Abstract
The administration of κ-opioid receptor antagonists, nor-binaltorphimine (norBNI) and 5′-guanidinonaltrindole (GNTI) enhanced allodynia in rats and mice after sciatic nerve ligation. In order to understand the mechanism underlying this effect, we examined the possible involvement of the endogenous ligand of κ-opioid receptor dynorphin.
The experiments were carried out on male Wistar rats and on Albino-Swiss mice. The rats had been implanted with a catheter 7 days earlier in the subarachnoid space of the spinal cord. Intrathecal (i.t.) administrations in mice were made by lumbar puncture. The animals were i.t. injected with norBNI, GNTI (κ-opioid receptor antagonists), dynorphin A1–17 antiserum (DYN A/S), ketamine (NMDA receptor antagonist) and their combinations. The nociceptive sensitivity was assessed using the mechanical (von Frey) and therma allodynia tests on days 2–4 and 8–10 after the sciatic nerve ligation.
Both antagonists, norBNI and GNTI, significantly enhanced mechanical and therma allodynia in rats and mice with neuropathic pain. The potentiation of allodynia after the administration of norBNI or GNTI was inhibited by earlier administration of DYN A/S or by ketamine.
Our results suggest that allodynia is mediated through nonopioid effect of the endogenous opioid peptide, dynorphin. The nonopioid action is potentiated by the blockade of κ-opioid receptors, and corresponding to the elevation of prodynorphin mRNA level in neuropathic pain. Furthermore, the potentiation of allodynia after the administration of the above drugs appears to be mediated through the activation of NMDA receptors directly by dynorphin.
Keywords: κ-Opioid receptor, norBNI, GNTI, dynorphin, allodynia, neuropathic pain
Introduction
Peripheral nerve injury can elicit abnormal pain characterized by allodynia, where normally non-noxious stimuli (mild warming, cooling or touch) elicit pain and hyperalgesia and where noxious stimuli (skin heating, cooling or strong mechanical stimuli) are perceived as more painful (Wang et al., 2001). The presence of allodynia and hyperalgesia in the neuropathic pain state is associated with neuroplastic alterations including changes in the spinal level of substance P, cholecystokinin and dynorphin. Identification of a precise mechanism underlying the development and maintenance of allodynia and hyperalgesia appears essential for the development of effective strategies for pharmacological treatment of the neuropathic pain state.
Our previous study analyzed the spinal antinociceptive and antiallodynic effects of opioid receptor agonists and antagonists after their acute and chronic intrathecal (i.t.) administration in a neuropathic pain model in the rat. We demonstrated that opioid receptor agonists dose-dependently antagonized allodynia, which developed after sciatic nerve injury, whereas μ-, δ-receptor antagonists did not show any effects (Przewlocka et al., 1999; Mika et al., 2001). Interestingly, we observed that the κ-opioid receptor antagonist norBNI significantly enhanced allodynia, and exhibited pronociceptive action. Also, Hao et al. (1998) observed similar results after norBNI administration. The effect seems to be associated with dynorphin, an endogenous ligand of the κ-opioid receptor, which may play an important role in the pathology of the neuropathic pain state. The following findings substantiate this statement: (1) neuropathic and other chronic (e.g., inflammatory) pain states are associated with the elevated spinal dynorphin content (Kajander et al., 1990; Bian et al., 1999; Malan et al., 2000), and elevated levels of endogenous dynorphin (Cox et al., 1985) or of prodynorphin mRNA (Przewlocki et al., 1988) are also a result of spinal cord trauma; (2) dynorphin is located in intrinsic neurons in laminae I, II and V of the spinal cord dorsal horn (Ruda et al., 1988), the locations which overlap the areas of occurrence of neurons responding to noxious input; (3) dynorphin may play a physiological role (antinociceptive action) via the opioid receptor (Przewlocki et al., 1983b; Stevens & Yaksh, 1986), as well as induce pathological effects (pronociceptive action) by activation of the NMDA receptor, that is, by a nonopioid mechanism (Vanderah et al., 1996; Laughlin et al., 1997). Even though some studies on the contribution of dynorphin to nociceptive transmission have been published (Vanderah et al., 1996; Yamamoto et al., 1997; Bian et al., 1999), its role in the prolonged neuropathic pain is still relatively poorly understood.
In order to validate the hypothesis that the proallodynic effect of κ-opioid receptor inhibition can be attributed not only to the particular antagonist norBNI, we also used in our study a new, more selective and more potent antagonist, GNTI, and tested both antagonists in rat and mouse models of neuropathic pain. We investigated also, using an in situ hybridization method, the changes in prodynorphin and κ-opioid receptor mRNA level in the dorsal horn of rats after the sciatic nerve ligation.
Methods
Animals
Male Wistar rats (200–350 g) and Albino-Swiss mice (25–30 g) were housed individually (rats) or in groups of six (mice) in polycarbonate cages with sawdust bedding under a standard 12-h/12-h light/dark cycle (lights on at 0800 hours) with food and water available ad libitum. All experiments were carried out according to the NIH Guide for Care and Use of Laboratory Animals, and are approved by the Local Bioethics Committee.
Surgical preparation
The rats were chronically implanted with i.t. catheters under pentobarbital anesthesia (60 mg kg−1, i.p.). The catheter consisted of a polyethylene tubing 12 cm long (PE 10, INTRAMEDIC, Clay Adams, Becton Dickinson and Company, Rutherford, NJ, U.S.A.; o.d.=0.4 mm, dead space=10 μl), sterilized by immersion in 70% ethanol, and fully flushed with sterile water prior to insertion. Rats were placed on the stereotaxic table (David Kopf), and an incision was made in the atlanto-occipital membrane. The catheter (7.8 cm of its length) was carefully introduced into the subarachnoid space at the rostral level of the spinal cord lumbar enlargement (L4–L5) according to Yaksh & Rudy (1976).
The i.t. injection procedure by lumbar puncture in unanesthetized mice was adapted from the method of Hylden & Wilcox (1980). The i.t. injections were performed with disposable 30 gauge 1/2-inch needles (Becton Dickinson and Company, Rutherford, NJ, U.S.A.) mated to a 10-μl Luer tip syringe (Hamilton, Reno, NV, U.S.A.).
Sciatic nerve injury was performed under pentobarbital anesthesia (60 mg kg−1, i.p.) 7 days after i.t. implantation of catheters in rats and under ether anesthesia in mice. The biceps femoris and the gluteus superficialis were separated, and the right sciatic nerve was exposed. The injury was produced by tying four (rats) or three (mice) ligations around the sciatic nerve, as described in rats by Bennett & Xie (1988) and in mice by Sommer & Schafers (1998). The ligatures (4/0 silk) were tied loosely around the nerve with 1 mm spacing. They were tied until they elicited a brief twitch in the respective hindlimb. In sham-operated groups, used only for biochemical study, identical dissection was performed, except that the sciatic nerve was not ligated. Testing procedures were carried out on days 2–4 and 8–10 after the injury.
Drugs
Chemicals and their sources were as follows: norBNI-Sigma Chemical Co. (St Louis, MO, U.S.A.), GNTI-Tocris Cookson Limited (Elliseville, MO, U.S.A.) and ketamine-Sigma Chemical Co. (St Louis, MO, U.S.A.). Although the dynorphin A1–17 antiserum ‘Goldy' (DYN A/S) (Professor V. Höllt; Max Planck Institute, Munich, Germany) that we used in the experiment was raised against dynorphin A1–17, it exhibited the same crossreactivity for dynorphin A1–13. The antiserum exhibited a negligible avidity for α-neoendorphin, β-neoendorphin and Leu-enkephalin.
Drug administrations
All drugs and dynorphin antiserum were dissolved in sterile water and were injected in a volume of 5 μl. NorBNI (1.4, 6.8, 13.6 nmol) and GNTI (0.02, 0.2, 1.8, 8.8 nmol) were i.t. administered in rats and mice (each dose to one experimental group of animals, n=8–10) on days 2–4 and 8–10 after nerve injury. The doses were calculated on the basis of experimental in vivo data of other authors (Jewett et al., 2001), and the IC50 values published in the papers of Stevens et al. (2000) and Jones & Portoghese (2000).
DYN A/S (1 : 1) was administered i.t. 30 min before norBNI (13.61 nmol i.t.) or GNTI (1.8 nmol i.t.) administration, whereas ketamine (73 nmol) was administered 5 min earlier.
All control animals (rats with sciatic nerve ligation) for behavioral experiments were injected i.t. with sterile water, and tested according to the same schedule as described below for the experimental groups. After completion of the experiment, the animals were killed with an overdose of pentobarbital. For measurement of changes in prodynorphin and κ-opioid receptor mRNA in neuropathic pain, the sham-operated rats and mice were used as control animals.
Behavioral tests
Mechanical allodynia (von Frey test)
Foot-withdrawal threshold in response to a mechanical stimulus was measured by the use of the automatic von Frey method (mod 1601CE, IITC Inc./Life Science Instruments, Woodland Hills, CA, U.S.A.). von Frey filament (rigid tip) is used to apply a slight pressure to the skin, ranging to 80 g. Rats and mice were placed in plastic cages with a wire net floor, and allowed to habituate for 5 min before the experiment. The von Frey filament was applied to the midplantar surface of the paw, as described by Starowicz et al. (2002) and Przewlocka et al. (2002). Each probe was applied three times to the paw. The mean of the baselines measured in eight rats with an automatic von Frey filament was 19.9±0.9 g and measured for comparison with von Frey filaments (Stoelting, Chicago, IL, U.S.A.) was 4.2±0.8 g in the same group of rats. The measurements were taken 15, 30, 60 and 120 min after i.t. drug injection on days 2–4 and 8–10 after the sciatic nerve ligation. The reaction after the load of 0.1 g was regarded as maximal allodynia.
Thermal allodynia
For assessment of thermal allodynia in rats, the latency of hindlimb withdrawal evoked by thermal stimulation (cold water allodynia test) was determined according to the method that has been previously described in detail by Hunter et al. (1997). The animal responded by lifting the paw on the injury side out of the cold water. Each animal was placed onto a metal stage submerged to a depth of 1.5 cm in ice-cold water (0°C). The animal responded by lifting only the paw on the injury side out of the water. In no case rats responded by lifting the contralateral paw out of the water. The measurements were taken 15, 30, 60 and 120 min after i.t. drug injection on days 2–4 and 8–10 after the sciatic nerve ligation. The cutoff latency for the test was 30 s.
For assessment of thermal allodynia in mice, the hindlimb withdrawal response evoked by thermal stimulation (acetone test) was determined according to the method that has been previously described in detail by Choi et al. (1994). The response to a 10 μl drop of acetone from PE10 tubing applied to each hindpaw was ranked: 0=no response; 1=foot lifted and/or light shake; 2=hard/long shake, or squeaking. The measurements were taken 15 min after i.t. drug injection on days 2–4 and 8–10 after the sciatic nerve ligation. The animals were used only for one measurement.
Biochemical test
Probes
Specific cDNA fragments of rat prodynorphin (n.t. 60–795; Acc. No. M32784) and of rat κ-opioid receptor (n.t. 1224–2136; Acc. No. DI6829) subcloned in pGEM 4 and pGEM4Z were used for in vitro transcription as described previously (Schäfer et al., 1994). The probes for prodynorphin mRNA and κ-opioid receptor mRNA were labeled by 35S-UTP or 33P-UTP (Amersham, Braunschweig) respectively. After transcription, the probes were subjected to mild alkaline hydrolysis, as described by Angerer et al. (1987).
Tissue preparation for in situ hybridization
The spinal cord lumbar enlargement (L4–L5) was removed 2 and 8 days after sciatic nerve ligation (or sham operation) and frozen on dry ice. Then the tissue was cut into 12 μm thick slices on a cryostat microtome (Leica Microsystems, Nussloch, Germany).
In situ hybridization
In situ hybridization was performed as described previously (Schfer et al., 1993; 1994). Briefly, the sections were fixed with 4% paraformaldehyde, acetylate and dehydrated. They were hybridized at 60°C with radioactively labeled cRNA probes at a final concentration of 5 × 104 dpm μl−1. After hybridization, the slices were washed with SSC at decreasing concentrations, and treated with RNase T1 (Boehringer, Mannheim, Germany), and then exposed to Hyperfilm-βmax films (Amersham) for 1–4 days.
Data analysis
Behavioral data were presented as the per cent of maximal possible allodynia (%MPA±s.e.m.), using the equation %MPA=[(BL−TL)/(BL−CUTOFF)] × 100%, where BL is the baseline latency and TL is the respective test latency or as a per cent of maximal possible score (%MPS±s.e.m.), where the highest score was assumed to represent 100%.
Analysis of in situ hybridization autoradiograms was conducted using the MCID M4 image analysis system (Imaging Research Inc., Ontario, Canada). The measurements were carried out in the dorsal horn (laminae I–VI). The results from 30–60 slices (3–7 animals per group; approx. 10–20 slices per rat) were analyzed. The radioactive standard ARC 146B (American Radiolabeled Chemicals Inc., St Louis, MO, U.S.A.) was used for calibration. The final data are expressed as mean nCi g−1 tissue (±s.e.m.).
A group included 8–10 animals for the behavioral study and 3–7 animals for biochemical experiments. The results of the experiments were evaluated by one-way analysis of variance (ANOVA). The differences between groups were further analyzed by Bonferroni post hoc test.
Results
Behavioral study
Mechanical allodynia
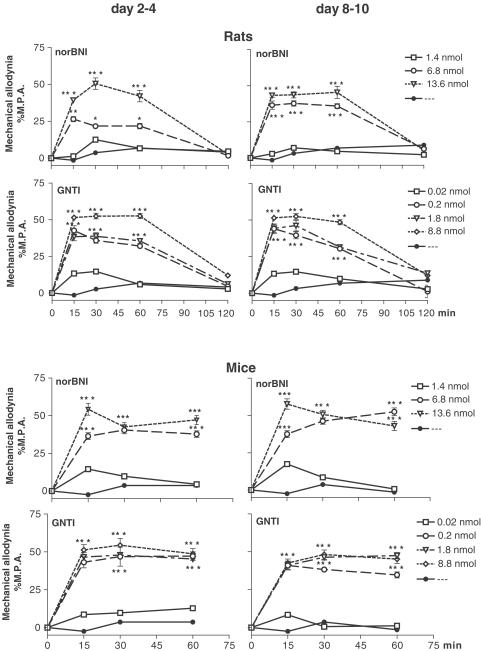
Both antagonists of the κ-opioid receptor, norBNI (6.8, 13.6 nmol i.t.) and GNTI (0.2, 1.8, 8.8 nmol i.t.), significantly and dose-dependently potentiated mechanical allodynia on days 2–4 and 8–10 after sciatic nerve ligation, as measured by von Frey test in rats and mice. The maximum effect was observed after 15 min. The enhancement of allodynia lasted 60 min after drug injection in both species (Figure 1).
Figure 1.
Effect of norBNI (1.4, 6.8, 13.6 nmol i.t.) and GNTI (0.02, 0.2, 1.8, 8.8 nmol i.t.) administration on days 2–4 and 8–10 after ligation of the sciatic nerve, as measured by mechanical allodynia test (von Frey test) in rats and mice. The reaction in the control group injected with solvent and subjected to sciatic nerve injury was 25.1±1.3 (rats) and 24.3±1.1 g (mice) on days 2–4; 27.5±2.3 (rats) and 25.5±3.6 g (mice) on days 8–10. The results are shown as the per cent of maximal possible allodynia (%MPA±s.e.m.) of 8–10 animals per group. *P<0.05, ***P<0.001 in comparison with the solvent-injected group of animals with the injured sciatic nerve (ANOVA, Bonferroni test).
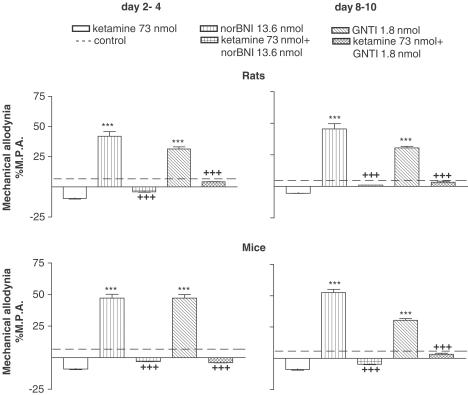
The above effects of norBNI (13.6 nmol i.t.) or GNTI (1.8 nmol i.t.) were inhibited by earlier administration of DYN A/S (Figure 2) as well as by ketamine (73 nmol i.t.), which is a NMDA receptor antagonist (Figure 3). These effects were observed 15, 30 and 60 min after drug injection in both species, but only the data obtained after 60 min were chosen for presentation in the graphs (Figures 2, 3).
Figure 2.
Effect of DYN A/S pretreatment on the norBNI (13.6 nmol i.t.) and GNTI (1.8 nmol i.t.)-induced enhancement of allodynia on days 2–4 and 8–10 after ligation of the sciatic nerve, as measured by mechanical allodynia test (von Frey test) in rats and mice. The results are shown as the per cent of maximal possible allodynia (%MPA±s.e.m.) of 8–10 animals per group. ***P<0.001 in comparison with the solvent-injected group of animals (broken line) with the injured sciatic nerve (ANOVA, Bonferroni test). +++P<0.001 in comparison with the group of animals with the injured sciatic nerve after i.t. administration of effective dose of norBNI (13.6 nmol i.t.) or GNTI (1.8 nmol i.t.) (ANOVA, Bonferroni test).
Figure 3.
Effect of ketamine (73 nmol i.t.) pretreatment on the norBNI- (13.6 nmol i.t.) and GNTI- (1.8 nmol i.t.)-induced enhancement of allodynia on days 2–4 and 8–10 after ligation of the sciatic nerve, as measured by mechanical allodynia test (von Frey test) in rats and mice. The results are shown as the per cent of maximal possible allodynia (%MPA±s.e.m.) of 8–10 animals per group. ***P<0.001 in comparison with the solvent-injected group of animals (broken line) with the injured sciatic nerve (ANOVA, Bonferroni test). +++P<0.001 in comparison with the group of animals with the injured sciatic nerve after i.t. administration of an effective dose of norBNI (13.6 nmol) and GNTI (1.8 nmol) (ANOVA, Bonferroni test).
Thermal allodynia
The significant, dose-dependent enhancement of thermal allodynia on days 2–4 and 8–10 after ligation of the sciatic nerve in rats and mice was observed after norBNI (6.8, 13.6 nmol. i.t.) and GNTI (0.2, 1.8, 8.8 nmol i.t.) administration (Table 1 ). Earlier injection of DYN A/S or ketamine (73 nmol i.t.), a NMDA receptor antagonist, reversed the pronociceptive action (Table 1). All the above effects were observed 15, 30 and 60 min after drug injection in both species, but only the data obtained after 60 min were chosen for presentation in Table 1.
Table 1.
(I) Effect of norBNI (1.4, 6.8, 13.6 nmol i.t.), GNTI (0.02, 0.2, 1.8, 8.8 nmol i.t.), ketamine (73 nmol i.t.), DYN A/S and sterile water (—) administration on days 2–4 and 8–10 after ligation of the sciatic nerve, as measured by thermal allodynia tests in rats and mice; (II) effect of pretreatment with DYN A/S and ketamine (73 nmol i.t.) on the effective dose of norBNI (13.6 nmol i.t.) and GNTI (1.8 nmol i.t.) on days 2–4 and 8–10 after ligation of the sciatic nerve, as measured by thermal allodynia tests in rats and mice
| Rats thermal allodynia %MPA | Mice thermal allodynia %MPS | |||
|---|---|---|---|---|
| Drugs | Days 2–4 | Days 8–10 | Days 2–4 | Days 8–10 |
| I | ||||
| — | 8.5±2.8 | 5.1±2.7 | 37.5±1.5 | 48.5±3.2 |
| norBNI 6.8 nmol | 42.1±3.3* | 49.2±6.8* | 58.5±1.5* | 75.0±8.1* |
| norBNI 13.6 nmol | 47.3±6.3* | 62.0±6.1* | 66.5±1.5* | 91.7±5.0* |
| GNTI 0.02 nmol | 1.2±0.3 | 1.1±0.3 | 41.6±5.3 | 41.5±3.8 |
| GNTI 0.2 nmol | 44.1±9.8* | 49.3±8.3* | 83.5±10.4* | 79.2±9.9* |
| GNTI 1.8 nmol | 46.1±7.1* | 51.7±7.5* | 91.5±8.5* | 87.5±8.5* |
| GNTI 8.8 nmol | 56.4±4.0* | 56.4±4.0* | 91.5±6.6* | 91.7±5.2* |
| DYN A/S | 7.1±4.7 | 9.4±1.1 | 37.5±1.5 | 41.5±1.4 |
| Ketamine 73 nmol | −9.6±2.2 | −5.6±1.2 | 37.5±1.0 | 37.5±1.6 |
| II | ||||
| DYN A/S+norBNI 13.6 nmol | −5.8±0.7† | −7.0±0.6† | 41.7±9.5† | 41.7±1.6† |
| Ketamine 73 nmol+norBNI 13.6 nmol | 0.3±0.0† | −2.4±0.6† | 33.3±6.2† | 41.7±8.7† |
| DYN A/S+GNTI 1.8 nmol | −2.6±0.6† | −14.7±2.1† | 50.0±10.7† | 54.2±13.5† |
| Ketamine 73 nmol+GNTI 1.8 nmol | −6.4±1.0† | −2.4±0.4† | 50.0±4.5† | 45.8±4.8† |
The results are shown as the per cent of maximal possible allodynia (%MPA±s.e.m.) or as the per cent of maximal possible score (%MPS±s.e.m.) of 8–10 animals per group.
P<0.05 in comparison with the solvent-injected group of animals with the injured sciatic nerve (ANOVA, Bonferroni test).
P<0.05 in comparison with the group of animals after i.t. administration of an effective dose of norBNI (13.6 nmol) and GNTI (1.8 nmol) with the injured sciatic nerve (ANOVA, Bonferroni test).
Biochemical study
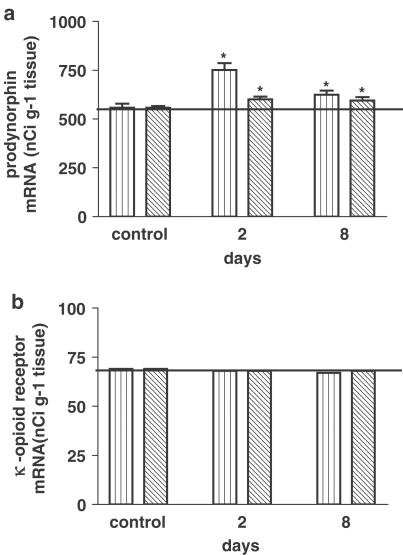
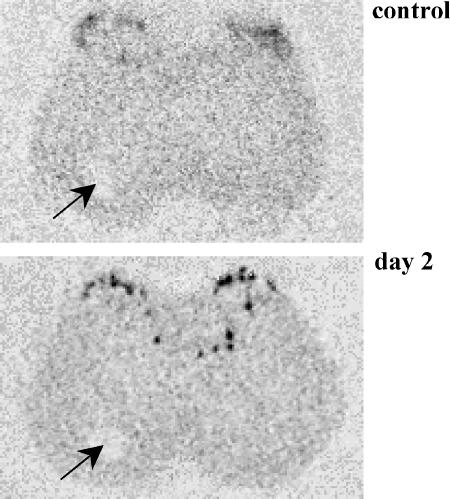
Prodynorphin mRNA level on the ipsilateral side 2 and 8 days following ligation of the sciatic nerve was significantly increased in laminae I–VI of the rat lumbar spinal cord (Figure 4a). Our results showed not only the increase in the level of prodynorphin mRNA in the lumbar part (L4–L6) of the spinal cord following ligation of the sciatic nerve, but also differences in the expression pattern of prodynorphin mRNA. In addition to the increase in the level of prodynorphin mRNA in laminae I–II, its increased expression in the deeper laminae IV–VI was observed (Figure 5), and no changes in the level of κ-opioid receptor mRNA were noted (Figure 4b).
Figure 4.
Influence of ligation of the sciatic nerve on the level of prodynorphin mRNA (a) and κ-opioid receptor mRNA (b) in laminae I–VI in the lumbar spinal cord on days 2 and 8 after the injury. The level of mRNA is presented as the mean nCi g−1 tissue ±s.e.m. from 30 to 60 slices (3–7 animals per group). *P<0.05 in comparison with the group of control (sham-operated) animals (ANOVA, Bonferroni test).
Figure 5.
Photomicrographs illustrating the pattern of prodynorphin expression (control and 2 days after sciatic nerve injury) in the lumbar part of the spinal cord after the injury. An increase in the level of prodynorphin mRNA was observed in the ipsilateral superficial dorsal horn, and was also evident in the deep dorsal horn. The contralateral side is marked by the hole made in frozen tissue (an arrow).
Discussion
The data presented here demonstrated that κ-opioid receptor antagonists, norBNI, as well as new more potent and more selective GNTI, profoundly enhanced mechanical and thermal allodynia in rats and mice after the sciatic nerve ligation. Hao et al. (1998) showed that i.t. norBNI provoked reversible augmentation of mechanical and cold allodynia response in spinally injured rats. Also, Ossipov et al. (1996) showed the enhancement of hyperalgesia after norBNI administration in inflammatory pain in rats. However, it was not clear if the effect is specific only for norBNI or rather for κ-opioid receptor blockade. Since some in vivo studies have shown partial selectivity of norBNI at the μ-receptor (Birch et al., 1987; Hao et al., 1998) and that the antagonism by norBNI at the κ-receptor is time-dependent (Butelman et al., 1993), we used a new κ-opioid receptor antagonist more selective and more potent than the prototypical norBNI (Jones & Portoghese, 2000; Stevens et al., 2000). Our present time course study of the effect of norBNI as well as GNTI revealed that the effect developed rapidly after i.t. administration and was maintained for 1 h. Thus, this finding validates the hypothesis that the enhancement of allodynia involves the antagonism at the κ-opioid receptors. The effect was observed at two time points, that is, on days 2–4 and 8–10 after the sciatic nerve injury.
Some authors have suggested that the activation of spinal κ-opioid receptors increases the excitability of the dorsal horn neurons (Hylden et al., 1991). Receptor-binding studies have also shown that the density of spinal κ-opioid receptors is low in the dorsal spinal cord (less than 10% of total opioid receptor density; Hao et al., 1998). Interestingly, we have recently found that ligation of the sciatic nerve had no influence on the level of the κ-opioid receptor mRNA. The observation confirms those of Wang et al. (2001), who showed the lack of changes in opioid receptor density and G-protein activation after the sciatic nerve injury. Agonists of the κ-opioid receptor, unlike μ and δ agonists, show no antiallodynic activity when applied i.t. to rats in L4–L5 tight nerve ligation model (Lee et al., 1995), as well as to rats with spinal injury. Taken together, the spinal κ-opioid receptors seem not to be involved in the development of chronic allodynia after nerve injury (spinal cord injury), and the effects that had been observed were not mediated directly via the κ-opioid receptor.
Thus, allodynia that was potently enhanced by κ-opioid receptor antagonists may be induced by nonopioid action of the endogenous ligand of κ-opioid receptor (Figure 6). Other authors demonstrated nonopioidergic mechanisms of dynorphin effects (Przewlocki et al., 1983a; Chen et al., 1995; Laughlin et al., 1997). I.t. dynorphin and related peptides have complex effects on the spinal cord function, including excitation, depression and neurotoxicity, which makes antinociceptive tests difficult to perform and can lead to divergent results. Single, i.t. dynorphin1–17 administration, especially at high doses, induced long-lasting mechanical and thermal allodynia and mechanical hyperalgesia in rats (Vanderah et al., 1996) and mice (Laughlin et al., 1997). Moreover, it was shown that the administration of naloxone, a nonselective opioid receptor antagonist, did not inhibit the allodynia development. Interestingly, the injection of dynorphin2–17, the peptide devoid of tyrosine required for the binding at opioid receptors, induced allodynia as well (Vanderah et al., 1996). Similarly, dynorphin-induced paralysis was reduced by NMDA receptor antagonist, but not by opioid receptor antagonists (Faden & Jacobs, 1984; Caudle & Isaac, 1987). The above-mentioned results indicate that dynorphin is capable of interacting also with nonopioidergic receptors, notably NMDA receptors, which was postulated by some authors (Chen et al., 1995; Vanderah et al., 1996; Laughlin et al., 1997). Dynorphin augmented [3H]glutamate binding to NMDA receptors, and blocked the increase in [3H]MK801 binding induced by glutamate and glycine (Massardier & Hunt, 1989). Patch-clamp analysis of isolated trigeminal neurons revealed that dynorphin attenuated NMDA receptor-mediated currents by shortening the mean open time and decreasing the probability of channel opening, apparently through interaction with the redox-modulatory site (Chen et al., 1995). Recently, however, it has been discovered that dynorphin and its fragments may directly bind to the closed/desensitized state of the NMDA receptor, although the physiological relevance of such binding remains to be established (Tang et al., 1999). In our experiment, ketamine, a selective NMDA receptor antagonist, as well as in another study MK-801, a noncompetitive NMDA receptor antagonist (Vanderah et al., 1996), or LY235959, a competitive NMDA receptor antagonist (Laughlin et al., 1997), reversed the enhancement of allodynia, apparently caused by dynorphin (Figure 6). Post-treatment with MK801 transiently blocked dynorphin-induced allodynia, which supported the existence of the specific interaction between dynorphin and NMDA receptor (Laughlin et al., 1997). These facts can lead to the conclusion that on the one hand, dynorphin may play a physiological role acting via opioid receptors, and on the other, it can cause pathological effects stimulating NMDA receptor both in acute and chronic pain (Laughlin et al., 1997).
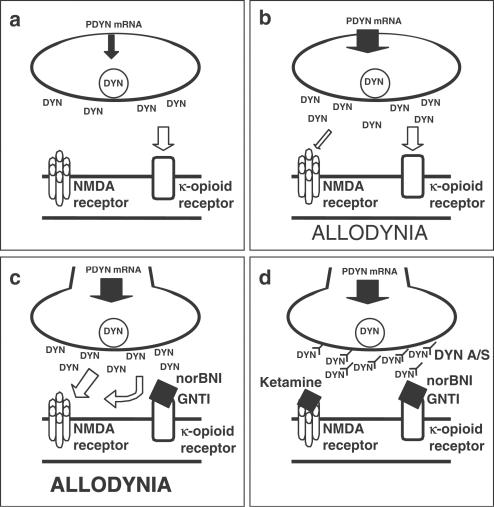
Figure 6.
Schematic illustration of the dynamic interaction between dynorphin and κ-opioid and NMDA receptors. (a) Normal state. Prodynorphin mRNA expression is at the physiological level. Dynorphin supply is sufficient only to activate κ-opioid receptors (opioid action). (b) Injury to the sciatic nerve. The level of prodynorphin mRNA is increased (as evidenced by the present study and literature data); therefore, more dynorphin is released and, apart from κ-opioid receptor activation (opiod action), the activation of NMDA receptors occurs (nonopioid action). As a result of this situation, the allodynia develops as a characteristic feature of neuropathic pain. (c) Injury to the sciatic nerve+administration of the κ-receptor antagonists norBNI or GNTI. The administration of κ-opioid antagonists blocks the opioid action of dynorphin and potentiates allodynia by a more potent activation of NMDA receptors by dynorphin. (d) Injury to the sciatic nerve+administration of the κ-receptor antagonists norBNI or GNTI+ketamine or DYN A/S. The effect described in (c) is antagonized by DYN A/S and by administration of ketamine, the NMDA receptor antagonist. These data explain that endogenous dynorphin is responsible for the abnormal pain sensations in neuropathic pain. Antagonists of the κ-opioid receptors worsen the situation because of potentiation of the nonopioid effects of dynorphin.
The endogenous peptide dynorphin might be pronociceptive in chronic pain states, and might play a role in the development of spinal neurological dysfunction (Faden, 1990; Bian et al., 1999; Malan et al., 2000). In the present study, DYN A/S (administrated 30 min prior to the administration of norBNI or GNTI) blocked the enhancement of allodynia (Figure 6d). These findings are in agreement with the results presented by Malan et al. (2000). The effect of DYN A/S, which parallels those seen with MK-801, suggests that dynorphin may interact with the NMDA receptor (Wegert et al., 1997; Bian et al., 1999), and this results in allodynia (Figure 6c). Moreover, the study in prodynorphin knockout mice indicated that although the action of dynorphin was not required for the initiation of the neuropathic pain state, the upregulation and presence of the peptide was pronociceptive and critical for the maintenance of such abnormal, nerve injury-evoked pain (Wang et al., 2001).
Dynorphin interaction with the NMDA receptor can be promoted by the elevation of prodynorphin level in neuropathic pain, as was demonstrated in the present study and by other authors, after spinal cord injury (Cox et al., 1985) and during peripheral inflammation (Iadarola et al., 1988). The elevated level of prodynorphin together with the administration of κ-opioid receptor antagonists may be a reason for strong nonopioid action of the endogenous peptide (Figure 6). Our in situ hybridization studies are in agreement with the results of Northern blot analysis performed by Draisci et al. (1991), who showed an increase in prodynorphin mRNA level after the sciatic nerve ligation. Moreover, our observations demonstrated that in neuropathic pain, prodynorphin mRNA expression was elevated not only in laminae I–II, but also in deeper laminae of the spinal cord (IV–VI), which can be considered an element of adaptive process in response to chronic nociceptive stimuli, and can reflect the changes in the organization of pain-transmitting nerve tracts. The highest prodynorphin level in laminae I–II and IV–VI would suggest a lesser role of dynorphin system in the transmission of acute painful stimuli, and its higher significance in the case of tactile stimuli. Nevertheless, this fact confirms its participation in chronic pain, accompanied by the changes in the perception of sensory stimuli. The changes in the prodynorphin system in neuropathic pain could be responsible for allodynia and hyperalgesia (Wang et al., 2001), and NMDA receptors appear to be crucial in this process.
In summary, in the present study we showed that the administration of κ-opioid receptor antagonists norBNI and GNTI enhanced allodynia in rats and mice after the sciatic nerve ligation. The results indicate that allodynia is mediated through nonopioid effect of the endogenous opioid peptide dynorphin. This nonopioid action of dynorphin is potentiated by the blockade of κ-opioid receptor and by the elevation of prodynorphin level in neuropathic pain. Furthermore, this effect appears to be mediated through the activation of NMDA receptor directly by dynorphin.
Acknowledgments
This research was supported by statutory funds from the State Committee for Scientific Research (KBN; Warsaw) and partly by the German Research Foundation (DFG, SFB 297). We thank Professor V. Höllt for generously providing us with dynorphin A1–17 antiserum and M.Sc. M. Maj for drawing Figure 6.
Abbreviations
- DYN A/S
dynorphin A1–17 antiserum
- GNTI
5′-guanidinonaltrindole
- i.p.
intraperitoneal
- i.t.
intrathecal
- %MPA
per cent of maximal possible allodynia
- %MPS
per cent of maximal possible score
- norBNI
nor-binaltorphimine
References
- ANGERER L.M., COX K.H., ANGERER R.C. Demonstration of tissue-specific gene expression by in situ hybridization. Methods Enzymol. 1987;152:649–661. doi: 10.1016/0076-6879(87)52071-7. [DOI] [PubMed] [Google Scholar]
- BENNETT G.J., XIE Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- BIAN D., OSSIPOV M.H., IBRAHIM M., RAFFA R.B., TALLARIDA R.J., MALAN T.P., JR, LAI J., PORRECA F. Loss of antiallodynic and antinociceptive spinal/supraspinal morphine synergy in nerve-injured rats: restoration by MK-801 or dynorphin antisera. Brain Res. 1999;831:55–63. doi: 10.1016/s0006-8993(99)01393-1. [DOI] [PubMed] [Google Scholar]
- BIRCH P.J., HAYES A.G., SHEEHAN M.J., TYERS M.B. Norbinaltorphimine: antagonist profile at kappa opioid receptors. Eur. J. Pharmacol. 1987;144:405–408. doi: 10.1016/0014-2999(87)90397-9. [DOI] [PubMed] [Google Scholar]
- BUTELMAN E.R., NEGUS S.S., AI Y., DE COSTA B.R., WOODS J.H. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J. Pharmacol. Exp. Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- CAUDLE R.M., ISAAC L. Intrathecal dynorphin(1–13) results in an irreversible loss of the tail-flick reflex in rats. Brain Res. 1987;435:1–6. doi: 10.1016/0006-8993(87)91579-4. [DOI] [PubMed] [Google Scholar]
- CHEN L., GU Y., HUANG L.Y. The mechanism of action for the block of NMDA receptor channels by the opioid peptide dynorphin. J. Neurosci. 1995;15:4602–4611. doi: 10.1523/JNEUROSCI.15-06-04602.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI Y., YOON Y.W., NA H.S., KIM S.H., CHUNG J.M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- COX B.M., MOLINEAUX C.J., JACOBS T.P., ROSENBERGER J.G., FADEN A.I. Effects of traumatic injury on dynorphin immunoreactivity in spinal cord. Neuropeptides. 1985;5:571–574. doi: 10.1016/0143-4179(85)90082-4. [DOI] [PubMed] [Google Scholar]
- DRAISCI G., KAJANDER K.C., DUBNER R., BENNETT G.J., IADAROLA M.J. Up regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Res. 1991;560:186–192. doi: 10.1016/0006-8993(91)91231-o. [DOI] [PubMed] [Google Scholar]
- FADEN A.I. Opioid and nonopioid mechanisms may contribute to dynorphin's pathophysiological actions in spinal cord injury. Ann. Neurol. 1990;27:67–74. doi: 10.1002/ana.410270111. [DOI] [PubMed] [Google Scholar]
- FADEN A.I., JACOBS T.P. Dynorphin-related peptides cause motor dysfunction in the rat through a non-opiate action. Br. J. Pharmacol. 1984;81:271–276. doi: 10.1111/j.1476-5381.1984.tb10074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAO J.X., YU W., XU X.J. Evidence that spinal endogenous opioidergic systems control the expression of chronic pain-related behaviors in spinally injured rats. Exp. Brain Res. 1998;118:259–268. doi: 10.1007/s002210050280. [DOI] [PubMed] [Google Scholar]
- HUNTER J.C., GOGAS K.R., HEDLEY L.R., JACOBSON L.O., KASSOTAKIS L., THOMPSON J., FONTANA D.J. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur. J. Pharmacol. 1997;324:153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- HYLDEN J.L., NAHIN R.L., TRAUB R.J., DUBNER R. Effects of spinal kappa-opioid receptor agonists on the responsiveness of nociceptive superficial dorsal horn neurons. Pain. 1991;44:187–193. doi: 10.1016/0304-3959(91)90136-L. [DOI] [PubMed] [Google Scholar]
- HYLDEN J.L., WILCOX G.L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- IADAROLA M.J., BRADY L.S., DRAISCI G., DUBNER R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- JEWETT D.C., GRACE M.K., JONES R.M., BILLINGTON C.J., PORTOGHESE P.S., LEVINE A.S. The kappa-opioid antagonist GNTI reduces U50,488-, DAMGO-, and deprivation-induced feeding, but not butorphanol- and neuropeptide Y-induced feeding in rats. Brain Res. 2001;909:75–80. doi: 10.1016/s0006-8993(01)02624-5. [DOI] [PubMed] [Google Scholar]
- JONES R.M., PORTOGHESE P.S. 5'-Guanidinonaltrindole, a highly selective and potent kappa-opioid receptor antagonist. Eur. J. Pharmacol. 2000;396:49–52. doi: 10.1016/s0014-2999(00)00208-9. [DOI] [PubMed] [Google Scholar]
- KAJANDER K.C., SAHARA Y., IADAROLA M.J., BENNETT G.J. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 1990;11:719–728. doi: 10.1016/0196-9781(90)90187-a. [DOI] [PubMed] [Google Scholar]
- LAUGHLIN T.M., VANDERAH T.W., LASHBROOK J., NICHOLS M.L., OSSIPOV M.H., PORRECA F., WILCOX G.L. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- LEE Y.W., CHAPLAN S.R., YAKSH T.L. Systemic and supraspinal, but not spinal, opiates suppress allodynia in a rat neuropathic pain model. Neurosci. Lett. 1995;199:111–114. doi: 10.1016/0304-3940(95)12034-2. [DOI] [PubMed] [Google Scholar]
- MALAN T.P., OSSIPOV M.H., GARDELL L.R., IBRAHIM M., BIAN D., LAI J., PORRECA F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- MASSARDIER D., HUNT P.F. A direct non-opiate interaction of dynorphin-(1–13) with the N-methyl-D-aspartate (NMDA) receptor. Eur. J. Pharmacol. 1989;170:125–126. doi: 10.1016/0014-2999(89)90149-0. [DOI] [PubMed] [Google Scholar]
- MIKA J., PRZEWLOCKI R., PRZEWLOCKA B. The role of δ-opioid receptor subtypes in neuropathic pain. Eur. J. Pharmacol. 2001;415:31–37. doi: 10.1016/s0014-2999(01)00814-7. [DOI] [PubMed] [Google Scholar]
- OSSIPOV M.H., KOVELOWSKI C.J., WHEELER-ACETO H., COWAN A., HUNTER J.C., LAI J., MALAN T.P., JR, PORRECA F. Opioid antagonists and antisera to endogenous opioids increase the nociceptive response to formalin: demonstration of an opioid kappa and delta inhibitory tone. J. Pharmacol. Exp. Ther. 1996;277:784–788. [PubMed] [Google Scholar]
- PRZEWLOCKA B., MIKA J., LABUZ D., TOTH G., PRZEWLOCKI R. Spinal analgesic action of endomorphins in acute, inflammatory and neuropathic pain in rats. Eur. J. Pharmacol. 1999;19:189–196. doi: 10.1016/s0014-2999(98)00956-x. [DOI] [PubMed] [Google Scholar]
- PRZEWLOCKA B., SIEJA A., STAROWICZ K., MAJ M., BILECKI W., PRZEWLOCKI R. Knockdown of spinal opioid receptors by antisense targeting beta-arrestin reduces morphine tolerance and allodynia in rat. Neurosci. Lett. 2002;25:107–110. doi: 10.1016/s0304-3940(02)00246-x. [DOI] [PubMed] [Google Scholar]
- PRZEWLOCKI R., HAARMANN I., NIKOLARAKIS K., HERZ A., HOLLT V. Prodynorphin gene expression in spinal cord is enhanced after traumatic injury in the rat. Mol. Brain Res. 1988;4:37–41. doi: 10.1016/0169-328x(88)90016-2. [DOI] [PubMed] [Google Scholar]
- PRZEWLOCKI R., SHEARMAN G.T., HERZ A. Mixed opioid/nonopioid effects of dynorphin and dynorphin releated peptides after their intrathecal injections in rats. Neuropeptides. 1983a;3:233–240. doi: 10.1016/0143-4179(83)90019-7. [DOI] [PubMed] [Google Scholar]
- PRZEWLOCKI R., STALA L., GRECZEK M., SHEARMAN G.T., PRZEWLOCKA B. Analgesic effect of mu, delta and kappa opiate agonists and particularly dynorphin at the spinal level. Life Sci. 1983b;33:649–652. doi: 10.1016/0024-3205(83)90586-6. [DOI] [PubMed] [Google Scholar]
- RUDA M.A., IADAROLA M.J., COHEN L.V., YOUNG W.S., III In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 1988;85:622–626. doi: 10.1073/pnas.85.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHÄFER M.K., BETTE M., ROMEO H., SCHWAEBLE W., WEIHE E. Localization of kappa-opioid receptor mRNA in neuronal subpopulations of rat sensory ganglia and spinal cord. Neurosci. Lett. 1994;167:137–140. doi: 10.1016/0304-3940(94)91046-4. [DOI] [PubMed] [Google Scholar]
- SCHÄFER M.K., DAY R., CULLINAN W.E., CHRETIEN M., SEIDAH N.G., WATSON S.J. Gene expression of prohormone and proprotein convertases in the rat CNS: a comparative in situ hybridization analysis. J. Neurosci. 1993;13:1258–1279. doi: 10.1523/JNEUROSCI.13-03-01258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMMER C., SCHAFERS M. Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- STAROWICZ K., PRZEWLOCKI R., GISPEN W.H., PRZEWLOCKA B. Modulation of melanocortin-induced changes in spinal nociception by mu-opioid receptor agonist and antagonist in neuropathic rats. Neuroreport. 2002;13:2447–2452. doi: 10.1097/00001756-200212200-00015. [DOI] [PubMed] [Google Scholar]
- STEVENS C.W., YAKSH T.L. Dynorphin A and related peptides administered intrathecally in the rat: a search for putative kappa opiate receptor activity. J. Pharmacol. Exp. Ther. 1986;238:833–838. [PubMed] [Google Scholar]
- STEVENS W.C., JR, JONES R.M., SUBRAMANIAN G., METZGER T.G., FERGUSON D.M., PORTOGHESE P.S. Potent and selective indolomorphinan antagonists of the kappa-opioid receptor. J. Med. Chem. 2000;43:2759–2769. doi: 10.1021/jm0000665. [DOI] [PubMed] [Google Scholar]
- TANG Q., GANDHOKE R., BURRITT A., HRUBY V.J., PORRECA F., LAI J. High-affinity interaction of (des-Tyrosyl)dynorphin A(2–17) with NMDA receptors. J. Pharmacol. Exp. Ther. 1999;291:760–765. [PubMed] [Google Scholar]
- VANDERAH T.W., LAUGHLIN T., LASHBROOK J.M., NICHOLS M.L., WILCOX G.L., OSSIPOV M.H., MALAN T.P., JR, PORRECA F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68:275–281. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- WANG Z., GARDELL L.R., OSSIPOV M.H., VANDERAH T.W., BRENNAN M.B., HOCHGESCHWENDER U., HRUBY V.J., MALAN T.P., JR, LAI J., PORRECA F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J. Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEGERT S., OSSIPOV M.H., NICHOLS M.L., BIAN D., VANDERAH T.W., MALAN T.P., JR, PORRECA F. Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats. Pain. 1997;71:57–64. doi: 10.1016/s0304-3959(97)03337-x. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., RUDY T.A. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N., KIMURA S. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by unilateral constriction injury to the sciatic nerve in the rat. Neurosci. Lett. 1997;224:107–110. doi: 10.1016/s0304-3940(97)13475-9. [DOI] [PubMed] [Google Scholar]