Abstract
Δ9-Tetrahydrocannabinol (THC), the main psychoactive ingredient of marijuana, induces apoptosis in cultured cortical neurons. THC exerts its apoptotic effects in cortical neurons by binding to the CB1 cannabinoid receptor. The CB1 receptor has been shown to couple to the stress-activated protein kinase, c-Jun N-terminal kinase (JNK). However, the involvement of specific JNK isoforms in the neurotoxic properties of THC remains to be established.
The present study involved treatment of rat cultured cortical neurons with THC (0.005–50 μM), and combinations of THC with the CB1 receptor antagonist, AM 251 (10 μM) and pertussis toxin (PTX; 200 ng ml−1). Antisense oligonucleotides (AS) were used to deplete neurons of JNK1 and JNK2 in order to elucidate their respective roles in THC signalling.
Here we report that THC induces the activation of JNK via the CB1 receptor and its associated G-protein, Gi/o. Treatment of cultured cortical neurons with THC resulted in a differential timeframe of activation of the JNK1 and JNK2 isoforms.
Use of specific JNK1 and JNK2 AS identified activation of caspase-3 and DNA fragmentation as downstream consequences of JNK1 and JNK2 activation.
The results from this study demonstrate that activation of the CB1 receptor induces JNK and caspase-3 activation, an increase in Bax expression and DNA fragmentation. The data demonstrate that the activation of both JNK1 and JNK2 isoforms is central to the THC-induced activation of the apoptotic pathway in cortical neurons.
Keywords: Δ9-Tetrahydrocannabinol, antisense, c-Jun N-terminal kinase, apoptosis, caspase-3, bax
Introduction
Δ9-Tetrahydrocannabinol (THC), the predominant psychoactive ingredient in preparations of marijuana (Cannabis saliva), exerts a broad spectrum of central effects, such as alterations in cognition (Howlett, 1990) and impairment of short-term memory consolidation (Abood & Martin, 1992). THC exerts these effects by binding to G-protein-coupled receptors (Howlett, 1995). While two different subtypes of cannabinoid receptor have been characterised from mammalian tissues, CB1 (Matsuda et al., 1990) and CB2 (Munro et al., 1993), the CB1 receptor is associated with brain regions responsible for mediating the psychoactive effects of THC, such as the cortex and hippocampus (Twitchell et al., 1997; Tsou et al., 1998).
At a cellular level, THC has been shown to modulate a number of neuronal functions, including ion channel activity (Turkanis et al., 1991; Mackie et al., 1995; Twitchell et al., 1997) and neurotransmitter release (Shen et al., 1996; Gessa et al., 1998; Jentsch et al., 1998), and there is increasing evidence suggesting a role for THC in the regulation of neuronal viability. THC has a toxic effect on cultured hippocampal neurons (Chan et al., 1998), glioma cells (Sanchez et al., 1998) and cortical neurons (Campbell, 2001), and inhibits glioma cell growth in vivo (Galve-Roperh et al., 2000). In contrast, there is also evidence that THC and other cannabinoids act as neuroprotectants in certain systems. Cannabinoid receptor agonists are neuroprotective in excitotoxic cell death in vivo (Panikashvili et al., 2001; van der Stelt et al., 2001) and in vitro (Shen & Thayer, 1998; Abood et al., 2001), which is dependent on CB1 receptor activation. Furthermore, cannabinoids have been shown to protect cultured neurons against glutamate-induced toxicity (Hampson et al., 1998) and in vitro hypoxia and glucose deprivation (Nagayama et al., 1999). However, the precise role of the cannabinoid receptors in regulating neuronal viability is poorly understood, although some of the in vitro protective effects of cannabinoids (Hampson et al., 1998; Nagayama et al., 1999) are receptor-independent while the neurotoxic effects of THC on cortical (Campbell, 2001) and hippocampal (Chan et al., 1998) cultures are blocked by CB1 receptor antagonists.
We have recently reported that THC activates the caspase-3 cell death pathway in cultured cortical neurons, resulting in DNA fragmentation and programmed cell death (Campbell, 2001). Furthermore, we have shown that the activation of the central CB1 cannabinoid receptor is vital in the execution of this cell death cascade (Downer et al., 2001). It has been established that the CB1 receptor couples to several signal transduction pathways (Dill & Howlett, 1988; Bouaboula et al., 1995; Bouaboula et al., 1996; Sanchez et al., 1998; Gomez del Pulgar et al., 2000; Derkinderen et al., 2001) including the stress-activated protein kinase pathway (Rueda et al., 2000). While there is growing evidence that the c-Jun N-terminal kinase (JNK) pathway is central to cell death in the nervous system (Mielke & Herdegen, 2000), the role of JNK in the CB1 receptor-dependent induction of neuronal apoptosis remains to be established. The present work was therefore undertaken to examine the nature of the link between the CB1 receptor, activation of JNK and downstream apoptotic consequences in cultured cortical neurons. Furthermore, three JNK isoforms, JNK1, JNK2 and JNK3, have been identified in the mammalian brain (Gupta et al., 1996), and current commercially available JNK inhibitors are unable to distinguish between JNK1-, JNK2- and JNK3-mediated effects. We therefore treated neurons with specific antisense oligonucleotides (AS) that target rat JNK1 or JNK2 mRNA in order to investigate the upstream roles of these JNK isoforms in the THC-induced activation of caspase-3 and subsequent DNA fragmentation.
Methods
Culture of cortical neurons
Primary cortical neurons were prepared from 1-day-old Wistar rats and maintained in neurobasal medium (Gibco BRL, Paisley, U.K.). Rats were decapitated, the cerebral cortices dissected and the meninges removed. The cortices were incubated in phosphate-buffered saline (PBS) with trypsin (0.25 μg ml−1) for 25 min at 37°C. The cortical tissue was then triturated (× 5) in PBS containing soyabean trypsin inhibitor (0.2 μg ml−1) and DNAse (0.2 mg ml−1) and gently filtered through a sterile mesh filter (40 μm). The suspension was centrifuged at 2000 × g for 3 min at 20°C and the pellet resuspended in warm neurobasal medium, supplemented with heat-inactivated horse serum (10%), penicillin (100 U ml−1), streptomycin (100 U ml−1) and glutamax (2 mM). Suspended cells were plated at a density of 0.25 × 106 cells on circular 10 mm diameter coverslips, coated with poly-L-lysine (60 μg ml−1), and incubated in a humidified atmosphere containing 5% CO2 : 95% air at 37°C for 2 h prior to being flooded with prewarmed neurobasal medium. After 48 h, 5 ng ml−1 cytosine-arabino-furanoside was added to the culture medium to suppress the proliferation of non-neuronal cells and maintain the purity of the cortical neuronal culture. This ensures that microglia and astrocyte contamination is less than 5% in culture preparations. Medium was exchanged for fresh medium every 3 days and cells were grown in culture for up to 14 days.
Oligonucleotide treatment
JNK antisense (AS) and scrambled control (SC) phosphorothioated oligonucleotides were synthesized by Biognostik (Gottingen, Germany). The sequences used were according to previously published work (Hreniuk et al., 2001) and are complementary to the mRNA encoding rat JNK1 and JNK2 protein: JNK1 AS, 5′-CTCATGATGGCAAGCAATTA-3′; JNK1 SC, 5′-ACTACTACACTAGACTAC-3′; JNK2 AS, 5′-GCTCAGTGGACATGGATGAG-3′ and JNK2 SC, 5′-GGACTACTACACTAGACTAC-3′. Neurons were maintained in supplemented media in the presence of the oligonucleotides for 48 h prior to treatment with THC. N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium/dioleoyl-phosphatidylethanolamine (DOTMA/DOPE 5 μg ml−1; Life Technologies, Paisley, U.K.) was incorporated into serum-free media for the first 4 h of oligonucleotide treatment to ensure sufficient oligonucleotide uptake. Downregulation of JNK1 and JNK2 protein expression was achieved by using JNK1 AS and JNK2 AS at a final concentration of 2 μM.
Terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labelling (TUNEL)
Apoptotic cell death was determined in cultures using TUNEL staining according to the manufacturer's instructions (DeadEnd Colorimetric Apoptosis Detection System; Promega Corporation, Madison, MD, U.S.A.). Following treatment with THC (5 μM) for 3 h, cells were fixed with paraformaldehyde (4%) for 30 min at room temperature. Neurons were permeabilised with Triton X-100 (0.2%) and Proteinase K (0.2%) and biotinylated nucleotide was incorporated at the 3′-OH DNA ends using the enzyme terminal deoxynucleotidyl transferase (TdT). Endogenous peroxidases were blocked using 0.3% H2O2, and horseradish-peroxidase-labelled streptavidin was bound to the biotinylated nucleotides. Apoptotic cells (TUNEL-positive) were detected using diaminobenzidine, a stable chromagen that stains the apoptotic nuclei of TUNEL-positive cells dark brown. After mounting coverslips on slides, the neurons were viewed under light microscopy at × 100 magnification. Cells displaying apoptotic nuclei were counted and expressed as a percentage of the total number of cells examined (400–500 cells coverslip−1).
Western blot analysis
Cortical neurons were harvested in lysis buffer (25 mM HEPES, 5 mM MgCl2, 5 mM dithiothreitol, 5 mM EDTA, 2 mM PMSF, 5 μg ml−1 leupeptin, 5 μg ml−1 pepstatin, 5 μg ml−1 aprotinin, pH 7.4) and homogenised on ice. Lysates were then centrifuged (13,000 × g for 15 min at 4°C) and the supernatant containing the cytosolic fraction was prepared for SDS–polyacrylamide gel electrophoresis. The cytosolic fraction was diluted to 50 μg ml−1 protein concentration with sodium dodecyl sulphate (SDS) sample buffer (150 mM Tris-HCl pH 7.4, 10% w v−1 glycerol, 4% w v−1 SDS, 5% v v−1 β-mercaptoethanol, 0.002% w v−1 bromophenol blue) and samples heated to 100°C for 5 min. Samples were separated by electrophoresis on 10 and 12% polyacrylamide minigels and cytosolic proteins were transferred from the gel onto nitrocellulose membrane (Sigma, U.K.). The blots were blocked with 2% BSA for 1 h at 37°C and then incubated with the primary antibody overnight at 4°C. The blots were washed thoroughly with Tris-buffered saline (TBS) containing 0.05% Tween and immunoreactive bands detected using horseradish-peroxidase-conjugated anti-mouse IgG (Sigma, U.K.) or anti-rabbit IgG (Sigma, U.K.) and an enhanced chemiluminescence (ECL) system (Amersham, U.K.). Primary antibodies used were: monoclonal JNK1 and JNK2 antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, U.S.A.) purified from mouse serum that recognise JNK1 (46 kDa) and JNK2 (54 kDa) protein expression; monoclonal anti-phospho-specific JNK antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, U.S.A.) purified from mouse serum that recognises phosphorylated JNK1, JNK2 and JNK3 isoforms; polyclonal Bax antibody (DAKO Corporation, Carpinteria, CA, U.S.A.) purified from rabbit serum that recognises amino acids 43–61 of human Bax. Molecular weight markers were used to confirm the molecular weight of protein bands. Bandwidths were quantified using densitometric analysis (D-Scan PC software).
Measurement of caspase-3 activity
Cleavage of the fluorogenic caspase-3 substrate (Ac-DEVD-7-amino-4-trifluoromethylcoumarin peptide (AFC); Alexis Corporation, U.S.A.) to its fluorescent product was used as a measure of caspase-3 activity. Following treatment with THC (5 μM) for 2 h, cells were harvested in lysis buffer (25 mM HEPES, 5 mM MgCl2, 5 mM dithiothreitol, 5 mM EDTA, 2 mM PMSF, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin, 10 μg ml−1 aprotinin, pH 7.4) and homogenised on ice. Samples of supernatant (50 μl) were incubated with the DEVD peptide (10 μM; 4 μl) or incubation buffer (50 μl; 50 mM HEPES, 10 mM dithiothreitol, 20% w v−1 glycerol, pH 7.4) for 1 h at 37°C and the fluorescence assessed (excitation, 400 nm; emission, 505 nm) using a Fluoroscan Ascent FL plate reader (MSC Medical Supply Co. Ltd, U.K.).
Drug treatment
THC was obtained from Sigma-Aldrich Company Ltd (Dorset, U.K.). The drug was initially dissolved in methanol and stored at −20°C as an 80 mM stock solution. For use, the stock drug was diluted to a final concentration of 5 μM in prewarmed culture media and 0.007% methanol was used as vehicle control. The selective CB1 receptor antagonist AM 251 (Tocris Cookson Ltd, Bristol, U.K.) was stored as a 10 mM stock solution in dimethylsulphoxide (DMSO) and diluted to a final concentration of 10 μM in warm culture media for addition to cultures; 0.0001% DMSO was used as a vehicle control for AM 251. Neurons were preincubated with AM 251 for 30 min before treating with THC. Pertussis toxin (PTX; Sigma, Dorset, U.K.) was reconstituted in PBS and neurons were incubated with 200 ng ml−1 PTX for 24 h prior to THC treatment.
Statistical analysis
Data are reported as the mean±s.e.m. of the number of experiments indicated in every case. Statistical analysis was performed by Student's t-tests and a one-way ANOVA. A post hoc analysis was made by the Student–Neumann–Keuls test. Our criterion for significance was P<0.05. Extreme significance differences were expressed by probability values of P<0.01 and P<0.001.
Results
THC activates JNK1 and JNK2 isoforms within a differential timeframe
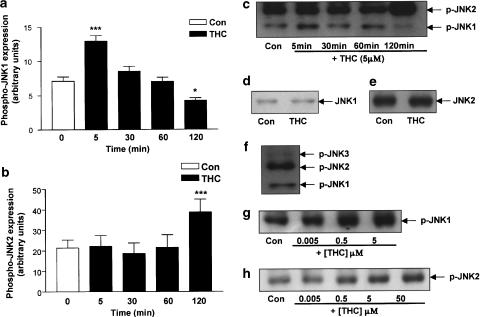
Exposure of cultured cortical neurons to THC (5 μM) resulted in the activation of the JNK protein within the cytosol in a time-dependent manner (Figure 1). Cytosolic expression levels of the phosphorylated (active) forms of JNK1 and JNK2 were measured by Western immunoblot and bandwidths were quantified using densitometry. Interestingly, there was a differential timeframe of activation for JNK1 and JNK2. In terms of the JNK1 time course of activation (Figure 1a), in control cells phospho-JNK1 expression was 7.28±0.80 (arbitrary units; mean bandwidth±s.e.m.) and this was significantly increased to 13.41±1.63 following treatment with THC for 5 min (P<0.001, ANOVA; n=7). However, no change in phospho-JNK1 expression was observed at subsequent time points, and phospho-JNK1 expression was in fact significantly reduced to 4.21±0.40 following treatment with THC for 2 h (P<0.05, ANOVA, n=7; Figure 1a).
Figure 1.
Time course of THC-induced activation of JNK. Cortical neurons were exposed to THC for 5–120 min, then cells were harvested and the cytosolic fractions analysed for the expression levels of the phosphorylated active forms of JNK 1 and JNK2 using Western immunoblot. (a) A significant increase in phospho-JNK1 expression was found following treatment with THC (5 μM) for 5 min. Exposure to THC for 30 and 60 min had no effect on phospho-JNK1 expression. However, treatment with THC for 120 min significantly decreased phospho-JNK1 expression. Results are expressed as mean±s.e.m. for seven observations, ***P<0.001, *P<0.05. (b) Phospho-JNK2 expression was significantly increased when neurons were treated with THC (5 μM) for 120 min. Exposure to THC for 5, 30 and 60 min had no effect on phospho-JNK2 expression. Results are expressed as mean±s.e.m. for seven observations, ***P<0.001. (c) A sample Western immunoblot demonstrating the increase in phospho-JNK1 expression at 5 min of THC treatment and the corresponding increase in phospho-JNK2 expression following treatment with THC for 120 min. (d) Neurons were treated with THC (5 μM) for 5 min, then cells were harvested in lysis buffer and the cytosolic fractions analysed for total JNK1 protein expression using Western immunoblot. THC had no effect on JNK1 protein expression at 5 min. Inset: A sample Western immunoblot showing that THC treatment for 5 min had no effect on JNK1 protein expression as compared to vehicle-treated neurons. (e) Cortical neurons were treated with THC (5 μM) for 2 h, then cells were harvested and analysed for total JNK2 protein expression by Western blot analysis. Incubation of cells with THC for 2 h did not affect JNK2 protein expression. Inset: A sample Western immunoblot demonstrating total JNK2 protein expression in vehicle-treated neurons and neurons treated with THC for 2 h. (f) Untreated cortical neurons were harvested and analysed for phosphorylated JNK1, JNK2 and JNK3 expression. JNK3 expression was moderate compared to JNK1/2 expression. (g) A sample Western immunoblot showing that a 5 min treatment with THC increases phospho-JNK1 expression in a dose-dependent manner. Similar results were observed in five separate experiments. (h) A sample Western immunoblot showing that a 120 min treatment with THC increases phospho-JNK2 expression in a dose-dependent manner. Similar results were observed in five separate experiments.
In contrast, Figure 1b demonstrates that expression of phospho-JNK2 in control neurons was 21.44±3.89 (arbitrary units; mean bandwidth±s.e.m.) and this was significantly increased to 38.79±6.25 when neurons were cultured in media containing THC (5 μM) for 2 h (P<0.001, ANOVA; n=7). At earlier time points, treatment with THC had no effect on phospho-JNK2 expression. A sample immunoblot demonstrating the THC-induced activation of JNK1 at 5 min and JNK2 at 2 h is shown in Figure 1c.
Total JNK1 and JNK2 expression was unaffected by THC (5 μM; sample immunoblots shown). In vehicle-treated cells, JNK1 total protein expression was 46.53±1.15 (arbitrary units; mean bandwidth±s.e.m.) and exposure of neurons to THC for 5 min had no effect on total JNK1 expression (46.92±2.66, n=5; Figure 1d). In neurons treated with vehicle alone for 2 h, mean total JNK2 expression was 56.32±7.31 and this was unaffected by exposure to THC for 2 h (56.26±9.09; n=5; Figure 1e). Thus, the increase in expression of phosphorylated JNK1 (Figure 1a) and phosphorylated JNK2 (Figure 1b) is not attributable to a THC-induced increase in total JNK protein expression. Furthermore, in our cell culture model, JNK3 expression was very moderate (Figure 1f), hence the relative contribution of JNK1 and JNK2 to the apoptotic cascade was assessed.
Since we have previously shown that 5 μM is the concentration of THC required to induce maximal neuronal degeneration in a CB1 receptor-dependent manner (Downer et al., 2001), we performed a dose–response analysis of THC-induced JNK activation. Figure 1(g and h) represents sample immunoblots which demonstrate that treatment of cells with THC (0.005–50 μM) evoked a dose-dependent increase in phospho-JNK1 (Figure 1g) and phospho-JNK2 (Figure 1h) expression at 5 and 120 min, respectively.
AM 251 pretreatment prevents THC-induced JNK activation
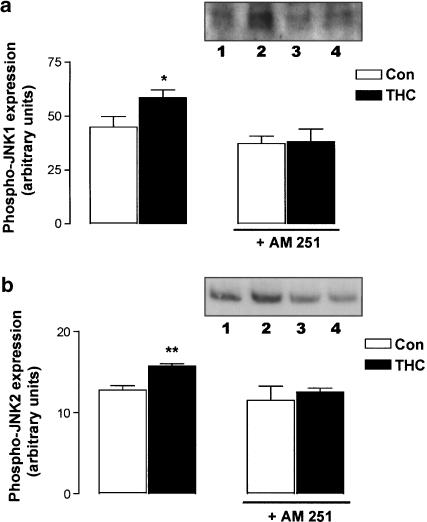
To determine whether THC-induced JNK activation was dependent on the CB1 receptor, we employed the use of the selective CB1 receptor antagonist AM 251 (10 μM). Figure 2 demonstrates that AM 251 prevented the THC-induced activation of JNK1 and JNK2. Thus, in neurons treated with vehicle for 5 min, phosphorylated JNK1 expression was 44.95±4.86 (arbitrary units; mean bandwidth±s.e.m.) and this was significantly increased following THC treatment (5 μM; 5 min) to 58.67±3.59 (P<0.05, ANOVA, n=6; Figure 2a). While pretreatment with AM 251 alone had no effect on phosphorylated JNK1 expression (37.35±3.43), it prevented the THC-induced increase in phospho-JNK1 expression (38.14±5.99). Although it has been shown that AM 251 behaves as an inverse agonist (New & Wong, 2003), we were unable to detect any effect of AM 251 on basal JNK activity (Figure 2).
Figure 2.
THC-induced activation of JNK is mediated by the CB1 receptor. (a) Treatment of neurons with THC (5 μM) for 5 min evoked a significant increase in phospho-JNK1 expression. Preincubation of cortical neurons with the CB1 receptor antagonist AM 251 (10 μM) for 30 min abolished the THC-induced increase in phospho-JNK1 expression. Results are expressed as mean±s.e.m. for six observations, *P<0.05. Inset: A sample Western immunoblot demonstrating the increase in phospho-JNK1 expression after 5 min of THC treatment (lane 2) compared to control cells (lane 1). While AM 251 had no effect on phospho-JNK1 expression (lane 3), it abolished the ability of THC to increase phospho-JNK1 expression (lane 4). (b) Treatment of neurons with THC (5 μM) for 120 min evoked a significant increase in phospho-JNK2 expression. Preincubation of cortical neurons with the CB1 receptor antagonist AM 251 (10 μM) for 30 min abolished the THC-induced increase in phospho-JNK2 expression. Results are expressed as mean±s.e.m. for six observations, **P<0.01. Inset: A sample Western immunoblot demonstrating the increase in phospho-JNK2 expression after 5 min of THC treatment (lane 2) compared to control cells (lane 1). While AM 251 had no effect on phospho-JNK2 expression (lane 3), it abolished the ability of THC to increase phospho-JNK2 expression (lane 4).
Figure 2b demonstrates that phosphorylated JNK2 expression was significantly increased from 12.83±0.54 (arbitrary units; mean bandwidth±s.e.m.) to 15.72±0.34 following treatment with THC (5 μM) for 2 h (P<0.01, Student's t-test, n=6). Exposure to AM 251 (10 μM) alone had no effect on basal phosphorylated JNK2 expression (11.55±1.75) and it prevented the THC-induced increase in phosphorylated JNK2 expression (12.55±0.52; Figure 2b). Sample immunoblots demonstrating the activation of JNK1 and JNK2 following THC treatment, and the abolition of these effects in AM 251-treated cells are shown as insets in Figure 2a and b. This result provides evidence of a role for the CB1 cannabinoid receptor in the THC-induced activation of both JNK1 and JNK2.
PTX abrogates THC-induced JNK1/2 activation
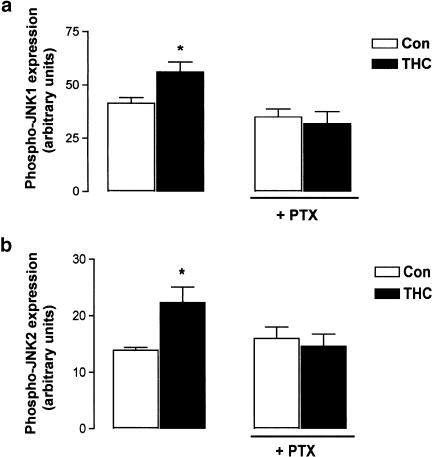
The central CB1 cannabinoid receptor is coupled to Gi/o-proteins (Howlett, 1995). The involvement of Gi/o in coupling the CB1 receptor to JNK activation in this system was assessed using PTX (200 ng ml−1). As shown in Figure 3a, PTX pretreatment prior to THC (5 μM) treatment for 5 min (31.92±5.59 arbitrary units; mean bandwidth±s.e.m.) prevented THC-induced JNK1 activation at 5 min (55.98±4.74; P<0.05, ANOVA, n=5). Incubation of neurons with methanol vehicle for 5 min (41.44±2.72) or PTX alone for 24 h (35.06±3.71) had no effect on JNK1 activity (Figure 3a).
Figure 3.
THC-induced JNK activation is mediated by G-protein subtypes Gi/o. (a) THC (5 μM; 5 min) significantly increased phospho-JNK1 expression and this was prevented by pretreatment with PTX (200 ng ml−1; 24 h). Results are expressed as mean±s.e.m. for five observations, *P<0.05. (b) THC (5 μM; 120 min) significantly increased phospho-JNK2 expression and this was prevented by pretreatment with PTX (200 ng ml−1; 24 h). Results are expressed as mean±s.e.m. for five observations, *P<0.05.
Similarly, Figure 3b demonstrates that phospho-JNK2 expression was significantly increased from 13.90±0.54 to 22.34±2.75 (arbitrary units; mean bandwidth±s.e.m.) following treatment with THC (5 μM) for 2 h (P<0.05, ANOVA, n=5). Treatment with PTX (200 ng ml−1) alone had no effect on phosphorylated JNK2 expression (16.05±1.99), but it prevented the THC-induced increase in JNK2 activity at 2 h (14.65±2.12; Figure 3b). These data suggest that the coupling of the CB1 receptor to G-protein subtypes Gi/o facilitates THC-induced JNK1 and JNK2 activation.
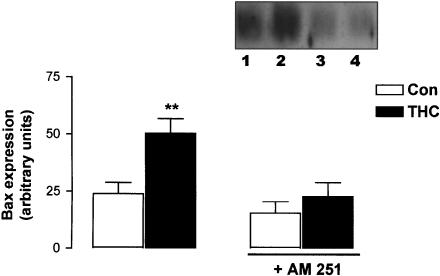
AM 251 prevents THC-induced Bax expression
JNK kinases have been linked to neuronal apoptosis by altering the expression of the Bcl family of mitochondrial-associated proteins (Mielke & Herdegen, 2000). In this study, expression levels of the proapoptotic protein Bax were measured by Western immunoblot in cells treated with THC (5 μM) for 2 h and the effect of the CB1 antagonist AM 251 (10 μM) was assessed. Figure 4 demonstrates that Bax expression in control cells was 23.82±4.89 (arbitrary units; mean±s.e.m.) and this was significantly increased following THC treatment for 2 h to 50.18±6.43 (P<0.01, ANOVA, n=5). AM 251 alone had no effect on Bax expression (15.39±4.96); however, it prevented the THC-induced increase in Bax expression (22.67±6.01). A sample Western immunoblot demonstrating the CB1-dependent increase in Bax expression is shown as an inset in Figure 4.
Figure 4.
THC increases Bax expression via the CB1 receptor. Neurons were preincubated with AM 251 (10 μM) for 30 min, treated with THC (5 μM) for 2 h and analysed for the expression levels of Bax using Western immunoblotting. THC significantly increased Bax expression and this was abolished by AM 251. Results are expressed as mean±s.e.m. for five observations, **P<0.01. Inset: A sample Western immunoblot showing that THC increases Bax expression (lane 2) compared to vehicle-treated neurons (lane 1). The stimulatory effect of THC on Bax expression was prevented by AM 251 (lane 4). Exposure of cells to AM 251 alone had no effect on Bax expression (lane 3).
JNK AS downregulates JNK protein expression
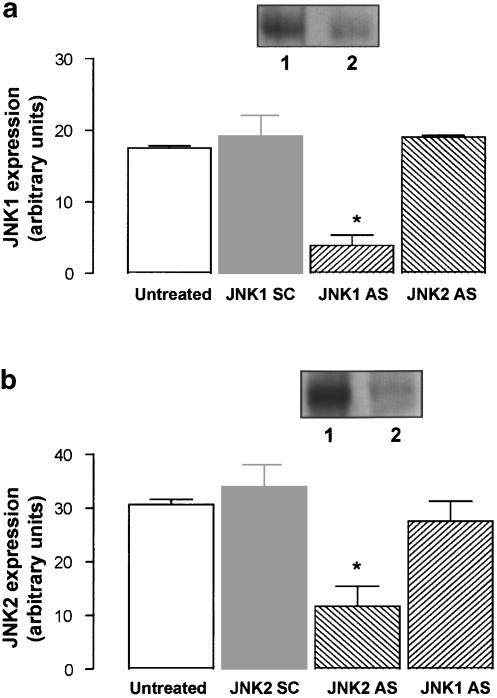
Since JNK activation was observed following THC treatment, we employed the use of antisense technology to analyse whether JNK has an upstream role in the regulation of apoptotic effectors. Cells were exposed to 2 μM of either JNK1 AS or JNK2 AS to downregulate JNK1 and JNK2 protein expression in order to delineate the specific role of each isoform in the cell death pathway. Upon treatment with JNK1 and JNK2 AS, cellular levels of JNK1 and JNK2 protein were markedly reduced (Figure 5a, b). In cells treated with JNK1 SC, JNK1 expression was 19.20±2.89 (arbitrary units; mean±s.e.m.) and this was significantly decreased by 79% when JNK1 AS was incorporated into the media for 48 h (P<0.05, Student's t-test, n=4; Figure 5a). Similarly, JNK2 AS significantly decreased JNK2 protein expression by 70%, compared to the cells treated with JNK2 SC for 48 h (P<0.05, Student's t-test, n=5; Figure 5b). The antisense-mediated depletion of JNK1 and JNK2 was specific since JNK2 AS had no effect on JNK1 protein expression (Figure 5a) and, similarly, JNK1 AS had no effect on JNK2 protein expression (Figure 5b). Sample immunoblots demonstrating the antisense-mediated downregulation of JNK1 and JNK2 protein expression are shown as insets in Figure 5a and b.
Figure 5.
JNK AS effectively reduces JNK protein expression. Neurons were incubated with 2 μM JNK1/JNK2 antisense (AS) or JNK1/JNK2 scrambled control (SC) for 48 h. Cells were harvested in lysis buffer and the total expression of JNK1 and JNK2 protein was examined by Western blot analysis. (a) Treatment with JNK1 AS significantly decreased JNK1 protein expression in comparison to untreated cells and cells treated with JNK1 SC. JNK2 AS had no effect on JNK1 protein expression. Results are expressed as mean±s.e.m. for four experiments, *P<0.05. Inset: A sample immunoblot demonstrating that JNK1 AS significantly reduces JNK1 protein expression (lane 2) compared to JNK1 SC-treated neurons (lane 1). (b) JNK2 AS significantly reduced JNK2 protein expression. JNK1 AS had no effect on JNK2 protein expression. Results are expressed as mean±s.e.m. for four experiments, *P<0.05. Inset: A sample immunoblot demonstrating the significant reduction in JNK2 protein expression in JNK2 AS-treated neurons (lane 2) compared to JNK2 SC-treated cells (lane 1).
JNK AS prevents THC-induced caspase-3 activation
Apoptotic cell death is typically accompanied by the activation of the cysteine protease caspase-3 (Janicke et al., 1998). Our lab has previously shown the pivotal role of this enzyme in the cell death pathway triggered by THC within the rat cortex (Campbell, 2001; Downer et al., 2001). Here we analysed whether JNK1 or JNK2 is involved in the THC-induced activation of caspase-3. Following downregulation of each of the JNK isoforms using appropriate AS, the role of JNK1 and JNK2 in THC-induced caspase-3 activation was assessed (Figure 6a, b).
Figure 6.
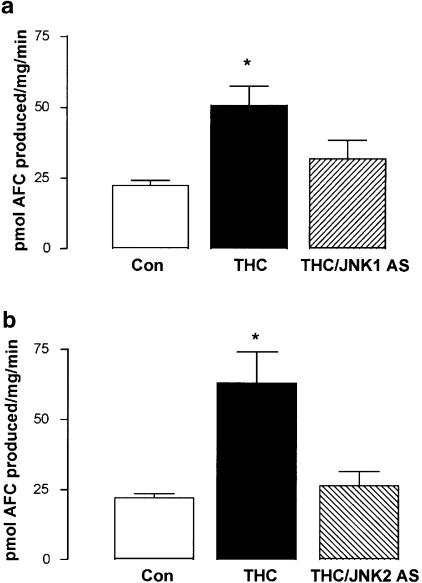
JNK is required for caspase-3 activity in THC-induced apoptosis. (a) Treatment of cortical neurons with THC (5 μM) for 2 h significantly increased caspase-3 activity, as assessed by the cleavage of the fluorogenic DEVD substrate. The stimulatory effect of THC on caspase-3 activity was prevented by pretreatment with JNK1 AS (2 μM; 48 h). Results are expressed as mean±s.e.m. for eight observations, *P<0.05. (b) Similarly, pretreatment of cells with JNK2 AS (2 μM; 48 h) prior to THC treatment abolished the stimulatory effect of THC on caspase-3 activity. Results are expressed as mean±s.e.m. for seven observations, *P<0.05.
The data presented in Figure 6a indicate that treatment with THC (5 μM) for 2 h significantly increased caspase-3 activity from 22.50±1.67 (mean±s.e.m.) to 50.66±6.86 pmol AFC produced (mg protein)−1 min−1 (P<0.05, ANOVA; n=8). In cells treated with JNK1 SC (2 μM) alone for 48 h, caspase-3 activity was comparable to control values (25.09 ±5.80 pmol AFC produced (mg protein)–1 min–1). Pretreatment with JNK1 AS (2 μM) for 48 h prior to THC treatment prevented the THC-induced increase in caspase-3 activity (31.79±6.53 pmol AFC produced (mg protein)−1 min−1; Figure 6a).
Similarly, Figure 6b demonstrates that a 2 h exposure of cortical neurons to THC (5 μM) significantly increased caspase-3 activity from 22.05±1.48 (mean±s.e.m.) to 63.05±11.10 pmol AFC produced (mg protein)−1 min−1 (P<0.05, ANOVA, n=7). Neurons incubated with JNK2 SC (2 μM) for 48 h showed a level of caspase-3 activity similar to that measured in vehicle-treated neurons (32.73±8.74 pmol AFC produced (mg protein)−1 min−1). Pretreatment with JNK2 AS (2 μM) for 48 h prior to THC treatment prevented the THC-induced increase in caspase-3 activity (26.06±5.20 pmol AFC produced (mg protein)−1 (min)−1; Figure 6b). These results demonstrate a role for both JNK1 and JNK2 in the THC-induced activation of caspase-3.
JNK AS prevents THC-induced DNA fragmentation
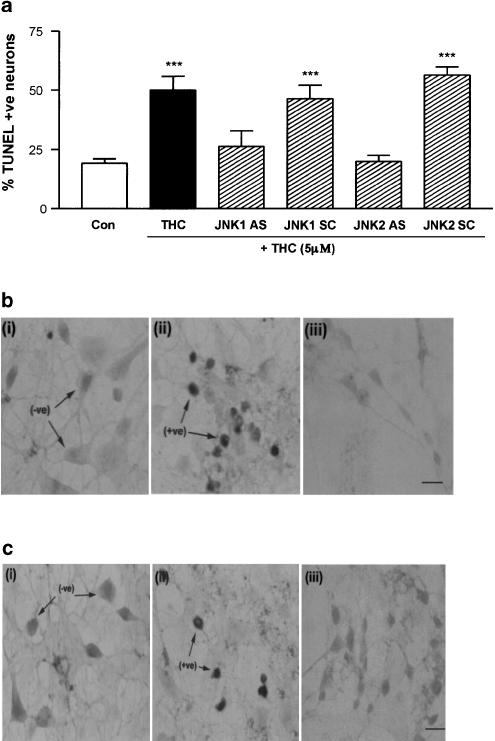
The TUNEL technique was used to demonstrate that the THC-induced DNA fragmentation that we have previously reported (Campbell, 2001) involves JNK1 and JNK2 (Figure 7). In control cells, 19±2% (mean±s.e.m.) of cells displayed fragmented DNA (TUNEL-positive), and this was significantly increased to 50±6% in cells treated with THC (5 μM) for 3 h (P<0.001, ANOVA, n=5 coverslips; Figure 7a). AS-mediated depletion of JNK1 or JNK2 prevented the THC-induced increase in DNA fragmentation. Thus, exposure of cells to JNK1 AS (2 μM) for 48 h prior to THC treatment prevented the THC-induced increase in TUNEL staining (26±7% TUNEL-positive cells; Figure 7a, b). In contrast, in cells pretreated with JNK1 SC (2 μM), the THC-induced increase in DNA fragmentation was retained (46±6% TUNEL-positive cells, P<0.001, ANOVA, n=5 coverslips; Figure 7a). Treatment with JNK1 AS (2 μM) alone for 48 h had no effect of the percentage of neurons displaying fragmented DNA (26±6% TUNEL-positive cells.
Figure 7.
JNK activity is required for THC-induced DNA fragmentation. (a) Exposure of neurons to THC (5 μM) for 3 h significantly increased the percentage of cells with DNA fragmentation. Treatment with JNK1 AS or JNK2 AS (2 μM; 48 h) prior to THC treatment prevented the THC-induced increase in DNA fragmentation. In contrast, in neurons treated with JNK1 SC or JNK2 SC (2 μM; 48 h), THC still evoked a significant increase in DNA fragmentation. Results are expressed as mean±s.e.m. for five observations, ***P<0.001. (b) Representative images of (i) control, (ii) THC-treated and (iii) THC/JNK1 AS-treated cortical neurons following TUNEL staining for DNA fragmentation. Arrows indicate TUNEL-negative (−ve) and TUNEL-positive (+ve) cells. Scale bar is 50 μM. (c) Representative images of TUNEL staining of (i) control, (ii) THC-treated and (iii) THC/JNK2 AS-treated cortical neurons. Scale bar is 50 μM.
Similarly, in cells pretreated with JNK2 AS (2 μM) for 48 h before THC (5 μM) treatment, 20±3% of cells were TUNEL-positive (Figure 7a, c). In contrast, cells preincubated with JNK2 SC oligonucleotides (2 μM) for 48 h prior to treatment with THC showed a significant increase in DNA fragmentation (56±3% TUNEL-positive cells, P<0.001, ANOVA, n=5 coverslips; Figure 7a). Treatment of neurons with JNK2 AS (2 μM) alone for 48 h did not have a neurotoxic effect (20±3% TUNEL-positive cells.
These results demonstrate that depletion of either JNK1 or JNK2 prevents the THC-induced DNA fragmentation. These data are consistent with the caspase-3 result and suggest that both JNK1 and JNK2 are intricately involved in regulating the THC-induced activation of caspase-3 and resultant DNA fragmentation in the apoptotic pathway.
JNK1 AS pretreatment has no effect on THC-induced JNK2 activity
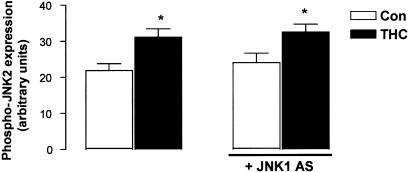
Since THC was found to increase JNK1 activity (at 5 min) prior to JNK2 activity (at 2 h), we employed the use of antisense technology to analyse whether JNK1 is involved upstream in the regulation of JNK2 activity (Figure 8). Neurons were treated with JNK1 AS (2 μM; 48 h) to downregulate JNK1 protein expression prior to exposure to THC (5 μM) for 2 h. Treatment of cells with THC for 2 h significantly increased phospho-JNK2 expression from 21.87±2.38 (arbitrary units; mean bandwidth±s.e.m.) to 31.14±2.85 (P<0.05, ANOVA, n=4). Neurons treated with JNK1 AS alone for 48 h showed a level of JNK2 activity comparable to that found in vehicle-treated neurons (23.99±2.31, n=4) and JNK1 AS failed to prevent the THC-induced increase in phospho-JNK2 expression (32.49±1.90, P<0.05, ANOVA, n=4). These data suggest that JNK1 does not occupy an upstream role in the regulation of JNK2 activity, despite the differential time course of activation of these JNK isoforms.
Figure 8.
JNK1 is not upstream of JNK2 in THC-induced apoptosis. Neurons were preincubated with JNK1 AS (2 μM) for 48 h, treated with THC (5 μM) for 2 h and analysed for phosphorylated JNK2 expression using Western immunoblot. THC evoked a significant increase in phospho-JNK2 expression. In cells exposed to JNK1 AS, THC was still capable of inducing a significant increase in phospho-JNK2. Results are expressed as mean±s.e.m. for four observations, *P<0.05.
Discussion
The aim of this study was to examine the ability of the CB1 cannabinoid receptor to couple to the JNK signalling pathway, and to assess the role of JNK in THC-induced neurotoxicity in cultured cortical neurons. THC was found to induce the activation of JNK1 and JNK2 within 5 min and 2 h, respectively. The ability of THC to induce activation of both JNK isoforms was blocked by AM 251 and PTX. THC significantly increased Bax expression in an AM 251-sensitive manner. Treatment of cortical neurons with specific AS targeted to rat JNK1 or JNK2 mRNA effectively reduced the protein expression of the respective JNK isoform. AS-mediated reduction of JNK1 and JNK2 protein expression prevented the THC-induced activation of caspase-3 and downstream DNA fragmentation. Data herein suggest that the CB1 cannabinoid receptor is coupled to JNK in cortical neurons, and that both JNK1 and JNK2 are involved in the regulation of the downstream effectors that are pertinent in the THC-induced apoptotic pathway.
Recently, there has been a growing interest in the proclivity of cannabinoids to control the cell survival/death decision, particularly in neurons. Several studies have revealed that THC can induce neurotoxic effects in a number of cultured cell systems. Specifically, THC has a toxic effect on cultured hippocampal neurons (Chan et al., 1998), cultured cortical neurons (Campbell, 2001) and glioma cells (Sanchez et al., 1998). THC has also been shown to inhibit neuronal cell growth in vivo (Galve-Roperh et al., 2000), in addition to its antiproliferative action in neuronal cultures. Furthermore, anandamide, an endogenous ligand of cannabinoid receptors (Devane et al., 1992), induces apoptosis in PC12 cells (Sarker et al., 2003) and lymphocytes (Schwarz et al., 1994). THC-induced apoptosis in hippocampal (Chan et al., 1998) and cortical (Downer et al., 2001) cultures is CB1 receptor-dependent. Proposed mechanisms of cannabinoid-induced neurotoxicity have included the generation of reactive oxygen species (Chan et al., 1998), activation of the caspase-3 cell death pathway (Campbell, 2001; Downer et al., 2001), sphingomyelin hydrolysis (Sanchez et al., 1998), sustained ceramide accumulation (Galve-Roperh et al., 2000) and activation of the JNK cascade (Sarker et al., 2003).
It should be considered that in contrast to the data supporting cannabinoid-induced neurodegeneration, the bulk of the experimental evidence indicates that cannabinoids may protect neurons from toxic insults. However, it is not clear if this is a CB1 receptor-dependent process. Nagayama et al. (1999) have shown that synthetic cannabinoid receptor agonists decrease hippocampal loss following transient global cerebral ischaemia via the CB1 receptor, and increase cell viability in cerebral cortical cultures subjected to 8 h of hypoxia and glucose deprivation in a CB1 receptor-independent manner. Cannabinoid receptor agonists are neuroprotective against excitotoxicity in vivo (Panikashvili et al., 2001; van der Stelt et al., 2001) and in vitro (Shen & Thayer, 1998; Abood et al., 2001), which is prevented by CB1 antagonists. Furthermore, THC and nonpsychotropic cannabinoids decrease glutamate toxicity in rat cortical neuronal cultures in a receptor-independent process that is not blocked by CB1 receptor antagonists (Hampson et al., 1998). Overall, it appears that neurotoxic and neuroprotective effects of cannabinoids can be observed, and these differences are likely to depend on a variety of influences, including the nature of the toxic insult, the cell type under study and the particular cannabinoid used.
The cannabinoid CB1 receptor is distributed mainly in the central nervous system and is localised in brain regions most likely involved in contributing to the psychoactive effects of THC (Twitchell et al., 1997). The rat cortex shows an intense pattern of CB1 receptor expression, in particular in frontal regions where the CB1 receptor subtype is found in cortical axons, cell bodies and dendrites (Tsou et al., 1998).
The JNK protein kinases belong to the family of mitogen-activated protein kinases (MAPK) and represent a group of enzymes that are activated by cytokines and environmental stresses (Ip & Davis, 1998). The function of these kinases is to convert extracellular stimuli to intracellular signals that, in turn, control the expression of genes that are essential for many cellular responses, including cell growth and death (Marshall, 1995). These widely distributed kinases are activated by dual phosphorylation on threonine and tyrosine residues by upstream kinases as part of the cellular response to stress (Derkinderen et al., 1999). JNKs are encoded by three different genes jnk1, jnk2 and jnk3, and the products of each gene reveal isoforms with approximate molecular weights of 46 (JNK1), 54 (JNK2) and 57 kDa (JNK3), all of which are found in the mammalian brain (Gupta et al., 1996). Although it is recognised that the JNK3 isoform is distributed throughout the rat cerebral cortex (Carboni et al., 1998), JNK3 protein expression was moderate in our cell culture model, hence the relative contribution of the JNK1 and JNK2 isoforms to the apoptotic process was investigated.
In this study, the time course of THC-induced activation of JNK1 and JNK2 in cultured cortical neurons was assessed. JNK activity was assessed up to the 2 h time point as this is within the timeframe at which we have found significant levels of THC toxicity in cortical neuronal cultures (Campbell, 2001). It was found that THC increases JNK1 and JNK2 activity after 5 min and 2 h of treatment, respectively. Half-maximal stimulation of JNK1 occurred at a lower dose of THC than JNK2, indicating that THC-induced JNK2 activation was more reluctant than JNK1 activation in our cell culture model. It has been established that JNK activation may occur early (Xia et al., 1995) or late (Virdie et al., 1997) in apoptosis, and the finding that THC activates JNK1 and JNK2 within dissimilar timeframes suggests that the JNK isoforms may mediate different signals in cultured cortical neurons. A potential function of JNK may be to initiate programmed cell death (Johnson et al., 1996), and our data demonstrating the early activation of JNK1 (within 5 min), may reflect a role for JNK1 in regulating downstream apoptotic effectors. However, our finding that THC activates JNK2 at 2 h of treatment is consistent with the time point at which maximal neuronal cell death has been previously shown in cortical neurons (Campbell, 2001). It is therefore possible that JNK2 activation may have occurred in response to DNA fragmentation, which is consistent with other studies (Ghahremani et al., 2002). Furthermore, considering that JNK activity may be regulated by caspase-3 (Ozaki et al., 1999; Hatai et al., 2000), it is possible that JNK2 activity is modulated by caspase-3 in this system. In refute of these hypotheses, antisense-mediated downregulation of JNK2 prevented the THC-induced increase in caspase-3 activation and DNA fragmentation, suggesting that JNK2 is upstream of these components of the apoptotic cascade. It is possible that an early cellular redistribution of JNK2, as opposed to an increase in overall JNK2 activity, may be involved in the apoptotic process. It is of note that although JNK2 activity was increased at 2 h, a concomitant decline in JNK1 activity was observed at this time point. This suggests a functional interaction between JNK1 and JNK2 isoforms and this is supported by evidence that JNK2 negatively regulates JNK1 phosphorylation (Hochedlinger et al., 2002). However, our finding that AS-induced depletion of JNK1 failed to prevent the THC-induced increase in JNK2 activity suggests that JNK1 does not occupy an upstream role in regulating JNK2.
The involvement of JNK cascades in the regulation of cell proliferation via G-protein-coupled receptors has been demonstrated (Coso et al., 1996). Our previous studies have demonstrated that the toxic effects of THC involve a PTX-sensitive G-protein (Campbell, 2001). This is consistent with evidence that links the central CB1 receptor to activation of G-protein subtypes Gi/o (Howlett, 1995). To date, the role of G-proteins in neuronal cell death has not been completely clarified since PTX-sensitive G-proteins have been found to exert both proapoptotic (Farkas et al., 1998) and antiapoptotic effects (Jakob & Kreiglsten, 1997). However, data presented here demonstrate that coupling of the CB1 receptor to PTX-sensitive G-proteins facilitates JNK activation.
JNK has the proclivity to phosphorylate a variety of nuclear and cytoplasmic substrates, some of which are vital for the apoptotic actions of JNK. It has been reported that JNK promotes cell death by promoting cytochrome c release from the mitochondria (Tournier et al., 2000). In the nervous system, the proapoptotic mitochondrial-associated protein Bax acts downstream of JNK in regulating the translocation of mitochondrial cytochrome c into the cytosol (Kang et al., 1998), and several studies have demonstrated an interaction between JNK and Bax in the cell death cascade (Lei et al., 2002). The apoptotic events that are evoked by THC in cortical neurons include the release of mitochondrial cytochrome c into the cytosol following CB1 receptor activation (Downer et al., 2001), and our finding that THC induces Bax expression following CB1 activation is a likely mechanism for this event.
Once in the cytosol, cytochrome c complexes with a cytosolic factor designated as apoptotic protease activating factor-1 (APAF-1), which in turn triggers the activation of the cysteine protease caspase-3, which contributes to the drastic morphological changes associated with apoptosis by disabling a number of key substrates (Zou et al., 1997). We have previously shown that caspase-3 is central to the apoptotic cascade triggered by THC in cortical neurons (Campbell, 2001; Downer et al., 2001). To elucidate the respective roles of JNK1 and JNK2 in THC-induced caspase-3 activation, we employed antisense technology to down-regulate temporarily JNK1 and JNK2 expression. In neurons treated with JNK1 and JNK2 AS, JNK1 and JNK2 protein expression was selectively downregulated. Depletion of JNK1 and JNK2 prevented the THC-induced activation of caspase-3, indicating that both JNK1 and JNK2 isoforms are upstream of caspase-3 in the THC-induced apoptotic cascade.
We have recently shown that THC promotes degeneration of cultured cortical neurons in a time-dependent manner, with a maximal effect occurring 3 h post-treatment (Campbell, 2001). The evidence presented here and by others (Rueda et al., 2000) supports an early interaction between THC and JNK, indicating that JNK activation is an upstream event in the degenerative pathway. The finding that the downregulation of JNK1 and JNK2 using antisense technology effectively blocked THC-induced activation of caspase-3 and downstream DNA fragmentation indicates that both JNK1 and JNK2 are intricately involved in the transduction of THC-induced apoptotic signals. It is of particular note that depletion of either JNK1 or JNK2 isoforms completely abrogated the THC-induced caspase-3 activation and DNA fragmentation. It may be expected that, since THC has the proclivity to couple to both JNK1 and JNK2, depletion of one isoform may result in THC coupling to the remaining isoform to evoke caspase-3 activation and resultant DNA fragmentation. This clearly was not the case and our result may reflect an interaction between JNK1 and JNK2 with respect to the regulation of upstream effectors involved in caspase-3 activation. Indeed, a requirement for both JNK1 and JNK2 has been implicated in the phosphorylation of the p53 tumour suppressor protein (Buschmann et al., 2001), a potential upstream event in the regulation of caspase-3 activity. Furthermore, it has been shown that inhibition of the active forms of both JNK1 and JNK2 prevents cytochrome c release from the mitochondria and activation of enzymes upstream of caspase-3 (Bozyczko-Coyne et al., 2001). This study provides evidence of a requirement for both JNK1 and JNK2 in the THC-induced activation of caspase-3 and DNA fragmentation, and may reflect cooperativity between JNK1 and JNK2 in relation to these aspects of the cell death cascade.
To date, little is known about the role of JNK in THC signalling. Rueda et al. (2000) have found that JNK1/2 are activated within 5 min of THC treatment and that sustained JNK activation over 3 days of THC insult is required to induce cell death in glioma cells. This suggests that the duration of JNK induction may be the determining factor in the cell death/survival decision. These differences may reflect differential cellular responses of neuronal populations to THC, with cortical neurons being susceptible to early insult of cannabinoid exposure due to the differential activation of the JNK1/2 signalling cascades. We are aware that in the same study of Rueda et al. (2000), rat cortical primary neurons were not susceptible to a 3-day exposure to THC, which may reflect the lower dose of THC used in that study. It has recently been found that the endogenous cannabinoid, anandamide, induces apoptosis in PC12, and this is accompanied by the activation of the JNK pathway (Sarker et al., 2003). Furthermore, JNK activity is not affected by THC in hippocampal slices prepared from the adult rat brain (Derkinderen et al., 2001). Thus, the effects of cannabinoids may be dependent on neuronal maturity, with neonatal cultures being susceptible to JNK activation following cannabinoid challenge.
The dose of THC used in the present study to induce the activation of JNK1 and JNK2 was within the concentration range we have previously shown to be neurotoxic to cultured cortical neurons (Campbell, 2001) and is also consistent with the human plasma concentrations of THC (low μM; Chiang & Barnett, 1984). Furthermore, the data demonstrate that THC is toxic for cortical cultures obtained from neonatal rats. Interestingly to date, there is no evidence to suggest that THC is neurotoxic in the adult rat central nervous system (Galve-Roperh et al., 2000). Our findings support the proposal that the apoptotic effects of THC may be operative early during development and may play a modulatory role in neural development (Fernandez-Ruiz et al., 1999). Indeed the consumption of marijuana by women during pregnancy and/or lactation has been shown to affect the neurobehavioural development of their children (Fried, 1995).
In summary, treatment of cortical cultures with THC activates the JNK pathway via the CB1 cannabinoid receptor. An antisense approach revealed that JNK1 and JNK2 are required for the THC-induced activation of caspase-3 and DNA fragmentation in cortical neurons. These findings identify a cannabinoid receptor-dependent transduction pathway associated with THC-induced neurotoxicity.
Acknowledgments
This study was supported by the Health Research Board Ireland and Enterprise Ireland.
Abbreviations
- AS
antisense oligonucleotides
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- SC
scrambled control oligonucleotides
- THC
Δ9-tetrahydrocannabinol
- TUNEL
terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labelling
References
- ABOOD M.E., MARTIN B.R. Neurobiology of marijuana abuse. Trends Pharmacol. Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- ABOOD M.E., RIZVI G., SALLAPUDI N., MCALLISTER S. Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neurosci. Lett. 2001;309:197–201. doi: 10.1016/s0304-3940(01)02065-1. [DOI] [PubMed] [Google Scholar]
- BOUABOULA M., POINOT-CHAZEL C., BOURRIE B., CANAT X., CALANDRA B., RINALDI-CARMONA M., LE FUR G., CASELLAS P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem. J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUABOULA M., POINOT-CHAZEL C., MARCHAND J., CANAT X., BOURRIE B., RINALDI-CARMONA M., CALANDRA B., LE FUR G., CASELLAS P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor: involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur. J. Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- BOZYCZKO-COYNE D., O'KANE T.M., WU Z.L., DOBRZANSKI P., MURTHY S., VAUGHT J.L., SCOTT R.W. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Aβ-induced cortical neuron apoptosis. J. Neurochem. 2001;77:849–863. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- BUSCHMANN T., POTAPOVA O., BAR-SHIRA A., IVANOV V.L., FUCHS S.Y., HENDERSEN S., FRIED V.A., MINAMOTO T., ALARCON-VARGAS D., PINCUS M.R., GAARDE W.A., HOLBROOKE N.J., SHILOH Y., RONAI Z. Jun NH2-terminal kinase phophorylation of p53 on thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol. Cell. Biol. 2001;21:2743–2754. doi: 10.1128/MCB.21.8.2743-2754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL V.A. Tetrahydrocannabinol-induced apoptosis in cultured cortical neurones is associated with cytochrome c release and caspase-3 activation. Neuropharmacology. 2001;40:702–709. doi: 10.1016/s0028-3908(00)00210-0. [DOI] [PubMed] [Google Scholar]
- CARBONI L., CARLETTI R., TACCONI S., CORTI C., FERRAGUTI F. Differential expression of SAPK isoforms in the rat brain. An in situ hybridisation study in the adult rat brain and during post-natal development. Mol. Brain Res. 1998;60:57–68. doi: 10.1016/s0169-328x(98)00166-1. [DOI] [PubMed] [Google Scholar]
- CHAN G.C., HINDS T.R., IMPEY S., STORM D.R. Hippocampal neurotoxicity of Δ9-tetrahydrocannabinol. J. Neurosci. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIANG C.W., BARNETT G. Marijuana effect and delta-9-tetrahydrocannabinol plasma level. Clin. Pharmacol. Ther. 1984;36:234–238. doi: 10.1038/clpt.1984.168. [DOI] [PubMed] [Google Scholar]
- COSO O.A., TERAMOTO H., SIMONDS W.F., GUTKIND J.S. Signaling from G protein-coupled receptors to c-Jun kinase involves βγ subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J. Biol. Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- DERKINDEREN P., ENSLEN H., GIRAULT J.-A. The ERK/MAP-kinases cascade in the nervous system. Neuroreport. 1999;10:R24–R34. [PubMed] [Google Scholar]
- DERKINDEREN P., LEDENT C., PARMENTIER M., GIRAULT J.-A. Cannabinoids activate p38 mitogen-activated protein kinases through CB1 receptors in hippocampus. J. Neurochem. 2001;77:957–960. doi: 10.1046/j.1471-4159.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFF C., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DILL J.A., HOWLETT A.C. Regulation of adenylate cyclase by chronic exposure to cannabimimetic drugs. J. Pharmacol. Exp. Ther. 1988;244:1157–1163. [PubMed] [Google Scholar]
- DOWNER E., BOLAND B., FOGARTY M., CAMPBELL V. Δ9-Tetrahydrocannabinol induces the apoptotic pathway in cultured cortical neurones via activation of the CB1 receptor. Neuroreport. 2001;12:3973–3978. doi: 10.1097/00001756-200112210-00024. [DOI] [PubMed] [Google Scholar]
- FARKAS I., BARANYI L., TAKAHASHI M., FUKUDA A., LIPOSITS Zs., YAMAMOTO T., OKADA H. A neuronal C5a receptor and an associated apoptotic signal transduction pathway. J. Physiol. 1998;507:679–687. doi: 10.1111/j.1469-7793.1998.679bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-RUIZ J.J., BERRENDERO F., HERNANDEZ M.L., ROMERO J., RAMOS J.A. Role of endocannabinoids in brain development. Life Sci. 1999;65:725–736. doi: 10.1016/s0024-3205(99)00295-7. [DOI] [PubMed] [Google Scholar]
- FRIED P.A. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings – Its easy to throw the baby out with the bath water. Life Sci. 1995;56:2159–2168. doi: 10.1016/0024-3205(95)00203-i. [DOI] [PubMed] [Google Scholar]
- GALVE-ROPERH I., SANCHEZ C., LUISA CORTES M., GOMEZ DEL PULGAR T., IZQUIERDO M., GUZMAN M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- GESSA G.L., CASU M.A., CARTA G., MASCIA M.S. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur. J. Pharmacol. 1998;355:119–124. doi: 10.1016/s0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- GHAHREMANI M.H., KERAMARIS E., SHREE T., XIA Z., DAVIS R.J., FLAVELL R., SLACK R.S., PARK D.S. Interaction of the c-Jun/JNK pathway and cyclin-dependent kinase in death of embryonic cortical neurones evoked by DNA damage. J. Biol. Chem. 2002;277:35586–35596. doi: 10.1074/jbc.M204362200. [DOI] [PubMed] [Google Scholar]
- GOMEZ DEL PULGAR T., VELASCO G., GUZMAN M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem. J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUPTA S., BARRETT T., WHITMARSH A.J., CAVANAGH J., SLUSS H.K., DERIJARD B., DAVIS R.J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- HAMPSON A.J., GRIMALDI M., AXELROD J., WINK D. Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATAI T., MATSUZAWAI A., INOSHITA S., MOCHHIDA Y., KURODA T., SAKAMAKI K., KUIDA K., YONEHARA S., ICHIJO H., TAKEDA K. Execution of Apoptosis Signal-regulating Kinase 1 (ASK1)-induced apoptosis by mito-chondria-dependent caspase activation. J. Biol. Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- HOCHEDLINGER K., WAGNER E.F., SABAPATHY K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–2445. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C. Reverse pharmacology of the cannabinoid receptor. Trends Pharmacol. Sci. 1990;11:395–397. doi: 10.1016/0165-6147(90)90142-u. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C. Pharmacology of cannabinoid receptors. Annu. Rev. Pharmacol. Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- HRENIUK D., GARAY M., GAARDE W., MONIA B.P., MCKAY R.A., CIOFFI C.L. Inhibition of c-Jun N-terminal kinase 1, but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by ischemia/reoxygenation in rat cardiac myocytes. Mol. Pharmacol. 2001;59:867–874. doi: 10.1124/mol.59.4.867. [DOI] [PubMed] [Google Scholar]
- IP Y.T., DAVIS R.J. Signal transduction by the c-Jun N-terminal kinase (JNK) – from inflammation to development. Curr. Opin. Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- JAKOB R., KREIGLSTEN J. Influence of flupirtine on a G-protein coupled inwardly rectifying potassium current in hippocampal neurones. Br. J. Pharmacol. 1997;122:1333–1338. doi: 10.1038/sj.bjp.0701519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANICKE R.U., SPRENGART M.L., WATU M.R., PORTER A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- JENTSCH J.D., VERRICO C.D., LE D., ROTH R.H. Repeated exposure to Δ9-tetrahydrocannabinol reduces prefrontal cortical dopamine metabolism in the rat. Neurosci. Lett. 1998;246:169–172. doi: 10.1016/s0304-3940(98)00254-7. [DOI] [PubMed] [Google Scholar]
- JOHNSON N.L., GARDNER A.M., DIENER K.M., LANGE-CARTER C.A., GLEAVY J., JARPE M.B., MINDEN A., KAR1N M., ZON L.I., JOHNSON G.L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J. Biol. Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- KANG C.-D., JANG J.-H., KIM K.-W., LEE H.-J., JEONG C.-S., KIM C.-M., KIM S.-H., CHUNG B.-S. Activation of c-Jun N-terminal kinase/stress-activated protein kinase and the decreased ratio of Bcl-2 to Bax are associated with the auto-oxidized dopamine-induced apoptosis in PC12 cells. Neurosci. Lett. 1998;256:37–40. doi: 10.1016/s0304-3940(98)00751-4. [DOI] [PubMed] [Google Scholar]
- LEI K., NIMUAL A., ZONG W.X., KENNEDY N.J., FLAVELL R.A., THOMPSON C.B., BAR-SAGI D., DAVIS R.J. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol. Cell. Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKIE K., LAI Y., WESTENBROEK R., MITCHELL R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL C.J. Specificity of receptor protein kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., LOIAIT S.J., BROWNSTEIN M.J., YOUNG A.C., BONNER T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MIELKE K., HERDEGEN T. JNK and p38 stress kinases – degenerative effectors of signal-transduction-cascades in the nervous system. Prog. Neurobiol. 2000;61:46–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- NAGAYAMA T., SINOR A.D., SIMON R.P., CHEN J., GRAHAM S.H., JIN K., GREENBER D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEW D.C., WONG Y.H. BML-190 and AM251 act as inverse agonists at the human cannabinoid CB2 receptor: signalling via camp and inositol phosphate. FEBS Lett. 2003;536:157–160. doi: 10.1016/s0014-5793(03)00048-6. [DOI] [PubMed] [Google Scholar]
- OZAKI I., TANI E., IKEMOTO H., KITAGAWA H., FUJIKAWA H. Activation of stress-activated protein kinase/c-Jun NH2-terminal kinase and p38 kinase in calphostin c-induced apoptosis requires caspase-3-like proteases but is dispensable for cell death. J. Biol. Chem. 1999;274:5310–5317. doi: 10.1074/jbc.274.9.5310. [DOI] [PubMed] [Google Scholar]
- PANIKASHVILI D., SIMEONIDOU C., BEN-SHABAT S., HANUS L., BREUER A., MECHOULAM R., SHOHAMI E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- RUEDA D., GALVE-ROPERH I., HARO A., GUZMAN M. The CB1 cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol. Pharmacol. 2000;58:814–820. doi: 10.1124/mol.58.4.814. [DOI] [PubMed] [Google Scholar]
- SANCHEZ C., GALVE-ROPERH I., CANOVA C., BRACHET P., GUZMAN M. Δ9-Tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- SARKER K.P., BISWAS K.K., YAMAKUCHI M., LEE K.-Y., HAHIGUCHI T., KRACHT M., KITAJIMA I., MARUYAMA I. ASK1-p38 MAPK/JNK signaling cascade mediates anandamide-induced PC 12 cell death. J. Neurochem. 2003;85:50–61. doi: 10.1046/j.1471-4159.2003.01663.x. [DOI] [PubMed] [Google Scholar]
- SCHWARZ H., BLANCO F.J., LOTZ M. Anandamide, an endogenous cannabinoid receptor agonist inhibits lymphocytes proliferation and induces apoptosis. J. Neuroimmunol. 1994;55:107–115. doi: 10.1016/0165-5728(94)90152-x. [DOI] [PubMed] [Google Scholar]
- SHEN M., PISER T.M., SEYBOLD V.S., THAYER S.A. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J. Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN M., THAYER S.A. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol. Pharmacol. 1998;54:459–462. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- TOURNIER C., HESS P., YANG D.D., XU J., TURNER T.K., NIMUAL A., BAR-SAGI D., JONES S.N., FLAVELL R.A., DAVIS R.J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–873. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- TSOU K., BROWN S., SANUDO-PENA M.C., MACKIE K., WALKER J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- TURKANIS S.A., PARTLOW L.M., KARLER R. Delta-9-tetrahydrocannabinol depresses inward sodium current in mouse neuroblastoma cells. Neuropharmacology. 1991;30:73–77. doi: 10.1016/0028-3908(91)90045-d. [DOI] [PubMed] [Google Scholar]
- TWITCHELL W., BROWN S., MACKIE K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- VAN DER STELT M., VELDHEIS W.B., BAR P.R., VELDINK G.A., VLIEGENTHART J.F.G., NICOLAY K. Neuroprotection by Δ9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. J. Neurosci. 2001;21:6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRDIE K., BANNISTER A.J., HUNT S.P., TOLKOVSKL A.M. Comparison between the timing of JNK activation, c-Jun phosphorylation, and the onset of death commitment in sympathetic neurones. J. Neurochem. 1997;66:550–561. doi: 10.1046/j.1471-4159.1997.69020550.x. [DOI] [PubMed] [Google Scholar]
- XIA Z., DICKENS M., RAINGEAUD J., DAVIS R.J., GREENBERG M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- ZOU H., HENZEL W.J., LIU X., LUTSCHG A., WANG X. Apaf-1, a human protein homologous C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]