Abstract
We studied whether cannabinoid CB1 receptor gene disruption (to yield CB1−/− mice) affects the electrically evoked tritium overflow from vas deferens and atrial pieces preincubated with [3H]-noradrenaline (NA) (‘noradrenaline release') and from cerebral cortex slices preincubated with [3H]-choline (‘acetylcholine release').
NA release was higher by 37% in vas deferens from CB1−/− mice than in vas deferens from CB1+/+ mice. The cannabinoid receptor agonist WIN 55,212-2 inhibited, and the CB1 receptor inverse agonist/antagonist SR 141716, increased NA release in vas deferens from CB1+/+ mice without affecting it in vas deferens from CB1−/− mice.
Atrial NA release did not differ between CB1+/+ and CB1−/− mice nor did WIN 55,212-2 affect NA release in either strain.
Cortical acetylcholine (Ach) release did not differ between CB1+/+ and CB1−/− mice. WIN 55,212-2 inhibited, but SR 141716 did not affect, Ach release in the cortex from CB1+/+ mice. Both drugs did not alter Ach release in the cortex from CB1−/− mice.
Tritium content did not differ between CB1+/+ and CB1−/− mice in any preparation.
In conclusion, the increase in NA release associated with CB1 receptor deficiency in the vas deferens, which cannot be ascribed to an alteration of tritium content of the preparations, suggests an endogenous tone at the CB1 receptors of CB1+/+ mice in this tissue. Furthermore, the effect of WIN 55,212-2 on NA release in the vas deferens and on cortical Ach release involves CB1 receptors, whereas the involvement of non-CB1–non-CB2 receptors can be excluded.
Keywords: Cerebral cortex, vas deferens, atrium, acetylcholine release, noradrenaline release, cannabinoid CB1 receptors, CB1 receptor-deficient mouse, presynaptic receptors, SR 141716
Introduction
Cannabinoid CB1 receptors serve as presynaptic inhibitory receptors on a variety of central and peripheral neurones (for review, see Schlicker & Kathmann, 2001; Howlett et al., 2002). These receptors are activated by endocannabinoids like anandamide, 2-arachidonoylglycerol, noladin ether and virodhamine (Hanus̆ et al., 2001; Porter & Felder, 2001; Porter et al., 2002). Many types of presynaptic CB1 receptors appear to be subject to an endogenous tone as suggested by the fact that CB1 receptor inverse agonists/antagonists like SR 141716 facilitate the release of the respective neurotransmitter (for review, see Pertwee, 1999; Schlicker & Kathmann, 2001). It is of interest in this context whether lack of CB1 receptors will lead to the same result.
We have indeed found recently (Kathmann et al., 2001b) that in hippocampal slices, in which SR 141716 increases acetylcholine (Ach) release (Kathmann et al., 2001a), the release of this transmitter was also increased by CB1 receptor deficiency (CB1 receptor knockout mouse (CB1−/−) generated by Zimmer et al. (1999) from C57BL/6J mice). This alteration in transmitter release is very specific inasmuch as hippocampal noradrenaline (NA) release and striatal Ach release (both of which are not subject to modulation via CB1 receptors; Schlicker et al., 1997; Kathmann et al., 2001a) did not differ between both strains (Kathmann et al., 2001b). The aim of the present study was to examine whether parallel effects of SR 141716 and of CB1 receptor deficiency also occur in other isolated tissues of the mouse. For this purpose, we determined the influence of CB1 receptor gene disruption on NA release in the vas deferens and in the atrium. NA release in the vas deferens is inhibited via presynaptic CB1 receptors subject to an endogenous tone (Rinaldi-Carmona et al., 1994; Pertwee et al., 1996), whereas atrial NA release is not affected by presynaptic CB1 receptors at all (Trendelenburg et al., 2000). Our study was extended to cerebral cortex slices in which Ach release was determined. This experimental model differs from the latter two and from the models considered in our previous study (Kathmann et al., 2001b) in that the release of the transmitter is inhibited via presynaptic CB1 receptors, which are, however, not subject to an endogenous tone, that is, Ach release is not facilitated by SR 141716 (Kathmann et al., 2001a).
Methods
C57BL/6J mice with disrupted CB1 receptor gene (CB1−/− mice) were obtained from A. Zimmer (Klinik für Psychiatrie und Psychotherapie, Rheinische Friedrich-Wilhelms-Universität, Bonn, Germany) and C57BL/6J mice of the wild-type strain (CB1+/+ mice) were purchased from Jackson (Bar Harbor, ME, U.S.A.). Animals were bred in the animal facilities of our department where they were housed in a temperature- and humidity-controlled environment under a 12-h dark–light cycle with food and water available ad libitum. The following tissues were prepared from 2- to 4-month-old animals: pieces from the vas deferens and the atrium and slices (0.3 mm thick, 3 mm diameter) from the cerebral cortex. Pieces from the atrium and cerebral cortex slices were obtained from animals of either sex. The preparations were incubated (37°C) for 30 min with a physiological salt solution (PSS) containing [3H]-choline 0.1 μM or [3H]-NA 0.025 μM (see below). The PSS was composed as follows (mM): NaCl 118, KCl 4.8, NaHCO3 25, KH2PO4 1.2, MgSO2 1.2, CaCl2 1.3 (if not stated otherwise), glucose 10, ascorbic acid 0.06, Na2EDTA 0.03; it was aerated with 95% O2 and 5% CO2.
The preparations were then superfused at 1 ml min−1 (37°C; 110 min) and the superfusate was collected in 5-min samples; the CaCl2 concentration in the superfusion fluid was 3.25 mM. The vas deferens and atrium pieces preincubated with [3H]-NA were superfused with PSS containing desipramine 1 μM and rauwolscine 1 μM. Cortical slices preincubated with [3H]-choline were superfused with PSS containing hemicholinium-3 10 μM. (Desipramine and rauwolscine were used for the blockade of the neuronal NA transporter and the presynaptic α2-adrenoceptor, respectively, whereas hemicholinium-3 was used to block the high-affinity choline uptake.) Tritium overflow was evoked by two 2-min periods of electrical field stimulation after 40 and 90 min (S1, S2); the stimulation parameters were 3 Hz, 200 mA, and 2 ms. In each of the three preparations and regardless of the mouse strain, addition of tetrodotoxin 1 μM or omission of Ca2+ ions (from 62 min of superfusion onward) inhibited the electrically evoked tritium overflow by 88% or more (results not shown), suggesting that the electrically evoked tritium overflow represents quasi-physiological release of NA or Ach, as appropriate.
At the end of superfusion, the tritium remaining in a given preparation and the tritium of all superfusion samples collected from this preparation were added up to allow for determination of the tritium content at any time of the superfusion experiment. Tritium efflux was calculated as the fraction of the tritium content in the preparations at the beginning of the respective collection period (fractional rate of tritium efflux). To quantify basal tritium efflux, the fractional rates in the 5-min collection period from 85 to 90 min were determined (t2). The electrically evoked tritium overflow was calculated by subtraction of basal from total efflux during stimulation and the subsequent 13 min and expressed as percent of the tritium present in the preparation at the onset of stimulation (basal tritium efflux was assumed to decline linearly from the 5-min collection period before to that 15–20 min after the onset of stimulation). To quantify the stimulated tritium overflow, the tritium overflow evoked by S1 or the ratio of the overflow evoked by S2 over that evoked by S1 was determined, as explained in the Results section. As a measure for tritium accumulation, the tritium remaining in the preparation at the end of the superfusion was determined.
Statistics
Results are given as mean±s.e.m. of n experiments. For the t2 and S1 values and the tritium content (Figure 1, Table 1 and text), n refers to the number of mice; for the S2/S1 values (Figures 2 and 3), n is higher than the number of mice since in some instances two values were obtained from the same animal. The Student's t-test was used for comparison of mean values; the Bonferroni correction was used when two or more values were compared to the same control.
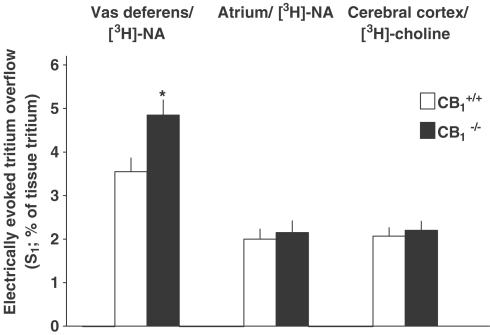
Figure 1.
Electrically (3 Hz) evoked tritium overflow from superfused vas deferens, atrium and cerebral cortex preparations from CB1+/+ and CB1−/− mice. Vas deferens and atrium pieces preincubated with [3H]-NA were superfused with a medium containing desipramine 1 μM and rauwolscine 1 μM. Cortex slices preincubated with [3H]-choline were superfused with a medium containing hemicholinium-3 10 μM. Tritium overflow was evoked after 40 min (S1) of superfusion (and again after 90 min, not shown). Mean±s.e.m. of 25 experiments (cerebral cortex) and 12–13 experiments (vas deferens, atrium). *P<0.05, compared to the corresponding value from CB1+/+ mice.
Table 1.
Tritium content in vas deferens and atrium pieces (preincubated with [3H]-noradrenaline) and in cerebral cortex slices (preincubated with [3H]-choline) from CB1+/+ and CB1−/− mice
| Tritium content (nCi) | ||||
|---|---|---|---|---|
| Tissue | Preincubation with | n | CB1+/+ | CB1−/− |
| Vas deferens | [3H]-NA | 16 | 6.5±0.8 | 6.8±0.6 |
| Atrium | [3H]-NA | 13 | 5.8±1.1 | 5.2±1.1 |
| Cerebral cortex | [3H]-choline | 26 | 46.8±4.1 | 46.0±3.4 |
Tissues were superfused for 110 min with a medium containing desipramine 1 μM plus rauwolscine 1 μM (tissues preincubated with [3H]-NA) or hemicholinium-3 10 μM (tissues preincubated with [3H]-choline). The results represent mean±s.e.m. of the tritium content present in the preparations after completion of the experiments.
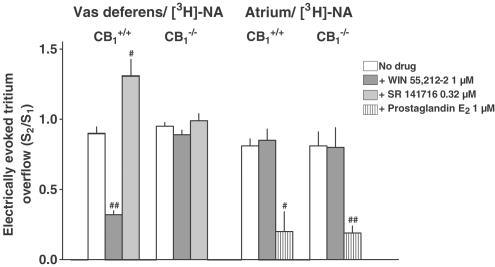
Figure 2.
Effect of cannabinoid receptor ligands and of prostaglandin E2 on the electrically (3 Hz) evoked tritium overflow from superfused vas deferens and atrium pieces from CB1+/+ and CB1−/− mice. The preparations were preincubated with [3H]-NA. Tritium overflow was evoked twice, after 40 min (S1) and 90 min (S2) of superfusion, and the ratio of the overflow evoked by S2 over that evoked by S1 was determined. Desipramine 1 μM and rauwolscine 1 μM were present in the medium throughout superfusion, whereas the cannabinoid receptor agonist WIN 55,212-2, the CB1 receptor inverse agonist/antagonist SR 141716 or prostaglandin E2 was added from 28 min before S2 onward. Mean±s.e.m. of 5–16 experiments. #P<0.05, # #P<0.001, compared to the corresponding control.
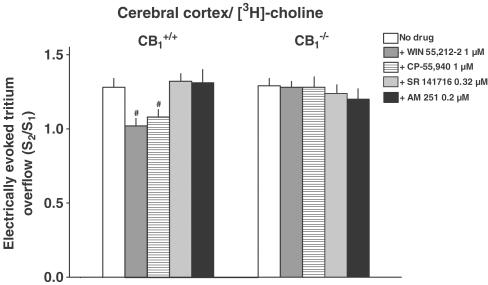
Figure 3.
Effect of cannabinoid receptor ligands on the electrically (3 Hz) evoked tritium overflow from superfused cerebral cortex slices from CB1+/+ and CB1−/− mice. Slices were preincubated with [3H]-choline. Tritium overflow was evoked twice, after 40 min (S1) and 90 min (S2) of superfusion, and the ratio of the overflow evoked by S2 over that evoked by S1 was determined. Hemicholinium-3 10 μM was present in the medium throughout superfusion, whereas a cannabinoid receptor agonist (WIN 55,212-2, CP-55,940) or a CB1 receptor inverse agonist/antagonist (SR 141716, AM 251) was added from 28 min before S2 onward. Mean±s.e.m. of 5–20 experiments. #P<0.05, compared to the corresponding control.
Drugs used
The following drugs were used: [methyl-3H]-choline chloride (specific activity 75–86 Ci mmol−1); R-(-)-[ring-2, 5, 6-3H]-noradrenaline (specific activity 51.8–56.3 Ci mmol−1) (NEN, Zaventem, Belgium); AM 251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide); CP-55,940 ((-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol) (Toc-ris/Biotrend, Köln, Germany); desipramine hydrochloride (Ciba-Geigy, Wehr, Germany); hemicholinium-3 (ChemCon, Freiburg, Germany); prostaglandin E2 (Sigma, München, Germany); rauwolscine hydrochloride (Roth, Karlsruhe, Germany); SR 141716 (N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide; Sanofi, Montpellier, France); tetrodotoxin (ICN, Eschwege, Germany or Roth, Karlsruhe, Germany); WIN 55,212-2 (R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo-[1,2,3-de]-1,4-benzoxazin-yl](1-naphthalenyl)methanone mesylate; RBI/Sigma, München, Germany). Drugs were dissolved in dimethylsulfoxide (DMSO) (AM 251, CP-55,940, SR 141716 and WIN 55,212-2), ethanol (prostaglandin E2), citrate buffer (0.1 mM, pH 4.8; tetrodotoxin) or water (other drugs) and diluted with PSS to obtain the concentration required. Diluted DMSO, ethanol and citrate buffer did not affect basal or evoked tritium overflow by themselves.
Results
Basal tritium efflux was expressed as a fractional rate of tritium efflux in the collection period from 85 to 90 min of superfusion (t2; i.e., in the 5-min period preceding the second stimulation). The t2 value in vas deferens and atrium pieces and in cortical slices from CB1+/+ mice was 0.0038±0.0004 (n=15), 0.0056±0.0003 (n=13) and 0.0021±0.0002 min−1 (n=26), respectively. Basal efflux was not affected by the strain (CB1+/+ vs CB1−/−) and by the drugs under study (AM 251, CP-55,940, prostaglandin E2, SR 141716, WIN 55,212-2; results not shown).
In the first series of experiments, we examined how the lack of the CB1 receptor affects the electrically evoked tritium overflow in pieces of the vas deferens and the atrium and in cortical slices. Tritium overflow from superfused tissues was evoked twice, after 40 and 90 min of superfusion (S1, S2), and the amount of tritium overflow evoked by S1 (expressed as percent of tissue tritium) was determined. Tritium overflow in vas deferens pieces (preincubated with [3H]-NA) from CB1−/− mice exceeded that in vas deferens pieces from wild-type mice by 37% (Figure 1). On the other hand, both strains did not differ with respect to the evoked overflow from atrial pieces preincubated with [3H]-NA and from cortical slices preincubated with [3H]-choline (Figure 1).
In the second series, the influence of CB1 receptor deficiency on the effects of various drugs on the evoked overflow was examined. The drugs were added to the medium before and during S2 and the ratio of the overflow evoked by S2 over that evoked by S1 was determined. In vas deferens pieces preincubated with [3H]-NA, the evoked overflow (S2/S1) was inhibited by the cannabinoid receptor agonist WIN 55,212-2 by 64% and facilitated by the CB1 receptor inverse agonist/antagonist SR 141716 by 45% in tissues from CB1+/+ mice, but was not affected in pieces from CB1−/− mice, (Figure 2). In atrial pieces (preincubated with [3H]-NA) from either strain, WIN 55,212-2 failed to affect the evoked tritium overflow whereas prostaglandin E2 caused an inhibition by about 75% (Figure 2). With respect to the third model, that is, cortical slices preincubated with [3H]-choline, WIN 55,212 and another cannabinoid receptor agonist, CP-55,940, inhibited the evoked overflow by about 15–20% in slices from CB1+/+ mice, but failed to do so in slices from CB1−/− mice (Figure 3). SR 141716 and another CB1 receptor inverse agonist/antagonist, AM 251, did not affect the evoked overflow in slices from either strain (Figure 3).
In the third series, we examined whether the lack of CB1 receptors affects the tritium content determined at the end of superfusion. However, for each of the three experimental models the tritium content did not differ between preparations obtained from either mouse strain (Table 1).
Discussion
The aim of the present study was to examine how CB1 receptor deficiency affects transmitter release in three isolated tissues differing qualitatively and quantitatively with respect to modulation of transmitter release by presynaptic CB1 receptors. In the first model, that is, the vas deferens, NA release is inhibited via presynaptic CB1 receptors, as suggested by studies in which transmitter release was determined directly (Trendelenburg et al., 2000) or via the endorgan response (electrically induced twitch response; Rinaldi-Carmona et al., 1994; Pertwee et al., 1996). The presynaptic CB1 receptors in this tissue are subject to an endogenous tone since the CB1 receptor inverse agonist/antagonist SR 141716 increases NA release (present study) or the electrically induced twitch response (Pertwee et al., 1996). The stimulatory effect of SR 141716 may be related to its antagonistic effect against endogenously formed endocannabinoids. Evidence that endocannabinoids can be formed and degraded in vas deferens tissue has been presented recently (Ates et al., 2003). Since SR 141716 is an inverse CB1 receptor agonist, another explanation for the stimulatory effect is that part of the CB1 receptors is precoupled.
In vas deferens pieces from CB1 receptor knockout mice, the cannabinoid receptor agonist WIN 55,212-2 and the CB1 receptor inverse agonist/antagonist SR 141716 failed to affect NA release, confirming that the effects in wild-type animals are indeed related to CB1 receptors. These results, in addition, exclude the involvement of a non-CB1–non-CB2 receptor identified in the hippocampus of CB1 receptor knockout mice, which is activated by WIN 55,212-2 and blocked by SR 141716 (Hájos et al., 2001). The fact that NA release is higher in the vas deferens from knockout mice when compared to wild-type animals (Table 2) is an additional argument that the presynaptic CB1 receptors are subject to an endogenous tone. The possibility that the difference in NA release between both strains is related to an alteration of the neuronal NA transporter, or the presynaptic α2-autoreceptor can be excluded since both mechanisms were routinely blocked by desipramine and rauwolscine, respectively. Furthermore, the possibility has to be considered that the increase in NA release in the vas deferens from CB1 receptor-deficient mice is related to a higher density of the noradrenergic nerve terminals. However, the manner of calculation of the release (S1) in our experiments as the ratio of the electrically evoked tritium overflow over the tritium content of the preparations a priori excludes this possibility. Nonetheless, it was of interest to examine whether the alteration in release might be accompanied by an alteration of the tritium content of the preparations. However, the amount of tritium present in the preparations at the end of superfusion did not differ between wild-type and knockout mice.
Table 2.
Effects of cannabinoid receptor ligands and of cannabinoid CB1 receptor deficiency on transmitter release in superfused tissues from C57BL/6J mice
| Transmitter | Tissue | Effects of cannabinoid receptor ligands on transmitter release in wild-type mice (CB1+/+) | Effect of CB1 receptor deficiency on transmitter release (CB1−/− vs CB1+/+) | Source | |
|---|---|---|---|---|---|
| WIN 55,212-2 1 μM | SR 141716 0.32 μM | ||||
| NA | Vas deferens | ↓ | ↑ | ↑ | Present study |
| NA | Atrium | 0 | ND | 0 | Present study |
| NA | Hippocampus | 0 | 0 | 0 | Kathmann et al. (2001b) |
| Ach | Hippocampus | ↓ | ↑ | ↑ | Kathmann et al. (2001b) |
| Ach | Cerebral cortex | (↓) | 0 | 0 | Present study |
| Ach | Striatum | 0 | 0 | 0 | Kathmann et al. (2001b) |
Ach, acetylcholine; NA, noradrenaline; ND, not determined. The symbols ↓, (↓) and ↑ indicate marked decrease, slight decrease and marked increase.
The possibility that the increase in NA release in the vas deferens from CB1 receptor-deficient mice may be a more general phenomenon also has to be considered (e.g., in sympathetically innervated tissues). To exclude this possibility, we used our second model, that is, atrial NA release. Transmitter release in this tissue is not inhibited via CB1 receptors in NMRI (Trendelenburg et al., 2000) and C57BL/6J mice (present study). CB1+/+ and CB1−/− mice did not differ with respect to atrial NA release (Table 2), arguing against the view that CB1 receptor deficiency generally leads to an increase in transmitter release. The latter conclusion was also reached previously since hippocampal NA release and striatal Ach release, both of which are not modulated by CB1 receptors, did not differ between CB1+/+ and CB1−/− mice (Table 2; Kathmann et al., 2001b). In order to prove that atrial NA release can be modified via presynaptic inhibitory receptors under the experimental conditions of the present study, we used prostaglandin E2, which caused a strong inhibition in wild-type and knockout mice. A direct inhibitory effect of prostaglandin E2 on NA release in the mouse atrium has so far not been shown. However, the fact that diclofenac (an inhibitor of prostaglandin synthesis) increases the facilitatory effect of bradykinin on atrial NA release is an indirect evidence that prostaglandins are capable of inhibiting NA release in the atrium of this species (Chulak et al., 1998).
The vas deferens (NA release) and the hippocampus (Ach release) resemble each other in that in both tissues transmitter release is increased by SR 141716 and by CB1 receptor gene disruption (Table 2). In this context, one might wonder how transmitter release will be affected by CB1 receptor deficiency in a tissue in which transmitter release is inhibited via CB1 receptors, but not facilitated by SR 141716. Our third model, that is, Ach release in the cerebral cortex, is such a tissue. It has previously been found that WIN 55,212-2 inhibits, whereas SR 141716 does not affect, Ach release in the cerebral cortex from NMRI mice (Kathmann et al., 2001a; Steffens et al., 2002) and the same results were obtained in the present study for C57BL/6J mice. To be very sure, we also examined another cannabinoid receptor agonist, CP-55,940, and another CB1 receptor inverse agonist/antagonist, AM 251, which, as expected, inhibited and failed to affect Ach release in the cortex from wild-type mice, respectively. The fact that cortical Ach release did not differ between wild-type and knockout mice (Table 2) suggests that the lack of CB1 receptors leads to an increase in transmitter release only in those tissues in which these receptors are subject to an endogenous tone.
Since the presynaptic CB1 receptors causing inhibition of NA release in the mouse vas deferens are subject to an endogenous tone, the question might arise as to the physiological and/or pathophysiological role played by this receptor. The effects of cannabinoid receptor agonists on sexual functions are not uniform; both aphrodisiac effects and sexual dysfunction have been described in humans and animals (for a review, see Dewey, 1986; Stella, 2001). In a recent study on the sexual behavior of male rats, the cannabinoid receptor agonist HU-210 inhibited the latencies to mounting, intromission and ejaculation (Ferrari et al., 2000). These effects may be related to activation of cannabinoid receptors in the brain, but the possibility should be taken into consideration that the inhibitory effect on ejaculation is partially due to an impairment of NA release from the sympathetic neurones innervating the vas deferens (which is also endowed with presynaptic CB1 receptors in the rat; Christopoulos et al., 2001).
In conclusion, the fact that NA release is higher in vas deferens from CB1 receptor-deficient mice than in vas deferens from wild-type animals is an additional piece of evidence that these presynaptic CB1 receptors are subject to an endogenous tone. This view is supported by our finding that in cortical slices, in which the presynaptic inhibitory CB1 receptors according to findings with SR 141716 are not subject to an endogenous tone, Ach release is not facilitated by CB1 receptor deficiency. The study also shows that the effects of WIN 55,212-2 and/or of SR 141716 on transmitter release in either tissue are related to CB1 receptors and, in particular, excludes the involvement of non-CB1–non-CB2 receptors.
Acknowledgments
We thank Professor A. Zimmer for donation of CB1 receptor knockout mice and the companies Ciba-Geigy and Sanofi for gifts of drugs. The skilled technical assistance of Mrs Doris Petri and Mrs Petra Zeidler is gratefully acknowledged. This study was supported by grants from the Deutsche Forschungsgemeinschaft (GRK 246, Schl 266/5-3 and -/5-4) and from the Land Nordrhein-Westfalen (BONFOR programme).
Abbreviations
- Ach
acetylcholine
- AM 251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- C57BL/6J
mouse strain
- CB1−/−, CB1+/+ mouse
CB1 receptor-deficient and wild-type mouse
- CP-55,940
(–)-cis-3-[2-hydroxy-4-(1, 1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- DMSO
dimethylsulfoxide
- NA
noradrenaline
- NMRI
mouse strain
- PSS
physiological salt solution
- S1, S2, first and second stimulation
respectively
- SR 141716
N-piperidino-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide
- t2
collection period in which basal tritium efflux was determined
- WIN 55,212-2
R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-yl](1-naphthalenyl)methanone
References
- ATES M., HAMZA M., SEIDEL K., KOTALLA C.E., LEDENT C., GÜHRING H. Intrathecally applied flurbiprofen produces an endocannabinoid-dependent antinociception in the rat formalin test. Eur. J. Neurosci. 2003;17:597–604. doi: 10.1046/j.1460-9568.2003.02470.x. [DOI] [PubMed] [Google Scholar]
- CHRISTOPOULOS A., COLES P., LAY L., LEW M.J., ANGUS J.A. Pharmacological analysis of cannabinoid receptor activity in the rat vas deferens. Br. J. Pharmacol. 2001;132:1281–1291. doi: 10.1038/sj.bjp.0703930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHULAK C., COUTURE R., FOUCART S. Modulatory effect of bradykinin on noradrenaline release in isolated atria from normal and B2 knockout transgenic mice. Eur. J. Pharmacol. 1998;346:167–174. doi: 10.1016/s0014-2999(98)00060-0. [DOI] [PubMed] [Google Scholar]
- DEWEY W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- FERRARI F., OTTANI A., GIULIANI D. Inhibitory effects of the cannabinoid agonist HU 210 on rat sexual behaviour. Physiol. Behav. 2000;69:547–554. doi: 10.1016/s0031-9384(00)00214-6. [DOI] [PubMed] [Google Scholar]
- HÁJOS N., LEDENT C., FREUND T.F. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- HANUŠ L., ABU-LAFI S., FRIDE E., BREUER A., VOGEL Z., SHALEV D.E., KUSTANOVICH I., MECHOULAM R. 2-Arachidonoyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- KATHMANN M., WEBER B., SCHLICKER E. Cannabinoid CB1 receptor-mediated inhibition of acetylcholine release in the brain of NMRI, CD-1 and C57BL/6J mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001a;363:50–56. doi: 10.1007/s002100000304. [DOI] [PubMed] [Google Scholar]
- KATHMANN M., WEBER B., ZIMMER A., SCHLICKER E. Enhanced acetylcholine release in the hippocampus of cannabinoid CB1 receptor-deficient mice. Br. J. Pharmacol. 2001b;132:1169–1173. doi: 10.1038/sj.bjp.0703987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., GRIFFIN G., RYAN W., RAZDAN R.K., COMPTON D.R., MARTIN B.R. Agonist–antagonist characterization of 6′-cyanohex-2′-yne-Δ8-tetrahydrocannabinol in two isolated tissue preparations. Eur. J. Pharmacol. 1996;315:195–201. doi: 10.1016/s0014-2999(96)00631-0. [DOI] [PubMed] [Google Scholar]
- PORTER A.C., FELDER C.C. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol. Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- PORTER A.C., SAUER J.M., KNIERMAN M.D., BECKER G.W., BERNA M.J., BAO J., NOMIKOS G.G., CARTER P., BYMASTER F.P., LEESE A.B., FELDER C.C. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HÉAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NÉLIAT G., CAPUT D., FERRARA P., SOUBRIÉ P., BRELIÈRE J.C., LE FUR G. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., KATHMANN M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., TIMM J., ZENTNER J., GÖTHERT M. Cannabinoid CB1 rceptor-mediated inhibition of noradrenaline release in the human and guinea-pig hippocampus. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:583–589. doi: 10.1007/pl00005093. [DOI] [PubMed] [Google Scholar]
- STEFFENS M., KLAR M., SZABO B., ZENTNER J., FEUERSTEIN T.J. Modulation of evoked [3H]-acetylcholine release in neocortical slices of humans and mice through CB1 receptors – evidence of an endogenous tone in humans. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365 Suppl. 1:R23. [Google Scholar]
- STELLA N. How might cannabinoids influence sexual behavior. Proc. Natl. Acad. Sci. 2001;98:793–795. doi: 10.1073/pnas.98.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENDELENBURG A.U., COX S.L., SCHELB V., KLEBROFF W., KHAIRALLAH L., STARKE K. Modulation of 3H-noradrenaline release by presynaptic opioid, cannabinoid and bradykinin receptors and β-adrenoceptors in mouse tissues. Br. J. Pharmacol. 2000;130:321–330. doi: 10.1038/sj.bjp.0703305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMER A., ZIMMER A.M., HOHMANN A.G., HERKENHAM M., BONNER T.J. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad Sci. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]