Abstract
Agonists for protease-activated receptor-2 (PAR-2) cause hypotension and an increase in gastric mucosal blood flow (GMBF) in vivo. We thus studied the mechanisms underlying the circulatory modulation by PAR-2 activation in vivo, especially with respect to involvement of endothelium-derived hyperpolarizing factor (EDHF).
Arterial blood pressure and GMBF were measured in anesthetized rats in vivo. Vascular relaxation was assessed in the precontracted rat gastric arterial rings in vitro.
The PAR-2-activating peptide SLIGRL-NH2 and/or trypsin, administered i.v., produced largely NO-independent hypotension and increase in GMBF accompanied by decreased gastric mucosal vascular resistance (GMVR) in rats.
Combined administration of apamin and charybdotoxin, but not each of them, specifically abolished the hypotension, increased GMBF and decreased GMVR caused by the PAR-2 agonists.
In the isolated rat gastric artery, SLIGRL-NH2 elicited endothelium-dependent relaxation even in the presence of an NO synthase inhibitor and indomethacin, which was abolished by apamin plus charybdotoxin.
Our data suggest involvement of apamin/charybdotoxin-sensitive K+ channels in the PAR-2-triggered hypotension and increased GMBF, predicting a role of EDHF-like factors.
Keywords: PAR-2, hypotension, gastric mucosal blood flow, EDHF, protease
Introduction
Protease-activated receptor-2 (PAR-2) (Nystedt et al., 1994) is a G-protein-coupled, seven-trans-membrane-domain receptor activated by certain endogenous proteases including trypsin, tryptase and coagulation factors VIIa and Xa, and also possibly by unknown proteases (Macfarlane et al., 2001). Activation of PAR-2 is achieved by proteolytic unmasking of the N-terminal cryptic tethered ligand that subsequently binds to the extracellular second loop of the receptor, and also directly by exogenously applied synthetic PAR-2-activating peptides based on the aminoacid sequences of the tethered ligand, such as SLIGKV-NH2 and SLIGRL-NH2 derived from human and murine PAR-2, respectively (Nystedt et al., 1994; Macfarlane et al., 2001).
PAR-2 is widely distributed throughout the mammalian body, modulating a variety of physiological functions possibly during inflammation or tissue injury (Macfarlane et al., 2001). Especially, in the alimentary systems, PAR-2 is involved in salivary and pancreatic exocrine secretion (Nguyen et al., 1999; Kawabata et al., 2000), modulation of gastrointestinal smooth muscle motility (Saifeddine et al., 1996; Kawabata et al., 2001c), gastric mucosal protection (Kawabata et al., 2001b), gastric pepsin secretion (Kawao et al., 2002), etc. PAR-2 present in the capsaicin-sensitive sensory neurons appears to play roles in neurogenic inflammation (Steinhoff et al., 2000), nociception/hyperalgesia (Kawabata et al., 2001a; Vergnolle et al., 2001) and gastric mucosal protection (Kawabata et al., 2001b). In the vascular system, endothelial PAR-2, upon activation by PAR-2-activating peptides, trypsin or factor Xa, triggers NO-dependent relaxation in many of the isolated blood vessels (Saifeddine et al., 1996; Sobey et al., 1999; Kawabata et al., 2001d, 2001e), although endothelium-derived hyperpolarizing factor (EDHF) is also involved in PAR-2-mediated relaxation in the rat coronary and murine mesenteric arteries in vitro (McGuire et al., 2002; McLean et al., 2002). PAR-2 agonists produce prompt hypotension in vivo (Cicala et al., 1999; 2001), implying that PAR-2 might be implicated in the pathogenesis of hypotension during septic shock that is often accompanied by disseminated intravascular coagulation (DIC) in which potential PAR-2 activators including factors X and VII would become activated. This PAR-2-mediated hypotension in vivo is only slightly attenuated by NO synthase inhibition (Cicala et al., 1999; 2001). Most recently, we have found that the PAR-2-activating peptide SLIGRL-NH2 at 1 μmol kg−1, a mucosal protective dose, elevated gastric mucosal blood flow (GMBF) in a manner independent of NO or calcitonin gene-related peptide (CGRP) in anesthetized rat (Kawabata et al., 2001b). Thus, the mechanisms responsible for NO-independent vascular control by PAR-2 in vivo remain to be elucidated. Considering recent evidence that PAR-2 agonists produce EDHF-dependent relaxation responses in some isolated blood vessels (McGuire et al., 2002; McLean et al., 2002), the NO-independent hypotension and increased GMBF following PAR-2 activation in vivo might involve the EDHF-mediated mechanisms. Since a combination of apamin, an inhibitor of small-conductance Ca2+-activated K+ channels, and charybdotoxin, an inhibitor of large- and intermediate-conductance Ca2+-activated K+ channels, is capable of abolishing the EDHF-dependent vascular relaxation in vitro (Edwards & Weston, 2001), the present study examined the effects of apamin and charybdotoxin on the hypotension in response to PAR-2 activation in anesthetized rats. We then examined dose-dependent effects of the PAR-2-activating peptide on GMBF in vivo, and also tested the involvement of apamin/charybdotoxin-sensitive K+ channels. Finally, we evaluated and characterized the effect of the PAR-2 agonist on the isolated rat gastric artery.
Methods
Animals
Male Wistar rats (6–7 week old, Japan SLC. Inc., Japan) were used throughout the experiments. All procedures involving the use of animals conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Chemicals
The PAR-2-activating peptide SLIGRL-NH2 and the control peptide LSIGRL-NH2 were synthesized by a standard solid-phase method, and the concentration, purity and composition of the peptides were determined by high-performance liquid chromatography, mass spectrometry and quantitative amino-acid analysis. Trypsin (from porcine pancreas), NG-nitro-L-arginine methyl ester hydrochloride (L-NAME), apamin, phentolamine and capsaicin were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.), and charybdotoxin and CGRP were from Peptide Institute, Inc. (Minoh, Japan). Indomethacin was obtained from Wako Pure Chem. (Osaka, Japan). Capsaicin was suspended in a saline solution containing 10% ethanol and Tween 80, and indomethacin was dissolved in 5 mM Na2CO3 for the in vitro study or 4% NaHCO3 (480 mM) for the in vivo study. All other chemicals were dissolved in saline.
Measurement of arterial blood pressure
Under anesthesia with i.p. urethane at 1.5 g kg−1, the left carotid artery was cannulated with a polyethylene tube containing isotonic heparin saline solution (5 U ml−1), which was connected with a pressure transducer (DTX Plus DT-XXAD, Becton Dickinson Critical Care System. Pte Ltd, Singapore) for continuous monitoring of mean arterial pressure (MAP). During the experiments, the rats received a continuous infusion of saline. The right jugular vein was exposed for i.v. administration of drugs and peptides. The body temperature was controlled by a Thermocouple Temperature Controller® (Harvard Apparatus Inc., Holliston, U.S.A.).
Measurement of GMBF and vascular resistance
GMBF was measured by a laser Doppler flow meter (ALF-21, Advance Co., Tokyo, Japan), as described previously (Kawabata et al., 2001b). After exposure to a 24-h fast, the rat was anesthetized with urethane and laparotomized, and a probe (type N, Advance Co.) was placed lightly on the surface of the corpus mucosa through a balancer (ALF-B, Advance Co.). Experiments were performed after a 1-h stabilization period. Gastric mucosal vascular resistance (GMVR; pressure divided by flow) was also calculated.
Drug administration schedules in vivo
SLIGRL-NH2 at 3–1000 nmol kg−1, LSIGRL-NH2 at 1000 nmol kg−1, trypsin at 6.56–65.6 nmol kg−1, phentolamine at 5 mg kg−1 (16 μmol kg−1) or CGRP at 2.8 nmol kg−1 were administered i.v. through the femoral vein over 15 s. A bolus dose of L-NAME at 30 or 100 mg kg−1 (111 or 370 μmol kg−1), as described elsewhere (Kawabata et al., 2001b), was given i.v. 15 min before i.v. challenge with PAR-2 agonists. Indomethacin at 20 mg kg−1 (56 μmol kg−1) was administered s.c. 30 min before i.v. PAR-2 agonists. Bolus doses of apamin at 0.05–0.2 mg kg−1 (25–100 nmol kg−1) and/or charybdotoxin at 0.05–0.4 mg kg−1 (12–93 nmol kg−1), as reported elsewhere (Tanaka et al., 2000), were given i.v. 20 min before i.v. administration of PAR-2 agonists, phentolamine or CGRP. Ablation of sensory nerves was performed by preadministration of large doses of capsaicin as described previously (Kawabata et al., 2001b). Briefly, capsaicin at 25, 50 and 50 mg kg−1 (82, 164 and 164 μmol kg−1) was administered s.c. to the rat three times under anesthesia with i.p. pentobarbital (50 mg kg−1), at 0, 6 and 32 h, respectively, and the rat was used for experiments 10 days after the last dose. The effectiveness of this treatment was verified by counting wiping movements in response to instillation of one drop of 0.1 mg ml−1 capsaicin into the eye as described (Steinhoff et al., 2000).
Isometric measurement of tension oscillation of isolated rat gastric artery
The rat was killed by exsanguinations under urethane (1.5 g kg−1, i.p.) anesthesia, and small gastric arteries were rapidly removed and carefully cleaned of all fat and connective tissue. Ring segments (0.3–0.36 mm in diameter × 1 mm long) with the endothelium were prepared and mounted in a microtissue organ bath (2 ml) (MTOB-1, Labo-Support, Co., Suita, Japan) for measurements of isometric tension oscillation. In some experiments, the arterial rings were stripped off the endothelium by gently rubbing the inner surface with a cotton string. The preparations were then equilibrated under a 5 mN load for 30 min at 37°C in a gassed (95% O2/5% CO2) Krebs–Henseleit solution of the following composition (mM): NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; KH2PO4, 1.2; glucose, 10. The ring preparations were exposed twice to high K+ (100 mM) solution that was prepared by replacing NaCl in the Krebs–Henseleit solution with KCl. Afterwards, the preparations were contracted with 10 μM phenylephrine, and the presence and absence of a functional endothelium were ascertained by monitoring a relaxation response to 10 μM acetylcholine. The PAR-2-activating peptide SLIGRL-NH2 was cumulatively applied to the ring preparations precontracted with 10 μM phenylephrine, and thereafter 100 μM papaverine was added to the organ bath. L-NAME at 100 μM, indomethacin at 10 μM, apamin at 0.1 μM and charybdotoxin at 0.1 μM were applied 5 min before addition of 10 μM phenylephrine to the bath.
Statistics
Changes in MAP, GMBF and GMVR are represented as differences (% change) from the baseline values before i.v. administration of test compounds. All data are expressed as mean±s.e.m., and were analyzed with ANOVA followed by Tukey's test or by Bonferroni's test for multiple comparisons, and with Student's t-test for comparison of two-group data. A value of P<0.05 was considered statistically significant.
Results
Dose-dependent depressor effects of the PAR-2-activating peptide and trypsin in anesthetized rats and their resistance to NO inhibition and ablation of sensory neurons
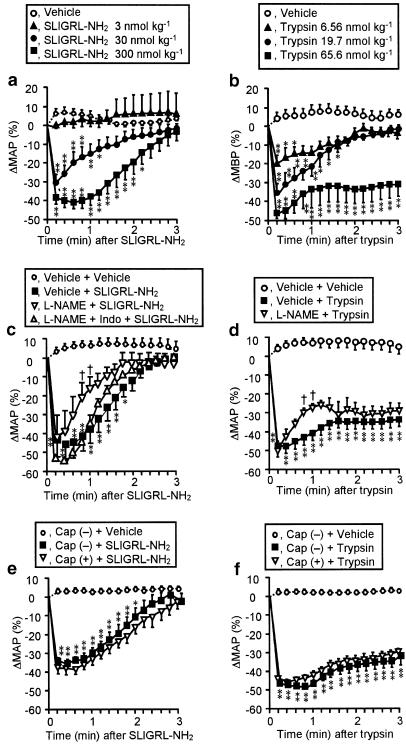
The PAR-2-activating peptide SLIGRL-NH2, administered i.v. at 3–300 nmol kg−1, rapidly decreased MAP in a dose-dependent manner, disappearing within 3 min (Figure 1a). These depressor responses were followed by transient slight hypertension (about 10 mmHg) (data not shown), being possibly secondary to a sympathetic reflex (Cicala et al., 2001). Of note is that the inactive control peptide LSIGRL-NH2 at corresponding doses had no effect (data not shown). Trypsin, administered i.v. at 6.56–65.6 nmol kg−1, also produced dose-dependent decreases in MAP (Figure 1b). The depressor effect of trypsin at the maximal dose of 65.6 nmol kg−1 lasted for approximately 10 min (data not shown), while its effects at low doses, 6.56 and 19.7 nmol kg−1 disappeared within 3 min (Figure 1b). No delayed hypertension was observed after disappearance of the hypotension following i.v. trypsin (data not shown).
Figure 1.
Characterization of the depressor effects of the PAR-2 activators SLIGRL-NH2 (left) and trypsin (right) in rats. (a, b) SLIGRL-NH2 and trypsin were administered i.v. to rats. n=10 (vehicle) or 4–6 (others). The basal MAP (mmHg) was 96.9±5.0 (a) and 115.8±2.7 (b). (c, d) L-NAME (30 mg kg−1) was administered i.p. 15 min before i.v. SLIGRL-NH2 (300 nmol kg−1) or trypsin (65.6 nmol kg−1). Indomethacin (Indo) (20 mg kg−1) was given s.c. 30 min before SLIGRL-NH2. n=4–6. L-NAME continuously elevated MAP (mmHg) from 106.0±6.6 to 146.3±4.8 (c) and from 103.5±3.2 to 154.8±3.0 (d). (e, f) The same doses of PAR-2 activators as (c, d) were administered i.v. 10 days after capsaicin treatment. Cap (+) and (−), treated with capsaicin and vehicle, respectively, n=4–7. Capsaicin treatment unaffected the basal MAP. *P<0.05, **P<0.01 vs vehicle in (a, b), vehicle+vehicle in (c, d), or Cap (−)+vehicle in (e, f); †P<0.05 vs vehicle+PAR-2 activator in (c, d).
The maximal hypotensive responses to the PAR-2-activators, SLIGRL-NH2 at 300 nmol kg−1 and trypsin at 65.6 nmol kg−1, were resistant to pretreatment with L-NAME at 30 mg kg−1 (Figure 1c, d) that, by itself, persistently elevated MAP by 40–50 mmHg (see legend for Figure 1), although slight inhibition by L-NAME was noted in the recovery phase after the maximal hypotension was achieved (Figure 1c, d). A supramaximal dose, 100 mg kg−1, of L-NAME produced neither additional hypertension by itself nor additional inhibition of the depressor responses to PAR-2 activators (data not shown). L-NAME in combination with indomethacin at 20 mg kg−1 had no effect on the depressor effect of SLIGRL-NH2 (Figure 1c). The hypotension in response to SLIGRL-NH2 at 300 nmol kg−1 or trypsin at 65.6 nmol kg−1 was also unaffected by ablation of capsaicin-sensitive sensory neurons (Figure 1e, f). Collectively, it was ascertained in our experimental conditions that the PAR-2-mediated hypotension in vivo was largely independent of endogenous NO, in agreement with the previous studies (Cicala et al., 1999; 2001), and was not mediated by capsaicin-sensitive sensory neurons.
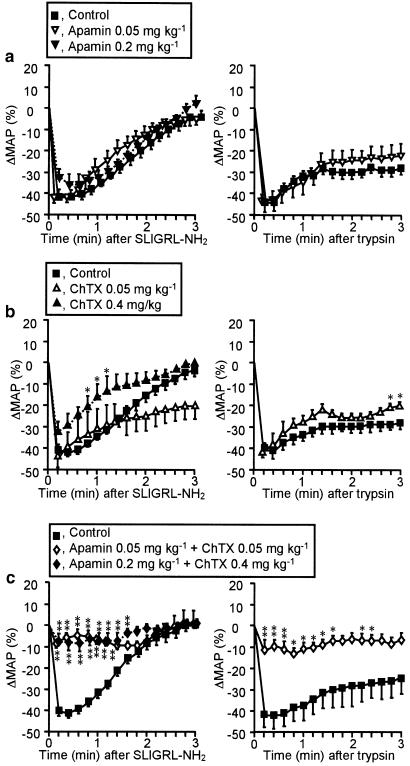
Effects of apamin and/or charybdotoxin on the hypotension in response to PAR-2 activators
Apamin, administered i.v. at 0.05 or 0.2 mg kg−1, temporarily increased MAP by 10–15 mmHg, which returned to the basal level within 10 min (see legend for Figure 2). The depressor response to the PAR-2-activating peptide SLIGRL-NH2 at 300 nmol kg−1 was unaffected by pretreatment with apamin at 0.05 or 0.2 mg kg−1 (Figure 2a, left). Charybdotoxin, administered i.v. at 0.05 or 0.4 mg kg−1, also transiently elevated MAP by 30–50 mmHg, which returned to the basal level within 15 min (see legend for Figure 2). Charybdotoxin at 0.4, but not 0.05 mg kg−1 only slightly reduced the depressor response to SLIGRL-NH2 (Figure 2b, left). Combined preadministration of apamin and charybdotoxin at 0.2 and 0.4 mg kg−1, respectively, or each at 0.05 mg kg−1, transiently elevated MAP by 25–50 mmHg, which returned to the basal level within 15 min (see legend for Figure 2). Apamin plus charybdotoxin at either combination of the doses employed abolished the hypotension in response to i.v. SLIGRL-NH2 (Figure 2c, left). Similarly, the hypotension caused by i.v. trypsin at 65.6 nmol kg−1 was resistant to preadministration of apamin at 0.05 mg kg−1 (Figure 2a, right) or charybdotoxin at 0.05 mg kg−1 (Figure 2b, right), but almost completely blocked by their combined administration (Figure 2c, right). In contrast, the persistent hypotension following i.v. phentolamine at 5 mg kg−1 was not altered by combined administration of apamin at 0.05 mg kg−1 plus charybdotoxin at 0.05 mg kg−1; ΔMAP (%) after phentolamine administration was −46.8±3.0 and −45.9±1.6 in the control and apamin-/charybdotoxin-treated rats, respectively.
Figure 2.
Effects of apamin and charybdotoxin on the depressor responses to the PAR-2 activators SLIGRL-NH2 (left) and trypsin (right) in rats. Apamin (0.05 or 0.2 mg kg−1) (a), charybdotoxin (ChTX; 0.05 or 0.4 mg kg−1) (b), or their combination (c) were administered i.v. to rats 20 min before i.v. SLIGRL-NH2 (300 nmol kg−1) or trypsin (65.6 nmol kg−1). Apamin at 0.05 and 0.2 mg kg−1 increased MAP (mmHg) from 107.0±7.5 to 119.0±6.1 and from 96.3±11.2 to 108.8±8.8, respectively, at 2–3 min, which returned to the basal level within 10 min (a). ChTX at 0.05 and 0.4 mg kg−1 increased MAP from 109.5±8.9 to 140.3±7.3 and from 85.5±6.1 to 137.8±7.8, respectively, at 3–5 min, which returned to the basal level within 15 min (b). Combination of apamin at 0.05 and 0.2 mg kg−1 and ChTX at 0.05 and 0.4 mg kg−1 respectively, increased MAP from 108. 4±7.3 to 132.6±5.4 and from 103.0±7.6 to 153.8±7.2, which returned to the basal level within 15 min (c). n=4. *P<0.05, **P<0.01 vs the control.
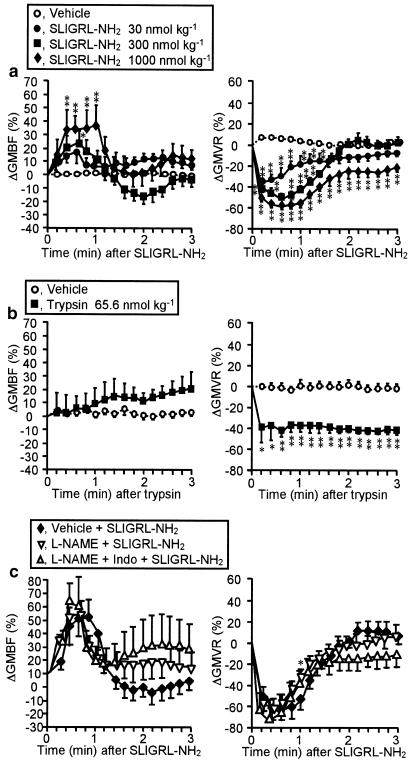
Dose-dependent alterations in GMBF and GMVR caused by the PAR-2-activating peptide and their resistance to NO inhibition
The PAR-2-activating peptide SLIGRL-NH2, administered i.v. at 30–1000 nmol kg−1, caused transient increase in GMBF accompanied by decrease in GMVR, in a dose-dependent manner (Figure 3a). The magnitude and duration of the GMBF increase due to SLIGRL-NH2 at the maximal dose of 1000 nmol kg−1 (Figure 3a) were consistent with those in our previous study (Kawabata et al., 2001b), in which the lack of effect of the control peptide LSIGRL-NH2 at the same dose had been demonstrated. In contrast, trypsin at 65.6 nmol kg−1 did not alter GMBF, although it caused long-lasting decrease in GMVR (Figure 3b). The NO synthase inhibitor L-NAME at 30 mg kg−1 slightly reduced the evoked decrease in GMVR, whereas it failed to significantly reduce the elevation in GMBF caused by SLIGRL-NH2 (Figure 3c). L-NAME in combination with indomethacin at 20 mg kg−1 had no effect on the SLIGRL-NH2-evoked increase in GMBF and decrease in GMVR (Figure 3c).
Figure 3.
Characterization of effects of SLIGRL-NH2 or trypsin on GMBF (left) and GMVR (right) in rats. (a, b): SLIGRL-NH2 (a) or trypsin (b) was administered i.v. to rats. n=25 (vehicle) or 8–9 (peptide) in (a), and 6 in (b). The basal GMBF (ml min−1 100 g−1) was 8.3±0.4. (c) L-NAME (30 mg kg−1) was administered i.p. 15 min before i.v. SLIGRL-NH2 (1000 nmol kg−1). Indomethacin (Indo) (20 mg kg−1) was given s.c. 30 min before SLIGRL-NH2. L-NAME unaffected the basal GMBF. n=4–7. *P<0.05, **P<0.01 vs vehicle in (a, b), or vehicle+SLIGLR-NH2 in (c).
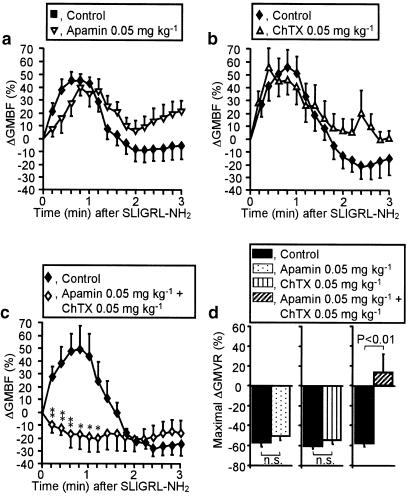
Effects of apamin and/or charybdotoxin on the increased GMBF and decreased GMVR caused by the PAR-2 agonist
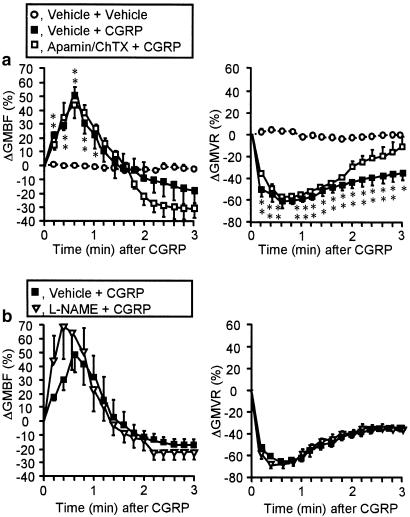
Neither apamin at 0.05 mg kg−1 nor charybdotoxin at 0.05 mg kg−1 significantly altered the basal levels of GMBF in rats (data not shown). Apamin or charybdotoxin at 0.05 mg kg−1 failed to modify the elevated GMBF and decreased GMVR produced by the PAR-2 agonist SLIGRL-NH2 at 1000 nmol kg−1 (Figure 4a, b, d). However, apamin and charybdotoxin, coadministered at the same dose, completely abolished the increase in GMBF and decrease in GMVR caused by PAR-2 activation (Figure 4c, d). In contrast, CGRP at 2.8 nmol kg−1 produced a prompt increase in GMBF and a persistent decrease in GMVR that were resistant to apamin plus charybdotoxin at 0.05 mg kg−1 or L-NAME at 30 mg kg−1 (Figure 5). It is also of note that CGRP-evoked hypotension was also unaffected by apamin plus charybdotoxin or L-NAME (data not shown). CGRP would thus appear to produce the in vivo effects through an endothelium-independent direct mechanism on vascular smooth muscle that involves elevated cyclic AMP levels, as described elsewhere (Yoshimoto et al., 1998).
Figure 4.
Effects of apamin and charybdotoxin on the increase in GMBF and decrease in GMVR caused by the PAR-2 activator SLIGRL-NH2. Apamin (0.05 mg kg−1) (a), charybdotoxin (ChTX; 0.05 mg kg−1) (b), or their combination (c) were administered i.v. to rats 20 min before i.v. SLIGRL-NH2 (1000 nmol kg−1). Apamin and/or ChTX unaffected the basal GMBF. Maximal changes in GMVR (42 s after SLIGRL-NH2) are shown in (d). n=5–7. *P<0.05, **P<0.01 vs the control. n.s., not sigficant.
Figure 5.
Lack of effects of apamin plus charybdotoxin or L-NAME on the increase in GMBF (left) and decrease in GMVR (right) caused by CGRP in rats. (a) Apamin (0.05 mg kg−1) plus charybdotoxin (ChTX; 0.05 mg kg−1) were administered i.v. to rats 20 min before i.v. CGRP (2.8 nmol kg−1). n=10 (vehicle+vehicle) or 4 (others). *P<0.05, **P<0.01 vs vehicle+vehicle. (b) L-NAME (30 mg kg−1) was administered i.p. 15 min before i.v. CGRP. n=5–7.
Relaxation responses of rat gastric arteries to the PAR-2-activating peptide in vitro
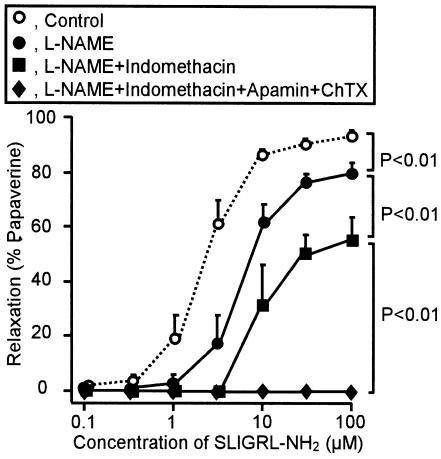
The PAR-2-activating peptide SLIGRL-NH2 at 0.1–100 μM induced concentration-dependent relaxation of the isolated rat gastric artery precontracted by 10 μM phenylephrine (Figure 6). This relaxant effect of the PAR-2 agonist was abolished by removal of the endothelium (data not shown). L-NAME at 100 μM slightly inhibited the relaxation caused by SLIGRL-NH2 and indomethacin at 10 μM produced additional inhibition, whereas the large component of the PAR-2-mediated relaxation still remained in the presence of L-NAME and indomethacin (Figure 6). The residual responses to SLIGRL-NH2 in the preparations were completely inhibited by apamin at 0.1 μM plus charybdotoxin at 0.1 μM (Figure 6). Thus, the PAR-2-triggered relaxation in rat gastric arteries was largely dependent on activation of apamin/charybdotoxin-sensitive potassium channels, indicating the involvement of EDHF, although it also included some components that involved NO- and prostanoid-dependent mechanisms.
Figure 6.
The relaxation caused by the PAR-2 activator SLIGRL-NH2 in isolated rat gastric artery. SLIGRL-NH2 (0.1–100 μM) was cumulatively applied to the preparation precontracted with 10 μM phenylephrine. L-NAME (100 μM), indomethacin (10 μM), apamin (0.1 μM) and charybdotoxin (ChTX; 0.1 μM) were added to the bath 5 min before phenylephrine. Data show the mean with s.e.m. from four experiments.
Discussion
Our study provides novel evidence that the NO-independent hypotension caused by PAR-2 activation in vivo is predominantly mediated by activation of apamin/charybdotoxin-sensitive K+ channels, predicting a critical role of EDHF in PAR-2-triggered relaxation of resistant blood vessels. Further, our data demonstrate, for the first time to our knowledge, contribution of apamin/charybdotoxin-sensitive K+ channels and possibly EDHF to the PAR-2-mediated increase in GMBF that may play a role in the gastric mucosal cytoprotection by PAR-2 activation (Kawabata et al., 2001b). The data from in vitro experiments also strongly suggest that endothelial PAR-2, upon activation, produces relaxation in gastric arteries, an effect being largely dependent on EDHF. Collectively, EDHF appears to play a critical role in the circulatory modulation by PAR-2, especially in the stomach.
It is noteworthy that the effective dose range of the PAR-2-activating peptide SLIGRL-NH2, 3–300 nmol kg−1, was not greatly different from that of trypsin, 6.56–65.6 nmol kg−1, in producing hypotension in vivo, while the latter agonist was approximately 1000-fold potent than the former in the previous in vitro relaxation assay (Kawabata et al., 2001d). This inconsistency is attributable to abundance of endogenous trypsin inhibitors in the blood stream. Some difference in the effective dose ranges of SLIGRL-NH2 in decreasing MAP and increasing GMBF might reflect the variance of expression levels of endothelial PAR-2 in distinct blood vessels. The reason why trypsin that triggered prompt hypotension failed to produce clear increase in GMBF, as SLIGRL-NH2 did, is still open to question. PAR-1, -3 and -4 that can also be activated by trypsin at relatively high concentrations might overcome the effect of trypsin on PAR-2 in gastric arterioles.
Tanaka et al. (2000) have reported that apamin in combination with charybdotoxin partially inhibited the depressor responses to platelet-activating factor (PAF), but not sodium nitroprusside, in rats in vivo, suggesting the possible involvement of EDHF, to a certain extent, in the vascular effect of PAF. The present finding that apamin plus charybdotoxin almost completely inhibited the PAR-2-triggered hypotension, without affecting the phentolamine-evoked hypotension, suggests that EDHF is even more important in the modulation of blood pressure by PAR-2 in vivo. PAR-2 might be abundant in smaller arterioles where EDHF would play a predominant role as an endothelial relaxant. Consistently, the fact that apamin plus charybdotoxin almost completely abolished the increase in GMBF caused by the PAR-2 agonist, but not by CGRP, strongly suggests a critical role of EDHF in regulating GMBF. This is also supported by our in vitro data showing a major role of EDHF in the production of endothelial PAR-2-mediated relaxation in isolated gastric arteries, which is consistent with the study of Van de Voorde & Vanheel (2000) demonstrating the involvement of EDHF in acetylcholine-evoked relaxation in isolated gastric arteries. Nevertheless, our in vitro data suggest that NO and prostaglandins are also involved, to a certain extent, in the PAR-2-mediated relaxation of gastric arteries, being inconsistent with the possibly exclusive role of EDHF in the PAR-2-mediated increase in GMBF. It is possible that EDHF might play a greater role in the relaxation of smaller gastric arterioles than the preparations used in our study, which is more important in regulation of GMBF.
Apamin and charybdotoxin are known to abolish the EDHF pathway in vitro, whereas each of them, given alone, is incapable of producing sufficient inhibition (Edwards & Weston, 2001), suggesting that distinct K+ channels sensitive to apamin or charybdotoxin compensate each other in mediating the EDHF pathway. Such synergistic inhibitory effects of apamin and charybdotoxin were also observed in our in vivo experiments, further supporting the involvement of EDHF in vivo. Our finding that charybdotoxin, but not apamin, given alone, showed slight but significant inhibitory effect on the hypotension in response to the PAR-2 agonist, might imply greater importance of charybdotoxin-sensitive K+ channels in activation of the EDHF pathway in systemic circulation.
PAR-2 is also expressed in capsaicin-sensitive sensory neurons. Most recently, McLean et al. (2002) have shown the involvement of activation of sensory neurons in the PAR-2-triggered vasodilation of rat coronary artery. However, our data indicate that sensory neurons are not involved in the PAR-2-triggered hypotension. Similarly, the PAR-2-triggered increase in GMBF has also been shown to be independent of sensory neurons in our previous study (Kawabata et al., 2001b). This is also in agreement with the present result that the CGRP-induced increase in GMBF was resistant to apamin plus charybdotoxin, differing from the PAR-2-triggered increase in GMBF.
Vascular control by CGRP is well described, especially in the stomach (Holzer, 1995). Our finding that CGRP-evoked hypotension was resistant to L-NAME is consistent with the study of Holzer et al. (1993). They also found that L-NAME significantly reduced the increase in GMBF caused by low doses, 0.1–0.3 nmol kg−1, of CGRP, whereas it had no effect on the GMBF increase by a high dose, 1 nmol kg−1, of CGRP, in agreement with our present study in which 2.8 nmol kg−1 of CGRP was administered i.v. to the rat. In other words, our data may reveal that large doses of CGRP increase GMBF by a direct mechanism independent of NO or EDHF.
In conclusion, our data suggest that EDHF-like factors play a major role in the hypotension and enhancement of GMBF caused by PAR-2 activation in vivo. Given the upregulation/induction of endothelial PAR-2 in response to inflammatory stimuli (Cicala et al., 1999; Kawabata et al., 2001e), the protease-PAR-2-EDHF pathway might be potentially important in septic hypotension and also in mucosal cytoprotection during gastric tissue injury.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research from the Japan Society of the Promotion of Science.
Abbreviations
- DIC
disseminated intravascular coagulation
- EDHF
endothelium-derived hyperpolarizing factor
- GMBF
gastric mucosal blood flow
- L-NAME
NG-nitro-L-arginine methyl ester
- MAP
mean arterial pressure
- PAR-2
protease-activated receptor-2
References
- CICALA C., MORELLO S., SANTAGADA V., CALIENDO G., SORRENTINO L., CIRINO G. Pharmacological dissection of vascular effects caused by activation of protease-activated receptors 1 and 2 in anesthetized rats. FASEB J. 2001;15:1433–1435. doi: 10.1096/fj.00-0633fje. [DOI] [PubMed] [Google Scholar]
- CICALA C., PINTO A., BUCCI M., SORRENTINO R., WALKER B., HARRIOT P., CRUCHLEY A., KAPAS S., HOWELLS G.L., CIRINO G. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999;99:2590–2597. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. EDHF – are there gaps in the pathway. J. Physiol. 2001;531:299. doi: 10.1111/j.1469-7793.2001.0299i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T., JOCIC M., WACHTER C., ERB R., HEINEMANN A. Nitric oxide-dependent and -independent hyperaemia due to calcitonin gene-related peptide in the rat stomach. Br. J. Pharmacol. 1993;110:404–410. doi: 10.1111/j.1476-5381.1993.tb13824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P. Chemosensitive afferent nerves in the regulation of gastric blood flow and protection. Adv. Exp. Med. Biol. 1995;371B:891–895. [PubMed] [Google Scholar]
- KAWABATA A., KAWAO N., KURODA R., TANAKA A., ITOH H., NISHIKAWA H. Peripheral PAR-2 triggers thermal hyperalgesia and nociceptive responses in rats. Neuroreport. 2001a;12:715–719. doi: 10.1097/00001756-200103260-00020. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KINOSHITA M., NISHIKAWA H., KURODA R., NISHIDA M., ARAKI H., ARIZONO N., ODA Y., KAKEHI K. The protease-activated receptor-2 agonist induces gastric mucus secretion and mucosal cytoprotection. J. Clin. Invest. 2001b;107:1443–1450. doi: 10.1172/JCI10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NAGATA N., KAWAO N., MASUKO T., NISHIKAWA H., KAWAI K. In vivo evidence that protease-activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br. J. Pharmacol. 2001c;133:1213–1218. doi: 10.1038/sj.bjp.0704211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NAKAYA Y., KAWAI K., NISHIKAWA H., KAWAO N. Factor Xa-evoked relaxation in rat aorta: involvement of PAR-2. Biochem. Biophys. Res. Commun. 2001d;282:432–435. doi: 10.1006/bbrc.2001.4597. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NAKAYA Y., KAWAO N., NISHIKAWA H. Ex vivo evidence that the phosphodi-esterase inhibitor IBMX attenuates the up-regulation of PAR-2 in the endotoxemic rat aorta. Thromb. Res. 2001e;101:513–515. doi: 10.1016/s0049-3848(00)00425-4. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., NISHIKAWA H., KURODA R., KAWAI K., HOLLENBERG M.D. Proteinase-activated receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in vivo in rats and mice. Br. J. Pharmacol. 2000;129:1808–1814. doi: 10.1038/sj.bjp.0703274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAO N., SAKAGUCHI Y., TAGOME A., KURODA R., NISHIDA S., IRIMAJIRI K., NISHIKAWA H., KAWAI K., HOLLENBERG M.D., KAWABATA A. Protease-activated receptor-2 (PAR-2) in the rat gastric mucosa: immunolocalization and facilitation of pepsin/pepsinogen secretion. Br. J. Pharmacol. 2002;135:1292–1296. doi: 10.1038/sj.bjp.0704562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACFARLANE S.R., SEATTER M.J., KANKE T., HUNTER G.D., PLEVIN R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MCGUIRE J.J., HOLLENBERG M.D., ANDRADE-GORDON P., TRIGGLE C.R. Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles. Br. J. Pharmacol. 2002;135:155–169. doi: 10.1038/sj.bjp.0704469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN P.G., ASTON D., SARKAR D., AHLUWALIA A. Protease-activated receptor-2 activation causes EDHF-like coro-nary vasodilation: selective preservation in ischemia/reperfusion injury: involvement of lipoxygenase products, VR1 receptors, and C-fibers. Circ. Res. 2002;90:465–472. doi: 10.1161/hh0402.105372. [DOI] [PubMed] [Google Scholar]
- NGUYEN T.D., MOODY M.W., STEINHOFF M., OKOLO C., KOH D.S., BUNNETT N.W. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J. Clin. Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBEY C.G., MOFFATT J.D., COCKS T.M. Evidence for selective effects of chronic hypertension on cerebral artery vasodilatation to protease-activated receptor-2 activation. Stroke. 1999;30:1933–1940. doi: 10.1161/01.str.30.9.1933. [DOI] [PubMed] [Google Scholar]
- STEINHOFF M., VERGNOLLE N., YOUNG S.H., TOGNETTO M., AMADESI S., ENNES H.S., TREVISANI M., HOLLENBERG M.D., WALLACE J.L., CAUGHEY G.H., MITCHELL S.E., WILLIAMS L.M., GEPPETTI P., MAYER E.A., BUNNETT N.W. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., HAYAKAWA S., IMAI T., AKUTSU A., HIRANO H., TANAKA H., NAKAHARA T., ISHII K., SHIGENOBU K. Possible involvement of endothelium-derived hyperpolarizing factor (EDHF) in the depressor responses to platelet activating factor (PAF) in rats. Br. J. Pharmacol. 2000;131:1113–1120. doi: 10.1038/sj.bjp.0703681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DE VOORDE J., VANHEEL B. EDHF-mediated relaxation in rat gastric small arteries: influence of ouabain/Ba2+ and relation to potassium ions. J. Cardiovasc. Pharmacol. 2000;35:543–548. doi: 10.1097/00005344-200004000-00005. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., BUNNETT N.W., SHARKEY K.A., BRUSSEE V., COMPTON S.J., GRADY E.F., CIRINO G., GERARD N., BASBAUM A.I., ANDRADE-GORDON P., HOLLENBERG M.D., WALLACE J.L. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat. Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- YOSHIMOTO R., MITSUI-SAITO M., OZAKI H., KARAKI H. Effects of adrenomedullin and calcitonin gene-related peptide on contractions of the rat aorta and porcine coronary artery. Br. J. Pharmacol. 1998;123:1645–1654. doi: 10.1038/sj.bjp.0701805. [DOI] [PMC free article] [PubMed] [Google Scholar]