Abstract
Statins are mainly used to control hypercholesterolemia; however, recent studies have also ascribed anti-inflammatory effects to the statins. LFA703 is a novel statin-derived compound, which potently inhibits lymphocyte function antigen-1 (LFA-1, CD11a/CD18) but does not affect HMG-CoA reductase activity.
The objective of this study was to examine the anti-inflammatory mechanisms of LFA703 in ischemia/reperfusion (I/R)-induced leukocyte–endothelium interactions in the colon. For this purpose, the superior mesenteric artery was occluded for 30 min and leukocyte responses were analyzed in colonic venules after 120 min of reperfusion in mice using inverted intravital fluorescence microscopy.
First, the inhibitory mechanisms of LFA703 on leukocyte adhesion were investigated in vitro using a mouse CD4+8+ thymocyte cell line. Immunoneutralization of LFA-1 and ICAM-1 abolished leukocyte adhesion, whereas inhibition of VLA-4 had no effect in this in vitro assay. Indeed, it was found that LFA703 dose-dependently reduced LFA-1-dependent leukocyte adhesion to mouse endothelial cells in vitro with an IC50 of 3.2 μM.
I/R caused an increase in leukocyte rolling and adhesion in colonic venules. Immunoneutralization of LFA-1 significantly reduced I/R-induced leukocyte adhesion by 89% in colonic venules. In contrast, I/R-provoked leukocyte rolling was insensitive to inhibition of LFA-1 function.
Administration of 30 mg kg−1 of LFA703 decreased reperfusion-induced leukocyte adhesion by more than 91%, while the level of leukocyte rolling was unchanged, suggesting that LFA703 effectively blocked LFA-1-dependent firm adhesion of leukocyte in the colon. However, LFA703 did not decrease the expression of LFA-1 on circulating leukocytes.
This study demonstrates that LFA-1 is indeed a critical adhesion molecule in mediating postischemic leukocyte adhesion in the colon. Moreover, this is the first study showing that a statin-based synthetic compound has the capacity to abolish LFA-1-dependent leukocyte adhesion in I/R. These novel findings may have great implications in the clinical treatment of conditions associated with I/R-induced tissue injury, such as organ transplantation, trauma and major surgery.
Keywords: Adhesion, CD11a, colon, ischemia, intravital microscopy, reperfusion
Introduction
Intestinal ischemia is a common feature in trauma, shock, bowel strangulation and organ transplantation (Haglund, 1994). Reperfusion of ischemic tissues is associated with enhanced production of oxygen radicals, endothelial barrier dysfunction and tissue injury. A rate-limiting step in the pathophysiology of ischemia–reperfusion (I/R) damage is the activation and recruitment of leukocytes (Carden & Granger, 2000; Colvin & Thomson, 2002). The extravasation process of leukocytes is a multistep process mediated by a coordinated expression of specific adhesion molecules regulating the interactions between leukocytes and endothelial cells in the microcirculation (Butcher, 1991; Springer, 1994). At first, leukocyte rolling reduces the velocity of the circulating leukocytes and allows time for these cells to detect chemotactic signals from the local environment and the endothelial cell surface (Jung et al., 1999). In response to a chemoattractant, leukocytes adhere firmly to the vascular wall and subsequently transmigrate out into the extravascular space (Butcher, 1991). This firm adhesion of leukocytes is a critical precondition for tissue accumulation of leukocytes. Numerous studies have demonstrated that firm adhesion of leukocytes to endothelial cells is mediated by β2-integrins expressed on leukocytes, which in turn bind to members of the immunoglobulin gene superfamily, such as intercellular adhesion molecule-1 (ICAM-1), thereby promoting a strong adhesive interaction and arrest of rolling cells (Butcher, 1991; Springer, 1994). The β2-integrins are heterodimeric molecules consisting of a common β-subunit (CD18), which is noncovalently associated with one α-subunit (CD11a–d) (Springer, 1994; Hynes, 2002). The α-subunits of the β2-integrin family contain a domain of about 200 amino acids, known as an inserted (I) domain. A region of the I-domain of CD11 referred to as the metal-ion-dependent adhesion site (MIDAS) is the likely site of interaction with ICAM-1 (Lee et al., 1995). Indeed, the adhesive mechanisms underlying reperfusion-induced leukocyte–endothelium interactions in several tissues and organs, including the liver, mesentery, small bowel, striated muscle and skin, are well known (Thorlacius et al., 1994; Molla et al., 2001; Klintman et al., 2002). However, the knowledge about I/R-provoked inflammation in the large bowel is limited due to the previous lack of an appropriate animal model. We have recently developed and validated a new approach to study the molecular mechanisms behind leukocyte–endothelium interactions in the colon in vivo, based on the use of an inverted intravital fluorescence microscopic technique (Riaz et al., 2002a).
The functional significance of CD18 is illustrated by the leukocyte adhesion deficiency-1 syndrome, in which a mutation in the gene encoding for the common CD18 subunit results in the absence of β2-integrins. This autosomal recessive disorder is characterized by an inability to recruit neutrophils, leukocytosis and an increased susceptibility to bacterial infections (Harlan, 1993). Although the early literature on the role of individual β2-integrins in supporting leukocyte adhesion in vivo has been controversial, an accumulating body of investigations forwards a key role of LFA-1 (CD11a/CD18) in mediating leukocyte adhesion. In fact, using LFA-1-deficient mice, we and others have documented that LFA-1 plays an important role in supporting firm leukocyte adhesion in striated muscle (Thorlacius et al., 2000; Dunne et al., 2002), colon (Riaz et al., 2002b) and liver (unpublished data).
Statins are mainly used to control hypercholesterolemia in order to decrease the risk of cardiovascular disease (Corsini et al., 1995). However, an accumulating body of evidence indicates that statins also exert anti-inflammatory effects, including reduction of cytokine and chemokine production and inhibition of class II major histocompatibility complex expression on antigen-presenting cells (Kwak et al., 2000; Romano et al., 2000). These protective effects of statins are reversed by administration of mevalonate and, thus, are related to the inhibition of HMG-CoA reductase (Weitz-Schmidt, 2002). Indeed, several lipid intermediates in the synthesis of cholesterol, such as nonsteroidal isoprenoids, exert potent biological effects, which help to explain these specific anti-inflammatory effects (Bellosta et al., 2000). Nonetheless, it has recently been demonstrated that statins, such as lovostatin and simvastatin, also exert distinct anti-inflammatory effects independent of HMG-CoA reductase inhibition, such as interference with LFA-1 function (Weitz-Schmidt et al., 2001). One important discovery was made by Kallen et al. (1999), showing that lovastatin blocked the interaction between LFA-1 and ICAM-1 in vitro. Based on these findings and the crystal structure of the complex formed by the LFA-1 I domain and lovastatin, Weitz-Schmidt et al. (2001) designed a compound, LFA703, which effectively and specifically inhibits the function of LFA-1. Moreover, it was shown that LFA703 does not interfere with the activity of HMG-CoA reductase (Weitz-Schmidt et al., 2001). LFA703 inhibits LFA-1 by binding to a novel regulatory integrin site (L-site) of the I-domain distant to the MIDAS and, thus, inhibits LFA-1 function in an allosteric manner (Weitz-Schmidt et al., 2001; Welzenbach et al., 2002). However, the potential effects of LFA703 on leukocyte–endothelium interactions and I/R in vivo have not been examined.
Based on the above considerations, we wanted to define the effect of LFA703 on I/R-induced leukocyte rolling and adhesion in vivo. For this purpose, we used intravital fluorescence microscopy of the microcirculation in the mouse colon.
Methods
Cells
The polyoma-transformed murine endothelioma cell line eEnd.2 (Williams et al., 1989) and the mouse CD4+8+ thymocyte cell line DPK (Kaye & Ellenberger, 1992) were cultured in RPMI 1640, 10% fetal calf serum, 1% Glutamax I, 1% minimal essential medium, 1% penicillin/streptomycin and 10 mM HEPES, pH 7.4.
Adhesion assay
The CD4+8+ thymocyte cell line DPK cells were fluorescently labeled in 20 mM Tris, 150 mM NaCl, 5 mM glucose, 2 mM MgCl2, 2 mM MnCl2 and 0.25% bovine serum albumin (assay buffer) containing 15 μg/ml 3′-O-acetyl-2′,7′-bis(carboxyethyl)-4 or 5-carboxyfluorescein diacetoxy-methyl ester (BCECF)-AM at 37°C for 35–40 min. After labeling, the DPK cells were washed and resuspended in assay buffer. eEnd.2 cells were grown in microtiter plates until confluency was reached, and then further incubated for 1–2 days. Compounds, antibodies and controls diluted in assay buffer were added to the microtiter plates containing confluent eEnd.2 cell monolayers, followed by the transfer of labeled DPK cells to the plates (105 cells/well). The DPK cells were allowed to adhere for 30 min at 37°C. After the washing step, the bound cells were quantified by measuring fluorescence. Cell adhesion is expressed as percentage of vehicle control.
Animals
Male C57Bl/6J mice (M & B As, Skensved, Denmark) weighing 21–27 g were kept under standard laboratory conditions, maintained on a 12 h light and 12 h dark cycle, and were allowed free access to animal chow and tap water ad libitum. All experimental procedures were performed in accordance with legislation on the protection of animals, and were approved by the Regional Ethical Committee for Animal Experimentation.
Anaesthetic and surgical preparation
The mice were anesthesized with 7.5 mg ketamine and 2.5 mg xylazine per 100 g body weight by intraperitoneal (i.p.) injection. The animals were placed in supine position on a heating pad (37°C) for maintenance of body temperature. A polyethylene catheter (PE-10 with an internal diameter of 0.28 mm) was placed into the right internal jugular vein. A midline laparotomy was performed and the abdominal contents were deflected to the left side. The aorta and the vena cava were identified along with the celiac axis and the superior mesenteric artery (SMA). A nontraumatic vascular clamp was carefully placed across the SMA at the aortic origin in order to induce gut ischemia. In rodents, the SMA supports the most of the colon, and clamping of the SMA reduces microvascular perfusion in the colon by more than 85% (Riaz et al., 2002a). Following 30 min of ischemia, the clamp was removed and reperfusion was continued for 120 min.
Experimental protocol
The animals were divided into different groups, negative control/sham-operated group, which underwent all the surgical procedures except clamping of the SMA, whereas the ischemic group underwent 30 min of ischemia and 120 min of reperfusion. In order to delineate the role of LFA-1 in the leukocyte response to I/R, we injected monoclonal antibodies (Abs) directed against mouse LFA-1 (M17/4.11.9, 100 μg per mouse) intravenously (i.v.), and an isotype-matched control antibody (9-A2, 100 μg per mouse) immediately before ischemia. LFA703 was dissolved in ethanol/cremaphor EL and diluted 1 : 3 in PBS. The compound was given i.p. 2 h prior to ischemia at 10 and 30 mg kg−1. Following I/R, the animals were prepared for inverted intravital microscopy and leukocyte rolling and adhesion were determined as previously described (Riaz et al., 2002a). The antibodies were injected i.v. in order to assure access to the circulation and LFA703, which is a small molecule, was administered i.p.
Inverted intravital microscopy
Observations of the colonic microcirculation were made using an inverted Olympus microscope (IX70, Olympus Optical Co. GmbH, Hamburg, Germany) equipped with different lenses (× 10/NA 0.25 and × 40/NA 0.60). The microscopic image was televized using a charge-coupled device video camera (FK 6990 Cohu, Pieper GmbH, Schwerte, Germany), and recorded on videotape (Sony SVT-S3000P S-VHS recorder) for subsequent off-line analysis. After positioning under the microscope, a 5-min equilibration period preceded quantitative measurements in the colon 3 cm proximal to the anus. Analysis of leukocyte–endothelium interactions (rolling and adhesion) was made in colonic venules (inner diameter 15–30 μm) with stable resting blood flow. Blood perfusion within individual microvessels was studied after contrast enhancement by i.v. administration of (0.05 ml, 5 mg ml−1) fluorescein isothiocyanate (FITC)-labeled dextran (mw 150000). In vivo labeling of leukocytes with rhodamine-6G (0.1 ml, 0.5 mg ml−1) enabled quantitative analysis of leukocyte flow behavior in the colonic microcirculation. Quantification of microcirculatory parameters was performed off-line by frame-to-frame analysis of the videotaped images. Microcirculatory analysis included determination of leukocyte rolling by counting the number of leukocytes passing a reference point in the venule per 20 s, and is expressed as cells min−1. Firm adhesion was measured by counting the number of cells adhering to the venular endothelium (220 μm long segments) and remaining stationary for 20 s, and was given as cells mm−1 venule length. Blood flow velocities were measured off-line by using CapImage software (Zeintl, Heidelberg, Germany). The velocity was calculated as a mean value from five to eight measurements per venule and is expressed as mm s−1. Venular wall shear rate was determined based on the Newtonian definition: wall shear rate=8((red blood cell velocity/1.6)/venular diameter), as described previously (House & Lipowsky, 1987). Wall shear stress was calculated according to the relationship, wall shear stress=wall shear rate × 0.025 (dyn cm−1), assuming a blood viscosity of 0.025 poise.
Systemic leukocyte counts
Blood (20 μl) was mixed with Turks solution (0.2 mg gentian violet in 1 ml glacial acetic acid, 6.25% v v−1) in a 1 : 10 dilution. Leukocytes were counted and differentiated as polymorphonuclear (PMNL) or monomorphonuclear (MNL) in a Burker chamber.
Flow cytometry
Blood was collected from mice by cardiac puncture in EDTA tubes. Then, 50 μl of blood samples was stained using an FITC-labeled rat anti-mouse CD11a antibody (M17/4, 1 μg per 106 leukocytes) as well as an FITC-labeled isotype-matched control antibody (R3-34, 1 μg per 106 leukocytes). After antibody incubation for 30 min at room temperature in the dark, the red blood cells were lyzed (2 ml of NH4Cl-EDTA for 10 min) and washed (1500 rpm) for 5 min. The cell pellet was resuspended with 0.5 ml PBS and put on ice until analysis, which was performed within 45 min. Neutrophils were gated based on forward and side scatter characteristics.
Materials
The hybridoma cell lines used for the production of the anti-mouse LFA-1 Ab M17/4.4. 11.9, the anti-mouse LFA-1 Ab M18/2.a.12.7, the anti-mouse ICAM-1 Ab YN1/1.7 and the anti-mouse VLA-4 Ab R1-2 were obtained from the American Type Culture Collection, Rockville, MD, U.S.A. The isotype control antibody (9-A2) was from Bio-Express, West Lebanon, NH, U.S.A. FITC-labeled M17/4 against murine CD11a and FITC-labeled R3-34 were from Pharmingen, San Diego, CA, U.S.A. FITC-labeled dextran and rhodamine-6G were from Sigma Chemical Co., St Louis, MO, U.S.A. BCECF was purchased from Molecular Probes, Leiden, Netherlands. LFA703 and ethanol/cremaphor EL were from Novartis Pharma AG, Basel, Switzerland.
Statistical analysis
Statistical evaluations were performed using the Kruskal–Wallis one-way analysis of variance on ranks for unpaired samples (Dunn's post hoc test was used). The results are presented as mean values±s.e.m. and n represents the number of animals. Differences were considered to be significant at P<0.05.
Results
LFA703 abolishes LFA-1-mediated leukocyte adhesion in vitro
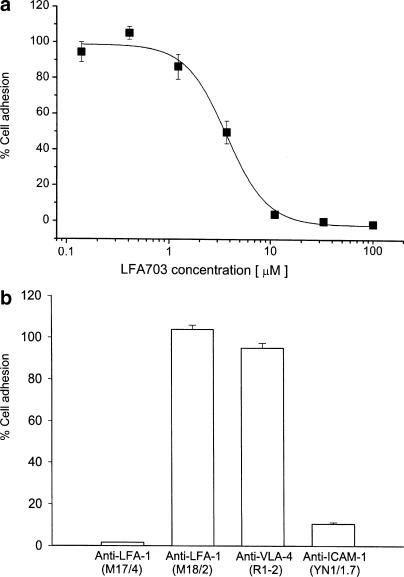
We found that LFA703 dose-dependently reduced DPK T-cell adhesion to mouse endothelial cells in vitro (Figure 1a). It was observed that the IC50 of LFA703 was 3.2 μM in this mouse system, which is within the range of IC50 values determined in human cell-based assays, that is, 0.7 μM (Welzenbach et al., 2002). In separate experiments, the function-blocking anti-LFA-1 Ab M17/4 was incubated in the assay, which completely inhibited the adhesion of leukocytes to the endothelial cells (Figure 1b). Moreover, immunoneutralization of ICAM-1 markedly inhibited leukocyte adhesion (Figure 1b). In contrast, the anti-mouse LFA-1 Ab M18/2 known to be inactive in ICAM-1-dependent adhesion assays (Driessens et al., 1996) and the anti-VLA-4 Ab R1-2 had no effect on leukocyte adhesion (Figure 1b). These results demonstrate that cell binding in this assay is entirely mediated by LFA-1, and strongly suggest that LFA703 inhibits murine LFA-1 function.
Figure 1.
Leukocyte adhesion to endothelial cells. The adhesion of fluorescently labeled murine DPK T cells to endothelial cells was quantified in the presence of (a) LFA703 or (b) different Abs (20 μg/ml), as described in Methods. Data are given as per cents of control, and represent the mean of triplicates±s.e.m. A representative experiment out of four experiments is shown.
LFA703 abolishes LFA-1-mediated leukocyte adhesion in vivo
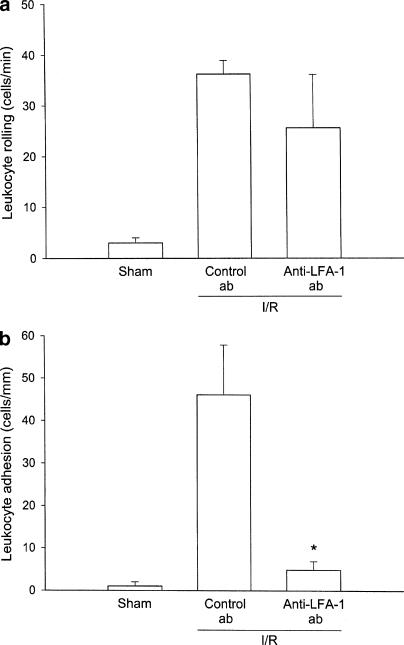
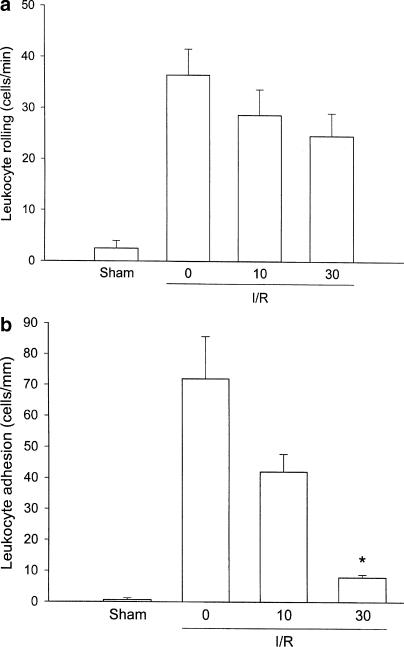
Intravital microscopic observation in colonic postcapillary venules in sham-operated mice demonstrated only occasional interactions between leukocytes and the microvascular endo-thelium, that is, the number of rolling and adherent leukocytes was 0.6±0.6 cells min−1 and 2.5±1.5 cells mm−1, respectively (Figure 2a, b). Indeed, 30 min of ischemia followed by 120 min of reperfusion induced a substantial increase in leukocyte rolling and adhesion (Figure 2a, b). In order to examine the role of LFA-1 in postischemic leukocyte–endothelium interactions, we first investigated the effect of an antibody directed against LFA-1 (M17/4). Leukocyte rolling was 36.2±2.7 cells min−1 and adhesion was 46.1±11.8 cells mm−1 in mice treated with a control antibody (Figure 2a, b). Notably, administration of the anti-LFA-1 antibody greatly decreased leukocyte adhesion in the colon down to 4.9±2.0 cells mm−1, corresponding to a more than 89% reduction (Figure 2b, P<0.05 vs control antibody, n=6). However, leukocyte rolling induced by I/R was insensitive to passive immunization of LFA-1 (Figure 2a, P>0.05 vs control antibody, n=6). These findings establish that stationary adhesion of leukocytes along the endothelium in colonic venules is mediated by LFA-1. In agreement with the results obtained with the anti-LFA-1 Ab, I/R-induced leukocyte rolling was not significantly altered by LFA703 (Figure 3a, P>0.05 vs vehicle, n=4–7). Next, we evaluated the effect of LFA703 in this LFA-1-dependent model of leukocyte adhesion. Interestingly, it was observed that the number of firmly adherent leukocytes after I/R was 71.9±13.7 cells mm−1 in vehicle-treated mice, whereas leukocyte adhesion was significantly lower, that is, 7.9±0.9 cells mm−1, in animals receiving 30 mg kg−1 of LFA703, corresponding to an 89% reduction exerted by LFA703 (Figure 3b, P<0.05 vs vehicle, n=7). We observed that 10 mg kg−1 of LFA703 had no significant influence on I/R-induced leukocyte responses in the colon (Figure 3a, b).
Figure 2.
Venular leukocyte (a) rolling and (b) adhesion in the mouse colon after 30 min of ischemia and 120 min of reperfusion. Mice were pretreated i.v. with a control antibody (Control ab) and an antibody against LFA-1 (anti-LFA-1 ab). Sham-operated (sham) mice received phosphate-buffered saline (PBS). Data represent mean±s.e.m. Asterisks indicate significant difference (P<0.05 vs control antibody, n=4–7).
Figure 3.
Venular leukocyte (a) rolling and (b) adhesion in the mouse colon after 30 min of ischemia and 120 min of reperfusion. Mice were pretreated i.v. with vehicle (0) and LFA703 (10 and 30 mg kg−1). Sham-operated (sham) mice received vehicle (0). Data represent mean±s.e.m. Asterisks indicate significant difference (P<0.05 vs vehicle, n=4–8).
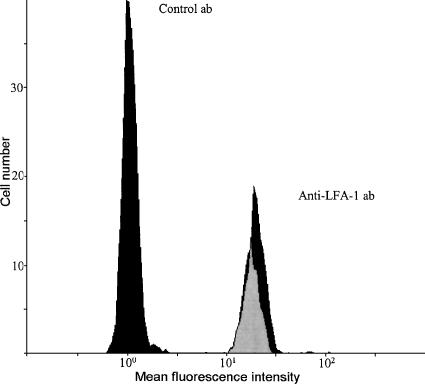
Administration of the monoclonal antibody directed against LFA-1 or LFA703 had no effect on systemic leukocyte differential counts (Table 1). There was no difference in the hemodynamic parameters between the different experimental groups (Table 2). Moreover, flow cytometric analysis revealed that peripheral neutrophils from wild-type mice were uniformly and highly positive for LFA-1. Administration of LFA703 did not decrease the level of anti-LFA-1 antibody binding to neutrophils (Figure 4). In fact, the mean fluorescence intensity on neutrophils from control mice and LFA703-treated mice was 19.0 and 19.8, respectively.
Table 1.
Systemic leukocyte differential counts
| PMNL | MNL | TOTAL | |
|---|---|---|---|
| Sham | 1.1±0.1 | 3.3±0.3 | 4.4±0.3 |
| Vehicle | 1.6±0.2 | 3.1±0.3 | 4.8±0.4 |
| LFA703, 10 mg kg−1 | 1.9±0.2 | 3.5±0.3 | 5.5±0.5 |
| LFA703, 30 mg kg−1 | 1.9±0.2 | 3.9±0.2 | 5.7±0.3 |
| Control Ab | 1.5±0.3 | 4.9±0.3 | 6.4±0.4 |
| Anti-LFA-1 Ab | 1.4±0.4 | 3.4±0.4 | 4.8±0.9 |
Ischemia was induced by clamping the superior mesenteric artery (SMA) for 30 min followed by 120 min of reperfusion. Sham-operated controls underwent the identical procedures except clamping of the SMA (n=4). Animals were pretreated with vehicle (n=7), a monoclonal antibody against LFA-1 (M17/4, 100 μg per mouse, n=6), a control antibody (100 μg per mouse, n=7) or LFA 703 (10 and 30 mg kg−1, n=7–8). Blood was collected from the mice after the experiments, and the cells were defined as polymorphonuclear (PMNL) or mononuclear (MNL) leukocytes. Data are mean±s.e.m. and represent 106 cell ml−1.
Table 2.
Hemodynamic parameters in colonic venules
| Diameter (μm) | Red blood cell velocity (mm s−1) | Wall shear rate (s−1) | Wall shear stress (dyn cm−1) | |
|---|---|---|---|---|
| Sham | 18.0±1.1 | 0.8±0.1 | 214±22 | 5.3±0.6 |
| Vehicle | 23.0±1.1 | 1.3±0.2 | 308±61 | 7.7±1.5 |
| LFA 703, 10 mg kg−1 | 22.2±0.5 | 1.2±0.2 | 272±72 | 6.7±1.4 |
| LFA 703, 30 mg kg−1 | 20.7±0.7 | 1.3±0.2 | 282±32 | 7.1±0.8 |
| Control Ab | 24.6±1.5 | 1.2±0.1 | 278±51 | 7.0±1.2 |
| Anti-LFA-1 Ab | 20.6±0.4 | 1.4±0.3 | 320±59 | 7.8±1.9 |
Ischemia was induced by clamping the superior mesenteric artery (SMA) for 30 min followed by 120 min of reperfusion. Sham-operated controls underwent the identical procedures except clamping of the SMA (n=4). Animals were pretreated with vehicle (n=7), a monoclonal antibody against LFA-1 (M17/4, 100 μg per mouse, n=6), a control antibody (100 μg per mouse, n=7) or LFA 703 (10 and 30 mg kg−1, n=7–8). Blood flow velocities were measured off-line by frame-to-frame analysis of the videotaped images. Data are mean±s.e.m.
Figure 4.
Surface expression of lymphocyte function antigen-1 (LFA-1) on peripheral neutrophils from mice treated with LFA703 (30 mg kg−1, black) and vehicle (gray) for 120 min. Peripheral blood samples were stained using an FITC-labeled anti-mouse CD11a antibody (M17/4) as well as an FITC-labeled isotype-matched control antibody. In each sample, 104 cells were counted and gated for neutrophils based on forward and side scatter characteristics.
Discussion
An accumulating body of evidence suggests that statins exert several anti-inflammatory effects besides reducing cholesterol levels. The anti-inflammatory effects of statins have been ascribed to both HMG-CoA reductase-dependent and -independent mechanisms. LFA703 is a novel statin-derived compound that has no inhibitory effect on HMG-CoA reductase (Weitz-Schmidt et al., 2001). This study demonstrates that LFA703 is an effective inhibitor of LFA-1-dependent adhesion of leukocytes to endothelial cells both in vitro and in vivo. In fact, we found that LFA703 abolished I/R-induced leukocyte adhesion in the colon. On the other hand, LFA703 has no effect on leukocyte rolling in the colon in vivo. Taken together, this is the first study showing that a statin-based molecule has the capacity to inhibit leukocyte adhesion in vivo, and that such compounds may be useful to control pathological inflammation in the gut.
Anti-inflammatory properties of the statins have generated an increasing interest in the molecular actions of statins in the immune system, and based on the crystal structure of the complex formed by the LFA-1 I-domain and lovastatin, a new class of synthetic inhibitors of LFA-1 has been generated (Weitz-Schmidt et al., 2001; Welzenbach et al., 2002). One of these is LFA703, which allosterically inhibits LFA-1 function by binding to the I-domain (Weitz-Schmidt et al., 2001). In the present study, we show that LFA703 is an effective inhibitor of LFA-1-mediated adhesion of murine leukocytes in vitro. The IC50 of LFA703 in the murine adhesion assay was found to be similar to the IC50 values obtained using human cells (Welzenbach et al., 2002). In this context, it is also important to note that LFA703 does not interfere with the function of the human integrins Mac-1 (CD11b/CD18) and VLA-4 (CD49d/CD29), indicating a high selectivity for LFA-1 (Weitz-Schmidt et al., 2001). The interaction between LFA703 and murine integrins other than LFA-1 has not been examined yet and it cannot, at present, be excluded that LFA703 may also exert other effects than only inhibition of LFA-1 in mice.
It is widely held that leukocyte–endothelial cell adhesive interactions constitute an integral part of the inflammatory response associated with reperfusion of previously ischemic tissues. Herein, we found that administration of a monoclonal antibody directed against LFA-1 abolished (89% reduction) I/R-provoked leukocyte adhesion in the colon. On the other hand, immunoneutralization of LFA-1 had no effect on leukocyte rolling triggered by colonic I/R. These findings are in line with previous findings of ours, showing that postischemic leukocyte adhesion, but not rolling, is markedly reduced (95% reduction) in LFA-1-deficient mice (Riaz et al., 2002b). Considered collectively, these findings using both function-blocking antibodies and gene-targeted animals strongly suggest that leukocyte adhesion associated with I/R in the colon is mainly mediated by LFA-1. Notably, in this LFA-1-dependent system, it was found that administration of 30 mg kg−1 of LFA703 completely inhibited I/R-induced leukocyte adhesion in the colon, while leukocyte rolling was not significantly decreased by LFA703. Thus, the effects of LFA703 mimicked very well the effects observed with the anti-LFA-1 antibody, suggesting that the inhibitory mechanisms of LFA703 are indeed related to interference with LFA-1 function. In this context, it is important to note that we observed that leukocyte rolling in vivo was not reduced by treatment with LFA703. Salas et al. (2002) reported recently that LFA703 reduced a rolling adhesive interaction of leukocytes on coated ICAM-1 using an in vitro flow chamber. Our present findings do not support such an effect on leukocyte rolling of LFA703 in vivo and one explanation is that leukocyte rolling on endothelial cells in vivo is mainly mediated by the selectin family of adhesion molecules (Butcher, 1991; Springer, 1994). Notably, this is the first study showing that a statin-based compound has the capacity to reduce firm adhesion of leukocyte in vivo, and based on these findings, it may be predicted that LFA703 will be protective in other models of LFA-1-dependent leukocyte accumulation. Furthermore, it is noticeable that one great advantage using synthetic compounds, such as LFA703, is that the adverse side effects frequently associated with the use of antibodies, including formation of anti-idiotype antibodies, leukopenia and systemic activation of the complement system, can be circumvented.
Taken together, this study documents for the first time that a synthetic statin-derived compound has the ability to completely inhibit LFA-1 function in vitro and in vivo. In fact, we demonstrated that LFA703 abolished I/R-induced leukocyte adhesion in vivo. These novel findings suggest that statin-based inhibitors of LFA-1, such as LFA703, may be useful to control pathological inflammation in the gastrointestinal tract pharmacologically.
Acknowledgments
We thank Dr J. Kaye (The Scripps Research Institute, La Jolla, CA, U.S.A.) for providing the DPK cell line and Dr. H.G. Zerwes (Novartis Pharma, Basel, Switzerland) for providing the eEnd.2 cells. This work was supported by grants from the Swedish Medical Research Council (K2000-04P-13411-01A, K2002-73-X-14273-01A), Cancerfonden (4265-B99-01XAB), Crafoordska stiftelsen (20010968), Blaceflors stiftelse, Einar och Inga Nilssons stiftelse, Harald och Greta Jaenssons stiftelse, Greta och Johan Kocks stiftelser, Fröken Agnes Nilssons stiftelse, Franke and Margareta Bergqvists stiftelse för främjande av cancerforskning, Nanna Svartz stiftelse, Ruth och Richard Julins stiftelse, Svenska Läkaresällskapet (2001-907), Teggers stiftelse, Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder, Malmö University Hospital and Lund University.
Abbreviations
- Ab
antibody
- BCECF
3′-O-acetyl-2′,7′-bis(carboxyethyl)-4 or 5-carboxyfluorescein diacetoxy-methyl ester
- FITC
fluorescein isothiocyanate
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-coenzyme A
- I
inserted
- ICAM-1
intercellular adhesion molecule-1
- i.p.
intraperitoneally
- I/R
ischemia/reperfusion
- i.v.
intravenously
- LFA-1
lymphocyte function antigen-1
- MIDAS
metal-ion-dependent adhesion site
- MNL
monomorphonuclear leukocytes
- PBS
phosphate-buffered saline
- PMNL
polymorphonuclear leukocytes
- SMA
superior mesenteric artery
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late antigen-4
References
- BELLOSTA S., FERRI N., BERNINI F., PAOLETTI R., CORSINI A. Non-lipid-related effects of statins. Ann. Med. 2000;32:164–176. doi: 10.3109/07853890008998823. [DOI] [PubMed] [Google Scholar]
- BUTCHER E.C. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- CARDEN D.L., GRANGER D.N. Pathophysiology of ischaemia–reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- COLVIN B.L., THOMSON A.W. Chemokines, their receptors, and transplant outcome. Transplantation. 2002;74:149–155. doi: 10.1097/00007890-200207270-00001. [DOI] [PubMed] [Google Scholar]
- CORSINI A., MAGGI F.M., CATAPANO A.L. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol. Res. 1995;31:9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- DRIESSENS M.H., VAN HULTEN P., ZUURBIER A., LA RIVIERE G., ROOS E. Inhibition and stimulation of LFA-1 and Mac-1 functions by antibodies against murine CD18. Evidence that the LFA-1 binding sites for ICAM-1, -2, and -3 are distinct. J. Leukoc. Biol. 1996;60:758–765. doi: 10.1002/jlb.60.6.758. [DOI] [PubMed] [Google Scholar]
- DUNNE J.L., BALLANTYNE C.M., BEAUDET A.L., LEY K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- HAGLUND U. Gut ischaemia. Gut. 1994;35:S73–S76. doi: 10.1136/gut.35.1_suppl.s73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARLAN J.M. Leukocyte adhesion deficiency syndrome: insights into the molecular basis of leukocyte emigration. Clin. Immunol. Immunopathol. 1993;67:S16–S24. doi: 10.1006/clin.1993.1079. [DOI] [PubMed] [Google Scholar]
- HOUSE S.D., LIPOWSKY H.H. Leukocyte endothelium adhesion: microhaemodynamics in the mesentery of the cat. Microvasc. Res. 1987;34:3363–3379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- HYNES R.O. Integrins: bi-directional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- JUNG U., NORMAN K.E., SCHARFFETTER-KOCHANEK K., BEAUDET A.L., LEY K. Transit time of leukocytes through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Invest. 1999;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLEN J., WELZENBACH K., RAMAGE P., GEYL D., KRIWACKI R., LEGGE G., COTTENS S., WEITZ-SCHMIDT G. Hommel U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J. Mol. Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- KAYE J., ELLENBERGER D.L. Differentiation of an immature T cell line: a model of thymic positive selection. Cell. 1992;71:423–435. doi: 10.1016/0092-8674(92)90512-b. [DOI] [PubMed] [Google Scholar]
- KLINTMAN D., SCHRAMM R., MENGER M.D., THORLACIUS H. Leukocyte recruitment in hepatic venules: selectin-mediated rolling is a prerequisite for CD18-dependent firm adhesion in vivo. J. Hepatol. 2002;36:53–59. doi: 10.1016/s0168-8278(01)00226-4. [DOI] [PubMed] [Google Scholar]
- KWAK B., MULHAUPT F., MYIT S., MACH F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- LEE J.O., RIEU P., ARNAOUT M.A., LIDDINGTON R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- MOLLA M., GIRONELLA M., SALAS A., MIQUEL R., PEREZ-DEL-PULGAR S., CONILL C., ENGEL P., BIETE A., PIQUE J.M., PANES J. Role of P-selectin in radiation-induced intestinal inflammatory damage. Int. J. Cancer. 2001;96:99–109. doi: 10.1002/ijc.1009. [DOI] [PubMed] [Google Scholar]
- RIAZ A.A., WAN M.X., SCHAFER T., SCHRAMM R., DAWSON P.M., MENGER M.D., JEPPSSON B., THORLACIUS H. Allopurinol and superoxide dismutase protect against leukocyte–endothelium interactions in a novel model of ischaemia/reperfusion in the mouse colon. Br. J. Surg. 2002a;89:1572–1580. doi: 10.1046/j.1365-2168.2002.02279.x. [DOI] [PubMed] [Google Scholar]
- RIAZ A.A., WAN M.X., SCHAFER T., SCHRAMM R., EKBERG H., MENGER M.D., JEPPSSON B., THORLACIUS H. Fundamental and distinct roles of P-selectin and LFA-1 in ischemia/reperfusion-induced leukocyte–endothelium interactions in the colon. Ann. Surg. 2002b;236:777–784. doi: 10.1097/00000658-200212000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMANO M., DIOMEDE L., SIRONI M., MASSIMILIANO L., SOTTOCORNO M., POLENTARUTTI N., GUGLIELMOTTI A., ALBANI D., BRUNO A., FRUSCELLA P., SALMONA M., VECCHI A., PINZA M., MANTOVANI A. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Invest. 2000;80:1095–1100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- SALAS A., SHIMAOKA M., CHEN S., CARMAN S.C., SPRINGER T. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin lymphocyte function-associated antigen-1. J. Biol. Chem. 2002;277:50255–50262. doi: 10.1074/jbc.M209822200. [DOI] [PubMed] [Google Scholar]
- SPRINGER T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- THORLACIUS H., RAUD J., ROSENGREN-BEEZLEY S., FORREST M.J., HEDQVIST P., LINDBOM L. Mast cell activation induces P-selectin-dependent leukocyte rolling and adhesion in postcapillary venules in vivo. Biochem. Biophys. Res. Commun. 1994;203:1043–1049. doi: 10.1006/bbrc.1994.2287. [DOI] [PubMed] [Google Scholar]
- THORLACIUS H., VOLLMAR B., GUO Y., MAK T.W., PFREUNDSCHUH M.M., MENGER M.D., SCHMITS R. LFA-1 (lymphocyte function antigen-1) mediates early tumor necrosis factor-α-induced leukocyte adhesion in venules. Br. J. Hematol. 2000;110:424–429. doi: 10.1046/j.1365-2141.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- WEITZ-SCHMIDT G. Statins as anti-inflammatory agents. Trends Pharmacol. Sci. 2002;23:482–486. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- WEITZ-SCHMIDT G., WELZENBACH K., BRINKMANN V., KAMATA T., KALLEN J., BRUNS C., COTTENS S., TAKADA Y., HOMMEL U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- WELZENBACH K., HOMMEL U., WEITZ-SCHMIDT G. Small molecule inhibitors induce conformational changes in the I domain and the I-like domain of lymphocyte function-associated antigen-1. Molecular insights into integrin inhibition. J. Biol. Chem. 2002;277:10590–10598. doi: 10.1074/jbc.M110521200. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R.L., RISAU W., ZERWES H.G., DREXLER H., AGUZZI A., WAGNER E.F. Endothelioma cells expressing the polyoma middle T oncogene induce hemangiomas by host cell recruitment. Cell. 1989;57:1053–1063. doi: 10.1016/0092-8674(89)90343-7. [DOI] [PubMed] [Google Scholar]