Abstract
Caspases play a critical role in apoptosis, and are considered to be key targets for the design of cytoprotective drugs. As part of our antiapoptotic drug-discovery effort, we have synthesized and characterized Z-VD-fmk, MX1013, as a potent, irreversible dipeptide caspase inhibitor.
MX1013 inhibits caspases 1, 3, 6, 7, 8, and 9, with IC50 values ranging from 5 to 20 nM. MX1013 is selective for caspases, and is a poor inhibitor of noncaspase proteases, such as cathepsin B, calpain I, or Factor Xa (IC50 values >10 μM).
In several cell culture models of apoptosis, including caspase 3 processing, PARP cleavage, and DNA fragmentation, MX1013 is more active than tetrapeptide- and tripeptide-based caspase inhibitors, and blocked apoptosis at concentrations as low as 0.5 μM.
MX1013 is more aqueous soluble than tripeptide-based caspase inhibitors such as Z-VAD-fmk.
At a dose of 1 mg kg−1 i.v., MX1013 prevented liver damage and the lethality caused by Fas death receptor activation in the anti-Fas mouse-liver apoptosis model, a widely used model of liver failure.
At a dose of 20 mg kg−1 (i.v. bolus) followed by i.v. infusion for 6 or 12 h, MX1013 reduced cortical damage by approximately 50% in a model of brain ischemia/reperfusion injury.
At a dose of 20 mg kg−1 (i.v. bolus) followed by i.v. infusion for 12 h, MX1013 reduced heart damage by approximately 50% in a model of acute myocardial infarction.
Based on these studies, we conclude that MX1013, a dipeptide pan-caspase inhibitor, has a good combination of in vitro and in vivo properties. It has the ability to protect cells from a variety of apoptotic insults, and is systemically active in three animal models of apoptosis, including brain ischemia.
Keywords: Apoptosis, brain ischemia/stroke, caspase inhibitor, cytoprotective, liver failure, myocardial infarction
Introduction
Apoptosis is a highly regulated process involving the systematic disassembly and death of cells. The ability to undergo apoptosis appears to be a general property of metazoan cells, and the essential features of the process are conserved from worms to man (Aravind et al., 1999). Apoptosis is a normal part of tissue homeostasis during embryonic and post-embryonic development, and also plays an important role in the remodeling of adult tissues. However, as with any complex physiological pathway, defects in apoptosis signaling, including inappropriate apoptosis induction or failure of apoptosis induction when required for normal growth, can cause disease (Thompson, 1995).
The basic molecular framework underlying apoptosis is reasonably well established. It comprises a complex system of signal transduction pathways (Zimmermann & Green, 2001) consisting of cell surface and intracellular death receptors and their ligands; a network of protein–protein interactions extending from the plasma membrane to the nucleus; the participation of organelles, such as mitochondria, which act as both intracellular stress sensors and a source of amplifying apoptotic signals; and the activation of caspases, a group of cysteine proteases. The caspases are a family of structurally related cysteine proteases, which mediate an array of death-promoting proteolytic reactions within the cell and are considered to play a pivotal role in the apoptotic program (Thornberry & Lazebnik, 1998). These enzymes are responsible for both the induction of apoptosis signaling (carried out by initiator caspases, such as caspase 8), as well as the myriad phenotypic changes that characterize cell death (carried out by effector caspases such as caspase 3). More importantly, the activation of the caspase proenzymes is largely controlled by the caspases themselves, either by the proteolytic action of one family member on another or through autocatalytic activation via an induced proximity mechanism (Salvesen & Dixit, 1999). The result is a caspase-mediated cascade of molecular events that maintains and amplifies the original apoptotic stimulus. Since the caspases play such an important role in initiating, regulating, and carrying out apoptosis, as well as in their own biochemical activation, they represent a key molecular target for the discovery and development of antiapoptotic drugs (Talanian et al., 2000).
There is now a large body of data that show that caspase-mediated apoptosis accounts for at least part of the cell death associated with a variety of diseases, such as ischemic stroke (Schulz et al., 1999; Han et al., 2002), myocardial infarction (Haunstetter & Izumo, 1998), and neurodegenerative disorders (e.g., Huntington's disease, Alzheimer's disease, and amyotrophic lateral sclerosis) (Wellington & Hayden, 2000). Moreover, evidence from several laboratories shows that genetic or pharmacological inhibition of caspases can reduce or even prevent the cell death caused by endogenous and exogenous apoptosis stimuli (Yaoita et al., 1998; Braun et al., 1999; Cursio et al., 1999; Grobmyer et al., 1999; Schulz et al., 1999; Lee et al., 2000; Mocanu et al., 2000). Taken together, these studies suggest that drugs that block apoptosis may be able to halt or reduce disease progression.
In this paper, we describe our search for a peptide-based caspase inhibitor with good in vitro and in vivo activities and our discovery of MX1013 as a potent and broad-spectrum caspase inhibitor containing a dipeptide scaffold and a fluoromethyl ketone warhead. Despite being 10–100-fold less potent in caspase enzyme inhibition assays than caspase inhibitors with tripeptide or tetrapeptide scaffolds, MX1013 has unexpectedly strong activity as a cytoprotectant. MX1013 also is more water-soluble than the commonly used caspase inhibitor Z-VAD(OMe)-fmk. Previously, we showed that MX1013 (CV1013) was effective in blocking apoptosis and death in a rodent model of endotoxemia (Jaeschke et al., 2000). In the current report, we present data showing that the dipeptide scaffold of MX1013 provides a good combination of in vitro and in vivo activities for a caspase inhibitor. We show that MX1013 has antiapoptotic activity in three cell culture models of apoptosis, where it prevents the appearance of the main biochemical markers of apoptosis and blocks cell death, and that it is efficacious by intravenous (i.v.) administration in three rodent models of apoptosis: anti-Fas-induced liver failure, transient focal brain ischemia/reperfusion, and myocardial ischemia (MCI)/reperfusion. These in vitro and in vivo studies provide a comprehensive analysis of this broad-spectrum caspase inhibitor, and suggest that MX1013 may be useful in treating human apoptosis-related disorders, which fulfills the need for cell death inhibitors that show efficacy in whole-cell models of apoptosis and are active in animal models of apoptosis (Thornberry, 1998).
Methods
Materials
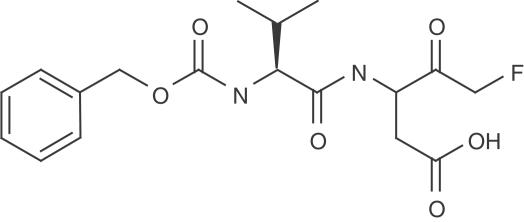
MX1013 (Z-VD-fmk; Figure 1) was prepared by coupling Z-Val-CO2H with t-butyl 3-amino-5-fluoro-4-hydroxy-pentanoate (Revesz et al., 1994). The coupling product was oxidized by Dess-Martin reagent to give the t-butyl ester of MX1013, which was then cleaved by trifluoroacetic acid to yield MX1013 as the free acid. The structure of MX1013 was confirmed by 1H-NMR, mass spectrometry, and elemental analysis. Other caspase inhibitors, including Z-D(OMe)E(OMe)VD(OMe)-fmk, Z-E(OMe)VD(OMe)-fmk, Z-VD(OMe)-fmk, Boc-D(OMe)-fmk, and Z-VAD(OMe)-fmk, were obtained from Enzyme System Products (Livermore, CA, U.S.A.). The Animal Care Committee of CoCensys and Maxim approved all procedures for in vivo experiments.
Figure 1.
Structure of MX1013.
Protease inhibition assays
The ability of MX1013 to inhibit the activity of human recombinant caspases was determined using a standard fluorometric microplate assay (Thornberry, 1994). Briefly, caspase enzyme (Pharmingen, San Diego, CA, U.S.A.) was incubated at 37°C with 5 μM of the fluorogenic caspase 3 substrate Ac-DEVD-AMC (BioMol, Plymouth Meeting, PA, U.S.A.) in a buffer containing 10 mM of either HEPES or PIPES (pH 7.4), with 100 mM NaCl, 10% sucrose, 1 mM EDTA, 0.1% CHAPS, and 10 mM DTT. At the end of the incubation period, the amount of cleaved AMC was measured on a fluorescent microplate reader, using an excitation wavelength of 355 nm and an emission wavelength of 460 nm. IC50 values were calculated from 12-point inhibition curves using drug concentrations ranging from 1 × 10−5 to 5 × 10−11 M. Values for kinactivation were determined as described (Morrison and Walsh, 1988; Thornberry et al., 1994). The ability of MX1013 to inhibit the activity of the noncaspase proteases was conducted with the following proteases (Calbiochem) and substrates (Bachem or Molecular Probes): Cathepsin B and Z-Phe-Arg-AMC (Tchoupe et al., 1991), calpain I and suc-Leu-Tyr-AMC (Meyer et al., 1996), cathepsin D and BODIPY FL casein (Molecular Probes), renin and suc-Arg-Pro-Phe-His-Leu-Leu-Val-Tyr-AMC (Murakami et al., 1981), Factor Xa and Boc-Ile-Glu-Gly Arg-AMC (Morita et al., 1977), and thrombin and Boc-Asp(OBzl)-Pro-Arg-AMC (Laura et al., 1980). Substrate cleavage was measured in the presence and absence of drug, using the fluorogenic substrates and assay conditions, as described in the references.
HeLa cell protection assays
HeLa S3 cells (ATCC-American Type Culture Collection, Manassas, VA, U.S.A.) were grown in minimal essential medium with 2 mM glutamine and 10% fetal bovine serum. For cell protection assays, the cells were seeded in multiwell plates and allowed to grow for 24 h. The cells were then preincubated with inhibitors for 2 h and incubated with 25 ng ml−1 TNF-α (Roche Molecular Biochemicals, Indianapolis, IN, U.S.A.) and 30 μg ml−1 cycloheximide (CHX) (Sigma, St Louise, MO, U.S.A.) for 18–24 h. All incubations were performed at 37°C in a CO2 incubator. The viability of the cells was assessed by phase-contrast microscopy, or quantified by uptake of calcein AM (Molecular Probes, Eugene, OR, U.S.A.). For the latter assay, the cultures were washed twice with PBS to remove nonadherent cells, and incubated at room temperature for 30–45 min with 4 μM calcein AM in serum-free and phenol red-free culture medium. The amount of calcein AM fluorescence in each well, which gives a quantitative measure of the number of remaining cells, was then measured using an excitation wavelength of 485 nm, and an emission wavelength of 525 nm using a Wallac fluorescence microplate reader. Data were expressed as percent control, with control values taken from cultures incubated with CHX, but without TNF-α.
Western blots
Jurkat cells (ATCC) were seeded in 2 ml of RPMI 1640 media with 10% FCS in 35 mm dishes, at a density of 0.5 × 106–1 × 106 per culture. The cells were preincubated at 37°C in a CO2 incubator for 2 h with inhibitor or vehicle (DMSO), followed by incubation for an additional 4 h with 500 ng ml−1 anti-Fas monoclonal antibody (clone CH1) (PanVera, Madison, WI, U.S.A.). At the end of the incubation period, the cells were harvested by centrifugation, washed with PBS and lysed in a buffer containing 50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and a cocktail of protease inhibitors (Complete Protease Inhibitor tablets, Roche Molecular Systems). The amount of protein in each sample was measured by the method of Bradford, using a BioRad Protein Assay Kit. Samples of lysate containing equivalent amounts of protein were separated by SDS—PAGE, and transferred to a PVDF membrane. The membranes were probed with either a monoclonal anticaspase 3 antibody coupled to HRP (Transduction Technologies) or a polyclonal antibody to poly(ADP)ribose polymerase (PARP; Enzyme Systems Products), followed by goat-anti-rabbit HRP second antibody. Antibody binding was visualized by chemiluminescence (Pierce SuperSignal).
Jurkat cell protection assay with doxorubicin as activator of apoptosis
Jurkat cells were grown in RPMI 1640 media with 10% FCS at 37°C in a CO2 incubator maintained at a cell density between 4 and 8 × 105 cells ml−1. Cells (1 × 105 in 0.1 ml of media) were placed into a well of a 96-well plate, and were treated with doxorubicin (1 μM) at 37°C for 18 h. MX1013 (10 μM) in DMSO was added to the appropriate samples at the time of compound addition. Control samples were treated with the same volume of solvent (DMSO). After 18 h, cells were observed by phase-contrast microscopy to visualize morphologic changes induced by the apoptosis stimuli.
Mouse liver apoptosis model
In vivo apoptosis protection assays, using the mouse liver failure model, were run as described (Rodriguez et al., 1996). Female ND4 Swiss Webster mice (16.5–21 g, Harlan Sprague–Dawley, San Diego, CA, U.S.A.) were injected i.v. with 8 μg of anti-Fas monoclonal antibody (clone JO-2; Pharmingen), followed 5 min later by i.v. administration of various doses of MX1013 formulated in an aqueous vehicle containing 50 mM Tris-HCl, pH 8.0. Control animals received anti-Fas antibody, followed by vehicle. The number of surviving animals in each treatment group was determined at 1, 3, 24 h and 5 days postinjection, and was expressed as a percentage of the total number of animals in each group. To assess the prevention of liver failure by MX1013 quantitatively, a separate experiment was run, where serum levels of two liver enzymes, SGPT (serum glutamic pyruvic transaminase) and SGOT (serum glutamic oxaloacetic transaminase), were measured using an Ames Seralyzer.
Rat transient focal brain ischemia model
Male Fischer-344 rats (200–240 g, Harlan Sprague–Dawley, San Diego, CA, U.S.A.) were anesthetized with halothane and implanted with two catheters: one through the left femoral vein to the inferior vena cava for drug administration, and the other in the left femoral artery for monitoring blood pressure and blood gasses. In addition, a PhysioTel Transmitter was implanted in the peritoneal cavity to remotely monitor the body temperature. Following preparative surgery, a ventral midline cervical incision was made to expose both common carotid arteries (CCA). The right CCA was permanently ligated with 4-O silk suture, and the left CCA was clamped with an atraumatic aneurysm clip. In order to gain access to the right middle cerebral artery (MCA), a 1-cm incision was made between the lateral canthus of the right eye and the external auditory canal, and the underlying temporalis muscle was excised and retracted. A 2 mm burr hole was drilled 2–3 mm rostral to the fusion of the zygomatic arch and the squamosal bone, and the dura was cut and retracted to expose the MCA proximal to the rhinal fissure. The right MCA was occluded using a Codman microaneurysm clip (No. 1), and interruption of blood flow was verified visually using a dissecting microscope. The incisions were then closed with surgical clips, anesthesia was discontinued, and the animals returned to their cages. After 2.5 h, the animals were reanesthetized, the surgical clips removed, and the aneurysm clips occluding the right MCA and left CCA were removed. Restoration of blood flow was confirmed visually. The incisions were closed and the animals returned to their cages for 24 h. MX1013, formulated in the aqueous with 50 mM Tris-HCl, pH 8.0, was tested in two different dosing regimens: one in which the drug was administered as a 20 mg kg−1 i.v. bolus 10 min after the onset of ischemia, followed by a continuous i.v. infusion of 5 mg kg−1 h−1 for 6 h, and the other in which the infusion time was extended to 12 h. For determination of infarct volumes, animals were killed by overanesthetization and the brains removed. Then, 2 mm coronal sections of the right hemispheres were prepared and stained with 2,3,5-triphenyltetrazolium chloride (TTC). Infarct areas were measured in each section using image analysis software, and the infarct volume calculated by summing the areas in adjacent sections. Statistical analyses were conducted using Sigmastat software (Jandel Scientific Software, San Rafael, CA, U.S.A.). Student's t-tests were used for unpaired data, and ANOVA for multiple comparisons. A P-value of <0.05 was considered significant.
Rat myocardial infarction model
Male Fischer-344 rats (200–250 g, Harlan Sprague–Dawley, Indianapolis, IN, U.S.A.) were intubated by insertion of an endotracheal tube, and ventilated with a rodent respirator. A left thoracotomy was performed, and the proximal branch of the left descending coronary artery (LDCA) was exposed, following procedures described previously (Anversa et al., 1986). An atraumatic balloon device (prepared with a length of 5 mm silastic tubing connected to prolonged teleflex tubing) was introduced to allow occlusion and reperfusion of the LDCA while the animal was conscious. Two rounds of suture loop were wound loosely around the LDCA to allow enclosure of both the balloon and the artery. The volume of balloon inflation necessary for interruption of blood was determined, and the teleflex tubing was exteriorized through the back of the animal. Mechanical ventilation was discontinued, and the animals regained spontaneous respiration within seconds. Using this procedure, MCI was effected for 1 h by inflating the implanted balloon to the predetermined volume with sterile saline injected through the teleflex tubing. Reperfusion was achieved by removing the saline and deflating the implanted balloon. MX1013, formulated in the aqueous with 50 mM Tris-HCl, pH 8.0, or its vehicle, was administered as a single i.v. bolus of 20 mg kg−1 upon initiation of ischemia, followed by continuous infusion at 5 mg kg−1 h−1 for 12 h. Infarcts were assessed 24 h after surgery by TTC staining. Briefly, the heart tissue was sliced into sections, stained with TTC, and the extent of ischemic injury determined for the volume of the infarcted area. The infarct was computed as the ratio of injured/total volume of the left ventricle. Data are expressed as percent tissue injury. Statistical differences between groups were determined using Student's t-test.
Results
Z-VD-fmk (MX1013) is a potent cytoprotectant
Previous studies of caspase 1 inhibitors of different peptide lengths indicated that mono and dipeptides showed equivalent potencies, whereas a tripeptide had approximately 50 × greater potency, which suggested that the longer peptides with the appropriate recognition sequences were preferred due to their increased potency (Prasad et al., 1995; Linton et al., 2002). We examined the efficacy of different length peptides in a cell assay where cytoprotective activity against TNF-α-induced apoptosis of HeLa cells was measured. Methyl ester forms of the acidic amino acids were used, since this modification had been proposed to enhance the cell-permeation properties of peptides with acidic amino acids. Enzyme-inhibitory activity, though, depends upon removal of the methyl groups (Rotonda et al., 1996) by ubiquitous cellular esterases. Using HeLa cells and a TNF-α-induced apoptosis model, we compared the potencies of the following four methyl ester peptide inhibitors whose peptide scaffolds were designed based on the caspase 3 recognition site present in PARP (Nicholson et al., 1995): (1) Z-D(OMe)E(OMe)VD(OMe)-fmk, a tetrapeptide which contains the P1–P4 amino acids of the PARP site; (2) Z-E(OMe)VD(OMe)-fmk, a methyl ester tripeptide compound which contains the P1–P3 amino acids; (3) Z-VD(OMe)-fmk, a dipeptide which contains the P1 and P2 amino acids; and, (4) Boc-D(OMe)-fmk, a monopeptide which contains only the P1 amino acid.
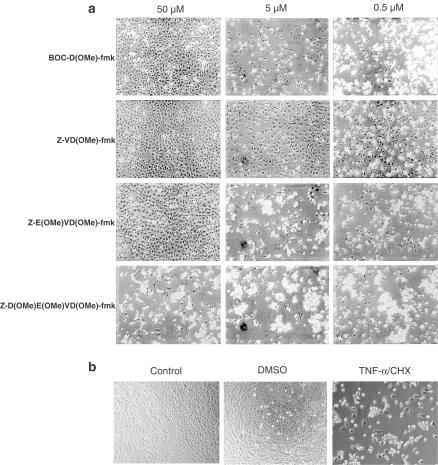
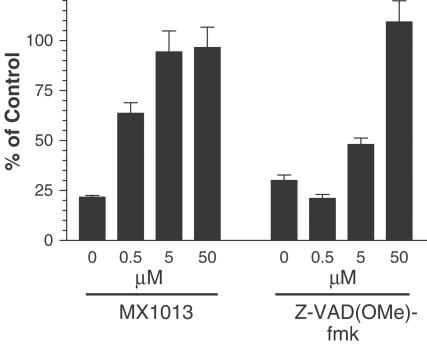
At a concentration of 50 μM, the methyl ester mono, di, and tripeptides effectively protected HeLa cells from TNF-α, while the methyl ester tetrapeptide was almost completely ineffective (Figure 2), as assessed by the round floating or apoptotic cells. At a concentration of 5 μM, the methyl ester mono and tripeptides were only marginally effective, and they were completely ineffective at 0.5 μM (Figure 2). In contrast, the methyl ester dipeptide retained substantial cytoprotective activity at both 5 and 0.5 μM. We also compared the methyl ester dipeptide to the tripeptide pan-caspase inhibitor Z-VAD(OMe)-fmk, a compound that is widely used in cell culture apoptosis experiments and which has been found to be an effective cytoprotectant in a variety of animal models of apoptosis (Yaoita et al., 1998; Braun et al., 1999; Cursio et al., 1999; Mocanu et al., 2000). Z-VAD(OMe)-fmk was an effective cytoprotectant at 50 μM, but like the methyl ester tetra, tri, and monopeptides, it had little activity at 0.5 μM (Figure 3), whereas the dipeptide was effective. Based on these results, we selected the dipeptide as a preferred scaffold for optimal caspase inhibition in cells. We next examined the difference between the free acid and methyl ester derivative of the dipeptide Z-VD-fmk on cytoprotection. We found that the IC50's of both compounds were very similar, approximately 0.1 μM, indicating that the monocharged Z-VD-fmk has good cell-permeation properties, compared to tetra- and tripeptide-based inhibitors. Therefore, we chose the free acid dipeptide Z-VD-fmk (MX1013) (Figure 1) as a lead compound for further in vitro and in vivo studies.
Figure 2.
Effect of caspase inhibitors containing different peptide lengths on TNF-α/CHX-induced apoptosis of HeLa cells. HeLa cells were preincubated for 2 h with inhibitors as indicated, followed by treatment for 20 h with 25 ng ml−1 TNF-α and 30 μg ml−1 cycloheximide to induce apoptosis. Cells were photographed using phase-contrast optics at × 10 magnification. Protected cells can be seen as a normal adherent monolayer, whereas apoptotic cells can be seen as rounded, phase-bright, floating cells in the medium. The results shown are representative examples of at least two experiments. (a) Apoptotic cells treated with different concentration of inhibitors. (b) Control panel including control cells, DMSO-treated control cells, and apoptotic cells without treatment by inhibitors.
Figure 3.
Comparison of the cytoprotective effects of MX1013 and Z-VAD(OMe)-fmk. HeLa cells were treated with inhibitors at the concentrations indicated, followed by TNF-α/cycloheximide, as described in Figure 2. Plates were washed and processed for calcein AM assays as described in Methods. The values measured at the different concentrations of MX1013 or Z-VAD(OMe)-fmk tested in triplicate were expressed as a percentage of the control cells, which were not treated with apoptosis inducer. The results are shown as the mean±s.e.m.
Caspase inhibition studies
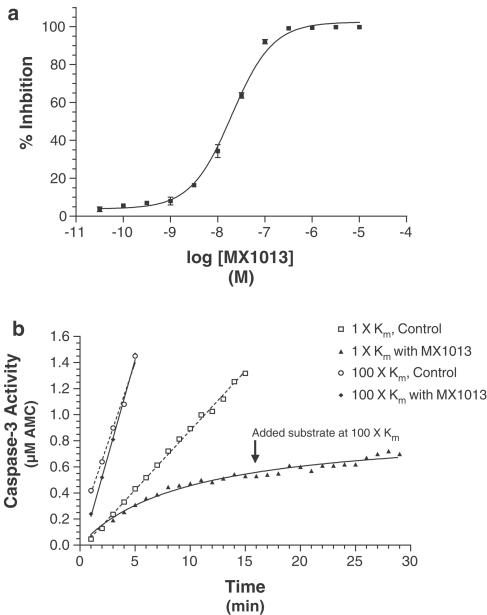
In order to characterize the basic in vitro properties of MX1013, we examined its ability to inhibit the activity of recombinant human caspases, particularly the principal effector caspase caspase 3. MX1013 inhibited recombinant human caspase 3 with an IC50 of 30 nM (Figure 4a) and Kinactivation of 18 700 M−1 S−1. Progress curves, run in the absence and presence of compound, were used to determine the nature of inhibition. In the absence of MX1013, substrate cleavage increased linearly with time, and the reaction rate increased as the substrate concentration was shifted from 5 μM (1Km) to 500 μM (100Km) (Figure 4b). By contrast, in the presence of MX1013, enzyme activity rapidly tailed off, approaching a plateau starting at about 15 min. Incubation of the enzyme with an excess (100Km) of substrate prior to addition of MX1013 protected it from inhibition, indicating that the drug is competitive for substrate. However, the addition of excess substrate 15 min after MX1013 addition did not result in an increase in activity, indicating that MX1013 acts irreversibly. These findings are consistent with the known characteristics of peptide-halomethylketone inhibitors, and show that decreasing the size of the peptide scaffold from tetra to dipeptide does not change the basic characteristics of the inhibitor.
Figure 4.
Effect of MX1013 on recombinant human caspase 3 activity. (a) A representative dose–response curve of caspase 3 inhibition by MX1013. Each concentration of drug was tested in triplicate in a standard caspase 3 enzyme fluorometric assay, using Ac-DEVD-AMC as the substrate. The percent inhibition was calculated using control samples in which activity was measured in the absence of drug. Each data point represents the mean, and the error bars represent the s.e.m.; in some cases the error bars are too small to be seen. (b) Progress curves from competition experiments with MX1013. Standard fluorometric caspase 3 enzyme assays were run in the presence or absence of MX1013 (30 nM), and the activity was determined as a function of assay time. Substrate was added at 5 μM (1Km) or 500 μM (100Km). In the experiment with 5 μM substrate, an excess of substrate (500 μM) was added 15 min after the assay was initiated, in order to determine whether the inhibition was reversible. The figure shown is a representative example of three experiments.
We extended these enzyme-inhibition studies by testing the ability of MX1013 to block other members of the caspase family, as well as six noncaspase proteases. MX1013 was roughly equipotent against caspases 1 (IC50=20 nM) and 3 (IC50=30 nM) and caspases 6–9 (IC50=5–18 nM). In contrast, the compound was a poor inhibitor of calpain I, cathepsin B, cathepsin D, and renin (IC50>10 μM), as well as thrombin and Factor Xa (IC50>100 μM). These results indicate that MX1013 is a broad-spectrum caspase inhibitor, but not a general protease inhibitor.
MX1013 inhibits three key markers of apoptosis
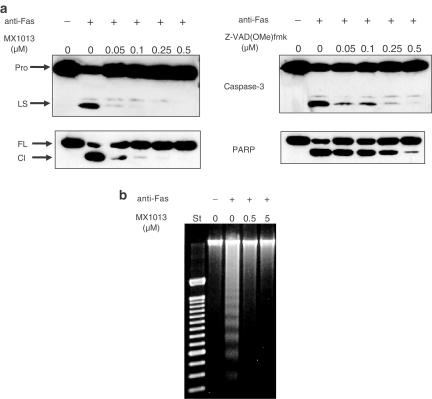
Apoptosis is characterized by a series of biochemical events, which ultimately lead to cell death. We tested the ability of MX1013 to block three of these events: the proteolytic maturation of caspase 3, the caspase-mediated cleavage of PARP, and the fragmentation of genomic DNA. During apoptosis, caspase 3 is proteolytically processed from a single-chain zymogen to its mature subunits, which consist of a 17 kDa large subunit and a 12 kDa small subunit (Nicholson et al., 1995). In addition, PARP, one of the key substrates for caspase 3 (Lazebnik et al., 1994), is cleaved to form 25 and 85 kDa fragments. Both proteolytic events can be monitored by Western blotting. In anti-Fas-treated Jurkat cells preincubated with 0.5 μM MX1013, neither caspase 3 processing, nor PARP cleavage, could be detected (Figure 5a). At concentrations of MX1013 as low as 0.05 μM, caspase 3 processing and PARP cleavage were still markedly reduced. In contrast, 0.5 μM Z-VAD(OMe)-fmk only partially inhibited caspase 3 processing and PARP cleavage, and these apoptotic events were readily detectable at lower concentrations (0.25, 0.1, and 0.05 μM) of Z-VAD(OMe)-fmk. An analysis of DNA fragmentation confirmed these results: concentrations of MX1013 as low as 0.5 μM prevented the formation of DNA ladders (Figure 5b).
Figure 5.
The effect of MX1013 on three biochemical markers of apoptosis. Jurkat T-lymphocytes were treated with MX1013 or Z-VAD(OMe)-fmk, at the indicated concentrations, followed by an agonistic anti-Fas antibody, to initiate apoptosis. (a) The cells were processed for Western blotting in order to visualize the processing of caspase 3 (top panels) or PARP (bottom panels). Pro indicates the migration position of caspase 3 proenzyme, LS the caspase 3 large subunit, FL the full-length PARP, and Cl indicates the position of cleaved PARP. (b) Samples were processed for agarose gel electrophoresis, and stained with ethidium bromide in order to visualize DNA laddering. St lane indicates DNA size standards and the other lanes indicate the concentration of MX1013 inhibitor. The results shown are representative examples of at least three experiments.
MX1013 protects cells from doxorubicin-induced apoptosis
To determine if MX1013 can protect cells against another activator of apoptosis, Jurkat cells were treated with doxorubicin in the absence or presence of MX1013. Apoptosis was monitored for membrane blebbing by microscopy. Membrane blebbing, a characteristic effect of apoptosis, was blocked by addition of MX1013 along with doxorubicin (data not shown).
MX1013 is an effective antiapoptotic agent in vivo
To test the cytoprotective activity of MX1013 in vivo, we utilized three rodent disease models involving apoptosis: anti-Fas-induced liver failure, transient focal brain ischemia/reperfusion, and MCI/reperfusion. The liver failure model is a straightforward method for determining the in vivo antiapoptotic efficacy of caspase inhibitors (Rodriguez et al., 1996), and is based on the fact that hepatocytes express high levels of Fas, a cell death receptor, which can be activated by agonistic antibodies. To measure drug efficacy in this model, the number of surviving animals as a function of time after challenge with anti-Fas antibody was determined. When mice were challenged with 8 μg of anti-Fas antibody followed by vehicle, all the mice died by 3 h time point (Table 1 ). In contrast, the lowest dose of MX1013 (0.25 mg kg−1, i.v. injection) protected 66% (4/6) of the mice from the lethal effects of anti-Fas antibody at the 3 h time point, and 1 and 10 mg kg−1 dose of MX1013 protected 100% of the mice at the 3 h time point. Moreover, animals that were protected by MX1013 at the 3 h time point were all alive at 24 h post-antibody challenge, and were alive after 5 days at which time the experiment was terminated, indicating that MX1013 truly protected the mice from the initial injury cause by anti-Fas antibody. These results are similar to what have been reported for another irreversible caspase inhibitor (IDN-1965), which at a dose of 1 mg kg−1 i.p. completely blocked lethality measured up to 7 days after anti-Fas antibody administration (Hoglen et al., 2001). These results show that the cytoprotective activity of MX1013 observed in cell culture studies extends to apoptosis in vivo, and that MX1013 not only inhibits local tissue apoptosis, but also can protect animals against its lethal effects. In separate experiments, the levels of SGOT and SPGT activity in the serum (markers for liver toxicity) were also drastically reduced when treated with drug (data not shown), in accord with the survival data.
Table 1.
Survival of mice with MX1013 treatment after induction of apoptosis by anti-Fas antibody
| % Survival (n=6) | ||||
|---|---|---|---|---|
| Dose of MX1013 (mg kg−1) | 1 h | 3 h | 24 h | 5 days |
| 0 | 100 | 0 | 0 | 0 |
| 0.25 | 100 | 66 | 66 | 66 |
| 1 | 100 | 100 | 100 | 100 |
| 10 | 100 | 100 | 100 | 100 |
Mice were injected intravenously (i.v.) with 8 μg of anti-Fas antibody, followed 5 min later with i.v. injection of MX1013 at the indicated doses or by vehicle. Six mice were treated in each group, and the number surviving was monitored for up to 5 days.
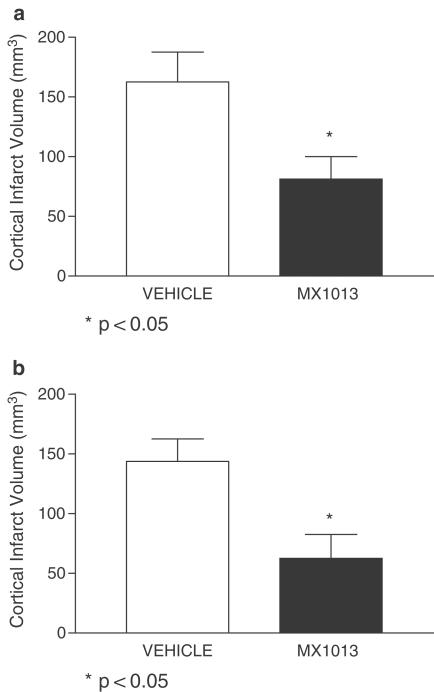
The rat transient middle cerebral artery occlusion (MCAO) model has been used to evaluate the efficacy of caspase inhibitors as anti-stroke agents. This model involves the transient induction of brain ischemia by MCAO, and an estimate of the ability of a test agent to reduce the size of the resulting infarct after reperfusion. We tested MX1013 in this model in two separate experiments, one in which the drug was administered as a 20 mg kg−1 i.v. bolus 10 min after the onset of ischemia, followed by a continuous i.v. infusion of 5 mg kg−1 h−1 for 6 h (Figure 6a), and the other in which the infusion was extended to 12 h (Figure 6b). MX1013 significantly reduced cortical infarct size in both cases (by 46 and 57%, respectively; P<0.05). The drug did not cause any changes in blood pressure, blood gasses, or body temperature (data not shown). The efficacy of MX1013 administered by i.v. injection in the MCAO model is comparable with Z-VAD(OMe)-fmk administered by i.c.v. injection in a MCAO model, which was reported to reduce infarct volume by approximately 40–50% (Hara et al., 1997).
Figure 6.
Effects of MX1013 treatment in the cortical infarct volume of animals in a transient MCAO. The MCA of rats was occluded for 2.5 h before release of the occlusion. MX1013 was administered i.v. with a bolus of 20 mg kg−1 10 min after the onset of occlusion, followed by an i.v. infusion of 5 mg kg−1 h−1 for 6 h (Figure 6a, 162±26, n=16 for vehicle, and 86±18, n=15 for MX1013 treated) or 12 h (Figure 6b, 143±18, n=12 for vehicle, and 62±20, n=10 for MX1013 treated) by the jugular vein. At 24 h after occlusion, the brain was sectioned and stained with TTC. Sections were made and the infarct area was determined, and the volume of the infarct calculated. The average for the MX1013-treated and the control vehicle-treated group is shown as the mean±standard error of mean (s.e.m.). The statistical significance value is indicated.
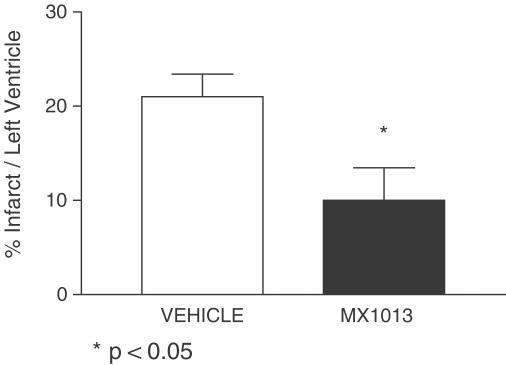
The rat model of acute MCI is a widely used model for studying the effects of cytoprotective drugs on cardiac ischemia/reperfusion injury. Similar in principle to the rat brain ischemia model, it involves interruption of the blood supply to the heart by occlusion of the LDCA, followed by restoration of blood flow (reperfusion) and determination of the size of the resultant infarct. When administered as a single i.v. bolus of 20 mg kg−1 at the time of ischemia, followed by continuous infusion at 5 mg kg−1 h−1 for 12 h, MX1013 treatment resulted in a reduction of infarct size (by 52%, P<0.05) (Figure 7). In comparison, Z-VAD(OMe)-fmk was reported to be active in the MCI model by i.v. administration and produced 21% reduction of infarct volume (Yaoita et al., 1998), which is less efficacious than that of MX1013.
Figure 7.
Effects of MX1013 treatment in an acute myocardial infarction and reperfusion model in rats. Ischemia was effected by occlusion of the left anterior descending artery of rat hearts for 1 h, followed by reperfusion for 23 h. Drug was administered i.v. at the time of occlusion as a bolus (20 mg kg−1), followed by i.v. infusion (5 mg kg−1 h−1) for 12 h. At the end of the reperfusion, the hearts were sectioned, stained, and analyzed for infarct volume, as described in Methods. The average of 12 animals (10±3.5) in the MX1013-treated group and 10 animals (21±2.4) in the vehicle control group is shown as the mean±s.e.m. The statistical significance value is indicated.
Taken together, these results show that MX1013 is an effective antiapoptotic agent in vivo. It can reduce the tissue damage and lethality associated with activation of death receptor pathways, as well as the amount of cellular damage found in two different models of ischemia/reperfusion injury.
Discussion
Our studies of MX1013 show that it is an effective cytoprotective agent, in several tissue culture models of apoptosis, in a mouse liver apoptosis model, and in two different animal models of ischemia/reperfusion. These results suggest that, although tetrapeptide-based inhibitors may be more potent inhibitors of caspases under cell-free conditions, dipeptide inhibitors have superior in vivo properties. It is likely that the strong in vivo activity of MX1013 is due to its ability to permeate the plasma membrane and reach the caspases, which are intracellular enzymes (Reed, 2002). This enhanced permeation may be a function of both the size and structure of MX1013: it contains only two amino acids, only one of which is charged. In contrast, caspase inhibitors containing more than one charged or polar amino acid (such as Z-DEVD-fmk, which has three free carboxy acids) probably do not readily pass through the plasma membrane. Based on the fact that charged molecules penetrate cell membranes poorly, conversion of the free carboxy acids of acidic amino acids to methyl esters has been used to enhance cell permeation. We have not observed that the methyl ester version of Z-DEVD-fmk demonstrates such an enhancement, as it is still a relatively poor cytoprotectant, possibly due to the requirement to remove the methyl esters.
Despite containing a peptide scaffold with only two amino acids, MX1013 still has reasonable potency in cell-free enzyme assays, which is probably due to the fact that the compound retains the essential features of a caspase-recognition sequence (an aspartic acid residue at the P1 position and a small hydrophobic residue in the P2 position) and utilizes an irreversible warhead. The crystal structures of caspases 1, 3, 7, and 8 bound to the peptide aldehyde inhibitor Ac-DEVD-CHO reveal that the P1 aspartic acid forms a hydrogen bond network with the side chains of Arg341, Arg179, and Gln283 of the enzyme (Wei et al., 2000). Moreover, the P1 amide NH group forms hydrogen bonds with the backbone carbonyl of Ser339. The crystal structures also show that the P2 valine of Ac-DEVD-CHO lies against a hydrophobic portion of the binding pocket, and its backbone carbonyl forms a hydrogen bond with Arg341. The P1 and P2 amino acids of MX1013 would be expected to participate in all of these interactions. The methyl ester version of MX1013 was found to be >70-fold less active than MX1013 in the caspase-3 enzyme assay, supporting the notion that the carboxylic acid in P1 is critical for interaction with caspases. It is also likely that the fluoromethylketone-leaving group of MX1013, which is known to bind irreversibly to the active site cysteine of caspases (Mittl et al., 1997), plays a major role in the compound's high potency. The reversible aldehyde form of MX1013 has an IC50 of 16 μM against caspase 3. Thus, the loss of potency resulting from truncation of the peptide scaffold can apparently be compensated for by use of an irreversible warhead, but this truncation does not decrease its specificity against caspases.
The P4 amino acid in tetrapeptide-based caspase inhibitors appears to be a key determinant of specificity between caspases (Thornberry et al., 1997). Charged or polar residues in this position are preferred by caspases 3 and 7, while large hydrophobic residues are preferred by caspases 1 and 4 (Thornberry et al., 1997). In MX1013, the absence of the P4 and P3 amino acids would be expected to reduce the selectivity of the compound, allowing it to inhibit most, if not all, caspases. Our data, which show an IC50 value in the low to mid-nanomolar range for inhibition of caspases 1, 3, and 6 to 9, support this prediction. Since apoptosis signal transduction involves the activation of multiple caspases, it can be argued that, in order to achieve optimal inhibition of apoptosis, the ability to inhibit most or all of the caspases is a desirable feature in a cytoprotective drug. However, it is important that a pan-caspase inhibitor does not inhibit all proteases. MX1013 is not a general protease inhibitor, as shown by its relatively low potency as an inhibitor of calpain I, cathepsin B, cathepsin D, renin, thrombin, and Factor Xa. MX 1013 is not a general protease inhibitor, as shown by its relatively low potency as an inhibitor of calpain I, cathepsin B, cathepsin D, renin thrombin and Factor Xa. The selectivity of MX 1013 for caspases vs other proteases is probably due to the free carboxylic acid group of the P1 Asp.
Due to its relatively small size and the free carboxylic acid group, MX1013 is more water soluble than the commonly used caspase inhibitor Z-VAD(OMe)-fmk. For in vivo studies, MX1013 can be formulated in a slightly basic pH 8.0 aqueous solution with 50 mM Tris-HCl, at a concentration of >10 mg ml−1 for i.v. administration. In comparison, Z-VAD(OMe)-fmk has low water solubility, and generally has been formulated in DMSO/water at a concentration of 1 mg ml−1 (Farber et al., 1999; Huang et al., 2000).
Our studies of MX1013 in rodent models of cell death show that the drug is effective in preventing anti-Fas-induced liver failure and the ensuing lethality, and also in reducing the size of brain and myocardial ischemic infarcts. In the mouse anti-Fas liver apoptosis model, MX1013 reduced the levels of two biochemical markers of liver damage 3 h after anti-Fas challenge, and reduced the number of deaths in a dose-dependent manner up to 5 days after antibody challenge. The efficacy of MX1013 was comparable to that of Z-VAD(OMe)-fmk in previously reported studies, although in those experiments Z-VAD(OMe)-fmk was administered in multiple doses (Rodriguez et al., 1996), while MX1013 was effective when given as a single dose. The mouse liver damage model has been widely used to test the in vivo efficacy of caspase inhibitors (Hoglen et al., 2001), and has been proposed as a model for human fulminant hepatic failure arising from viral hepatitis (Kondo et al., 1997). The cytoprotective activity of MX1013 in this model suggests that it may be useful in humans in the prevention of acute liver failure mediated by caspase-dependent mechanisms.
The ability of MX1013 to reduce the size of ischemic infarcts in the brain and heart is in agreement with the known role that apoptosis plays in ischemia/reperfusion injury (Gottlieb and Engler, 1999; Schulz et al., 1999). In both organs, ischemia/reperfusion triggers the activation of caspases and DNA fragmentation, two key biochemical markers of apoptosis. The development of these markers, as well as the resulting cell death, can be reduced by Z-VAD(OMe)-fmk and the tetrapeptide caspase inhibitors, as well as a peptidomimetic caspase inhibitor (Hara et al., 1997; Mocanu et al., 2000; Deckwerth et al., 2001). It is important to note that with MX1013 we were able to achieve large, statistically significant reductions in brain infarct size with i.v. administration, suggesting that MX1013 can pass the blood–brain barrier. In contrast, neuroprotection by tripeptide, tetrapeptide inhibitors, and a selective caspase 3 inhibitor appears to require direct injection into the cerebral ventricles (i.c.v.) (Loddick et al., 1996; Hara et al., 1997; Endres et al., 1998; Han et al., 2002). In general, these peptide-based inhibitors have low brain penetration, and they are given by i.c.v. injection to overcome this limitation (Robertson et al., 2000; Loetscher et al., 2001). For clinical application, i.c.v. administration is impractical, and systemic administration such as i.v. is preferred. However, there are a few caspase inhibitors that have been reported to be active by the i.v. route in the brain ischemia model (Deckwerth et al., 2001), and it has been suggested that clinical studies of caspase inhibitors for cerebral ischemia have not yet been performed due to the lack of synthetic caspase inhibitors that cross the blood–brain barrier (Schulz et al., 1999). Therefore, the ability of MX1013 to provide neuroprotection by i.v. administration, suggesting that it has improved brain penetration properties vs the earlier tetrapeptide and tripeptide based caspase inhibitors, is a critical advantage for any drug proposed for use in treating human stroke patients.
Based on these studies, it appears that the dipeptide structure of MX1013 provides a good combination of in vitro and in vivo activities for a caspase inhibitor, which are useful for the design of novel caspase inhibitors. MX1013 is effective in inhibiting the deleterious effects of apoptosis in a number of animal models, and is a leading candidate for additional preclinical studies.
Acknowledgments
We would like to thank Professor John Keana and Dr Eckard Weber for contributory discussions. We thank Lisa D'Arcy for her excellent technical assistance.
Abbreviations
- Ac-DEVD-AMC
acetyl-Asp-Glu-Val-Asp-7-amino-4-methyl-coumarin
- Ac-DEVD-CHO
acetyl-Asp-Glu-Val-Asp-carbaldehyde
- Boc-D(OMe)-fmk
t-butyloxycarbonyl-Asp(OMe)-fluoromethylketone
- CCA
common carotid arteries
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- EDTA
ethylenediaminetetraacetic acid
- FACS
fluorescence-activated cell sorting
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, sodium salt
- HRP
horseradish peroxidase
- LDCA
left descending coronary artery
- MCA
middle cerebral artery
- MCAO
middle cerebral artery occlusion
- MX1013, Z-VD-fmk
benzyloxycarbonyl-Val-Asp-fluoromethylketone
- PARP
poly(ADP)ribose polymerase
- PIPES
1,4-piperazinebis(ethanesulfonic acid, sodium salt)
- PVDF
polyvinylidene fluoride
- SGPT
serum glutamic pyruvic transaminase
- SGOT
serum glutamic oxaloacetic transaminase
- suc-Leu-Tyr-AMC
succinyl-Leu-Tyr-7-amino-4-methyl-coumarin
- TTC
2,3,5-triphenyltetrazolium chloride
- Z-D(OMe)E(OMe)VD(OMe)-fmk
benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone
- Z-E(OMe)VD(OMe)-fmk
benzyloxycarbonyl-Glu(OMe)-Val-Asp (OMe)-fluoromethylketone
- Z-VAD(OMe)-fmk
benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone
- Z-VD(OMe)-fmk
benzyloxycarbonyl-Val-Asp(OMe)-fluoromethylketone
References
- ANVERSA P., BEGHI C., KIKKAWA Y., OLIVETTI G. Myocardial infarction in rats. Infarct size, myocyte hypertrophy, and capillary growth. Circ. Res. 1986;58:26–37. doi: 10.1161/01.res.58.1.26. [DOI] [PubMed] [Google Scholar]
- ARAVIND L., DIXIT V.M., KOONIN E.V. The domains of death: evolution of the apoptosis machinery. Trends Biochem. Sci. 1999;24:47–53. doi: 10.1016/s0968-0004(98)01341-3. [DOI] [PubMed] [Google Scholar]
- BRAUN J.S., NOVAK R., HERZOG K.H., BODNER S.M., CLEVELAND J.L., TUOMANEN E.I. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 1999;5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- CURSIO R., GUGENHEIM J., RICCI J.E., CRENESSE D., ROSTAGNO P., MAULON L., SAINT-PAUL M.C., FERRUA B., AUBERGER A.P. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. Faseb J. 1999;13:253–261. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- DECKWERTH T.L., ADAMS L.M., WIESSNER C., ALLEGRINI P.R., RUDIN M., SAUTER A., HENGERER B., SAYERS R.O., ROVELLI G., AJA T., MAY R., NALLEY K., LINTON S., KARANEWSKY D.S., TERNANSKY R.J., WU J.C., ROGGO S., SCHMITZ A., CONTRERAS P.C., TOMASELLI K.J. Long-term protection of brain tissue from cerebral ischemia by peripherally administered peptidomimetic caspase inhibitors. Drug Dev. Res. 2001;52:579–586. [Google Scholar]
- ENDRES M., NAMURA S., SHIMIZU-SASAMATA M., WAEBER C., ZHANG L., GOMEZ-ISLA T., HYMAN B.T., MOSKOWITZ M.A. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J. Cereb. Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- FARBER A., CONNORS J.P., FRIEDLANDER R.M., WAGNER R.J., POWELL R.J., CRONENWETT J.L. A specific inhibitor of apoptosis decreases tissue injury after intestinal ischemia–reperfusion in mice. J. Vasc. Surg. 1999;30:752–760. doi: 10.1016/s0741-5214(99)70115-1. [DOI] [PubMed] [Google Scholar]
- GOTTLIEB R.A., ENGLER R.L. Apoptosis in myocardial ischemia–reperfusion. Ann. N.Y. Acad. Sci. 1999;874:412–426. doi: 10.1111/j.1749-6632.1999.tb09255.x. [DOI] [PubMed] [Google Scholar]
- GROBMYER S.R., ARMSTRONG R.C., NICHOLSON S.C., GABAY C., AREND W.P., POTTER S.H., MELCHIOR M., FRITZ L.C., NATHAN C.F. Peptidomimetic fluoromethylketone rescues mice from lethal endotoxic shock. Mol. Med. 1999;5:585–594. [PMC free article] [PubMed] [Google Scholar]
- HAN B.H., XU D., CHOI J., HAN Y., XANTHOUDAKIS S.R., TAM J., VAILLANCOURT J., COLUCCI J., SIMAN R., GIROUX A., ROBERTSON G.S., ZAMBONI R., NICHOLSON D.W., HOLTZMAN D.M. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic–ischemic brain injury. J. Biol. Chem. 2002;277:30126–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- HARA H., FRIEDLANDER R.M., GAGLIARDINI V., AYATA C., FINK K., HUANG Z., SHIMIZU-SASAMATA M., YUAN J., MOSKOWITZ M.A. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUNSTETTER A., IZUMO S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ. Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- HOGLEN N.C., HIRAKAWA B.P., FISHER C.D., WEEKS S., SRINIVASAN A., WONG A.M., VALENTINO K.L., TOMASELLI K.J., BAI X., KARANEWSKY D.S., CONTRERAS P.C. Characterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury. J. Pharmacol. Exp. Ther. 2001;297:811–818. [PubMed] [Google Scholar]
- HUANG J.Q., RADINOVIC S., REZAIEFAR P., BLACK S.C. In vivo myocardial infarct size reduction by a caspase inhibitor administered after the onset of ischemia. Eur. J. Pharmacol. 2000;402:139–142. doi: 10.1016/s0014-2999(00)00477-5. [DOI] [PubMed] [Google Scholar]
- JAESCHKE H., FARHOOD A., CAI S.X., TSENG B.Y., BAJT M.L. Protection against TNF-induced liver parenchymal cell apoptosis during endotoxemia by a novel caspase inhibitor in mice. Toxicol. Appl. Pharmacol. 2000;169:77–83. doi: 10.1006/taap.2000.9035. [DOI] [PubMed] [Google Scholar]
- KONDO T., SUDA T., FUKUYAMA H., ADACHI M., NAGATA S. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- LAURA R., ROBISON D.J., BING D.H. (p-Amidinophenyl) methanesulfonyl fluoride, an irreversible inhibitor of serine proteases. Biochemistry. 1980;19:4859–4864. doi: 10.1021/bi00562a024. [DOI] [PubMed] [Google Scholar]
- LAZEBNIK Y.A., KAUFMANN S.H., DESNOYERS S., POIRIER G.G., EARNSHAW W.C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- LEE D., LONG S.A., ADAMS J.L., CHAN G., VAIDYA K.S., FRANCIS T.A., KIKLY K., WINKLER J.D., SUNG C.M., DEBOUCK C., RICHARDSON S., LEVY M.A., DEWOLF W.E., JR, KELLER P.M., TOMASZEK T., HEAD M.S., RYAN M.D., HALTIWANGER R.C., LIANG P.H., JANSON C.A., MCDEVITT P.J., JOHANSON K., CONCHA N.O., CHAN W., ABDEL-MEGUID S.S., BADGER A.M., LARK M.W., NADEAU D.P., SUVA L.J., GOWEN M., NUTTALL M.E. Potent and selective nonpeptide inhibitors of caspases 3 and 7 inhibit apoptosis and maintain cell functionality. J. Biol. Chem. 2000;275:16007–16014. doi: 10.1074/jbc.275.21.16007. [DOI] [PubMed] [Google Scholar]
- LINTON S.D., KARANEWSKY D.S., TERNANSKY R.J., WU J.C., PHAM B., KODANDAPANI L., SMIDT R., DIAZ J.L., FRITZ L.C., TOMASELLI K.J. Acyl dipeptides as reversible caspase inhibitors. Part 1: initial lead optimization. Bioorg. Med. Chem. Lett. 2002;12:2969–2971. doi: 10.1016/s0960-894x(02)00629-7. [DOI] [PubMed] [Google Scholar]
- LODDICK S.A., MACKENZIE A., ROTHWELL N.J. An ICE inhibitor, z-VAD-DCB attenuates ischaemic brain damage in the rat. Neuroreport. 1996;7:1465–1468. doi: 10.1097/00001756-199606170-00004. [DOI] [PubMed] [Google Scholar]
- LOETSCHER H., NIEDERHAUSER O., KEMP J., GILL R. Is caspase-3 inhibition a valid therapeutic strategy in cerebral ischemia. Drug Discov. Today. 2001;6:671–680. doi: 10.1016/s1359-6446(01)01826-8. [DOI] [PubMed] [Google Scholar]
- MEYER S.L., BOZYCZKO-COYNE D., MALLYA S.K., SPAIS C.M., BIHOVSKY R., KAYWOOYA J.K., LANG D.M., SCOTT R.W., SIMAN R. Biologically active monomeric and heterodimeric recombinant human calpain I produced using the baculovirus expression system. Biochem. J. 1996;314:511–519. doi: 10.1042/bj3140511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITTL P.R., DI MARCO S., KREBS J.F., BAI X., KARANEWSKY D.S., PRIESTLE J.P., TOMASELLI K.J., GRUTTER M.G. Structure of recombinant human CPP32 in complex with the tetrapeptide acetyl-Asp-Val-Ala-Asp fluoromethyl ketone. J. Biol. Chem. 1997;272:6539–6547. doi: 10.1074/jbc.272.10.6539. [DOI] [PubMed] [Google Scholar]
- MOCANU M.M., BAXTER G.F., YELLON D.M. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br. J. Pharmacol. 2000;130:197–200. doi: 10.1038/sj.bjp.0703336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORITA T., KATO H., IWANAGA S., TAKADA K., KIMURA T. New fluorogenic substrates for alpha-thrombin, factor Xa, kallikreins, and urokinase. J. Biochem. (Tokyo) 1977;82:1495–1498. doi: 10.1093/oxfordjournals.jbchem.a131840. [DOI] [PubMed] [Google Scholar]
- MORRISON J.F., WALSH C.T. The behavior and significance of slow-binding enzyme inhibitors. Adv. Enzymol. Relat. Areas Mol. Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- MURAKAMI K., OHSAWA T., HIROSE S., TAKADA K., SAKAKIBARA S. New fluorogenic substrates for renin. Anal. Biochem. 1981;110:232–239. doi: 10.1016/0003-2697(81)90140-8. [DOI] [PubMed] [Google Scholar]
- NICHOLSON D.W., ALI A., THORNBERR N.A., VAILLANCOURT J.P., DING C.K., GALLANT M., GAREAU Y., GRIFFIN P.R., LABELLE M., LAZEBNIK Y.A., MUNDAY N.A., RAJU S.M., SMULSON M.E., YAMIN T.T., YU V.L., MILLER D.R. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- PRASAD C.V.C., PROUTY C.P., HOYER D., ROSS T.M., SALVINO S.M., AWAD M., GRAYBILL T.L., SCHMIDT S.J., OSIFO I.K., DOLLE R.E., HELASZEK C.T., MILLER R.E., ATOR M.A. Structural and stereochemical requirements of time-dependent inactivators of the interleukin-1 beta converting enzyme. Bioorg. Med. Chem. Lett. 1995;5:315–318. [Google Scholar]
- REED J.C. Apoptosis-based therapies. Nat. Rev. Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- REVESZ L., BRISWALTER C., HENG R., LEUTWILER A., MUELLER R., WUETHRICH H.J. Synthesis of P1 aspartate-based peptide acyloxymethyl and fluoromethyl ketones as inhibitors of interleukin-1 beta-converting enzyme. Tetrahedron Lett. 1994;35:9693–9696. [Google Scholar]
- ROBERTSON G.S., CROCKER S.J., NICHOLSON D.W., SCHULZ J.B. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–292. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ I., MATSUURA K., ODY C., NAGATA S., VASSALLI P. Systemic injection of a tripeptide inhibits the intracellular activation of CPP32-like proteases in vivo and fully protects mice against Fas-mediated fulminant liver destruction and death. J. Exp. Med. 1996;184:2067–2072. doi: 10.1084/jem.184.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTONDA J., NICHOLSON D.W., FAZIL K.M., GALLANT M., GAREAU Y., LABELLE M., PETERSON E.P., RASPER D.M., RUEL R., VAILLANCOURT J.P., THORNBERRY N.A., BECKER J.W. The three-dimensional structure of apopain/CPP32, a key mediator of apoptosis. Nat. Struct. Biol. 1996;3:619–625. doi: 10.1038/nsb0796-619. [DOI] [PubMed] [Google Scholar]
- SALVESEN G.S., DIXIT V.M. Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZ J.B., WELLER M., MOSKOWITZ M.A. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann. Neurol. 1999;45:421–429. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- TALANIAN R.V., BRADY K.D., CRYNS V.L. Caspases as targets for anti-inflammatory and anti-apoptotic drug discovery. J. Med. Chem. 2000;43:3351–3371. doi: 10.1021/jm000060f. [DOI] [PubMed] [Google Scholar]
- TCHOUPE J.R., MOREAU T., GAUTHIER F., BIETH J.G. Photometric or fluorometric assay of cathepsin B, L and H and papain using substrates with an aminotrifluoromethylcoumarin leaving group. Biochem. Biophys. Acta. 1991;1076:149–151. doi: 10.1016/0167-4838(91)90232-o. [DOI] [PubMed] [Google Scholar]
- THOMPSON C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A. Interleukin-1 beta converting enzyme. Methods Enzymol. 1994;244:615–631. doi: 10.1016/0076-6879(94)44045-x. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A. Caspases: key mediators of apoptosis. Chem. Biol. 1998;5:R97–R103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A., LAZEBNIK Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A., PETERSON E.P., ZHAO J.J., HOWARD A.D., GRIFFIN P.R., CHAPMAN K.T. Inactivation of interleukin-1 beta converting enzyme by peptide (acyloxy)methyl ketones. Biochemistry. 1994;33:3934–3940. doi: 10.1021/bi00179a020. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A., RANO T.A., PETERSON E.P., RASPER D.M., TIMKEY T., GARCIA-CALVO M., HOUTZAGER V.M., NORDSTROM P.A., ROY S., VAILLANCOURT J.P., CHAPMAN K.T., NICHOLSON D.W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- WEI Y., FOX T., CHAMBERS S.P., SINTCHAK J., COLL J.T., GOLEC J.M., SWENSON L., WILSON K.P., CHARIFSON P.S. The structures of caspases-1, -3, -7 and -8 reveal the basis for substrate and inhibitor selectivity. Chem. Biol. 2000;7:423–432. doi: 10.1016/s1074-5521(00)00123-x. [DOI] [PubMed] [Google Scholar]
- WELLINGTON C.L., HAYDEN M.R. Caspases and neurodegeneration: on the cutting edge of new therapeutic approaches. Clin. Genet. 2000;57:1–10. doi: 10.1034/j.1399-0004.2000.570101.x. [DOI] [PubMed] [Google Scholar]
- YAOITA H., OGAWA K., MAEHARA K., MARUYAMA Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998;97:276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN K.C., GREEN D.R. How cells die: apoptosis pathways. J. Allergy Clin. Immunol. 2001;108:S99–S103. doi: 10.1067/mai.2001.117819. [DOI] [PubMed] [Google Scholar]