Abstract
Ischemic preconditioning in the brain consists of reducing the sensitivity of neuronal tissue to further, more severe, ischemic insults. We recorded field epsps (fepsps) extracellularly from hippocampal slices to develop a model of in vitro ischemic preconditioning and to evaluate the role of A1, A2A and A3 adenosine receptors in this phenomenon.
The application of an ischemic insult, obtained by glucose and oxygen deprivation for 7 min, produced an irreversible depression of synaptic transmission. Ischemic preconditioning was induced by four ischemic insults (2 min each) separated by 13 min of normoxic conditions. After 30 min, an ischemic insult of 7 min was applied. This protocol substantially protected the tissue from the irreversible depression of synaptic activity.
The selective adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 100 nM), completely prevented the protective effect of preconditioning. The selective adenosine A2A receptor antagonist 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385, 100 nM) did not modify the magnitude of fepsp recovery compared to control slices. The selective A3 adenosine receptor antagonists, 3-propyl-6-ethyl-5[ethyl(thio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate (MRS 1523, 100 nM) significantly improved the recovery of fepsps after 7 min of ischemia.
Our results show that in vitro ischemic preconditioning allows CA1 hippocampal neurons to become resistant to prolonged exposure to ischemia. Adenosine, by stimulating A1 receptors, plays a crucial role in eliciting the cell mechanisms underlying preconditioning; A2A receptors are not involved in this phenomenon, whereas A3 receptor activation is harmful to ischemic preconditioning.
Keywords: Adenosine, cerebral ischemia, ischemic tolerance, hippocampal slices, synaptic potential, field epsp
Introduction
In the mammalian brain, oxygen and glucose deprivation (OGD) caused by an ischemic episode leads to the development of increasingly severe, with duration of ischemia, tissue damage. The available knowledge on the consequences of ischemic episodes indicates that synaptic transmission is particularly sensitive to OGD. Neurotransmission is rapidly impaired and, when the duration of the ischemic episode lasts for more than a few minutes, the disappearance of synaptic responses becomes irreversible (Pedata et al., 1993). Thus, disappearance of synaptic transmission is probably the first detectable functional sign of cell suffering that eventually results in neuron death.
Much work has been devoted to the study of the sequence and nature of the events caused by ischemic episodes in vivo (see Dirnagl et al., 1999). However, the in vivo study of the mechanisms involved in the early events causing the loss of neurotransmission is limited by obvious difficulties.
Therefore, the investigation of factors that play a major role in the disappearance of neurotransmission within the first few minutes of ischemia has been approached by using a variety of in vitro models aimed at reproducing either oxygen (Musleh et al., 1994; Furling et al., 2000) or glucose deprivation (Fowler, 1994; Calabresi et al., 1997; Alici et al., 1998) or both (Fowler, 1992; Latini et al., 1999a, 1999b).
In a variety of tissues, including the brain, exposure to brief periods of hypoxia or ischemia results in increased tolerance to severe ischemic insults (Ishida et al., 1997; Dawson & Dawson, 2000). This phenomenon, called ‘preconditioning', was first observed in the heart (Murry et al., 1986) and later shown in rat and gerbil brain (Kitagawa et al., 1990; Kato et al., 1991; Kirino et al., 1991; Liu et al., 1992; Heurteaux et al., 1995). In a pioneering work in vitro, Schurr et al. (1986) observed that, in the CA1 region of hippocampal slices, a brief episode of hypoxia produced increased tolerance of the excitatory neurotransmission to a second, otherwise lethal hypoxic episode. Since then, several experimental approaches have been developed in the attempt to establish reliable protocols for the study of cellular mechanisms of ischemic brain damage in the in vitro brain tissue. Cultured neuronal cells have been favored when the mechanisms of neuron damage and of cell survival were the principal objectives of investigation, because of the possibility of observing the parameters over days (Khaspekov et al., 1998; Paschen & Mies, 1999; Pringle et al., 1999; Miller et al., 2001; Raval et al., 2003).
In contrast, the study of mechanisms of neurotransmission impairment and of early adaptive cell changes leading to development of tolerance in the adult brain required the use of brain slices from adult animals. At present, only a few investigations have been conducted on preconditioning in brain slices and were mostly directed at the study of the effects of a brief anoxic episode on a subsequent longer anoxia in hippocampal slices (Schurr et al., 1986; Perez-Pinzon et al., 1996; Perez-Pinzon & Born, 1999).
Unlike the previous preconditioning in vitro conditions utilized in hippocampal slices (i.e. anoxic conditions), in this work we studied the effects of preconditioning under oxygen and glucose deprivation in the rat hippocampus in vitro. Such model may better mimic, within the limitations of an in vitro model, the occurrence of repeated, transient, ischemic attacks known to induce relative tolerance to severe ischemic episodes in vivo (Kitagawa et al., 1990; Kato et al., 1991, Miller et al., 2001). The subsequent, long episode of oxygen and glucose deprivation was aimed at reproducing the interruption of blood flow following cardiac arrest or occlusion of intracranial vessels, one of the most frequent causes of cerebral ischemia.
The second objective of the present work was the study of the involvement of adenosine in the preconditioning phenomenon. In fact, the extracellular concentration of adenosine is increased in those situations in which the supply of oxygen and energy substrates is not adequate to meet the energy demands of the brain, such as during hypoglycemia, hypoxia or ischemia (Latini et al., 1999a; Plaschke et al., 2000). Most evidence indicates that stimulation of adenosine A1 receptors is involved in protection from hypoxic/ischemic insults, while the role of A2A and/or A3 receptors is still controversial (Dunwiddie et al., 1997; Brand et al., 2001; Pedata et al., 2001).
In vivo, administration of adenosine or of selective A1 receptor agonists mimics a preconditioning effect in the brain (Heurteaux et al., 1995; Blondeau et al., 2000). Consistently, intraperitoneal treatment with A1-receptor antagonists attenuates the beneficial effects of ischemic preconditioning both in the rat brain (Nakamura et al., 2002) and in the CA1 gerbil hippocampus (Hiraide et al., 2001).
Adenosine is involved in anoxic (Perez-Pinzon et al., 1996; Perez-Pinzon & Born, 1999) and chemical preconditioning (Aketa et al., 2000) in the hippocampus in vitro and a role of A1 adenosine receptors in the preconditioning phenomenon was demonstrated (Blondeau et al., 2000; Hiraide et al., 2001; Nakamura et al., 2002).
In the present study, the role of A1, A2A and A3 adenosine receptors in the early mechanisms of protection exerted by ischemic preconditioning in the hippocampus in vitro was investigated, using selective antagonists.
Part of this work has been communicated (Latini et al., 2001).
Methods
All animal procedures were carried out according to the European Community Guidelines for Animal Care, DL 116/92, application of the European Communities Council Directive (86/609/EEC). Experiments were carried out on rat hippocampal slices, prepared as previously described (Latini et al., 1999a, 1999b).
Slice preparation
Male Wistar rats (Harlan Italy; Udine Italy, 140–180 g body weight), under anesthesia with ether, were killed with a guillotine and their hippocampi were rapidly removed and placed in ice-cold oxygenated (95% O2–5% CO2) artificial cerebral spinal fluid (aCSF) of the following composition (mM): NaCl 124, KCl 3.33, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 25 and D-glucose 10. Slices (400 μm thick) were cut by a McIlwain tissue chopper (The Mickle Lab. Engineering, Co. Ltd, Gomshall, U.K.) and kept in oxygenated aCSF for at least 1 h at room temperature. A single slice was then placed on a nylon mesh, completely submerged in a small chamber (0.5 ml) and superfused with oxygenated aCSF (30–32°C) at a constant flow rate of 2 ml min−1. The treated solutions reached the preparation in 90 s and this delay was taken into account in our calculations.
Extracellular recording
Test pulses (80 μs, 0.06 Hz) were delivered through a bipolar nichrome electrode positioned in the stratum radiatum. Evoked extracellular potentials were recorded with glass microelectrodes (2–10 MΩ, Clark Electromedical Instruments, Panghourne, U.K.) filled with 3 M NaCl, placed in the CA1 region of the stratum radiatum. Responses were amplified (Neurolog NL 104, Digitimer Ltd, Welwyn Garden City, U.K.), digitized (sample rate, 33.33 kHz), and stored for later analysis using pCLAMP 6 software facilities (Axon Instruments, Foster City, CA, U.S.A.). Stimulus–response curves, were obtained by gradual increases in stimulus strength at the beginning of each experiment. The test stimulus pulse was then adjusted to produce a field excitatory postsynaptic potential (fepsp) whose slope was 40–50% of the maximum and was kept constant throughout the experiment. The amplitude of fepsp was routinely measured and expressed as the percentage of the average amplitude of the potentials measured during 10 min after establishing a stable baseline of evoked response under normoxic conditions. In some experiments, both the amplitude and the initial slope of fepsp were quantified, but since no appreciable differences were observed in the effect of drugs and of in vitro ischemia, only the measure of the amplitude was expressed in figures.
Application of OGD and protocols of ischemic preconditioning
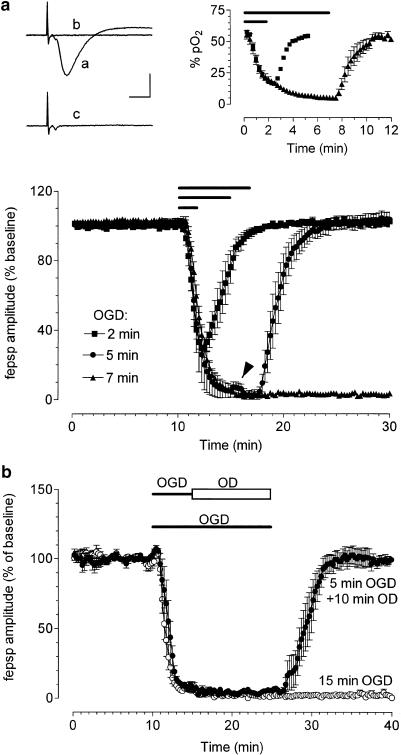
In vitro OGD was obtained by perfusing the slice with aCSF without glucose and gassed with nitrogen (95% N2–5% CO2) (Pedata et al., 1993), whereas in vitro oxygen deprivation (OD) was obtained by perfusing the slice with aCSF gassed with nitrogen. At the end of the ischemic or anoxic period, the slice was again superfused with normal (glucose-containing) oxygenated aCSF. pO2 values were measured with ISO2 and its associated OXEL-1 probe (WPI, Aston, U.K.). The instrument amperometrically measured the concentration of oxygen in aqueous solutions and gas mixtures. The oxygen probe houses a platinum working electrode and a polymer membrane fits over the end of the sleeve that allows oxygen to pass while blocking liquid ions and particulate matter. Dissolved oxygen calibration was corrected for the effect of water vapor by the following equation: pO2=21%(1-pH2O/760), where pH2O is the partial pressure of water vapor at standard atmospheric pressure in mmHg. The pO2 values obtained decreased from 58±0.7 (corresponding to a range of 420–500 mmHg) in control to 17±0.5 (corresponding to a range of 120–150 mmHg, P<0.05, n=4) after 2 min of OGD and to 5±1.3 (corresponding to a range of 35–75 mmHg, P<0.05, n=4) after 7 min of OGD (see Figure 1).
Figure 1.
Effects of ischemia or hypoxia on fepsps evoked by electrical stimulation of the stratum radiatum in the CA1 hippocampal region. (a) The graphs show the time course of fepsps amplitude, before, during different time durations of OGD and after reperfusion in normal oxygenated aCSF. Data, expressed as percent of baseline values, are means±s.e.m., n=20 for 2 and 5 min of OGD and n=50 for 7 min of OGD. Bars indicate the time duration of OGD. Note the transient reappearance of synaptic potential after superfusion in normal oxygenated aCSF only after 5-min OGD (arrowhead). Upper panel, left side: fepsps recorded from hippocampal slices in a typical 7-min OGD experiment: (a) control, (b) at the end of 7-min OGD, (c) after a 20-min washout in normal oxygenated aCSF. A 7-min OGD abolishes both fepsp and afferent volley amplitude. After reperfusion in normal oxygenated aCSF, only the recovery of afferent volley was recorded. Calibration bars: 0.5 mV, 5 ms. (b) Upper panel, right side: Values (mean±s.e.m.) in the graphs represent pO2 levels (expressed in percentage) during 2- or 7-min OGD and immediately after reperfusion in aCSF. The lowest pO2 values are obtained starting from the fifth minute of the 7-min OGD. (b) The graphs show the time-course of fepsps amplitude before, during OGD or OD application and after reperfusion in oxygenated aCSF. Data, expressed as percent of baseline values, are means±s.e.m. No electrical activity was recorded after 15 min of OGD (n=7), whereas a complete recovery of neurotransmission after 5 min of OGD, followed by 10-min anoxia, was observed (n=5). Closed and open bars indicate time duration of OGD and OD, respectively.
In a preliminary series of preconditioning experiments, we found that a single OGD lasting 2 min (n=3) or 5 min (n=3) did not modify the outcome of the 7-min OGD (not shown). Since the administration of repeated short ischemic insults appears to be the most effective protocol for preconditioning in the heart (Kloner & Jennings, 2001), we tested the possible effectiveness of a similar conditioning treatment in brain tissue. For this reason, the number of preconditioning ischemic stimuli was increased.
During ischemic preconditioning, hippocampal slices underwent four repeated short OGD episodes of 2 min, separated by 13 min of reperfusion in normal oxygenated aCSF. At 30 min following the fourth conditioning insult, a prolonged OGD of 7 min was induced. In most cases both a control protocol, in which the 7 min OGD was applied after 40 min of baseline, and a preconditioning protocol were performed on hippocampal slices taken from the same rat. Since in the absence of preconditioning, the largest fepsp recovery observed was 6% (see Results), only an fepsp amplitude recovery higher than 15% was taken into consideration for quantitative analysis after preconditioning.
In three different set of experiments, the effects of selective adenosine receptor antagonists on ischemic preconditioning were studied.
DPCPX (100 nM) a selective A1 receptor antagonist, ZM 241385 (100 nM) a selective A2A antagonist and MRS 1523 (100 nM) a selective A3 adenosine receptor antagonist were applied for 25 or 20 min before and during the application of the four brief repeated ischemic insults and then washed for 33 min before the application of the 7-min OGD. Experiments were carried out either in the absence or presence of the adenosine antagonists, in slices taken from the same rat in the same day. The 100 nM concentration of DPCPX was chosen because it maximally blocks the 2 min ischemia-induced fepsp depression (Latini et al., 1999a) and this action is reversible within a 30-min drug washout (see Results). The 100 nM concentration of ZM 241385 was chosen because it fully antagonizes the reduction of synaptic depression, induced by in vitro ischemia, produced by the selective adenosine A2A agonist CGS 21680 (Latini et al., 1999b). The 100 nM concentration of the selective adenosine A3 receptor antagonist, MRS 1523, was chosen on the basis of Ki values on rat A3 receptors (Li et al., 1998).
Drugs
DPCPX (8-cyclopentyl-1,3-dipropylxanthine) was purchased from Research Biochemicals International (Natik, MA, U.S.A.), MRS 1523 (3-propyl-6-ethyl-5[ethyl(thio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate) from Sigma (Milano, Italy), ZM 241385 (4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol) from Tocris (Bristol, U.K.). Each drug was dissolved in dimethylsulfoxide (DMSO) and stock solutions were made to obtain concentrations of DMSO of 0.05 and 0.01% in aCSF, respectively. Control experiments, carried out in parallel for an unrelated project, showed that this concentration of DMSO did not affect the depression of synaptic potential induced by the following OGD.
Statistical analysis
Data were analyzed using Clampfit (Axon) and Prism 3.02 software (Graphpad Software, San Diego, CA, U.S.A.). All numerical data are expressed as the mean±s.e.m. Data were tested for statistical significance with paired two-tailed Student's t-test or by analysis of variance (one-way ANOVA), as appropriate. When significant differences were observed, Newman–Keuls multiple comparison test was inferred. A value of P<0.05 was considered significant.
Results
The effects of in vitro OGD in the absence or after an ischemic preconditioning protocol on synaptic transmission were studied with extracellular recordings of fepsps in the CA1 region of rat hippocampal slices. Experiments were performed on a total of 150 slices taken from 102 rats.
7- or 15-min OGD irreversibly abolish fepsps
Figure 1 illustrates the time course of the fepsp depression caused by administration of OGD for 2, 5 or 7 min. As shown in Figure 1a, an OGD of 2 min caused a partial fepsp depression (−69±5%, n=20, paired Student's t-test P<0.01), while 5-min OGD abolished fepsps (−97±2%, n=20, paired Student's t-test P<0.01). In both cases, the synaptic depression induced by OGD was reversible after returning to normal oxygenated aCSF.
The application of 7-min OGD irreversibly abolished synaptic neurotransmission (−99±3.5%, n=50, paired Student's t-test P<0.01; Figure 1a).
At the end of 7-min OGD, the disappearance of the synaptic component of the fepsp was followed by the disappearance of the afferent fiber volley in all preparations (e.g. Figure 1a, upper panel, left side). The afferent fiber volley amplitude, unlike the fepsp, recovered within a few minutes of superfusion in normal oxygenated aCSF.
The pO2 levels (mean±s.e.m.) measured during 2 or 7 min of OGD and at the beginning of superfusion in normal oxygenated aCSF are also shown in Figure 1a (upper panel, right side). The depression of synaptic activity was temporally coincident with a decrease in pO2 values measured in the slice. It should be noted that the complete loss of synaptic activity was observed starting at the 5-min OGD, when pO2 reached minimum values.
The application of a longer, 15-min OGD irreversibly reduced the amplitude of fepsp (−98±1%, n=7, paired Student's t-test P<0.01, Figure 1b). Under this condition, within superfusion in normal oxygenated aCSF, the recovery of fiber volley amplitude was observed in four, out of seven, slices examined (data not shown).
During the first 2 min of recovery from the 5-min OGD episode in normal, oxygenated aCSF, a transient reappearance of the fepsp was observed (Figure 1a and see also Latini et al., 1999a). This phenomenon was time-locked to readmission normal oxygenated aCSF and was neither present during or after the 7- or 15-min OGD episodes.
In a previous study on the effects of 10-min anoxia in hippocampal slices (Sick et al., 1987), a transient reappearance of synaptic responses was observed after about 5–6 min of anoxia and was ascribed to the increase in extracellular potassium concentration (to about 8 mM) caused by the anoxic episode. In our experience, superfusion of aCSF containing 8 mM potassium at the fifth minute of an OGD lasting 15 min did not cause any reappearance of the fepsp (n=5, not shown).
To investigate whether anoxia had a predominant role in the irreversible disappearance of neurotransmission, a group of hippocampal slices was first exposed for 5 min to OGD and was then superfused for a further 10 min with OD solution. As illustrated in Figure 1b, under these conditions, fepsp amplitude fully recovered (102±6%, n=5, paired Student's t-test P<0.01) after a few minutes of reperfusion in normal oxygenated aCSF. Nevertheless, no transient appearance of the fepsp was found.
Since 7-min OGD application induced an irreversible depression of synaptic activity without affecting the recovery of fiber volley amplitude in all the slices tested, this indicated that in the time-window a substantial damage of neurotransmission machinery occurred, which did not involve an irreversible impairment of nerve conduction.
This 7-min OGD duration was therefore chosen to test the effects of ischemic preconditioning on an ischemic insult otherwise capable of irreversibly compromising synaptic transmission.
Effects of ischemic preconditioning on a 7-min OGD
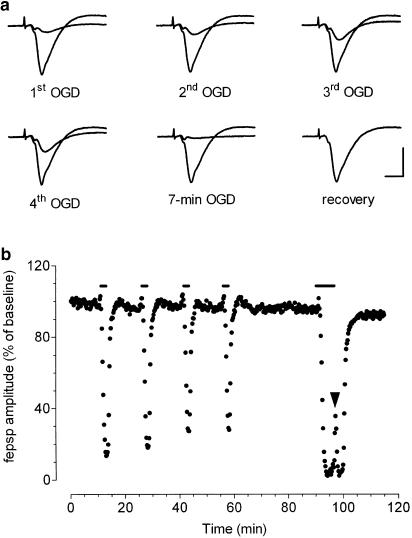
As illustrated in Figure 2, the preconditioning treatment consisted of a sequence of four, brief (2-min) OGD episodes, separated by 13-min intervals of superfusion in normal oxygenated aCSF. This preconditioning protocol was followed by 33 min of reperfusion in normal oxygenated aCSF before testing the effects of a 7-min OGD. In a typical experiment shown in Figure 2a and b this preconditioning protocol resulted in a complete recovery of fepsps after a few minutes of reperfusion in normal oxygenated aCSF. After a 7-min OGD application, immediately after starting superfusion with normal oxygenated aCSF, a transient reappearance of synaptic potentials was observed (Figure 2b, arrowhead). As shown in Figure 2b, the depressions induced by the four conditioning ischemic episodes were progressively smaller and a statistical significant difference among the groups was observed (P<0.05, one-way ANOVA, n=22, Table 1).
Figure 2.
Ischemic preconditioning induces tolerance against the depression of synaptic transmission induced by 7 min of OGD in the CA1 hippocampal region. (a) Traces are fepsps recorded from CA1 stratum radiatum at representative times of the corresponding experiment shown in (b) Synaptic potentials recorded in normoxic aCSF and at the point of maximal depression are superimposed to show the maximal effect of ischemic episodes. Calibration bars: 0.5 mV, 5 ms. (b) The graph shows the time course of fepsp amplitude, expressed as percent of baseline responses, in a typical experiment, during the four preconditioning 2-min OGD episodes and the 7 min of OGD ‘test' application. Bars indicate the time duration of OGD episodes. Note the complete recovery of synaptic potential, after 7-min OGD, occurring within 10 min of superfusion in normal oxygenated aCSF and the transient reappearance of synaptic potentials at the end of the 7 min of OGD (arrowhead).
Table 1.
Reduction of fepsp amplitude at the end of the four brief OGD episodes during preconditioning and at the corresponding time during 7-min OGD
| Preconditioning | 7-min OGD | ||||
|---|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | 2.5 min (%) | |
| No drug (n=22) | 72±4 | 59±4 | 52±4 | 48±4* | 65±5 |
| DPCPX (n=12) | 13±3 | 9±3 | 8±2 | 7±3 | 63±10 |
| MRS 1523 (n=10) | 62±12 | 61±9 | 55±10 | 53±10 | 65±12 |
| ZM 241385 (n=8) | 66±11 | 53±10 | 51±11 | 43±7* | 65±5 |
The peak of fepsps depression was expressed as percentage of the respective control values. Values (mean±s.e.m.) were measured at the time of maximal peak depression (2.5 min) induced by brief OGD of preconditioning protocol and at 2.5 min of the 7-min OGD application.
P<0.05 compared with the first brief OGD and at 2.5 min of 7-min OGD (one-way ANOVA).
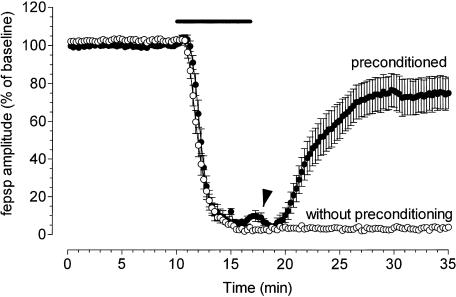
Figure 3 illustrates the effects of ischemic preconditioning on the time course of the fepsp amplitude depression caused by a 7-min OGD episode (n=22) compared to that observed in control ‘nonpreconditioned' slices (n=50) conducted in parallel in slices taken from the same rats. A substantial recovery (80±9%, paired Student's t-test P<0.05) of fepsps from the depression induced by 7-min OGD, after superfusion in normal oxygenated aCSF was obtained only in preconditioned slices (22 out of 27), whereas no recovery of fepsps was recorded in nonpreconditioned, control slices (3.3±0.75%, paired Student's t-test, P<0.05). The transient reappearance of synaptic potentials was observed, soon after superfusion with normal oxygenated aCSF, in preconditioned slices (Figure 3).
Figure 3.
Ischemic preconditioning protects hippocampal slices from the fepsp depression induced by the 7 min of ischemia. The graphs show the time course of fepsp amplitude, expressed as percent of baseline, 10 min before, during 7 min of OGD and superfusion in normal oxygenated aCSF in the absence (mean±s.e.m., n=50) and after ischemic preconditioning (mean±s.e.m., n=22). A transient reappearance of synaptic potentials was observed in preconditioned slices at the end of 7-min OGD (arrowhead). Bars indicate the time duration of OGD episodes.
Role of A1, A2A and A3 adenosine receptor subtypes in preconditioning-induced recovery of neurotransmission
We investigated the possibility that a stimulation of adenosine receptors by the endogenous adenosine released during the four brief episodes of OGD was involved in the induction of the ‘preconditioning' effect. For this reason, the preconditioning protocol was administered in the presence of selective antagonists for each adenosine receptor type (A1, A2A and A3), which was washed out immediately after the fourth conditioning episode.
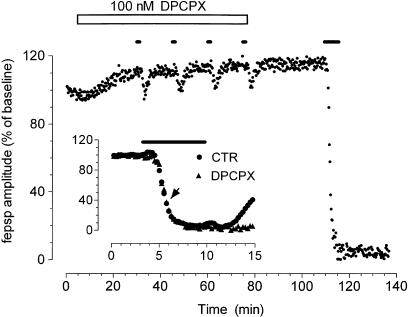
A first series of experiments was addressed to assess whether the block of adenosine A1 receptors, by a selective adenosine A1 receptor antagonist DPCPX, can prevent the protective effects of ischemic preconditioning on synaptic transmission. DPCPX (100 nM), applied 25 min before and during the four conditioning OGD episodes, almost completely blocked fepsp depression induced by each short OGD. The 7-min OGD, administered after a 33-min washout of DPCPX, irreversibly abolished the evoked fepsps, showing that a block of A1 receptors prevented the protection induced by preconditioning against the 7-min OGD (Figure 4). The application of DPCPX caused, per se, an increase in fepsp amplitude (on average from 0.92±0.02 to 1.06±0.05 mV, Student's t-test P<0.05, n=12), confirming the existence of a tonic inhibition of fepsps by the endogenous adenosine present in control conditions (Dunwiddie & Diao, 1994; Latini et al., 1999a). The effect of DPCPX is washed out of the preparation in 33 min, as demonstrated by the fact that the magnitude of fepsps depression at 2.5 min of the 7-min OGD application is comparable to that obtained in preconditioned slices in the absence of the drug (Table 1 and inset of Figure 4).
Figure 4.
DPCPX prevents the protective effect of ischemic preconditioning on fepsp depression induced by 7 min of ischemia. The graph shows the time course of fepsp amplitude recorded from a typical experiment carried out in the presence of DPCPX (100 nM, open bar), an A1 adenosine selective antagonist, applied 25 min before and during the preconditioning OGD episodes. (Inset) The time course of fepsp depression caused by 7-min OGD in nonconditioned (control) slices and in slices in which preconditioning was performed in the presence of DPCPX. Given are mean±s.e.m. values of fepsp amplitude (not conditioned: n=50; conditioned n=12). Note that the entity of the fepsp depression recorded at 2.5 min of the 7-min OGD application in slices preconditioned in the presence of DPCPX was similar (arrowhead) to that observed in controls (see also Table 1). Bars in the figure and in the inset indicate the time duration of OGD episode.
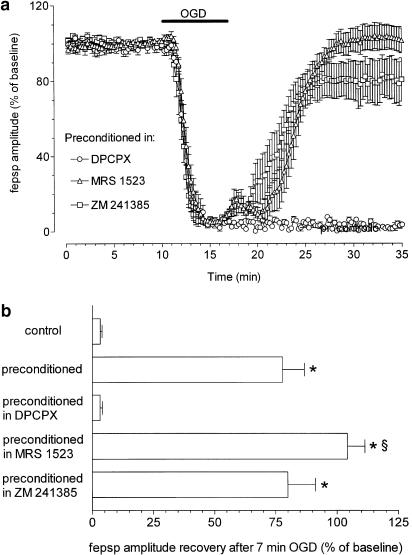
In Figure 5 the effects of adenosine A1, A2A and A3 antagonists on the recovery of synaptic potential amplitude after 7-min OGD are compared.
Figure 5.
Role of A1, A2A and A3 receptor activation in ischemic preconditioning in rat hippocampal slices. (a) Graphs show the time course of fepsp amplitude 10 min before, during 7 min of OGD and superfusion in normal oxygenated aCSF, after preconditioning in the presence of different adenosine receptor antagonists (DPCPX, 100 nM, n=12; ZM 241385, 100 nM, n=8; MRS 1523, 100 nM, n=10). Data, expressed as percent of baseline values, are means±s.e.m. of fepsp amplitude. Bar indicates the time duration of ischemic episodes. (b) Column bars in the graph summarize the average recovery (mean±s.e.m.) of fepsp after 7 min of OGD, recorded in hippocampal slices at 15 min reperfusion in normal, oxygenated aCSF in control and preconditioned slices. *P<0.05 versus control; §P<0.05 versus all experimental conditions. The selective adenosine antagonists, DPCPX, ZM 241385, MRS 1523, were applied during ischemic preconditioning at a concentration of 100 nM. Differences between data were analyzed by one way ANOVA, followed by Newman–Keuls multiple comparison post hoc test.
As shown in Figure 5, the 7-min OGD episode, administered after a 30-min washout of DPCPX (100 nM), irreversibly abolished (−97±1%, n=12, paired Student's t-test P<0.01) the preconditioning effect. In contrast, ischemic preconditioning in the presence of a selective A3 adenosine receptor antagonist (MRS 1523, 100 nM), substantially improved the recovery of synaptic potential amplitude (Figure 5a). As shown in Figure 5b, the recovery of fepsp, after 7-min OGD episode in these conditions was of 104±7% (n=10). This effect was statistically significant when compared to that obtained with any other treatment, including control preconditioning (P<0.05, one-way ANOVA, Newman–Keuls multiple comparison post hoc test). Finally, no apparent involvement of A2A receptors was found when the ischemic preconditioning was carried out in the presence of the selective A2A adenosine receptor antagonist, ZM 241385 (100 nM, Figure 5a), that did not affect fepsp depression during the preconditioning protocol (Table 1). The recovery of neurotransmission (80±11%, n=8) was similar to that obtained after ischemic preconditioning in the absence of the antagonist (Figure 5b).
Discussion
Our work demonstrates that exposure of in vitro brain tissue to repeated, brief ischemic episodes elicits rapid adaptive mechanisms that prevent the irreversible disruption of synaptic transmission caused by a severe ischemic episode. The second finding is that adenosine, by stimulating A1 receptors, plays a crucial and permissive role in eliciting the cell mechanism(s) induced by preconditioning and underlying the functional recovery of neurotransmission in the hippocampal CA1 area after ischemic insults. The third finding is that the block of A3 adenosine receptors during the preconditioning episodes enhances the effectiveness of preconditioning and suggests that the stimulation of A3 receptors by endogenous adenosine may be one of the negative stimuli that eventually leads to the irreversible loss of neurotransmission during prolonged ischemic episodes.
Extended work has been directed to study the impairment of neurotransmission by anoxic/ischemic insults of various duration in vitro (Schurr & Rigor, 1989). More recently, the in vivo demonstration that transient ischemic episodes may induce tolerance to severe brain ischemia (Kitagawa et al., 1990; Kato et al., 1991; Heurteaux et al., 1995) generated several attempts to establish a model for preconditioning brain tissue in vitro.
Available results converge in indicating that a preconditioning effect can be obtained against in vitro ischemic insults either by preceding transient ischemic episodes (Tokunaga et al., 1998) in vivo, or by administration of anoxic/episodes in vitro (Schurr et al., 1986; Perez-Pinzon et al., 1996). Nevertheless, none of the models proposed provides exhaustive means of investigation of all aspects of preconditioning and/or ischemic damage. In fact, the experimental protocols proposed by different authors have been directed to study aspects of anoxic/ischemic damage that are very dissimilar in terms of investigated parameters and therefore of possible conclusions regarding the cellular mechanisms involved and their presynaptic or postsynaptic location.
For instance, cultured neuronal cells or organotypic hippocampal slices have been used by several groups (Khaspekov et al., 1998; Paschen & Mies 1999; Pringle et al., 1999; Miller et al., 2001). Although these preparations undoubtedly present advantageous features for anatomofunctional studies, they have the intrinsic limitation of being at an unknown stage of cell maturation and therefore of expressing naturally occurring cellular mechanisms.
Accordingly, a large number of investigators have elected to use brain slices from adult animals for the study of anoxic/ischemic damage and more recently, for the investigation of preconditioning in vitro (Schurr et al., 1986; Perez-Pinzon et al., 1996). From this point of view, the model we present is endowed with several characteristics and advantages that are unprecedented and complementary to those of other models and that may eventually be of particular interest for multidisciplinary investigations.
Characteristics of the experimental conditions chosen for reproducing ischemia and preconditioning in vitro
It is obvious that none of the in vitro models would fully reproduce the damage caused by in vivo ischemic episodes. Nevertheless, OGD, instead of anoxia alone, may better mimic an episode of severe brain ischemia when the study is directed at the investigation of the effects of a transient vascular occlusion followed by reperfusion. Indeed, previous work in vitro shows that the availability of glucose is of great relevance for the early functional outcome of an associated anoxia (Schurr et al., 1997). Accordingly, in our study we have used episodes of OGD in submerged hippocampal slices versus those taken at interface for several theoretical and practical considerations: (i) the changes in pO2/glucose are likely to be more homogeneous in the preparation, and the application/washout of drugs is faster; (ii) the slice is better preserved and, in perspective, may be used to assess neurochemical parameters (e.g. by immunohistochemistry) in parallel studies in the same conditions or even after electrophysiological recording as previously carried out for different purposes (Giovannini et al., 2001).
Extracellular recording from the dendritic region of CA1 pyramidal cells has been performed because it has the advantage of monitoring neurotransmission at its source (i.e. records the epsp generation due to glutamate receptor activation). This minimizes the influence of modulatory mechanisms possibly acting at a somatic level and able to alter the cell discharge recorded with the population spike (e.g. sodium channel inactivation). Therefore, a decrease in fepsps predominantly reflects a decrease in the release of the excitatory neurotransmitter.
On the other hand, alterations in the postsynaptic response (e.g. dendrite depolarization) may be involved in the disappearance of the fepsp during the ischemic period. When present, a substantial or full recovery of fepsps also ensures that those alterations are recovered after reperfusion with control aCSF.
The first set of experiments devoted to the identification of the type and duration of the ischemic insult to be used for the induction of the adaptive changes (preconditioning) and of the outcome of an ischemic episode allow several conclusions.
The first conclusion is that, in the early phases of the ischemic episode, the disappearance of the fepsps parallels the decrease in pO2, but that the irreversible damage observed for OGD episodes longer than 5 min is due primarily to the decrease in glucose availability. In fact, the readmission of glucose in the absence of oxygen for a further 10 min allows for full recovery of synaptic transmission. This is consistent with previous findings (Zhu & Krnjevic, 1999; Tian & Baker, 2002) showing the crucial role of glucose levels for the recovery of neurotransmission in CA1 and the relatively longer resistance of hippocampal slices to oxygen deprivation (Pearson et al., 2001).
Furthermore, in our conditions, a transient reappearance of neurotransmission was observed only at reperfusion with normal oxygenated aCSF after 5-min OGD, but not during more prolonged OGD episodes (up to 15 min). The transient reappearance of synaptic potentials is probably linked to hyperexcitability phenomena and was described by several researchers in the hippocampus during hypoxia (Zhu & Krnjevic, 1999), ischemia (Fowler, 1990), spreading depression (Stone, 2000) or recovery from prolonged hypoxia (Luhmann & Heinemann, 1992; Doolette & Kerr, 1995).
Therefore, it appears that the absence of glucose also hampers the development of the transient phase of hyperexcitability caused by the rise in extracellular potassium concentration shown in purely anoxic episodes (Sick et al., 1987). On the other hand, a constant finding in our experiments was the transient reappearance of synaptic potentials at the end of the 7-min OGD in preconditioned slices, which was absent in controls. Regardless of what mechanism(s) are responsible for this phenomenon, this transient phase, observed only after ischemic preconditioning, further points to an increased tissue resistance and its presence may be considered predictive of a functional recovery of neurotransmission during reperfusion.
The second conclusion is that, within a narrow time frame (between 5 and 7 min of OGD), cellular mechanisms are activated and lead to the irreversible disappearance of neurotransmission. This time frame can be widened by repeated exposure of the tissue to brief OGD episodes. In fact, the recovery of fepsp is an unambiguous sign of cell survival in the face of an otherwise lethal insult. It appears therefore that preconditioning triggers cellular mechanisms able to protect, to some extent, neurotransmission from disruption caused by the ischemic episode. Further investigation is needed to understand whether these mechanisms may also be responsible for long-lasting neuroprotection.
Role of adenosine receptors in the in vitro preconditioning
During in vitro brain ischemic episodes, inhibition of excitatory synaptic transmission is mainly ascribed to the increase in extracellular adenosine (Fowler, 1990; Gribkoff et al., 1990; Pedata et al., 1993; Latini et al., 1998) and subsequent stimulation of A1 receptors (Latini et al., 1999a) that inhibit glutamate release (Corradetti et al., 1984).
Accordingly, during the OGD episodes of the preconditioning protocol, substantial outflow of adenosine produced a depression of fepsp amplitude. However, it should be noted that, in the preconditioning phase in controls, a significant decrease in depression of fepsps at the fourth preconditioning episode was observed, compared to the first episode. The simplest interpretation of the decreased effectiveness of brief OGD episodes in depressing fepsp is that the mechanisms activated by preconditioning and able to counteract the effect of OGD are increasingly apparent over time and with reinforcement of the preconditioning stimuli. Nevertheless, in the presence of the A3 adenosine receptor antagonist, this phenomenon was partially blocked. One can speculate that the decreased effectiveness of brief OGD episodes is due to A3 receptor stimulation and subsequent desensitization of A1 adenosine receptor, as suggested by Dunwiddie et al. (1997). Alternatively, it can be envisaged that repeated OGD causes a substantial decrease in availability of adenosine and therefore the smaller depression in fepsp amplitude is a mere reflection of a decreased adenosine outflow. Indeed, it has been shown that adenosine outflow during the hypoxic/ischemic episode was reduced in hippocampal slices previously exposed to an initial hypoxic/ischemic period (Pearson et al., 2001 and personal, unpublished data). However, although the amount released at the second episode is smaller, it is sufficient to block neurotransmission completely (Latini et al., 1999a; Pearson et al., 2001). On the other hand, this possibility cannot be completely excluded on the basis of the present results, because at the second minute of the lethal episode the depression is similar to that found with the first and larger than that caused by the fourth. The fact that the fepsp depression during the first minute of the 7-min OGD was similar to that observed with the 2-min conditioning OGD, suggested that the time (>30 min) between preconditioning and the 7-min OGD may have allowed refilling of adenosine stores to be released upon OGD insult.
In both cases, however, it should be noted that the effects of preconditioning are most probably due to the activation of adenosine receptors during preconditioning and not to altered release of adenosine during the 7-min OGD. In fact, regardless of the mechanisms causing the progressive decrease in fepsp amplitude depression during the preconditioning protocol, the cause for the decrease in excitatory neurotransmission was predominantly produced by the stimulation of A1 receptors, as confirmed by the antagonism of fepsp depression exerted by 100 nM DPCPX. Our experiments demonstrate that when A1 receptor stimulation is antagonized by the presence of DPCPX during the preconditioning phase, no recovery of excitatory neurotransmission is observed after the 7-min OGD episode. Since the removal of the A1 receptor block appears to be complete, as judged by the time course and magnitude of fepsp depression during the first minutes of the lethal OGD, the lack of recovery does not seem to be due to a substantial impairment of A1 receptor-mediated responses during the test ischemia. Therefore, it can be concluded that stimulation of A1 receptors during the conditioning OGD episodes is necessary and permissive for the activation of cell mechanisms responsible for the tolerance to a severe ischemic insult. This result also shows that our experimental protocol shares this fundamental mechanism with other models and confirms the overall consistency of interpretation of A1 receptor role in preconditioning.
In regard to adenosine receptor-mediated mechanisms involved in preconditioning and neuroprotection, our results add the novel finding that the selective block of A3 adenosine receptors during preconditioning facilitates the full recovery of CA1 hippocampal neurotransmission from a 7-min OGD.
This is in line with observations in vivo and in vitro (Abbracchio & Cattabeni, 1999; Von Lubitz, 1999) on the potential role of A3 receptor stimulation in physiological neurotransmission and during ischemic episodes in vivo. In particular, the possibility that one of the effects of A3 receptor stimulation counteracts those of A1 receptors through desensitization of intracellular cross-talk has been suggested (Dunwiddie et al., 1997). Blockade of A3 receptors would conceivably allow full development of A1 receptor-dependent favorable effects. On the other hand, it is possible that A3 receptor stimulation produces cellular negative effects that are completely independent of those monitored through electrophysiological methods, but that limit the expression of the cellular adaptive mechanisms, peculiar to preconditioning. The antagonism of these effects, limiting full expression of preconditioning favorable mechanisms, would result in enhanced recovery of neurotransmission. Finally, from our results it appears that A2A receptors do not play any role in the mechanisms involved in preconditioning, at least relative to immediate protection of neurotransmission. It should be noted, however, that their stimulation has been involved as a negative factor contributing to neuronal cell death following brain ischemia in vivo (Melani et al., 2003) and may play a considerable role in triggering/sustaining deleterious cellular mechanisms that are not manifest in the early phase of cell damage.
In conclusion, the relationship between the increased tolerance of neurotransmission to ischemic episodes produced by preconditioning in vitro and the mechanisms of cell survival and neuroprotection in vivo remains to be established. However, the present results demonstrate the existence of several adenosine-dependent adaptive mechanisms responsible for the increase in tolerance to ischemic episodes, which can be activated and studied using preconditioning protocols in hippocampal tissue in vitro.
Acknowledgments
This work was supported by the University of Florence (MURST ex 60% and COFIN, 2001) and by grants from the Ente Cassa di Risparmio Firenze (ECRF).
Abbreviations
- aCSF
artificial cerebral spinal fluid
- DMSO
dimethylsulfoxide
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- fepsp
field excitatory postsynaptic potential
- MRS 1523
3-propyl-6-ethyl-5[ethyl(thio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate
- OGD
oxygen glucose deprivation
- OD
oxygen deprivation
- ZM 241385
4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
References
- ABBRACCHIO M.P., CATTABENI F. Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann. N.Y. Acad. Sci. 1999;890:79–92. doi: 10.1111/j.1749-6632.1999.tb07983.x. [DOI] [PubMed] [Google Scholar]
- AKETA S., NAKASE H., KAMADA Y., HIRAMATSU K., SAKAKI T. Chemical preconditioning with 3-nitropropionic acid in gerbil hippocampal slices: therapeutic window and the participation of adenosine receptor. Exp. Neural. 2000;166:385–391. doi: 10.1006/exnr.2000.7507. [DOI] [PubMed] [Google Scholar]
- ALICI K., WEBER-LUXENBURGER G., HEINEMANN U. Effects of glucose deprivation in area CA1 of hippocampal slices from adult and juvenile rats. Brain Res. Dev. Brain Res. 1998;107:71–80. doi: 10.1016/s0165-3806(97)00222-8. [DOI] [PubMed] [Google Scholar]
- BLONDEAU N., PLAMONDON H., RICHELME C., HEURTEAUX C., LAZDUNSKI M. K(ATP) channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience. 2000;100:465–474. doi: 10.1016/s0306-4522(00)00304-3. [DOI] [PubMed] [Google Scholar]
- BRAND A., VISSIENNON Z., ESCHKE D., NIEBER K. Adenosine A(1) and A(3) receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- CALABRESI P., CENTONE D., PISANI A., BERNARDI G. Endogenous adenosine mediates the presynaptic inhibition induced by aglycemia at corticostriatal synapses. J. Neurosci. 1997;17:4509–4516. doi: 10.1523/JNEUROSCI.17-12-04509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRADETTI R., MORONI F., PASSANI M.B., PEPEU G. 8-Phenyltheophylline potentiates the electrical activity evoked in hippocampal slices. Eur. J. Pharmacol. 1984;103:177–180. doi: 10.1016/0014-2999(84)90208-5. [DOI] [PubMed] [Google Scholar]
- DAWSON V.L., DAWSON T.M. Neuronal ischaemic preconditioning. Trends Pharmacol. Sci. 2000;21:423–424. doi: 10.1016/s0165-6147(00)01560-1. [DOI] [PubMed] [Google Scholar]
- DIRNAGL U., IADECOLA C., MOSKOWITZ M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- DOOLETTE D.J., KERR D.I. Hyperexcitability in CA1 of the rat hippocampal slice following hypoxia or adenosine. Brain Res. 1995;677:127–137. doi: 10.1016/0006-8993(95)00139-h. [DOI] [PubMed] [Google Scholar]
- DUNWIDDIE T.V., DIAO L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J. Pharmacol. Exp. Ther. 1994;268:537–545. [PubMed] [Google Scholar]
- DUNWIDDIE T.V., DIAO L., KIM H.O., JIANG J.L., JACOBSON K.A. Activation of hippocampal adenosine A3 receptors produces a desensitization of A1 receptor-mediated responses in rat hippocampus. J. Neurosci. 1997;17:607–614. doi: 10.1523/JNEUROSCI.17-02-00607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER J.C. Adenosine antagonists alter the synaptic response to in vitro ischemia in the rat hippocampus. Brain Res. 1990;509:331–334. doi: 10.1016/0006-8993(90)90560-x. [DOI] [PubMed] [Google Scholar]
- FOWLER J.C. Escape from inhibition of synaptic transmission during in vitro hypoxia and hypoglycemia in the hippocampus. Brain Res. 1992;573:169–173. doi: 10.1016/0006-8993(92)90128-v. [DOI] [PubMed] [Google Scholar]
- FOWLER J.C. Glucose deprivation results in a lactate preventable increase in adenosine and depression of synaptic transmission in rat hippocampal slices. J. Neurochem. 1994;60:572–576. doi: 10.1111/j.1471-4159.1993.tb03187.x. [DOI] [PubMed] [Google Scholar]
- FURLING D., GHRIBI O., LAHSAINI A., MIRAULT M.E., MASSICOTTE G. Impairment of synaptic transmission by transient hypoxia in hippocampal slices: improved recovery in glutathione peroxidase transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4351–4356. doi: 10.1073/pnas.060574597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIOVANNINI M.G., BLITZER R.D., WONG T., ASOMA K., TSOKAS P., MORRISON J.H., IYENGAR R., LANDAU E.M. Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. Neuroscience. 2001;21:7053–7062. doi: 10.1523/JNEUROSCI.21-18-07053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIBKOFF V.K., BAUMAN L.A., VANDERMAELEN C.P. The adenosine antagonist 8-cyclopentyltheophylline reduces the depression of hippocampal neuronal responses during hypoxia. Brain Res. 1990;512:353–357. doi: 10.1016/0006-8993(90)90648-U. [DOI] [PubMed] [Google Scholar]
- HEURTEAUX C., LAURITZEN I., WIDMANN C., LAZDUNSKI M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc. Natl. Acad. Sci. U.S.A. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAIDE T., KATSURA K., MURAMATSU H., ASANO G., KATAYAMA Y. Adenosine receptor antagonists cancelled the ischemic tolerance phenomenon in gerbil. Brain Res. 2001;910:94–98. doi: 10.1016/s0006-8993(01)02647-6. [DOI] [PubMed] [Google Scholar]
- ISHIDA T., YARIMIZU K., GUTE D.C., KORTHUIS R.J. Mechanisms of ischemic preconditioning. Shock. 1997;8:86–94. doi: 10.1097/00024382-199708000-00003. [DOI] [PubMed] [Google Scholar]
- KATO H., LIU Y., ARAKI T., KOGURE K. Temporal profile of the effects of pretreatment with brief cerebral ischemia on the neuronal damage following secondary ischemic insult in the gerbil: cumulative damage and protective effects. Brain Res. 1991;553:238–242. doi: 10.1016/0006-8993(91)90831-f. [DOI] [PubMed] [Google Scholar]
- KHASPEKOV L., SHAMLOO M., VICTOROV I., WIELOCH T. Sublethal in vitro glucose–oxygen deprivation protects cultured hippocampal neurons against a subsequent severe insult. Neuroreport. 1998;9:1273–1276. doi: 10.1097/00001756-199805110-00003. [DOI] [PubMed] [Google Scholar]
- KIRINO T., TSUJITA Y., TAMURA A. Induced tolerance to ischemia in gerbil hippocampal neurons. J. Cereb. Blood Flow Metab. 1991;11:299–307. doi: 10.1038/jcbfm.1991.62. [DOI] [PubMed] [Google Scholar]
- KITAGAWA K., MATSUMOTO M., TAGAYA M., HATA R., UEDA H., NIINOBE M., HANDA N., FUKUNAGA R., KIMURA K., MIKOSHIBA K. Ischemic tolerance phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- KLONER R.A., JENNINGS R.B. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–3167. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- LATINI S., BORDONI F., CORRADETTI R., PEPEU G., PEDATA F. Temporal correlation between adenosine outflow and synaptic potential inhibition in rat hippocampal slices during ischemia-like conditions. Brain Res. 1998;794:325–328. doi: 10.1016/s0006-8993(98)00304-7. [DOI] [PubMed] [Google Scholar]
- LATINI S., BORDONI F., PEDATA F., CORRADETTI R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br. J. Pharmacol. 1999a;127:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATINI S., BORDONI F., CORRADETTI R., PEPEU G., PEDATA F. Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices. Br. J. Pharmacol. 1999b;128:1035–1044. doi: 10.1038/sj.bjp.0702888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATINI S., PUGLIESE A.M., CORRADETTI R., PEDATA F. A model of in ‘vitro' preconditioning in the rat hippocampus. Soc. Neurosci. Abstr. 2001;208:16. [Google Scholar]
- LI A.H., MORO S., MELMAN N., JI X.D., JACOBSON K.A. Structure–activity relationships and molecular modeling of 3,5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J. Med. Chem. 1998;41:3186–3201. doi: 10.1021/jm980093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y., KATO H., NAKATA N., KOGURE K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain Res. 1992;586:121–124. doi: 10.1016/0006-8993(92)91380-w. [DOI] [PubMed] [Google Scholar]
- LUHMANN H.J., HEINEMANN U. Hypoxia-induced functional alterations in adult rat neocortex. J. Neurophysiol. 1992;67:798–811. doi: 10.1152/jn.1992.67.4.798. [DOI] [PubMed] [Google Scholar]
- MELANI A., PANTONI L., BORDONI F., GIANFRIDDO M., BIANCHI L., VANNUCCHI M.G., BERTORELLI R., MONOPOLI A., PEDATA F. The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959:243–250. doi: 10.1016/s0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- MILLER B.A., PEREZ R.S., SHAH A.R., GONZALES E.R., PARK T.S., GIDDAY J.M. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia–reperfusion. Neuroreport. 2001;2:1663–1669. doi: 10.1097/00001756-200106130-00030. [DOI] [PubMed] [Google Scholar]
- MUSLEH W., BRUCE A., MALFROY B., BAUDRY M. Effects of EUK-8, a synthetic catalytic superoxide scavenger, on hypoxia- and acidosis-induced damage in hippocampal slices. Neuropharmacology. 1994;33:929–934. doi: 10.1016/0028-3908(94)90191-0. [DOI] [PubMed] [Google Scholar]
- MURRY C.E., JENNINGS R.B., REIMER K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- NAKAMURA M., NAGAKIMURA K., MATSUMOTO M., SAKABE T. Rapid tolerance to focal cerebral ischemia in rats is attenuated by adenosine A1 receptor antagonist. J. Cereb. Blood Flow Metab. 2002;22:161–170. doi: 10.1097/00004647-200202000-00004. [DOI] [PubMed] [Google Scholar]
- PASCHEN W., MIES G. Effect of induced tolerance on biochemical disturbances in hippocampal slices from the gerbil during and after oxygen/glucose deprivation. Neuroreport. 1999;10:1417–1421. doi: 10.1097/00001756-199905140-00006. [DOI] [PubMed] [Google Scholar]
- PEARSON T., NURITOVA F., CALDWELL D., DALE N., FRENGUELLI B.G. A depletable pool of adenosine in area CA1 of the rat hippocampus. J. Neurosci. 2001;21:2298–2307. doi: 10.1523/JNEUROSCI.21-07-02298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEDATA F., CORSI C., MELANI A., BORDONI F., LATINI S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann. N.Y. Acad. Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- PEDATA F., LATINI S., PUGLIESE A.M., PEPEU G. Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J. Neurochem. 1993;61:284–289. doi: 10.1111/j.1471-4159.1993.tb03566.x. [DOI] [PubMed] [Google Scholar]
- PEREZ-PINZON M.A., MUMFORD P.L., ROSENTHAL M., SICK T.J. Anoxic preconditioning in hippocampal slices: role of adenosine. Neuroscience. 1996;75:687–694. doi: 10.1016/0306-4522(96)00311-9. [DOI] [PubMed] [Google Scholar]
- PEREZ-PINZON M.A., BORN J.G. Rapid preconditioning neuroprotection following anoxia in hippocampal slices: role of the K+ ATP channel and protein kinase C. Neuroscience. 1999;89:453–459. doi: 10.1016/s0306-4522(98)00560-0. [DOI] [PubMed] [Google Scholar]
- PLASCHKE K., WEIGAND M.A., MICHEL A., MARTIN E., BARDENHEUER H.J. Permanent cerebral hypoperfusion: ‘preconditioning-like' effects on rat energy metabolism towards acute systemic hypotension. Brain Res. 2000;858:363–370. doi: 10.1016/s0006-8993(00)01950-8. [DOI] [PubMed] [Google Scholar]
- PRINGLE A.K., THOMAS S.J., SIGNORELLI F., IANNOTTI F. Ischaemic preconditioning in organotypic hippocampal slice cultures is inversely correlated to the induction of the 72 kDa heat shock protein (HSP72) Brain Res. 1999;845:152–164. doi: 10.1016/s0006-8993(99)01916-2. [DOI] [PubMed] [Google Scholar]
- RAVAL A.P., DAVE K.R., MOCHLY-ROSEN D., SICK T.J., PEREZ-PINZON M.A. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J. Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHURR A., REID K.H., TSENG M.T., WEST C., RIGOR B.M. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res. 1986;374:244–248. doi: 10.1016/0006-8993(86)90418-x. [DOI] [PubMed] [Google Scholar]
- SCHURR A., RIGOR B.M. Cerebral ischemia revisited: new insights as revealed using in vitro brain slice preparations. Experientia. 1989;45:684–695. doi: 10.1007/BF01974560. [DOI] [PubMed] [Google Scholar]
- SCHURR A., PAYNE R.S., MILLER J.J., RIGOR B.M. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res. 1997;744:105–111. doi: 10.1016/s0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- SICK T.J., SOLOW E.L., ROBERTS E.L. Extracellular potassium ion activity and electrophysiology in the hippocampal slice: paradoxical recovery of synaptic transmission during anoxia. Brain Res. 1987;418:227–234. doi: 10.1016/0006-8993(87)90090-4. [DOI] [PubMed] [Google Scholar]
- STONE T.W. Effects of clomethiazole on spreading depression in the rat hippocampal slice. Eur. J. Pharmacol. 2000;399:29–34. doi: 10.1016/s0014-2999(00)00331-9. [DOI] [PubMed] [Google Scholar]
- TIAN G.F., BAKER A.J. Protective effect of high glucose against ischemia-induced synaptic transmission damage in rat hippocampal slices. J. Neurophysiol. 2002;88:236–248. doi: 10.1152/jn.00572.2001. [DOI] [PubMed] [Google Scholar]
- TOKUNAGA H., HIRAMATSU K., SAKAKI T. Effect of preceding in vivo sublethal ischemia on the evoked potentials during secondary in vitro hypoxia evaluated with gerbil hippocampal slices. Brain Res. 1998;784:316–320. doi: 10.1016/s0006-8993(97)01237-7. [DOI] [PubMed] [Google Scholar]
- VON LUBITZ D.K. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept. Eur. J. Pharmacol. 1999;365:9–25. doi: 10.1016/s0014-2999(98)00788-2. [DOI] [PubMed] [Google Scholar]
- ZHU P.J., KRNJEVIC K. Persistent block of CA1 synaptic function by prolonged hypoxia. Neuroscience. 1999;90:759–770. doi: 10.1016/s0306-4522(98)00495-3. [DOI] [PubMed] [Google Scholar]