Abstract
Adrenomedullin (AM) has two known receptors formed by the calcitonin receptor-like receptor (CL) and receptor activity-modifying protein (RAMP) 2 or 3: We report the effects of the antagonist fragments of human AM and CGRP (AM22–52 and CGRP8–37) in inhibiting AM at human (h), rat (r) and mixed species CL/RAMP2 and CL/RAMP3 receptors transiently expressed in Cos 7 cells or endogenously expressed as rCL/rRAMP2 complexes by Rat 2 and L6 cells.
AM22–52 (10 μM) antagonised AM at all CL/RAMP2 complexes (apparent pA2 values: 7.34±0.14 (hCL/hRAMP2), 7.28±0.06 (Rat 2), 7.00±0.05 (L6), 6.25±0.17 (rCL/hRAMP2)). CGRP8–37 (10 μM) resembled AM22–52 except on the rCL/hRAMP2 complex, where it did not antagonise AM (apparent pA2 values: 7.04±0.13 (hCL/hRAMP2), 6.72±0.06 (Rat2), 7.03±0.12 (L6)).
On CL/RAMP3 receptors, 10 μM CGRP8–37 was an effective antagonist at all combinations (apparent pA2 values: 6.96±0.08 (hCL/hRAMP3), 6.18±0.18 (rCL/rRAMP3), 6.48±0.20 (rCL/hRAMP3)). However, 10 μM AM22–52 only antagonised AM at the hCL/hRAMP3 receptor (apparent pA2 6.73±0.14).
BIBN4096BS (10 μM) did not antagonise AM at any of the receptors.
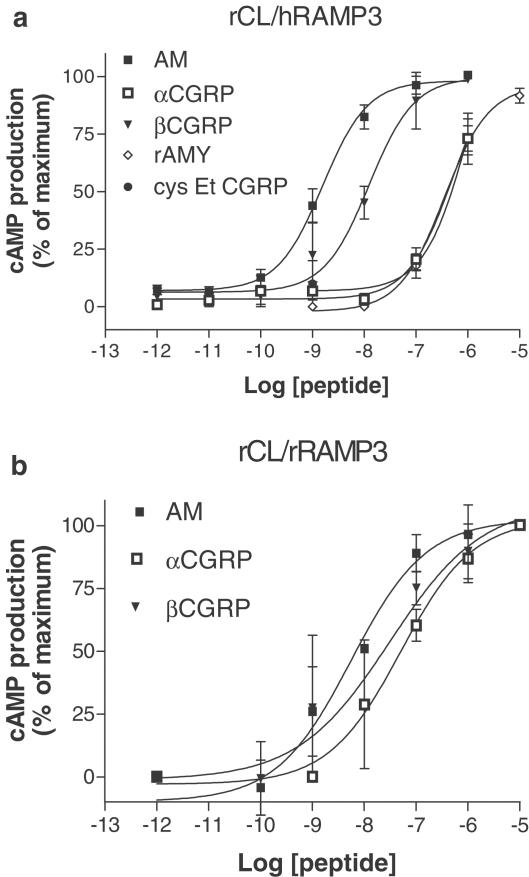
Where investigated (all-rat and rat/human combinations), the agonist potency order on the CL/RAMP3 receptor was AM∼βCGRP>αCGRP.
rRAMP3 showed three apparent polymorphisms, none of which altered its coding sequence.
This study shows that on CL/RAMP complexes, AM22–52 has significant selectivity for the CL/RAMP2 combination over the CL/RAMP3 combination. On the mixed species receptor, CGRP8–37 showed the opposite selectivity. Thus, depending on the species, it is possible to discriminate pharmacologically between CL/RAMP2 and CL/RAMP3 AM receptors.
Keywords: CGRP, CGRP8–37, adrenomedullin, adrenomedullin22–52, calcitonin receptor-like receptor, CL, RAMP2, RAMP3
Introduction
AM is an essential vascular peptide; its presence in the developing foetus governs the formation of intact vasculature and thus foetal survival (Caron & Smithies, 2001; Shindo et al., 2001). These studies support the well-recognised role of AM in cell growth, and further the concept that AM may be involved in angiogenesis (Miller et al., 1996; Withers et al., 1996; Nikitenko et al., 2002). The pharmacology of receptors responsive to AM has been examined in many tissues and cell lines (see Hinson et al., 2000; Hay & Smith, 2001 for reviews). Specific AM receptors can be characterised by high affinity for AM and ⩾100-fold lower affinity for the other members of the calcitonin family of peptides (Coppock et al., 1999). The effects of AM at such receptors can be inhibited by the AM antagonist fragment AM22–52 (Eguchi et al., 1994). The αCGRP antagonist fragment CGRP8–37 and the amylin receptor antagonist AC187 can also antagonise specific AM receptors, but only at high concentrations (>5 μM) (Coppock et al., 1999). AM also activates CGRP receptors, and these effects can be inhibited by CGRP8–37 (Nagoshi et al., 2002). However, this description is likely to be an oversimplification. AM22–52 is the only antagonist available which is specific for AM receptors, and this is a relatively low-affinity peptide. Without better antagonists, it is difficult to separate receptor subtypes which may exist in tissues that are likely to contain very complex mixtures of receptors, for example, vas deferens (Poyner et al., 1999; Wu et al., 2000).
Generally, it seems that AM22–52 antagonises the effects of AM but not CGRP. However, high concentrations of AM22–52 are often required due to the low affinity of this antagonist, and observations of unusual pharmacology, potentially attributable to the existence of subtypes of AM receptors, have been noted. In the rat vas deferens, the effects of AM or [Cys(Et)2,7]αCGRP (a putative ‘CGRP2'-receptor selective agonist; Dumont et al., 1997) were more potently antagonised by BIBN4096BS than those of either α or βCGRP (Wu et al., 2000). In the hind limb vascular bed of the cat, AM22–52 could not antagonise the effects of AM, but could inhibit the effects of CGRP (Champion et al., 1997). In this system, CGRP8–37 could inhibit responses to CGRP but not AM.
Two AM receptor subtypes have now been defined in molecular terms: AM1, composed of CL with RAMP2, and AM2, composed of CL and RAMP3 (McLatchie et al., 1998; Poyner et al., 2002). RAMP2 and RAMP3 can be differentially regulated in in vivo models of the disease (Ono et al., 2000). For example, in a rat model of obstructive neuropathy, CL, RAMP1 and RAMP2 mRNA levels were upregulated, but RAMP3 levels were unchanged (Nagae et al., 2000). At present, there is no pharmacological separation of AM1 and AM2 receptors although it has been reported that a mouse RAMP3/rat CL (rCL) complex is more sensitive to the effects of CGRP than its RAMP2 counterpart (Husmann et al., 2000). The mouse RAMP3/rat CL receptor is considered a mixed AM/CGRP receptor, but in terms of the effects of antagonists, specific AM1 and AM2 receptors have never been thoroughly characterised. Recent studies have examined the effects of CGRP8–37 and AM22–52 at human, bovine and porcine CL complexes with RAMPs1–3 (Aiyar et al., 2001; 2002). However, these studies were performed in HEK293 cells which are known to express endogenous RAMPs (particularly RAMP2) and/or CL (Aiyar et al., 1996; Kuwasako et al., 2001). Most other analyses have been based on binding studies. Therefore, the functional effects of these antagonists at exclusive CL/RAMP3 complexes have never been examined. Furthermore, although CL has been cloned from several species (Elshourbagy et al., 1998; Aiyar et al., 2001; 2002), these have usually been coexpressed with human RAMPs (hRAMPs). There has been no study of a non-human CL expressed with a RAMP from the same species. It is not known how well these mixed species receptors reflect the pharmacology of the homologous receptors. In turn, this means that there is no reliable information on species variation from exogenously expressed, recombinant receptors.
RAMP2 and RAMP3 are divergent in sequence (Sexton et al., 2001), and the regions of RAMP2 and RAMP3 with which AM interacts are not conserved between the two proteins (Kuwasako et al., 2001; 2002). This suggests that there could be pharmacological differences between these receptors. Therefore, this study was designed to make a detailed comparison of the highest affinity antagonists available for studying AM/CGRP receptors. At the same time, the effect of species composition on the observed pharmacology was also investigated. AM22–52 (Eguchi et al., 1994), CGRP8–37 (Chiba et al., 1989; Dennis et al., 1990) and the novel CGRP receptor antagonist BIBN4096BS (Doods et al., 2000) were used to inhibit AM responses at AM receptors composed of various combinations of rat or human CL, RAMP2 or RAMP3. A more limited investigation into agonist potency ratios on CL/RAMP3 receptors was also carried out. This work was done using Cos 7 cells which have a null CL/RAMP background, making it an ideal cell line for studying the pharmacology of single populations of AM1 or AM2 receptors.
Methods
Cell culture
Cos 7, Rat-2 and L6 cells were cultured in Dulbecco's modified Eagles medium supplemented with 10% foetal bovine serum and 5% penicillin/streptomycin in a humidified 95% air/5% CO2 atmosphere. The cells were subcultured by removing the growth medium and washing the cells with cell culture-grade phosphate-buffered saline for 1–2 min. The cells were removed from the flasks with a small volume of trypsin/EDTA solution. Fresh growth medium was added to the cell suspension to neutralise the trypsin, and the cells were centrifuged at 500 × g for 5 min. The supernatant was removed, and the cell pellet was resuspended in fresh growth medium. The cells were transferred to fresh flasks, or plated onto 48-well plates.
Cloning of rat RAMP3 (rRAMP3)
rRAMP3 was cloned from a rat lung cDNA library (Invitrogen) using primers based on the published sequence (Oliver et al., 2001). These were 5′-CTCGAGATGGCGACCCCGGCACAGCGGCTGCACC-3′ and 5′-GAATTCTCACAGAAGCCGGTCAGTGTGCTTGCTACG-3′. After 30 rounds of polymerase chain reaction (92°C, 60 s; 60°C, 60 s; 72°C, 60 s), using Pfu polymerase (Promega), the amplified product was identified as a 0.48 kilobase band on a 1.4% agarose gel. Its identity was confirmed by sequencing (Alta Biosciences, Birmingham, U.K.). The product was subcloned into pcDNA3 using restriction enzyme sites EcoRI and BamHI, which were included in the primer design.
Transient transfection
Cells were transfected with various combinations of hRAMP2, hRAMP3, rRAMP3, N-terminally HA epitope-tagged hCL (kindly donated by Dr S.M. Foord, GSK, Stevenage, U.K.) or rCL (Njuki et al., 1993), using the calcium phosphate (Clontech) method of transient transfection. Transfections were undertaken essentially according to the manufacturer's instructions, but with minor modifications. Test DNA (1 μg total per well of a 48-well plate) was mixed with sterile water and 2 M calcium chloride solution. This was mixed and left at room temperature for 10 min. The DNA mix was then added dropwise to an equal volume of HEPES-buffered saline. The HEPES-buffered saline was continually agitated as the DNA mix was added to it. This transfection solution was left at room temperature for 30 min. Ten times the volume of the normal growth medium was then added to the transfection solution. The old growth medium was replaced with the transfection solution. After a 5–16 h incubation period, the transfection mix was removed from the cells and replaced with fresh growth medium. The plates were used for cyclic AMP assay 48–72 h after the medium was replaced.
Assay of cyclic AMP production
The growth medium was removed from the cells and replaced with serum and antibiotic-free Dulbecco's modified Eagle medium containing 500 μM isobutyl methyl xanthine for 30 min. All drugs were diluted in the same medium. Antagonists were added for 15 min before the addition of agonists in the range 1 pM–1 μM for a further 15 min. Cyclic AMP was extracted with ice-cold 95–100% ethanol. Cyclic AMP was measured by radio-receptor assay as previously described (Poyner et al., 1992).
Analysis of data
For cyclic AMP studies, the data from each concentration–response curve were fitted to a sigmoidal concentration–response curve to obtain the maximum response, Hill coefficient and EC50, using the fitting routine PRISM Graphpad. From the individual curves, dose ratios were calculated. Where three antagonist concentrations were used, a Schild plot was constructed; after confirming that the slope was not significantly different from unity, it was constrained to 1 to obtain the pKb. Where only one or two antagonist concentrations were used, an apparent pA2 was calculated from the formula log[antagonist]−log(dose ratio−1), after first confirming that there were no significant differences in the Hill coefficient or maximum response between the concentration–response curves in the presence and absence of antagonist.
Statistical analysis was carried out by Student's t-test, or by one-way ANOVA followed by Tukey's test (where every value was compared against each other), or Dunnett's test (where several values were being compared against a single control). The significance was accepted at P<0.05; two-tailed tests were used throughout. All values are quoted as means±s.e.m.
Materials
Rat AM and human AM22–52 were obtained from Bachem (St Helens, Merseyside, U.K.). Human αCGRP (hαCGRP) and human αCGRP8–37 (hαCGRP8–37) were from Calbiochem (Beeston, Nottingham, U.K.) or Neosystems (Strasbourg, France). [cys(ACM)2,7]αCGRP, rat amylin and rat calcitonin were from Bachem (St Helens, U.K.). [cys(Et)2,7]αCGRP was from Phoenix Pharmaceuticals (Mountain View, CA, U.S.A.), and humanβCGRP (hβCGRP) was from Sigma (Gillingham, Dorset, U.K.). Salmon calcitonin was purchased from Cambridge Research Biochemicals (Northwich, Cheshire, U.K.). All peptides were dissolved in distilled water and stored as aliquots at −20°C or −70°C (AM and AM22–52) in nonstick microcentrifuge tubes (Thermo Life Sciences, Basingstoke, U.K.). BIBN4096BS was a gift from Dr M. Schindler (Boehringer-Ingelheim, Biberach, Germany), and was prepared as previously described (Hay et al., 2002). Unless otherwise specified, chemicals were from Sigma or Fisher (Loughborough, U.K.). Cell culture reagents were from Gibco BRL (Paisley, Renfrewshire, U.K.) or Sigma.
Results
Characterisation of baseline receptor expression in Cos 7 cells
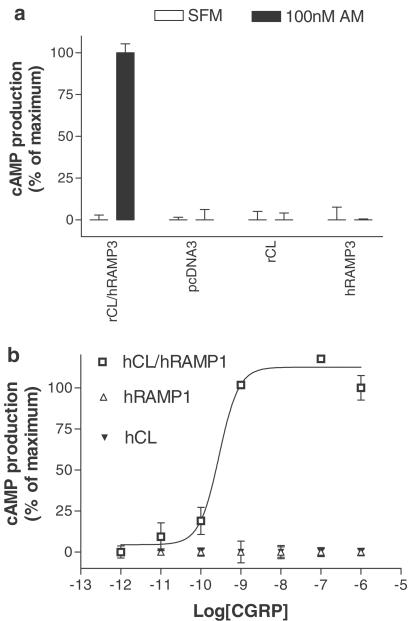
Careful characterisation of AM1 and AM2 receptors required the use of a batch of cells for transfection studies, which do not express CL or RAMPs endogenously. Cos 7 cells have previously been reported to contain only low levels of RAMPs (Tilakaratne et al., 2000). In agreement with this, Figure 1 shows that in cells transfected with CL alone, 100 nM AM (Figure 1a) or concentrations of CGRP up to 1 μM (Figure 1b) failed to cause any increase in cyclic AMP production. This demonstrates that the cells lack any endogenous RAMPs. The cells also failed to respond to these concentrations of AM and CGRP when transfected with RAMP1 or RAMP3 alone, showing the absence of any endogenous CL (Figure 1). By way of positive controls, the cells did respond to AM when transfected with hRAMP3 and rCL, and to CGRP when transfected with hCL and hRAMP1 (Figure 1). These cells were cultured for over 50 passages and tested in this way every few passages. On no occasion were endogenous receptor components evident, making this a suitable cell line for characterising transfected AM1 and AM2 receptors.
Figure 1.
Characterisation of Cos 7 cells, (a) Responses of cells transfected with rCL/hRAMP3, cloning vector (pcDNA3), rCL and hRAMP3, and subsequently challenged with either 100 nM AM or serum-free medium (SFM). (b) Concentration–response curves to hαCGRP in cells transfected with hCL/hRAMP1, hCL and hRAMP1. Points are the mean±s.e.m. of triplicate determinations. These are representative data from experiments repeated 10 times.
Attempts were made to further characterise the putative AM receptor L1 (Kapas et al., 1995) in transfection experiments using COS 7 cells. However, on no occasion was elevation of cyclic AMP evident in response to 1 μM of either AM or CGRP (data not shown). The effects of transfection of L1 in the presence of RAMPs were not examined in this study. Concurrent transfection of RAMPs with L1 was reported to be without effect in a previous study (Chakravarty et al., 2000).
Characterisation of rRAMP3
rRAMP3 showed three differences from the previously reported sequence (Oliver et al., 2001). Codon 128, previously reported as CTG, was TTG; codon 134, previously reported as GGC, was GGG, and codon 137, previously reported as GTG, was GTA. None of these alter the amino acids (i.e. L128, G134 and V137). As Pfu polymerase, used for the polymerase chain reaction, has stringent proofreading ability, the probability of obtaining three errors as a result of this process, none of which alter the coding sequence, is very remote. Accordingly, these are likely to be polymorphisms.
Effect of antagonists on AM responses in hCL/hRAMP2-transfected Cos 7 cells
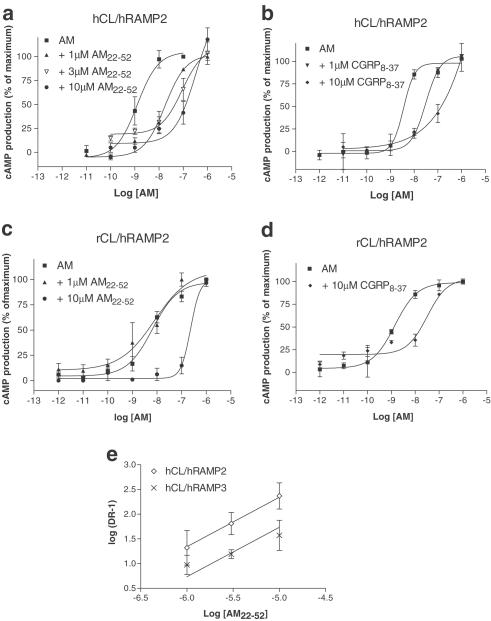
The effects of AM on cyclic AMP responses in hCL/hRAMP2 cotransfected cells in the presence or absence of AM22–52, CGRP8–37 and BIBN4096BS are shown in Table 1 . In the presence of AM22–52, the concentration–effect curve to AM was shifted to the right in a parallel fashion (Figure 2a). These data were used to generate a Schild plot (Figure 2e). As the slope of the line was not significantly different from unity, the slope was constrained to 1, and a pKB of 7.34±0.14 (n=11) estimated. CGRP8–37 also produced a significant change in the pEC50 to AM, with no significant change in Hill coefficient or maximum response (Table 1, Figure 2b). This antagonist was slightly less potent than AM22–52 (apparent pA2 7.04±0.15, n=9, Figure 2b), although the difference was not significant. BIBN4096BS at 10 μM had no significant effect on the response to AM (Table 1).
Table 1.
Actions of antagonists on CL/RAMP2 and CL/RAMP3 complexes
| Receptor | pEC50±s.e.m (n) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| +AM22–52 | +CGRP8–37 | +BIBN4096BS | ||||||||
| AM alone | 1 μM | 3 μM | 10 μM | AM alone | 1 μM | 10 μM | AM alone | 10 μM | ||
| hCL/hRAMP2 | 8.74±0.08 (6) | 7.28±0.33 (3)** | 6.83±0.14 (4)*** | 6.43±0.28 (4)*** | 9.06±0.2 (7) | 7.56±0.1 (3)** | 7.21±0.2 (6)*** | 8.61±0.72 (3) | 7.64±0.72 (3) | |
| rCL/hRAMP2 | 8.16±0.14 (6) | 8.32±0.42 (3) | – | 6.7±0.07 (3)** | 8.23±0.3 (4) | – | 7.36±0.22 (4) | 8.88±0.21 (3) | 8.83±0.13 (3) | |
| rCL/rRAMP2 | Rat-2 | 8.76±0.12 (3) | 7.45±0.15 (3)* | – | – | 8.75±0.11 (3) | 7.77±0.01 (3)* | – | 8.75±0.11 (3) | 9.01±0.09 (3) |
| L6 | 8.70±0.14 (3) | 7.64±0.14 (3)* | – | – | 8.39±0.08 (4) | 7.34±0.01 (3)* | – | 8.70±0.14 (3) | 8.55±0.13 (3) | |
| hCL/hRAMP3 | 9.26±0.21 (7) | 8.41±0.13 (3)* | 8.24±0.15 (3)* | 7.34±0.16 (4)*** | 9.43±0.22 (6) | 8.17±0.17 (3)** | 7.52±0.19 (6)*** | 8.49±0.21 (3) | 7.72±0.28 (3) | |
| rCL/hRAMP3 | 8.89±0.25 (6) | 8.79±0.47 (3) | – | 8.27±0.36 (3) | 8.56±0.25 (3) | – | 7.06±0.14 (3)** | 8.38±0.21 (4) | 7.9±0.20 (4) | |
| rCL/rRAMP3 | 8.30±0.17 (3) | 8.06±0.13 (3) | – | 7.91±0.24 (3) | 8.26±0.72 (5) | 7.43±0.07 (5) | 7.05±0.39 (5)* | ND | ND | |
Numbers in parentheses are n values. *, **, ***Significantly different from control pEC50, Dunnett's test at P<0.05, 0.01 and 0.001 levels, respectively. ND, not determined.
Figure 2.
Characterisation of the stimulation of cyclic AMP production by rat AM in Cos 7 cells transfected with CL/RAMP2 combinations. Points are the mean±s.e.m. of triplicate determinations. Concentration–response curves are representative of three to seven experiments. Data are expressed as the percentage of maximum cyclic AMP production, estimated by fitting each line to a logistic Hill equation, as described in Methods. Maximum cyclic AMP values were 250±20 pmol per 106 cells for hCL/hRAMP2, and 450±60 pmol per 106 cells for hCL/hRAMP3; basal values were all below 10 pmol per 106 cells, (a) hCL/hRAMP2, AM22–52; (b) hCL/hRAMP2, CGRP8–37; (c) rCL/hRAMP2, AM22–52; (d) rCL/hRAMP2, CGRP8–37; (e) Schild plot, antagonism of AM by AM22–52 on hCL/hRAMP2 and hCL/hRAMP3 receptors.
Effect of antagonists on AM responses in rCL/hRAMP2 Cos 7 cells
The effects of AM on cyclic AMP responses in rCL/hRAMP2 cotransfected cells are shown in Table 1. pEC50 values in the presence of AM22–52, CGRP8–37 and BIBN4096BS are also shown. In the presence of AM22–52, the concentration–effect curve to AM was shifted to the right in a parallel fashion (Hill slopes; control 0.77±0.5, 1 μM AM22–52 0.5±0.1, 10 μM AM22–52 1.25±0.5). An apparent pA2 of 6.25±0.17 (n=3) (Figure 2c) was estimated from the shift. There was no significant difference in the pEC50 value to AM obtained in the presence of BIBN4096BS or CGRP8–37 (Table 1, Figure 2d).
Effect of antagonists on rat AM1 receptors endogenously expressed in Rat-2 and L6 cell lines
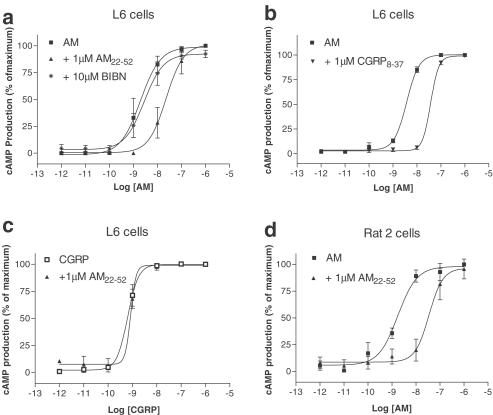
Rat-2 and L6 cell lines have previously been demonstrated to express CL and RAMP2 (Choksi et al., 2002), and are therefore good models of rat AM1 receptors. In L6 cells, AM22–52 (1 μM) produced a significant rightward shift in the concentration–effect curve to AM (Table 1, Figure 3a). From this shift, an apparent pA2 of 7.00±0.05 (n=3) was generated. CGRP8–37 (1 μM) was also effective at inhibiting the effects of AM, eliciting a significant change in the pEC50 (P<0.05, Table 1, Figure 3b). An apparent pA2 value of 7.03±0.12 (n=3) was calculated from these data. The effect of CGRP on cyclic AMP in these cells could not be inhibited by 1 μM AM22–52 (Figure 3c). BIBN4096BS was unable to antagonise the effects of AM in L6 cells up to concentrations of 10 μM (Table 1, Figure 3a). Slow kinetics of BIBN4096BS have previously been reported (Schindler & Doods, 2002); hence, the incubation time for pretreatment with BIBN4096BS was increased from 15 to 60 min. However, this antagonist was still unable to inhibit the effects of AM. The pEC50 values were 8.56±0.32 without BIBN4096BS, compared to 8.30±0.25 (both n=2) in the presence of the antagonist. Therefore, the lack of effect of this antagonist in the studies described above is unlikely to be due to the short (15 min) antagonist incubation time. BIBN4096BS inhibited the binding of 125I-iodohistidyl-CGRP to membranes made from COS 7 cells cotransfected with hCL and hRAMP1, with a pKi of 10.85±0.21. This is in line with its pKi, on SK-N-MC cells which also express hCL and hRAMP1 (Schindler & Doods, 2002), confirming that the antagonist was active.
Figure 3.
Characterisation of the stimulation of cyclic AMP production by rat AM in L6 and Rat 2 cells (endogenous rCL/rRAMP2). Points are the mean±s.e.m. of triplicate determinations. Concentration–response curves are representative of three or four experiments. Data are expressed as the percentage of maximum cyclic AMP production, estimated by fitting each line to a logistic Hill equation, as described in Methods. Maximum cyclic AMP values were 290±20 pmol per 106 cells for Rat 2 cells, and 2000±300 pmol per 106 cells for L6 cells; basal values were all below 10 pmol per 106 cells, (a) L6 cells, AM22–52 and BIBN4096BS against rAM; (b) L6 cells CGRP8–37 against rAM; (c) L6 cells, AM22–52 against αCGRP, (d) Rat 2 cells, AM22–52 against rAM.
CGRP was inactive on Rat-2 cells at concentrations of up to 1 μM, in accordance with published data (Coppock et al., 1999) (n=3, data not shown), but AM caused a concentration-dependent stimulation of cyclic AMP production, as shown in Table 1. AM22–52 (1 μM) caused a rightward shift in the concentration–effect curve to AM, with an apparent pA2 of 7.28±0.06 (n=3, Table 1, Figure 3d). We have previously demonstrated that the AM response in these cells can be antagonised by 1 μM CGRP8–37, but not by 10 μM BIBN4096BS (Hay et al., 2002). These data are included in Table 1 for comparison with the data presented here.
Effect of antagonists on AM responses in hCL/hRAMP3 Cos 7 cells
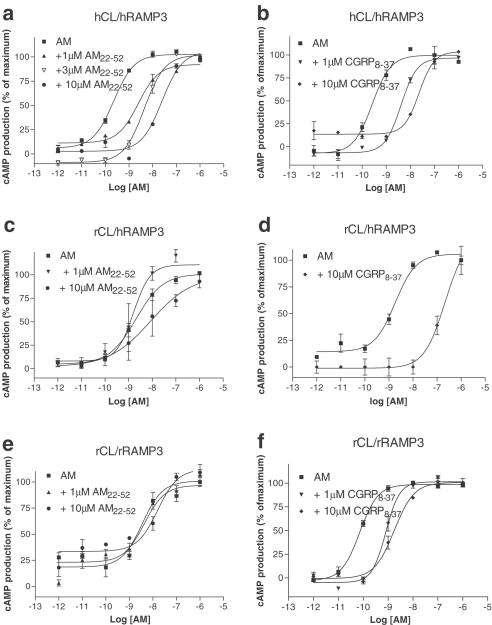
In hCL/hRAMP3 cotransfected cells, the concentration–effect curve to AM was shifted to the right in the presence of AM22–52 or CGRP8–37 (Table 1, Figure 4a, b). Figure 2e shows the Schild plot generated from the antagonist shifts with AM22–52, from which a pKB of 6.73±0.14 (n=10) was estimated. It was significantly less potent at this receptor (P<0.01) than at the hCL/hRAMP2 complex. An apparent pA2 of 6.96±0.08 (n=9, Figure 4b) was generated for CGRP8–37. This was not significantly different from its effects at the hCL/hRAMP2 complex. BIBN4096BS was inactive at up to 10 μM.
Figure 4.
Characterisation of the stimulation of cyclic AMP production by rat AM in Cos 7 cells transfected with CL/RAMP3 combinations. Points are the mean±s.e.m. of triplicate determinations. Concentration–response curves are representative of three to six experiments. Data are expressed as the percentage of maximum cyclic AMP production, estimated by fitting each line to a logistic Hill equation, as described in Methods. Maximum cyclic AMP values were 450±60 pmol per 106 cells for hCL/hRAMP3, 300±25 pmol per 106 cells for hCL/rRAMP3 and 630±70 pmol per 106 cells for rCL/rRAMP3; basal values were all below 10 pmol per 106 cells, (a) hCL/hRAMP3, AM22–52; (b) hCL/hRAMP3 CGRP8–37; (c) rCL/hRAMP3, AM22–52; (d) rCL/hRAMP3, CGRP8–37; (e) rCL/rRAMP3, AM22–52; (f) rCL/rRAMP3, CGRP8–37.
Effect of antagonists on AM responses in rCL/hRAMP3 Cos 7 cells
In the presence of CGRP8–37, the concentration–effect curve to AM was shifted to the right in a parallel fashion (Hill slopes; control 1.3±0. 16, 1 μM CGRP8–37 1.63±0.38, 10 μM CGRP8–37, 1.45±0.45), giving an apparent pA2 of 6.48±0.20 (n=3, Table 1, Figure 4d). AM22–52 up to 10 μM failed to cause any significant shift (Table 1, Figure 4c). In common with the rCL/hRAMP2-transfected cells, BIBN4096BS did not produce any significant effect on AM-stimulated cyclic AMP responses (Table 1).
Effect of antagonists on AM responses in rCL/rRAMP3 Cos 7 cells
At concentrations up to 10 μM, AM22–52 failed to antagonise the actions of AM (Table 1, Figure 4e). However, 10 μM CGRP8–37 did cause a significant shift in the AM concentration–response curve, from which an apparent pA2 of 6.18±0.18 (n=5, Figure 4f) was calculated.
Effect of AM, α and βCGRP and other peptides in rCL/hRAMP3 and rCL/rRAMP3 Cos 7 cells
AM, α and βCGRP, all elevated cyclic AMP with pEC50 values as indicated in Table 2 and Figure 5a and b. For both receptors, the rank potency order was AM>βCGRPzαCGRP. In particular, on the rCL/rRAMP3 complex, AM and βCGRP were not significantly different in potency from each other (Tukey's test, P>0.05). Other peptides were also tested on rCL/hRAMP3 receptor. Here, αCGRP, [Cys(Et)2,7]αCGRP and rat amylin were equipotent. [Cys(ACM)2,7] αCGRP, rat calcitonin, salmon calcitonin and the three antagonists used above were unable to elevate cyclic AMP when acting at the rCL/hRAMP3 receptor.
Table 2.
Peptide potency values for the rCL/hRAMP3 and rCL/rRAMP3 complex
| rCL/hRAMP3 | rCL/rRAMP3 | |||
|---|---|---|---|---|
| pEC50±s.e.m. (n) | Relative potency | pEC50±s.e.m. (n) | Relative potency | |
| rAM | 8.78±0.03 (12) | 1 | 8.56±0.12 (4) | 1 |
| αCGRP | 6.38±0.06 (5) | 0.0039 | 7.37±0.06 (3) | 0.064 |
| βCGRP | 7.94±0.1 (5) | 0.14 | 8.16±0.35 (5) | 0.40 |
| [Cys(Et)2,7]αCGRP | 6.37±0.1 (3) | 0.0038 | ||
| rAmylin | 6.4±0.05 (3) | 0.0041 | ||
[Cys(ACM)2,7] αCGRP, rat calcitonin, salmon calcitonin, AM22–52. CGRP8–37 and BIBN4096BS, all had no effect on cyclic AMP accumulation at concentrations up to 1 μM on the rCL/hRAMP3 receptor.
Figure 5.
Characterisation of the stimulation of cyclic AMP production by agonists in Cos 7 cells transfected with CL/RAMP3 combinations. Points are the mean±s.e.m. of triplicate determinations. Concentration–response curves are representative of three to 12 experiments. Data are expressed as the percentage of maximum cyclic AMP production to rAM, estimated by fitting each line to a logistic Hill equation, as described in Methods, (a) hCL/rRAMP3; (b) rCL/rRAMP3.
Discussion
The functional effects of antagonists at complexes of CL with RAMP2 and RAMP3 are little studied. The limited data previously available suggested that there were no differences between these receptors, despite the low homology between RAMP2 and RAMP3 (Aiyar et al., 2001; 2002; Sexton et al., 2001). Furthermore, the studies that have looked at the detailed pharmacology of these receptors have usually been performed in HEK293 cells which are known to express RAMP2 endogenously. CL/RAMP3 has often been overlooked in the literature. There is a considerable lack of information on this receptor complex although AM stimulates cyclic AMP production with similar potency to its actions at CL/RAMP2 (McLatchie et al., 1998; Aiyar et al., 2001; 2002; Kuwasako et al., 2002). While expression of RAMP3 mRNA is low in rats (Chakravarty et al., 2000), in humans it is at least as abundant as RAMP2 (McLatchie et al., 1998).
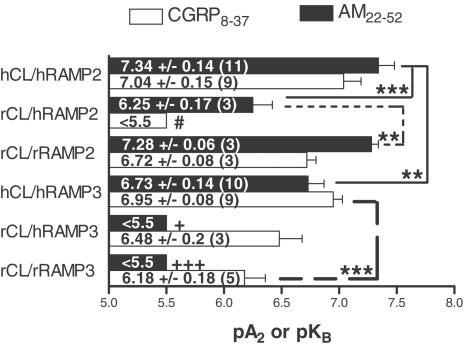
The data in this study suggest that there are pharmacological differences between CL/RAMP2 and CL/RAMP3 receptors (Figure 6). AM22–52 was a significantly more effective antagonist of AM at the CL/RAMP2 receptor compared to the CL/RAMP3 receptor, regardless of species composition. CGRP8–37 was less discriminating, but in the mixed rat/human receptor it had the opposite selectivity to AM22–52. As a consequence of these effects, AM22–52 was numerically more potent than CGRP8–37 at all CL/RAMP2 receptors (although the difference was only statistically significant for the mixed rat/human receptor), whereas the opposite was true for the CL/RAMP3 receptors (with statistically significant differences between the antagonists at the all-rat and rat/human receptors). BIBN4096BS showed no measurable antagonist activity at any of the CL/RAMP2 or CL/RAMP3 combinations, confirming its status as an extremely selective antagonist at CL/RAMP1 receptors (see Hay et al., 2002, for a full account of the pharmacology of this compound at CL/RAMP1 receptors expressed on L6 and SK-N-MC cells). We also determined agonist potency ratios at the mixed species and all-rat CL/RAMP3 receptor. This revealed that the potency of βCGRP approached that of AM (at the all-rat receptor they were statistically indistinguishable). This finding was similar to that reported by Fraser et al. (1999) for the all-human CL/RAMP3 receptor.
Figure 6.
Summary of apparent pA2 or pKb values for CGRP8–37 and AM22–52 on CL/RAMP combinations. Values that are significantly different from each other according to Tukey's test are indicated. Where the affinity of the antagonist was too low to be calculated, the following comparisons have been highlighted: +P<0.05; outside the 95% confidence limit for AM22–52 on rCL/hRAMP2. +++P<0.001; outside the 99.9% confidence limits for AM22–52 on rCL/rRAMP2. #P<0.05; outside the 95% confidence limit for CGRP8–37 on rCL/hRAMP3.
The data revealed marked differences depending upon the species composition of the CL/RAMP receptors. For CL/RAMP2 receptors, the AM22–52 and CGRP8–37 had significantly lower affinities at the mixed species receptor compared to the human and rat receptors. There were no differences between the human and rat receptors. For CL/RAMP3 receptors, a somewhat different pattern was seen; the pA2 for AM22–52 at both the mixed and rat receptors was too low to measure, and for CGRP8–37 there were no significant differences between any of the receptors.
Some caution is needed in interpreting these results. It was not possible to use concentrations of these antagonists greater than 10 μM. Thus, it was not possible to establish pA2 values where the antagonists were inactive at this concentration, and in most cases apparent pA2 values had to be calculated from one or two antagonist concentrations. It was also not possible to study potencies in radioligand-binding studies, due to low levels of specific binding in the RAMP3-transfected cells. However, in the past, we have found that apparent pA2 values calculated in this manner are in good agreement with pKi values obtained from radioligand-binding studies (Poyner et al., 1992; 1998; Howitt et al., 2003). Furthermore, the published radioligand-binding data on human and rat RAMP/CL combinations are in agreement with our findings (McLatchie et al., 1998; Buhlmann et al., 1999; Fraser et al., 1999; Aiyar et al., 2001; Aldecoa et al., 2000; Oliver et al., 2001). Thus, we feel that the patterns seen in our results do reflect the underlying pharmacological differences.
There is one apparent discrepancy between this work and our previous studies on L6 cells. While we previously described the presence of an AM receptor on these cells, we could not demonstrate any stimulation of adenylate cyclase as a result of activation of this receptor (Coppock et al., 1996). However, we subsequently demonstrated that L6 cells express mRNA for CL, RAMP1 and RAMP2, and so should express the CL/RAMP2 receptor that is normally linked to Gs and cyclic AMP production (Choksi et al., 2002). In the present study, it is clear that AM activates cyclic AMP production by a receptor that is distinct from that used by CGRP; the AM but not the CGRP response is blocked by 1 μM AM22–52, and CGRP8–37 is an order of magnitude more effective against CGRP (pA2∼8; Poyner et al., 1992; 1998; Hay et al., 2002; Howitt et al., 2003) compared to AM. The low potency of AM22–52 against CGRP acting at CL/RAMP1 has been seen in other studies (McLatchie et al., 1998; Husmann et al., 2000; Oliver et al., 2001; Nagoshi et al., 2002). It may be significant that the batch of L6 cells used for this study appears to have a much greater ratio of adrenomedullin receptors to CGRP receptors (as judged by the ratio of RAMP2 to RAMP1, see Choksi et al., 2002) compared to the L6 cells used by Coppock et al. We have previously noted considerable variability over time in the responses of cell lines to CGRP and AM (Choksi et al., 2002).
The differences in affinities seen between CL/RAMP2 and CL/RAMP3 may be rationalised by considering the structures of these proteins. RAMP3 shows greater similarities to RAMP1 than RAMP2. RAMP2 is N-terminally extended compared to RAMPs 1 and 3; on the other hand, the latter have an extra pair of conserved cysteines that may create a disulphide-bonded loop that is absent in RAMP2. The consensus sequences at either end of this putative loop (NH2CN/QE..GCY/FW..) are very different from the RAMP2 sequence (..GTV…DLGF..), and may contribute to the different pharmacologies shown by the CL/RAMP2 and CL/RAMP3 complexes. The very low affinity shown by BIBN4096BS for either CL/RAMP2 or CL/RAMP3 is consistent with it primarily interacting with human (but not rat) RAMP1 at a site not conserved in the other RAMPs (Sexton et al., 2001). Mallee et al. (2002) showed that a key residue for high-affinity binding of BIBN4096BS with hRAMP1 was W74; in both hRAMPs 2 and 3, the equivalent residue is a glutamate.
The reduced affinities demonstrated by the mixed species CL/RAMP2 receptors suggest a process of correlated mutagenesis in their evolution, where mutations in one component are compensated by mutations in the other partner. Rat and human CL are very similar, except at the extreme N-terminus. Ignoring the signal peptide, only three of the first 12 positions are identical, with a further four conservative substitutions. By contrast, there are only 24 nonidentical residues in the rest of the protein. The first 18 amino acids of hCL have been identified as necessary for AM and CGRP binding (Koller et al., 2002). Thus, this seems by far the most likely site on CL to explain species differences. Human and rat RAMP2 become progressively more divergent as their N-terminii are approached; the C-terminal thirds show 96% identity, the middle thirds 68% identity and N-terminal thirds only 37% identity. On this basis, it seems likely that there is an interaction between the extreme N-terminus of CL and the N-terminal third of RAMP2, which is disrupted in the rat/human mixed receptor. For RAMP3, the extracellular domains of the rat and human proteins are 86% identical, and the mismatches are randomly distributed over the sequence. Given this similarity, it is not surprising that the species effects are much less pronounced, and it implies that the variation at the extreme N-terminus of CL is of less significance in determining affinities. Nonetheless, differences can still be seen (e.g. between the apparent pA2 values for AM22–52 at rCL/rRAMP3 and hCL/hRAMP3), and these probably reflect the species divergence between human and rat CL at the extreme N-termini of the proteins.
The data in this paper have several implications for the study of CGRP and adrenomedullin pharmacology. They suggest that affinities obtained from mixed species CL/RAMP receptors need to be treated with caution if the RAMPs themselves show divergence. Caution should be applied when interpreting data from transfected cells where, for example, hRAMP1 (with CL?) is transfected into LLC-PK1 cells which endogenously express a porcine CT receptor. What would the result of these complex interactions be? Such considerations have been taken into account for amylin receptors (Tilakaratne et al., 2000). The multiphasic curves obtained in these studies suggest very complex interactions of exogenously applied receptors with endogenous cellular components (Tilakaratne et al., 2000). Our data also show that species differences can result in significant differences to antagonist potencies, as has previously been shown for BIBN4096BS at CL/RAMP1 receptors (Doods et al., 2000). To fully interpret data from studies on non-human tissues using the currently available agonists and antagonists, it is desirable to have data on the properties of these agents against recombinant receptors from the species under investigation. A comprehensive analysis might also need to consider the possible actions of AM and CGRP against CT receptor/RAMP complexes (Tilakaratne et al., 2000).
The pharmacology of the CL/RAMP3 receptor is interesting. The all-rat receptor shows a moderate EC50 for CGRP in our system, and it has a pA2 below 7 for CGRP8–37. Although [Cys(Et)2,7]αCGRP was not tested on rCL/rRAMP3, on the mixed species equivalent it was equipotent with hαCGRP. These are characteristics of a CGRP2 receptor (Juaneda et al., 2000). It is not suggested that this is the basis of all reports of CGRP2 receptors; the high affinity for adrenomedullin should be a distinctive feature of a CL/RAMP3 complex. However, it is possible that in tissues that express CL with a mixture of RAMPs, this may be one contribution to heterogeneity in receptors that respond to CGRP. Indeed, the pA2 of CGRP8–37 on the CL/RAMP2 complexes is also within the range of CGRP2-like receptors (Juaneda et al., 2000). While the CL/RAMP2 receptors have a low affinity for CGRP, it is not impossible that in tissues with a high receptor reserve, they could also make a contribution to the CGRP response that showed modest sensitivity to CGRP8–37.
The CL/RAMP3 receptor showed a clear preference for β over αCGRP and this is reflected in radioligand-binding studies (Fraser et al., 1999; Aiyar et al., 2001). At the all-rat CL/RAMP3 combination, βCGRP was statistically indistinguishable from AM. A similar rank potency order has also been reported for CL/RAMP2 (McLatchie et al., 1998; Buhlmann et al., 1999; Fraser et al., 1999; Aldecoa et al., 2000; Aiyar et al., 2001; Oliver et al., 2001), although the relative potency of βCGRP to AM is less for this receptor. There are several reports of CGRP8–37 (Jansen-Olesen et al., 1996; Tomlinson & Poyner, 1996; Yoshimoto et al., 1998) and BIBN4096BS (Wu et al., 2000) antagonising αCGRP more effectively than βCGRP. It is possible that this reflects the expression of CL/RAMP1 with either CL/RAMP2 or CL/RAMP3; while both forms of CGRP would activate CL/RAMP1, βCGRP would preferentially activate CL/RAMP2 or CL/RAMP3, thus showing resistance to CGRP8–37.
CGRP8–37 appears in this study as a drug with only very limited selectivity. There is not much more than a 10-fold difference in its affinity on CL/RAMP1 receptors and the CL/RAMP2 and CL/RAMP3 receptors. The use of AM22–52 is also problematic. Radioligand-binding studies have suggested that it has 100-fold greater affinity at CL/RAMP2 receptors compared to CL/RAMP1 receptors (see Poyner et al., 2002, for summary). This is consistent with our results comparing its effects at 1 μM on the endogenous CL/RAMP1 and CL/RAMP2 receptors expressed by L6 cells and also the studies of Nagoshi et al. (2002), where it was ineffective on all-human CL/RAMP1 receptors. Thus, where only these two receptors are present, it is a useful antagonist. However, as it shows an intermediate affinity for CL/RAMP3 receptors, in tissues where this is present, its selectivity will be impaired.
Although it has been shown that BIBN4096BS has low affinity for AM-binding sites in tissues (Doods et al., 2000), the functional effects of this antagonist have not been examined on AM receptors of known molecular composition (with the exception of the Rat 2 cells studied by Hay et al., 2002). It was by far the most selective of the antagonists used in our study. The inability to antagonise AM at the CL/RAMP2 complex expressed by L6 and Rat-2 cells demonstrates that BIBN4096BS shows at least a 1000-fold preference for CL/RAMP1 in rats (Hay et al., 2002). Curiously, BIBN4096BS antagonised AM responses in the rat vas deferens (Wu et al., 2000). The nature of this AM receptor remains unknown although CL and each RAMP are present in this tissue (Chakravarty et al., 2000). The data presented here suggest that BIBN4096BS is unlikely to interact with a simple CL/RAMP2 complex in rat vas deferens. This compound was also unable to inhibit the effects of AM at complexes of rCL and hRAMPs 2 or 3.
In conclusion, this study demonstrates that there are pharmacological differences between the AM1 and AM2 receptors formed by CL/RAMP2 and CL/RAMP3. The magnitude of these differences depends on the species, and the current peptide antagonists are unlikely to be selective enough to exploit the differences. However, it is possible that future antagonists may show more discrimination between the subtypes.
Acknowledgments
This work was supported by a grant from the Wellcome Trust. D.L.H. was funded by a Medical Research Council studentship.
Abbreviations
- AM
adrenomedullin
- BIBN4096BS
1-piperidinecarboxamide, N-[2-[[5amino-1-[[4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)
- CGRP
calcitonin gene-related peptide
- CL
calcitonin receptor-like receptor
- [cys(ACM)2,7]αCGRP
Cys-acetoamidomethyl2,7 human αCGRP
- [cys(Et)2,7]αCGRP
Cys-ethylamide2,7 human αCGRP
- r
rat
- RAMP
receptor activity-modifying protein
References
- AIYAR N., DISA J., AO Z., XU D., SURYA A., PILLARISETTI K., PARAMESWARAN N., GUPTA S.K., DOUGLAS S.A., NAMBI P. Molecular cloning and pharmacological characterization of bovine calcitonin receptor-like receptor from bovine aortic endothelial cells. Biochem. Pharmacol. 2002;63:1949–1959. doi: 10.1016/s0006-2952(02)00990-5. [DOI] [PubMed] [Google Scholar]
- AIYAR N., DISA J., PULLEN M., NAMBI P. Receptor activity modifying proteins interaction with human and porcine calcitonin receptor-like receptor (CRLR) in HEK-293 cells. Mol. Cell. Biochem. 2001;224:123–133. doi: 10.1023/a:1011907328682. [DOI] [PubMed] [Google Scholar]
- AIYAR N., RAND K., ELSHOURBAGY N.A., ZENG Z.Z., ADAMOU J.E., BERGSMA D.J., LI Y. A cDNA encoding the calcitonin-gene-related peptide type-1 receptor J. Biol. Chem. 1996;271:11325–11329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- ALDECOA A., GUJER R., FISCHER J., BORN W. Mammalian calcitonin receptor-like receptor/receptor modifying protein complexes define calcitonin gene-related peptide and adrenomedullin receptors in Drosophila Schneider 2 cells. FEBS Lett. 2000;471:156–160. doi: 10.1016/s0014-5793(00)01387-9. [DOI] [PubMed] [Google Scholar]
- BUHLMANN N., LEUTHAUSER K., MUFF R., FISCHER J.A., BORN W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- CARON K.M., SMITHIES O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional adrenomedullin gene. Proc. Natl. Acad. Sci. U.S.A. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKRAVARTY P., SUTHAR T.P., COPPOCK H.A., NICHOLL C.G., BLOOM S.R., LEGON S., SMITH D.M. CGRP and adrenomedullin binding correlates with transcript levels for calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) in rat tissues. Br. J. Pharmacol. 2000;130:189–195. doi: 10.1038/sj.bjp.0702975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMPION H.C., SANTIAGO J.A., MURPHY W.A., COY D.H., KADOWITZ P.J. Adrenomedullin-(22–52) antagonizes vasodilator responses to CGRP but not adrenomedullin in the cat. Am. J. Physiol. 1997;272:R234–R242. doi: 10.1152/ajpregu.1997.272.1.R234. [DOI] [PubMed] [Google Scholar]
- CHIBA T., YAMAGUCHI A., YAMATANI T., NAKAMURA A., MORISHITA T., INUI T., FUKASE M., NODA T., FUJITA T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8–37) Am. J. Physiol. 1989;256:E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- CHOKSI T., HAY D.L., LEGON L., POYNER D.R., HAGNER S., BLOOM S.R., SMITH D.M. Comparison of the expression of calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) with CGRP and adrenomedullin binding in cell lines. Br. J. Pharmacol. 2002;136:784–792. doi: 10.1038/sj.bjp.0704761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPPOCK H.A., OWJI A.A., AUSTIN C., UPTON P.D., JACKSON M.L., GARDINER J.V., GHATEI M.A., BLOOM S.R., SMITH D.M. Rat-2 fibroblasts express specific adrenomedullin receptors, but not calcitonin-gene-related-peptide receptors, which mediate increased intracellular cyclic AMP and inhibit mitogen-activated protein kinase activity. Biochem. J. 1999;338:15–22. [PMC free article] [PubMed] [Google Scholar]
- COPPOCK H.A., OWJI A.A., BLOOM S.R., SMITH D.M. A rat skeletal muscle cell line (L6) expresses specific adrenomedullin binding sites but activates adenylate cyclase via calcitonin gene-related peptide receptors. Biochem. J. 1996;318:241–245. doi: 10.1042/bj3180241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNIS T., FOURNIER A., CADIEUX A., POMERLEAU F., JOLICOEUR F.B., ST PIERRE S., QUIRION R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J. Pharmacol. Exp. Ther. 1990;254:123–128. [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., WU D., ENTZEROTH M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., ST PIERRE S., QUIRION R. A potent and selective CGRP2 agonist, [Cys(Et)2,7]hCGRP alpha: comparison in prototypical CGRP1 and CGRP2 in vitro bioassays. Can. J. Physiol. Pharmacol. 1997;75:671–676. [PubMed] [Google Scholar]
- EGUCHI S., HIRATA Y., IWASAKI H., SATO K., WATANABE T.X., INUI T., NAKAJIMA K., SAKAKIBARA S., MARUMO F. Structure–activity relationship of adrenomedullin, a novel vasodilatory peptide, in cultured rat vascular smooth muscle cells. Endocrinology. 1994;135:2454–2458. doi: 10.1210/endo.135.6.7988431. [DOI] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., ADAMOU J.E., SWIFT A.M., DISA J., MAO J., GANGULY S., BERGSMA D.J., AIYAR N. Molecular cloning and characterization of the porcine calcitonin gene-related peptide receptor. Endocrinology. 1998;139:1678–1683. doi: 10.1210/endo.139.4.5860. [DOI] [PubMed] [Google Scholar]
- FRASER N.J., WISE A., BROWN J., MCLACHTIE L.M., MAIN M.J., FOORD S.M. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol. Pharmacol. 1999;55:1054–1059. doi: 10.1124/mol.55.6.1054. [DOI] [PubMed] [Google Scholar]
- HAY D.L., HOWITT S.G., CONNER A.C., DOODS H., SCHINDLER M., POYNER D.R. A comparison of the actions of BIBN4096BS and CGRP(8-37) on CGRP and adrenomedullin receptors expressed on SK-N-MC, L6, Col 29 and Rat 2 cells. Br. J. Pharmacol. 2002;137:80–86. doi: 10.1038/sj.bjp.0704844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAY D.L., SMITH D.M. Knockouts and transgenics confirm the importance of adrenomedullin in the vasculature. Trends Pharmacol. Sci. 2001;22:57–59. doi: 10.1016/s0165-6147(00)01617-5. [DOI] [PubMed] [Google Scholar]
- HINSON J.P., KAPAS S., SMITH D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- HOWITT S.G., KILK K., WANG Y., SMITH D.M., LANGEL U., POYNER D.R. The role of the 8–18 helix of CGRP(8–37) in mediating high affinity binding to CGRP receptors; coulombic and steric interactions. Br. J. Pharmacol. 2003;138:325–332. doi: 10.1038/sj.bjp.0705040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSMANN K., SEXTON P.M., FISCHER J.A., BORN W. Mouse receptor-activity-modifying proteins 1, -2 and -3: amino acid sequence, expression and function. Mol. Cell. Endocrinol. 2000;162:35–43. doi: 10.1016/s0303-7207(00)00212-4. [DOI] [PubMed] [Google Scholar]
- JANSEN-OLESEN I., MORTENSEN A., EDVINSSON L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- JUANEDA C., DUMONT Y., QUIRION R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol. Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- KAPAS S., CATT K.J., CLARK A.J. Cloning and expression of cDNA encoding a rat adrenomedullin receptor. J. Biol. Chem. 1995;270:25344–25347. doi: 10.1074/jbc.270.43.25344. [DOI] [PubMed] [Google Scholar]
- KOLLER D., BORN W., LEUTHAUSER K., FLUHMANN B., MCKINNEY R.A., FISCHER J.A., MUFF R. The extreme N-terminus of the calcitonin-like receptor contributes to the selective interaction with adrenomedullin or calcitonin gene-related peptide. FEBS Lett. 2002;531:464–468. doi: 10.1016/s0014-5793(02)03585-8. [DOI] [PubMed] [Google Scholar]
- KUWASAKO K., KITAMURA K., ITO K., UEMURA T., YANAGITA Y., KATO J., SAKATA T., ETO T. The seven amino acids of human RAMP2 (86–92) and RAMP3 (59–65) are critical for agonist binding to human adrenomedullin receptors. J. Biol. Chem. 2001;276:49459–49465. doi: 10.1074/jbc.M108369200. [DOI] [PubMed] [Google Scholar]
- KUWASAKO K., KITAMURA K., ONITSUKA H., UEMURA T., NAGOSHI Y., KATO J., ETO T. Rat RAMP domains involved in adrenomedullin binding specificity. FEBS Lett. 2002;519:113–116. doi: 10.1016/s0014-5793(02)02721-7. [DOI] [PubMed] [Google Scholar]
- MALLEE J.J., SALVATORE C.A., LEBOURDELLES B., OLIVER K.R., LONGMORE J., KOBLAN K., KANE S.A. RAMP1 determines the species selectivity of non-peptide CGRP receptor antagonists. J. Biol. Chem. 2002;277:14294–14298. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MILLER M.J., MARTINEZ A., UNSWORTH E.J., THIELE C.J., MOODY T.W., ELSASSER T., CUTTITTA F. Adrenomedullin expression in human tumor cell lines: its potential role as an autocrine growth factor. J. Biol. Chem. 1996;271:23345–23351. doi: 10.1074/jbc.271.38.23345. [DOI] [PubMed] [Google Scholar]
- NAGAE T., MUKOYAMA M., SUGAWARA A., MORI K., YAHATA K., KASAHARA M., SUGANAMI T., MAKINO H., FUJINAGA Y., YOSHIOKA T., TANAKA I., NAKAO K. Rat receptor-activity-modifying proteins (RAMPs) for adreno-medullin/CGRP receptor: cloning and upregulation in obstructive nephropathy. Biochem. Biophys. Res. Commun. 2000;270:89–93. doi: 10.1006/bbrc.2000.2390. [DOI] [PubMed] [Google Scholar]
- NAGOSHI Y., KUWASAKO K., ITO K., UEMURA T., KATO J., KITAMURA K., ETO T. The calcitonin receptor-like receptor/receptor activity-modifying protein 1 heterodimer can function as a calcitonin gene-related peptide-(8–37)-sensitive adrenomedullin receptor. Eur. J. Pharmacol. 2002;450:237–243. doi: 10.1016/s0014-2999(02)02184-2. [DOI] [PubMed] [Google Scholar]
- NIKITENKO L.L., SMITH D.M., HAGUE S., WILSON C.R., BICKNELL R., REES M.C. Adrenomedullin and the microvasculature. Trends Pharmacol. Sci. 2002;23:101–103. doi: 10.1016/S0165-6147(00)01983-0. [DOI] [PubMed] [Google Scholar]
- NJUKI F., NICHOLL C.G., HOWARD A., MAK J.C., BARNES P.J., GIRGIS S.I., LEGON S. A new calcitonin-receptor like sequence in rat pulmonary blood vessels. Clin. Sci. Colch. 1993;85:385–388. doi: 10.1042/cs0850385. [DOI] [PubMed] [Google Scholar]
- OLIVER K.R., KANE S.A., SALVATORE C.A., MALLEE J.J., KINSEY A.M., KOBLAN K.S., KEYVAN-FOULADI N., HEAVENS R.P., WAINWRIGHT A., JACOBSON M., DICKERSON I.M., HILL R.G. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur. J. Neurosci. 2001;14:618–628. doi: 10.1046/j.0953-816x.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- ONO Y., OKANO I., KOJIMA M., OKADA K., KANGAWA K. Decreased gene expression of adrenomedullin receptor in mouse lungs during sepsis. Biochem. Biophys. Res. Commun. 2000;271:197–202. doi: 10.1006/bbrc.2000.2606. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., ANDREW D.P., BROWN D., BOSE C., HANLEY M.R. Pharmacological characterization of a receptor for calcitonin gene-related peptide on rat, L6 myocytes. Br. J. Pharmacol. 1992;105:441–447. doi: 10.1111/j.1476-5381.1992.tb14272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POYNER D.R., TAYLOR G.M., TOMLINSON A.E., RICHARDSON A.G., SMITH D.M. Characterization of receptors for calcitonin gene-related peptide and adrenomedullin on the guinea-pig vas deferens. Br. J. Pharmacol. 1999;126:1276–1282. doi: 10.1038/sj.bjp.0702437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POYNER D.R., SEXTON P.M., MARSHALL I., SMITH D.M., QUIRION R., BORN W., MUFF R., FISCHER J.A., FOORD S.M. International Union of Pharmacology. XXXII. The mammalian CGRP, adrenomedullin, amylin and calcitonin receptors. Pharm. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., SOOMETS U., HOWITT S.G., LANGEL U. Structural determinants for binding to CGRP receptors expressed by human SK-N-MC and Col-29 cells: studies with chimeric and other peptides. Br. J. Pharmacol. 1998;124:1659–1666. doi: 10.1038/sj.bjp.0702032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER M., DOODS H.N. Binding properties of the novel, non-peptide CGRP receptor antagonist radioligand, [(3)H]BIBN4096BS. Eur. J. Pharmacol. 2002;442:187–193. doi: 10.1016/s0014-2999(02)01544-3. [DOI] [PubMed] [Google Scholar]
- SEXTON P.M., ALBISTON A., MORFIS M., TILAKARATNE N. Receptor activity modifying proteins. Cell Signal. 2001;13:73–83. doi: 10.1016/s0898-6568(00)00143-1. [DOI] [PubMed] [Google Scholar]
- SHINDO T., KURIHARA Y., NISHIMATSU H., MORIYAMA N., KAKOKI M., WANG Y., IMAI Y., EBIHARA A., KUWAKI T., JU K.H., MINAMINO N., KANGAWA K., ISHIKAWA T., FUKUDA M., AKIMOTO Y., KAWAKAMI H., IMAI T., MORITA H., YAZAKI Y., NAGAI R., HIRATA Y., KURIHARA H. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104:1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- TILAKARATNE N., CHRISTOPOULOS G., ZUMPE E.T., FOORD S.M., SEXTON P.M. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J. Pharmacol. Exp. Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- TOMLINSON A.E., POYNER D.R. Multiple receptors for CGRP and amylin on guinea pig vas deferens and vas deferens. Br. J. Pharmacol. 1996;117:1362–1368. doi: 10.1111/j.1476-5381.1996.tb16737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITHERS D.J., COPPOCK H.A., SEUFFERLEIN T., SMITH D.M., BLOOM S.R., ROZENGURT E. Adrenomedullin stimulates DNA synthesis and cell proliferation via elevation of cyclic AMP in Swiss 3T3 cells. FEBS Lett. 1996;378:83–87. doi: 10.1016/0014-5793(95)01427-6. [DOI] [PubMed] [Google Scholar]
- WU D., EBERLEIN W., RUDOLF K., ENGEL W., HALLERMAYER G., DOODS H. Characterisation of calcitonin gene-related peptide receptors in rat atrium and vas deferens: evidence for a [Cys(Et)(2, 7)]hCGRP-preferring receptor. Eur. J. Pharmacol. 2000;400:313–319. doi: 10.1016/s0014-2999(00)00407-6. [DOI] [PubMed] [Google Scholar]
- YOSHIMOTO R., MITSUI-SAITO M., OZAKI H., KARAKI H. Effects of adrenomedullin and calcitonin gene-related peptide on contractions of the rat aorta and porcine coronary artery. Br. J. Pharmacol. 1998;123:1645–1654. doi: 10.1038/sj.bjp.0701805. [DOI] [PMC free article] [PubMed] [Google Scholar]