Abstract
ATP receptors present on rat alveolar macrophages (NR8383 cells) were identified by recordings of membrane current, measurements of intracellular calcium, RT–PCR and immunocytochemistry.
In whole-cell recordings with a sodium-based internal solution, ATP evoked an inward current at −60 mV. This reversed at 0 mV. The EC50 for ATP was 18 μM in normal external solution (calcium 2 mM, magnesium 1 mM). The currents evoked by 2′,3-O-(4-benzoyl)benzoyl-ATP were about five-fold smaller than those observed with ATP. ADP, UTP and αβ-methylene-ATP (αβmeATP) (up to 100 μM) had no effect. ATP-evoked currents were potentiated up to ten-fold by ivermectin and were unaffected by suramin (30–100 μM), pyridoxal-phosphate-6-azophenyl-(2,4-sulphonic acid) (30–100 μM), and brilliant blue G (1 μM).
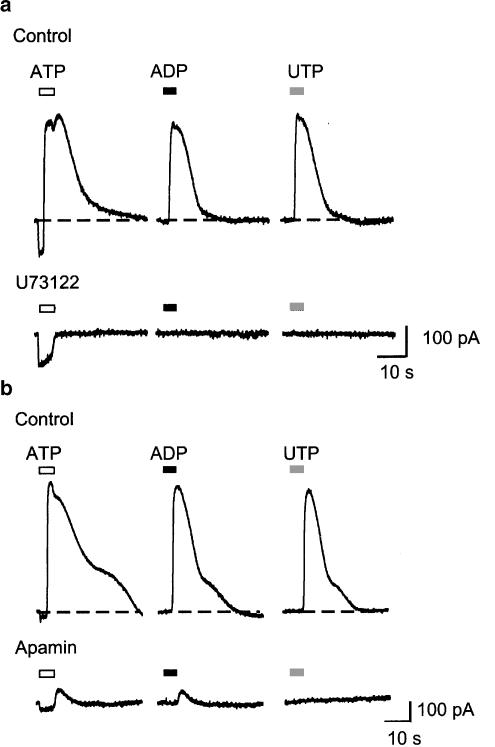
In whole-cell recordings with a potassium-based internal solution and low EGTA (0.01 mM), ATP evoked an inward current at −60 mV that was followed by larger outward current. ADP and UTP (1–100 μM) evoked only outward currents; these reversed polarity at the potassium equilibrium potential and were blocked by apamin (10 nM). Outward currents were also blocked by the phospholipase C inhibitor U73122 (1 μM), and they were not seen with higher intracellular EGTA (10 mM). Suramin (30 μM) blocked the outward currents evoked by ATP and UTP, but not that evoked by ADP. PPADS (10 μM) blocked the ADP-evoked outward current without altering the ATP or UTP currents.
RT–PCR showed transcripts for P2X subunits 1, 4 and 7 (not 2, 3, 5, 6) and P2Y receptors 1, 2, 4 and 12 (not 6). Immunocytochemistry showed strong P2X4 receptor expression partly associated with the membrane, weak P2X7 staining that was not associated with the cell membrane, and no P2X1 receptor immunoreactivity.
We conclude that rat alveolar macrophages express (probably homomeric) P2X4 receptors, but find no evidence for other functional P2X subtypes. The P2Y receptors are most likely P2Y1 and P2Y2 and these couple through phospholipase C to an increase in intracellular calcium and the opening of SK type potassium channels.
Keywords: P2X, purinergic receptor, P2Y, phospholipase C, SK channel, macrophages
Introduction
The alveolar macrophage is the first line of defence against inhaled pathogens, and they appear to be involved in the production and maintenance of airway inflammation in asthma and other inflammatory lung diseases (Holgate, 2002; Barnes, 2003). They produce and release inflammatory mediators such as interleukin-1β (IL-1β), TNF-α and macrophage inflammatory protein 1α (MIP-1α), which can contribute in the short term to bronchoconstriction and hyper-reactivity, and in the longer term to the development of pulmonary fibrosis (Kelly et al., 2003). One of the principal stimuli to the release of these inflammatory cytokines from lipopolysaccharide-(LPS) primed macrophages in other tissues is ATP acting at P2 receptors (Di Virgilio et al., 2001). ATP causes a rise in intracellular calcium concentration ([Ca]i) in macrophages; part of this action (μM in concentrations, mimicked by ADP, UTP and UDP) is independent of extracellular calcium, whereas part of it (mM in concentrations of ATP, not mimicked by other nucleotides) requires calcium entry from outside (Sung et al., 1985; Greenberg et al., 1988). Thus, both P2Y and P2X receptors have been implicated in these effects.
The preponderance of studies have since focused on the part played by the P2X7 (formerly P2Z) receptor (Surprenant et al., 1996). Activation of this receptor results in processing and release of IL-1β in macrophages (Perregaux & Gabel, 1994; Ferrari et al., 1997; Perregaux et al., 2000) by the shedding of membrane micovesicles (Mackenzie et al., 2001), and LPS-primed peritoneal macrophages from mice with a P2X7 gene disruption do not release IL-1β in response to ATP (Solle et al., 2001). The main aim of this work was to study the actions of ATP on lung macrophages and to identify the P2 receptor types involved.
The NR8383 cell line was originally cloned from resident macrophages lavaged from the lungs of Sprague–Dawley rats (Helmke et al., 1987). The cells synthesize and release IL-1β and TNF-α in response to activation of CD8α (Lin et al., 2000). TNF-α and MIP-1α are also released by agonists at leukotriene (LTD4) receptors (Menard & Bissonnette, 2000). The cells respond to ATP with an increase in intracellular calcium concentration ([Ca]i) (Zhang et al., 1997), but otherwise there is little known about the expression of P2 receptors by these cells, or the consequences of their activation. As an initial step in the characterization of the receptors we have studied the actions of some purines and pyrimidines on membrane currents using patch-clamp recording, we have measured the changes in [Ca]i that they cause, and we have sought the presence of P2 receptor transcripts by RT–PCR.
Methods
Immunocytochemistry and RT–PCR
The cells used were NR8383, a rat alveolar macrophage cell line (ATCC, Rockville, MD, U.S.A.). For immunostaining, cells were fixed with Zamboni's fixative (30 min), blocked with 5% goat serum in buffered saline with 2% Triton X-100 (30 min) and incubated with primary antibody for 2–3 h at room temperature. Cells were rinsed and FITC-labelled secondary antibody applied for 1 h, rinsed and mounted on slides for viewing under confocal microscope. Antibodies used were: rabbit polyclonal anti-P2X1 (10 μg ml−1); anti-P2X4 (0.6 μg ml−1), anti-P2X7 (0.6–2 μg ml−1) from Alomone Labs (Israel).
RNA was extracted from 1 × 106 cells using an RNeasy kit (Qiagen West Sussex UK.), including DNase I treatment according to the manufacturer's instructions. First-strand cDNA was prepared from 5 μg total RNA in a 20 μl reaction using an oligo(dT) primer and Superscript II First Strand Synthesis System for RT–PCR (Invitrogen Paisley UK). Reverse transcriptions were also performed in the absence of reverse transcriptase as a control for contaminating genomic DNA. PCR reactions were performed with mRNA-specific primer pairs for P2X and P2Y as listed below. The reaction contained 1 μl cDNA, 1 μl forward primer (10 pmol), 1 μl of reverse primer (10 pmol), 25 μl 2 × Reddymix PCR master mix (ABgene: contains 1.25 U Taq DNA polymerase, 1.5 mM MgCl2, 200 μM each of dATP, dCTP, dGTP and dTTP) and 22 μl H2O. Amplification conditions were 60 s at 94°C, 30 s at 55°C, 45 s at 72°C for 35 cycles and finally 5 min at 72°C. Products were resolved on a 1.5% agarose gel by electrophoresis. The primers used (forward, reverse and product size (bp)) were as follows: P2X1: CAGAAAGGAAAGCCCAAGGTATTC, TGACGACGGTTTGTCCCATTC, 508; P2X2 GTGACTACCTCAAGCATTG, CTGTTGGGAAGGCTCAGGGAC, 785; P2X3: GGACATAAAGAGGTGCCGCTTC, AACACTGGGTTGGTTGACGCAG, 534; P2X4: GGAACATCCTCCCCAACATCAC, TTCATCTCCCCCGAAAGACC, 553; P2X5: TGTCACGCTGGGGAGTCTGTTGTAG, TTGCTATTCTGCTTCCTGCCAC, 869; P2X6: AGAGTAGTGCTGTGCCCAGGAAAC, CCTCAAAGTCCCCTCCAGTCATAG, 228; P2X7: AATGAGTCCCTGTTCCCTGGCTAC, CAGTTCCAAGAAGTCCGTCTGG, 468; P2Y1: TCCTCTTCATTCCGATGTGCC, TCTTCTTCTTGAGCCTGCCCAG, 391; P2Y2: GGGACGAACTGGGTTACAAATGTC, GGTGTGGCAACTGAGGTCAAGTG, 785; P2Y4: CAACCAATGCCAATGGAACTACC, ACTTGTCCCCCGTGAAGAGATAG, 414; P2Y6: TGCTTGGGTGGTATGTGGAGTC, TGTTGTGTGAAGTAGAAGAGGATAGGG, 489; P2Y12: TCAGCCAACACCACCTCCATTC, CCAGACCAAACTCCGACTTCAAG, 544; β-actin: GGCTCTCTTCCAGCCTTCCTTCTTG, CACAGAGTACTTGCGCTCAGGAGG, 241.
Electrophysiology and data analysis
Whole-cell patch-clamp recordings were made at room temperature using an EPC9 amplifier and Pulse software (HEKA, Germany); agonists were applied by fast-flow using the RSC system (Bio-Logic Science Instruments, France). Patch pipettes were filled with high EGTA solution (mM): NaCl or KCl 145, HEPES 10, glucose 10, EGTA 10; or low EGTA solution (mM): NaCl or KCl 145, HEPES 10, glucose 10, EGTA 0.01, CaCl2 0.1. The external solution was NaCl 145, KCl 2, CaCl2 2, MgCl2 1, HEPES 10, glucose 10; solutions were maintained at pH 7.3 and osmolarity of 300–310mosmol l−1.
Results are expressed as mean ±s.e.m. for number of cells examined and curves were fit from pooled data using Kaleidagraph (Synergy Software, Reading, PA, U.S.A.). Agonist concentration–response curves for inward currents and changes in [Ca]i were fit by I/Imax=100 ([A]n/[A]n+EC50n), where I is the peak current evoked by concentration [A] as a percent of maximum current. ATP, 2′,3-O-(4-benzoyl)benzoyl-ATP (BzATP), α,β methylene ATP (αβmeATP), ivermectin and Coomassie brilliant blue G, were obtained from Sigma Dorset UK; suramin was from Bayer (Germany), pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) from Sigma, apamin from Sigma and U73122 was from Calbiochem Nottingham UK.
Fluo-4 measurements of intracellular calcium
NR8383 cells were incubated for 30 min at 37°C with Fluo-4 AM (3 μM; Molecular Probes, Eugene, OR, U.S.A.) in the following buffer (mM): NaCl 136, KCl 1.8, KH2PO4 1.2, MgSO4 1.2, NaHCO3 5, CaCl2 2, glucose 6, HEPES 20 and EGTA 5. Cells were washed three times, resuspended in the same buffer without EGTA after which 3 × 105 cells ml−1 were placed in 1 ml volumes into cuvettes for fluorescence measurements (488 nm) using the Cairn Integra fluorometer (Cairn Research, Kent, U.K.). Concentration–response curves were obtained from cumulative (UTP) and non-cumulative (UTP and ADP) additions of agonist; no differences in UTP dose–response curves were obtained using either type of agonist application and so results have been pooled.
Results
P2X receptor-mediated currents
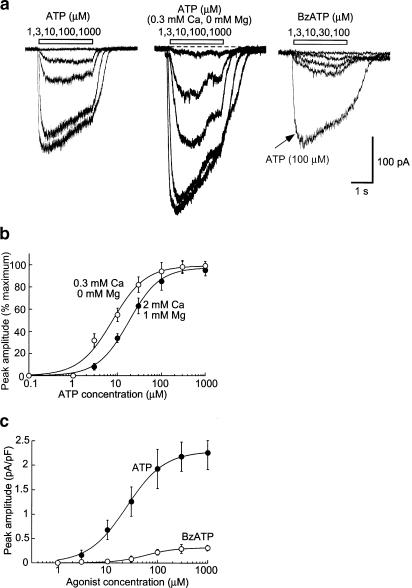
We first used a high EGTA NaCl-based internal solution (see Methods) in order to isolate potential P2X-mediated currents because the high EGTA would be expected to abrogate intracellular calcium rises due to activation of P2Y receptors and the internal Na blocks all potassium currents (see below). ATP (30 μM) evoked relatively sustained inward currents at the holding potential of −60 mV in all cells examined (n=56; Figure 1a); the current reversed at 0 mV and showed slight inward rectification (see Figure 4b), consistent with typical P2X-mediated cationic currents (North, 2002). ATP concentration–response curves were constructed in normal external solution and also in a low calcium/magnesium-free external solution, which is known to potentiate P2X7 receptor-mediated currents by several fold (North, 2002); EC50 values were 18±2 and 7±1.5 μM (n=10), respectively (Figure 1). Maximum current responses in low divalent external solution were 1.3–2-fold greater than in normal external solution (Figure 1a). A similar small increase in maximum ATP-mediated currents in low divalent solutions was observed from recordings made in HEK293 cells transfected with rat P2X4 receptors (data not shown). BzATP, which is several fold more potent than ATP at P2X7 receptors, was a weak partial agonist, evoking currents of about 15% that were evoked by ATP (Figure 1a, c); this relationship was not altered in low divalent external solutions (n=3). ADP, UTP, GTP and αβme-ATP (all at 30–100 μM, n=4–10) were without effect when recordings were made with this high EGTA NaCl internal solution.
Figure 1.
ATP-induced currents. (a) Currents evoked by ATP (left), ATP in solution with reduced divalent ion concentration (middle) and BzATP (right). (b) Concentration–response curves for ATP to cause inward current, both in normal solution and solution containing reduced divalent ion concentration. Current normalized to maximum current in each case: n=4–8 cells for each point. (c) Relative effectiveness of ATP and BzATP to evoke inward current. Currents normalized to cell capacitance.
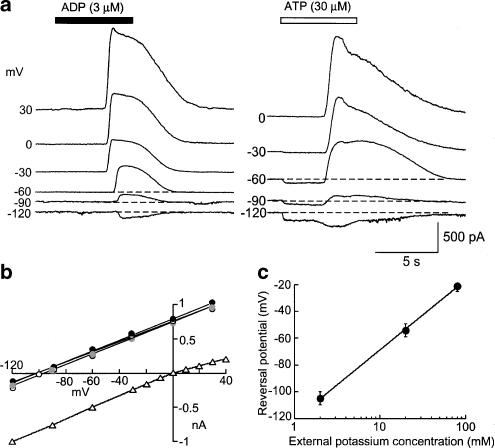
Figure 4.
P2 agonists open potassium-selective channels. (a) Examples of currents recorded in response to ADP (left) and ATP (right) at holding potentials from −120 to 30 mV. Note that ATP but not ADP evokes an inward current prior to the outward current. (b) Averaged current voltage plots from experiments such as those shown in (a) circles are ATP, UTP and ADP respectively with potassium-based internal solution (normalized to 1 at 30 mV). Open triangles are ATP-evoked currents with sodium-based internal solution (normalized to 1 at −120 mV). Standard error bars are smaller than symbol size. (c) Reversal potential (Erev) for outward current (pooled using ATP, UTP and ADP) as a function of extracellular potassium concentration ([K]o). The fitted line is Erev=58 log{[K]o/147}.
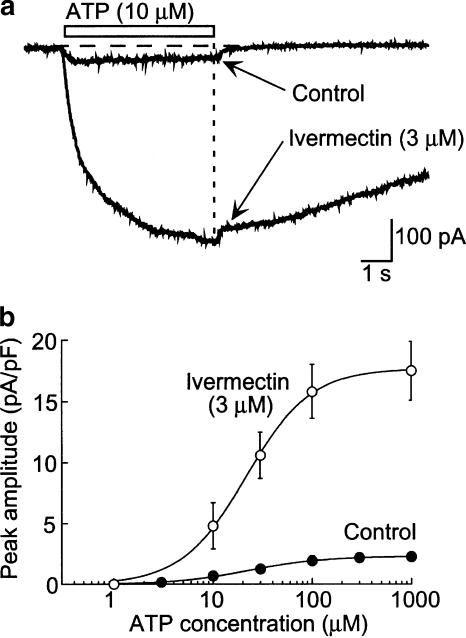
The rat P2X4 receptor is not inhibited by high concentrations of either suramin or PPADS, while the rat P2X7 receptor is fully inhibited by a concentration of BBG (0.3–1 μM) that has little action at P2X4 receptors (Buell et al., 1996; Jiang et al., 2001). Therefore, we examined these compounds on the ATP-evoked currents in NR8383 cells. Neither suramin (30, 100 μM) nor PPADS (30, 100 μM), nor BBG (1 μM) inhibited ATP- or BzATP-mediated currents (n=3–6). Ivermectin, a channel modulator that has been shown to enhance specifically P2X4-mediated currents in heterologous expression systems (Khakh et al., 1999), potentiated ATP-mediated currents in NR8383 cells by up to 20-fold without significantly altering the EC50 value (Figure 2).
Figure 2.
Ivermectin potentiates ATP-evoked current. (a) Representative traces of current before and after adding ivermectin (3 μM). (b) Averaged data show that ivermectin increased the ATP-evoked current about 10-fold but did not alter the EC50 (n=5).
These currents elicited by ATP were not obviously different when the EGTA concentration of the internal solution was 0.01 mM, or when a potassium-based internal solution was used containing 10 mM EGTA (in the latter condition, delayed rectifier potassium currents were obvious when the cells were depolarized).
P2Y receptor-mediated currents
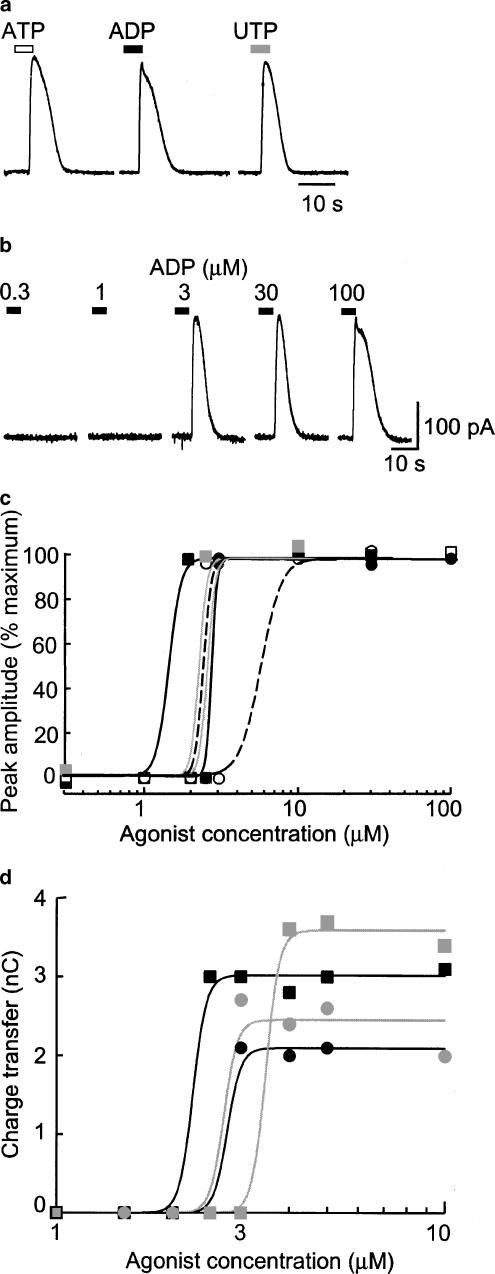
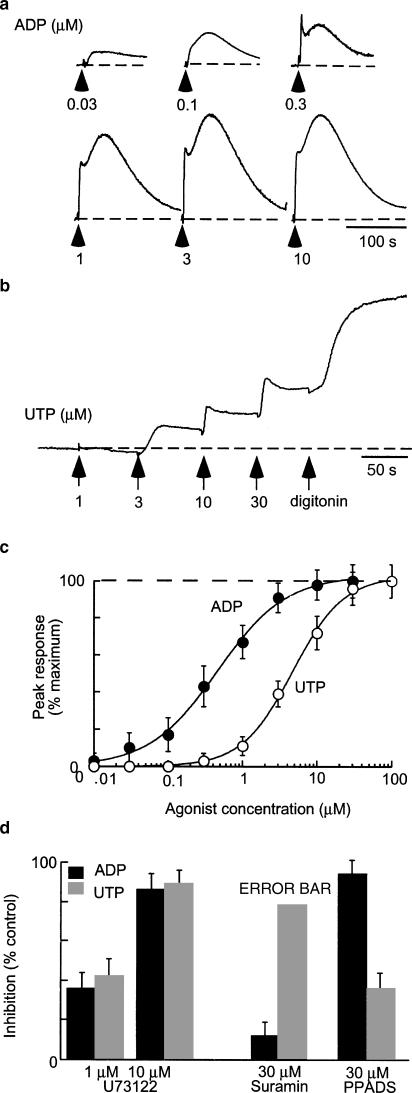
We next used a potassium-based internal solution with low EGTA (0.01 mM). At −60 mV ATP evoked an inward current, and this was similar in all respects to that described above. However, within a few seconds of beginning the application (5±0.4 s, n=10) this reversed to a large outward current (n>30; Figures 3, 4, 5 and 6). ADP and UTP did not elicit any inward current (see above) but were as effective as ATP to elicit the outward current (Figure 3a). The time to onset of the outward current induced by ADP or UTP at −60 mV was 3.8±0.1 s (n=20, e.g. Figure 4a). It was difficult to examine the concentration-dependence of the outward current because, in any given cell, a very small increase in concentration (typically from 1 to 3 μM) was sufficient to activate fully the current (Figure 3b–d).
Figure 3.
P2 agonists evoke outward currents. (a) Outward currents recorded at −60 mV in one cell in response to ATP, ADP and UTP (each 3 μM). Note that this concentration of ATP does not evoke any inward current. (b) Currents in response to increasing concentrations of ADP, as indicated. (c) Concentration–response curves plotted for individual cells from six experiments such as that as illustrated in (b) (ADP two cells: black circles, squares, line; UTP two cells: grey circles, squares, line; ATP two cells: open circles and squares, broken line). (d) Concentration–response curves plotted for individual cells from four experiments, using several concentrations in the 1–10 μM range. In this case, the time integral of the current is plotted. ADP two cells: black circles, squares, line; UTP: grey circles, squares, line.
Figure 5.
P2 agonists open calcium-activated, SK potassium channels. (a) Currents in one cell in response to ATP, ADP and UTP (each at 30 μM). The outward currents were completely blocked by the phospholipase C inhibitor U73122 (1 μM). (b) A similar experiment in another cell in which apamin (100 nM) blocked the outward currents. Note that the inward current evoked by ATP was not changed by U73122 or apamin.
Figure 6.
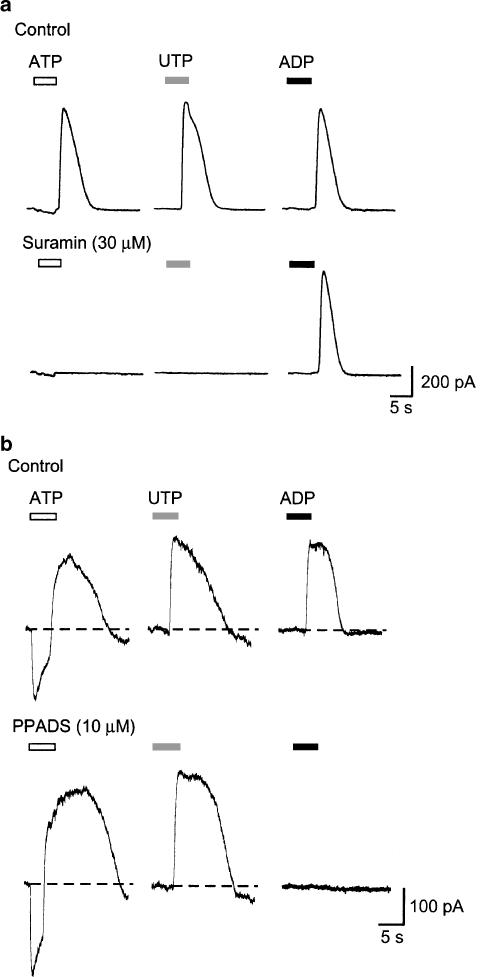
Antagonists selectively block the outward current evoked by ADP, and by ATP or UTP. (a) Currents recorded from one cell in response to 5 s applications of ATP, UTP and ADP (each 3 μM), first in control solution and then in suramin (3 μM). (b) Currents recorded from one cell in response to 5 s applications of ATP, UTP and ADP (each 30 μM), first in control solution and then in PPADS (10 μM). Note that ATP-evoked inward currents are unaffected by PPADS.
The currents evoked by ADP and ATP showed a linear current–voltage relationship with reversal potential at −105 mV in normal external solution (Figure 4a, b); the delayed component of the ATP-evoked current showed similar properties, even though the initial inward component (at −60 mV) reversed at 0 mV (Figure 4b). The reversal potential of the UTP- and ADP-evoked currents in external solutions containing 2, 20 and 80 mM potassium changed as expected for a potassium-selective current (Figure 4c).
The phospholipase C inhibitor U73122 (1 μM) abolished agonist-evoked outward currents without affecting the ATP-induced inward current (n=6; Figure 5a), while the DMSO vehicle was without effect on either inward or outward current (n=2). This concentration of U73122 was effective within 2 min of introduction into the superfusion solution and reached maximum effect within 4 min; no reversal of blockade was observed over a 10-min washout period (n=4). Agonist-evoked outward currents were also abolished by the SK2/SK3-channel blocker apamin (100 nM, n=4) (Figure 5b) but were unaltered by tetraethylammonium (1–10 mM). The inhibition by apamin was irreversible during a 20-min washout period.
Suramin (30 μM) abolished the currents elicited by ATP and UTP but had no effect on the ADP-evoked outward current (Figure 6b). Conversely, PPADS (10 μM) abolished currents evoked by ADP (3, 10, 30 μM) and had no significant effect on ATP or UTP-evoked currents (3–30 μM; Figure 5a). Neither ADP nor UTP evoked any currents when the high EGTA concentration (10 mM) was used with the potassium chloride internal solution (n=5).
Fluo-4 measurements of intracellular calcium
ADP and UTP consistently evoked concentration-dependent increases in intracellular calcium [Ca]i. The threshold for activation by ADP was 10–30 nM with an EC50 of 0.8±0.5 μM (n=4); for UTP the threshold was approximately 300 nM and the EC50 was 4±0.9 μM (n=4) (Figure 7). PPADS (10 μM) inhibited ADP-mediated responses but had no significant effect on UTP-evoked responses (Figure 7d). Suramin (100 μM) did not alter ADP responses but strongly inhibited UTP-evoked responses (Figure 7d). Preincubation with U73122 at the concentration (1 μM) that abolished ADP- and UTP-evoked potassium currents reduced calcium responses by about 40%, although a higher concentration (10 μM) inhibited responses by >85% (Figure 7).
Figure 7.
Rises in [Ca]i evoked by ADP and UTP. (a) Representative fluorescence traces recorded from Fluo-4 loaded cells in response to ADP (concentrations as indicated). Responses are from separate experiments to avoid desensitization that was observed with ADP; each is normalized to the signal observed with digotonin. (b) Fluorescence traces during cumulative additions of UTP; note minimal desensitization. (c) Summary of experiments such as those shown in (a) and (b) (ADP n=4, UTP n=3). (d) Inhibition of [Ca]i signals evoked by ADP (filled bars, 3 μM) and UTP (grey bars, 3 μM) by U73122, suramin and PPADS (n=5 for each histogram).
mRNA expression and immunocytochemistry
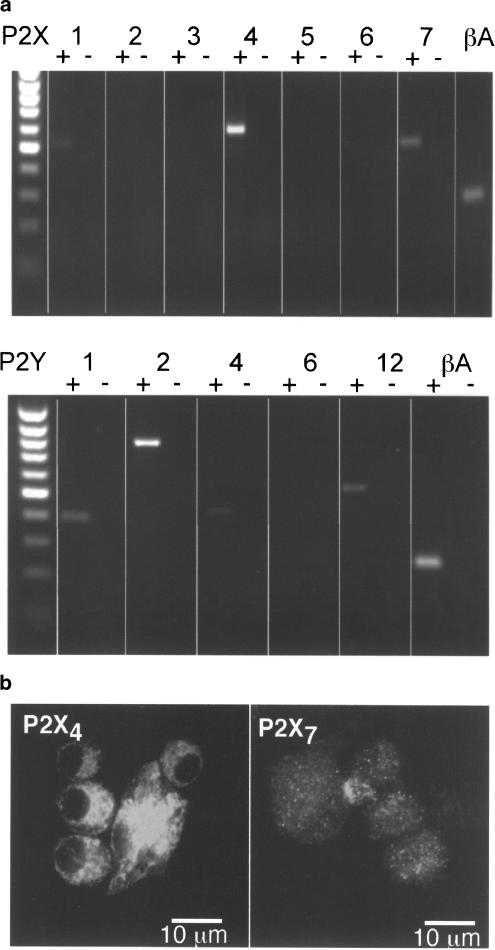
Figure 8 shows that NR8383 cells express mRNA transcripts for P2X1, P2X4, P2X7 as well as for P2Y1, P2Y2, P2Y4, and P2Y12, but not for P2X2, P2X3, P2X5 or P2X6 or P2Y6 (Figure 8a). Immunocytochemistry with P2X1-, P2X4- and P2X7-specific antibodies showed no staining for P2X1 subunits, strong immunoreactivity for P2X4 subunits (Figure 8b), and weaker staining for P2X7 subunits (Figure 8b). P2X4 immunoreactivty was present both in the plasma membrane and underlying cytoplasm, whereas the P2X7 immunoreactivity was weak and punctate throughout the cytoplasm with no clear labelling of the cell membrane apparent.
Figure 8.
Detection of P2 receptor mRNAs and protein. (a) RT–PCR shows amplification of appropriately sized products corresponding to P2X1, P2X4 and P2X7 subunits (top panel), and P2Y1, P2Y2, P2Y4 and P2Y12 (bottom panel). No P2X2, P2X3, P2X5, P2X6 or P2Y6 was detected. Vertical lines separate lanes for clarity. (b) Confocal microscope images of cells exhibiting immunoreactivity for P2X4 (left) and P2X7 (right) subunits.
Discussion
The main conclusion of this work is that the NR8383 cells express both P2X and P2Y receptors. It is convenient to discuss the evidence for this in turn.
P2X receptors
The rapid-onset inward current elicited by ATP but not UTP or ADP is indicative of P2X receptor involvement. The effective concentrations of ATP (EC50 about 15 μM), the time course of the current, and the reversal potential close to 0 mV are all fully consistent with P2X receptors. Several further distinguishing features of the current allow us to conclude that it results from activation of P2X4 receptors (see North, 2002). These are the lack of effect of αβmeATP, the potentiation by ivermectin and the lack of any blockade by suramin or PPADS (Buell et al., 1996; North, 2002). Three features, in particular, distinguish it from currents at P2X7 receptors: these are the relatively low effective concentrations of ATP (Figure 1), the finding that BzATP is much less potent than ATP (Figure 1c), and the weak potentiation by reducing the concentration of divalent cations (Figure 1b). We have observed similar small effects of these changes in divalent ion concentrations in HEK293 cells transfected with rat P2X4 subunit (data not shown), whereas the currents at rat P2X7 receptors are typically increased more than five-fold (Surprenant et al., 1996). Three features exclude the presence of a significant component through P2X1 receptors: these are the weak desensitization of the currents, the lack of action of αβmeATP and the absence of any block by suramin and PPADS. Together with the results from the RT–PCR and immunocytochemistry, these findings allow us to conclude that the current induced by ATP on NR8383 cells results from the activation of P2X4 receptors.
Membrane currents induced by ATP in macrophages and related cells have recently been reviewed (North, 2002). In some cases, the current described has properties that closely resemble those of the cloned P2X7 receptor, such as the requirement for mM concentrations of ATP, marked potentiation by low divalent ion concentrations, and the observation that BzATP is more potent than ATP. These include J774 cells (Buisman et al., 1988; Surprenant et al., 1996), NTW8 microglia (Chessell et al., 1997) and human monocyte-derived macrophages (Rassendren et al., 1997). However, there are other studies for which the agreement is much less good (monocyte-derived macrophages; Eschke et al., 2002; brain amoeboid microglia: Haas et al., 1996). In particular, rat microglia after 7–10 days in culture express two distinct components to the inward current activated by ATP and BzATP, the properties of which correspond very closely to those expected for P2X4 and P2X7 receptors (Visentin et al., 1999). Cells other than macrophages can exhibit responses to ATP that are very similar to those reported here for NR8383 cells. These include submandibular gland cells (Buell et al., 1996) and osteoclasts (Naemsch et al., 1999).
P2Y receptors
The outward current component observed in response to ATP is clearly a potassium-selective current (Figures 4 and 5). The block by apamin identifies it as a member of the SK family of potassium channels (Bond et al., 1999). The block by U73122 indicates that it is activated as a result of calcium released by inositol 1,4,5-trisphosphate generated by the action of phospholipase C on phosphatidyl inositol 4,5-bisphosphate. The finding that the outward current was not observed with a high (10 mM) EGTA concentration inside the cell is consistent with this interpretation. The delay in the onset of the current and its explosive ‘all-or-nothing' nature as a function of the agonist concentration might also be expected from an intracellular transduction mechanism providing considerable intracellular signal amplification.
Our results with Fluo-4 loaded cells show that ADP and UTP at concentrations that were effective to increase the potassium conductance also caused a substantial increase in [Ca]i. We have not attempted to quantify the [Ca]i in these experiments, or to probe in any detail its source within the cell. The main point is that neither ADP nor UTP activated any inward current similar to that caused by ATP, but both caused substantial rises in [Ca]i that were largely (≈85%) blocked by inhibition of phospholipase C with U73122 (Figure 7d). It is therefore likely that the P2 receptors activated by ADP and UTP in these experiments lead directly to stimulation of phospholipase C.
Potassium currents activated by P2Y receptor stimulation have been described in many cells and occur either by direct coupling to the channel (i.e. G protein βγ subunit) or through an increase in [Ca]i. The former case has been most widely described in neurons and cardiac muscle (e.g. Friel & Bean, 1990; Ikeuchi & Nishizaki, 1996; Matsuura & Ehara, 1996). The latter case has been best characterized in hepatocytes (Yamashita et al., 1996) and intestinal smooth muscle, where it brings about an ATP-mediated inhibitory postsynaptic potential (Koh et al., 1997). In all these tissues, an apamin-sensitive potassium conductance was involved. Microglia also exhibit an outward current in response to ATP (Langosch et al., 1994; Norenberg et al., 1994), but probably the cells having the response closest in its properties to NR8383 cells are rat osteoclasts (which of course have many of the properties and cell markers of macrophages). Weidema et al. (1997), (2001); describe that the inward current evoked by ATP is followed by calcium-activated potassium current. Only the potassium current was activated by UTP; the potassium current, but not the preceding inward current, was also blocked by suramin.
A main focus of the present work was the identification of the underlying P2Y receptor and this can be approached by comparing the currents with the known properties of rat P2Y receptors in heterologous expression systems (reviewed by von Kugelgen & Wetter, 2000; Sak & Webb, 2002). It is clear from the experiment illustrated in Figure 6 that PPADS (10 μM) and suramin (30 μM) distinguish two P2Y receptors. The current activated by ADP that was selectively blocked by PPADS, and the increase in [Ca]i with similar properties (Figure 7), probably results from activation of the P2Y1 receptor. PPADS (30 μM) has previously been shown to block the rat P2Y1 receptor (Schachter et al., 1997). PPADS did not affect the current activated by ATP and UTP, which therefore involves a different P2Y receptor. The rat P2Y4 receptor is activated by both ATP and UTP but is not blocked by suramin (100 μM) (Bogdanov et al., 1998) on the basis of our current measurements, we can therefore exclude its involvement. On the other hand, the rat P2Y2 receptor is activated equally well by ATP and UTP and this is sensitive to blockade by suramin (Chen et al., 1996). Involvement of the P2Y6 receptor, which is more selectively activated by UDP, is unlikely given that we failed to amplify its mRNA. The P2Y12 (formerly P2 T) receptor (Hollopeter et al., 2001), which is also expressed by NR8383 cells at the mRNA level, is most commonly linked to inhibition of adenyl cyclase (Gio coupled) and thus perhaps less likely to underlie the increase in [Ca]i and potassium current.
The most parsimonious explanation of the present results is therefore that NR8383 cells express both P2Y1 and P2Y2 receptors. Such coexpression has previously been reported for inter alia rat glioma (C6) cells (Jin et al., 2001; Sabala et al., 2001), rat hepatocytes (Dixon et al., 2000) and human keratinocytes (Burrell et al., 2003). However, in rat hepatocytes, the ADP-mediated calcium influx, which was attributed to activation of P2Y1 receptors, was suramin-sensitive (Dixon et al., 2000) in contrast to the suramin-insensitive ADP response in the present study on NR8383 cells. Thus, it remains possible that a distinct P2Y receptor, or perhaps a heteromeric metabotropic purine receptor, underlies the ADP-mediate response in either NR8383 cells or rat hepatocytes. In any event, given that the downstream signalling pathways are similar, at our present level of understanding, the advantage of such coexpression would be to generalize the sensitivity to a wider range of purine and pyrimidine nucleotides.
The significance of this to immune cells such as the lung macrophage is not yet clear. It has been widely held that macrophages release inflammatory cytokines such as IL-1β as a result of activation of P2X7 receptors. However, our failure in the present experiments to detect any functional P2X7 receptors makes it important to determine whether NR8383 release IL-1β in response to any purine nucleotide and, if they do, to investigate the underlying mechanism. It is possible that P2X7 receptors are upregulated under conditions of inflammation in these lung macrophages. This might occur in response to the activation of P2Y receptors. The P2 receptors might even interact in a more direct way, such that P2Y activation results in phosphorylation of P2X receptors, as has been suggested for a parallel pathway in the case of the P2X3 receptors on sensory neurons (see North, 2002). Future studies might distinguish these and other possibilities.
Abbreviations
- BzATP
2′3-O-(4-benzoyl)benzoyl-ATP
- αβmeATP
αβ-methylene-ATP
- PPADS
pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid
- LPS
lipopolysaccharide
- U73122
1-[6-[[17 β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1-H-pyrrole-2,5-dione
References
- BARNES P.J. New concepts in chronic obstructive pulmonary disease. Annu. Rev. Med. 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- BOGDANOV Y.D., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOND C.T., MAYLIE J., ADELMAN J.P. Small-conductance calcium-activated potassium channels. Ann. N. Y. Acad. Sci. 1999;868:370–378. doi: 10.1111/j.1749-6632.1999.tb11298.x. [DOI] [PubMed] [Google Scholar]
- BUELL G., LEWIS C., COLLO G., NORTH R.A., SURPRENANT A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- BUISMAN H.P., STEINBERG T.H., FISCHBARG J., SILVERSTEIN S.C., VOGELZANG S.A., INCE C., YPEY D.L., LEIJH P.C. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURRELL H.E., BOWLER W.B., GALLAGHER J.A., SHARPE G.R. Human keratinocytes express multiple P2Y-receptors: evidence for functional P2Y1, P2Y2, and P2Y4 receptors. J. Invest. Dermatol. 2003;120:440–447. doi: 10.1046/j.1523-1747.2003.12050.x. [DOI] [PubMed] [Google Scholar]
- CHEN Z.P., KRULL N., XU S., LEVY A., LIGHTMAN S.L. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- CHESSELL I.P., MICHEL A.D., HUMPHREY P.P. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br. J. Pharmacol. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI VIRGILIO F., CHIOZZI P., FERRARI D., FALZONI S., SANZ J.M., MORELLI A., TORBOLI M., BOLOGNESI G., BARICORDI O.R. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- DIXON C.J., WOODS N.M., WEBB T.E., GREEN A.K. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br. J. Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCHKE D., WUST M., HAUSCHILDT S., NIEBER K. Pharmacological characterization of the P2X7 receptor on human macrophages using the patch-clamp technique. Naunyn Schmiedebergs Arch. Pharmacol. 2002;365:168–171. doi: 10.1007/s00210-001-0501-2. [DOI] [PubMed] [Google Scholar]
- FERRARI D., CHIOZZI P., FALZONI S., DAL SUSINO M., MELCHIORRI L., BARICORDI O.R., DI VIRGILIO F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- FRIEL D.D., BEAN B.P. Dual control by ATP and acetylcholine of inwardly rectifying K+ channels in bovine atrial cells. Pflugers Arch. 1990;415:651–657. doi: 10.1007/BF02584001. [DOI] [PubMed] [Google Scholar]
- GREENBERG S., DI VIRGILIO F., STEINBERG T.H., SILVERSTEIN S.C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J. Biol. Chem. 1988;263:10337–10343. [PubMed] [Google Scholar]
- HAAS S., BROCKHAUS J., VERKHRATSKY A., KETTENMANN H. ATP-induced membrane currents in ameboid microglia acutely isolated from mouse brain slices. Neuroscience. 1996;75:257–261. doi: 10.1016/0306-4522(96)00270-9. [DOI] [PubMed] [Google Scholar]
- HELMKE R.J., BOYD R.L., GERMAN V.F., MANGOS J.A. From growth factor dependence to growth factor responsiveness: the genesis of an alveolar macrophage cell line. In Vitro Cell Dev. Biol. 1987;23:567–574. doi: 10.1007/BF02620974. [DOI] [PubMed] [Google Scholar]
- HOLGATE S.T. Airway inflammation and remodeling in asthma: current concepts. Mol. Biotechnol. 2002;22:179–189. doi: 10.1385/MB:22:2:179. [DOI] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.B., NURDEN P., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- IKEUCHI Y., NISHIZAKI T. P2 purinoceptor-operated potassium channel in rat cerebellar neurons. Biochem. Biophys. Res. Commun. 1996;218:67–71. doi: 10.1006/bbrc.1996.0013. [DOI] [PubMed] [Google Scholar]
- JIANG L.-H., MACKENZIE A.B., NORTH R.A., SURPRENANT A. Brilliant blue G selectively blocked ATP-gated P2X7 receptors. Mol. Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- JIN J., TOMLINSON W., KIRK I.P., KIM Y.B., HUMPHRIES R.G., KUNAPULI S.P. The C6-2B glioma cell P2YAC receptor is pharmacologically and molecularly identical to the platelet P2Y12 receptor. Br. J. Pharmacol. 2001;133:521–528. doi: 10.1038/sj.bjp.0704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M., KOLB M., BONNIAUD P., GAULDIE J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr. Pharm. Des. 2003;9:39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., PROCTOR W.R., DUNWIDDIE T.V., LABARCA C., LESTER H.A. Allosteric control of gating and kinetics at P2X4 receptor channels. J. Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., DICK G.M., SANDERS K.M. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am. J. Physiol. Cell Physiol. 1997;42:C2010–C2021. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- LANGOSCH J.M., GEBICKE-HAERTER P.J., NORENBERG W., ILLES P. Characterization and transduction mechanisms of purinoceptors in activated rat microglia. Br. J. Pharmacol. 1994;113:29–34. doi: 10.1111/j.1476-5381.1994.tb16169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN T.-J., HIRJI N., STENTON G.R., GILCHRIST M., GRILL B.J., SCHREIBER A.D., BEFUS A.D. Activation of macrophage CD8: pharmacological studies of TNF and IL-1β production. J. Immunol. 2000;164:1783–1792. doi: 10.4049/jimmunol.164.4.1783. [DOI] [PubMed] [Google Scholar]
- MACKENZIE A., WILSON H.L., KISS-TOTH E., DOWER S.K., NORTH R.A., SURPRENANT A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- MATSUURA H., EHARA T. Modulation of the muscarinic K+ channel by P2-purinoceptors in guinea-pig atrial myocytes. J. Physiol. 1996;497:379–393. doi: 10.1113/jphysiol.1996.sp021775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENARD G., BISSONNETTE E.Y. Priming of alveolar macrophages by leukotriene D4: potentiation of inflammation. Am. J. Respir. Cell Mol. Biol. 2000;23:572–577. doi: 10.1165/ajrcmb.23.4.4152. [DOI] [PubMed] [Google Scholar]
- NAEMSCH L.N., WEIDEMA A.F., SIMS S.M., UNDERHILL T.M., DIXON S.J. P2X4 purinoceptors mediate an ATP-activated, non-selective cation current in rabbit osteoclasts. J. Cell Sci. 1999;112:4425–4435. doi: 10.1242/jcs.112.23.4425. [DOI] [PubMed] [Google Scholar]
- NORENBERG W., LANGOSCH J.M., GEBICKE-HAERTER P.J., ILLES P. Characterization and possible function of adenosine 5′-triphosphate receptors in activated rat microglia. Br. J. Pharmacol. 1994;111:942–950. doi: 10.1111/j.1476-5381.1994.tb14830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. The molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- PERREGAUX D., GABEL C.A. Interleukin-1 beta maturation and release in response to ATP and nigericin Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- PERREGAUX D.G., MCNIFF P., LALIBERTE R., CONKLYN M., GABEL C.A. ATP acts as an agonist to promote stimulus-induced secretion of IL-1β and IL-18 in human blood. J. Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- RASSENDREN F., BUELL G.N., VIRGINIO C., COLLO G., NORTH R.A., SURPRENANT A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- SABALA P., CZAJKOWSKI R., PRZYBYLEK K., KALITA K., KACZMAREK L., BARANSKA J. Two subtypes of G protein-coupled nucleotide receptors, P2Y1 and P2Y2 are involved in calcium signalling in glioma C6 cells. Br. J. Pharmacol. 2001;132:393–402. doi: 10.1038/sj.bjp.0703843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAK K., WEBB T.E. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch. Biochem. Biophys. 2002;397:131–136. doi: 10.1006/abbi.2001.2616. [DOI] [PubMed] [Google Scholar]
- SCHACHTER J.B., BOYER J.L., LI Q., NICHOLAS R.A., HARDEN T.K. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br. J. Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLLE M., LABASI J., PERREGAUX D.G., STAM E., PETRUSHOVA N., KOLLER B.H., GRIFFITHS R.J., GABEL C.A. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- SUNG S.S., YOUNG J.D., ORIGLIO A.M., HEIPLE J.M., KABACK H.R., SILVERSTEIN S.C. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J. Biol. Chem. 1985;260:13442–13449. [PubMed] [Google Scholar]
- SURPRENANT A., RASSENDREN F., KAWASHIMA E., NORTH R.A., BUELL G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- VISENTIN S., RENZI M., FRANK C., GRECO A., LEVI G. Two different ionotropic receptors are activated in rat microglia. J. Physiol. 1999;519:723–736. doi: 10.1111/j.1469-7793.1999.0723n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON KUGELGEN I., WETTER A. Molecular pharmacology of P2Y receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WEIDEMA A.F., BARBERA J., DIXON S.J., SIMS S.M. Extracellular nucleotides activate non-selective cation and Ca2+-dependent K+ channels in rat osteoclasts. J. Physiol. 1997;503:303–315. doi: 10.1111/j.1469-7793.1997.303bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEMA A.F., DIXON S.J., SIMS S.M.Activation of P2Y but not P2X4 nucleotide receptors causes elevation of [Ca2+]i in mammalian osteoclasts Am. J. Physiol. Cell Physiol. 2001280C1531C1519 [DOI] [PubMed] [Google Scholar]
- YAMASHITA Y., OGAWA H., AKAIKE N. ATP-induced rise in apamin-sensitive Ca2+-dependent K+ conductance in adult rat hepatocytes. Am. J. Physiol. 1996;270:G307–G313. doi: 10.1152/ajpgi.1996.270.2.G307. [DOI] [PubMed] [Google Scholar]
- ZHANG G.H., HELMKE R.J., MORK A.C., MARTINEZ J.R. Regulation of cytosolic free Ca2+ in cultured rat alveolar macrophages (NR8383) J. Leukoc. Biol. 1997;62:341–348. doi: 10.1002/jlb.62.3.341. [DOI] [PubMed] [Google Scholar]