Abstract
Staurosporine is a broad-specificity kinase inhibitor, which has acted as lead compound for the development of some novel cytotoxic compounds for treatment of cancer. This study investigates the unexpected observation that staurosporine can also induce homotypic cellular aggregation.
In this study, staurosporine is shown to activate rapid homotypic aggregation of U937 cells, at concentrations below those required to induce cell death. This activity is a particular feature of staurosporine, and is not shared by a number of other kinase inhibitors. The proaggregating activity of staurosporine is inhibited by deoxyglucose, cytochalasin B and colchicine. Staurosporine-induced aggregation can be distinguished from that induced by the phorbol 12-myristate 13-acetate by faster kinetics and insensitivity to cycloheximide. Staurosporine induces translocation of conventional and novel, but not atypical isoforms of protein kinase C (PKC). Aggregation induced by staurosporine is inhibited by a number of inhibitors of PKC isoforms, and by inhibitors of protein tyrosine kinases. Staurosporine also induces rapid phosphorylation of ERK and p38, and inhibitors of both these enzymes block aggregation.
Staurosporine induces dysregulated activation of multiple kinase signaling pathways in U937 cells, and the combined activity of several of these pathways is essential for the induction of aggregation.
Keywords: Staurosporine, homotypic aggregation, protein kinase C, U937, monocyte, adhesion, ERK, kinase inhibitor
Introduction
Staurosporine is a bacterial alkaloid with broad inhibitory activities against a variety of serine/threonine kinases (Ruegg & Burgess, 1989), including protein kinase C (PKC), cAMP-dependent protein kinase, cGMP-dependent protein kinase, myosin light chain kinase and calcium/calmodulin-dependent protein kinase II. Staurosporine is also reported to suppress several tyrosine kinase activities including p60v-src (Nakano et al., 1987), epidermal growth factor receptor (Secrist et al., 1990) and insulin receptor (Fujita-Yamaguchi & Kathuria, 1988). Staurosporine is a weak tumor promotor in mouse skin (Yoshizawa et al., 1990), but a fairly potent inhibitor of PMA-induced tumor promotion (Yamada et al., 1988). It also induces apoptosis in a variety of transformed cell lines. Based predominantly on its anti-PKC activities, and its ability to induce apoptosis, staurosporine and its analogues are being tested in clinical trials, and have shown some promise as anticancer agents (Senderowicz, 2001).
In the course of studies on the signaling pathways associated with CD98-mediated aggregation of the human promonocytic cell line U937, it was noted that staurosporine alone induced rapid homotypic aggregation of these cells. Intercellular adhesion of leukocytes plays an essential role in many immunologically important phenomena such as leukocyte trafficking and activation, cytokine production and antigen presentation. Leukocyte homotypic aggregation can be observed with neutrophils (Rochon & Frojmovic, 1992), monocytes (Yue et al., 1999), lymphocytes (Zapata et al., 1995) and eosinophils (Teixeira et al., 1995), and is generally mediated by adhesion molecules such as integrins and intercellular adhesion molecules (ICAMs). However, excessive homotypic aggregation of immune cells is often associated with pathological consequences, such as granuloma formation, or blood vessel occlusion. The intracellular pathways that regulate adhesion/aggregation are many and complex. However, PKC is known to play an important role in regulating cell aggregation (Laudanna et al., 1998; Yue et al., 1999). PKC signals are believed to be linked to cytoskeleton changes via mitogen-activated protein kinase (MAPK) activation (Miranti et al., 1999). Furthermore, activated PKC can control integrin clustering on the cell surface, and the avidity of surface adhesion molecules (e.g. β2 integrins) (Sano et al., 2001).
This study therefore investigates the proaggregation activity of staurosporine, specifically in relation to the role of PKC and MAPK kinases in the process. The results highlight the ability of staurosporine to act as an activator, as well as inhibitor, of numerous kinase cascades in the cells. Uncoordinated activation of multiple kinase pathways appears to underlie the alterations in U937 adhesiveness observed.
Methods
Materials
Genistein, N-[2-(4-bromocinnamylamino) ethyl]-5-isoquinoline (H-89), 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H-7), [9R,10S,12S]-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3,2,1-kl]pyrro-lo[3,4-l][1,6]benzodiazocine-10-carboxylic acid hexyl ester (KT5720), C26H19N3O5, CAS [99570-78-2] (K-252b), (2-[1-(3-dimethylaminopropyl)-indol-3-yl-3-(indol-3-yl)]-maleimide (GF109203X), 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a) pyrrolo(3,4-c)-carbazole (Gö6976), rottlerin, 2′-amino-3′-methoxyflavone (PD98059), 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole (SB203580) and calphostin C were from Calbiochem (La Jolla, CA, U.S.A.). Herbimycin A, staurosporine, PMA, colchicine, cycloheximide, cytochalasin B, ethylene diamine tetra-acetic acid (EDTA), deoxyglucose and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (Poole, U.K.). Fetal calf serum (FCS) and RPMI1640 were obtained from GIBCO (Grand Island, NY, U.S.A.). U937, the human promonocytic cell line, was purchased from ATCC (American Tissue Culture Collection, Rockville, MD, U.S.A.).
Antibodies
The following antibodies were used in this study. CD18 (CLB-LFA1, IgG1, ascites, CLB); fluorescein-conjugated anti-CD18 (Immunotech, Marseille, France); CD29 (P5D5, IgG1, purified antibody, gift from N. Hogg); anti-PKC (α, β, γ, δ, ɛ, θ, η, λ/ι) antibodies were obtained from Transduction Laboratories (Lexington, KY, U.S.A.). Anti-MAPK/JNK and anti-phospho MAPK/JNK-1 antibodies were from Santa Cruz Biotechnology (Beverly, MA, U.S.A.). Anti-MAPK/ERK and anti-phosphotyrosine antibody 4G10 were from Upstate Biotechnology Inc. (Lake Placid, NY, U.S.A.). Anti-MAPK/p38 antibody and anti-phospho MAPK/p38 are from Cell Signaling Technology (Beverly, MA, U.S.A.). Anti-phospho MAPK/ERK antibody was obtained from Promega (Madison, WI, U.S.A.).
Cell culture
U937 cells were maintained in RPMI 1640 supplemented with 10% FCS. Cells were grown at 37°C and 5% CO2 in humidified air.
Quantitative homotypic cell aggregation assay
RPMI 1640 medium (20 μl, supplemented with 10% FCS) in the presence or absence of staurosporine was placed in round-bottom wells of a 96-microwell plate. An equal volume of cells (106 cells ml−1) was added and incubated at 37°C for 4–7 h (Cho et al., 2001). The cells were resuspended gently, so as not to break up the clusters, and total and unaggregated cells were counted in a hemocytometer. Very few cells (less than 5%) remained in the wells. The percentage of cells in aggregates was determined by the following equation: % of cells in aggregates=[total cells−free cells/total cells] × 100. In each experiment, aggregation for each experimental group was measured in 3–6 replicate cultures, and each experiment was repeated at least three times. The results are expressed either as % cells in aggregates, or as % aggregation relative to control cultures without addition of inhibitor, but with aggregating inducer. Aggregation in medium alone was <10% in all experiments. The Student's t-test for unpaired observations, or a one-way ANOVA with post hoc Dunnett's two-sided test was used for statistical evaluation of differences, as shown in legends to each figure.
Cytofluorometric analysis
Expression of cell surface molecules on U937 cells was determined by flow cytometry according to standard protocols.
MTT assay (colorimetric assay) for measurement of cell viability
Cell viability was measured by standard MTT assay. MTT solution (10 μl; 10 mg ml−1 in phosphate-buffered saline) was added to each well of U937 cultures for 3 h before the end of the culture period. The cells were lysed by the addition of 15% SDS for solubilization of formazan, and the optical density (OD) at 570 nm (OD570–630) was measured using a Spectramax 250 microplate reader.
Subcellular fractionation for PKC translocation assay
U937 cells (5 × 106) were cultured in 4 ml serum-free RPMI (similar results were obtained in the presence of serum, although the basal level of PKC in the membrane fractions was slightly higher). After 3 h recuperation, staurosporine or PMA was added for 20 min, and the cells were then transferred onto ice, and washed three times with ice-cold phosphate-buffered saline. Homogenisation buffer A (800 μl; 20 mM Tris-HCl, pH 7.4, 2 mM EDTA, 2 mM EGTA, 50 mM β-glycerophosphate, 1 mM DTT, 1 mM Na3VO4, 10 μg ml−1 leupeptin, aprotinin and pepstatin, 0.4 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM benzimide, 2 mM hydrogen peroxide, 0.25 M sucrose) was added and the cell lysate was sonicated for 10 s three times. The homogenate was centrifuged at 2000 × g for 5 min at 4°C. The supernatant was further centrifuged at 100,000 × g for 25 min at 4°C to obtain the cytosolic fraction. The pellet was resuspended in 300 μl of homogenization buffer B (2% Triton X-100 in buffer A), and sonicated for 10 s twice. After incubation on ice for a further 25 min, the suspension was centrifuged at 12,000 × g for 10 min at 4°C. The supernatant was collected and used as membrane fraction. The protein concentration of each sample was determined by the Bradford method and 2 × Laemmli sample buffer was added before boiling for 5 min.
For preparing whole-cell lysates, cells (5 × 106 cells ml−1) were washed three times in cold PBS containing 1 mM sodium orthovanadate, and lysed in lysis buffer (20 mM Tris-HCl, pH 7.4, 2 mM EDTA, 2 mM EGTA, 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1% Triton X-100, 10% glycerol, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 10 μg ml−1 pepstatin, 1 mM benzimide and 2 mM hydrogen peroxide) for 30 min with rotation at 4°C. Hydrogen peroxide was added as an additional inhibitor of phosphatases. The lysates were clarified by centrifugation at 16,000 × g for 10 min at 4°C.
Western blotting
Samples containing equal amounts (40 or 80 μg per lane) of protein were loaded in each lane, and separated on a 10% SDS–polyacrylamide gel. Proteins were transferred by electroblotting to nitrocellulose membrane. Membranes were blocked for 60 min in 3% bovine serum albumin (BSA) Tris-buffered saline (TTBS containing 20 mM NaF, 2 mM EDTA, 0.2% Tween 20) at room temperature. Immunoblots for phosphorylated or nonphosphorylated proteins of six PKC isoforms, MAPK/p38, MAPK/ERK and MAPK/JNK, and phosphotyrosine proteins were carried out using specific antibodies against phosphorylated or nonphosphorylated sites and secondary horseradish peroxidase-conjugated rabbit anti-mouse or swine anti-rabbit immunoglobulins. Peroxidase activity was visualized by chemiluminescence detection (ECL reagents, Amersham, Little Chalfont, Buckinghamshire, U.K.). The intensity of bands on the film was quantified using Synoptic Ltd. (Cambridge, U.K.) image analysis system and software.
Results
Staurosporine selectively induces homotypic aggregation
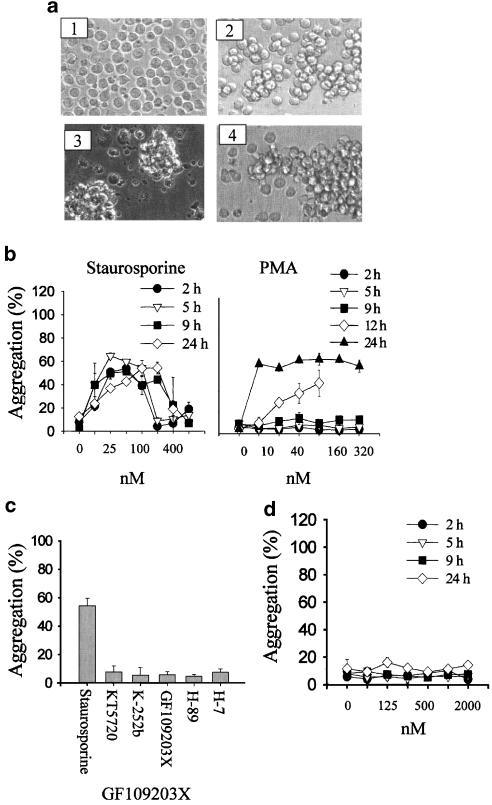
Clustering of U937 cells induced by staurosporine treatment for 4 h is shown in Figure 1a. PMA, a potent PKC agonist that has previously been shown to induce homotypic aggregation in a number of cell types, did not induce any clustering by 4 h. For comparison, the clustering induced by an anti-β1 integrin antibody is also shown in Figure 1a. A quantitative assay of clustering (Cho et al., 2001) was used to analyze both concentration response and kinetics of the staurosporine effect. Staurosporine induced aggregation over a wide range of concentrations (12–200 nM), while higher concentrations induced cell death. Staurosporine did not affect cell viability as measured by MTT assay at concentrations below 100 nM (not shown). Clustering could be observed after 2 h, and was maintained for up to 24 h (Figure 1b). In contrast, PMA induced clustering only after 12 and 24 h (Figure 1b). Viability of cells after PMA treatment was 70% at 24 h. Staurosporine-induced clustering was also observed using a number of other cell lines, including the macrophage lines RAW264.7 and J774, and the EL4 thymoma.
Figure 1.
Staurosporine induces rapid homotypic aggregation of U937 cells, which is blocked by protein kinase inhibitors. (a) U937 cells were incubated with medium alone (1), staurosporine (100 nM, 4 h) (2), PMA (80 nM, 24 h) and (3) anti-CD29 (MEM101A) (0.5 μg ml−1 4 h) (panel 4), as described in Methods. Images of cells in culture at this time point were obtained using an inverted phase contrast microscope, attached to a video camera, and captured using NIH image software. (b) Concentration response and kinetics of U937 aggregation in response to staurosporine or PMA. U937 cells were incubated in the presence or absence of staurosporine or PMA, at a range of concentrations for various times as indicated. Mean±s.e.m. of three experiments. (c) Selectivity of U937 aggregation response to staurosporine and other protein kinase inhibitors. U937 cells were incubated in the presence of inhibitors (staurosporine (100 nM), KT5720 (500 nM), K-252b (500 nM), GF109203X (1 μM), H-89 (50 μM) and H-7 (50 μM)) for 3 h. Aggregation was measured as described in Methods. Mean±s.e.m. of three experiments. (d) Lack of response to GF109203X at a range of concentrations and time points (as for panel b).
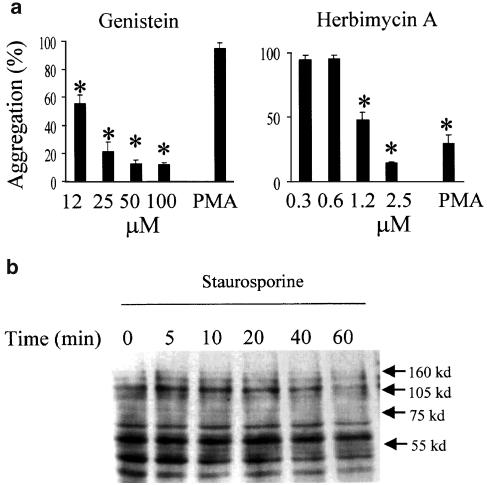
Staurosporine is best known as a potent inhibitor of many intracellular kinases. The ability of a panel of other protein kinase inhibitors was therefore tested (Figure 1c). There was no aggregation induced by KT5720 and K252b (two kinase inhibitors structurally related to staurosporine), or H89 and H-7 (two protein kinase inhibitors unrelated in structure to staurosporine). These inhibitors show variable selectivity for various different subclasses of protein kinases at lower concentrations, but at the higher concentrations used in Figure 1c inhibit a broad range of kinases similar to staurosporine. The concentrations shown are the maximum that were used (usually limited by cellular cytotoxicity), but no effect was observed over a wide range of lower concentrations (not shown). GF109203X, an inhibitor of all isotypes of PKCs, also showed no proaggregation activity (Figure 1c and d). Similarly, neither genistein nor herbimycin A, two broad spectrum inhibitors of tyrosine protein kinases, induced U937 aggregation (aggregation in the presence of 25 μM genistein was 7.7±2.8% and in the presence of 2.5 μM herbimycin was 5.1±2.9%, mean±s.e.m., n=3).
Staurosporine-induced aggregation is dependent on energy, temperature and cytoskeleton, but not protein synthesis
In order to probe the events underlying staurosporine-induced aggregation, a number of inhibitors of various aspects of cell function were tested in this model. As shown in Table 1, staurosporine-induced aggregation shares many properties with PMA-induced aggregation, including sensitivity to ATP synthesis blockers (deoxyglucose), inhibitors of cytoskeleton (colchicine (microtubule) and cytochalasin B (actin)) and low temperature (4°C), suggesting that the aggregation is dependent on energy, temperature and cytoskeleton, but not protein synthesis. The pharmacological profile of staurosporine-induced aggregation is similar to that of PMA, except that PMA-induced aggregation is sensitive to cycloheximide, suggesting a requirement for protein synthesis.
Table 1.
Characterization of staurosporine-induced homotypic aggregation with metabolic inhibitors
| Aggregation (% of control: mean±s.e.m.) | ||
|---|---|---|
| Staurosporine | PMA | |
| Deoxyglucose | 21.0±3.9** | 27.4±3.2** |
| Cycloheximide | 97.5±8.0 | 15.2±5.1** |
| Colchicine | 30.1±5.0** | 22.5±2.6** |
| EDTA | 95.7±5.9 | NT |
| Cytochalasin B | 18.9±1.8** | 15.6±1.9** |
| 4°C | 6.1±0.5** | 5.9±0.8** |
U937 cells were treated with staurosporine (100 nM) or PMA (80 nM) for 3 or 12 h, respectively. The metabolic inhibitors (deoxyglucose (10 mM), cycloheximide (10 μg ml−1), colchicine (10 μM), EDTA (2 mM) and cytochalasin B (1 μM)) were added 1 h prior to the aggregation agonists and remained in the culture. Results are expressed as aggregation relative to control cultures in the presence of the aggregating agonist, but absence of inhibitor (mean±s.e.m., three independent experiments). In all, 47% of U937 cells aggregated in the presence of staurosporine alone, and 56% aggregated in the presence of PMA alone. Aggregation in medium alone was always less than 8%. All values which differ significantly from 100% (Student's t-test, P<0.01) are indicated by asterisks.
Staurosporine-induced aggregation is not mediated by either β1 or β2 integrins
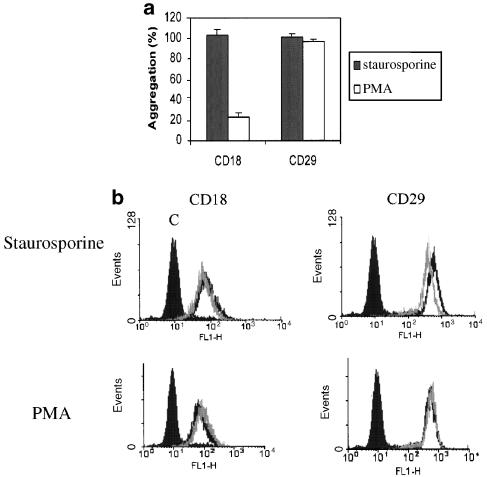
The involvement of adhesion molecules in U937 aggregation was tested by addition of blocking antibodies to β1 (CD29: P5D2) and β2 (CD18: CLB-LFA1) integrins. As shown in Figure 2a, integrin-blocking antibodies had no inhibitory effect on staurosporine-triggered aggregation. In contrast, PMA-induced aggregation was strongly suppressed by blocking mAbs to CD18. Blocking antibodies to CD98, CD147, CD43 and CD44 (other cell surface molecules previously implicated in cell–cell adhesion of U937; Cho et al., 2001) had no effect on staurosporine-induced aggregation (data not shown).
Figure 2.
The involvement of cell surface adhesion molecules in staurosporine-induced U937 homotypic aggregation. (a) U937 cells were pretreated with blocking antibodies to CD18 and CD29 (10 μg ml−1) for 1.5 h prior to the addition of staurosporine (100 nM) or PMA (80 nM). Aggregation was measured at 4 h for staurosporine and 12 h for PMA. The results show aggregation as a % of the aggregation in the presence of staurosporine or PMA alone (mean±s.e.m. of three experiments.) (b) U937 cells were incubated in the presence of staurosporine (100 nM) or PMA (80 nM) for 4 h, and then analyzed for cell surface expression of CD18 and CD29, as described in Methods. Dark line – medium; light line – staurosporine; solid histogram – isotype control.
The levels of CD18 and CD29 in response to both staurosporine and PMA were examined in parallel (Figure 2b). Staurosporine decreased CD18 and CD29 expression slightly. In contrast, PMA slightly increased the level of CD18 expression as reported previously (Nambu et al., 1992). The changes in surface expression levels were small, although consistent between experiments.
Stauroporine-induced aggregation is PKC dependent
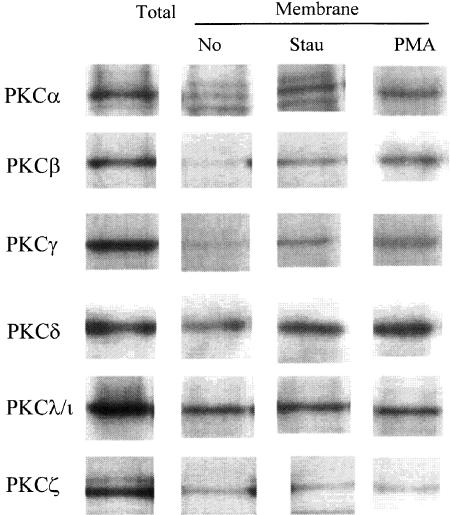
Although staurosporine acts predominantly as a kinase inhibitor, it is known to be a weak activator of novel PKC isoforms that can act as a tumor promotor (Yoshizawa et al., 1990; Jones et al., 1997). The membrane translocation (as a surrogate of activation) of several PKC isoforms (α, β, γ, δ, λ/ι and ζ) that are expressed in U937 cells (Laudanna et al., 1998) was carefully examined. As shown in Figure 3, staurosporine, at the same concentration as that which induces aggregation, induced translocation of both conventional and novel isoforms of PKC, similar to those induced by PMA. The membrane concentrations of atypical PKC isoforms (λ/ι and ζ) were not changed by staurosporine or PMA (Keenan et al., 1995; Miranti et al., 1999).
Figure 3.
Staurosporine-induced aggregation is accompanied by translocation of conventional and novel PKC isotypes. U937 were incubated with staurosporine (100 nM) or PMA (80 nM). After 20 min, the cells were lysed and analyzed for membrane-associated PKC (α, β, γ, δ, λ/ι, ζ), as described in Methods. All lanes are equalized for protein loading. The results show one representative experiment out of three. The first column confirms the presence of the various isotypes of PKC in unstimulated U937 by Western blot of the total cell lysate.
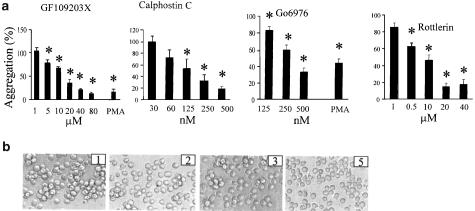
The role of PKC activation in mediating the aggregation response was explored by addition of a panel of other kinase inhibitors (since as shown in Figure 1, these inhibitors did not in themselves induce any aggregation). All PKC inhibitors tested showed strong inhibition of staurosporine-induced aggregation (Figure 4a, b). The inhibitors tested included both those with broad specificity for multiple isotypes (GF109203X), and more selective inhibitors including calphostin C (DAG-sensitive novel PKC), Gö6976 (Ca2+-sensitive conventional and novel PKC) and rottlerin (selectivity for PKCδ). As predicted, GF109203X and Gö6976 also blocked PMA-induced aggregation. The concentration of GF109203X required was relatively high (10 μM or above), perhaps reflecting the low sensitivity of some novel PKC isoforms such as PKCδ (Cho et al., 2003).
Figure 4.
Staurosporine-induced aggregation is inhibited by inhibitors of both conventional and novel PKC isotypes. U937 cells were incubated with various PKC inhibitors in the presence or absence of staurosporine (100 nM, 4 h) or PMA (80 nM, 12 h). Inhibitors were added 1.5 h prior to the agonists. (a) The inhibitors were added at the concentrations shown. Aggregation is expressed as a % of the aggregation obtained in the absence of inhibitor. PMA aggregation is shown in the presence of the highest concentration shown for each inhibitor. The figure shows the mean±s.e.m. of three experiments, and values that differ significantly from control (i.e. no inhibitor) (ANOVA with post hoc Dunnett's, P<0.05) are indicated by an asterisk. Aggregation in medium alone was less than 8%. (b) Representative images of aggregation in the presence of staurosporine (100 nM) alone (panel 1), staurosporine and calphostin C (500 nM; panel 2), staurosporine and Gö6976 (500 nM; panel 3) and staurosporine and rottlerin (10 μM; panel 4). Images of cells in culture point were obtained using an inverted-phase contrast microscope, attached to a video camera, and captured using NIH image software.
Tyrosine kinase inhibitors block staurosporine-induced aggregation
A previous study has suggested that staurosporine requires protein tyrosine kinase activation for β1 integrin-dependent spreading of a colon carcinoma line (Yoshimura et al., 1995). We therefore explored whether tyrosine phosphorylation is required as a part of the signaling pathway for aggregation. As shown in Figure 5a, two general protein tyrosine kinase inhibitors attenuated staurosporine-induced aggregation. Unexpectedly, PMA-induced clustering was sensitive to herbimycin but not genistein, perhaps reflecting the differential sensitivity to these two inhibitors of specific tyrosine kinases (Graber et al., 1992; Haraguchi et al., 2001). Western blot analysis with a pan-phosphotyrosine antibody (Figure 5b), however, did not show striking changes in the overall pattern of tyrosine-phosphorylated proteins. (Rather, staurosporine blocked tyrosine phosphorylation at 1 h incubation.) The change in tyrosine phosphorylation may be too small to pick up, or alternatively this antibody may not recognize the particular phosphorylated protein involved.
Figure 5.
Staurosporine-induced aggregation is blocked by protein tyrosine kinase inhibitors. (a) U937 cells were pretreated for 1.5 h with various concentrations of genistein and herbimycin A as indicated, before addition of staurosporine (100 nM, 4 h) or PMA (80 nM, 12 h). The concentrations of inhibitor used with PMA were 50 and 1.2 μM for genistein and herbimycin, respectively. Aggregation is expressed as a % of the aggregation obtained in the absence of inhibitor. Mean±s.e.m. of three experiments and values that differ significantly from control (i.e. no inhibitor) (ANOVA with post hoc Dunnett's, P<0.05) are indicated by an asterisk. Aggregation in the medium alone was less than 10%. (b) U937 cells (5 × 106) were incubated in the presence of staurosporine (100 nM) for various time periods indicated. Cells were lysed and analyzed for phospho-tyrosine proteins as described in Methods. Mean±s.e.m. of three experiments.
The activation of MAPK is linked with staurosporine-induced aggregation
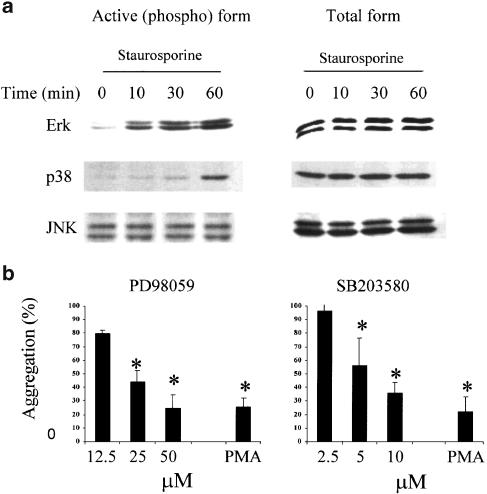
MAPK is a downstream target of activated PKC. MAPK activation in turn regulates cytoskeletal changes such as actin polymerization. Therefore, we examined the involvement of these three MAP kinase pathways in staurosporine-induced aggregation. Staurosporine induced strong phosphorylation (a measure of activation) of ERK1/2 and p38, as analysed by Western blot (Figure 6a). JNK/SAPK phosphorylation was not affected significantly. Two inhibitors (PD98059, an inhibitor of MAPKK1/2, and SB203580, an inhibitor of p38 MAPK) were tested in the staurosporine aggregation model (Figure 6b). Treatment of cells with either inhibitor concentration-dependently inhibited the aggregation triggered by staurosporine. Both inhibitors also reduced PMA-induced aggregation. The concentration of SB203580 required was rather high, and this inhibitor has previously been shown to inhibit PI3 kinase in some systems (Lali et al., 2000). However, wortmannin, a selective inhibitor of PI3 kinase signaling, did not inhibit staurosporine-induced aggregation at concentrations up to 150 nM.
Figure 6.
Staurosporine-induced aggregation is linked to MAPK activation. (a) U937 cells (5 × 106) were incubated in the presence of staurosporine (100 nM) for various time periods as indicated. Cells were lysed and analysed for total or phospho-ERK and p38, as described in Methods. (b) U937 cells were pretreated for 1.5 h with various concentrations of PD98059 or SB203580 as indicated, before addition of staurosporine (100 nM, 4 h) or PMA (80 nM, 12 h). PMA aggregation is in the presence of the highest concentration shown for each inhibitor. Aggregation is expressed as a % of the aggregation obtained in the absence of inhibitor. Mean±s.e.m. of three experiments, and values that differ significantly from control (i.e. no inhibitor) (ANOVA with post hoc Dunnett's, P<0.05) are indicated by an asterisk. Aggregation in medium alone was less than 10%.
Staurosporine has also been reported to enhance cAMP responses (Kawase et al., 1992), although the mechanisms remain unclear. The PKA selective inhibitor KT5720 (5 μM) was therefore tested, but did not affect staurosporine-induced aggregation (aggregation <10%). Furthermore, dibutyryl cAMP did not induce aggregation or inhibit staurosporine-dependent aggregation (data not shown).
Inhibition of staurosporine-induced aggregation is time dependent
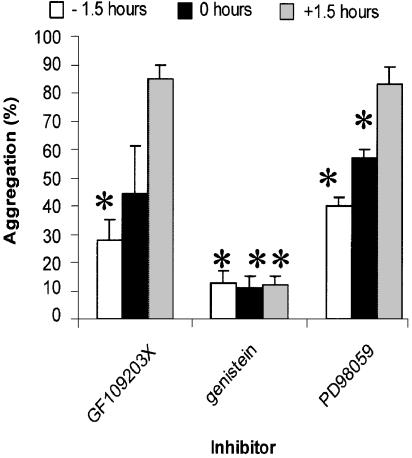
Pharmacological analysis suggested that staurosporine-induced aggregation required the activity of multiple families of kinases. To try and distinguish between enzymes involved in induction and effector (cell–cell binding) phases of aggregation, we examined the ability of the various enzyme inhibitors to block aggregation when added either prior to, simultaneously with, or after addition of staurosporine (Figure 7). The PKC inhibitor GF109203X and the ERK/MAPK inhibitor PD98059 blocked staurosporine-induced adhesion most effectively when added 1.5 h before staurosporine, and lost their inhibitory activities if added after staurosporine. In contrast, the tyrosine kinase inhibitor genistein inhibited to the same extent, whether added before staurosporine or up to 1.5 h afterwards.
Figure 7.
Inhibition of staurosporine-induced aggregation is time-dependent. U937 cells were incubated in the presence of inhibitors either 1.5 h before, at the same time as, or 1.5 h after staurosporine (100 nM). The inhibitors were used at the following concentrations: GF109203X (80 μM), PD98059 (50 μM) and genistein (100 μM). Aggregation was measured after 4 h. Aggregation is expressed as a % of the aggregation obtained in the absence of inhibitor. Mean±s.e.m. of three experiments, and values that differ significantly (Student's t-test, P<0.05) from 100% are indicated by an asterisk.
Discussion
Staurosporine has been used as a lead compound in the development of new cytotoxic protein kinase inhibitors, with potential anticancer activities (Senderowicz, 2001). In this study, we investigate the unexpected observation that this compound can induce homotypic cellular aggregation at concentrations below those required for the induction of cell death. This activity of staurosporine may be of importance in fully characterizing the pharmacological properties of staurosporine, since homotypic aggregation can be associated with several pathological events, including granuloma formation and blood vessel occlusion.
Cell aggregation induced by staurosporine was rapid, and was induced over a wide range of concentrations. Rapid clustering of cells is sometimes seen as a consequence of cell death, but the ability to induce aggregation occurred at low concentrations of drug, which had no effect on cell viability (as measured both by MTT assay and trypan blue exclusion). Aggregation required both metabolic and cytoskeletal activity, since it was blocked by deoxyglucose, cytochalasin B and colchicine.
Staurosporine is best known as a broad-spectrum kinase inhibitor, with some activity against most of the major classes of cellular kinases. However, close structural analogues of staurosporine (KT5720, K252b), as well as unrelated compounds with broad protein kinase inhibitor activity, failed to induce aggregation. Similarly, neither GF109203X, a broad-spectrum PKC inhibitor, nor genistein and herbimycin, protein tyrosine kinase inhibitors, had any proaggregating activity. The ability to induce aggregation is therefore a particular property of staurosporine, and must be distinguished both from its known proapoptotic and its kinase inhibitor activities.
Staurosporine has also been described as possessing weak PKC agonist activities (Jones et al., 1997). The relationship between PKC activity and aggregation was therefore investigated in more detail. PMA, a classical PKC activator, also induced aggregation, as previously described. However, aggregation induced by PMA was much slower, and was sensitive to cycloheximide, a protein synthesis inhibitor. Both the kinetics and the sensitivity to cycloheximide suggest that PMA-induced aggregation requires de novo protein synthesis, while staurosporine does not. PMA-induced aggregation was also blocked by antibodies to CD18 (Ikewaki et al., 1996; Cho et al., 2001), while staurosporine-induced aggregation is not. Indeed, the molecular interactions that mediate cell–cell attachment in the presence of staurosporine remain unknown. In view of the multiple signaling pathways activated by this compound (see below), it is likely that adhesion may be mediated by changes in the binding properties of many cell surface molecules acting in parallel, and identifying a single key molecular interaction by the use of blocking antibodies may not be possible.
Staurosporine, like PMA, also induced translocation of both conventional and novel PKC isoforms, but not the atypical forms PKCγ and ζ. Translocation of ɛ, δ and θ novel isoforms by staurosporine has been reported previously (Kiley et al., 1992; Jones et al., 1997), but the activation of the conventional forms has not been reported, and may be a particular property of the cell line used in this study. Inhibitors showing selectivity for either conventional or novel PKC isoforms, both blocked staurosporine-induced aggregation, suggesting either that both classes of enzyme were required for aggregation or that the inhibitors were not selective under the conditions used in these assays.
In addition to inducing translocation of PKCs, staurosporine induced rapid phosphorylation of the MAP kinase ERK, and a somewhat slower activation of p38. Furthermore, inhibitors of both these kinase pathways blocked aggregation, albeit partially. Inhibitors of both ERK and PKC blocked aggregation most effectively when added prior to staurosporine, and lost activity if added afterwards. ERK and PKC may therefore be required for the inductive phase of staurosporine-dependent aggregation, while protein tyrosine kinases may be involved in later phases, since aggregation remains sensitive to genistein up to 1.5 h after staurosporine addition.
In conclusion, staurosporine is a potent inducer of homotypic aggregation in the promonocyte cell line, U937, which is a widely used model of myeloid cell function. The activity of the compound does not appear directly related to its known activity as a kinase inhibitor. The proaggregating activity is also not simply related to the agonistic activity of staurosporine on PKCs. Rather, staurosporine induces a rapid and nonspecific activation of multiple kinase pathways in U937 cells. This uncoordinated activation of several key signaling pathways may result in changes in cell surface properties of the cells, leading to their aggregation. The molecular basis of this staurosporine activity requires further study, and may provide useful information for the further rational design of staurosporine analogues for use in cancer.
Abbreviations
- ATCC
American tissue culture collection
- BSA
bovine serum albumin
- ERK
extracellular-signal-regulated kinase
- FCS
fetal calf serum
- GF109203X
((2-[1-(3-dimethylaminopropyl)-indol-3-yl-3-(indol-3-yl)]-maleimide)
- Gö6976
(12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-apyrrolo(3,4-c)-carbazole)
- H-7
(1-(5-isoquinolinesulfonyl)-2-methylpiperazine)
- H-89
(N-[2-(4-bromocinnamylamino)ethyl]-5-isoquinoline)
- ICAM
intercellular adhesion molecule
- JNK
c-JUN N-terminal kinase
- K-252b
(C26H19N3O5, CAS [99570-78-2]
- KT5720
[9R,10S,12S]-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3,2,1-kl]pyrrolo[3,4-l][1,6]benzodiazocine-10-carboxylic acid hexyl ester)
- MAPK
mitogen-activated protein kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD
optical density
- PD98059
(2′-amino-3′-methoxyflavone)
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PMSF
phenylmethylsulfonyl fluoride
- SB203580
(4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole)
References
- CHO J.Y., FOX D.A., HOREJSI V., SAGAWA K., SKUBITZ K.M., KATZ D.R., CHAIN B.M. The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood. 2001;98:374–382. doi: 10.1182/blood.v98.2.374. [DOI] [PubMed] [Google Scholar]
- CHO J.Y., SKUBITZ K.M., KATZ D.R., CHAIN B.M. CD98-dependent homotypic aggregation is associated with translocation of protein kinase cdelta and activation of mitogen-activated protein kinases. Exp. Cell Res. 2003;286:1–11. doi: 10.1016/s0014-4827(03)00106-x. [DOI] [PubMed] [Google Scholar]
- FUJITA-YAMAGUCHI Y., KATHURIA S. Characterization of receptor tyrosine-specific protein kinases by the use of inhibitors. Staurosporine is a 100-times more potent inhibitor of insulin receptor than IGF-I receptor. Biochem. Biophys. Res. Commun. 1988;157:955–962. doi: 10.1016/s0006-291x(88)80967-7. [DOI] [PubMed] [Google Scholar]
- GRABER M., JUNE C.H., SAMELSON L.E., WEISS A. The protein tyrosine kinase inhibitor herbimycin A, but not genistein, specifically inhibits signal transduction by the T cell antigen receptor. Int. Immunol. 1992;4:1201–1210. doi: 10.1093/intimm/4.11.1201. [DOI] [PubMed] [Google Scholar]
- HARAGUCHI S., BRIGINO-BUENAVENTURA E.N., HITCHCOCK R., JAMES-YARISH M., GOOD R.A., DAY N.K. Involvement of a herbimycin A-sensitive protein tyrosine kinase in extracellular action of HIV-1 Nef. Immunol. Lett. 2001;75:97–101. doi: 10.1016/s0165-2478(00)00306-0. [DOI] [PubMed] [Google Scholar]
- IKEWAKI N., YAMADA A., SONODA A., INOKO H. A novel monoclonal antibody mNI-58A against the alpha-chain of leukocyte function-associated antigen-1 (LFA-1) blocks the homotypic cell aggregation and actively regulates morphological changes in the phorbol myristate acetate (PMA)-activated human monocyte-like cell line, U937. Tissue Antigens. 1996;48:161–173. doi: 10.1111/j.1399-0039.1996.tb02624.x. [DOI] [PubMed] [Google Scholar]
- JONES T., COURAGE C., HUBBARD A., GESCHER A. Cellular relocalisation of protein kinase C-theta caused by staurosporine and some of its analogues. Biochem. Pharmacol. 1997;53:1413–1418. doi: 10.1016/s0006-2952(96)00863-5. [DOI] [PubMed] [Google Scholar]
- KAWASE T., ORIKASA M., SUZUKI A. Phorbol ester-like action of staurosporine on the cAMP response to prostaglandin E2 in two macrophage-like cell lines at distinct differentiation stages. Cell Signal. 1992;4:479–485. doi: 10.1016/0898-6568(92)90017-3. [DOI] [PubMed] [Google Scholar]
- KEENAN C., KELLEHER D., LONG A. Regulation of non-classical protein kinase C isoenzymes in a human T cell line. Eur. J. Immunol. 1995;25:13–17. doi: 10.1002/eji.1830250104. [DOI] [PubMed] [Google Scholar]
- KILEY S.C., PARKER P.J., FABBRO D., JAKEN S. Selective redistribution of protein kinase C isozymes by thapsigargin and staurosporine. Carcinogenesis. 1992;13:1997–2001. doi: 10.1093/carcin/13.11.1997. [DOI] [PubMed] [Google Scholar]
- LALI F.V., HUNT A.E., TURNER S.J., FOXWELL B.M. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J. Biol. Chem. 2000;275:7395–7402. doi: 10.1074/jbc.275.10.7395. [DOI] [PubMed] [Google Scholar]
- LAUDANNA C., MOCHLY-ROSEN D., LIRON T., CONSTANTIN G., BUTCHER E.C. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J. Biol. Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- MIRANTI C.K., OHNO S., BRUGGE J.S. Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc. J. Biol. Chem. 1999;274:10571–10581. doi: 10.1074/jbc.274.15.10571. [DOI] [PubMed] [Google Scholar]
- NAKANO H., KOBAYASHI E., TAKAHASHI I., TAMAOKI T., KUZUU Y., IBA H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J. Antibiot. (Tokyo) 1987;40:706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- NAMBU M., MAYUMI M., MIKAWA H. Regulation of the expression of leukocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) on a human eosinophilic leukemia cell line EoL-3. Cell Immunol. 1992;143:335–347. doi: 10.1016/0008-8749(92)90030-s. [DOI] [PubMed] [Google Scholar]
- ROCHON Y.P., FROJMOVIC M.M. A model for the recruitment of neutrophils at sites of inflammation. Physiological relevance of in vivo neutrophil aggregation. Med. Hypotheses. 1992;38:132–138. doi: 10.1016/0306-9877(92)90086-r. [DOI] [PubMed] [Google Scholar]
- RUEGG U.T., BURGESS G.M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- SANO H., ZHU X., SANO A., BOETTICHER E.E., SHIOYA T., JACOBS B., MUNOZ N.M., LEFF A.R. Extracellular signal-regulated kinase 1/2-mediated phosphorylation of cytosolic phospholipase A(2) is essential for human eosinophil adhesion to fibronectin. J. Immunol. 2001;166:3515–3521. doi: 10.4049/jimmunol.166.5.3515. [DOI] [PubMed] [Google Scholar]
- SECRIST J.P., SEHGAL I., POWIS G., ABRAHAM R.T. Preferential inhibition of the platelet-derived growth factor receptor tyrosine kinase by staurosporine. J. Biol. Chem. 1990;265:20394–20400. [PubMed] [Google Scholar]
- SENDEROWICZ A.M. Development of cyclin-dependent kinase modulators as novel therapeutic approaches for hematological malignancies. Leukemia. 2001;15:1–9. doi: 10.1038/sj.leu.2401994. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., AU B.T., HELLEWELL P.G., ROSSI A.G. Characterization of eosinophil homotypic aggregation. J. Leukoc. Biol. 1995;57:226–234. doi: 10.1002/jlb.57.2.226. [DOI] [PubMed] [Google Scholar]
- YAMADA S., HIROTA K., CHIDA K., KUROKI T. Inhibition of phorbol ester-caused induction of ornithine decarboxylase and tumor promotion in mouse skin by staurosporine, a potent inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1988;157:9–15. doi: 10.1016/s0006-291x(88)80003-2. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA M., NISHIKAWA A., NISHIURA T., IHARA Y., KANAYAMA Y., MATSUZAWA Y., TANIGUCHI N. Cell spreading in Colo 201 by staurosporin is alpha 3 beta 1 integrin-mediated with tyrosine phosphorylation of Src and tensin. J. Biol. Chem. 1995;270:2298–2304. doi: 10.1074/jbc.270.5.2298. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA S., FUJIKI H., SUGURI H., SUGANUMA M., NAKAYASU M., MATSUSHIMA R., SUGIMURA T. Tumor-promoting activity of staurosporine, a protein kinase inhibitor on mouse skin. Cancer Res. 1990;50:4974–4978. [PubMed] [Google Scholar]
- YUE L., BAO Z., LI J. Phosphorylated form of MacMARCKS is essential to LFA-1-dependent cell–cell adhesion of U937 monocytic cells. J. Cell Physiol. 1999;181:355–360. doi: 10.1002/(SICI)1097-4652(199911)181:2<355::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- ZAPATA J.M., CAMPANERO M.R., MARAZUELA M., SANCHEZ-MADRID F., DE LANDAZURI M.O. B-cell homotypic adhesion through exon-A restricted epitopes of CD45 involves LFA-1/ICAM-1, ICAM-3 interactions, and induces coclustering of CD45 and LFA-1. Blood. 1995;86:1861–1872. [PubMed] [Google Scholar]