Abstract
The cytokine interleukin-1 (IL-1) has been implicated in ischaemic, excitotoxic and traumatic brain damage in rodents. The naturally occurring IL-1 receptor antagonist (IL-1ra) markedly reduces neuronal injury in these conditions. However, the effects of IL-1ra on focal, transient cerebral ischaemia in the rat, which is of major clinical relevance, have not been reported.
The objectives of this study were to test the effects of IL-1ra on cell death after temporary cerebral ischaemia, and to investigate the therapeutic time window for IL-1ra treatment.
Ischaemia was induced by temporary (60 min) occlusion of the middle cerebral artery (MCAO) in rats, via surgical insertion (and subsequent removal) of a thread into the internal carotid artery. Damage was quantified at various times after MCAO to investigate the temporal progression of damage and establish an appropriate time to assess the effects of IL-1ra on cell death.
Cell death was complete 18–24 h after temporary MCAO. Intracerebroventricular injection of IL-1ra (10 μg) at the time of MCAO and 60 min later reduced the lesion volume measured 24 h (57% reduction) or 48 h (52% reduction) after MCAO. Cell death was also significantly reduced when IL-1ra (20 μg) was administered as a single injection, 1 h (47%), 2 h (57%) or 3 h (46%) after MCAO, when compared to vehicle.
These data show that IL-1ra markedly reduces cell death even when administration is delayed until 3 h after induction of reversible, focal cerebral ischaemia in the rat, and support our proposal that IL-1ra may be of therapeutic benefit in stroke.
Keywords: Interleukin-1, interleukin-1 receptor antagonist, stroke, reversible, transient, protection, ischaemia
Introduction
Extensive data now implicate the cytokine interleukin-1 (IL-1) in ischaemic, excitotoxic and traumatic brain damage (see Touzani et al., 1999; Allan & Rothwell, 2001). IL-1 expression is upregulated rapidly in the brains of experimental animals exposed to these insults, and is increased in relevant clinical conditions (see Allan & Rothwell, 2001). Exogenous administration of IL-1 markedly exacerbates neuronal damage caused by ischaemic or excitotoxic insults in rodents (Yamasaki et al., 1995; Lawrence et al., 1998; Stroemer & Rothwell, 1998). Furthermore, deletion of IL-1α and IL-1β or caspase-1 (required to release active IL-1β) (Schielke et al., 1998; Boutin et al., 2001), or inhibition of IL-1, through administration of a recombinant form of the naturally occurring IL-1 receptor antagonist (IL-1ra) or neutralising antibodies to IL-1, all markedly reduce ischaemic brain damage in rodents (see Touzani et al., 1999).
The most extensive data exist for the neuroprotective effects of IL-1ra, a selective and highly effective antagonist of IL-1α and IL-1β, with no known agonist activities (Dinarello & Thompson, 1991). IL-1ra, administered intracerebroventricularly (i.c.v.) or peripherally, significantly reduces neuronal injury induced by permanent, middle cerebral artery occlusion (MCAO), global ischaemia, perinatal hypoxia, or by excitotoxic, traumatic, haemorrhagic or heat stroke damage in experimental animals (see Touzani et al., 1999; Allan & Rothwell, 2001). Significant protective effects of IL-1ra have been demonstrated by both histological (total lesion volume and neuronal counts) and behavioural analyses, and are sustained for at least 7 days after the injury (Garcia et al., 1995b; Loddick & Rothwell, 1996). These data, together with the apparent absence of side effects of IL-1ra and its safety in clinical trials for sepsis (Opal et al., 1997) and rheumatoid arthritis (Calabrese, 2002; Schwetz, 2002) suggest that IL-1ra may be of therapeutic benefit in stroke.
Many promising neuroprotective agents identified from preclinical studies have, however, failed in clinical trials in stroke (see Dirnagl et al., 1999; Grotta, 2002; Lees, 2002; Stroke Therapy Academic Industry Roundtable, 2001). While the reasons for these failures are undoubtedly complex, many appear to be associated with a mismatch between laboratory experiments and the design of subsequent clinical trials. In response to these issues, the STAIR group has made a series of recommendations for preclinical studies that neuroprotective agents should satisfy prior to clinical trials (Stroke Therapy Academic Industry Roundtable, 1999). IL-1ra meets many of these recommendations, but two important issues remain to be addressed: firstly, rigorous studies have not been reported on the effects of IL-1ra in focal, reversible cerebral ischaemia in the rat, which is of direct clinical relevance, and secondly, the ‘therapeutic time window' (i.e. the period of time after the induction of ischaemia during which administration of treatment is effective) of IL-1ra has not been reported in transient focal cerebral ischaemia.
The objective of this study was therefore to address these important issues by studying the effects of administration of IL-1ra at the time of, or at specific times after, induction of transient focal cerebral ischaemia (60 min MCAO) in the rat. The intraluminal thread method of MCAO is a commonly used and widely accepted method of inducing transient cerebral ischaemia in experimental animals. However, numerous variations in the technique exist (e.g. differences in the size and/or type of thread, or duration of occlusion), which can influence not only the extent of the final damage (see Abraham et al., 2002) but also the rate of progression of damage (our unpublished data). Given these observations, and the fact that we are aware of no published data describing the temporal progression of damage in this experimental paradigm, we characterised the temporal progression of damage after 60 min MCAO, prior to conducting intervention studies. To determine if IL-1ra could protect the brain from transient cerebral ischaemia, we assessed the effect of IL-1ra (using a treatment protocol shown to be effective previously) on lesion volume 24 and 48 h after MCAO. Subsequently, we tested the effect of delaying the administration of IL-1ra until after the induction of ischaemia. The results demonstrate that IL-1ra significantly reduces neuronal injury induced by temporary MCAO, and is effective when administered as a single treatment 3 h after the induction of ischaemia.
Methods
Reagents
Recombinant human IL-1ra (rhIL-1ra) was a generous gift from Amgen, U.S.A., supplied at 100 mg ml−1 (in: 10 mM citrate, 140 mM NaCl, 0.5 mM EDTA, pH 6.5) and diluted with sterile 0.9% NaCl prior to injection.
Surgical procedures
All experiments were conducted in male, Sprague–Dawley rats (Charles River, U.K.) in accordance with the United Kingdom Animals (Scientific Procedures) Act (1986).
All surgical procedures were performed on animals under halothane anaesthesia (Fluothane, Zeneca, U.K., 1–2% in a mixture of oxygen and nitrous oxide (1 : 2 ratio)). Body temperature was maintained (37.0±0.5°C) throughout anaesthesia by means of a homeothermic blanket (Harvard Apparatus, U.K.).
To permit i.c.v. administration of rhIL-1ra, guide cannulae were implanted stereotaxically into the left lateral ventricle of the brain (1.5 mm lateral and 0.8 mm posterior to bregma) 3–7 days before middle cerebral artery occlusion.
Transient focal cerebral ischaemia was induced in rats (290–390 g body weight) by the intraluminal thread method, as described previously (Longa et al., 1989). Briefly, the right carotid arteries were exposed, and a poly-L-lysine (0.1%, weight volume−1)-coated nylon monofilament thread (3/0 gauge with the tip heat blunted to a diameter of 0.29 mm) was inserted into the external carotid artery and manipulated to enter the internal carotid artery, where it was advanced until resistance was felt (approximately 20 mm) at the point where the filament blocks the middle cerebral artery. The animals were allowed to recover from anaesthesia under a heating lamp. At 1 h after MCAO, the animals were briefly re-anaesthetised and the thread withdrawn to permit reperfusion of the MCA.
Experimental design
Study 1: Time course of lesion development
At various times after MCAO (2, 4, 6, 8 h (n=4 per group), and 12 and 18 h (n=5 per group)), the animals were killed and the brains removed and frozen on dry ice prior to histological analysis (see below).
Study 2: Neuroprotective effect of rhIL-1ra
Animals were subjected to transient, focal, cerebral ischaemia and injected i.c.v. twice, with rhIL-1ra (10 μg in 2 μl) or vehicle (2 μl of 0.9% sterile NaCl): immediately after ischaemia, and immediately after reperfusion (total dose 20 μg). Laser doppler flowmetry (Moor Instruments, U.K.) was used to monitor cerebral blood flow (CBF) during the surgical procedure and for 5 min after MCAO. A small area of the skull was thinned to enable a 500 μm diameter, flexible, laser doppler probe to be positioned over the surface of the cortex (approx 8 mm lateral and 3 mm posterior to bregma) and secured using a small amount of cryanoacrylate glue. Body temperature was recorded (using the homeothermic blanket system) at the time of reperfusion (1 h after MCAO). The animals were killed either 24 or 48 h after MCAO and the brains frozen on dry ice.
Study 3: Therapeutic time window for the neuroprotective effect of rhIL-1ra
Animals were subjected to transient focal cerebral ischaemia and injected i.c.v. with rhIL-1ra (20 μg in 2 μl) or vehicle (2 μl) at various times after MCAO (1, 2 or 3 h). At 24 h after MCAO, the animals were killed and the brains frozen on dry ice.
Histological analysis
In all studies described above, damage was assessed histologically on a series of coronal sections (20 μm, taken at 500 μm intervals throughout the brain) stained with cresyl violet. Areas of dead tissue were clearly visualised by a complete lack of staining. Image analysis software (SigmaScan 5.0, SPSS Science, U.K.) was used to measure the lesion area in the cortical and subcortical (striatum and thalamus) regions individually from the brain sections. To correct for the effect of oedema on lesion measurements, a ‘swelling factor (SF)' was calculated for each brain section, where
This factor was then used to correct lesion areas for swelling using the formula:
The lesion volume was calculated by integration of the lesion areas with the known distance between each coronal section. The total lesion volumes were calculated by summing the cortical and subcortical lesion volumes. Values for the total lesion volume obtained using this method of calculation concurred with those obtained using the approach described by Swanson et al. (1990). All data are expressed as corrected volumes. Serial sections were taken around the injection site and stained with cresyl violet to confirm accurate i.c.v. injection. All measurements and analyses were performed by an individual blinded to the treatment.
Statistical analysis
Data are expressed as mean±s.e.m. Significant differences between groups in study 1 (six groups) were analysed using Kruskal–Wallis with Dunn's post test. Differences in studies 2 and 3 (two groups) were assessed using the student's t-test with Welch correction where appropriate. Comparison of vehicle groups across experiments was analysed using ANOVA.
Results
Study 1: time course of lesion development
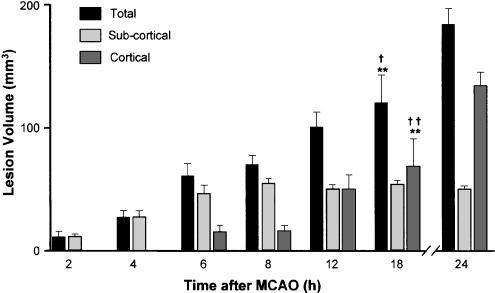
Lesion volumes are presented in Figure 1 as the total, cortical and subcortical (striatal and thalamic) components. Representative data from 24 h after MCAO are included for comparison, but were not included in the statistical analyses. Subcortical damage was evident as early as 2 h after MCAO, when it was restricted to the striatum. By 4 h, damage was also evident in the thalamus, and the subcortical lesion was maximal between 6 and 8 h after MCAO. Cortical damage progressed more slowly; it was detected first 6 h after MCAO, and by 18 h, it had not reached the maximum volume (51% of final cortical volume seen at 24 h). The total lesion volume 2 h after MCAO was significantly different from that at 12 and 18 h, and the total lesion at 4 h differed from that at 18 h. The cortical lesion volumes at both 2 and 4 h differed significantly from those at 12 and 18 h. The subcortical lesion at 2 h was significantly different from those at 8 and 18 h.
Figure 1.
Lesion development after temporary (60 min) middle cerebral artery occlusion in the rat. Animals were killed at various times after MCAO (2–12 h, n=4 per group, 12 and 18 h n=5 per group) and the areas of total cell death (as indicated by a complete lack of cresyl violet staining) in cortical and subcortical (striatal and thalamic) regions were measured as lesion. *P<0.05, **P<0.01 compared to appropriate group at 2 h, †P<0.05, ††P<01 compared to appropriate group at 4 h. Representative data from 24 h after MCAO (pooled values from experiments in study 3, n=24) are shown for comparison.
Study 2: neuroprotective effect of rhIL-1ra
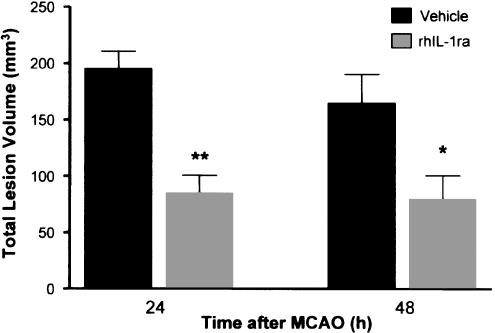
The administration of rhIL-1ra (10 μg, at the time of ischaemia and 10 μg 60 min later) significantly reduced (57%, P<0.01). lesion volume compared to vehicle, when assessed 24 h after MCAO (Figure 2). This protective effect of rhIL-1ra was very similar in magnitude (Figure 2) and pattern (Figure 3) when damage was assessed in a separate group of rats 48 h after MCAO (52% reduction, P<0.05). The volume of damage in vehicle-treated animals was also similar when assessed 24 h (195 mm3) or 48 h (164 mm3) after ischaemia, indicating that there was no progression of the lesion between 24 and 48 h in either vehicle or rhIL-1ra-treated animals. Therefore, in accordance with ethical and legal requirements to minimise the duration of subsequent experiments, 24 h was chosen as the end-point. Laser doppler monitoring indicated that the reductions in CBF at the time of MCAO were comparable in vehicle- and rhIL-1ra-treated animals in both the experiments (percentage decrease in CBF from the preischaemic value: 24 h survival group: vehicle 72±7%; rhIL-1ra, 64±10%; 48 h survival group: vehicle 73±3%; rhIL-1ra 70±3%). All animals were hyperthermic 60 min after MCAO, but there was no difference in body temperature between vehicle- and rhIL-1ra-treated animals (core temperature 60 min after MCAO: 24 h survival group: vehicle 38.5±0.1°C; rhIL-1ra 38.5±0.1°C; 48 h survival group: vehicle 38.6±0.1°C; rhIL-1ra 38.5±0.1°C).
Figure 2.
Effect of rhIL-1ra on total lesion volume assessed 24 or 48 h after transient (60 min) middle cerebral artery occlusion in the rat. Animals were injected i.c.v. with rhIL-1ra (10 μg) or vehicle (2 μl) immediately after MCAO and immediately after reperfusion (total dose injected=20 μg). Data are presented as mean ±s.d. of a group of animals (24 h: n=6 per group; 48 h: vehicle n=11, rhIL-1ra n=10). *P<0.05, **P<0.01 compared to appropriate vehicle-treated group (Student's t-test).
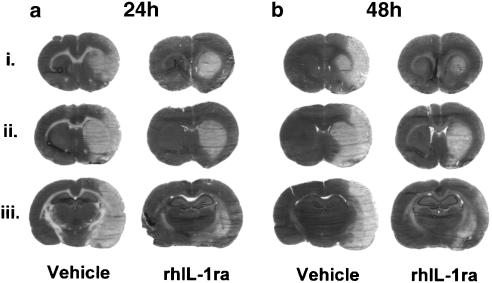
Figure 3.
Representative cresyl violet-stained brain sections showing the lesion 24 h (a) and 48 h (b) after transient (60 min) MCAO and injection of vehicle or rhIL-1ra (10 μg, i.c.v. immediately after MCAO and immediately after reperfusion). Each experimental group is represented by three sections taken from three different anterior–posterior levels (similar levels for each experimental group, approximately +1.4 mm (i), +0.7 mm (ii) and −2.3 mm (iii) relative to bregma). The pattern of injury in vehicle-treated animals was similar 24 and 48 h after MCAO. Damage was smaller in rhIL-1ra-treated animals, most notably in the cortex, and the pattern of damage was similar 24 and 48 h after MCAO.
Study 3: therapeutic time window for the neuroprotective effect of rhIL-1ra
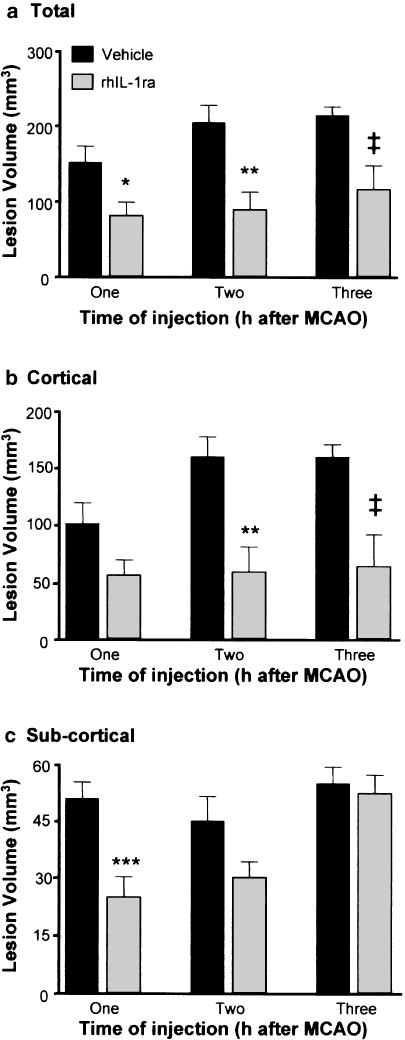
To characterise the therapeutic time window for rhIL-1ra, a single i.c.v. injection of rhIL-1ra (20 μg) or vehicle was administered, 1, 2 or 3 h after ischaemia (i.e. 0, 1 or 2 h after reperfusion). Damage was assessed histologically 24 h after MCAO in each of these three experiments, and the results are presented as the total (a), cortical (b) and subcortical (c, striatal and thalamic) lesion volumes (Figure 4). At all three time-points, rhIL-1ra caused a significant reduction of the total lesion volume (1 h: 47%; 2 h: 57%; 3 h: 46% versus respective vehicle-treated groups Figure 4a). The cortical lesion volume in rhIL-1ra-treated animals was similar in all three experiments (Figure 4b, 55, 58 and 61 mm3). However, the cortical lesion of animals injected with vehicle at 1 h was slightly smaller than for other vehicle-treated groups (151 versus 204 and 213 mm3 P=0.07, ANOVA). rhIL-1ra administered 1 h after MCAO caused a relatively small (45%) reduction of the cortical lesion that did not achieve statistical significance (P=0.06). At the later times of administration, rhIL-1ra significantly reduced the cortical lesion (2 h: 63%; 3 h: 60%, versus vehicle, Figure 4b). rhIL-1ra caused a significant reduction (51%) of the subcortical lesion when administered 1 h after ischaemia. Administration of rhIL-1ra at later time-points caused successively smaller reductions of the subcortical lesion (2 h: 33% and 3 h: 5%), which did not achieve statistical significance (P=0.05 and 0.66, respectively).
Figure 4.
Effect of delayed administration of rhIL-1ra on (a) total, (b) cortical and (c) subcortical lesion volume assessed 24 h after transient (60 min) middle cerebral artery occlusion. Animals were subjected to MCAO and injected (i.c.v.) with vehicle or rhIL-1ra (20 μg) either 1 h (vehicle n=10, rhIL-1ra n=10), 2 h (vehicle n=7, rhIL-1ra n=8) or 3 h (vehicle n=7, rhIL-1ra n=9) after MCAO. *P<0.05, **P<0.01 compared to vehicle (Student's t-test) ‡P<0.05 compared to vehicle (Student's t-test with Welch correction).
Discussion
The results reported here demonstrate that i.c.v. administration of rhIL-1ra significantly reduces cell death induced by temporary MCAO in the rat, and that rhIL-1ra is effective when administered 3 h after the induction of ischaemia.
The time course of development of injury induced by transient MCAO may vary depending on the experimental conditions (e.g. see Abraham et al., 2002). Thus, it is important to characterise the temporal development of damage, especially for the interpretation of delayed administration studies. We investigated the temporal progression of damage in cresyl violet-stained, frozen brain sections taken from animals killed at various times after MCAO. While cellular morphology is disrupted in frozen brains, this commonly used method enables the lesion to be delineated clearly from normal tissue. Lesions were detected in the striatum as early as 2 h after transient (60 min) MCAO in the rat (i.e. 1 h after reperfusion), and subcortical (striatal and thalamic) lesion development was complete within 6–8 h after MCAO. Cortical lesions developed more slowly, detected for the first time 6 h after MCAO, and were not complete until 18–24 h after MCAO. The more rapid development of neuronal death in the striatum is typical of MCAO damage, and is thought to occur because the cortex has a more extensive collateral blood supply than the striatum (Coyle & Jokelainen, 1982; Garcia et al., 1995a). Our subsequent study demonstrated no progression of the lesion volume between 24 and 48 h after MCAO (Figure 2 and Figure 3).
Peripheral injection of rhIL-1ra inhibits ischaemic brain damage (Relton et al., 1996); repeated dosing or constant infusion is, however, required due to the short half-life (approx. 3 min) of rhIL-1ra in the plasma (Kim et al., 1995). Therefore, due to the large doses of IL-1ra required for peripheral administration, to test the delayed neuroprotective effects of rhIL-1ra, we implanted guide cannulae into the brains of rats to permit the injection of rhIL-1ra into the lateral ventricles (Relton & Rothwell, 1992). Central injection of rhIL-1ra (10 μg i.c.v., at the time of MCAO and 60 min later) caused a significant reduction of lesion volume, and a similar protection was observed 24 h (57% reduction) and 48 h (52% reduction) after MCAO. Although it is possible that further progression of damage could occur after 48 h, our previous observations showing sustained protection up to 7 days after permanent MCAO, suggesting that this is unlikely (Garcia et al., 1995b; Loddick & Rothwell, 1996).
Our experiments do not directly address the efficacy of penetration of IL-1ra into brain tissue. However, previously published studies have reported that IL-1ra is actively transported across the blood–brain barrier in rodents (Gutierrez et al., 1994), and IL-1ra injected into the lateral ventricle rapidly diffuses from the CSF into the brain parenchyma (Konsman et al., 2000). In addition, our own unpublished studies have shown extensive penetration of biotin-labelled IL-1ra throughout the brain of normal rodents after i.c.v. administration.
Consistent with previous studies (Zhao et al., 1994; Li et al., 1999; Legos et al., 2002), we observed an increase in body temperature 1 h after transient MCAO. Changes in body temperature can influence ischaemic brain damage (Chen et al., 1991; Kim et al., 1996; Reglodi et al., 2000), and rhIL-1ra is antipyretic (Luheshi et al., 1996; Cartmell et al., 2001). However, in the present study rhIL-1ra had no effect on body temperature. A recent study reported that the increase in body temperature observed after transient (90 min) MCAO was evident only during ischaemia, and body temperature normalised on reperfusion (Legos et al., 2002). We have demonstrated previously that rhIL-1ra has no effect on the body temperature up to 24 h after permanent MCAO in the rat (Loddick &, Rothwell, 1996). Thus, it seems unlikely that the neuroprotective effects of rhIL-1ra are due to an effect on body temperature. Laser doppler flowmetry confirmed that all animals were subjected to a similar ischaemic insult.
To investigate the therapeutic time window for rhIL-1ra treatment, we tested the effect of a single injection of rhIL-1ra administered after MCAO. rhIL-1ra caused a significant reduction of the total lesion volume when injected 1, 2 or 3 h after MCAO. The reduction of the cortical lesion was similar when rhIL-1ra was injected 2 or 3 h after MCAO (63 and 60% reduction, respectively). The neuroprotective effect of rhIL-1ra in subcortical tissue was greatest when rhIL-1ra was injected 1 h after MCAO (51% reduction), with delayed treatment resulting in smaller, and not statistically significant reductions of the subcortical lesion (33 and 5% reduction for 2 and 3 h injection, respectively). Thus, whereas protection of subcortical tissue was significantly reduced as treatment was delayed, the degree of cortical protection elicited by rhIL-1ra was not impaired by delaying the injection until 3 h after MCAO. These data are consistent with the more rapid development of damage in subcortical tissue, and suggest an extended therapeutic time window for rhIL-1ra after ischaemia, where the lesion is complete by 18–24 h.
The amount of potentially salvageable tissue, and the duration of the potential therapeutic time window for neuroprotection in humans is difficult to determine and remains to be resolved. Nevertheless, it has been agreed that preclinical studies should investigate the therapeutic time window (Stroke Therapy Academic Industry Roundtable, 2001), and recent studies suggest that there is significant lesion expansion, and therefore possibly salvageable tissue, beyond 24 h after stroke in humans (Beaulieu et al., 1999; Touzani et al., 2001). Studies with tissue plasminogen activator have shown the feasibility of treating patients within 3 h of onset of symptoms, even when CT scans are required to exclude haemorrhage. Thus, the present finding that rhIL-1ra causes a significant reduction in brain damage when administered 3 h after ischaemia suggests that rhIL-1ra may have clinical potential for the treatment of stroke, and indeed IL-1ra is now in early clinical trials for acute stroke. These data have broad implications since rhIL-1ra is neuroprotective in experimental animals subjected to ischaemic, hypoxic, excitotoxic, traumatic and haemorrhagic brain injury.
Acknowledgments
We are grateful to Anthea Hughes and Sally Shepherd for technical assistance and to Dr Rosemary Gibson for critical discussion of the manuscript. This work was supported by the Medical Research Council, Research into Ageing, The Royal Society and Amgen.
Abbreviations
- CBF
cerebral blood flow
- i.c.v.
intracerebroventricular
- IL-1
interleukin-1
- IL-1ra
interleukin-1 receptor antagonist
- MCAO
middle cerebral artery occlusion
- STAIR
Stroke Therapy Academic Industry Roundtable
References
- ABRAHAM H., SOMOGYVARI-VIGH A., MADERDRUT J.L., VIGH S., ARIMURA A. Filament size influences temperature changes and brain damage following middle cerebral artery occlusion in rats. Exp. Brain. Res. 2002;142:131–138. doi: 10.1007/s00221-001-0909-4. [DOI] [PubMed] [Google Scholar]
- ALLAN S.M., ROTHWELL N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- BEAULIEU C., DE CRESPIGNY A., TONG D.C., MOSELEY M.E., ALBERS G.W., MARKS M.P. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann. Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- BOUTIN H., LEFEUVRE R.A., HORAI R., ASANO M., IWAKURA Y., ROTHWELL N.J. Role of IL-1alpha and IL-1beta in ischemic brain damage. J. Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALABRESE L.H. Anakinra treatment of patients with rheumatoid arthritis. Ann. Pharmacother. 2002;36:1204–1209. doi: 10.1345/aph.1A396. [DOI] [PubMed] [Google Scholar]
- CARTMELL T., LUHESHI G.N., HOPKINS S.J., ROTHWELL N.J., POOLE S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J. Physiol. 2001;531:171–180. doi: 10.1111/j.1469-7793.2001.0171j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN H., CHOPP M., WELCH K.M. Effect of mild hyperthermia on the ischemic infarct volume after middle cerebral artýry occlusion in the rat. Neurology. 1991;41:1133–1135. doi: 10.1212/wnl.41.7.1133. [DOI] [PubMed] [Google Scholar]
- COYLE P., JOKELAINEN P.T. Dorsal cerebral arterial collaterals of the rat. Anat. Res. 1982;203:397–404. doi: 10.1002/ar.1092030309. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A., THOMPSON R.C. Blocking IL-1: interleukin-1 receptor antagonist in vivo and in vitro. Immunol. Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- DIRNAGL U., IADECOLA C., MOSKOWITZ M.A. Pathobiology of ischaemic stroke: an integrated view. Trend Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- GARCIA J.H., LIU K.F., HO K.L. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995a;26:636–643. doi: 10.1161/01.str.26.4.636. [DOI] [PubMed] [Google Scholar]
- GARCIA J.H., LIU K.F., RELTON J.K. Interleukin-1 receptor antagonist decreases the number of necrotic neurons in rats with middle cerebral artery occlusion. Am. J. Pathol. 1995b;147:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- GROTTA J. Neuroprotection is unlikely to be effective in humans using current trial designs. Stroke. 2002;33:306–307. [PubMed] [Google Scholar]
- GUTIERREZ E.G., BANKS W.A., KASTIN A.J. Blood-borne interieukin-1 receptor antagonist crosses the blood–brain barrier. J. Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- KIM D.C., REITZ B., CARMICHAEL D.F., BLOEDOW D.C. Kidney as a major clearance organ for recombinant human interleukin-1 receptor antagonist. J. Pharm. Sci. 1995;84:575–580. doi: 10.1002/jps.2600840511. [DOI] [PubMed] [Google Scholar]
- KIM Y., BUSTO R., DIETRICH W.D., KRAYDIEH S., GINSBERG M.D. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke. 1996;27:2274–2280. doi: 10.1161/01.str.27.12.2274. [DOI] [PubMed] [Google Scholar]
- KONSMAN J.P., TRIDON V., DANTZER R. Diffusion and action of intracerebroventricularly injected interleukin-1 in the CNS. Neuroscience. 2000;101:957–967. doi: 10.1016/s0306-4522(00)00403-6. [DOI] [PubMed] [Google Scholar]
- LAWRENCE C.B., ALLAN S.M., ROTHWELL N.J. Interleukin-1beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur. J. Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- LEES K.R. Neuroprotection is unlikely to be effective in humans using current trial designs: an opposing view. Stroke. 2002;33:308–309. [PubMed] [Google Scholar]
- LEGOS J.J., MANGONI A.A., READ S.J., CAMPBELL C.A., IRVING E.A., ROBERTS J., BARONE F.C., PARSONS A.A. Programmable microchip monitoring of post-stroke pyrexia: effects of aspirin and paracetamol on temperature and infarct size in the rat. J. Neurosci. Methods. 2002;113:159–166. doi: 10.1016/s0165-0270(01)00488-5. [DOI] [PubMed] [Google Scholar]
- LI F., OMAE T., FISHER M. Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke. 1999;30:2464–2470. doi: 10.1161/01.str.30.11.2464. [DOI] [PubMed] [Google Scholar]
- LODDICK S.A., ROTHWELL N.J. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J. Cereb. Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- LONGA E.Z., WEINSTEIN P.R., CARLSON S., CUMMINS R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- LUHESHI G., MILLER A.J., BROUWER S., DASCOMBE M.J., ROTHWELL N.J., HOPKINS S.J. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am. J. Physiol. 1996;270:E91–E95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- OPAL S.M., FISHER C.J., JR, DHAINAUT J.F., VINCENT J.L., BRASE R., LOWRY S.F., SADOFF J.C., SLOTMAN G.J., LEVY H., BALK R.A., SHELLY M.P., PRIBBLE J.P., LABRECQUE J.F., LOOKABAUGH J., DONOVAN H., DUBIN H., BAUGHMAN R., NORMAN J., DEMARIA E., MATZEL K., ABRAHAM E., SENEFF M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1, Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- REGLODI D., SOMOGYVARI-VIGH A., MADERDRUT J.L., VIGH S., ARIMURA A. Postischemic spontaneous hyperthermia and its effects in middle cerebral artery occlusion in the rat. Exp. Neurol. 2000;163:399–407. doi: 10.1006/exnr.2000.7367. [DOI] [PubMed] [Google Scholar]
- RELTON J.K., MARTIN D., THOMPSON R.C., RUSSELL D.A. Peripheral administration of Interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp. Neurol. 1996;138:206–213. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- RELTON J.K., ROTHWELL N.J. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res. Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- SCHIELKE G.P., YANG G.Y., BETZ A.L. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J. Cereb. Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- SCHWETZ B.A. From the Food and Drug Administration (Rhematoid Arthritis Treatment) JAMA. 2002;287:33. [PubMed] [Google Scholar]
- STROEMER R.P., ROTHWELL N.J. Exacerbation of ischemic brain damage by localized striatal injection of interleukin-1beta in the rat. J. Cereb. Blood Flow Metab. 1998;18:833–839. doi: 10.1097/00004647-199808000-00003. [DOI] [PubMed] [Google Scholar]
- STROKE THERAPY ACADEMIC INDUSTRY ROUNDTABLE Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- STROKE THERAPY ACADEMIC INDUSTRY ROUNDTABLE Recommendations for clinical trial evaluation of acute stroke therapies. Stroke. 2001;3:1598–1606. doi: 10.1161/01.str.32.7.1598. [DOI] [PubMed] [Google Scholar]
- SWANSON R.A., MORTON M.T., TSAO-WU G., SAVALOS R.A., DAVIDSON C., SHARP F.R. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- TOUZANI O., BOUTIN H., CHUQUET J., ROTHWELL N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J. Neuroimmunol. 1999;100:203–215. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- TOUZANI O., ROUSSEL S., MACKENZIE E.T. The ischaemic penumbra. Curr. Opin. Neurol. 2001;14:83–88. doi: 10.1097/00019052-200102000-00013. [DOI] [PubMed] [Google Scholar]
- YAMASAKI Y., MATSUURA N., SHOZUHARA H., ONODERA H., ITOYAMA Y., KOGURE K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- ZHAO Q., MEMEZAWA H., SMITH M.L., SIESJÖ B.K. Hyperthermia complicates middle cerebral artery occlusion induced by an intraluminal filament. Brain Res. 1994;649:253–259. doi: 10.1016/0006-8993(94)91071-5. [DOI] [PubMed] [Google Scholar]