These consisted of a rapid and transient first phase, reaching its maximum within 2 s, followed by a second phase with a slower onset time and a longer duration. As previously described, the rapid relaxation reached the maximal amplitude at 8 Hz, whereas the delayed prolonged relaxation was enhanced in amplitude and duration as the stimulation frequency was increased (Mulè & Serio, 2002). L-NAME (300 μM), which increased the resting endoluminal pressure (1–2 cm H2O), caused the abolition of the fast relaxation and often unmasked a NANC contraction. L-NAME also reduced the second long-lasting relaxant phase evoked at all frequencies tested (Figure 1). This suggests that NO is responsible for the fast relaxation and it is also involved in the long-lasting relaxation.
Figure 1.
Original tracings illustrating the effect of L-NAME on the EFS-evoked NANC relaxations. EFS (0.5 ms, supramaximal voltage, for 5 s, 2–32 Hz) evoked biphasic responses, consisting of a fast relaxation followed by a slow relaxation. L-NAME (300 μM) abolished the early fast component and reduced the slow relaxation. Arrows indicate EFS.
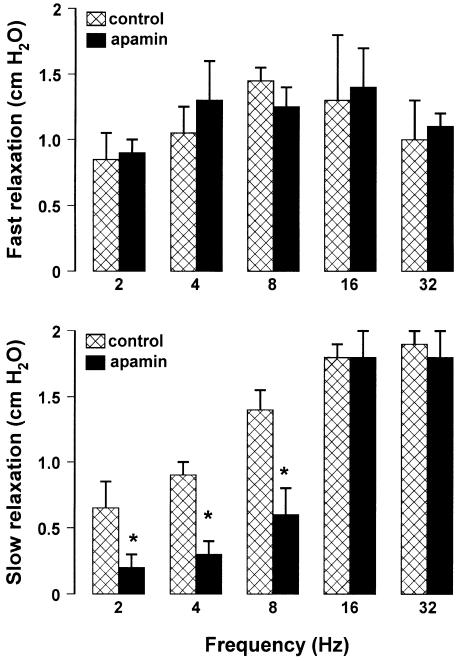
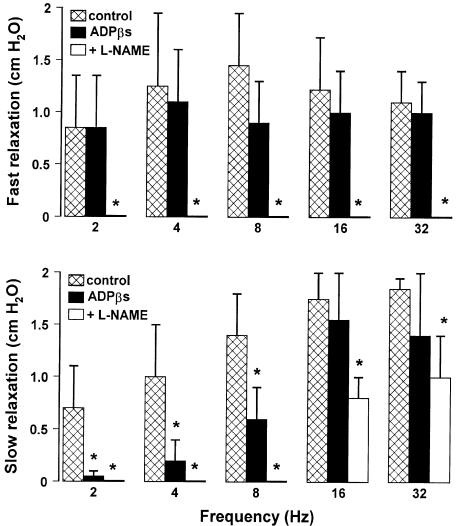
To investigate the involvement of a purinergic pathway in mediating the gastric response to EFS, we examined the effects induced by apamin (0.1 μM) or by desensitisation of the P2y purinergic receptors with ADPβs (10 μM). Neither drug affected the resting endoluminal pressure. In the presence of apamin (0.1 μM), the slow relaxation evoked by EFS at frequencies up to 8 Hz was significantly reduced, whereas the slow relaxation evoked by EFS at frequencies higher than 8 Hz as well as the fast relaxation were not modified (Figure 2). The desensitisation of P2y purinergic receptors with ADPβs (10 μM) significantly reduced only the slow component evoked by low frequencies of stimulation (up to 8 Hz). The addition to the bath of L-NAME (300 μM) after ADPβs (10 μM) abolished the slow relaxation to 2–8 Hz, and significantly reduced the slow component to 16–32 Hz (Figure 3).
Figure 2.
Effects of apamin on the amplitude of the two components of NANC relaxation evoked by different stimulation frequencies. Apamin (0.1 μM) did not modify the fast relaxation, but it significantly reduced the slow relaxation to 2–8 Hz, without affecting that to higher frequencies of stimulation. All values are mean±s.e.m., n=4. *P<0.05 when compared to the respective control conditions (using Student's t-test).
Figure 3.
Effects of the desensitisation of the P2y purinergic receptors with ADPβs on the amplitude of the two components of NANC relaxation evoked by different stimulation frequencies. ADPβs (10 μM for 30 min) did not modify the fast relaxation, but it significantly reduced the slow relaxation to 2–8 Hz, without affecting that to higher frequency of stimulation. The subsequent addition of L-NAME (300 μM) abolished the fast relaxation and the slow relaxation to 2–8 Hz, and significantly reduced the second slow component to 16–32 Hz. All values are mean±s.e.m., n=4. *P<0.05 when compared to the respective control conditions (using ANOVA followed by Bonferroni's t-test).
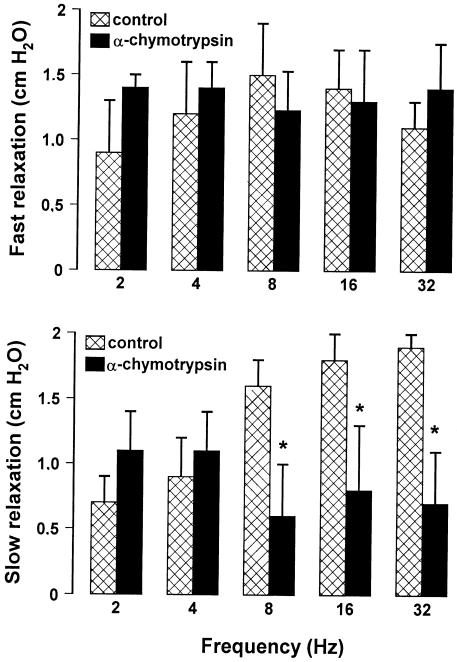
To investigate whether VIP participates in the evoked gastric relaxation, we performed some experiments using α-chymotrypsin, an enzyme that cleaves peptides at the level of tyrosine residues (present in VIP and also in other peptides). α-chymotrypsin (10 U ml−1), which increased the resting endoluminal pressure (about 1 cm H2O), significantly decreased only the slow component in response to high frequencies (8–32 Hz), suggesting an involvement of a peptide (Figure 4). The VIP receptor antagonist, VIP6-28 (10 μM), enhanced the resting endoluminal pressure (1–1.5 cm H2O) and significantly reduced only the slow relaxation evoked by high frequencies (8–32 Hz). The subsequent addition of L-NAME (300 μM) in the continuous presence of VIP6–28 (10 μM) abolished the slow component to high frequencies (16–32 Hz) and reduced partially the slow component to low frequencies (2–8 Hz) (Figure 5).
Figure 4.
Effects of α-chymotrypsin on the amplitude of the two components of NANC relaxation evoked by different stimulation frequencies. α-chymotrypsin (10 U ml−1) did not modify the fast relaxation and the slow relaxation to low frequencies, but it significantly reduced that to high frequencies of stimulation. All values are mean±s.e.m., n=6. *P<0.05 when compared to the respective control conditions (using Student's t-test).
Figure 5.
Effects of VIP6–28, a selective antagonist of VIP receptors, on the amplitude of the two components of NANC relaxation evoked by different stimulation frequencies. VIP6–28 (10 μM) did not modify the fast relaxation and the slow relaxation to low frequencies, but it significantly reduced that to high frequencies of stimulation. The subsequent addition of L-NAME (300 μM) abolished the fast relaxations and the slow relaxations to 16–32 Hz, and significantly reduced the second slow component to 2–8 Hz. All values are mean±s.e.m., n=4. *P<0.05 when compared to the respective control conditions (using ANOVA followed by Bonferroni's t-test).
Relaxation to SNP, VIP and ATP
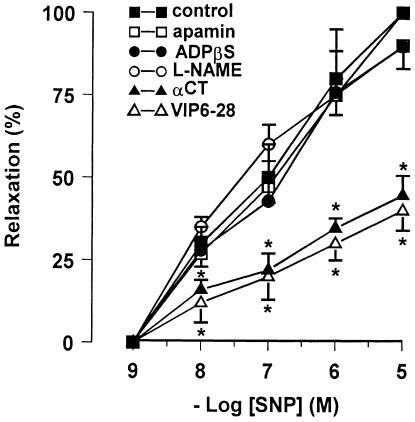
The NO donor, SNP (1 nM–10 μM) produced a concentration-dependent well-maintained relaxation. The magnitude of relaxation to SNP was not affected by L-NAME (300 μM), apamin (0.1 μM) or ADPβS (10 μM), but it was significantly reduced by exposure to α-chymotrypsin (10 U ml−1) or VIP6–28 (10 μM) (Figure 6).
Figure 6.
Concentration–response curves for SNP-induced relaxation in mouse gastric preparations before and after different pharmacological treatment. Apamin (0.1 μM, n=4), ADPβs (10 μM, n=5) and L-NAME (300 μM, n=5) did not alter the responses to SNP. α-Chymotrypsin (αCT) (10 U ml−1, n=5) and VIP6–28 (10 μM, n=5) reduced the effectiveness of SNP. All values are mean±s.e.m., and are reported as a percentage of the maximum effect induced by 10 μM SNP. *P<0.05 vs control (using paired Student's t-test).
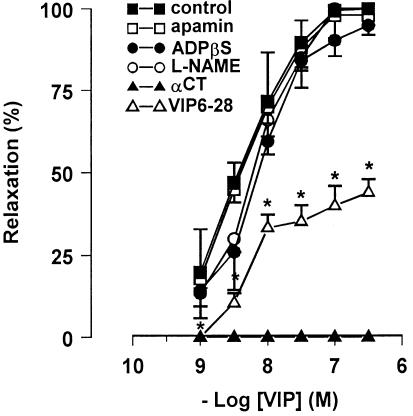
VIP (0.1–100 nM) produced a slowly developing relaxation that was not affected by L-NAME (300 μM), apamin (0.1 μM) or ADPβS (10 μM), but was antagonised by VIP6–28 (10 μM) and abolished by α-chymotrypsin (10 U ml−1) (Figure 7).
Figure 7.
Concentration–response curves for VIP-induced relaxation in mouse gastric preparations before and after different pharmacological treatment. Apamin (0.1 μM, n=4), ADPβs (10 μM, n=5) and L-NAME (300 μM, n=5) did not alter the responses to VIP, whereas α-chymotrypsin (αCT) (10 u ml−1, n=5) abolished and VIP6–28 (10 μM, n=5) antagonised the response to VIP. All values are mean±s.e.m., and are reported as a percentage of the maximum effect induced by 0.3 μM VIP. *P<0.05 vs control (using Student's t-test).
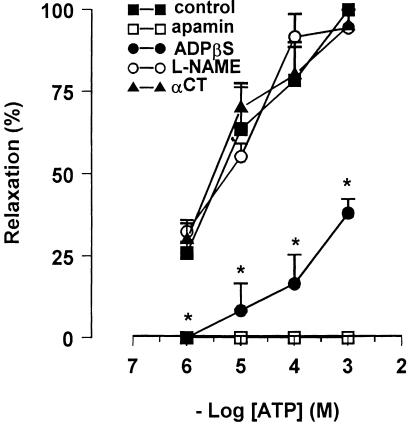
ATP (1 μM–1 mM) produced a sustained relaxation, which was abolished by apamin (0.1 μM), greatly reduced by desensitisation of the P2y purinergic receptors with ADPβs (10 μM), but it was not affected by L-NAME (300 μM) or by α-chymotrypsin (10 U ml−1) (Figure 8).
Figure 8.
Concentration–response curves for ATP-induced relaxation in mouse gastric preparations before and after different pharmacological treatment. The response to ATP was abolished by apamin (0.1 μM, n=4), antagonised by ADPβs (10 μM, n=5) and was not altered by L-NAME (300 μM, n=5) or by α-chymotrypsin (αCT) (10 U ml−1, n=5). All values are mean±s.e.m., and are reported as a percentage of the maximum effect induced by 1 μM ATP. *P<0.05 vs control (using paired Student's t-test).
Discussion
The results of the present study suggest that several neurotransmitters are responsible for the inhibitory mechanical responses of the mouse stomach. NO, VIP and ATP are differently involved in the nerve-evoked NANC relaxation. The NO inhibitory action appears to be due, at least in part, to VIP release, but no other interactions between the neurotransmitters were evident.
Previous studies in mouse gastric smooth muscle have shown that NANC inhibitory nerve stimulation evoked a biphasic relaxation, characterised by a rapid and transient ‘first' phase followed by a ‘second' phase, slower in onset and long-lasting, which was more evident in response to high frequencies of stimulation (Pfeifer et al., 1998; Baccari et al., 2000; Dick et al., 2002). The first component has been reported to be nitrergic in nature (Pfeifer et al., 1998; Baccari et al., 2000; Dick et al., 2002; Mulè & Serio, 2002), whereas the ‘second' phase has been suggested to be peptidergic, likely mediated by VIP (Baccari et al., 2000). These results were obtained in strips precontracted by carbachol or endothelin, but it should be kept in mind that contractile agonists, used to raise basal tension, lead to stimulation of protein kinase C and inactivation of smooth muscle NOS (Murthy et al., 1994), thus possibly masking the production of NO. Our results using the noncontracted whole stomach confirm that the fast component of the NANC relaxation is mediated exclusively by NO irrespective of the frequency of stimulation. In fact, fast relaxation was abolished by L-NAME, but it was not affected by desensitisation of P2y purinoceptors with ADPβs, by α-chymotrypsin or by VIP6–28. Moreover, L-NAME reduced the slow relaxation evoked by all frequencies of stimulation, suggesting that NO is also partly responsible for this component, together with other inhibitory transmitter(s). The results from our experiments indicate that ATP participates in the slow relaxation evoked by low frequencies of stimulation, whereas VIP contributes to the slow relaxation evoked by high frequencies of stimulation. Since we recorded the pressure changes of whole stomach, our methods do not permit the recognition of regional differences within the stomach.
ATP has been long demonstrated to act as a NANC inhibitory neurotransmitter in the mammalian intestine (Burnstock, 1990), but up to date the role of purinergic transmission and its possible interactions with NO and VIP have not been investigated in mouse stomach. Classically, it is generally accepted that ATP acting on P2x purinoceptors mediate muscle contractions, whereas P2y purinoceptors mediate muscle relaxations (Burnstock & Kennedy, 1985). In the present study to investigate the role of ATP in the gastric inhibitory NANC responses, we have used ADPβs, which desensitise P2y purinoceptors, and apamin, considered to antagonise the purinergic NANC inhibitory responses (Costa et al., 1986; Koh et al., 1997). The slow component of relaxation evoked at frequencies up to 8 Hz was abolished by apamin, suggesting that it is mediated by the opening of small conductance, Ca2+-dependent K+ channels. The observation that the desensitisation of P2y purinoceptors with ADPβs markedly reduced the slow component of the relaxation induced by frequencies up to 8 Hz suggests that ATP mediates this component by acting on P2y purinoreceptors to induce opening of apamin-sensitive K+ channels. In support of this hypothesis, there is the observation that the relaxation to exogenous ATP was antagonised by apamin in our preparation. ADPβs antagonised the relaxation to exogenous ATP without affecting relaxations to either SNP or VIP, demonstrating its selectivity for purinoceptors in this tissue. However, it has been reported that apamin can reduce the muscular responses to NO (Jenkinson & Reid, 2000; Xue et al., 2000; Serio et al., 2003). In our preparation, this possibility can be ruled out because apamin was without any effect on the evoked nitrergic relaxation. In addition, apamin did not affect the responses to exogenous SNP or VIP, suggesting that these mediators do not act through the activation of apamin-sensitive K+ channels. Moreover, in the present study, the relaxation induced by ATP was not affected by L-NAME or α-chymotrypsin, thus excluding the possibility that ATP stimulates the release of NO or a peptide. Also, the lack of effect of ADPβS on SNP or VIP-evoked relaxation rules out any interaction between NO and purines as reported in other preparations (Boeckxstaens et al., 1991; Christinck et al., 1991; Plujà et al., 1999; Xue et al., 2000) or between VIP and purines.
To our knowledge, these data represent the first experimental evidence that ATP or a related purine is involved in the mechanical relaxation in response to nerve NANC activation in mouse stomach. Actually, Mashimo et al. (1996), in an electrophysiological study, suggested that in mouse gastric fundus ATP acting on P2 receptors opens apamin-sensitive potassium channels to produce fast inhibitory junction potentials. However, the type of junction potential does not always relate to the mechanical response, and it is therefore important to measure mechanical responses directly to assess the role of putative neurotransmitters in the modifications of mechanical activity.
α-Chymotrypsin decreased the slow component in response to high frequencies of stimulation, suggesting the involvement of a peptide. Since the enzyme cleaves peptides at the level of tyrosine residues, present in VIP and also in other peptides, we also used VIP6–28, a VIP receptor antagonist (Fishbein et al., 1994). The finding that the VIP receptor antagonist significantly reduced the slow relaxation evoked by high frequencies of stimulation suggests that VIP is involved in the slow component of NANC relaxation. These results are in agreement with other reports indicating that VIP is released either by brief stimuli at high frequency or by sustained stimuli at low frequency (Li & Rand, 1990; D'Amato et al., 1992a,1992b; Lefebvre et al., 1995; Takahashi & Owyang, 1995; Currò & Preziosi, 1998), and thus they confirm that the stimulation frequency of the myenteric neurones is important, as recently shown in the human gastric fundus: low frequency of stimulation caused only NO release, whereas high frequency of stimulation induced both NO and VIP release (Tonini et al., 2000).
A matter of debate in the discussion of NO-mediated effects in the gastrointestinal tract has been whether or not VIP is crucial for NO-mediated effects and vice versa (Makhlouf & Grider, 1993; Keef et al., 1994; Bayguinov et al., 1999). In the present experiments, the finding that the relaxation induced by VIP was insensitive to L-NAME gives evidence that in mouse stomach, as in human, pig, guinea-pig or canine gastric preparations (Desai et al., 1994; Lefebvre et al., 1995; Bayguinov et al., 1999; Tonini et al., 2000), VIP does not cause its relaxant effect by producing NO, and it excludes a sequential mechanism between VIP and NO in the NANC relaxation of mouse stomach. These results are in contrast with Mashimo et al. (1996), who, in an electrophysiological study, suggested that in mouse gastric fundus VIP, acting presynaptically, releases NO. Nevertheless, no evidence for this serial interaction between VIP and NO in mouse gastric preparation was obtained in other studies from measuring smooth muscle contractile activity, because VIP-induced relaxation was not influenced by inhibitors of NOS and was still present in nNOS(−/−) mice (Dick et al., 2002).
The possibility that VIP release might be activated by NO has been also considered in our study, because NO has been shown to stimulate the release of VIP in different intestinal preparations (Allescher et al., 1996; Matsuyama et al., 2002) including guinea-pig gastric fundus (Grider et al., 1992; Grider & Jin, 1993). The observation that, in our preparation, the relaxation to exogenous SNP was specifically reduced by α-chymotrypsin or VIP6–28 implies a role for VIP in the SNP-evoked relaxation. We suggest that NO acts as a primary neurotransmitter mediating smooth muscle relaxation, and as a modulator enhancing VIP release. Our data do not allow us to clarify the cellular source of NO responsible for this neuromodulatory action. Colocalisation of NOS and VIP immunoreactivity in the enteric neurones has been described at gastric level (Lefebvre et al., 1995; Tonini et al., 2000); therefore, NO of neural origin could exert this effect. Alternatively, NO could be produced by the smooth muscle cells (Grider et al., 1992; Murthy & Makhlouf, 1994; Mulè et al., 2001) or by interstitial cells of Cajal (Xue et al., 1994). Further experiments are needed to clarify this point.
In conclusion, depending on the stimulation frequency, different neurotransmitters appear to be involved in the inhibitory mechanical response of the mouse stomach. NO is responsible for the rapid relaxation and appears to mediate partly the slow relaxation. ATP or a related purine, acting on P2y purinoceptors through opening of apamin-sensitive K+ channels, is involved in the slow relaxation evoked by low frequencies of stimulation. VIP is responsible for the sustained relaxation evoked by high frequencies of stimulation. The different neurotransmitters appear to work in a parallel manner, although NO could also serve as a neuromodulator substance that facilitates release of VIP.
Acknowledgments
This work was supported by grant from the Comitato Telethon Fondazione Onlus, Italy.
Abbreviations
- ADPβS
adenosine 5′-O-2-thiodiphosphate
- ATP
adenosine 5′-triphosphate
- EFS
electrical field stimulation
- L-NAME
Nω-nitro-L-arginine methyl ester
- NANC
nonadrenergic, noncholinergic
- NO
nitric oxide
- NOS
NO synthase
- SNP
sodium nitroprusside
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
References
- ABRAHAMSSON H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol. Scand. 1973;390:1–38. [PubMed] [Google Scholar]
- ALLESCHER H.D., KURJAK M., HUBER A., TRUDRUNG P., SCHUSDZIARRA V. Regulation of VIP release from rat enteric nerve terminals: evidence for a stimulatory effect of NO. Am. J. Physiol. 1996;271:G568–G574. doi: 10.1152/ajpgi.1996.271.4.G568. [DOI] [PubMed] [Google Scholar]
- BACCARI M.C., ROMAGNANI P., CALAMAI F. Impaired nitrergic relaxations in the gastric fundus of dystrophic (mdx) mice. Neurosci. Lett. 2000;282:105–108. doi: 10.1016/s0304-3940(00)00879-x. [DOI] [PubMed] [Google Scholar]
- BARBIER A.J., LEFEBVRE R.A. Involvement of the L-arginine: nitric oxide pathway in nonadrenergic noncholinergic relaxation of the cat gastric fundus. J. Pharmacol. Exp. Ther. 1993;266:172–178. [PubMed] [Google Scholar]
- BAYGUINOV O., KEEF K.D., HAGEN B., SANDERS K.M. Parallel pathways mediate inhibitory effects of vasoactive intestinal polypeptide and nitric oxide in canine fundus. Br. J. Pharmacol. 1999;126:1543–1552. doi: 10.1038/sj.bjp.0702450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELAI A., BURNSTOCK G. Evidence for coexistence of ATP and nitric oxide in non-adrenergic non-cholinergic (NANC) inhibitory neurones in the rat ileum, colon and anococcygeus muscle. Cell Tissue Res. 1994;278:197–200. doi: 10.1007/BF00305792. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., BULT H., DE MAN J.G., HERMAN A.G., VAN MAERCKE Y.M. Evidence for nitric oxide as mediator of non-adrenergic non-cholinergic relaxations induced by ATP and GABA in the canine gut. Br. J. Pharmacol. 1991;102:434–438. doi: 10.1111/j.1476-5381.1991.tb12191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. Overview. Purinergic mechanisms. Ann. N.Y. Acad. Sci. 1990;603:1–17. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KENNEDY C. Is there a basis for distinguishing two types of P2-purinoceptor. Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- CHRISTINCK F., JURY J., CAYABYAB F., DANIEL E.E. Nitric oxide may be the final mediator of nonadrenergic noncholinergic inhibitory junction potentials in the gut. Can. J. Physiol. Pharmacol. 1991;69:1448–1458. doi: 10.1139/y91-217. [DOI] [PubMed] [Google Scholar]
- COSTA M., FURNESS J.B., HUMPHREYS C.M.S. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn Schmiedeberg's Arch. Pharmacol. 1986;332:79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- CURRÒ D., PREZIOSI P. Non-adrenergic non-cholinergic relaxation of the rat stomach. Gen. Pharmacol. 1998;31:697–703. doi: 10.1016/s0306-3623(98)00096-2. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., CURRÒ D., MONTUSCHI P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J. Auton. Nerv. Syst. 1992a;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., CURRÒ D., MONTUSCHI P., CIABATTONI G., RAGAZZONI E., LEFEBVRE R.A. Release of vasoactive intestinal polypeptide from the rat gastric fundus. Br. J. Pharmacol. 1992b;105:691–695. doi: 10.1111/j.1476-5381.1992.tb09040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESAI K.M., SESSA W.C., VANE J.R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- DESAI K.M., WARNER T.D., BISHOP A.E., POLAK J.M., VANE J.R. Nitric oxide, and not vasoactive intestinal peptide, as the main neurotransmitter of vagally induced relaxation of the guinea pig stomach. Br. J. Pharmacol. 1994;113:1197–1202. doi: 10.1111/j.1476-5381.1994.tb17124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICK J.M.C., LEFEBVRE R.A. Interplay between nitric oxide and vasoactive intestinal polypeptide in the pig gastric fundus smooth muscle. Eur. J. Pharmacol. 2000;397:389–397. doi: 10.1016/s0014-2999(00)00299-5. [DOI] [PubMed] [Google Scholar]
- DICK J.M.C., VAN GELDRE L.A., TIMMERMANS J.P., LEFEBVRE R.A. Investigation of the interaction between nitric oxide and vasoactive intestinal polypeptide in the guinea-pig gastric fundus. Br. J. Pharmacol. 2000;129:751–763. doi: 10.1038/sj.bjp.0703089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICK J.M.C., VAN MOLLE W., BROUCKAERT P., LEFEBVRE R.A. Relaxation by vasoactive polypeptide in the gastric fundus of nitric oxide synthase-deficient mice. J. Physiol. 2002;538:133–144. doi: 10.1113/jphysiol.2001.012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERGÜN Y., ÖĞÜLENER N. Evidence for the interaction between nitric oxide and vasoactive intestinal polypeptide in the mouse gastric fundus. J. Pharmacol. Exp. Ther. 2001;299:945–950. [PubMed] [Google Scholar]
- ERGÜN Y., ÖĞÜLENER N., DIKMEN A. Involvement of nitric oxide in non-adrenergic non-cholinergic relaxation and action of vasoactive intestinal polypeptide in circular muscle strips of the rat gastric fundus. Pharmacol. Res. 2001;44:221–228. doi: 10.1006/phrs.2001.0844. [DOI] [PubMed] [Google Scholar]
- FISHBEIN V.A., COY D.H., HOCART S.J., JIANG N.Y., MROZINSKI J.E., JR, MANTEY S.A., JENSEN R.T. A chimeric VIP-PACAP analogue but not VIP pseudopeptides function as VIP receptor antagonists. Peptides. 1994;15:95–100. doi: 10.1016/0196-9781(94)90176-7. [DOI] [PubMed] [Google Scholar]
- GIARONI G., KNIGHT G.E., RUAN H.-Z., GLASS R., BARDINI M., LECCHINI S., FRIGO G., BURNSTOCK G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/s0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., CABLE M.B., SAID S.I., MAKHLOUF G.M. Vasoactive intestinal peptide as a neural mediator of gastric relaxation. Am. J. Physiol. 1985;248:G73–G78. doi: 10.1152/ajpgi.1985.248.1.G73. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., JIN J.G. VIP release and L-citrulline production from isolated ganglia of the myenteric plexus: regulation of VIP release by nitric oxide. Neuroscience. 1993;54:521–526. doi: 10.1016/0306-4522(93)90271-g. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., MURTHY K.S., JIN J.G., MAKHLOUF G.M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am. J. Physiol. 1992;262:G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- JENKINSON K.M., REID J.J. Evidence that adenosine 5′-triphosphate is the third inhibitory non-adrenergic non-cholinergic neurotransmission in the rat gastric fundus. Br. J. Pharmacol. 2000;130:1627–1631. doi: 10.1038/sj.bjp.0703481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J.G., MURTHY K.S., GRIDER J.R., MAKHLOUF G.M. Stoichiometry of neurally induced VIP-release, NO formation, and relaxation in rabbit and rat gastric muscle. Am. J. Physiol. 1996;271:G357–G369. doi: 10.1152/ajpgi.1996.271.2.G357. [DOI] [PubMed] [Google Scholar]
- KEEF K.D., SHUTTLEWORTH C.W.R., XUE C., BAYGUINOV O., PUBLICOVER N.G., SANDERS K.M. No relationship between nitric oxide and vasoactive intestinal polypeptide in enteric inhibitory neurotransmission. Neuropharmacology. 1994;33:1303–1314. doi: 10.1016/0028-3908(94)90030-2. [DOI] [PubMed] [Google Scholar]
- KOH S.D., DICK G.M., SANDERS K.M. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am. J. Physiol. 1997;273:C2010–C2021. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., BAERT E., BARBIER A.J. Influence of NG-nitro-L-arginine on non-adrenergic non-cholinergic relaxation in the guinea-pig gastric fundus. Br. J. Pharmacol. 1992;106:173–179. doi: 10.1111/j.1476-5381.1992.tb14311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A., SMITS G.J.M., TIMMERMANS J.P. Study of NO and VIP as non-adrenergic non-cholinergic neurotransmitters in the pig gastric fundus. Br. J. Pharmacol. 1995;116:2017–2026. doi: 10.1111/j.1476-5381.1995.tb16406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur. J. Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- MAKHLOUF G.M., GRIDER J.R. Nonadrenergic noncholinergic inhibitory neurotransmission of the gut. N. Physiol. Sci. 1993;8:195–199. [Google Scholar]
- MASHIMO H., HE X.D., HUANG P.L., FISHMAN M.C., GOYAL R.K. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J. Clin. Invest. 98:8–13. doi: 10.1172/JCI118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUYAMA H., UNNO T., EL-MAHMOUDY M., KOMORI S., KOBAYASHI H., THAPALIYA S., TAKEWAKI T. Peptidergic and nitrergic inhibitory neurotransmissions in the hamster jejunum: regulation of vasoactive intestinal polypeptide release by nitric oxide. Neuroscience. 2002;110:779–788. doi: 10.1016/s0306-4522(01)00580-2. [DOI] [PubMed] [Google Scholar]
- MULÉ F., SERIO R. Spontaneous mechanical activity and evoked responses in isolated gastric preparations from normal and dystrophic (mdx) mice. Neurogastr. Mot. 2002;14:1–10. doi: 10.1046/j.1365-2982.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- MULÉ F., VANNUCCHI G., CORSANI L., SERIO R., FAUSSONE-PELLEGRINI M.S. NOS and endogenous NO production are defective in colon from dystrophic (mdx) mice. Am. J. Physiol. 2001;281:G1264–G1270. doi: 10.1152/ajpgi.2001.281.5.G1264. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., JIN J.G., MAKHLOUF G.M. Inhibition of nitric oxide synthase activity in dispersed gastric muscle cells by protein kinase C. Am. J. Physiol. 1994;266:G161–G165. doi: 10.1152/ajpgi.1994.266.1.G161. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Vasoactive intestinal polypeptide/pituitary adenylyl cyclase-activating peptide-dependent activation of membrane-bound NO synthase in smooth muscle mediated by pertussis toxin-sensitive Gi1-2. J. Biol. Chem. 1994;269:15,977–15,980. [PubMed] [Google Scholar]
- NY L., PFEIFER A., ASZÓDI A., AHMAD M., ALM P., HEDLUND P., FÄSSLER R., ANDERSSON K.E. Impaired relaxation of stomach smooth muscle in mice lacking cyclic GMP-dependent protein kinase I. Br. J. Pharmacol. 2000;129:395–401. doi: 10.1038/sj.bjp.0703061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFER A., KLATT P., MASSBERG S., NY L., SAUSBIER M., HIRNEIβ C., WANG G.X., KORTH M., ASZÓDI A., ANDERSSON K.E., KROMBACH F., MAYERHOFER A., RUTH P., FÄSSLER R., HOFMANN F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUJÀ L., FERNÁNDEZ E., JIMÉNEZ M. Neural modulation of the cyclic electrical and mechanical activity in the rat colonic circular muscle: putative role of ATP and NO. Br. J. Pharmacol. 1999;126:883–892. doi: 10.1038/sj.bjp.0702363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELEMIDIS S., COCKS T. Nitrergic relaxation of the mouse gastric fundus is mediated by cyclic GMP-dependent and ryanodine-sensitive mechanisms. Br. J. Pharmacol. 2000;129:1315–1322. doi: 10.1038/sj.bjp.0703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERIO R., ZIZZO M.G., MULE' F. Nitric oxide induces muscular relaxation via cyclic GMP-dependent and -independent mechanisms in the longitudinal muscle of the mouse duodenum. Nitric Oxide. 2003;8:48–52. doi: 10.1016/s1089-8603(02)00144-1. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI Y., OWYANG C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J. Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONINI M., DE GIORGIO R., DE PONTI F., STERNINI C., SPELTA V., DIONIGI P., BARBARA G., STANGHELLINI V., CORINALDESI R. Role of nitric oxide- and vasoactive intestinal polypeptide-containing neurones in human gastric fundus strip relaxations. Br. J. Pharmacol. 2000;129:12–20. doi: 10.1038/sj.bjp.0702977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XUE L., FARRUGIA G., SZURSZEWSKI J.H. Effect of exogenous ATP on canine jejunal smooth muscle. Am. J. Physiol. 2000;278:G725–G733. doi: 10.1152/ajpgi.2000.278.5.G725. [DOI] [PubMed] [Google Scholar]
- XUE C., POLLOCK J., SCHMIDT H.H.H.W., WARD S.M., SANDERS K.M. Expression of nitric oxide synthase by interstitial cells of the canine proximal colon. J. Auton. Nerv. Sys. 1994;49:1–14. doi: 10.1016/0165-1838(94)90015-9. [DOI] [PubMed] [Google Scholar]
- YANO S., KIYOTA Y., YAMAMOTO M., WATANABE K. Pharmacological features of non-adrenergic non-cholinergic (NANC) relaxation induced by electrical vagal stimulation in isolated mouse stomach. Jpn. J. Pharmacol. 1995;69:9–15. doi: 10.1254/jjp.69.9. [DOI] [PubMed] [Google Scholar]