Abstract
The list of pathophysiological conditions associated with the overproduction of superoxide expands every day. Much of the knowledge compiled on the role of this radical in disease has been gathered using the native superoxide dismutase enzyme and, more recently, by the use of superoxide dismutase knockout models or transgenic models that overexpress the various isoforms of the enzyme. Although the native enzyme has shown promising anti-inflammatory properties in both preclinical and clinical studies, there were drawbacks and issues associated with its use as a therapeutic agent and pharmacological tool. Based on the concept that removal of superoxide modulates the course of inflammation, synthetic, low-molecular-weight mimetics of the superoxide dismutase enzymes that could overcome some of the limitations associated with the use of the native enzyme have been designed. In this review, we will discuss the advances made using various superoxide dismutase mimetics that led to the proposal that superoxide (and/or the product of its interaction with nitric oxide, peroxynitrite) is an important mediator of inflammation, and to the conclusion that superoxide dismutase mimetics can be utilized as therapeutic agents in diseases of various etiologies. The importance of the selectivity of such compounds in pharmacological studies will be discussed.
Keywords: Inflammation, free radicals, superoxide, superoxide dismutase mimetic, peroxynitrite
Superoxide dismutase
The recognition that Orgotein, a CuZn metalloprotein prepared from animal tissues, has potent anti-inflammatory activity (Huber et al., 1980) predates the recognition that the corresponding protein from bovine erythrocytes possesses an enzyme activity catalysing the dismutation of superoxide into hydrogen peroxide and molecular oxygen (Mc Cord & Fridovich, 1969). It is now well appreciated that, under normal circumstances, in healthy individuals, this radical burden is contained by superoxide dismutase (SOD) enzymes (Figure 1). SOD enzymes are a class of oxidoreductase enzymes, which, in mammals, contain either Cu or Mn at the active site and catalyse the dismutation of superoxide, the one-electron reduction product of molecular oxygen,
where Mn is the metalloenzyme in the reduced state and Mn+1 is the enzyme in the oxidized state to oxygen and hydrogen peroxide. Three different isoforms of SOD have been characterized in mammals. The SOD enzymes have a distinct genomic structure and are well compartmentalized (Figure 2).
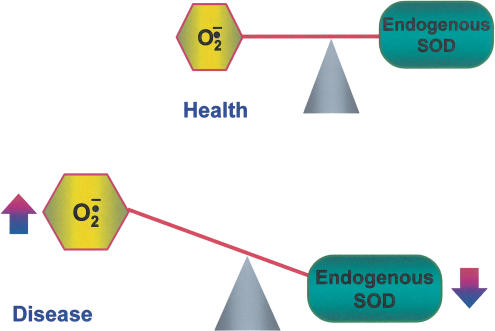
Figure 1.
Superoxide and SOD in health and disease. Endogenous SODs are critical in keeping superoxide under tight control. In diseases, there is an imbalance between the amount of superoxide formed and in the ability of the enzymes to remove it (activity of the enzyme is reduced).
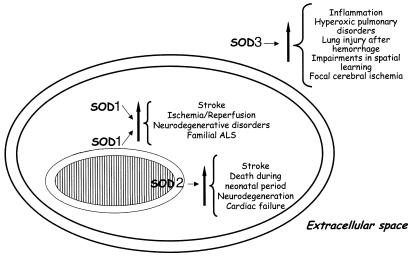
Figure 2.
Relative roles of SOD isoforms in disease. SOD isoforms are well compartmentalized inside the cell (SOD1, cytoplasm; SOD2 in the mitochondria) and in the extracellular space (SOD3). The involvement of the three isoforms in several pathological conditions has been unravelled by modulating the expression of the three enzymes using knockout and transgenic models.
SOD1 or Cu/Zn SOD and SOD3 or EC-SOD have Cu and Zn in their catalytic centre, while SOD2 or Mn SOD has Mn in the catalytic centre. SOD1 has been found in the cytoplasm, nuclear compartments and in the inter membrane space of the mitochondria (Chang et al., 1988; Keller et al., 1991; Crapo et al., 1992; Liou et al., 1993) (Figure 2). SOD2 is localized in the mitochondria matrix (Weisiger & Fridovich, 1973) of the cells and SOD3 has been found in the extracellular compartments (Marklund, 1984), where it binds the extracellular matrix through its high-affinity carboxyterminus (Oury et al., 1996; Bowler et al., 2001) (Figure 2). The mammalian ECSOD is a tetrameric glycosylated CuZnSOD (Marklund, 1982). The importance of SOD2 is highlighted by the findings that, in contrast to SOD1 (Reaume et al., 1996) and SOD3 (Carlsson et al., 1995), the SOD2 knockout is lethal to mice (Li et al., 1995; Lebovitz et al., 1996; Melov et al., 1999).
The use of the knockout models and systems where the SOD enzymes have been overexpressed by genetic manipulation have been crucial in understanding the importance of the SOD antioxidant system under physiological and pathological conditions (Figure 2). SOD1 maps to chromosome 21 (Tan et al., 1973), a finding that was crucial in understanding the contribution of this enzyme, for example, in Down's syndrome (Avraham et al., 1988; Groner et al., 1994). Using mutant SOD1-expressing transgenic mice, several studies have pointed to a variety of functions of mutant SOD1, which enhances the catalytic activity of the peroxynitrite-mediated tyrosine nitration, readily releases the reactive Cu ions, induces apoptotic cell death, enhances peroxidase activity, damages the mitochondria to release Ca2+, and forms SOD1-containing aggregates in the cytoplasm (Shibata et al., 2000). Many of these studies have obtained evidence for increased oxidative damage in neurodegenerative disorders such as amyotrophic lateral sclerosis (Gurney, 1994; Brown, 1995; Wong et al., 1995). In considering the findings of increased oxidative damage in mutant SOD1-expressing transgenic mice, it should be remembered that overexpression of mutant SOD1 may enhance oxidative stress generation from this enzyme (Shibata et al., 2000). Downregulation of SOD1 in vitro and in vivo models has been associated with neuronal death (Troy et al., 1996), while overexpression of SOD1 in transgenic mice has been associated with protection of the cerebral tissue in several pathological conditions such as ischaemia or Parkinson's disease (Chan et al., 1991; Przedborski et al., 1992; Nakao, 1995).
SOD2 maps to chromosome 6 and the knockout of this gene is lethal (Lebovitz et al., 1996). The deficiency of SOD2 resulted in an increased production of superoxide, which in turn inhibits the respiratory chain by inactivating complex I and complex II (Li et al., 1995; Lebovitz et al., 1996). Loss or reduction of the SOD2 activity has been associated with mitochondrial vacuolization and lipid peroxidation, which in turn leads to neurodegeneration and heart failure Figure 2. SOD2 gene has several polymorphisms which have been associated with a reduction of the enzyme activity and in turn to increased risk of sporadic motor neuron disease, nonfamilial idiopathic cardiomyopathy, breast cancer and reduction of the tumour-suppressive effect of SOD2 (Zelko et al., 2002). Several efforts have been made in order to determine whether an upregulation of SOD2 would be critical for the improvement of pathological conditions where the loss of SOD activity is known to contribute to the underlying pathology (for example, ischaemia and reperfusion injury). It has been shown that transgenic animals that overexpress SOD2 had a 35% reduction of infarct size compared to wild-type animals (Chen et al., 1998). The SOD2 overexpression, or induction of the enzyme activity, lead to a reduction of lipid peroxidation and tyrosine nitration, protecting tissues from cellular death (Keller et al., 1998). In addition, overexpression of SOD2 is critical for tissue protection against radiation-induced cytotoxicity, while HIV regulatory proteins induce a reduction of the expression and activity of SOD2 (Flores et al., 1993; Westendorp et al., 1995; Yan et al., 1999).
SOD3 is the least characterized among the SOD family enzymes. It maps to chromosome 4 and part of chromosome 5, and has been shown to have high affinity for heparin. To date, the only known mutation has been demonstrated to be localized to the heparin-binding site (Folz et al., 1994). Several polymorphisms have been described, but their roles need to be further clarified (Zelko et al., 2002). SOD3 has been detected in several compartments such as the plasma, lymph, ascites, cerebrospinal fluid and lung (this organ has the highest concentration of this enzyme) (Marklund, 1982). The studies performed using knockout animals (Figure 2) highlighted the role of SOD3 in focal cerebral ischaemia, where the implications of the loss of this enzyme activity are in impaired spatial learning and increased sensitivity of the null mice to hyperoxia exposure (Carlsson et al., 1995; Levin et al., 1998). Transgenic animals with increased expression of SOD3, in particular, in compartments such as alveolar type II and nonciliated bronchial epithelial cells demonstrated the critical role of this enzyme during hyperoxic pulmonary disease. Thus, enhancement of the SOD3 expression and activity in the lung attenuates the hyperoxic lung injury response by attenuating neutrophil infiltration (Folz et al., 1999). The overexpression of SOD3 seems to be critical during lung injury after haemorrhage (Bowler et al., 2001). Here, overexpression improves lung injury, as shown by the overall inhibition of neutrophil infiltration, lipid peroxidation and pulmonary oedema. ECSOD has a number of other important properties, which are not discussed here but can be found in recent reviews by Crapo and co-workers (Bowler & Crapo, 2002a,2002b).
In summary, a number of animal models of disease have shown that genetically engineered mice that lack SODs are more sensitive and those that overexpress SODs are resistant.
Superoxide
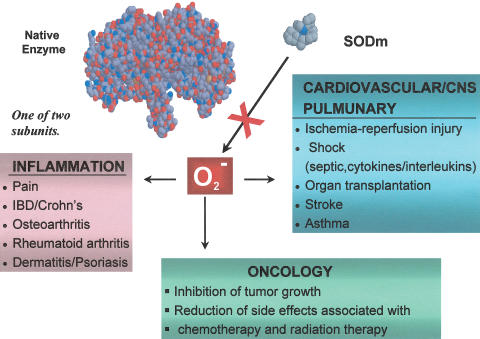
Superoxide is formed from various sources, including normal cellular respiration, activated polymorphonuclear leucocytes, endothelial cells and mitochondrial electron flux (Mc Cord & Omar, 1993; Lenaz, 2001). Superoxide generation by phagocytic NADPH oxidase is known to be important in bacterial killing. In host defence, free radicals can serve, in some instance, as microbicidal and parasiticidal agents (Peterhans, 1997; Rojkind et al., 2002), or they may facilitate/promote replication of parasites, depending on the cell and virus involved (Albrecht et al., 1992; Pace & Leaf, 1995). What is clear and what has been reported since the discovery of the native superoxide enzyme (Mc Cord & Fridovich, 1969) is that superoxide contributes to the pathogenesis of a wide array of diseases (Salvemini & Cuzzocrea, 2002a,2002b,2003). In acute and chronic inflammation, the production of superoxide is increased at a rate that overwhelms the capacity of the endogenous SOD enzyme defence system to remove it (Figure 1). The consequence of this imbalance results in superoxide-mediated damage (Figure 3).
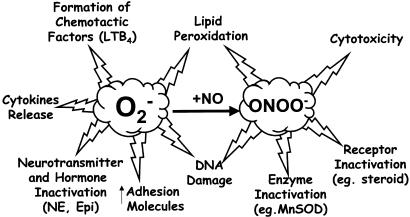
Figure 3.
Impact of superoxide generation in inflammation. Excessive production of superoxide can lead to inflammation through various pathways, including the generation of destruction of beneficial nitric oxide (NO) and simultaneous generation of cytotoxic and proinflammatory peroxynitrite (ONOO−).
Some important proinflammatory roles for superoxide include endothelial cell damage and increased microvascular permeability (Droy-Lefaix et al., 1991; Haglind et al., 1994), recruitment of neutrophils at sites of inflammation (Salvemini et al., 1999a), autocatalytic destruction of neurotransmitters and hormones such as noradrenaline and adrenaline, respectively (Macarthur et al., 2000), lipid peroxidation and oxidation, DNA damage (Dix et al., 1996), activation of poly-ADP-ribose polymerase (PARP; Szabó et al., 1996b; Szabó & Dawson, 1999a), inactivation of nitric oxide (Gryglewski et al., 1986) and formation of peroxynitrite (the reaction product of nitric oxide and superoxide, Beckman et al., 1990), a potent cytotoxic and proinflammatory molecule (Ischiropoulos et al., 1992; Beckman & Crow, 1993; Salvemini et al., 1998,1999a) (Figure 3). Peroxynitrite nitrates endogenous SOD, the enzyme that keeps superoxide under tight control. Once nitrated, MnSOD and/or CuZn SOD lose their enzymatic activity, an event favouring the accumulation of superoxide and superoxide-driven damage (Yamakura et al., 1998,2001; MacMillan-Crow & Thompson, 1999; Souza et al., 2000; Matsunaga et al., 2001). This is supported by the overwhelming body of evidence which shows that nitration of this enzyme is closely linked to those disease states driven by overt production of superoxide, for example, ischaemia and reperfusion, organ transplantation, shock and inflammation, neurodegeneration such as Alzheimer's disease, amyotrophic lateral sclerosis or AIDS dementia complex (MacMillan-Crow & Cruthirds, 2001; Mollace et al., 2001; Ischiropoulos & Beckman, 2003 for review).
SOD: preclinical experience
The list of pathophysiological conditions associated with the production of superoxide continues to expand. The most exciting realization is that there appears to be a commonality to the tissue injury observed in various disease states, namely, superoxide produces tissue injury (and associated inflammation) in most tissues in similar ways. Tissue injury and inflammation form the basis of many disease pathologies: ischaemia and reperfusion injuries, radiation injury, hyperoxic lung damage, asthma, atherosclerosis and so forth. This provides a unique opportunity to manipulate numerous disease states with an agent that selectively removes superoxide (vide infra). Protective and beneficial roles of SOD have been demonstrated in a broad range of diseases, both preclinically and clinically (Mc Cord, 1974; Halliwell & Gutteridge, 1985; Maxwell, 1995). For example, preclinical studies have revealed that SOD enzymes have a protective effect in animal models of ischaemia–reperfusion injury (including the heart, liver, kidneys and brain) (Werns et al., 1988; Ando et al., 1989; Chan et al., 1991; Omar & McCord, 1991; Yang et al., 1994), transplant-induced reperfusion injury (Zweier, 1997), inflammation (Oyanagui, 1976; Shingu et al., 1994), Parkinson's disease (Przedborski et al., 1992; Andreassen et al., 2001), cancer (Church et al., 1993; Safford et al., 1994; Yoshizaki et al., 1994), AIDS (Flores et al., 1993; Edeas et al., 1997; Mollace et al., 2001,2002), asthma (Bowler & Crapo, 2002a,2002b), chronic obstructive pulmonary diseases (Barnes, 2000; Salminen et al., 2001) and respiratory syncytial virus infections (Wyde et al., 1996). Furthermore, the native enzyme attenuates the intestinal injury induced by alcohol (Terano et al., 1989), Helicobacter Pylori (Lamarque et al., 2000) and nonsteroidal anti-inflammatory drugs including indomethacin, diclofenac and flurbiprofen (Evans & Whittle, 2001).
SOD: clinical experience
When tested in humans in various clinical trials, Orgotein® (bovine CuZnSOD) showed promising results in acute and chronic conditions associated with inflammation, including rheumatoid arthritis and osteoarthritis (Goebel et al., 1981; Goebel & Storck, 1983; Lund-Olesen & Menander-Huber, 1983; Gammer & Broback, 1984; Mc Ilwain et al., 1989; Mazieres et al., 1991; Borigini & Paulus, 1995) as well as side effects (acute and chronic) associated with chemotherapy and radiation therapy (Marberger et al., 1974,1975,1981; Edsmyr et al., 1976; Menander-Huber et al., 1978; Housset et al., 1989; Delanian et al., 1994; Sanchiz et al., 1996). Thus, in clinical trials, the use of the native enzyme supported the concept that removal of superoxide had a beneficial outcome. There were drawbacks associated with its use. The main problem was the nonhuman origin of the enzyme bovine. This inevitably gave rise to a variety of immunological problems, which eventually led to its removal from the market, except in Spain where it is still clinically used to prevent radiation-induced side effects.
Based on the concept that removal of superoxide modulates the course of inflammation, others and we have pursued the concept of designing synthetic, low-molecular-weight mimetics of the SOD enzymes, which could overcome some of the limitations associated with Orgotein. This allows the synthetic SOD mimetics (SODm) to serve as pharmaceutical candidates in a variety of diseases in which the native SOD enzyme was found to be effective. This concept has proven to be one which a number of researchers and companies have been pursuing in recent years. A review of the patent literature in this arena was published (Henke, 1999).
Pharmacological use of SODm
Manganese(III) Metalloporphyrins
Manganese-based metalloporphyrin complexes scavenge of superoxide, hydrogen peroxide, peroxynitrite and lipid peroxyl radicals (Faulkner et al., 1994; Day et al., 1995,1997,1999; Szabó et al., 1996a). The manganese moiety of the porphyrin-based SODm (Figure 4) functions in the dismutation reaction with superoxide by successive reduction followed by oxidation changes in its valence between Mn(III) and Mn(II), much like native SODs, whereas the catalase activity of metalloporphyrins could be attributed to their ability to also undergo oxidation to higher oxidation states as Mn(IV) or Mn(V) or Mn(IV) (porphyrin radical cation) oxidation levels. In general, metalloporphyrins with higher SOD activity possessed greater catalase activity. Metalloporphyrins have been shown to be protective in a wide variety of in vitro oxidative stress models involving the generation of superoxide, hydrogen peroxide and peroxynitrite alone or in concert. At micromolar levels, they protect cultured cells against the toxicity of superoxide generators paraquat (Day et al., 1995) and pyocyanine (Gardner et al., 1996), hydrogen peroxide generator, glucose oxidase (Day et al., 1997) and peroxynitrite injury produced by endotoxin (Szabó et al., 1996a) or peroxynitrite itself (Misko et al., 1998). Metalloporphyrins such as Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP) (Figure 4) are also potent inhibitors of lipid peroxidation (Day et al., 1999), exerting a protective effect against some of the detrimental effects associated with endotoxic and haemorrhagic shock (Zingarelli et al., 1997; Szabó, 1998), as well as in acute liver failure (Ferret et al., 2001; Malassagne et al., 2001). Since these SODm scavenge other reactive oxygen species including peroxynitrite, the efficacy of MnTBAP in these models probably relates to its peroxynitrite-scavenging activity in addition to its superoxide-scavenging activity (Zingarelli et al., 1997; Szabó, 1998).
Figure 4.
Structure of different classes of superoxide scavengers.
Manganese(III)salen complexes
The Mn(III)salen complexes (Figure 4) have been reported to be superoxide scavengers/SODm, and possess catalase activity (Doctrow et al., 1997). Biological studies have been published for two of these complexes, manganese N,N′-bis(salicyldene)ethylenediamine chloride (EUK-8) and manganese 3 methoxy N,N′-bis(salicyldene)ethylenediamine chloride (EUK-134), in several disease models related to oxidative stress including stroke, ischaemia–reperfusion injury, Parkinson's disease and experimental allergic encephalomyelitis (EAE) (Doctrow et al., 1997; Malfroy et al., 1997; Baker et al., 1998; Pong et al., 2000; Bianca et al., 2002). However, Mn(III)salen complexes, in the presence of peroxynitrite and/or hypochlorite, become oxidized to oxoMn-salen. OxoMn-salens are potent oxidants that rapidly oxidize NO to NO2 and also oxidize nitrite to nitrate (Sharpe et al., 2002). These data support the evidence that the Mn(III)salen complex is a nonselective free radical scavenger that protects cells from oxidative stress, but are not useful pharmacological tools to dissect the relative importance of superoxide in disease. Evidence is available to support that EUK-8 significantly attenuated many of the features of a porcine model of LPS-induced adult respiratory distress syndrome (ARDS), where the role of superoxide has been well described by Kinnula and co-workers (Gonzalez et al., 1995; Kinnula et al., 1995). EUK-134 attenuates the multiple organ injury and dysfunction caused by endotoxin in the rat (Bianca et al., 2002).
Furthermore, it has been shown that a small increase in myocyte oxidative stress due to partial inhibition of SOD1 leads to myocyte hypertrophy, whereas a higher level of oxidative stress caused by total inhibition of SOD1 results in apoptosis (Siwik et al., 1999). The response of cardiac myocytes to low and high levels of mechanical stretch is similar to the effect of directly increasing myocyte oxidative stress, and the phenotype shift at each level of stretch is inhibited by EUK-8 (Pimentel et al., 2001). EUK-8 and EUK-134 have been used to investigate the role of oxidative stress in several neurodegenerative diseases, and it has been shown, for example, that these compounds are able to protect microglial cells from beta-amyloid peptide (1–42 fragment) injury and to limit the output of prostaglandin E2 produced from activated microglial cells (Anderson et al., 2001). Furthermore, the compounds reduce the levels of oxidative stress and prolong the survival of mice expressing human mutant SOD1 G93A, which is a polymorphism of familiar amyotrophic lateral sclerosis (FALS; Jung et al., 2001). Recently, it has been demonstrated that manganese (salen) complex compounds, when administered to MnSOD knockout mice, double the lifespan of these animals and inhibit the cardiomyopathies associated with the knockout phenotype (Melov et al., 2001). These results suggest that these compounds enter the mitochondria, the intracellular location where the majority of superoxide is generated and where endogenous MnSOD enzyme is localized, to protect against the damage elicited by the deregulation of superoxide radical. Transforming growth factor β (TGF β) is directly involved in the development of tubulointerstitial fibrosis in chronic renal disease, and oxygen radicals can modulate the expression of TGF β. EUK-8 stimulates the transcription of TGF β gene as well as protein expression in treated mouse proximal tubular cells, whereas exogenous hydrogen peroxide suppressed the transcription of TGF β (Wolf et al., 2001). EUK-8 has been shown to reduce arrythmias induced by a 10 min regional ischaemia/reperfusion injury induced by the left coronary artery ligation and release (Tanguy et al., 1996). Therefore, although these agents are protective in some models of diseases, the results obtained from the studies cannot be used to support the nature of the radical involved.
Nitroxides
Tempol(4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) (Figure 4) is a water-soluble analogue of the spin label TEMPO, which is widely employed in electron spin resonance spectroscopy. Tempol is a stable piperidine nitroxide of low molecular weight (MW: 172), which permeates biological membranes. There is now good evidence that tempol exerts beneficial effects in animal models of shock, ischaemia–reperfusion injury, inflammation, hypertension, diabetes and endothelial cell dysfunction (see Thiemermann (2003) for review). There is a controversy as to whether tempol and other stable nitroxides are ‘SOD-mimetics' or act as stoichiometric scavengers of superoxide anions (Weiss et al., 1993; Krishna et al., 1996). Nevertheless, there is evidence that tempol does attenuate the effects of superoxide in vitro (Samuni et al., 1991; Reddan et al., 1992; Laight et al., 1997,1998). However, tempol also reduces the formation of hydroxyl radicals (Mitchell et al., 1990; Monti et al., 1996) and attenuates the cytotoxic effects of hydrogen peroxide, which is mediated by hydroxyl radicals (Bowes et al., 1998,1999), supporting its nonselectivity. Tempol also inhibits the peroxynitrite-mediated nitration of phenolic compounds in the presence of a large molar excess of peroxynitrite, suggesting a catalytic-like mechanism (Carroll et al., 2000). Reactive oxygen species and peroxynitrite cause strand breaks in DNA, which in turn results in activating PARP. Activation of PARP catalyses the transfer of ADP-ribose moieties from NAD+ to various nuclear proteins including histones and PARP (automodification domain) itself. Continuous or excessive activation of PARP produces extended chains of ADP-ribose on nuclear proteins (poly-ADP-ribose chains or PAR chains), and results in a substantial depletion of intracellular NAD+ and subsequently ATP, which may ultimately cause cell death (Szabó, 1998; Szabó et al., 1996b; Szabó & Dawson, 1999a). Tempol prevents the increase in PARP activity caused by hydrogen peroxide in cultured human cardiomyoblasts (Thiemermann (2003) for review).
There is now good evidence that tempol exerts beneficial effects in conditions associated with ischaemia–reperfusion of many organs including the heart, kidney and brain. For instance, administration of tempol prior to the onset of reperfusion reduces the infarct size caused by regional myocardial ischaemia and reperfusion in the rat and rabbit in vivo. In addition, reperfusion of hearts with buffer containing tempol causes a 37% reduction in infarct size. These data provide convincing evidence that the administration of tempol prior to the onset of reperfusion reduces myocardial infarct size in rodents.
Renal ischaemia/reperfusion injury is one of the most common causes of acute renal failure. In a rat model of renal ischaemia and reperfusion (bilateral clamping of both renal arteries), tempol significantly reduced the glomerular dysfunction and the tubular injury and dysfunction. Tempol also reduced the histological evidence of renal damage associated with ischaemia/reperfusion, and caused a substantial reduction in the staining for nitrotyrosine and PARP, suggesting reduced nitrosative and oxidative stress. In primary proximal tubule cells of the rat, tempol significantly attenuates the H2O2-mediated decrease in mitochondrial respiration and increase in LDH release, indicating a reduction in cell injury and death.
Tempol also reduces the tissue injury associated with ischaemia and reperfusion of the brain. In the anaesthetized rat, tempol reduces the infarct volume caused by middle cerebral artery occlusion and reperfusion without affecting the regional cerebral blood flow or blood pressure, indicating that the observed protective effects are independent of any local or systemic haemodynamic effects (Cuzzocrea et al., 2000a). In a gerbil model of bilateral occlusion and reperfusion of both common carotid arteries, tempol (30 mg kg−1 i.p. at 30 min prior to reperfusion, and at 1 and 6 h after the onset of reperfusion) reduced the formation of postischaemic brain oedema, attenuated the increase in the cerebral levels of malondialdehyde and the hippocampal levels of myeloperoxidase. The immunohistochemical analysis of the hippocampal region of brains subjected to ischaemia-reperfusion exhibited a positive staining for nitrotyrosine (an indicator of the generation of the potent oxidant peroxynitrite) and for PARP. In gerbils subjected to both common carotid arteries, which were treated with tempol, the degree of staining for nitrotyrosine and PARP was markedly reduced. Tempol increased survival and reduced the hyperactivity (secondary to the ischaemia-induced neurodegeneration) caused by cerebral ischaemia and reperfusion. The loss of neurons from the pyramidal layer of the CA1 region caused by ischaemia and reperfusion was also attenuated by treatment of gerbils with tempol. This study, hence, provides evidence that the membrane-permeable, radical scavenger tempol reduces the cerebral injury caused by transient, cerebral ischaemia in vivo (Cuzzocrea et al., 2000b).
Rats subjected to severe splanchnic ischaemia develop a significant decrease in the mean arterial blood pressure, a significant increase in tissue myeloperoxidase activity and a marked injury to the distal ileum. There is now evidence that tempol (30 mg kg−1 bolus injection 5 min prior to reperfusion, followed by an infusion of 30 mg kg−1 h−1 intravenously) reduces (i) the infiltration of the reperfused intestine with neutrophils, (ii) the lipid peroxidation, (iii) the production of peroxynitrite, (iv) the degree of P-selectin and ICAM-1 staining in tissue sections from SAO-shocked rats, (v) histological signs of bowel injury and (vi) mortality at 2 h after reperfusion (Cuzzocrea et al., 2000a).
There is good evidence that (i) both septic and haemorrhagic shock are associated with the generation of superoxide anions, hydrogen peroxide and peroxynitrite, and (ii) that tempol reduces the multiple organ injury in animal models of shock. For instance, endotoxemia in the rat causes within 6 h hypotension, acute renal dysfunction, hepatocellular injury, pancreatic injury and an increase in the plasma levels of nitrite/nitrate. Pretreatment of rats with tempol (100 mg kg−1 i.v. bolus injection, 15 min prior to LPS, followed by an infusion of 30 mg kg−1) did not affect the circulatory failure, but attenuated the renal dysfunction and the hepatocellular injury/dysfunction caused by LPS. Tempol did not affect the rise in nitrite/nitrate caused by endotoxin (Leach et al., 1998). Similarly, tempol also reduces the renal and liver injury and dysfunction caused by wall fragments of Gram-positive bacteria in the anaesthetized rat. Haemorrhage (sufficient to lower the mean arterial blood pressure to 50 mmHg for 90 min) and subsequent resuscitation with shed blood (in the rat) result within 4 h after resuscitation in a delayed fall in blood pressure, renal and liver injury, and dysfunction as well as lung and gut injury. In all organs, haemorrhage and resuscitation resulted in the nitrosylation of proteins (determined by immunohistochemistry for nitrotyrosine), suggesting the formation of peroxynitrite and/or reactive oxygen species. Treatment of rats upon resuscitation with tempol (30 mg kg−1 bolus injection followed by an infusion of 30 mg kg−1 h−1 i.v.) attenuated the delayed circulatory failure as well as the multiple organ injury and dysfunction associated with haemorrhagic shock (Mota-Filipe et al., 1999).
Following the observation by Sledzinski et al. (1997) that tempol exerts beneficial effects in a rodent model of pancreatitis, there is now good evidence that tempol reduces the degree of inflammation and the associated tissue injury in animal models of diseases associated with local or systemic inflammation. These include rodent models of carrageenan-induced pleurisy, colitis, zymosan-induced multiple organ injury and uveoretinitis (Thiemermann (2003) for review). The doses of tempol necessary to protect tissues against the injury associated with the above disorders are relatively high (ranging from 10 to 100 mg kg−1 for i.v. bolus administration).
Thus, tempol is a membrane-permeable, radical scavenger, which interferes with the formation and/or the effects of many radicals including superoxide anions, hydroxyl radicals and peroxynitrite. There is now a substantial body of evidence from preclinical studies showing that tempol may be useful in the therapy of ischaemia-reperfusion injury, shock and inflammation.
Manganese(II)(pentaazamacrocyclic ligand)-based complexes
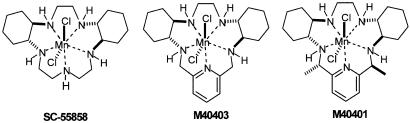
Our research group set out many years ago to design highly active and stable mimetics of the mammalian SOD enzymes for use as possible therapeutic agents in human disease. Initially, we sought to mimic the function of the enzymes with little emphasis on selectivity, but soon we found that many of the metal complexes capable of a fast dismutation of superoxide anion can also react with the hydrogen peroxide product of the dismutation in a Fenton-like reaction producing hydroxyl radical, an even more reactive and potentially destructive radical than superoxide anion. Thus, our earliest focus was to design functional SODm that would not react with oxidants like hydrogen peroxide or hypochlorite. An additional paradigm that we were working under was the constraint that we wanted to develop agents that could be used as human pharmaceutical agents, if they were indeed efficacious in animal models of disease. To this end, we focused on metal complexes that would be stable under physiological conditions. From our research efforts, we indeed discovered and developed a class of agents that met the four major criteria that we felt were critical: high SOD activity, high stability, selectivity only for superoxide and in vivo efficacy. We focused on the design and synthesis of Mn(II) and Fe(III) complexes which possess high inherent chemical and thermodynamic stability, and at the same time are highly effective catalysts for the dismutation of superoxide anion (Figure 4). As can be seen by EPR studies, M40403 is very effective in removing superoxide generated by, for example, phorbol 12-myristate 13-acetate-stimulated human neutrophils (Figure 5). This dual-design goal of high stability and high SOD activity was achieved by utilizing a combination of computer-aided modelling studies (Henke, 1999; Riley et al., 1999) and synthesis activities, and has led to the development of a novel class of highly active SOD catalysts which are also very stable complexes. These synthetic SODm are exemplified by the prototypical complex M40403 (Figure 4; Table 1 ), derived from the 15-membered macrocyclic ligand 1,4,7,10,13-pentaazacyclopentadecane, containing the added bis(cyclohexylpyridine) functionalities (Riley et al., 1999). M40403 is a stable, low molecular weight, manganese-containing, nonpeptidic molecule, possessing the function and catalytic rate of native SOD enzymes, but with the advantage of being a much smaller molecule (MW 483 vs 30,000 for the mimetic and native enzymes, respectively) (Salvemini et al., 1999b). Another important advantage of these synthetic enzymes is that they do not possess the bell-shaped curve that is a common characteristic to the native SOD enzyme. At low doses, the SOD enzymes are anti-inflammatory, whereas at high doses they exhibit proinflammatory effects (Dowling et al., 1993). The proinflammatory effects of the SOD enzyme are not well understood, but it is speculated to be due to its reaction with the dismutation product hydrogen peroxide, to generate hydroxyl radicals via Fenton chemistry (Mao et al., 1993). The lack of a bell-shaped dose–response curve with the SODm may be related to the selective reactivity of the SOD mimics with superoxide and the complexes' inability to react with hydrogen peroxide.
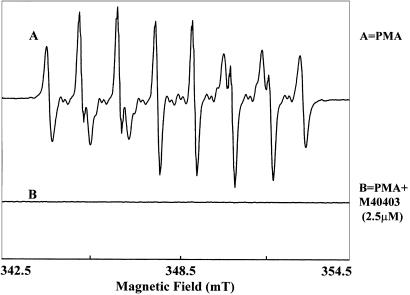
Figure 5.
Effect of SOD mimic M40403 on superoxide production from human neutrophils. Spin-trapping measurements were performed using the spin trap DEPMPO, 10 mM, with 0.4 M PMN stimulated with PMA, 200 ng/ml. The spectra shown were recorded 30 min post activation. (a) Control-activated PMNs; (b) in the presence of 2.5 M M40403. EPR spectra were recorded in a flat cell on a Bruker ESP300 spectrometer, using a TM110 cavity with 100 kHz modulation frequency.
Table 1.
Properties of Mn(II)-based selective superoxide dismutase mimetics (e.g. M40403)
| Manganese-containing biscyclohexylpyridine |
| Catalytic activity equivalent to that of the native enzyme |
| Penetrates cells and wide organ distribution |
| Selective for superoxide (no interaction with other biologically relevant molecules, for example, nitric oxide, hydrogen peroxide, peroxynitrite) |
| Stable in vivo: no loss of manganese and excreted intact |
| Not deactivated by peroxynitrite |
| Suitable pharmacological tool to dissect the role of superoxide in physiopathological conditions |
| Pharmacologically efficacious in numerous animal models of disease |
The Mn(II)-based SODm such as M40403 and M40401 (Figure 6) described herein are catalytic agents (drugs); that is, the compounds do not involve a stoichiometric interaction with a biological target, such as a receptor, but instead enhance the rate of conversion of superoxide to molecular oxygen and hydrogen peroxide, without the complex itself being consumed. The SODm have been rigorously characterized for SOD activity by stopped-flow kinetic analysis, for in vitro stability by kinetic and thermodynamic assays, and for in vivo stability by electron spin resonance and radiolabel studies using Mn-, H- and C-labelled mimics. The ability of the SODm to scavenge superoxide in vivo has also been demonstrated by electron spin resonance studies (Table 1). Their inability to react with hydrogen peroxide or peroxynitrite has been screened and demonstrated with a variety of techniques, including spectrophotometric diode array detection reactivity studies (for Mn(III) production from OCl−, OONO− and H2O2), via oxygen electrodes (for catalase activity), and in stopped flow experiments designed to follow the loss of peroxynitrite (Table 1).
Figure 6.
Structures of Mn(II)-based selective SODm (for example, M40403).
We have published extensively in chemical literature on the mechanism and subsequent computer-aided design (CAD) (Riley et al., 1999; Aston et al., 2001) of these highly active and stable mimetics (Riley et al., 1996). Three of the mimetics that we have used extensively in pharmacology studies are shown in Figure 6, and span a range of catalytic rate constants at pH=7.4 (M−1 s−1) (SC-55858=1.2 × 108, M40403=1.6 × 107 and M40401=1.6 × 109), with similar hydrophobicities. It should be noted that each of these catalysts has a proton first-order dependence on the rate; that is, the rate increases as the pH drops. All the three agents have a catalytic rate greater than the native MnSOD enzyme at pH=6.5, for example. Thus, by utilizing the CAD principles that we have developed, we have been able to synthesize not only highly active catalysts but also agents such as M40403 and M40401 that are stable in vivo for extended periods, and are amphoteric with log P-values of ∼0.3–0.4 (slightly hydrophilic). The recently developed M40401 (the S,S-dimethyl-substituted derivative of the M40403 biscyclohexylpyridyl class of mimetic) actually possesses a higher catalytic activity at pH=7.4 than the native Mn SOD enzyme (Aston et al., 2001). In fact, its catalytic rate exceeds 1 × 10+9 M−1 s−1, comparable to the native Cu/Zn SOD enzymes.
In the light of the critical roles of superoxide in disease, these new selective, potent and stable synthetic enzymes of SOD, as represented by M40403, have broad potential as pharmacological tools to dissect the role of superoxide in disease models where other such relevant biological oxidants may be present and are expected to play a role.
Others and we have shown over the last several years that SODm are anti-inflammatory (Salvemini et al., 1999b,2001a,2001b; Cuzzocrea et al., 2001a), and protective in models of septic shock (Cuzzocrea et al., 2001a; Salvemini & Cuzzocrea, 2002a) and ischaemia-reperfusion injury (Cuzzocrea et al., 2001a,2001b; Salvemini & Cuzzocrea, 2002b,2003). The anti-inflammatory profile of these agents is shown in Table 2 .
Table 2.
Summary of the anti-inflammatory properties of Mn(II)-based selective superoxide mimetics (e.g. M40403)
| Inhibition of the upregulation of adhesion molecules (ICAM-1, P-selectin) and PMNs infiltration at the inflamed site |
| Attenuation of proinflammatory cytokine release (TNFα, IL-1β, IL-6) |
| Protection of the inactivation of nitric oxide and preservation of its beneficial effects |
| Inhibition of lipid peroxidation and cellular protection |
| Attenuation of DNA damage and subsequent activation of poly-ADP-ribose polymerase |
| Attenuation of peroxynitrite formation and subsequent peroxynitrite-mediated damage |
| Protects against superoxide anion-driven deactivation of catecholamines – important immunoregulators |
| Inhibition of the activation of transcription factors (NFκB) |
An important mechanism by which SODm attenuate inflammation is by reducing peroxynitrite formation by simply removing the superoxide before it can react with nitric oxide. This is important since the proinflammatory and cytotoxic effects of peroxynitrite are numerous (Squadrito & Pryor, 1995). For instance, removal of peroxynitrite by agents such as FeTMPS, a porphyrin-containing molecule, which increases the rate of isomerization of peroxynitrite to nitrate (Stern et al., 1996) is cytoprotective (Misko et al., 1998) and anti-inflammatory (Salvemini et al., 1998). Peroxynitrite nitrates tyrosine residues in proteins, and nitrotyrosine formation, as monitored by immunofluorescence, has been used as a marker for the detection of the endogenous formation of peroxynitrite (Beckman, 1996). Using nitrotyrosine as a marker for the presence of ONOO− has been challenged by the demonstration that other reactions can also induce tyrosine nitration; for example, the reaction of nitrite with hypochlorous acid and the reaction of myeloperoxidase with hydrogen peroxide can lead to the formation of nitrotyrosine (Eiserich et al., 1998). Thus, increased nitrotyrosine staining is considered as an indicator of ‘increased nitrosative stress' rather than as a specific marker of the generation of peroxynitrite (Eiserich et al., 1998). Therefore, our recent study (Fries et al., 2003) and the studies with the peroxidase-deficient mice (Brennan et al., 2002; Gaut et al., 2002) reinforce previous discussions that a number of pathways apparently contribute to the nitration of tyrosine residues in proteins in vivo independently, or even simultaneously (Ischiropoulos, 1998; Hurst, 2002). With the combined judicious use of both pharmacological agents and molecular approaches, and depending upon the inflammatory stimuli and cell type, the sources and nature of nitrating agents in vivo are now being elucidated. Therefore, the conclusion of our recent publication is that superoxide contributes in a precise pattern of intracellular protein tyrosine nitration (Fries et al., 2003).
In this regard, we have also found that SODm of the M40403 class block nitrotyrosine staining in models of inflammation, suggesting that superoxide anion-driven peroxynitrite formation is in fact responsible for the formation of nitrotyrosine, and that its inhibition could account for the anti-inflammatory effects of SODm (Salvemini et al., 1999b,2001a,2001b; Cuzzocrea et al., 2001c). This in fact was the first evidence to show in vivo a superoxide-dependent nitration, since the M40403 class of SODm does not react with nitric oxide or peroxynitrite. A similar pattern of immunoreactivity for nitrotyrosine is observed in a lung model of pleurisy (Ischiropoulos et al., 1995; Salvemini et al., 2001a; Bianca et al., 2002).
A substantial amount of data have been generated to support the concept that peroxynitrite generation plays an important part in the proinflammatory roles that have been ascribed to nitric oxide (Salvemini et al., 1998). Based on this premise, we propose that selective SODm should be considered as a therapeutic means to attenuate inflammatory responses. In addition, superoxide, by interacting with nitric oxide, destroys the biological activity of this mediator (Gryglewski et al., 1986), attenuating important antiinflammatory and tissue-protective properties of nitric oxide; namely, its maintenance of blood vessel tone, platelet reactivity, cytoprotective effects in numerous organs (including heart, intestine and kidney) (Moncada et al., 1991; Szabo & Billiar, 1999b; Liaudet et al., 2000) and release of anti-inflammatory and cytoprotective prostacyclin (via activation of the constitutive cyclooxygenase enzyme) (Salvemini et al., 1993,1996).
In addition, it has been demonstrated that NO derived from iNOS is necessary for a substantial antiviral effect of interferon-γ and for the ability of the body to fight viruses. Thus, inhibition of iNOS enhances viral replication and leads to an increase in lethality in systemic viral infection models (Karupiah et al., 1993). In other words, the potential beneficial anti-inflammatory effects of iNOS inhibition are cancelled out by independent, deleterious actions of iNOS inhibition. However, in other types of viral infection, where clearance of the virus is independent of interferon-γ, the anti-inflammatory effect of iNOS inhibition becomes prominent. For example, viral pneumonitis induced by influenza A virus is abolished in iNOS-deficient mice (Karupiah et al., 1998). Therefore, we have clearly demonstrated that M40403 or M40401 did not interfere with the activation of iNOS during acute inflammation, and did not reduce the NO production after ischaemia and reperfusion. Thus, removal of superoxide reduces the formation of the cytotoxic peroxynitrite and does not interfere with the beneficial effects of NO.
As indicated earlier, superoxide anion and peroxynitrite induce DNA single-strand damage that is the obligatory trigger for PARP activation (Inoue & Kawanishi, 1995; Salgo et al., 1995), eventually leading to cell death (Szabò et al., 1996b). Furthermore, substantial evidence exists to support the fact that PARP activation is important in inflammation (Szabó & Dawson, 1999a). PARP is the target for therapeutic intervention, and its inhibitors such as nicotinamide and 3-aminobenzamide attenuate both acute and chronic inflammatory processes (Cuzzocrea et al., 1998a,1998b; Virag & Szabo, 2002). SODm reduce PARP immunofluorescence and attenuate the reduction of NAD+ in models of acute and chronic inflammation (Salvemini et al., 2001a,2001b; Cuzzocrea et al., 2001c). In light of the role of PARP in inflammation, it is possible that PARP inhibition by these SODm accounts for their anti-inflammatory response.
Superoxide increases neutrophil adhesion and infiltration (Warren et al., 1990; Salvemini et al., 1999a,2001a; Cuzzocrea et al., 2001c), and generates potent chemotactic mediators such as leukotriene B4 (Al-Shabanah et al., 1999). Removal of superoxide inhibits the infiltration of neutrophils at sites of inflammation, as shown by the use of the native SOD enzyme (Niwa et al., 1985), experiments performed in transgenic mice that overexpress the human CuZnSOD enzyme (Chan et al., 1991) and by the use of SODm such as SC-55858 and M40403 (Lowe et al., 1996; Salvemini et al., 1999b,2001a). This correlates well with an attenuation of lipid peroxidation and overall attenuation of acute and chronic inflammation. A possible mechanism by which SODm attenuate neutrophil infiltration is by downregulating adhesion molecules such as ICAM-1 and P-selectin. Thus, inhibition of neutrophil infiltration at sites of inflammation and reperfusion injury correlated well with the inhibition of both ICAM-1 and P-selectin (Hardy et al., 1994; Cuzzocrea et al., 2001c), supporting the involvement of superoxide in the regulation of adhesion molecules (through mechanisms yet to be defined). In addition to ICAM-1 and P-selectin, other adhesion molecules may be affected by superoxide. The release of a variety of proinflammatory cytokines is also regulated by superoxide. Thus, SODm inhibit a number of inflammatory cytokines, including tumour necrosis factor-α, interleukin-1β and interleukin-6 (TNFα, IL-1β and IL-6, respectively), as shown in models of acute and chronic inflammation (Cuzzocrea et al., 2001c; Salvemini et al., 2001a; Salvemini & Cuzzocrea, 2002b). The mechanism(s) through which superoxide regulates cytokines is under evaluation in various research groups. Recent data demonstrate that superoxide anions (generated from xanthine–xanthine oxidase) can directly release TNFα from macrophages (Volk et al., 1999). Interestingly, the anti-inflammatory cytokine IL-10 is not affected (Salvemini et al., 2001a). A role for reactive oxygen species in signal transduction during inflammatory stimuli is becoming appreciated. For example, the MAPK pathway and MAPK-mediated regulation of TNFα production (post LPS-stimulated alveolar epithelial cells) is redox-dependent, requiring at least in part a NFκB/ROS-sensitive mechanism (Haddad & Land, 2002). McInnis and co-workers reported that N-methyl-D-aspartate-mediated neurotoxicity is associated with activation of the NFκB system, an effect that is blocked by M40403, suggestive of a role for superoxide in the activation of this important transcription factor (Mc Innis et al., 2002).
Thus, removal of superoxide impacts the inflammatory cascade through at least three major pathways: (1) inhibition of peroxynitrite formation and sparing of nitric oxide, (2) inhibition of neutrophil infiltration at the site of inflammation, and (3) inhibition of proinflammatory cytokine release.
Although limited information is currently available on this topic, it is possible that another mechanism that may reveal itself as being important through which superoxide might modulate the inflammatory response is through the deactivation of catecholamines (noradrenaline and adrenaline). Catecholamines are released as part of the body's ‘survival' response to numerous exogenous and endogenous stimuli including, of course, inflammation. Besides their well-known vasoconstrictor (for noradrenaline) and bronchodilator (for adrenaline) properties, catecholamines are also well known in regulating the immune system (Van der Poll et al., 1994,1996; Van der Poll & Lowry, 1997). Once deactivated, catecholamines are no longer able to exert their effect. Deactivation of catecholamines accounts for the hyporeactivity and hypotension associated with shock (Macarthur et al., 2000,2003), contributing therefore to the development of both the vascular abnormalities with this disease. In addition to their pivotal role in transducing the sequential cardiovascular and haemodynamic crisis following severe Gram-negative bacteremic sepsis, endogenous catecholamines are instrumental in controlling the expression of cytokines. Nerve-derived noradrenaline and circulating adrenaline act via β-adrenergic receptors on macrophages and neutrophils downregulating inflammatory responses mediated specifically by TNFα and IL-1β (Severn et al., 1992; Monastra & Secchi, 1993; Van der Poll et al., 1996; Guirao et al., 1997). Deactivated catecholamines are no longer able to exert this effect, contributing to the increase in cytokine release during shock. M40403 or M404041 inhibits cytokine production by removing superoxide, at least in part through protection and preservation of endogenous catecholamines (Macarthur et al., 2000,2003). Furthermore, the production of superoxide by activated neutrophils and macrophages is also negatively modulated by catecholamines through β-adrenergic receptor activation (Baciu, 1988; Weiss et al., 1996), underlining the potential immunoregulatory effects of catecholamines. This mechanism is potentially relevant to sepsis and multiorgan failure (Salvemini & Cuzzocrea, 2002a,2003).
SODm- and hydrogen peroxide-driven toxicity
It is often asked whether SODm, which lack catalase activity, increase cellular damage by enhancing the local concentration of the so-called ‘toxic' hydrogen peroxide. We will take this opportunity to discuss that the contention that H2O2 is more toxic than superoxide is not neccessarily correct. This is based on the following. Hydrogen peroxide is itself not a radical but actually quite an inert oxidant, whose cellular toxicity is probably in the 100 μM–mM range. The hydrogen peroxide's toxicity is likely due to the generation of reduced iron (Fe(III) is the oxidation state of iron in iron-storage sites) which, as Fe(II) reacts with hydrogen peroxide (Fenton reaction), undergoing homolytic cleavage to generate Fe(III)(OH) and hydroxyl radical. Iron(III) must first be reduced to ‘free' soluble Fe(II) for this reaction to occur; one of the best reductants available in inflammatory or reperfusion disease states is superoxide, which has been shown to be an excellent kinetically competent reductant of Fe(III) in iron stage sites liberating Fe(II) (Keyer & Imlay, 1996). Thus, superoxide becomes (not hydrogen peroxide) the culprit leading to generation of conditions favourable for Fenton chemistry to be initiated. The statement that we would be creating a more toxic condition by generating more hydrogen peroxide is also not correct when one inspects the stochiometry of the reactions involved. Nearly all of the oxidizing reactions which superoxide enters into involve hydrogen atom abstraction from a biological target molecule such as a catecholamine, DNA, RNA, and an allylic CH of a fatty acid, steroids, and so forth. These oxidation reactions are free radical chain reactions and produce at least one hydrogen peroxide per oxidation; in fact, these are all free radical chain reactions that in the presence of oxygen will yield many molecules of hydrogen peroxide with one initiation from superoxide. When superoxide is dismuted, the stoichiometry is such that two superoxides and two protons generate one oxygen molecule and one hydrogen peroxide as the net reaction; thus, in effect, each mole of superoxide now leads to ½ a mole of hydrogen peroxide. So, in effect, by dismuting superoxide one actually decreases the potential H2O2 burden – not increasing it. Furthermore, to date there is no evidence that in vivo Fenton reaction occurs (Koppenol, 1998; Toyokuni, 2002; Blokhina et al., 2003). In fact, Koppenol and colleagues have indicated that no iron complex has been identified in vivo that participates in the Fenton reaction (Koppenol, 1998). Thus, the use of SODm does not lead to a toxic condition by generating more hydrogen peroxide. Futhermore, when these molecules have been tested in the in vitro study of neutrophil-mediated injury of human aortic endothelial cells, no toxic effect has been observed (Hardy et al., 1994). This study clearly demonstrates not only that the compounds protect against the activated neutrophil-mediated killing of the human aortic cells, but also that the coadministration with catalase or glutathione peroxidase did not have any additional benefit either in the presence of the mimetics or in their absence (Hardy et al., 1994). Thus, our data support that hydrogen peroxide toxicity is not an issue when efficient and selective superoxide dismutation is achieved. Futhermore, the chronic treatment in vivo with M40403 (10 days in a model of arthritis) did not exert any toxic effect (Salvemini et al., 2001b).
Conclusions
There is no doubt that superoxide plays a role in various disorders. That superoxide plays a role in human disease has also been substantiated by encouraging results obtained in various clinical trials performed with Orgotein® (Niwa et al., 1985; Flohe, 1988). Interestingly, the first clinical pilot studies with the native enzyme were carried out as early as the 1970s in rheumatoid arthritis (RA) and osteoarthritis (OA), with preliminary results demonstrating efficacy. Further studies then showed that Orgotein®, when given by intra-articular injection, attenuates signs and symptoms (inflammation and pain) of RA and OA (Goebel et al., 1981; Goebel & Storck, 1983; Lund-Olesen & Menander-Huber, 1983; Gammer & Broback, 1984; Mc Ilwain et al., 1989; Mazieres et al., 1991). Interestingly, in these studies, Orgotein® led to some 60% decrease in the consumption of analgaesics. Furthermore, Orgotein® was found to be effective when given by intra-articular injection in patients with temporomandibular joint (TMJ) dysfunction, who failed to respond to standard therapy (Lin et al., 1994), and was found to reduce pain in patients with duodenal ulcer pain (Pascu & Dejica, 1987). Possibly, the most compelling data for the efficacy of SOD in human disease come from a large body of data gathered since the early 1980s, showing the protective effects of Orgotein® (given by intramuscular injection) against acute and chronic side effects associated with chemotherapy and radiation therapy (Marberger et al., 1974,1975,1981; Edsmyr et al., 1976; Menander-Huber et al., 1978; Housset et al., 1989; Delanian et al., 1994; Sanchiz et al., 1996). It is of major importance that Orgotein® is able to reverse fibrosis once it is established (Niwa et al., 1985; Flohe, 1988). Other clinical settings in which SOD (whether of recombinant or bovine origin) was used include patients with Crohn's disease, various forms of periarticular inflammation and Peyronie's disease (Babior, 1997). Despite the apparent clinical benefit, SOD enzymes suffer as drug candidates due to a number of reasons described in the previous sections, and were removed from the markets primarily because of immunogenic response. In certain cases where an enzyme of potential therapeutic benefit does not have the appropriate properties for a drug, a synthetic, small-molecule enzyme mimetic can conceivably be designed with chemical and physical characteristics suitable for a therapeutic.
In this review, we have discussed a number of agents that may fulfill this purpose: these provide an example of a unique approach for the development of artificial enzymes as future drugs. The Mn(II)-based SODm are true mimetics of the native enzyme – they are catalysts and are selective for superoxide. These, like the native SOD enzymes, are catalysts, and remove superoxide at a high rate, selectively, without reacting with other biologically relevant oxidizing species, such as nitric oxide, peroxynitrite, hydrogen peroxide, hypochlorite or oxygen (Riley et al., 1996,1997; Salvemini et al., 1999b). The unique selectivity of mimetics such as M40403 resides in the nature of the manganese(II) centre in the complex. The resting oxidation state of the complex is the reduced Mn(II) ion; as a consequence, the complex has no reactivity with reducing agents until it is oxidized to Mn(III) by protonated superoxide, whereupon, the complex is rapidly reduced back to the Mn(II) state by the superoxide anion at diffusion-controlled rates. Since the complex is so difficult to oxidize (>+0.74 V (vs SHE)), many one-electron oxidants cannot oxidize this and its related complexes (including nitric oxide and oxygen). Further, since the SODm operate via a facile one-electron oxidation pathway, other two-electron nonradical, but nevertheless, potent oxidants are not kinetically competent to oxidize the Mn(II) complex; for example, peroxynitrite, hydrogen peroxide or hypochlorite. It is important to realize that this property is not shared by other ‘so-called and claimed classes of SODm' discussed in sections A through C. Although these agents are clearly protective (as discussed in this review) in animal models, one general limitation of these agents (if one is interested in dissecting the contribution of superoxide in physiopathological situations versus other reactive species) is that they react not only with superoxide but also with a wide variety of reactive oxygen species. From a pharmacological standpoint, such nonselectivity makes the interpretation of the result difficult to make, and caution needs to be taken before making general assumptions and claims on the type of the reactive oxygen species in the disease state under investigation. In general, such agents should not be accepted as pharmacological tools with which to investigate or validate the role(s) of the superoxide in in vivo model systems.
We propose that compounds that have been developed, and that will be developed in the future with SOD activity and selectivity for superoxide, will find clinical utility in diseases mediated, in part, by superoxide (Figure 7). To this end, M40403 has been developed with the intent to utilize it as a human pharmaceutical agent for the treatment of various diseases. M40403 has successfully completed a Phase I safety clinical trial in healthy human subjects.
Figure 7.
Therapeutic opportunities for SODm.
Acknowledgments
We would like to thank Dr Alexandre Samouilov for his help in generating EPR data to support Figure 5.
Abbreviations
- NFκB
nuclear factor κB
- OONO–
peroxynitrite
- PARP
poly-ADP-ribose polymerase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SODm
superoxide dismutase mimetics
- TNFα
tumour necrosis factor α
References
- ALBRECHT T., BOLDOGH I., FONS M.P. Receptor-initiated activation of cells and their oncogenes by herpes-family viruses. J. Invest. Dermatol. 1992;98:29S–35S. doi: 10.1111/1523-1747.ep12462169. [DOI] [PubMed] [Google Scholar]
- AL-SHABANAH O.A., MANSOUR M.A., ELMAZAR M.M. Enhanced generation of leukotriene B4 and superoxide radical from calcium ionophore (A23187) stimulated human neutrophils after priming with interferon-alpha. Res. Commun. Mol. Pathol. Pharmacol. 1999;106:115–128. [PubMed] [Google Scholar]
- ANDERSON I., ADINOLFI C., DOCTROW S., HUFFMAN K., JOY K.A., MALFROY B., SODEN P., RUPNIAK H.T., BARNES J.C. Oxidative signalling and inflammatory pathways in Alzheimer's disease. Biochem. Soc. Symp. 2001;67:141–149. doi: 10.1042/bss0670141. [DOI] [PubMed] [Google Scholar]
- ANDO Y., INOUE M., HIROTA M., MORINO Y., ARAKI S. Effect of superoxide dismutase derivative on cold-induced brain edema. Brain Res. 1989;477:286–291. doi: 10.1016/0006-8993(89)91416-9. [DOI] [PubMed] [Google Scholar]
- ANDREASSEN O.A., FERRANTE R.J., DEDEOGLU A., ALBERS D.W., KLIVENYI P., CARLSON E.J., EPSTEIN C.J., BEAL M.F. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol. 2001;167:189–195. doi: 10.1006/exnr.2000.7525. [DOI] [PubMed] [Google Scholar]
- ASTON K., RATH N., NAIK A., SLOMCZYNSKA U., SCHALL O.F., RILEY D.P. Computer-aided design (CAD) of Mn(II) complexes: superoxide dismutase mimetics with catalytic activity exceeding the native enzyme. Inorg. Chem. 2001;40:1779–1789. doi: 10.1021/ic000958v. [DOI] [PubMed] [Google Scholar]
- AVRAHAM K.B., SCHICKLER M., SAPOZNIKOV D., YAROM R., GRONER Y. Down's syndrome: abnormal neuromuscular junction in tongue of transgenic mice with elevated levels of human Cu/Zn-superoxide dismutase. Cell. 1988;54:823–829. doi: 10.1016/s0092-8674(88)91153-1. [DOI] [PubMed] [Google Scholar]
- BABIOR B.M. Superoxide: a two-edged sword braz. J. Med. Biol. Res. 1997;30:141–155. doi: 10.1590/s0100-879x1997000200001. [DOI] [PubMed] [Google Scholar]
- BACIU I. Nervous control of the phagocytic system. Intern. J. Neurosci. 1988;41:127–141. doi: 10.3109/00207458808985749. [DOI] [PubMed] [Google Scholar]
- BAKER K., MARCUS C.B., HUFFMAN K., KRUK H., MALFROY B., DOCTROW S.R. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- BARNES J.P. Chronic obstructive pulmonary disease. Med. Prog. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALLAND P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S., CROW L.P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- BIANCA R., WAYMAN N., MCDONALD M., PINTO A., SHAPE M., CHATTERJEE P., THIEMERMANN C. Superoxide dismutase mimetic with catalase activity, EUK-134, attenuates the multiple organ injury and dysfunction caused by endotoxin in the rat. Med. Sci. Monit. 2002;8:BR1–BR7. [PubMed] [Google Scholar]
- BLOKHINA O., VIROLAINEN E., FAGERSTEDT K.V.Antioxidants, oxidative damage and oxygen deprivation stress: a review Ann. Bot. 200391179–194.(review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORIGINI M.J., PAULUS H.E. Innovative treatment approaches for rheumatoid arthritis. Combination therapy. Baillieres Clin. Rheumatol. 1995;9:689–710. doi: 10.1016/s0950-3579(05)80309-7. [DOI] [PubMed] [Google Scholar]
- BOWES J., MCDONALD M.C., PIPER J., THIEMERMANN C. Inhibitors of poly (ADP-ribose) synthetase protect rat cardiomyocytes against oxidant stress. Cardiovasc. Res. 1999;41:126–134. doi: 10.1016/s0008-6363(98)00221-1. [DOI] [PubMed] [Google Scholar]
- BOWES J., PIPER J., THIEMERMANN C. Inhibitors of the activity of poly (ADP-ribose) synthetase reduce the cell necrosis caused by hydrogen peroxide in human cardiac myoblasts. Br. J. Pharmacol. 1998;124:1760–1766. doi: 10.1038/sj.bjp.0702009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWLER R.P., ARCAROLI J., CRAPO J.D., ROSS A., SLOT J.W., ABRAHAM E. Extracellular superoxide dismutase attenuates lung injury after hemorrhage. Am. J. Crit. Care Med. 2001;164:290–294. doi: 10.1164/ajrccm.164.2.2011054. [DOI] [PubMed] [Google Scholar]
- BOWLER R.P., CRAPO J.D. Oxidative stress in allergic respiratory diseases. J. Allergy Immunol. 2002a;110:349–356. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- BOWLER R.P., CRAPO J.D.Oxidative stress in airways: is there a role for extracellular superoxide dismutase Am. J. Respir. Crit. Care Med. 2002b166S38–S43.(review) [DOI] [PubMed] [Google Scholar]
- BRENNAN M.L., WU W., FU X., SHEN Z., SONG W., FROST H., VADSETH C., NARINE L., LENKIEWICZ E., BORCHERS M.T., LUSIS A.J., LEE J.J., LEE N.A., ABU-SOUD H.M., ISCHIROPOULOS H., HAZEN S.L. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- BROWN R.H., JRSuperoxide dismutase in familial amyotrophic lateral sclerosis: models for gain of function Curr. Opin. Neurobiol. 19955841–846.(review) [DOI] [PubMed] [Google Scholar]
- CARLSSON L.M., JONSSON J., EDLUNDAND T., MARKLUND S.L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL R.T., GALATSIS P., BOROSKY S., KOPEC K.K., KUMAR V., ALTHAUS J.S., HALL E.D. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem. Res. Toxicol. 2000;13:294–300. doi: 10.1021/tx990159t. [DOI] [PubMed] [Google Scholar]
- CHAN P.H., YANG G.Y., CHEN S.F., CARLSON E., EPSTEIN C.J. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing superoxide dismutase. Ann. Neurol. 1991;29:482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- CHANG L.-Y., SLOT J.W., GEUZE H.P., CRAPO J.D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J. Cell Biol. 1988;107:2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Z., SIU B., HO Y.S., VINCENT R., CHUA C.C., HAMDY R.C., CHUA B.H. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J. Mol. Cell. Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- CHURCH S.L., GRANT J.W., RIDENOUR L.A., OBERLEY L.W., SWANSON P.E., MELTZER P.S., TRENT J.M. Increased manganese superoxide dismutase expression suppresses the malignant human melanoma cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3113–3117. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAPO J.D., OURY T., RABOUILLE C., SLOT J.W., CHANG L.W. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., MAZZON E., DUGO L., CAPUTI A.P., ASTON K., RILEY D.P., SALVEMINI D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 2001a;132:19–29. doi: 10.1038/sj.bjp.0703775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., MAZZON E., DUGO L., CAPUTI A.P., RILEY D.P., SALVEMINI D. Protective effects of M40403, a superoxide dismutase mimetic, in a rodent model of colitis. Eur. J. Pharmacol. 2001b;432:79–89. doi: 10.1016/s0014-2999(01)01427-3. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., MCDONALD M.C., MAZZON E., FILIPE H.M., COSTATINO G., CAPUTI A.P., THIEMERMANN C. Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of splanchnic artery occlusion and reperfusion. Shock. 2000a;14:150–156. doi: 10.1097/00024382-200014020-00013. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., MCDONALD M.C., MAZZON E., SIRIWARDENA D., COSTANTINO G., FULIA F., CUCINOTTA G., GITTO E., CORDARO S., BARBERI I., DE SARRO A., CAPUTI A.P., THIEMERMANN C. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 2000b;875:96–106. doi: 10.1016/s0006-8993(00)02582-8. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., RILEY D.P., CAPUTI A.P., SALVEMINI D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001c;53:135–159. [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., GILAD E., HAKE P., SALZMAN A.L., SZABO C. Protective effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthase in a carrageenan-induced model of local inflammation. Eur. J. Pharmacol. 1998a;342:67–76. doi: 10.1016/s0014-2999(97)01417-9. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., HAKE P., SALZMAN A.L., SZABO C. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic. Biol. Med. 1998b;24:450–459. doi: 10.1016/s0891-5849(97)00280-3. [DOI] [PubMed] [Google Scholar]
- DAY B.J., BATINIC-HABERLE J., CRAPO J.D. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic. Biol. Med. 1999;26:730–736. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- DAY B.J., FRIDOVICH I., CRAPO J.D. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- DAY B.J., SHAWEN S., LIOCHEV S.I., CRAPO J.D. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J. Pharmacol. Exp. Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- DELANIAN S., BAILLET F., HUART J., LEFAIX J.L., MAULARD C., HOUSSET M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother. Oncol. 1994;32:12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- DIX T.A., HESS K.M., MEDINA M.A., SULLIVAN R.W., TILLY S.L., WEBB T.L.L. Mechanism of site-selective DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry. 1996;35:4578–4583. doi: 10.1021/bi952010w. [DOI] [PubMed] [Google Scholar]
- DOCTROW S.R., HUFFMAN K., MARCUS C.B., MUSLEH W., BRUCE A., BAUDRY M., MALFROY B. Salen–manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv. Pharmacol. 1997;38:247–269. doi: 10.1016/s1054-3589(08)60987-4. [DOI] [PubMed] [Google Scholar]
- DOWLING E.J., CHANDER C.L., CLAXSON A.W., LILLIE C., BLAKE D.R. Assessment of a human recombinant manganese superoxide dismutase in models of inflammation. Free Radic. Res. Commun. 1993;18:291–298. doi: 10.3109/10715769309147496. [DOI] [PubMed] [Google Scholar]
- DROY-LEFAIX M.T., DROUET Y., GERAUD G., HOSFOD D., BRAQUET P. Superoxide dimutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia–reperfusion. Free Radic. Res. Commun. 1991;12:725–735. doi: 10.3109/10715769109145852. [DOI] [PubMed] [Google Scholar]
- EDEAS M.A., EMERIT I., KHALHOUN Y., LAZIZI Y., CERNJAVSKI L., LEVY A., LINDENBAUM A. Clastogenic factors in plasma of HIV-1 infected patients activate HIV-1 replication in vitro: inhibition by superoxide dismutase. Free Radic. Biol. Med. 1997;23:571–578. doi: 10.1016/s0891-5849(97)00002-6. [DOI] [PubMed] [Google Scholar]
- EDSMYR F., HUBER W., MENANDER K.B. Orgotein efficacy in ameliorating side effects due to radiation therapy. I. Double-blind, placebo-controlled trial in patients with bladder tumors. Curr. Ther. Res. Clin. Exp. 1976;19:198–211. [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- EVANS S.M., WHITTHLE B.J. Interactive roles of superoxide and inducible nitric oxide synthase in rat intestinal injury provoked by non-steroidal anti-inflammatory drugs. Eur. J. Pharmacol. 2001;429:287–296. doi: 10.1016/s0014-2999(01)01327-9. [DOI] [PubMed] [Google Scholar]
- FAULKNER K.M., LIOCHEV S.I., FRIDOVICH I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- FERRET P.J., HAMMOUD R., TULLIEZ M., TRAN A., TREBEDEN H., JAFFRAY P., MALASSAGNE B., CALMUS Y., WEILL B., BATTEUX F. Detoxification of reactive oxygen species by a nonpeptidyl mimic of superoxide dismutase cures acetaminophen-induced acute liver failure in the mouse. Hepatology. 2001;33:1173–1180. doi: 10.1053/jhep.2001.24267. [DOI] [PubMed] [Google Scholar]
- FLOHE L. Superoxide dismutase for therapeutic use: clinical experience, dead ends and hopes. Mol. Cell. Biochem. 1988;84:123–131. doi: 10.1007/BF00421046. [DOI] [PubMed] [Google Scholar]
- FLORES S.C., MARECKI J.C., HARPER K.P., BOSE S.K., NELSON S.K., MCCORD J.M. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLZ R.J., ABUSHAMAA A.M., SULIMAN H.B. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J. Clin. Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLZ R.J., PENO-GREEN L., CRAPO J.D. Identification of a homozygous missense mutation (Arg to Gly) in the critical binding region of the human EC-SOD gene (SOD3) and its association with dramatically increased serum enzyme levels. Hum. Mol. Genet. 1994;3:2251–2254. doi: 10.1093/hmg/3.12.2251. [DOI] [PubMed] [Google Scholar]
- FRIES D.M., PAXINOU E., THEMISTOCLEOUS M., SWANBERG E., GRIENDLING K.K., SALVEMINI D., SLOT J.W., HEIJNEN H.F., HAZEN S.L., ISCHIROPOULOS H.Expression of inducible nitric oxide synthase and intracellular protein tyrosine nitration in vascular smooth muscle cells: role of reactive oxygen species J. Biol. Chem. 200327822901–22907.(April 10 as manuscript M210806200, in press) [DOI] [PubMed] [Google Scholar]
- GAMMER W., BROBACK L.G. Clinical comparison of orgotein and methylprednisolone acetate in the treatment of osteoarthrosis of the knee joint. Scand. J. Rheumatol. 1984;13:10E–112. doi: 10.3109/03009748409100372. [DOI] [PubMed] [Google Scholar]
- GARDNER P.R., NGUYEN D.D., WHITE C.W. Superoxide scavenging by Mn(II/III) tetrakis (1-methyl-4-pyridyl) porphyrin in mammalian cells. Arch. Biochem. Biophys. 1996;325:20–28. doi: 10.1006/abbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- GAUT J.P., BYUN J., TRAN H.D., LAUBER W.M., CARROLL J.A., HOTCHKISS R.S., BELAAOUAJ A., HEINECKE JW. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Invest. 2002;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEBEL K.M., STORCK U. Effect of intra-articular orgotein versus a corticosteroid on rheumatoid arthritis of the knees. Am. J. Med. 1983;74:124–128. doi: 10.1016/0002-9343(83)91128-2. [DOI] [PubMed] [Google Scholar]
- GOEBEL K.M., STORCK U., NEURATH F. Intrasynovial orgotein therapy in rheumatoid arthritis. Lancet. 1981;1:1015–1017. doi: 10.1016/s0140-6736(81)92185-1. [DOI] [PubMed] [Google Scholar]
- GONZALEZ P.K., ZHUANG J., DOCTROW S.R., MALFROY B., BENSON P.F., MENCONI M.J., FINK M.P. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J. Pharmacol. Exp. Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- GRONER Y., ELROY-STEIN O AVRAHAM K.B., SCHICKLER M., KNOBLER H., MINC-GOLOMB D., BAR-PELED O., YAROM R., ROTSHENKER S. Cell damage by excess CuZnSOD and Down's syndrome. 1994;48:231–240. doi: 10.1016/0753-3322(94)90138-4. [DOI] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- GUIRAO X., KUMAR A., KATZ J., SMITH M., LIN E., KEOGH C., CALVANO S.E., LOWRY S.F. Catecholamines increase monocyte TNF receptors and inhibit TNF through A2-adrenoceptor activation. Am. J. Physiol. 1997;273:E1203–E1208. doi: 10.1152/ajpendo.1997.273.6.E1203. [DOI] [PubMed] [Google Scholar]
- GURNEY M.E. Transgenic-mouse model of amyotrophic lateral sclerosis. N. Engl. J. Med. 1994;331:1721–1722. doi: 10.1056/NEJM199412223312516. [DOI] [PubMed] [Google Scholar]
- HADDAD J.J., LAND S.C. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-alpha biosynthesis. Br. J. Pharmacol. 2002;135:520–536. doi: 10.1038/sj.bjp.0704467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGLIND E., XIA G., RYLANDER R. Effects of antioxidants and PAF receptor antagonist in intestinal shock in the rat. Circ. Shock. 1994;42:83–91. [PubMed] [Google Scholar]
- HALLIWELL B., GUTTERIDGE J.M.C. Free radicals in biology and medicine 1985Oxford: Oxford University Press; 89–193.Baum, H., Gergely, J. & Fanburg, B.L. (eds.) pp [Google Scholar]
- HARDY M.M., FLICKINGER A.G., RILEY D.P., WEISS R.H., RYAN U.S. Superoxide dismutase mimetics inhibit neutrophil-mediated human aortic endothelial cell injury in vitro. J. Biol. Chem. 1994;269:18535–18540. [PubMed] [Google Scholar]
- HENKE S.L. Superoxide dismutase mimics as future therapeutics. Exp. Opin. Ther. Patents. 1999;9:169. [Google Scholar]
- HOUSSET M., BAILLET F., MICHELSON A.M., PUGET K. Action of liposomal superoxide dismutase on measurable radiation-induced fibrosis. Ann. Med. Interne. (Paris) 1989;140:365–367. [PubMed] [Google Scholar]
- HUBER W., MENANDER-HUBER K.B., SAIFER M.G.P., WILLIAMS L.D. Bioavailability of superoxide dismutase: implications for the anti-inflammatory action mechanism of orgotein. A.A.S. 1980;7:185–195. [PubMed] [Google Scholar]
- HURST J.K. Whence nitrotyrosine. J. Clin. Invest. 2002;109:1287–1289. doi: 10.1172/JCI15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE S., KAWANISHI S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., AL-MEHDI A.B., FISHER A.B.Reactive species in ischemic rat lung injury: contribution of peroxynitrite Am. J. Physiol. 1995269L158–L164.(2 Pt 1) [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., BECKMAN J.S. Oxidative stress and nitration in neurodegeneration: cause, effect, or association. J. Clin. Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., BECKMAN I.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- JUNG C., RONG Y., DOCTROW S., BAUDRY M., MALFROY B., XU Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci. Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- KARUPIAH G., CHEN J.H., MAHAHNGARN S., NATHAN C.F., MACMICKING J.D. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARUPIAH G., XIE Q.W., BULLER R.M., NATHAN C., DUARTE C., MACMICKING J.D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- KELLER J.N., KINDY M.S., HOLTSBERG F.W., ST CLAIR D.K., YEN H.C., GERMEYER A., STEINER S.M., BRUCE-KELLER A.J., HUTCHINS J.B., MATTSON M.P. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLER G.A., WARNER T.G., STEIMER K.S., HALLEWELL R.A. CuZn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7381–7385. doi: 10.1073/pnas.88.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYER K., IMLAY J.A. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINNULA V.L., CRAPO J.D., RAIVIO K.O. Generation and disposal of reactive oxygen metabolites in the lung. Lab. Invest. 1995;73:3–19. [PubMed] [Google Scholar]
- KOPPENOL W.H. The basic chemistry of nitrogen monoxide and peroxynitrite. Free Radic. Biol. Med. 1998;25:385–391. doi: 10.1016/s0891-5849(98)00093-8. [DOI] [PubMed] [Google Scholar]
- KRISHNA M.C., RUSSO A., MITCHELL J.B., GOLDSTEIN S., DAFNI H., SAMUNI A. Do nitroxide antioxidants act as scavengers of superoxide anions or as SOD mimetics. J. Biol. Chem. 1996;271:26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- LAIGHT D.W., ANDREWS T.J., HAJ-YEHIA A. Microassay of superoxide anion scavenging activity in vitro. Environ. Toxicol. Pharm. 1997;3:65–68. doi: 10.1016/s1382-6689(96)00143-3. [DOI] [PubMed] [Google Scholar]
- LAIGHT D.W., KAW A.V., CARRIER M.J., ANGGARD E.E. Interaction between superoxide anion and nitric oxide in the regulation of vascular endothelial function. Br. J. Pharmacol. 1998;124:238–244. doi: 10.1038/sj.bjp.0701814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMARQUE D., MORAN A.P., SZEPES Z., DELCHIER J.C., WHITTLE B.J. Cytotoxicity associated with induction of nitric oxide synthase in rat duodenal epithelial cells in vivo by lipopolysaccharide of Helicobacter pylori: inhibition by superoxide dismutase. Br. J. Pharmacol. 2000;130:1531–1538. doi: 10.1038/sj.bjp.0703468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEACH M., FRANK S., OLBRICH A., PFEILSCHIFTER J., THIEMERMANN C. Decline in the expression of copper/zinc superoxide dismutase in the kidney of rats with endotoxic shock: effects of the superoxide anion radical scavenger, tempol, on organ injury. Br. J. Pharmacol. 1998;125:817–825. doi: 10.1038/sj.bjp.0702123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBOVITZ R.M., ZHANG H., VOGEL H., CARTWRIGHT J., JR, DIONNE L., LU N., HUANG S., MATZUK M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENAZ G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- LEVIN E.D., BRADY T.C., HOCHREIN E.C., OURY T.D., JONSSON L.M., MARKLUND S.L., CRAPO J.D. Molecular manipulations of extracellular superoxide dismutase: functional importance for learning. Behav. Genet. 1998;28:381–390. doi: 10.1023/a:1021673703129. [DOI] [PubMed] [Google Scholar]
- LI Y., HUANG T.T., CARLSON E.J., MELOV S., URSELL P.C., OLSON J.L., NOBLE L.J., YOSHIMURA M.P., BERGER C., CHAN P.H. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- LIAUDET L., SORIANO F.G., SZABO C.Biology of nitric oxide signaling Crit. Care Med. 200028N37–N52.(review) [DOI] [PubMed] [Google Scholar]
- LIN Y., PAPE H.D., FRIEDRICH R. Use of superoxide dismutase (SOD) in patients with temporomandibular joint dysfunction–a preliminary study. Int. J. Oral Maxillofac. Surg. 1994;23:428–429. doi: 10.1016/s0901-5027(05)80038-4. [DOI] [PubMed] [Google Scholar]
- LIOU W., CHANG L.-Y., GEUZE H.P., STROUS G.P., CRAPO J.D., SLOT J.W. Distribution of CuZn superoxide dismutase in rat liver. Free Radic. Biol. Med. 1993;14:201–207. doi: 10.1016/0891-5849(93)90011-i. [DOI] [PubMed] [Google Scholar]
- LOWE D., PAGEL P.S., MCGOUGH M.F., HETTRICK D.A., WARLTIER D.C. Comparison of the cardiovascular effects of two novel superoxide dismutase mimetics, SC-55858 and SC-54417, in conscious dogs. Eur. J. Pharmacol. 1996;304:81–86. doi: 10.1016/0014-2999(96)00120-3. [DOI] [PubMed] [Google Scholar]
- LUND-OLESEN K., MENANDER-HUBER K.B. Intra-articular orgotein therapy in osteoarthritis of the knee. A double-blind, placebo-controlled trial. Arzneimittelforschung. 1983;33:1199–1203. [PubMed] [Google Scholar]
- MACARTHUR H., COURI D.M., WILKEN G.H., WESTFALL T.C., LECHNER A.J., MATUSCHAK G.M., CHEN Z., SALVEMINI D. Modulation of serum cytokine levels by a novel superoxide dismutase mimetic, M40401, in an Escherichia coli model of septic shock: correlation with preserved circulating catecholamines. Crit. Care Med. 2003;31:237–245. doi: 10.1097/00003246-200301000-00037. [DOI] [PubMed] [Google Scholar]
- MACARTHUR H., WESTFALL T.C., RILEY D.P., MISKO T.P., SALVEMINI D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9753–9758. doi: 10.1073/pnas.97.17.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACMILLAN-CROW L.A., CRUTHIRDS D.L. Manganese superoxide dismutase in disease. Free Radic. Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- MACMILLAN-CROW L.A., THOMPSON J.A. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (y34f) by peroxynitrite. Arch. Biochem. Biophys. 1999;66:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- MALASSAGNE B., FERRET P.J., HAMMOUD R., TULLIEZ M., BEDDA S., TREBEDEN H., JAFFRAY P., CALMUS Y., WEILL B., BATTEUX F. The superoxide dismutase mimetic MnTBAP prevents Fas-induced acute liver failure in the mouse. Gastroenterology. 2001;121:1451–1459. doi: 10.1053/gast.2001.29590. [DOI] [PubMed] [Google Scholar]
- MALFROY B., DOCTROW S.R., ORR P.L., TOCCO G., FEDOSEYEVA E.V., BENICHOU G. Prevention and suppression of autoimmune encephalomyelitis by EUK-8, a synthetic catalytic scavenger of oxygen-reactive metabolites. Cell. Immunol. 1997;177:62–68. doi: 10.1006/cimm.1997.1091. [DOI] [PubMed] [Google Scholar]
- MAO G.D., THOMAS P.D., LOPASCHUK G.D., POZNANSKY M.J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J. Biol. Chem. 1993;268:416. [PubMed] [Google Scholar]
- MARBERGER H., BARTSCH G., HUBER W., MENANDER K.B., SCHULTE T.L. Orgotein: a new drug for the treatment of radiation cystitis. Curr. Ther. Res. Clin. Exp. 1975;18:466–475. [PubMed] [Google Scholar]
- MARBERGER H., HUBER W., BARTSCH G., SCHULTE T., SWOBODA P. Orgotein: a new antiinflammatory metalloprotein drug evaluation of clinical efficacy and safety in inflammatory conditions of the urinary tract. Int. Urol. Nephrol. 1974;6:61–74. doi: 10.1007/BF02081999. [DOI] [PubMed] [Google Scholar]
- MARBERGER H., HUBERT W., MENANDER-HUBER K.B., BARTSCH G. Orgotein: a new drug for the treatment of radiation cystitis. Eur. J. Rheumatol. Inflamm. 1981;4:244–249. [PubMed] [Google Scholar]
- MARKLUND S.L. Human copper-containing superoxide dismutase of high molecular weight. Proc. Natl. Acad. Sci. U.S.A. 1982;79:7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKLUND S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUNAGA T., NAKAJIMA T., SONODA M., KOYAMA I., KAWAI S., INOUE I., KATAYAMA S., HIRANO K., HOKARI S., KOMODA T. Modulation of reactive oxygen species in endothelial cells by peroxynitrite-treated lipoproteins. J. Biochem. 2001;130:285–293. doi: 10.1093/oxfordjournals.jbchem.a002984. [DOI] [PubMed] [Google Scholar]
- MAXWELL S.R.Prospects for the use of antioxidant therapies Drugs 199549345–361.(review) [DOI] [PubMed] [Google Scholar]
- MAZIERES B., MASQUELIER A.M., CAPRON M.H. A French controlled multicenter study of intmarticular Orgotein versus intraartlcular corticosteroids in the treatment of knee osteoarthrftis: a one-year followup. J. Rheumatol. 1991;18:134–137. [PubMed] [Google Scholar]
- MC CORD J.M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- MC CORD J.M., FRIDOVICH I. Superoxide dismutase: an enzymatic function for erythrocuprein. J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- MC CORD J.M., OMAR B.A. Sources of free radicals. Toxicol. Ind. Health. 1993;9:23–37. doi: 10.1177/0748233793009001-204. [DOI] [PubMed] [Google Scholar]
- MC INNIS J., WANG C., ANASTASIO N., HULTMAN M., YE Y., SALVEMINI D., JOHNSON K.M. The role of superoxide and nuclear factor-kappaB signaling in N-methyl-D-aspartate-induced necrosis and apoptosis. J. Pharmacol. Exp. Ther. 2002;301:478–487. doi: 10.1124/jpet.301.2.478. [DOI] [PubMed] [Google Scholar]
- MC ILWAIN H., SILVERFIELD J.C., CHEATUM D.E., POILEY J., TABORN J., IGNACZAK T., MULTZ C.V. Intra-articular orgotein in osteoarthritis of the knees: a placebo-controlled efficacy, safety, and dosage comparison. Am. J. Med. 1989;87:295–300. doi: 10.1016/s0002-9343(89)80154-8. [DOI] [PubMed] [Google Scholar]
- MELOV S., COSKUN P., PATEL M., TUINSTRA R., COTTRELL B., JUN A.S., ZASTAWNY T.H., DIZDAROGLU M., GOODMAN S.I., HUANG T.T., MIZIORKO H., EPSTEIN C.J., WALLACE D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELOV S., DOCTROW S.R., SCHNEIDER J.A., HABERSON J., PATEL M., COSKUN P.E., HUFFMAN K., WALLACE D.C., MALFROY B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENANDER-HUBER K.B., EDSMYR F., HUBER W. Orgotein (superoxide dismutase): a drug for the amelioration of radiation-induced side effects. A double-blind, placebo-controlled study in patients with bladder tumours. Urol. Res. 1978;6:255–257. doi: 10.1007/BF00262630. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., HIGHKIN M.K., VEENHUIZEN A.W. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 1998;273:15646–15653. doi: 10.1074/jbc.273.25.15646. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.B., SAMUNI A., KRISHNA M.C. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990;29:2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- MOLLACE V., NOTTET H.S., CLAYETTE P., TURCO M.C., MUSCOLI C., SALVEMINI D., PERNO C.F.Oxidative stress and neuro AIDS: triggers, modulators and novel antioxidants Trends Neurosci. 200124411–416.(review) [DOI] [PubMed] [Google Scholar]
- MOLLACE V., SALVEMINI D., RILEY D.P., MUSCOLI C., IANNONE M., GRANATO T., MASUELLI L., MODESTI A., ROTIROTI D., NISTICO R., BERTOLI A., PERNO C.F., AQUARO S. The contribution of oxidative stress in apoptosis of human-cultured astroglial cells induced by supernatants of HIV-1-infected macrophages. J. Leukoc. Biol. 2002;71:65–72. [PubMed] [Google Scholar]
- MONASTRA G., SECCHI E.F. Beta-adrenergic receptors mediate in vivo the adrenaline inhibition of lipopolysaccharide-induced tumor necrosis factor release. Immunol. Lett. 1993;38:127–130. doi: 10.1016/0165-2478(93)90177-4. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A.Nitric oxide: physiology, pathophysiology, and pharmacology Pharmacol. Rev. 199143109–142.(review) [PubMed] [Google Scholar]
- MONTI E., COVA D., GUIDO E., MORELLI R., OLIVA C. Protective effect of the nitroxide tempol against the cardiotoxicity of adriamycin. Free Radic. Biol. Med. 1996;21:463–470. doi: 10.1016/0891-5849(96)00124-4. [DOI] [PubMed] [Google Scholar]
- MOTA-FILIPE H., MCDONALD M.C., CUZZOCREA S. A membrane-permeable radical scavenger reduces the organ injury in hemorrhagic shock. Shock. 1999;12:255–261. doi: 10.1097/00024382-199910000-00002. [DOI] [PubMed] [Google Scholar]
- NAKAO N. Overexpressing Cu/Zn superoxide dismutase enhances survival of transplanted neurons in a rat model of Parkinson's disease. Nat. Med. 1995;1:226–231. doi: 10.1038/nm0395-226. [DOI] [PubMed] [Google Scholar]
- NIWA Y., SOMIYA K., MICHELSO A.M., PUGET K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free Radic. Res. Comms. 1985;1:137–153. doi: 10.3109/10715768509056547. [DOI] [PubMed] [Google Scholar]
- OMAR B.A., MCCORD J.M. Interstitial equilibration of superoxide dismutase correlates with it protective effect in the isolated rabbit heart. J. Mol. Cell. Cardiol. 1991;23:149–159. doi: 10.1016/0022-2828(91)90102-r. [DOI] [PubMed] [Google Scholar]
- OURY T.D., CRAPO J.D., VALNICKOVA Z., ENGHILD J.J. Human extracellular superoxide dismutase is a tetramer composed of two disulfide-linked dimers: a simplified, high yield purification of extracellular superoxide dismutase. Biochem. J. 1996;317:51–57. doi: 10.1042/bj3170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYANAGUI Y. Participation of superoxide anions at the prostaglandin phase of carrageenan foot-oedema. Biochem. Pharmacol. 1976;25:1465–1472. [PubMed] [Google Scholar]
- PACE G.W., LEAF C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995;19:523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- PASCU O., DEJICA D. Oxygen free radicals and duodenal ulcer pain. Preliminary data. Med. Interne. 1987;25:81–84. [PubMed] [Google Scholar]
- PETERHANS E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J. Nutr. 1997;127:962S–965S. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- PIMENTEL D.R., AMIN J.K., XIAO L., MILLER T., VIERECK J., OLIVER-KRASINSKI J., BALIGA R., WANG J., SIWIK D.A., SINGH K., PAGANO P., COLUCCI W.S., SAWYER D.B. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- PONG K., DOCTROW S.R., BAUDRY M. Prevention of 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced nitration of tyrosine hydroxylase and neurotoxicity by EUK-134, a superoxide dismutase and catalase mimetic, in cultured dopaminergic neurons. Brain Res. 2000;881:182–189. doi: 10.1016/s0006-8993(00)02841-9. [DOI] [PubMed] [Google Scholar]
- PRZEDBORSKI S., KOSTIC V., JACKSON-LEWIS V., NAINI A.B., SIMONETTI S., FAHN S., CARLSON E., EPSTEIN C.J., CADET J.L. Transgenic mice with increased Cu/Zn superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REAUME A.G., ELLIOTT J.T., HOFFMAN E.K., KOWALL N.W., FERRANTE R.J., SIWEK D.F., WILCOX H.M., FLOOD D.G., BEAL M.F., BROWN RH JR., SCOTT R.W., SNIDER W.D. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- REDDAN J., SEVILLA M., GIBLIN F. Tempol and deferoxamine protect cultured rabbit lens epithelial cells from H2O2 insult: insight into the mechanism of H2O2-induced injury. Lens Eye Toxicol. Res. 1992;9:385–393. [PubMed] [Google Scholar]
- RILEY D.P., HENKE S.L., LENNON P.J., ASTON K. Computer-aided design (cad) of synzymes: use of molecular mechanics (mm) for the rational design of superoxide dismutase mimics. Inorg. Chem. 1999;38:1908–1917. doi: 10.1021/ic981319b. [DOI] [PubMed] [Google Scholar]
- RILEY D.P., HENKE S.L., LENNON P.J., WEISS R.H., NEUMANN W.L., RIVERS W.J., ASTON K.W., SAMPLE K.R., LING C.S., SHIEH J.J., BUSCH D.H., SZULBINSKI W. Synthesis, characterization and stability of manganese (ii) c-substituted 1,4,7,10,13-pentaazacyclopentadecane complexes exhibiting superoxide dismutase activity. Inorg. Chem. 1996;35:5213–5231. [Google Scholar]
- RILEY D.P., LENNON P.J., NEUMANN W.L., WEISS R.H. Toward the rational design of superoxide dismutase mimics: mechanistic studies for the elucidation of substituent effects on the catalysis activity of macrocyclic manganese (ii) complexes. J. Am. Chem. Soc. 1997;119:6522. [Google Scholar]
- ROJKIND M., DOMINGUEZ-ROSALES J.A., NIETO N., GREENWEL P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell. Mol. Life Sci. 2002;59:1872–1891. doi: 10.1007/PL00012511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFFORD S.E., OBERLEY T.D., URANO M., ST CLAIR D.K. Suppression of fibrosarcoma metastasis by elevated expression of manganese dismutase. Cancer Res. 1994;54:4261–4265. [PubMed] [Google Scholar]
- SALGO M.G., BERMUDEZ E., SQUADRITO G., PRYOR W. DNA damage and oxidation of thiols peroxynitrite causes in rat thymocytes. Arch. Biochem. Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- SALMINEN U., HARJULA A.L., MAASILTA P.K., ROMANSKA H.M., BISHOP A.E., POLAK J.M. Superoxide dismutase in development of obliterative bronchiolitis. Transplant Proc. 2001;33:2477. doi: 10.1016/s0041-1345(01)02066-8. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., CUZZOCREA S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic. Biol. Med. 2002a;33:1173–1185. doi: 10.1016/s0891-5849(02)00961-9. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., CUZZOCREA S. Superoxide, superoxide dismutase and ischemic injury. Curr. Opin. Investig. Drugs. 2002b;3:886–895. [PubMed] [Google Scholar]
- SALVEMINI D., CUZZOCREA S. Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit. Care Med. 2003;31:S29–S38. doi: 10.1097/00003246-200301001-00005. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MAZZON E., DUGO L., RILEY D.P., SERRAINO I., CAPUTI A.P., CUZZOCREA S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br. J. Pharmacol. 2001a;132:815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MAZZON E., DUGO L., SERRAINO I., DE SARRO A., CAPUTI A.P., CUZZOCREA S. Amelioration of joint disease in a rat model of collagen-induced arthritis by M40403, a superoxide dismutase mimetic. Arthrit. Rheum. 2001b;44:2909–2922. doi: 10.1002/1529-0131(200112)44:12<2909::aid-art479>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., RILEY D.P., LENNON P.J., WANG Z.Q., CURRIE M.G., MACARTHUR H., MISKO T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999a;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., BOURDON D.M., STERN M.K., CURRIE M.G., MANNING P.T. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 1996;303:217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.-Q., STERN M.K., CURRIE M.G., MISKO T.P. Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., ZWEIER J.L., SAMOUILOV A., MACARTHUR H., MISKO T.P., CURRIE M.G., CUZZOCREA S., SIKORSKI J.A., RILEY D.P. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999b;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- SAMUNI A., WINKELSBERG D., PINSON A. Nitroxide stable radicals protect beating cardiomyocytes against oxidative damage. J. Clin. Invest. 1991;87:1526–1530. doi: 10.1172/JCI115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHIZ F., MILLA A., ARTOLA N., JULIA J.C., MOYA L.M., PEDRO A., VILA A. Prevention of radioinduced cystitis by orgotein: a randomized study. Anticancer Res. 1996;16:2025–2028. [PubMed] [Google Scholar]
- SEVERN A., RAPSON N.T., HUNTER C.A., LIEW F.Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J. Immunol. 1992;148:3441–3445. [PubMed] [Google Scholar]
- SHARPE M.A., OLLOSSON R., STEWART V.C., CLARK J.B.Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics Biochem. J. 200236697–107.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA N., HIRANO A., YAMAMOTO T., KATO Y., KOBASHI M. Superoxide dismutase-1 mutation-related neurotoxicity in familial amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:143–161. doi: 10.1080/14660820050515151. [DOI] [PubMed] [Google Scholar]
- SHINGU M., TAKAHASHI S., ITO M., HAMAMATU N., SUENAGA Y., ICHIBANGASE Y., NOBUNAGA M. Anti-inflammatory effects of recombinant human manganese superoxide dismutase on adjuvant arthritis in rats. Rheumatol. Int. 1994;14:77–81. doi: 10.1007/BF00300251. [DOI] [PubMed] [Google Scholar]
- SIWIK D.A., TZORTZIS J.D., PIMENTAL D.R., CHANG D.L., PAGANO P.J., SINGH K., SAWYER D.B., COLUCCI W.S. Inhibition of copper–zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ. Res. 1999;85:147–153. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- SLEDZINSKI Z., ANTOSIEWICZ J., WOZNIAK M., OSTROWSKI M.M., STANEK A., WAJDA Z. Modification of proteins in the course of oxidative stress in acute experimental pancreatitis. Wiad Lek. 1997;2:115–118. [PubMed] [Google Scholar]
- SQUADRITO G.L., PRYOR W.A. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem. Biol. Interact. 1995;96:203–206. doi: 10.1016/0009-2797(94)03591-u. [DOI] [PubMed] [Google Scholar]
- SOUZA J.M., CHOI I., CHEN Q., WEISSE M., DAIKHIN E., YUDKOFF M., OBIN M., ARA J., HORWITZ J., ISCHIROPOULOS H. Proteolytic degradation of tyrosine nitrated proteins. Arch. Biochem. Biophys. 2000;380:360–366. doi: 10.1006/abbi.2000.1940. [DOI] [PubMed] [Google Scholar]
- STERN M.K., JENSEN M.P., KRAMER K. Peroxynitrite decomposition catalysts. J. Am. Chem. Soc. 1996;118:8735. [Google Scholar]
- SZABÓ C. Potential role of the peroxynitrate-poly(ADP-ribose) synthetase pathway in a rat model of severe hemorrhagic shock. Shock. 1998;9:341–344. doi: 10.1097/00024382-199805000-00005. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., BILLIAR T.R.Novel roles of nitric oxide in hemorrhagic shock Shock 1999b121–9.(review) [DOI] [PubMed] [Google Scholar]
- SZABÓ C., DAWSON V.L. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia–reperfusion. Trends Pharmacol. Sci. 1999a;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., DAY B.J., SALZMAN A.L. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages, using a novel mesoporphyrin superoxide dismutase analog and peroxynitrite scavenger. FEBS Lett. 1996a;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- SZABÒ C., ZINGARELLI B., O'CONNOR M., SALZMAN A.L. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc. Natl. Acad. Sci. U.S.A. 1996b;9:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN Y.H., TISCHIFIELD J, RUDDLE F.H. The linkage of genes for the human interferon induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J. Exp. Med. 1973;137:317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANGUY S., BOUCHER F.R., MAFROY B., DE LEIRIS J. Free radicals in reperfusion-induced arrhytihmias: study with EUK-8, a novel nonprotein catalytic antioxidant. Free Radic. Biol. Med. 1996;21:945–954. doi: 10.1016/s0891-5849(96)00231-6. [DOI] [PubMed] [Google Scholar]
- TERANO A., HIRAISHI H., OTA S., SHIGA J., SUGIMOTO T. Role of superoxide and hydroxyl radicals in rat gastric mucosal injury induced by ethanol. Gastroenterol. Jpn. 1989;24:488–493. doi: 10.1007/BF02773874. [DOI] [PubMed] [Google Scholar]
- THIEMERMANN C. Membrane-permeable radical scavengers (tempol) for shock, ischemia–reperfusion injury, and inflammation. Crit. Care Med. 2003;31 1 Suppl.:S76–S84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- TOYOKUNI S.Iron and carcinogenesis: from Fenton reaction to target genes Redox Rep. 20027189–197.(review) [DOI] [PubMed] [Google Scholar]
- TROY C.M., DEROSSI D., PROCHIANTZ A., GREENE L.A., SHELANSKI M.L. Down regulation of Cu/Zn superoxide dismutase leads to cell death via the nitric oxideperoxynitrite pathway. J. Neurosci. 1996;17:4180–4189. doi: 10.1523/JNEUROSCI.16-01-00253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER POLL T., COYLE S.M., BARBOSA K., BRAXTON C., LOWRY S.F. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J. Clin. Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER POLL T., JANSEN J., ENDERT E., SAUERWEIN H.P., VAN DEVENTER S.J.H. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect. Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER POLL T., LOWRY S.F. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor-alpha and IL-10. Am. J. Physiol. 1997;273:R1885–R1889. doi: 10.1152/ajpregu.1997.273.6.R1885. [DOI] [PubMed] [Google Scholar]
- VIRAG L., SZABO C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 2002;54:75–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- VOLK T., GERST J., FAUST-BELBE G., STROEHMANN A., KOX W.J. Monocyte stimulation by reactive oxygen species: role of superoxide and intracellular Ca2+ Inflamm. Res. 1999;48:544–549. doi: 10.1007/s000110050501. [DOI] [PubMed] [Google Scholar]
- WARREN J.S., YABROFF K.R., MANDEL D.M., JOHNSON K.J., WARD P.A. Role of O2− in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic. Biol. Med. 1990;8:163–172. doi: 10.1016/0891-5849(90)90089-2. [DOI] [PubMed] [Google Scholar]
- WEISIGER R.A., FRIDOVICH I. Mitochondrial superoxide dismutase: site of synthesis and intramitochondrial localization. J. Biol. Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- WEISS R.H., FLICKINGER A.G., RIVERS W.J. Evaluation of activity of putative superoxide dismutase mimics. direct analysis by stopped-flow kinetics. J. Biol. Chem. 1993;268:23049–23054. [PubMed] [Google Scholar]
- WEISS M., SCHNEIDER E.M., TARNOW J., METTLER S., KRONE M., TESCHEMACHER A., LEMOINE H. Is inhibition of oxygen radical production of neutrophils by sympathomimetics mediated via beta-2 adrenoceptors. J. Pharmacol. Exp. Ther. 1996;278:1105–1113. [PubMed] [Google Scholar]
- WERNS S.W., SIMPSON P.J., MICKELSON J.K., SHEA M.J., PITT B., LUCCHESI B. Sustained limitation by superoxide dismutase of canine myocardial injury due to ischemia followed by reperfusion. J. Cardiovasc. Pharmacol. 1988;11:36–44. doi: 10.1097/00005344-198801000-00006. [DOI] [PubMed] [Google Scholar]
- WESTENDORP M.O., SHATROV V.A., SCHULZE-OSTHOFF K., FRANK R., KRAFT M., LOS M., KRAMMER P.H., DROGE W., LEHMANN V. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF G., HANKKEN T., SCHROEDER R., ZAHNER G., ZIYADEH F.N., STAHL R.A. Antioxidant treatment induces transcription and expression of transforming growth factor beta in cultured renal proximal. FEBS Lett. 2001;488:154–159. doi: 10.1016/s0014-5793(00)02403-0. [DOI] [PubMed] [Google Scholar]
- WONG P.C., PARDO C.A., BORCHELT D.R., LEE M.K., COPELAND N.G., JENKINS N.A., SISODIA S.S., CLEVELAND D.W., PRICE D.L. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- WYDE P.R., MOORE D.K., PIMENTEL D.M., GILBERT B.E., NIMROD R., PANET A. Recombinant superoxide dismutase (SOD) administered by aerosol inhibits respiratory syncytial virus infection in cotton rats. Antiviral Res. 1996;31:173–184. doi: 10.1016/0166-3542(95)06967-4. [DOI] [PubMed] [Google Scholar]
- YAMAKURA F., MATSUMOTO T., FUJIMURA T., TAKA H., MURAYAMA K., IMAI T., UCHIDA K. Modification of a single tryptophan residue in human Cu,Zn-superoxide dismutase by peroxynitrite in the presence of bicarbonate. Biochim. Biophys. Acta. 2001;1548:38–46. doi: 10.1016/s0167-4838(01)00212-6. [DOI] [PubMed] [Google Scholar]
- YAMAKURA F., TAKA H., FUJIMURA T., MURAYAMA K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- YAN T., LI S., JIANG X., OBERLEY L.W. Altered levels of primary antioxidant enzymes in progeria skin fibroblasts. Biochem. Biophys. Res. Commun. 1999;257:163–167. doi: 10.1006/bbrc.1999.0423. [DOI] [PubMed] [Google Scholar]
- YANG G., CHAN P.H., CHEN J., CARLSON E., CHEN S.F., WEINSTEIN P., EPSTEIN C.J., KAMII H. Human copper–zinc superoxide dismutase transgenic mice are highly resistant to injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- YOSHIZAKI N., MOGI Y., MURAMATSU H., KOIKE K., KOGAWA K., NIITSU Y. Suppressive effect of recombinant human Cu,Zn-superoxide dismutase on lung metastasis of murine tumor cells. Int. J. Cancer. 1994;57:287–292. doi: 10.1002/ijc.2910570226. [DOI] [PubMed] [Google Scholar]
- ZELKO I.N., MARIANI T.J., FOLZ R.J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., DAY B.J., CRAPO J.D., SALZMAN A.L., SZABO C. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br. J. Pharmacol. 1997;120:259–267. doi: 10.1038/sj.bjp.0700872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZWEIER J.L. Prevention of reperfusion-induced, free radical-mediated acute endothelial injury by super oxide dismutase as an effective tool to delay/prevent chronic renal allograft failure: a review. Transplant. Proc. 1997;29:2567–2568. doi: 10.1016/s0041-1345(97)00509-5. [DOI] [PubMed] [Google Scholar]