Abstract
A missense mutation of the CACNA1A gene that encodes the α1A subunit of the voltage-dependent P/Q-type calcium channel has been discovered in patients suffering from familial hemiplegic migraine. This suggested that calcium channelopathies may be involved in migraine more broadly, and established the importance of genetic mechanisms in migraine.
Channelopathies share many clinical characteristics with migraine, and thus exploring calcium channel functions in the trigeminovascular system may give insights into migraine pathophysiology. It is also known that drugs blocking the P/Q- and N-type calcium channels have been successful in other animal models of trigeminovascular activation and head pain.
In the present study, we used intravital microscopy to examine the effects of specific calcium channel blockers on neurogenic dural vasodilatation and calcitonin gene-related peptide (CGRP)-induced dilation.
The L-type voltage-dependent calcium channel blocker calciseptine significantly attenuated (20 μg kg−1, n=7) the dilation brought about by electrical stimulation, but did not effect CGRP-induced dural dilation.
The P/Q-type voltage-dependent calcium channel blocker ω-agatoxin-IVA (20 μg kg−1, n=7) significantly attenuated the dilation brought about by electrical stimulation, but did not effect CGRP-induced dural dilation.
The N-type voltage-dependent calcium channel blocker ω-conotoxin-GVIA (20 μg kg−1, n=8 and 40 μg kg−1, n=7) significantly attenuated the dilation brought about by electrical stimulation, but did not effect CGRP-induced dural dilation.
It is thought that the P/Q-, N- and L-type calcium channels all exist presynaptically on trigeminovascular neurons, and blockade of these channels prevents CGRP release, and, therefore, dural blood vessel dilation. These data suggest that the P/Q-, N- and L-type calcium channels may be involved in trigeminovascular nociception.
Keywords: Calcium channels, missense mutations, CGRP, intravital microscopy, trigeminovascular, presynaptic
Introduction
Migraine pathophysiology is beginning to be understood, and it is likely to involve the activation of trigeminal afferents (Goadsby et al., 2002). Trigeminal sensory nerve fibres that innervate the cranial vasculature contain calcitonin gene-related peptide (CGRP), substance P and neurokinin A (Uddman et al., 1999). Trigeminal ganglion stimulation, which recruits both Aδ- and C-fibres (Lee et al., 1985; O'Connor & van der Kooy, 1988), results in the release of CGRP and substance P from perivascular trigeminal sensory nerves in humans (Goadsby et al., 1988). In acute migraine, only CGRP and neurokinin A (Goadsby et al., 1990; Gallai et al., 1995), but not substance P (May & Goadsby, 2001) levels, are increased. Moreover, CGRP and not substance P is elevated in acute cluster headache (Goadsby & Edvinsson, 1994). Taken together, these data suggest that CGRP has a pivotal role in trigeminovascular nociception.
Calcium influx into cells is believed to be responsible for neurotransmitter release via the voltage-dependent calcium channels (Katz & Miledi, 1967; Dunlap et al., 1995). It has recently been established that a rare form of migraine, familial hemiplegic migraine, is due to either a missense mutation of the CACNA1A gene that encodes the α1A subunit of the voltage-dependent P/Q-type calcium channel (Ophoff et al., 1996), or due to mutations in ATP1A2 that encode the α2 subunit of the Na+/K+ pump (De Fusco et al., 2003). These findings, and the clinical similarities between other channel-opathies (Griggs & Nutt, 1995) and migraine (Ferrari, 1998), have suggested that migraine may involve a genetic mutation to one or more of these calcium channels.
The P/Q-type calcium channels are located neuronally, and have been found to be involved in presynaptic calcium influx into cells and neurotransmitter release in the mammalian brain (Llinas et al., 1992; Dunlap et al., 1995). The P/Q-type calcium channel is present in the rat trigeminal ganglion (Westenbroek et al., 1995) and in a small number of cells in the rat spinal trigeminal nucleus (Westenbroek et al., 1995; Craig et al., 1998), a key area that is believed to be involved in the pain of acute migraine. The N-type calcium channel, so called for its neuronal localisation, is also involved in the presynaptic regulation of neurotransmitter release in the rat and guinea-pig (Maggi & Giuliani, 1991; Olivera et al., 1994; Wheeler et al., 1994). A mutation of the CACNA1B gene encoding the α1B subunit of the N-type calcium channel has been identified (Ino et al., 2001; Miller, 2001), although the phenotype of the mutation is unclear. There is evidence of α1B immunoreactivity in the trigeminal nucleus and trigeminal ganglion in the rat brain (Kim & Chung, 1999; Chung et al., 2000).
A third type of voltage-dependent calcium channel is the ‘long-lasting' L-type calcium channel. The L-type calcium channel is sensitive to drugs, such as verapamil and diltiazem, and has also been found to be involved in transmitter release in the skin and knee joint (Just et al., 2001; Kress et al., 2001). The L-type calcium channel has been found to be present on smooth muscle, more specifically on mammalian cardiac and brain vascular smooth muscle (Nakazawa et al., 1992; Dunlap et al., 1995; Matsuoka et al., 1997; Seisenberger et al., 2000). L-type calcium channels have also been found in rat trigeminal ganglion neurons, and are involved in long-term synaptic changes in the sensory nucleus of the rat trigeminal nerve after trigeminal ganglion stimulation (Kim & Chung, 1999; Guido et al., 2001).
In this study, we used the model of intravital microscopy (Williamson et al., 1997a, 1997b) that allows continuous measurement of dural blood vessel diameter, while inducing dilation both prejunctionally with electrical stimulation, and post-junctionally with intravenous CGRP injection. It has been shown that electrical stimulation of a cranial window results in dural vessel dilation inhibited by the CGRP antagonist CGRP8–37 (Williamson et al., 1997a). Antagonists at both the neurokinin 1 and 2 receptors were not able to produce any inhibition of neurogenic dural vasodilatation (Williamson et al., 1997a). Several antimigraine agents, sumatriptan, naratriptan, rizatriptan (Ferrari et al., 2002) and dihydroergotamine (Ferrari, 1998), have been shown to inhibit the dural dilation brought about by electrical stimulation (Williamson et al., 1997a,1997b,1997c). Exogenous CGRP also causes dural vasodilatation, presumably by activating CGRP receptors on the smooth muscle of dural arteries (Williamson et al., 1997a). We investigated the role of P/Q-, N- and L-type voltage-dependent calcium channel blockers with both neurogenic dural vasodilatation and CGRP-induced dural dilation, to characterise the pharmacology of these channels in the trigeminovascular innervation of the dura mater.
Methods
Surgical preparation
All experiments were conducted under the U.K. Home Office (Scientific Procedures) Act (1986). Male Sprague–Dawley rats (220–385 g) were anaesthetised throughout the experiments with sodium pentobarbitone (60 mg kg−1 i.p. initially and then 18 mg kg−1 h i.v. infusion). The left femoral artery and vein were cannulated for blood pressure recording, and intravenous infusion of anaesthetic and test compounds, respectively.
Temperature was maintained throughout, using a homeothermic blanket system. The rats were placed in a stereotaxic frame and ventilated with oxygen-enriched air, 3–5 ml, 60–80 strokes min−1 (Small Rodent Ventilator—Model 683, Harvard Instruments, U.K.). End-tidal CO2 was monitored (Capstar-100, CWE Inc., U.S.A.) and kept between 3.5 and 4.5%, and blood pressure was monitored continually. The skull was exposed and the right or left parietal bone thinned by drilling with a saline-cooled drill until the blood vessels of the dura were clearly visible through the intact skull.
Intravital microscopy
The cranial window was covered with mineral oil (37°C) and a branch of the middle meningeal artery viewed using an intravital microscope (Microvision MV2100, U.K.), and the image displayed on a television monitor. Dural blood vessel diameter was continuously measured using a video dimension analyser (Living Systems Instrumentation, U.S.A.) and displayed with blood pressure on a data analysis system (Spike4 v2, Cambridge Electronic Design, Cambridge, U.K.).
Experimental protocols
Defining electrical stimulation parameters
Electrical stimulation was used to evoke dilation of the dural blood vessels with a bipolar stimulating electrode (NE 200X, Clark Electromedical) that was placed on the surface of the cranial window approximately 200 μm from the vessel of interest. The surface of the cranial window was stimulated at 5 Hz, 1 ms for 10 s (Grass Stimulator S88, Grass Instrumentation, MA, U.S.A.) with increasing voltage, until maximal dilation was observed. Subsequent electrically induced responses in the same animal were then evoked using the same voltage (Williamson et al., 1997a; Akerman et al., 2002).
Effects of calcium channel blockers on electrical stimulation and CGRP-induced dilation
The effects of the L-type calcium channel blocker calciseptine were studied. Calciseptine (7 μg kg−1) was administered intravenously at least 10 min after a control response to electrical stimulation or bolus CGRP (1 μg kg−1; it has previously been defined that this dose provides maximum dilation of dural blood vessels when given intravenously (Williamson et al., 1997a)); the electrical stimulation or CGRP bolus was repeated after 5–10 min. Then, at least a further 10 min later, an increased dosage of calciseptine (10 μg kg−1) was administered, followed by electrical stimulation or CGRP bolus after 10 min. A final dose of calciseptine (20 μg kg−1) was administered after another 10 min, and a final repeat electrical stimulation or CGRP bolus given after a further 10 min. The effects of an N-type (ω-conotoxin-GVIA – 10, 20 and 40 μg kg−1) and a P/Q-type (ω-agatoxin-IVA – 3, 10 and 20 μg kg−1) calcium channel blockers were also studied using the same protocol. A P/Q-type (ω-agatoxin-TK – 3, 10 and 20 μg kg−1) calcium channel blocker used the same protocol, but was only tested against electrical stimulation. Up to three doses of each test substance was given during a single experiment; not all animals had every dose.
Data analysis
The peak effects of electrical stimulation and CGRP infusion on dural vessel diameter were calculated as a percentage increase from the prestimulation baseline diameter. The nature of the experimental set-up, where the magnification of the dural vessel selected for study was different in each set-up, made it impractical to standardise the dural vessel measurement; therefore, the dural vessel diameter was measured in arbitrary units, and all calculations related to the premanipulation baseline. All data are expressed as mean±s.e.m. Statistical analysis was performed using an ANOVA for repeated measures and post hoc comparison was made with a Student's paired t-test, to examine the level of dilation after administration of test compounds at specific doses compared to the control dilation, if repeated measures proved significant (SPSS v10.0). The reproducibility of both the neurogenic vasodilator and CGRP responses has been tested previously using four consecutive saline-controlled stimuli (Akerman et al., 2002) in the same experimental set-up. For the control studies, the consecutive determinations of the neurogenic vasodilator response were compared to each other, and also the baseline dilations obtained for the studies where there was pharmacological intervention. This was done using an ANOVA for repeated measures. Significance was assessed at the P<0.05 levels.
Drugs
The infusion of anaesthetic, CGRP and calcium channel blocker was via the same catheter, after flushing with saline for several minutes between each compound. CGRP, calciseptine (IC50 27 nM), which is isolated from the black mamba (Yasuda et al., 1994), ω-agatoxin-IVA and ω-agatoxin-TK, which are equipotent (IC50 20 nM) (Mintz et al., 1992), which are both isolated in the funnel web spider (all Sigma-Aldrich, U.K.), and ω-conotoxin-GVIA (IC50 3 nM) (Grantham et al., 1994), which is isolated in the marine snail (Peptide Institute, U.K.), were all dissolved in deoxygenated water, aliquotted and frozen until required. They were then redissolved in 0.9% saline, to make up to a volume of 0.2 ml before intravenous administration. The doses that were given for each calcium channel blocker were determined by the fact that a 10 μg kg−1 dose is effective in inhibiting the N-type calcium channel in vivo (Pruneau & Belichard, 1992), without being toxic, and the IC50 values for each blocker are similarly in the nM region.
Results
Control groups
The results for the series of control dilations using electrical stimulation and CGRP-induced dilation with saline intervention have been published, and showed no difference across the cohort (Akerman et al., 2002). These control effects were compared using an ANOVA to the control dilations used in the pharmacological studies, and there were no significant differences.
Effect of the L-type calcium channel blocker on electrical stimulation and CGRP-induced dilation
In rats treated with calciseptine (7 and 10 μg kg−1, n=8 and 20 μg kg−1, n=7), the dilation brought about by electrical stimulation was significantly reduced with the 20 μg kg−1 dose of calciseptine from 122±14 to 85±19% (P<0.05, t6=5.0). Increases in dural blood vessel diameter evoked by CGRP (1 μg kg−1, i.v.) showed no significant difference between the control CGRP-induced dilation and the dilation produced after calciseptine (7, 10 and 20 μg kg−1, n=7; F4,20=0.384, P=0.82; Figure 1). Calciseptine injections caused a slight reduction in blood pressure, which was accompanied by a slight increase in blood vessel diameter; neither were significant for each dose (Table 1 ). Both returned to preinjection levels by the time of the repeated vasodilator challenge.
Figure 1.
The effects of repeated electrical stimulation or CGRP injection (1 μgkg−1) with calciseptine treatment on dural blood vessel diameter. Following control responses, rats were injected with calciseptine (7, 10 and 20 μg kg−1) and electrical stimulation or CGRP injection repeated. *P<0.05 significance compared to the control response used in the calciseptine series of experiments.
Table 1.
Summary of blood pressure and dural blood vessel diameter changes under the different drug regimens
| Drug | Dosage (n) | Change in blood pressure (mmHg) | Change in dural blood vessel diameter (%) |
|---|---|---|---|
| Calciseptine | 7 μg kg−1 (n=10) | −1±1 | +6±4 |
| Calciseptine | 10 μg kg−1 (n=10) | −4±2 | +4±6 |
| Calciseptine | 20 μg kg−1 (n=10) | −8±4 | +3±2 |
| ω-Agatoxin-TK | 3 μg kg−1 (n=7) | −2±2 | −4±2 |
| ω-Agatoxin-TK | 10 μg kg−1 (n=7) | −3±2 | −7±1* |
| ω-Agatoxin-TK | 20 μg kg−1 (n=7) | −2±2 | −8±2 |
| ω-Agatoxin-IVA | 3 μg kg−1 (n=13) | −2±1 | −2±1 |
| ω-Agatoxin-IVA | 10 μg kg−1 (n=13) | −0.3±1 | +1±2 |
| ω-Agatoxin-IVA | 20 μg kg−1 (n=13) | −2±1 | +3±3 |
| ω-Conotoxin-GVIA | 10 μg kg−1 (n=14) | −44±2# | +20±6* |
| ω-Conotoxin-GVIA | 20 μg kg−1 (n=14) | −7±3 | +9±6 |
| ω-Conotoxin-GVIA | 40 μg kg−1 (n=14) | −6±3 | +23±12 |
Effects of calcium channel blockers on blood pressure and dural blood vessel diameter.
P<0.05 significance compared to blood vessel diameter prior to calcium channel blocker administration.
P<0.05 significance compared to blood pressure prior to calcium channel blocker injection.
Effect of the P/Q-type calcium channel blocker ω-agatoxin-IVA on electrical stimulation and CGRP-induced dilation
In rats treated with ω-agatoxin-IVA (3, 10 and 20 μg kg−1, n=7), the dilation brought about by electrical stimulation was significantly reduced with all doses of ω-agatoxin-IVA: 111±13–91±10% (3 μg kg−1, P<0.05, t6=3.9), 111±13–81±12% (10 μg kg−1, P<0.05, t6=3.6) and 111±13–63±11% (20 μg kg−1, P<0.05, t6=3.9). Increases in dural blood vessel diameter evoked by CGRP (1 μg kg−1, i.v.) showed no significant difference between the control CGRP-induced dilation and the dilation produced after ω-agatoxin-IVA (3, 10 and 20 μg kg−1, n=6, F4.20=0.05, P=0.46, Figure 2). ω-agatoxin-IVA injections had no significant effect on blood pressure or blood vessel diameter (Table 1).
Figure 2.
The effects of repeated electrical stimulation or CGRP injection (1 μg kg−1) with ω-agatoxin-IVA treatment on dural blood vessel diameter. Following control responses, rats were injected with ω-agatoxin-IVA (3, 10 and 20 μg kg−1) and electrical stimulation or CGRP injection repeated. *P<0.05 significance compared to the control response used in the ω-agatoxin-IVA series of experiments.
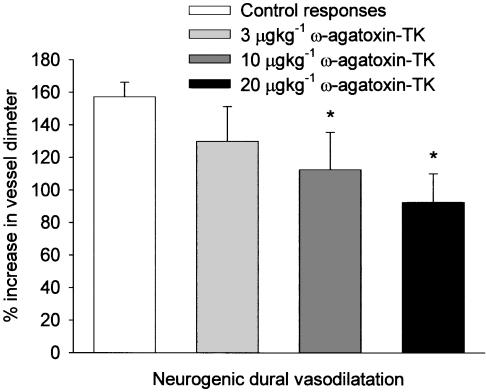
Effect of the P/Q-type calcium channel blocker ω-agatoxin-TK on electrical stimulation
In rats treated with ω-agatoxin-TK (3, 10 and 20 μg kg−1, n=8) the dilation brought about by electrical stimulation was significantly reduced with the 10 and 20 μg kg−1 doses of ω-agatoxin-TK: 157±9–112±23% (P<0.05, t7=2.4) and 157±9–92±18%, respectively (P<0.05, t7=3.6) (see Figure 3). ω-agatoxin-TK injections caused a slight decrease in blood pressure, which was accompanied by a small increase in blood vessel diameter (Table 1); this was only significant for the 10 μg kg−1 dose. Both returned to preinjection levels by the time of the repeated vasodilator challenge.
Figure 3.
The effects of repeated electrical stimulation with ω-agatoxin-TK treatment on dural blood vessel diameter. Following control responses, rats were injected with ω-agatoxin-TK (3, 10 and 20 μg kg−1) and electrical stimulation repeated. *P<0.05 significance compared to the control response used in the ω-agatoxin-TK series of experiments.
Effect of the N-type calcium channel blocker ω-conotoxin-GVIA on electrical stimulation and CGRP-induced dilation
In rats treated with ω-conotoxin-GVIA (10 and 20 μg kg−1, n=8 and 40 μg kg−1, n=7), the dilation brought about by electrical stimulation was significantly reduced with all doses: 102±13–83±17% (10 μg kg−1, P<0.05, t7=3.0), 102±13–82±17% (20 μg kg−1, P<0.05, t7=3.0) and 91±7–74±9% (40 μg kg−1, P<0.05, t6=3.8). Increases in dural blood vessel diameter evoked by CGRP (1 μg kg−1, i.v.) showed no significant difference between the control CGRP-induced dilation and the dilation produced after ω-conotoxin-GVIA (Figure 4; 10, 20 and 40 μg kg−1, n=6, F4,20=0.27, P=0.9). The initial (10 μg kg−1) ω-conotoxin-GVIA injection caused a significant decrease in blood pressure of 44±2 mmHg, accompanied by a 20±6% (n=14, P<0.01, t13=20.65) increase in vessel diameter (Table 1). The increase in vessel diameter returned to its preinjection level by the time of the repeated vasodilator challenge. The blood pressure drop did not return completely to its preinjection level, remaining 31±3 mmHg below the initial level. There was no significant change in either the blood pressure or vessel diameter with the second and third doses of ω-conotoxin-GVIA.
Figure 4.
The effects of repeated electrical stimulation or CGRP injection (1 μg kg−1) with ω-conotoxin-GVIA treatment on dural blood vessel diameter. Following control responses, rats were injected with ω-conotoxin-GVIA (10, 20 and 40 μg kg−1) and electrical stimulation and CGRP injection repeated. *P<0.05 significance compared to the control response used in the ω-conotoxin-GVIA series of experiments.
Effect of calcium channel blockers on the hypotensive effect of CGRP injections
The four consecutive CGRP injections (as described in Akerman et al., 2002) and the control group of the Results section produced decreases in mean blood pressure of 41±7, 38±5, 37±7 and 33±3 mmHg, respectively (Table 2 ). There was no difference across the cohort (F3,12=1.492, P=0.283). We compared these effects with an ANOVA for repeated measures to the control dilations used in the pharmacological studies, and there were no significant differences. Changes in blood pressure caused by CGRP (1 μg kg−1, i.v.) showed no significant difference between the control CGRP change and the change produced after calciseptine (7, 10 and 20 μg kg−1, n=5, F3,12=2.34, P=0.17) or ω-agatoxin-IVA (3, 10 and 20 μg kg−1, n=7, F3,18=2.91, P=0.11). The changes in blood pressure with CGRP injection after ω-conotoxin-GVIA (n=14) were significantly reduced for each dose from 40±6 to 16±2 mmHg (10 μg kg−1, P<0.05, t5=3.15), 22±3 mmHg (20 μg kg−1, P<0.05, t5=2.6) and 17±3 mmHg (40 μg kg−1, P<0.05, t5=4.1). There was no difference in the blood pressure drop with CGRP between the given different doses of ω-conotoxin-GVIA.
Table 2.
Summary of blood pressure changes with CGRP injection under different drug regimens
| CGRP injection (1 μg kg−1) | Saline (0.2ml) drop in BP (mmHg) | Calciseptine (dosage and drop in BP) | ω-Agatoxin-IVA (dosage and drop in BP) | ω-Conotoxin-GVIA (dosage and drop in BP) |
|---|---|---|---|---|
| 1 | 41±7 | (Control) 32±2 mmHg | (Control) 41±3 mmHg | (Control) 40±6 mmHg |
| 2 | 38±5 | (7 μg kg−1) 31±4 mmHg | (3 μg kg−1) 42±3 mmHg | (10 μg kg−1) 16±2 mmHg* |
| 3 | 37±7 | (10 μg kg−1) 22±2 mmHg | (10 μg kg−1) 36±5 mmHg | (20 μg kg−1) 22±3 mmHg* |
| 4 | 33±3 | (20 μg kg−1) 27±2 mmHg | (20 μg kg−1) 31±2 mmHg | (40 μg kg−1) 17±3 mmHg* |
BP=blood pressure. Control represents with initial CGRP injection prior to any drug intervention. There was no difference in the change in BP across all CGRP injections in the saline group. There was no difference when the controls used for each drug were compared using an ANOVA for repeated measures to the changes in the saline group.
P<0.05 significance compared to the control blood pressure drop.
Discussion
The data suggest that each of the P/Q-, N- and L-type voltage-dependent calcium channels are involved in neurogenic trigeminovascular dural vasodilatation. The specific employed blockers were all able to attenuate dural vasodilatation, but none had effects on CGRP-induced dural vasodilatation. This is likely to be due to presynaptic inhibition of calcium influx preventing the release of CGRP. It is thus likely that these calcium channels are not present postsynaptically and, therefore, were unable to affect exogenous CGRP.
P/Q-type calcium channel blockers and neurogenic dural extravasation
The P/Q-type calcium channel blockers ω-agatoxin-TK and ω-agatoxin-IVA were able to attenuate neurogenic dural vasodilatation in the rat, brought about by electrical stimulation. It seems likely that blockade of the P/Q-type voltage-dependent calcium channels prevents CGRP release presynaptically from perivascular trigeminal sensory nerve fibres, since we saw no effect on the dural dilation elicited by exogenously administered CGRP. This effect is analogous to that seen with triptans, serotonin 5-HT1B/1D agonists (Williamson et al., 1997b, 1997c) and the opioid receptor agonists (Williamson et al., 2001). The P/Q-type calcium channel blocker α-eudesmol inhibits plasma protein extravasation in the rat dura, and attenuated the vasodilatation in the facial skin, as monitored by laser Doppler flowmetry after trigeminal ganglion stimulation, and is thought to use the same mechanism, blocking the presynaptic release of neuropeptides from perivascular trigeminal terminals (Asakura et al., 2000).
N-type calcium channel blockers and neurogenic dural extravasation
The N-type calcium channel blocker, ω-conotoxin-GVIA was able to attenuate neurogenic dural vasodilatation in the rat. It is likely that this attenuation is via a similar mechanism to the P/Q-type calcium channel inhibition. The N-type calcium channel blocker is, therefore, preventing CGRP release from perivascular trigeminal sensory nerve fibres and inhibiting the dilation of the dural blood vessels. Indeed, ω-conotoxin-GVIA, the recognised N-type calcium channel blocker (Olivera et al., 1985), has been shown to inhibit presynaptic neuropeptide release, including CGRP, after electrical stimulation of the ureter in the guinea-pig and KCl-evoked CGRP release in the rat spinal cord (Maggi et al., 1990a, 1990b; Maggi & Giuliani, 1991). Also, recently, blockade of the N-type calcium channel by bathing the brain stem area with ω-conotoxin-GVIA (Richter et al., 2002) inhibited the central sensitisation of trigeminal nucleus caudalis neurons after dilation of the meningeal blood vessels with CGRP (Cumberbatch et al., 1999; 2001). Calcium influx into nerve terminals contributes to the release of neurotransmitters; therefore, it seems likely that ω-conotoxin-GVIA is preventing the release of CGRP by inhibiting calcium influx into nerve terminals, and thus preventing exocytosis and the mechanism for transmitter release (Dunlap et al., 1995).
L-type calcium channel blockade and neurogenic dural extravasation
The L-type calcium channel blocker calciseptine was also able to attenuate the neurogenic dural vasodilatation that occurs after electrical stimulation of the cranial window. We could not see evidence in the literature that L-type calcium channels contribute to transmitter release specifically on trigeminal neurons, although there is evidence that they are involved in dopaminergic mechanisms in the striatum and caudate putamen in the brain, to promote neurotransmitter release from presynaptic nerve endings (Galarraga et al., 1997; Okita et al., 2000; Baufreton et al., 2003), and they are also involved in transmitter release in the periphery (Just et al., 2001; Kress et al., 2001). There are no data for calciseptine acting on any other sites in the brain or periphery at the doses used in these experiments, although we cannot discount an action at another site at this concentration. From the evidence available, it seems that calciseptine is acting on a receptor or channel site presynaptically, attenuating the release of CGRP, and therefore the subsequent dilation. Without direct evidence that the L-type calcium channel is involved in presynaptic neurotransmitter release in the trigeminovascular region, or whether calciseptine may be acting on another non-L-type calcium channel site, we cannot be sure if this is the mechanism involved. It is worth noting that the dose required to produce any inhibition with the L-type calcium channel blocker is higher than the lowest dose required with the P/Q- and N-type calcium channel blockers, despite the fact that all the compounds have IC50 values in the nm region. This discrepancy may reflect a lower distribution of L-type calcium channels on trigeminal nerves.
Although each calcium channel blocker was able to attenuate to some degree the vasodilatation caused by electrical stimulation, none of them were able to totally block the effect. Earlier studies using this model have shown that only the CGRP receptor blocker CGRP8−37 was able to fully block the neurogenic dural vasodilatation, while the triptans were never able to fully inhibit the response (Williamson et al., 1997b, 1997c). The triptans were, however, more effective than the calcium channel blockers used in this study. This would seem to imply that there is not a single channel that governs the release of CGRP from sensory trigeminal nerve fibres. It is more likely that two or more contribute to the transmitter release in this region, which would make any attempted therapeutic intervention complex. It may be useful to apply a nonspecific calcium channel blocker in the model, or even the individual channel blockers simultaneously to see if this is more effective at fully blocking the neurogenic dural vaso-dilatation.
The mechanism by which the calcium channel blockers attenuate neurogenic dural vasodilatation may be by preventing presynaptic release of CGRP from trigeminal sensory terminals, although our data cannot confirm this. Electrical stimulation of dural sites provokes presynaptic CGRP release from trigeminal sensory nerve fibres that innervate the dura mater. This CGRP release causes vasodilatation by acting on postsynaptic CGRP receptors on the smooth muscle of dural arteries. If the dilation is blocked or attenuated by a mechanism that is not postsynaptic, then it seems likely to be via a presynaptic mechanism. It is necessary to study this point more thoughtfully, so that one can be certain that the mechanism involved is due to the blockade of presynaptic CGRP release rather than some other mechanism. This argument also stands for the data produced by the Williamson group.
One possibility would be to look at the peptide content of blood samples taken from the external jugular vein after electrical stimulation of the cranial window, before and after calcium channel blocker treatment, mirroring the studies done by Goadsby et al. (1988); (1990) on both migraine patients and experimental animals after trigeminal ganglion stimulation. It would also be useful to repeat the trigeminal ganglion stimulation studies using calcium channel blockers, to see if the different channels are preferentially situated on neurons that release either CGRP, substance P or neurokinin A, and also looking at the response of the different calcium channel blockers on extravasation, compared to vasodilatation, as the response of α-eudesmol on extravasation was more profound than on the vasodilatation of facial skin (Asakura et al., 2000).
Effect of calcium channel blockers on CGRP-induced dilation
Intravenous CGRP induces dural vessel dilation in the rat, and CGRP blockers inhibit this by acting postjunctionally at CGRP receptors in the smooth muscle (Williamson et al., 1997a). The P/Q-, N- and L-type calcium channel inhibitors were all unable to block the CGRP-induced dilation, when given intravenously. It is unlikely then that the P/Q-, N- and L-type calcium channel blockers are situated postsynaptically, as they are unable to prevent the dural vessel dilation caused by exogenous CGRP.
Blood pressure effects of calcium channel blockers
Neither calciseptine nor ω-agatoxin had any profound effects on blood pressure per se, and they had no significant effect on dural blood vessel diameter, except the 10 μg kg−1 dose of ω-agatoxin-TK. This seems a rather erroneous result, as the higher dose had no great effect on blood vessel diameter, and the three doses of ω-agatoxin-IVA had no great effect either, and the two compounds are equipotent as P/Q-type calcium channel blockers. CGRP, as well as being a potent vasodilator, is also known to cause a dose-dependent drop in blood pressure (Brain et al., 1993; Williamson et al., 1997a). We have shown previously that this vasodilatation is reproducible (Akerman et al., 2002), and, in this study, that the blood pressure response is also reproducible. Both calciseptine and ω-agatoxin-IVA were unable to alter the effects of CGRP on blood pressure.
ω-Conotoxin-GVIA had a profound effect on blood pressure; this was only the case on its first injection where it produced a significant drop in blood pressure and a corresponding significant increase in vessel diameter. It would seem that the blood pressure effect was irreversible as the blood pressure only slightly increased after this, and subsequent injections did not further affect the blood pressure. The blood vessel diameter was restored to its original level after 5 min postinjection; therefore, this response was not irreversible. The vasodilator response would seem to be linked to the blood pressure change, as subsequent injections of ω-conotoxin-GVIA did not affect either blood vessel diameter or blood pressure. This may reflect the fact that N-type calcium channels appear to be essential for the proper functioning of the sympathetic nervous in circulatory regulation (Pruneau & Belichard, 1992; Ino et al., 2001). On first inspection of the effect of ω-conotoxin on the CGRP-induced blood pressure drop, it would seem that all doses of the peptide had a significant effect on this response. In reality, it is more likely that the drop in blood pressure caused by the initial ω-conotoxin-GVIA, which was maintained throughout the remainder of the experiment, meant that CGRP was unable to drop the blood pressure that much more. It is important to note that the CGRP was still able to cause a consistent dural vasodilatation despite the limited blood pressure response. This indicates that it is the response of CGRP on the smooth muscle of meningeal vessels that cause the vasodilatation, and not a response to the blood pressure change.
There is considerable evidence for the involvement of the P/Q-type voltage-gated calcium channel in more common forms of migraine (May et al., 1995; Nyholt et al., 1998), in addition to clear evidence for its involvement in familial hemiplegic migraine (Ophoff et al., 1996). It is likely that the phenotype of these P/Q-type calcium channel mutations takes the form of opening the presynaptic calcium channels of sensory nerve terminals, although the functional consequences are variable (Hans et al., 1999). Several mutations of the gene that encodes the α1B subunit of the N-type calcium channel have been identified (Ino et al., 2001; Miller, 2001), although none are specifically related to migraine. Interpreting the data for the L-type calcium channel is slightly more difficult, as there is no evidence of their existence causing transmitter release in the CNS, although they are present in the trigeminal system (Kim & Chung, 1999; Guido et al., 2001). The fact that they are situated in the trigeminal ganglion means that it is possible that a mutation of the genes that encode subunits that make up the L-type calcium channels may cause the channels to remain open and evoke exocytosis and transmitter release. This may influence responses to cortical spreading depression (Choudhuri et al., 2002), which is believed to represent the aura phase of migraine, where L-type channel expression is altered.
The therapeutic possibilities of using specific calcium channel blockers in the acute treatment of migraine are limited as their effects in this model, while being significant, did not completely block the neurogenic dural vasodilatation, and were less effective than the triptans. However, there may be possibilities as a combination therapy, if it is found that the use of two or more calcium channel blockers is better able to inhibit the neurogenic dural vasodilatation. The limiting factor may be the effect they have on blood pressure, especially the N-type calcium channel blocker. Most calcium channel blockers that are used in the clinic are used to prevent angina and hypertension; therefore, an inevitable side effect, or consequence, of their use is the reduction of blood pressure. If one can establish an ideal combination that limits the impact on blood pressure, calcium channel blockers may yet be of use in the treatment of acute migraine. It is also important to note that all the studies mentioned above were done in either mice, rats or guinea-pig. The affinity and efficacy of the compounds used may differ in the human and, therefore, this issue will need to be addressed if a combination therapy were to be of any use.
In summary, we have shown the involvement of P/Q-, N- and L-type voltage-gated calcium channels in neurogenic trigeminovascular dural vasodilatation, which is not directly related to the activation of postsynaptic CGRP receptors. We can suggest that each of the P/Q-, N- and L-type calcium channels may represent novel therapeutic targets in migraine. Further pharmacological and human genetic studies in migraine may elucidate a role for these channels in primary headache.
Acknowledgments
We thank Thorsten Bartsch, Kevin Shields, Yolande Knight, James Storer and Paul Hammond of the Headache Group at the Institute of Neurology for both assistance and technical support during these experiments. This work has been supported by the Wellcome Trust. PJG is a Wellcome Senior Research Fellow.
Abbreviations
- ANOVA
analysis of variance
- CGRP
calcitonin gene-related peptide
- KCL
potassium chloride
- s.e.m.
standard error of the mean
References
- AKERMAN S., WILLIAMSON D.J., KAUBE H., GOADSBY P.J. Nitric oxide synthase inhibitors can antagonise neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002;137:62–68. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASAKURA K., KANEMASA T., MINAGAWA K., KAGAWA K., YAGAMI T., NAKAJIMA M., NINOMIYA M. α-Eudesmol, a P/Q-type Ca2+ channel blocker, inhibits neurogenic vasodilation and extravasation following electrical stimulation of trigeminal ganglion. Brain Res. 2000;873:94–101. doi: 10.1016/s0006-8993(00)02527-0. [DOI] [PubMed] [Google Scholar]
- BAUFRETON J., GARRET M., RIVERA A., DE LA CALLE A., GONON F., DUFY B., BIOULAC B., TAUPIGNON A. D5 (not D1) dopamine receptors potentiate burst-firing in neurons of the subthalamic nucleus by modulating an L-type calcium conductance. J. Neurosci. 2003;23:816–825. doi: 10.1523/JNEUROSCI.23-03-00816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAIN S.D., HUGHES S.R., CAMBRIDGE H., O'DRISCOLL G. The contribution of calcitonin gene-related peptide (CGRP) to neurogenic vasodilator responses. Agents Actions. 1993;38 Spec No:C19–C21. doi: 10.1007/BF01991124. [DOI] [PubMed] [Google Scholar]
- CHOUDHURI R., CUI L., YONG C., BOWYER S., KLEIN R.M., WELCH K.M., BERMAN N.E. Cortical spreading depression and gene regulation: relevance to migraine. Ann. Neurol. 2002;51:499–506. doi: 10.1002/ana.10158. [DOI] [PubMed] [Google Scholar]
- CHUNG Y.H., SHIN C., PARK K.H., CHA C.I. Immunohistochemical study on the distribution of the voltage-gated calcium channel alpha(1B) subunit in the mature rat brain. Brain Res. 2000;866:274–280. doi: 10.1016/s0006-8993(00)02289-7. [DOI] [PubMed] [Google Scholar]
- CRAIG P.J., MCAINSH A.D., MCCORMACK A.L., SMITH W., BEATTIE R.E., PRIESTLEY J.V., YIP J.L., AVERILL S., LONGBOTTOM E.R., VOLSEN S.G. Distribution of the voltage-dependent calcium channel α1A subunit throughout the mature rat brain and its relationship to neurotransmitter pathways. J. Comp. Neurol. 1998;397:251–267. [PubMed] [Google Scholar]
- CUMBERBATCH M.J., HORWOOD J.M., HILL R.G. The effects of voltage gated calcium channel blockers on trigeminal sensitisation responses to dural artery dilation in the rat. Cephalalgia. 2001;21:236. [Google Scholar]
- CUMBERBATCH M.J., WILLIAMSON D.J., MASON G.S., HILL R.G., HARGREAVES R.J. Dural vasodilatation causes a sensitization of rat caudal trigeminal neurones in vivo that is blocked by a 5-HT1B/1D agonist. Br. J. Pharmacol. 1999;126:1478–1486. doi: 10.1038/sj.bjp.0702444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE FUSCO M., MARCONI R., SILVESTRI L., ATORINO L., RAMPOLDI L., MORGANTE L., BALLABIO A., ARIDON P., CASARI G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 2003;33:192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- DUNLAP K., LUEBKE J.I., TURNER T.J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- FERRARI M.D. Migraine. Lancet. 1998;351:1043–1051. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- FERRARI M.D., GOADSBY P.J., ROON K.I., LIPTON R.B. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: methods and detailed results of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–658. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- GALARRAGA E., HERNANDEZ-LOPEZ S., REYES A., BARRAL J., BARGAS J. Dopamine facilitates striatal EPSPs through an L-type Ca2+ conductance. Neuroreport. 1997;8:2183–2186. doi: 10.1097/00001756-199707070-00019. [DOI] [PubMed] [Google Scholar]
- GALLAI V., SARCHIELLI P., FLORIDI A., FRANCESCHINI M., CODINI M., TREQUATTRINI A., PALUMBO R. Vasoactive peptides levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15:384–390. doi: 10.1046/j.1468-2982.1995.1505384.x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. Human in vivo evidence for trigeminovascular activation in cluster headache. Brain. 1994;117:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Release of vasoactive peptides in the extracerebral circulation of man and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., LIPTON R.B., FERRARI M.D. Migraine – current understanding and treatment. N. Engl. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- GRANTHAM C.J., BOWMAN D., BATH C.P., BELL D.C., BLEAKMAN D. Omega-conotoxin MVIIC reversibly inhibits a human N-type calcium channel and calcium influx into chick synaptosomes. Neuropharmacology. 1994;33:255–258. doi: 10.1016/0028-3908(94)90017-5. [DOI] [PubMed] [Google Scholar]
- GRIGGS R.C., NUTT J.G. Episodic ataxias as channelopathies. Ann. Neurol. 1995;37:285–287. doi: 10.1002/ana.410370302. [DOI] [PubMed] [Google Scholar]
- GUIDO W., LO F.S., ERZURUMLU R.S. Synaptic plasticity in the trigeminal principal nucleus during the period of barrelette formation and consolidation. Brain Res. Dev. Brain Res. 2001;132:97–102. doi: 10.1016/s0165-3806(01)00283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANS M., LUVISETTO S., WILLIAMS M.E., SPAGNOLO M., URRUTIA A., TOTTENE A., BRUST P.F., JOHNSON E.C., HARPOLD M.M., STAUDERMAN K.A., PIETROBON D. Functional consequences of mutations in the human alpha(1A) calcium channel subunit linked to familial hemiplegic migraine. J. Neurosci. 1999;19:1610–1619. doi: 10.1523/JNEUROSCI.19-05-01610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INO M., YOSHINAGA T., WAKAMORI M., MIYAMOTO N., TAKAHASHI E., SONODA J., KAGAYA T., OKI T., NAGASU T., NISHIZAWA Y., TANAKA I., IMOTO K., AIZAWA S., KOCH S., SCHWARTZ A., NIIDOME T., SAWADA K., MORI Y. Functional disorders of the sympathetic nervous system in mice lacking the alpha 1B subunit (Cav 2.2) of N-type calcium channels. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5323–5328. doi: 10.1073/pnas.081089398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUST S., LEIPOLD-BUTTNER C., HEPPELMANN B. Histological demonstration of voltage dependent calcium channels on calcitonin generelated peptide-immunoreactive nerve fibres in the mouse knee joint. Neurosci. Lett. 2001;312:133–136. doi: 10.1016/s0304-3940(01)02199-1. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. The timing of calcium action during neuromuscular transmission. J. Physiol. 1967;189:535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM H.C., CHUNG M.K. Voltage-dependent sodium and calcium currents in acutely isolated adult rat trigeminal root ganglion neurons. J. Neurophysiol. 1999;81:1123–1134. doi: 10.1152/jn.1999.81.3.1123. [DOI] [PubMed] [Google Scholar]
- KRESS M., IZYDORCZYK I., KUHN A. N- and L- but not P/Q-type calcium channels contribute to neuropeptide release from rat skin in vitro. Neuroreport. 2001;12:867–870. doi: 10.1097/00001756-200103260-00048. [DOI] [PubMed] [Google Scholar]
- LEE Y., KAWAI Y., SHIOSAKA S., TAKAMI K., KIYAMA H., HILLYARD C.J., GIRGIS S., MACINTYRE I., EMSON P.C., TOHYAMA M. Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: immunohistochemical analysis. Brain Res. 1985;330:194–196. doi: 10.1016/0006-8993(85)90027-7. [DOI] [PubMed] [Google Scholar]
- LLINAS R., SUGIMORI M., HILLMAN D.E., CHERKSEY B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S. The neurotransmitter role of calcitonin gene-related peptide in the rat and guinea-pig ureter: effect of a calcitonin gene-related peptide antagonist and species-related differences in the action of omega conotoxin on calcitonin gene-related peptide release from primary afferents. Neuroscience. 1991;43:261–268. doi: 10.1016/0306-4522(91)90433-o. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., SANTICIOLI P., TRAMONTANA M., MELI A. Effect of omega conotoxin on reflex responses mediated by activation of capsaicin-sensitive nerves of the rat urinary bladder and peptide release from the rat spinal cord. Neuroscience. 1990a;34:243–250. doi: 10.1016/0306-4522(90)90318-x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., TRAMONTANA M., CECCONI R., SANTICIOLI P. Neurochemical evidence for the involvement of N-type calcium channels in transmitter secretion from peripheral endings of sensory nerves in guinea pigs. Neurosci. Lett. 1990b;114:203–206. doi: 10.1016/0304-3940(90)90072-h. [DOI] [PubMed] [Google Scholar]
- MATSUOKA T., NISHIZAKI T., NOMURA T. The voltage-dependent non-selective cation channel sensitive to the L-type calcium channel blocker efonidipine regulates Ca2+ influx in brain vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1997;240:484–487. doi: 10.1006/bbrc.1997.7624. [DOI] [PubMed] [Google Scholar]
- MAY A., GOADSBY P.J. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Invest. Drugs. 2001;10:1–6. doi: 10.1517/13543784.10.4.673. [DOI] [PubMed] [Google Scholar]
- MAY A., OPHOFF R.A., TERWINDT G.M., URBAN C., VANEIJK R., HAAN J., DIENER H.C., LINDHOUT D., FRANTS R.R., SANDKUIJL A., FERRARI M.D. Familial hemiplegic migraine locus on chromosome 19p13 is involved in common forms of migraine with and without aura. Hum. Genet. 1995;96:604–608. doi: 10.1007/BF00197420. [DOI] [PubMed] [Google Scholar]
- MILLER R.J. Rocking and rolling with Ca2+ channels. Trends Neurosci. 2001;24:445–449. doi: 10.1016/s0166-2236(00)01859-2. [DOI] [PubMed] [Google Scholar]
- MINTZ I.M., VENEMA V.J., SWIDEREK K.M., LEE T.D., BEAN B.P., ADAMS M.E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- NAKAZAWA K., SAITO H., MATSUKI N. Effects of calcitonin generelated peptide (CGRP) on Ca(2+)-channel current of isolated smooth muscle cells from rat vas deferens. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;346:515–522. doi: 10.1007/BF00169006. [DOI] [PubMed] [Google Scholar]
- NYHOLT D.R., LEA R.A., GOADSBY P.J., BRIMAGE P.J., GRIFFITHS L.R. Familial typical migraine: linkage to chromosome 19p13 and evidence for genetic heterogeneity. Neurology. 1998;50:1428–1432. doi: 10.1212/wnl.50.5.1428. [DOI] [PubMed] [Google Scholar]
- O'CONNOR T.P., VAN DER KOOY D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in trigeminal sensory projection to the intracranial arteries. J. Neurosci. 1988;8:2468–2476. doi: 10.1523/JNEUROSCI.08-07-02468.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKITA M., WATANABE Y., TAYA K., UTSUMI H., HAYASHI T. Presynaptic L-type Ca(2)+ channels on excessive dopamine release from rat caudate putamen. Physiol. Behav. 2000;68:641–649. doi: 10.1016/s0031-9384(99)00227-9. [DOI] [PubMed] [Google Scholar]
- OLIVERA B.M., GRAY W.R., ZEIKUS R., MCINTOSH J.M., VARGA J., RIVIER J., DE SANTOS V., CRUZ L.J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985;230:1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- OLIVERA B.M., MILJANICH G.P., RAMACHANDRAN J., ADAMS M.E. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu. Rev. Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- OPHOFF R.A., TERWINDT G.M., VERGOUWE M.N., VAN EIJK R., OEFNER P.J., HOFFMAN S.M.G., LAMERDIN J.E., MOHRENWEISER H.W., BULMAN D.E., FERRARI M., HAAN J., LINDHOUT D., VAN OMMEN G.-J.B., HOFKER M.H., FERRARI M.D., FRANTS R.R. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- PRUNEAU D., BELICHARD P. Haemodynamic and humoral effects of omega-conotoxin GVIA in normotensive and spontaneously hypertensive rats. Eur. J. Pharmacol. 1992;211:329–335. doi: 10.1016/0014-2999(92)90389-l. [DOI] [PubMed] [Google Scholar]
- RICHTER F., EBERSBERGER A., MIKULIK O., SCHAIBLE H.-G. P/Q-type channels in the brainstem regulate activity of neurons with input from the dura by controlling GABA release from inhibitory interneurons. Soc. Neurosci. Abstr. 2002;28:313–349. [Google Scholar]
- SEISENBERGER C., SPECHT V., WELLING A., PLATZER J., PFEIFER A., KUHBANDNER S., STRIESSNIG J., KLUGBAUER N., FEIL R., HOFMANN F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J. Biol. Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- UDDMAN R., TAJTI J., MOLLER S., SUNDLER F., EDVINSSON L. Neuronal messengers and peptide receptors in the human sphenopalatine and otic ganglia. Brain Res. 1999;826:193–199. doi: 10.1016/s0006-8993(99)01260-3. [DOI] [PubMed] [Google Scholar]
- WESTENBROEK R.E., SAKURAI T., ELLIOTT E.M., HELL J.W., STARR T.V.B., SNUTCH T.P., CATTERALL W.A. Immunochemical identification and subcellular distribution of the alpha(1a) subunits of brain calcium channels. J. Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER D.B., RANDALL A., TSIEN R.W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural blood vessel diameter in the anaesthetized rat. Cephalalgia. 1997a;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat – intravital microscope studies. Cephalalgia. 1997b;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., COOK D.A., HARGREAVES R.J., HILL R.G., CUMBERBATCH M.J. Role of opioid receptors in neurogenic dural vasodilation and sensitization of trigeminal neurones in anaesthetized rats. Br. J. Pharmacol. 2001;133:807–814. doi: 10.1038/sj.bjp.0704136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., HILL R.G., HARGREAVES R.J. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur. J. Pharmacol. 1997c;328:61–64. doi: 10.1016/s0014-2999(97)83028-2. [DOI] [PubMed] [Google Scholar]
- YASUDA O., MORIMOTO S., JIANG B., KURODA H., KIMURA T., SAKAKIBARA S., FUKUO K., CHEN S., TAMATANI M., OGIHARA T. FS2. A mamba venom toxin, is a specific blocker of the L-type calcium channels. Artery. 1994;21:287–302. [PubMed] [Google Scholar]