Abstract
The pharmaceutical compound, dihydroergotamine (DHE) is dispensed to prevent and reduce the occurrence of migraine attacks. Although still controversial, the prophylactic effect of this drug is believed to be caused through blockade and/or activation of numerous receptors including serotonin (5-HT) receptors of the 5-HT2 subtype.
To elucidate if 5-HT2 receptors (5-HT2Rs) may be involved in DHE prophylactic effect, we performed investigations aimed to determine the respective pharmacological profile of DHE and of its major metabolite 8′-hydroxy-DHE (8′-OH-DHE) at the 5-HT2B and 5-HT2CRs by binding, inositol triphosphate (IP3) or cyclic GMP (cGMP) coupling studies in transfected fibroblasts.
DHE and 8′-OH-DHE are competitive compounds at 5-HT2B and 5-HT2CRs. 8′-OH-DHE interaction at (5-HT2BRs) was best fitted by a biphasic competition curve and displayed the highest affinity with a Ki of 5 nM. These two compounds acted as agonists for both receptors in respect to cGMP production with pEC50 of 8.32±0.09 for 8′-OH-DHE at 5-HT2B and 7.83±0.06 at 5-HT2CRs.
Knowing that the antimigraine prophylactic effect of DHE is only observed after long-term treatment, we chronically exposed the recombinant cells to DHE and 8′-OH-DHE. The number of 5-HT2BR-binding sites was always more affected than 5-HT2CRs. At 5-HT2BRs, 8′-OH-DHE was more effective than DHE, with an uncoupling that persisted for more than 40 h for IP3 or cGMP. By contrast, the 5-HT2CR coupling was reversible after either treatment.
Chronic exposure to 8′-OH-DHE caused a persistent agonist-mediated desensitisation of 5-HT2B, but not 5-HT2CRs. This may be of relevance to therapeutic actions of the compound.
Keywords: 5-HT2, cGMP, desensitisation, DHE, IP3, serotonin, receptors
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) receptors are highly heterogeneous and they have been regrouped within different families (5-HT1–5-HT7). With the exception of the 5-HT3 receptors, which are ligand-gated ion channels, all others are G-protein-coupled receptors with each family sharing structural, pharmacological and transductional characteristics (Hoyer et al., 2002). 5-HT receptors (5-HTRs) have been implicated in the regulation of several psychiatric and neurological disorders related to serotonergic neurotransmission, and specific receptor subtypes have recently been associated with either the pathogenesis or the treatment of migraine headache (Villalon et al., 1997).
A role for 5-HT in migraine has been supported by sudden changes in circulating levels of 5-HT and its metabolites observed during the early phases of a migraine attack (Goadsby, 2000), along with the ability of 5-HT-releasing agents to induce migraine-like symptoms (Kalkman, 1994; Panconesi & Sicuteri, 1997). Migraine patients have a systemic disturbance of 5-HT metabolism, associated with low 5-HT plasma levels between attacks and increased levels and release of platelet 5-HT during attacks (Ferrari, 1998). Contrary to earlier belief, the rise of plasma 5-HT is not the cause of migraine but probably represents a self-defence mechanism. Earlier observations showed that injection of 5-HT can abort migraine attacks, but at the cost of significant side effects. These findings led to the hypothesis that selective stimulation of 5-HTRs might safely abort attacks (Ferrari, 1998). The development of 5-HTR agonists with efficacy in the clinic for the alleviation of migraine pain further implicates 5-HT as a key molecule in migraine (Goadsby et al., 2002).
Several theories regarding the aetiology of migraine have been proposed (Ferrari, 1998; Johnson et al., 1998; Goadsby et al., 2002). The vasodilatory theory of migraine suggested that extracranial arterial dilation during an attack was related to migraine pain; a theory supported when vasoconstrictors such as sumatriptan alleviated migraine pain. The neurological theory of migraine proposed that migraine resulted from abnormal firing in brain neurons. Cortical spreading depression, one facet of the neurological theory, could explain the prodrome of migraine. The neurogenic dural inflammation theory of migraine supposed that the dural membrane surrounding the brain became inflamed and hypersensitive due to release of neuropeptides from primary sensory nerve terminals. A role for 5-HT is supported by the efficacy in treating acute migraine pain and associated symptoms of 5-HTR ligands such as triptans, which are agonists at 5-HT1D and 5-HT1BR subtypes. In addition, the prophylactic effect of several pharmaceuticals frequently dispensed to prevent the occurrence of migraine attacks has been suggested to be caused through blockade of 5-HTRs of the 5-HT2 type (Kalkman, 1994; Panconesi & Sicuteri, 1997). Migraine headache is thought to be transmitted by the trigeminal nerve from the meninges and their blood vessels. Various human meningeal tissues express 5-HT1B/1D, 5-HT2A, 5-HT2B, 5-HT4 and 5-HT7R mRNAs (Schmuck et al., 1996). 5-HT2CR mRNA was found in neurons involved in the central processing of nociceptive neurotransmission (Molineaux et al., 1989).
The ergot alkaloids and their semisynthetic derivatives including dihydroergotamine (DHE) are families of chemical entities that have many different pharmacological effects. This diversity results from their interaction with multiple receptors, their variable receptor affinity and intrinsic activity, and their variable organ-specific receptor access. DHE exhibits alpha-adrenergic antagonist activity with only low arterial vasoconstriction potential. The vasoconstrictor activities of these compounds have long been believed to be the basis of their clinical effects, but recent evidence suggests that its antimigraine action may result in part from their inhibitory effects on neurogenic inflammation and neuronal transmission and in part from vasodilatation inhibition and/or correction (Hoskin et al., 1996; Panconesi & Sicuteri, 1997; Goadsby, 2000). The long duration of action of DHE appears to result from its major active metabolite 8′-OH-DHE which is present, in case of oral repeated administration, at a concentration five to seven times greater than DHE (Cmax for DHE 5–35 nM) and its long half-life (10–13 h) has been proposed to account for the low rate of headache recurrence observed with DHE (Silberstein, 1997). The antimigraine drug DHE produces selective vasoconstriction in the external carotid artery (Villalon et al., 1999). Both sumatriptan and DHE are effective in aborting migraine headaches. However, headache recurrence is two and a half time as likely with sumatriptan as with DHE (Winner et al., 1996).
To elucidate if 5-HT2Rs could be involved in the long-term antimigraine prophylactic action of DHE via its metabolite 8′-OH-DHE, we determined their pharmacological profiles toward the human 5-HT2B and 5-HT2CRs. We, then, investigated the effects of chronic exposure to these compounds of cells expressing these receptors. We observed different responses to these compounds, the 5-HT2BRs being the most responsive and chronically affected by 8′-OH-DHE.
Methods
Materials
DHE and 8′-OH-DHE were obtained from CEA (Saclay, France). A 1 mM stock solution of each of the two compounds was prepared by first dissolving in 200 μl ethanol and then making up the volume with distilled water. The subsequent dilutions were performed in the binding buffer. All other chemicals of the purest grade available were from classical commercial sources. [125I]DOI (81.4 TBq mmol−1) was from DuPont-New England Nuclear (Boston, U.S.A.).
Cell cultures
The previously described transfection method to obtain clones of human 5-HT2BR-expressing LMTK− fibroblasts (Choi et al., 1994) was applied to obtain clones of human 5-HT2CR-expressing fibroblasts. Stable clonal cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% 5-HT-depleted foetal calf serum (FCS). Single clones expressing similar amount of receptors were selected and used at similar passages.
Radioligand binding experiments
They were performed on crude membranes of transfected cells prepared using [I25I]DOI as radiolabelled compound as previously detailed (Loric et al., 1995; Kellermann et al., 1996). Briefly, to prepare crude membranes for binding assays, cells were washed twice with cold phosphate buffer saline (PBS), then harvested with a rubber policeman in PBS containing 1 μg ml−1 pepstatin, 1 μg ml−1 antipain, 15 μg ml−1 benzamidine, and 0.1 mM phenylmethylsulphonyl fluoride. After centrifugation, the resulting pellet was frozen at −70°C before homogenisation. Frozen cell pellets were thawed at 37°C, resuspended with 10 ml of cold 4 mM EDTA, 1 mM EGTA, 0.1 mM phenylmethylsulphonyl fluoride, 10 mM imidazole buffer, pH 7.30, and centrifuged for 10 min at 5000 × g. The supernatant obtained from this centrifugation was collected, poured onto a 20% sucrose cushion, and then centrifuged for 90 min at 100,000 × g. The membrane-containing pellet was resuspended in 75 mM KCl, 5 mM MgCl2, 1 mM EGTA, 10 mM imidazole buffer, pH 7.30, for use in binding assays. Protein contents were determined using the bicinchoninic acid protein assay (Pierce, Chichester, U.K.). Binding experiments were carried out at room temperature under shaking in a total volume of 1 ml. The specific binding was defined as the binding that was inhibited by 1 μM levels of ritanserin. Assays were initiated by the addition of 50 μl of 50 mM Tris buffer, pH 7.40, containing 25 nM of radiolabelled ligand and appropriate competing ligands (12 different concentrations of compounds DHE, and 8′-OH-DHE) to 50 μl of membranes (20 μg of protein). A 30-min incubation period was followed by the addition of 5 ml of ice-cold 10 mM Tris buffer, pH 7.40. Samples were filtered on polyethyleneimine-treated filters and the radioactivity retained by the filters was determined as previously described (Bruns et al., 1983). This was repeated independently at least three times.
Determination of intracellular cGMP levels
Cells were washed twice in fresh serum-free medium and incubated for various times at 37°C with 100 mM isobutylmethylxanthine and test agents. The reaction was stopped by aspiration of the medium followed by addition of 500 ml ice-cold 95% ethanol/5% formic acid (1 : 1, v/v). After 1 h at 4°C, the ethanolic phase was collected and lyophilised. cGMP was quantified using an iodinated radioimmunoassay kit (cGMP RIA kit RPA 525, Amersham Pharmacia Biotech, Bucking hamshire, U.K.).
Desensitisation, receptor expression
Transfected cells maintained in DMEM containing 10% 5-HT-depleted FCS and 300 μg ml−1 of hygromycin were seeded in 12- or 24- well at a density of 4 × 104 cells cm−2. After 24-h, cells were rinsed in HBSS and incubated 24-h in serum-free medium [DMEM/F-12 (1 : 1) with 5 μg ml−1 insulin, 5 μg ml−1 transferrin, 30 nM selenium, 20 nM progesterone, and 100 μM putrescine]. Stably transfected mouse fibroblasts LMTK were exposed to saturating concentration of DHE (1 μM) (or 8′-OH-DHE) for various time (1, 2, 5, 10, 20 and 40 h) and the variation in receptor (Bmax) was determined by binding of [123I]DOI. Three to four independent experiments were performed, each measure determined in triplicate.
Desensitisation, receptor transduction
Transfected cells were rinsed, incubated 24-h in serum-free medium and exposed to a saturating concentration of DHE (1 μM) (or 8′-OH-DHE) for various time (1, 2, 5, 10, 20 and 40 h) and the cGMP levels were determined as described before (Manivet et al., 2000) using an iodinated radioenzymatic assay kit (cGMP RIA kit RPA 525, Amersham Pharmacia Biotech). Three to four independent experiments were performed with each measure determined in triplicate.
Similarly treated cells maintained in DMEM containing 10% 5-HT-depleted FCS and exposed to saturating concentration of DHE (1 μM) (or 8′-OH-DHE) for various time (1, 2, 5, 10, 20 and 40 h) were used to determine the IP3 levels as described before (Loric et al., 1995) using an iodinated radio receptor assay kit (IP3 kit TRK 1000, Amersham). Three to four independent experiments were performed, each measure determined in triplicate.
Data analysis
Binding data were analysed using the iterative nonlinear fitting software Graphpad-Prism 2.0. This allowed to determine IC50 values and to calculate inhibition constants (Ki) according to the Cheng–Prusoff equation. Data points were F-tested to fit a single- or two-site model with or without an additional parameter regarded as nonspecific binding.
Results
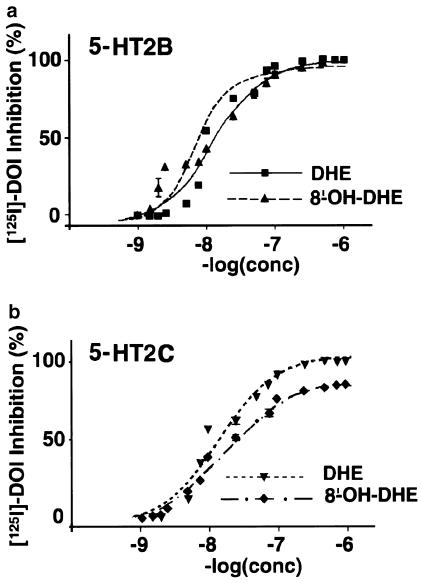
We have generated clones of stably transfected LMTK cells expressing human 5-HT2B or 5-HT2CRs to assess their respective affinity for DHE and 8′-OH-DHE by competition towards [125I]DOI. Mouse fibroblasts, LMTK− cells, do not expressed endogenous 5-HTRs. After transfection, we selected clonal cell lines expressing similar physiological levels of receptors. Clones expressing 205±2 fmol, (mean±s.e.m., n=4) of human 5-HT2BR per mg of protein or 195±3 fmol (mean±s.e.m., n=4) of human 5-HT2CR per mg of protein were selected, when measured against [125I]DOI on crude membrane extracts as previously described (Loric et al., 1995; Kellermann et al., 1996). Affinities for standard 5-HT2R agonists (Table 1) were found similar to previously published values (Cussac et al., 2002), indicating that they are not affected by expression levels of these receptors in the cell lines at passages used in this study. DHE and 8′-OH-DHE interacted with these receptors at high affinity. However, iterative curve fitting suggested the presence of more than one site for 8′-OH-DHE (Hill number of 1.79, P<0.01, Table 2) at 5-HT2BRs that displayed the highest affinity (Ki=8.85±0.13) (Figure 1).
Table 1.
Binding affinities (Ki, in nM)
| Ki | 5-HT2BR | 5-HT2CR |
|---|---|---|
| DOI | 24±5 | 63±6 |
| mCPP | 18±2 | 126±11 |
| MK212 | 151±18 | 891±18 |
| BW723C86 | 2.3±0.4 | 115±13 |
Affinities (Ki, values) were determined by competition binding experiments with [I25I]DOI. Ki values are expressed as means±s.e.m. of four independent experiments performed in triplicates.
Table 2.
Hill numbers determined by iterative curve fitting
| H number | 5-HT2BR | 5-HT2CR |
|---|---|---|
| DHE | 0.99 | 1.38 |
| 8′-OH-DHE | 1.79* | 0.89 |
Hill numbers determined by iterative curve fitting after competition binding experiments with [125I]DOI.
P<0.01.
Figure 1.
Competition curves against [125I]DOI at 5-HT2B and 5-HT2CRs. Data points represent the percentage of specific [125I]DOI binding inhibition at each concentration of DHE or 8′-OH-DHE at 5-HT2B (a) and 5-HT2C (b) receptors and are the average of at least three experiments performed in triplicate. Squares-plain line: 5-HT2BR DHE; upper triangle-dashed line: 5-HT2BR 8′-OH-DHE; lower triangle-dotted line: 5-HT2CR DHE; diamond-dotted/dashed line: 5-HT2CR 8′-OH-DHE.
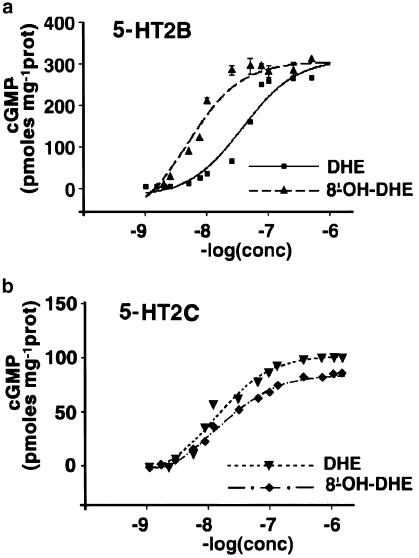
Intracellular levels of IP3 have been reported to be increased in cells expressing 5-HT2B or 5-HT2CRs in response to agonist stimulation (Loric et al., 1995; Lucaites et al., 1996). As previously shown for 5-HT (Manivet et al., 2000), cGMP levels were elevated upon stimulation by DHE or 8′-OH-DHE of LMTK− cells expressing 5-HT2B or 5-HT2CRs, thus, both compounds behaved as agonists. The EC50 values observed corresponded to the high-affinity sites previously identified, the pEC50 of 8′-OH-DHE being 8.32±0.09 at 5-HT2B and 7.83±0.06 at 5-HT2CRs for cGMP. In addition, 8′-OH-DHE acts as a partial agonist towards 5-HT2CRs (Figure 2).
Figure 2.
Concentration–response curves for cGMP production at 5-HT2B and 5-HT2CRs. Data points represent the intracellular cGMP level for each concentration of DHE or 8′-OH-DHE for 5-HT2B (a) and 5-HT2C (b) receptor-expressing cells. Values are expressed as pmol mg−1 of protein and are the average of at least three experiments performed in triplicate.
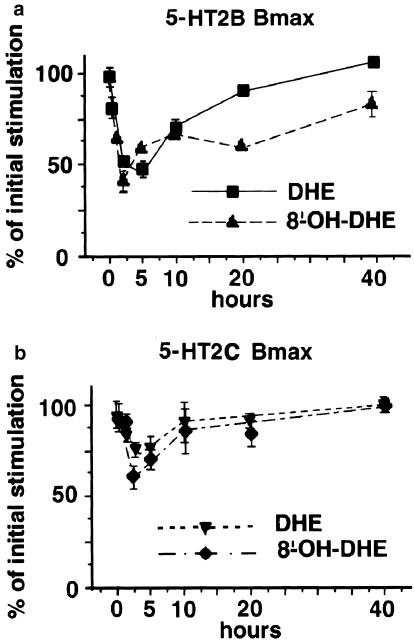
To assess the effect of long-term exposure to these compounds, stably transfected LMTK− cells expressing 5-HT2B or 5-HT2CRs were exposed to saturating concentration (1 μM) of DHE or 8′-OH-DHE for various time (0, 1, 2, 5, 10, 20 and 40 h) and the number of sites (Bmax) was measured by competition against [125I]DOI binding. This pharmacological evaluation of receptor expression indicated that 5-HT2B and 5-HT2CRs responded differently to these two compounds, the 5-HT2BRs being more sensitive to chronic stimulation by 8′-OH-DHE than 5-HT2CRs. After 2 h exposure to 8′-OH-DHE, 60% of the initial stimulated level of 5-HT2CR was still detected, whereas only 43% of the 5-HT2BR initial level remained. Moreover, after 40 h of exposure to 8′-OH-DHE, 5-HT2CR expression level returned to the initial value but this level was only 86% for 5-HT2BRs (Figure 3). The number of sites of 5-HT2BR and 5-HT2CR was much less affected by chronic exposure to DHE and returned to initial values within 10–15 h. At 20 h, the number of sites of 5-HT2BR and 5-HT2CR was variably affected by exposure to other 5-HT2R agonists (Table 3).
Figure 3.
Time course of the Bmax obtained at 5-HT2B and 5-HT2CRs after chronic exposure. Data points expressed as per cent of the initial stimulation (maximum) represent the average of the number of specific binding sites remaining for 5-HT2B (a) and 5-HT2C (b) receptor-expressing cells at each time point after exposure to saturating concentrations of DHE or 8′-OH-DHE and are the average of at least three experiments performed in triplicate.
Table 3.
Number of sites (Bmax after 20 h in per cent of 0 h)
| Bmax | 5-HT2BR | 5-HT2CR |
|---|---|---|
| 5-HT | 26±7 | 54±4 |
| DOI | 38±4 | 56±5 |
| mCPP | 93±6 | 51±5 |
| MK212 | 36±9 | 46±5 |
| BW723C86 | 54±4 | 25±6 |
Number of sites (Bmax) were determined by competition binding experiments with [125I]DOI after 20 h of stimulation by saturating concentrations of the different agonists and are expressed in percent of the initial Bmax at 0 h. Values are expressed as means±s.e.m. of four independent experiments performed in triplicates.
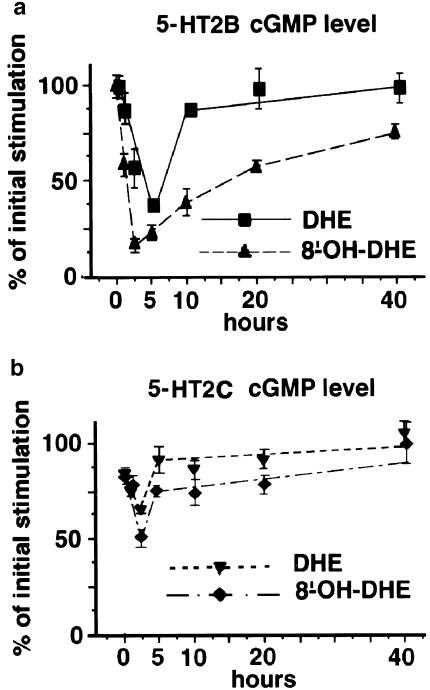
Stably transfected LMTK− cells expressing 5-HT2B or 5-HT2CRs were exposed to saturating concentration (1 μM) of DHE or 8′-OH-DHE for various time (0, 1, 2, 5, 10, 20 and 40 h) and the cGMP levels were assessed by radioimmunoassay. This experiment indicated that coupling of 5-HT2B and 5-HT2CRs to cGMP also behaved differently in response to these two compounds. The 5-HT2BR stimulation of cGMP was more reduced after chronic stimulation by 8′-OH-DHE than 5-HT2CRs as previously observed for Bmax (Figures 3 and 4). The lowest cGMP level was recorded after 2 h exposure of 5-HT2BRs-expressing cells to 8′-OH-DHE, in which only 14% of the initial stimulated level of cGMP remained, while 50% of the initial level of stimulated cGMP was detected in 5-HT2CRs-expressing cells. After 40 h of exposure to 8′-OH-DHE, the cGMP level returned to the initial value for 5-HT2CRs but this level was only 75% for 5-HT2BRs (Figure 4). The cGMP coupling of 5-HT2B and 5-HT2CRs was much less affected by chronic exposure to DHE than to 8′-OH-DHE, as initial desensitisation was subsequently completely reversed.
Figure 4.
Time course of the cGMP levels obtained on 5-HT2B and 5-HT2CRs-expressing cells after chronic exposure. Data points expressed as per cent of maximal stimulation represent the average of the cGMP level measured at 5-HT2B (a) and 5-HT2C (b) receptor-expressing cells at each time point after exposure to saturating concentrations of DHE or 8′-OH-DHE and are the average of at least three experiments performed in triplicate.
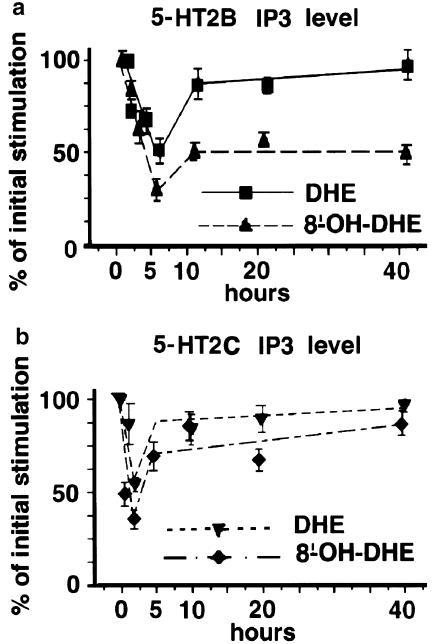
Since IP3 is the classical transduction pathways to both receptors, we also evaluated the coupling to IP3 production by stably transfected LMTK− cells expressing 5-HT2B or 5-HT2CRs after exposure to saturating concentration (1 μM) of DHE or 8′-OH-DHE for various time (0, 1, 2, 5, 10, 20 and 40 h). The IP3 coupling of 5-HT2B and 5-HT2CRs was indeed found but with different responses to chronic exposure to these two compounds. The 5-HT2BRs stimulation of IP3 levels was also more affected after chronic stimulation by 8′-OH-DHE than 5-HT2C as previously observed for Bmax and cGMP. The lowest IP3 stimulation level was recorded after 5 h exposure of 5-HT2BR-expressing cells to 8′-OH-DHE, in which only 30% of the initial levels of IP3 remained. In 5-HT2CR-expressing cells, the lowest IP3 level was 35% of the initial level and was detected after 2 h exposure to 8′-OH-DHE. Strikingly, after 40 h, 5-HT2CR IP3-coupling reached 86% of the initial value but this level remained stable at 50% of the initial values for 5-HT2BRs (Figure 5). As for cGMP, the IP3 coupling of 5-HT2B and 5-HT2CRs was much less affected by chronic exposure to DHE than to 8′-OH-DHE, as initial desensitisation was subsequently completely reversed. These results indicate that chronic exposure to DHE leads to permanent impairment of the coupling efficiency of 5-HT2BRs through its metabolite 8′-OH-DHE.
Figure 5.
Time course of the IP3 levels obtained on 5-HT2B and 5-HT2CR-expressing cells after chronic exposure. Data points expressed as per cent of maximal stimulation represent the average of the IP3 level measured at 5-HT2B (a) and 5-HT2C (b) receptors at each time point after exposure to saturating concentrations of DHE or 8′-OH-DHE and are the average of at least three experiments performed in triplicate.
Discussion
To our knowledge, this is the first functional characterisation of DHE and its major metabolite 8′-OH-DHE at the 5-HT2B and 5-HT2CRs described to date. Unfortunately, it is not possible to study receptors in native tissues due to the lack of complete segregation of receptors in discrete areas. The use of recombinant expression systems is therefore a useful approach, and also allows the study of human receptors. The two human receptor subtypes, 5-HT2B and 5-HT2CRs were transfected into LMTK− cells thus ensuring they were studied in an identical background. Parental nontransfected cells when examined under an identical experimental paradigm did not exhibit a concentration-dependent rise in second messengers in response to agonists. The above cell lines were therefore chosen as they express a single receptor of interest at relatively low and close to physiological levels and they were used at nearly similar passages to ensure stable expression levels. DHE had previously been reported to exhibit alpha-adrenergic antagonist and 5-HT1A, 5-HT1B, 5-HT1D, and 5-HT1FR agonist activity (EC50 around 1.5, 10, 0.5, and 10 nM, respectively) (Buzzi & Moskowitz, 1991; Boess & Martin, 1994; Silberstein, 1997). No data about 8′-OH-DHE have been published. Observed values for 5-HT2BR are within the same range suggesting that its stimulation is physiologically likely.
External carotid vasoconstrictor responses to DHE are mediated by 5-HT1B/1DRs as well as alpha2-adrenoceptors (Villalon et al., 1999). The moderate vasodilator response of cranial arteriovenous anastomoses to the ergot derivatives seems to be mediated, at least in part, by 5-HT1B/1DRs (De Vries et al., 1998). DHE does not contract isolated rat aorta rings, but insurmountably blocks contractions in response to 5-HT. As this response has been reported to be potently (pKB 8.1) and competitively antagonised by the selective 5-HT2AR antagonist, ketanserin (Kalkman & Schneider, 1996), it is thought to be mediated by the HT2AR. Thus, DHE appears to be an antagonist of the HT2AR. The 5-HT2A, 5-HT2B, and 5-HT2CRs share a high degree of sequence homology and have very similar pharmacological profiles (Rothman et al., 2000). DHE inhibits [3H]-mesulergine binding to HT2CRs in piglet choroid plexus with a pKD of 7.1, rather less than that of 5-HT (pKD 8.1) and is a full agonist (pEC50 7.6) but with potencies similar to that of 5-HT (pEC50 7.7) (Brown et al., 1991). Concerning the 5-HT2BR, there was only indirect information originating from pharmacological studies of endothelium-mediated relaxation of pig pulmonary or cerebral arteries, which show a pharmacological profile very close to that observed on transfected cells expressing the 5-HT2BR (Glusa & Roos, 1996; Schmuck et al., 1996; Glusa & Pertz, 2000). The 5-HT2B and 5-HT2CR-expressing cell lines used in the present study display very similar affinities for previously characterised agonists (Table 1) (Cussac et al., 2002). However, the 8′-OH-DHE presents complex interactions toward the 5-HT2BRs with a Hill number of 1.79, suggesting multiple interaction sites (Table 2), the highest affinity site for 5-HT2BRs being the most efficient. Our results clearly show that DHE and for the first time 8′-OH-DHE act as agonists vs both 5-HT2B and 5-HT2CRs.
Although it is also generally believed that the cellular signal transduction mechanisms activated by the 5-HT2Rs are indistinguishable, recent data suggest also significant differences in their signalling cascades. 5-HT-stimulated IP3 production has been previously observed at the 5-HT2A, 5-HT2B, and 5-HT2CRs (Loric et al., 1995; Briddon et al., 1998). In response to 5-HT stimulation, 5-HT2AR-mediated cGMP generation was reported together with calcium (Ca2+) mobilisation in C6 glioma cells through NO-dependent pathway (Kagaya et al., 1995; Miyoshi et al., 2001). The 5-HT2CRs expressed in the choroid plexus trigger phosphoinositide turnover and also the formation of cGMP formation in a calcium-sensitive manner (Kaufman et al., 1995). Concerning the 5-HT2BR, the pharmacological study of endothelial-mediated relaxation of pig pulmonary arteries (Glusa & Roos, 1996; Glusa & Pertz, 2000) showed that DHE, as well as 5-HT and alpha-methyl-5-HT, elicited a reversible endothelium-dependent relaxation of precontracted arterial ring segments, associated with an increase in intracellular cGMP production through direct activation of NO synthase (Manivet et al., 2000). The present results clearly identify DHE and 8′-OH-DHE as agonist for both IP3 and cGMP second messengers at 5-HT2B and 5-HT2CRs.
Knowing that DHE is generally prescribed for long-term use, we investigated the effect of chronic treatments of cells expressing 5-HT2B or 5-HT2CRs exposed to saturating concentrations (1 μM) of these compounds to ensure their complete desensitisation. Desensitisation is an adaptive mechanism in biological systems thought to facilitate responsiveness of the cell to successive multiple extracellular stimuli over time. The receptor, first uncoupled from the G protein is then sequestered in an intracellular compartment. This step is now viewed as a mechanism that allows dephosphorylation by specific phosphatases and resensitisation of the receptor. Degradation of the protein and reduction of the steady-state mRNA (mostly due to decreased stability of the mRNA) are the concomitant mechanisms leading to the decrease of receptor number (downregulation) (Chuang et al., 1996). In vivo, when the stimulation is chronically persistent, the receptor transcription is downregulated, but due to the use of a strong promoter containing expression vectors this step cannot be observed in transfected cells. To date, little is known about 5-HT2BRs desensitisation. The human 5-HT2A and 5-HT2CRs have been both reported to exhibit receptor uncoupling at the level of IP3, with the 5-HT2CR being more sensitive than the 5-HT2AR (Briddon et al., 1998). More recently, the desensitisation characteristics of recombinant human 5-HT2A, 5-HT2B, and 5-HT2CRs stably expressed in CHO-K1 cells, investigated by calcium fluorimetry showed that 5-HT2BRs exhibited the most dramatic degree of desensitisation to 5-HT, with a rapid time course. The partial agonists metachlorophenylpiperazine (mCPP) and DOI also elicited desensitisation, generally in line with their relative efficacies at each receptor (Berg et al., 2001; Porter et al., 2001). For the first time, we have studied long-term effects of DHE exposure and found that 5-HT2BRs exhibit the most dramatic degree of desensitisation in respect to both IP3 and cGMP coupling by its metabolite 8′-OH-DHE, which is more effective and permanent. Further experiments are necessary to elucidate the molecular mechanism involved in this phenomenon.
Both neuronal and vascular mechanisms have been proposed on the basis of actions of 5-HT in migraine. Blockade of neural transmission and the neurogenic inflammatory response provides a mechanism by which sumatriptan and ergot alkaloids alleviate vascular headaches (Moskowitz, 1992). The vasodilatory theory of migraine suggests that extracranial arterial dilation during an attack is related to migraine pain, whereas in the neurogenic dural inflammation theory of migraine, inflammation of the dural membrane surrounding the brain is due to release of neuropeptides from primary sensory nerve terminals. Substance P, calcitonin gene-related peptide and NO all play a role in the dural inflammatory cascade. Pretreatment with the antimigraine drugs DHE and sumatriptan markedly attenuates plasma protein extravasation induced by electrical trigeminal ganglion stimulation (Buzzi & Moskowitz, 1991; Hoskin et al., 1996). Pharmacological agents such as mCPP and NO donors have also been used to induce dural extravasation in animals.
NO is suspected to play a key role in migraine since NO donors cause a dose-dependent headache with several migrainous characteristics. A cause of migraine could be increased amounts and/or affinity of an enzyme in the NO-triggered cascade of reactions (Olesen et al., 1994). NO, which may be released from blood vessels, perivascular nerve endings or from brain tissue, is an important molecular trigger mechanism in spontaneous headache pain (Thomsen & Olesen, 2001). It has been shown that 5-HT2BRs stimulate the NO production in cell lines (Manivet et al., 2000) and relaxation of the pig cerebral artery (Schmuck et al., 1996). Thus, 5-HT2BRs located on endothelial cells of meningeal blood vessels have been proposed to trigger migraine headache through the formation of NO. Recent data are consistent with this hypothesis, since activation by mCPP of peripheral 5-HT2BRs initiates dural plasma protein extravasation via NO (Johnson et al., 2003).
In conclusion, the DHE long half-life may account for the low rate of headache recurrence at least partially through permanent inhibition of vascular 5-HT2B-dependent second messengers (NO) via its major active metabolite 8′-OH-DHE. Whether the antimigraine action of DHE involves a combination of acute agonist action at 5-HT1B/1DRs, a long-lasting uncoupling of 5-HT2BRs via 8′-OH-DHE and an insurmountable antagonist action at 5-HT2ARs remains to be established on cerebral vasculature.
Acknowledgments
This work has been supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Hôpital Universitaire de Strasbourg, the Université Louis Pasteur, and by grants from the Fondation de France, the Association pour la Recherche centre le Cancer and by a Region Alsace fellowship to B.S.
Abbreviations
- cGMP
cyclic GMP
- DHE
dihydroergotamine
- DOI
(±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- DMEM
Dulbecco's modified Eagle's medium
- FCS
foetal calf serum
- IP3
inositol triphosphate
- mCPP
meta-chlorophenylpiperazine
- NO
nitric oxide
- PBS
phosphate buffer saline
- 5-HT
serotonin, 5-hydroxytryptamine
References
- BERG K.A., STOUT B.D., MAAYANI S., CLARKE W.P. Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J. Pharmacol. Exp. Ther. 2001;299:593–602. [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- BRIDDON S.J., LESLIE R.A., ELLIOTT J.M. Comparative desensitization of the human 5-HT2A and 5-HT2C receptors expressed in the human neuroblastoma cell line SH-SY5Y. Br. J. Pharmacol. 1998;125:727–734. doi: 10.1038/sj.bjp.0702145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A.M., PATCH T.L., KAUMANN A.J. The antimigraine drugs ergotamine and dihydroergotamine are potent 5- HT1C receptor agonists in piglet choroid plexus. Br. J. Pharmacol. 1991;104:45–48. doi: 10.1111/j.1476-5381.1991.tb12382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNS R.F., LAWSON-WENDLING K., PUGSLEY T.A. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal. Biochem. 1983;132:74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- BUZZI M.G., MOSKOWITZ M.A. Evidence for 5-HT1B/1D receptors mediating the antimigraine effect of sumatriptan and dihydroergotamine. Cephalalgia. 1991;11:165–168. doi: 10.1046/j.1468-2982.1991.1104165.x. [DOI] [PubMed] [Google Scholar]
- CHOI D.-S., BIRRAUX G., LAUNAY J.-M., MAROTEAUX L. The human serotonin 5-HT2B receptor: pharmacological link between 5-HT2 and 5-HT1D receptors. FEBS Lett. 1994;352:393–399. doi: 10.1016/0014-5793(94)00968-6. [DOI] [PubMed] [Google Scholar]
- CHUANG T.T., IACOVELLI L., SALLESE M., DE BLASI A. G protein-coupled receptors: heterologous regulation of homologous desensitization and its implications. Trends Pharmacol. Sci. 1996;17:416–421. doi: 10.1016/s0165-6147(96)10048-1. [DOI] [PubMed] [Google Scholar]
- CUSSAC D., NEWMAN-TANCREDI A., QUENTRIC Y., CARPENTIER N., POISSONNET G., PARMENTIER J.G., GOLDSTEIN S., MILLAN M.J. Characterization of phospholipase C activity at h5-HT2C compared with h5-HT2B receptors: influence of novel ligands upon membrane-bound levels of [3H]phosphatidylinositols. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:242–252. doi: 10.1007/s00210-001-0505-y. [DOI] [PubMed] [Google Scholar]
- DE VRIES P., VILLALON C.M., HEILIGERS J.P., SAXENA P.R. Characterization of 5-HT receptors mediating constriction of porcine carotid arteriovenous anastomoses; involvement of 5-HT1B/1D and novel receptors. Br. J. Pharmacol. 1998;123:1561–1570. doi: 10.1038/sj.bjp.0701770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI M.D. Migraine. Lancet. 1998;351:1043–1051. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- GLUSA E., PERTZ H.H. Further evidence that 5-HT-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT2B receptors. Br. J. Pharmacol. 2000;130:692–698. doi: 10.1038/sj.bjp.0703341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLUSA E., ROOS A. Endothelial 5-HT receptors mediate relaxation of porcine pulmonary arteries in response to ergotamine and dihydroergotamine. Br. J. Pharmacol. 1996;119:330–334. doi: 10.1111/j.1476-5381.1996.tb15990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOADSBY P.J. The pharmacology of headache. Prog. Neurobiol. 2000;62:509–525. doi: 10.1016/s0301-0082(00)00010-1. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., LIPTON R.B., FERRARI M.D. Migraine – current understanding and treatment. N. Engl. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., KAUBE H., GOADSBY P.J. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain. 1996;119:249–256. doi: 10.1093/brain/119.1.249. [DOI] [PubMed] [Google Scholar]
- HOYER D., HANNON J.P., MARTIN G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- JOHNSON K.W., NELSON D.L., DIECKMAN D.K., WAINSCOTT D.B., LUCAITES V.L., AUDIA J.E., OWTON W.M., PHEBUS L.A. Neurogenic dural protein extravasation induced by meta-chlorophenylpiperazine (mCPP) involves nitric oxide and 5-HT2B receptor activation. Cephalalgia. 2003;23:117–123. doi: 10.1046/j.1468-2982.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON K.W., PHEBUS L.A., COHEN M.L. Serotonin in migraine: theories, animal models and emerging therapies. Prog. Drug Res. 1998;51:219–244. doi: 10.1007/978-3-0348-8845-5_6. [DOI] [PubMed] [Google Scholar]
- KAGAYA A., MOTOHASHI N., KUGAYA A., YAMAJI T., HAYASHI T., OKAMOTO Y., SHINNO H., TAKEBAYASHI M., UCHTTOMI Y., YAMAWAKI S. Cyclic GMP generation mediated by 5-HT2 receptors via nitric oxide-dependent pathway and its effect on the desensitization of 5-HT2 receptors in C6 glioma cells. J. Neurol Transm. 1995;100:27–38. doi: 10.1007/BF01276863. [DOI] [PubMed] [Google Scholar]
- KALKMAN H.O. Is migraine prophylactic activity caused by 5-HT2B or 5-HT2C receptor blockade. Life Sci. 1994;54:641–644. doi: 10.1016/0024-3205(94)00546-x. [DOI] [PubMed] [Google Scholar]
- KALKMAN H.O., SCHNEIDER F. Effects of ergotamine and dihydroergotamine on 5-hydroxytryptamine-2A receptors in the isolated rat aorta. Pharmacology. 1996;53:351–355. doi: 10.1159/000139450. [DOI] [PubMed] [Google Scholar]
- KAUFMAN M.J., HARTIG P.R., HOFFMAN B.J. Serotonin 5-HT2C receptor stimulates cyclic GMP formation in choroid plexus. J. Neurochem. 1995;64:199–205. doi: 10.1046/j.1471-4159.1995.64010199.x. [DOI] [PubMed] [Google Scholar]
- KELLERMANN O., LORIC S., MAROTEAUX L., LAUNAY J.-M. Sequential onset of three 5-HT receptors during the 5-hydroxytryptaminergic differentiation of the murine 1C11 cell line. Br. J. Pharmacol. 1996;118:1161–1170. doi: 10.1111/j.1476-5381.1996.tb15519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORIC S., MAROTEAUX L., KELLERMANN O., LAUNAY J.-M. Functionnal serotonin-2B receptors are expressed by a teratocarcinoma-derived cell line during serotoninergic differentiation. Mol. Pharmacol. 1995;47:458–466. [PubMed] [Google Scholar]
- LUCAITES V.L., NELSON D.L., WAINSCOTT D.B., BAEZ M. Receptor subtype and density determine the coupling repertoire of the 5-HT2 receptor subfamily. Life Sci. 1996;59:1081–1095. doi: 10.1016/0024-3205(96)00423-7. [DOI] [PubMed] [Google Scholar]
- MANIVET P., MOUILLET-RICHARD S., CALLEBERT J., NEBIGIL C.G., MAROTEAUX L., HOSODA S., KELLERMANN O., LAUNAY J.-M. PDZ-dependent activation of nitric-oxide synthases by the serotonin 2B receptor. J. Biol. Chem. 2000;275:9324–9331. doi: 10.1074/jbc.275.13.9324. [DOI] [PubMed] [Google Scholar]
- MIYOSHI I., KAGAYA A., KOHCHI C., MORINOBU S., YAMAWAKI S. Characterization of 5-HT2A receptor desensitization and the effect of cycloheximide on it in C6 cells. J. Neurol Transm. 2001;108:249–260. doi: 10.1007/s007020170070. [DOI] [PubMed] [Google Scholar]
- MOLINEAUX S.M., JESSELL T.M., AXEL R., JULIUS D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSKOWITZ M.A. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol. Sci. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- OLESEN J., THOMSEN L., IVERSEN H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol. Sci. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- PANCONESI A., SICUTERI R. Headache induced by serotonergic agonists – a key to the interpretation of migraine pathognesis. Cephalagia. 1997;17:3–14. doi: 10.1046/j.1468-2982.1997.1701003.x. [DOI] [PubMed] [Google Scholar]
- PORTER R.H., MALCOLM C.S., ALLEN N.H., LAMB H., REVELL D.F., SHEARDOWN M.J. Agonist-induced functional desensitization of recombinant human 5-HT2 receptors expressed in CHO-K1 cells. Biochem. Pharmacol. 2001;62:431–438. doi: 10.1016/s0006-2952(01)00677-3. [DOI] [PubMed] [Google Scholar]
- ROTHMAN R.B., BAUMANN M.H., SAVAGE J.E., RAUSER L., MCBRIDE A., HUFEISEN S.J., ROTH B.L. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- SCHMUCK K., ULLMER, C., KALKMAN H., PROBST A., LÜBBERT H. Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache. Eur. J. Neurosci. 1996;8:959–967. doi: 10.1111/j.1460-9568.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- SILBERSTEIN S.D. The pharmacology of ergotamine and dihydroergotamine. Headache. 1997;37:S15–S25. [PubMed] [Google Scholar]
- THOMSEN L.L., OLESEN J. Nitric oxide in primary headaches. Curr. Opin. Neurol. 2001;14:315–321. doi: 10.1097/00019052-200106000-00009. [DOI] [PubMed] [Google Scholar]
- VILLALON C.M., DE VRIES P., RABELO G., CENTURION D., SANCHEZ-LOPEZ A., SAXENA P. Canine external carotid vasoconstriction to methysergide, ergotamine and dihydroergotamine: role of 5-HT1B/1D receptors and alpha2-adrenoceptors. Br. J. Pharmacol. 1999;126:585–594. doi: 10.1038/sj.bjp.0702324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLALON C.M., DE VRIES P., SAXENA P.R. Serotonin receptors as cardiovascular targets. Drug Discovery Today. 1997;2:294–300. [Google Scholar]
- WINNER P., RICALDE O., LE FORCE B., SAPER J., MARGUL B. A double-blind study of subcutaneous dihydroergotamine vs subcutaneous sumatriptan in the treatment of acute migraine. Arch. Neurol. 1996;53:180–184. doi: 10.1001/archneur.1996.00550020092020. [DOI] [PubMed] [Google Scholar]