Abstract
The effect of preconditioning with U50,488 H (UP), a selective kappa-opioid receptor (κ-OR) agonist, on infarct size and intracellular Ca2+ ([Ca2+]i) in the heart subjected to ischaemic insults were studied and evaluated. U50,488 H administered intravenously reduced the infarct size 18–48 h after administration in isolated hearts subjected to regional ischaemia/reperfusion (I/R). The effect was dose dependent. A peak effect was reached at 10 mg kg−1 U50,488 H and at 24 h after administration. The effect of 10 mg kg−1 U50,488 H at 24 h after administration was abolished by nor-binaltorphimine (nor-BNI), a selective κ-OR antagonist, indicating the effect was κ-OR mediated.
The infarct reducing effect of U50,488 H was attenuated when a selective blocker of mitochondrial (5-hydroxydecanoic acid, 5-HD) or sarcolemmal (HRM-1098) ATP-sensitive potassium channel (KATP) was coadministered with U50,488 H 24 h before ischaemia or when 5-HD was administered just before ischaemia.
U50,488 H also attenuated the elevation in [Ca2+]i and reduction in electrically induced [Ca2+]i transient in cardiomyocytes subjected to ischaemic insults. The effects were reversed by blockade of KATP channel, which abolished the protective effect of preconditioning with U50,488 H.
The results indicated that mitochondrial KATP channel serves as both a trigger and a mediator, while sarcolemmal KATP channel as a trigger only, of delayed cardioprotection of κ-OR stimulation. The effects of these channels may result from prevention/attenuation of [Ca2+]i overload induced by ischaemic insults.
Keywords: Kappa-opioid receptor, preconditioning, ATP-sensitive potassium channel, intracellular Ca2+, U50;488H, ischaemia and reperfusion, metabolic inhibition and anoxia
Introduction
Brief periods of ischaemia lead to a reduced severity of cardiac injury following a subsequent or more sustained ischaemia, a mechanism termed ischaemic preconditioning (IP). IP has two phases – an early phase or first window of protection that lasts for 1–3 h after the IP and a delayed phase or second window of protection that appears 12–24 h after the IP and may last for 24–72 h. Previous studies have shown that pharmacological preconditioning by stimulation of cardiac kappa-opioid receptor (κ-OR) with trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]-benzeneacetamide (U50,488H), a selective κ-OR agonist, confers both immediate (Wang et al., 2001a; Ho et al., 2002; Valtchanova-Matchouganska & Ojewole, 2002) and delayed (Wu et al., 1999; Zhou et al., 2001; Wang et al., 2001b) cardioprotection. Both protein kinase C (PKC)-epsilon (Wang et al., 2001b) and stress-inducible heat shock protein 70 (Zhou et al., 2001) play important roles in delayed cardioprotection of preconditioning with κ-OR stimulation with U50,488 H. The roles of ATP-sensitive potassium (KATP) channels, another component likely involved in IP, are however not well known. There are two types of KATP channels, namely mitochondrial KATP chanel (mitoKATP) and sarcolemmal KATP channel (sarcKATP). The role of mitoKATP channel has been well established to play an important role in various types of preconditioning, whereas that of sarcKATP channel is controversial (Oldenburg et al., 2002).
Intracellular Ca2+ ([Ca2+]i) overload is well known to be an immediate cause that precipitates cardiac injury (Ylitalo et al., 2000). Ischaemia increases [Ca2+]i while IP attenuates the elevation in [Ca2+]i (Ylitalo et al., 2000), suggesting that IP may confer cardioprotection by attenuating the [Ca2+]i overload. It has been shown that opening of the mitoKATP channel depolarizes the intramitochondrial membrane, leading to a transient swelling and an increased production of ATP (Halestrap, 1994). ATP is essential to support the ATP-dependent ionic pumps that include the Ca2+ pumps. Opening of the KATP channel with pinacidil, a nonselective opener of the channel, has been shown to prevent the [Ca2+]i overload induced by severe metabolic stress (Jovanovic & Jovanovic, 2001). It has also been shown that activation of PKC prevented the [Ca2+]i overload and conferred cardioprotection against hypoxic insults, and blockade of the mitoKATP channel attenuated the effects of activating of PKC (Light et al., 2001). SarcKATP channel may also prevent [Ca2+]i overload by shortening the action potential duration, which reduces the Ca2+ entry (Gross & Fryer, 1999). It is expected that the opening of KATP channels, which triggers or mediates delayed cardioprotection of preconditioning, also attenuates the [Ca2+]i overload induced by ischaemic insults. Furthermore, blockade of the channels, which attenuates/abolishes the delayed cardioprotection, also attenuates/abolishes the attending action of opening of the channels or preconditioning on [Ca2+]i overload induced by ischaemic insults.
The present study attempted to define the roles of KATP channels in delayed cardioprotection of preconditioning with U50,488 H (UP) in order to provide more evidence on the roles of these channels, particularly the sarcKATP channel. Secondly, we determined the roles of these channels in [Ca2+]i homeo-stasis altered by ischaemic insults. We studied delayed cardioprotection, because its mechanism is less well understood and it may be of greater clinical importance in view of longer duration of protection. We administered intravenously U50,488 H to rats, which provides pharmacological preconditioning. After 24 h, a duration required for maximum protection as determined in previous (Wu et al., 1999) and present studies, we determined myocardial infarct in the heart upon sublethal ischaemia/reperfusion (I/R) and alterations in [Ca2+]i and electrically induced [Ca2+]i transient in isolated ventricular myocytes upon metabolic inhibition and anoxia (MI/A), which are the consequences of ischaemia. Results showed that UP conferred delayed cardioprotection in vivo. The mitoKATP channel acts as a trigger and a mediator, while sarcKATP channel as a trigger in delayed cardioprotection against ischaemic insults. The cardioprotective actions of these channels were accompanied by attenuation of [Ca2+]i overload in the heart.
Methods
Animals
Male Sprague–Dawley rats weighing 250–300 g supplied by The Laboratory Animal Unit, The University of Hong Kong, were used. All animal experiments were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong.
Pharmacological preconditioning with U50,488H (UP)
U50,488H dissolved in saline solution was given intravenously to the rat. At different time intervals (12–72 h) after administration of the drug, the rat was killed and the heart was removed for studies on cardioprotection and [Ca2+]i homeostasis.
Langendorff-perfused isolated rat heart preparation
The isolated perfused heart preparation was described previously (Wang et al., 2001a). Briefly, the heart was removed immediately after the rat was killed. It was mounted to the Langendorff apparatus and perfused retrogradely under a constant pressure of 100 cmH2O with a Krebs–Ringer solution containing (in mM) 115 NaCl, 5 KCl, 1.2 MgSO4, 1.2 KH2PO4, 1.25 CaCl2, 25 NaHCO3, and 1 l glucose. The solution was aerated with 95% O2–5% CO2 at pH 7.4. The temperature of the perfusion solution was maintained at 36°C. Total coronary arterial flow (CF) was measured by timed collection of the coronary venous effluent in a graduated cylinder. A 2-0 silk suture was passed around the left main coronary artery close to its origin with a taper needle, and the ends were passed through a small vinyl tube to form a snare. The coronary artery was occluded by pulling the snare, which produced myocardial ischaemia. Ischaemia was confirmed by regional cyanosis and a substantial fall in CF. Reperfusion was achieved by releasing the snare. In the first 15 min of perfusion, the heart was allowed to stabilize, and any heart exhibiting arrhythmia during this period was discarded.
Measurement of ischaemic (risk) zone and infarct size
At the end of the experiment, 0.25% Evans blue was infused into the heart to determine the myocardial risk zone. The heart was weighed, frozen, and cut into 2-mm slices. Alter removal of the right ventricle and the connective tissue, the slices were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC) in pH 7.4 buffer for 15 min at 37°C. The slices were immersed in 10% formalin overnight. The areas of infarct (TTC negative) and risk zone (TTC stained) were determined by a computerized planimetry technique (SigmaScan program 4). The volumes of left ventricle, infarct size, and risk zone were calculated by multiplying each area with slice thickness and summing products. Infarct size was expressed as a percentage of the area at risk (IS/AAR).
Isolation of rat ventricular myocytes and measurement of [Ca2+]i in the single ventricular myocyte
Myocytes were isolated from rat hearts with a collagenase method described previously (Wu et al., 1999). After isolation, they were allowed to stabilize for at least 30 min before experiments. A spectrofluorometric method with fura-2/AM as the Ca2+ indicator was used for measurement of [Ca2+]i. Loading of cells with fura-2/AM was performed as described previously (Wu et al., 1999). Briefly, ventricular myocytes were incubated with 4 μM fura-2/AM for 30 min in a modified Eagle's medium solution containing 1.25 mM CaCl2. Fluorescent signals obtained at 340 nm (F340) and 380 nm (F380) excitation wavelengths were recorded and stored in a computer for data processing and analysis. The F340/F380 ratio was used to represent cytosolic [Ca2+]i in the ventricular myocyte. To measure the electrically induced [Ca2+]i transient, myocytes were electrically stimulated at 0.2 Hz.
Metabolic inhibition and anoxia
MI/A are two of the consequences of ischaemia. In our previous study, we showed that MI/A causes myocardial injury and preconditioning with mild MI/A confers cardioprotection in a similar way as myocardial ischaemia (Ho et al., 2002). So in the present study, we subjected ventricular myocytes to MI/A as ischaemic insults. Before MI/A, ventricular myocytes were superfused with Krebs solution for 100 s followed by perfusing for 10 min with a glucose-free Krebs solution containing 10 mM 2-deoxy-D-glucose (2-DOG), an inhibitor of glycolysis (Macianskiene et al., 2001; Overend et al., 2001; Sugiyama et al., 2001), and 10 mM sodium dithionite (Na2S2O4), an oxygen scavenger (McKenna et al., 1991; Otter & Austin, 2000; Tateishi et al., 2001), known to cause MI/A. Then the ventricular myocytes were resuperfused with normal Krebs solution for 15 min-reperfusion (RE). During MI/A and RE, the resting [Ca2+]i and electrically induced [Ca2+]i transient were measured.
Drugs and chemicals
U50,488 H, 2-DOG, fura-2/AM, modified Eagle's medium, type 1 collagenase, 5-hydroxydecanoic acid (5-HD), glibenclamide (Glib), TTC, and Evans blue dye were purchased from Sigma (St Louis, U.S.A.), Na2S2O4 and nor-binaltorphimine (nor-BNI) were from Merck (Darmstadt, F.R., Germany) and Tocris Cookson Ltd (Bristol, U.K.), respectively. HMR-1098 was a generous gift from Aventis Pharma Deutschland GmbH (Frankfurt, Germany). All chemicals were dissolved in saline or Krebs solution, except fura-2/AM, which was dissolved in dimethyl sulphoxide, at a final concentration <0.1%, at which no effect can be observed (Wu et al., 1999). The concentrations of 2-DOG and sodium dithionite (Ho et al., 2002) and nor-BNI (Lishmanov Yu et al., 1999) used were based on previous studies. 5-HD and HMR-1098 were used as selective inhibitors of mito- and sarc-KATP channels, respectively, according to previous studies (Light et al., 2001; Patel et al., 2002: Tonkovic-Capin et al., 2002).
Statistical analysis
Two types of data were evaluated. One was expressed as mean±s.e.m. and two-tailed unpaired Student's t-test was used to determine the difference between two groups. Another type was enumerative data for comparison for which χ2 test of contingency was used. P<0.05 was considered statistically significant.
Results
Heart rate and coronary flow in the isolated perfused rat heart
Coronary artery occlusion resulted in a marked reduction in CF in all of experimental groups. The CF tended to return towards the baseline levels after reperfusion in all groups. There were no significant differences in the heart rate and CF between treatment groups and control group at 5 min after regional ischaemia and 120 min after reperfusion (Table 1).
Table 1.
Heart rate and coronary flow in the isolated perfused rat heart
| Baseline | Ischaemia | Reperfusion | |||||
|---|---|---|---|---|---|---|---|
| Treatment | N | HR | CF | HR | CF | HR | CF |
| CON | 8 | 241±06 | 13.1±0.9 | 217±10 | 5.9±0.6 | 211±11 | 8.1±1.1 |
| UP | 9 | 232±10 | 11.6±1.6 | 215±08 | 5.2±1.7 | 209±07 | 7.8±0.9 |
| BNI+UP | 6 | 237±13 | 12.6±1.4 | 212±11 | 6.7±1.1 | 207±10 | 10.2±2.1 |
| BNI | 6 | 229±09 | 11.8±1.1 | 208±07 | 4.8±0.8 | 210±08 | 9.8±0.7 |
| HMR+UP | 9 | 239±07 | 13.2±0.8 | 211±09 | 7.2±1.5 | 209±12 | 11.2±0.6 |
| 5-HD+UP | 8 | 247±12 | 14.3±1.0 | 220±10 | 7.8±1.2 | 212±07 | 12.8±1.1 |
| UP+HMR | 9 | 251±08 | 14.1±0.6 | 228±08 | 6.9±0.7 | 200±05 | 13.2±0.5 |
| UP+5-HD | 6 | 226±16 | 10.7±1.2 | 210±12 | 4.9±0.9 | 211±09 | 8.7±1.6 |
| UP+Glib | 8 | 235±06 | 12.5±0.6 | 218±10 | 7.1±1.0 | 222±09 | 8.3±0.8 |
| 5-HD(before) | 6 | 242±09 | 11.6±0.8 | 230±14 | 4.9±0.6 | 232±05 | 10.1±1.6 |
| HMR (end) | 6 | 238±11 | 12.1±0.9 | 224±13 | 6.4±1.3 | 221±06 | 9.7±0.9 |
| 5-HD(end) | 6 | 245±10 | 14.5±1.0 | 219±10 | 7.0±0.4 | 213±12 | 12.4±1.8 |
| Glib(end) | 6 | 233±09 | 13.2±1.1 | 212±10 | 7.7±1.2 | 223±11 | 9.8±1.2 |
The experimental protocol was described in Figures 1, 2,3,4,5. Baseline, at 10 min of perfusion; Ischaemia, 5 min after regional ischaemia; Reperfusion, 120 min after reperfusion; CON: administration of saline 24 h before I/R; UP: administration of U50,488 H (10 mg kg−1) 24 h before I/R; BNI+UP: administration of nor-BNI (10 mg kg−1) 10 min before U50,488 H (10 mg kg−1) administration; BNI: nor-BNI (10 mg kg−1) was given intravenously 10 min before administration of saline 24 h before I/R; HMR+UP or 5-HD+UP: administration of U50,488 H (10 mg kg−1) together with HMR-1098 (6 mg kg−1) or 5-HD (10 mg kg−1) 24 h before I/R; UP+HMR, UP+5-HD or UP+Glib: perfusion with HMR-1098 (30 μM), 5-HD (100 μM) or Glib (10 μM) alone 20 min before I/R at 24 h after administration of U50,488 H (10 mg kg−1); HMR (before) or 5-HD (before): administration of HMR-1098 (6 mg kg−1) or 5-HD (10 mg kg−1) with saline 24 h before I/R; HMR (end), 5-HD (end) or Glib (end): perfusion with HMR-1098 (30 μM), 5-HD (100 μM) or Glib (10 μM) alone for 20 min before I/R at 24 h after intravenous administration of saline. HR, heart rate in beats per min; CF, coronary flow in ml min−1. Values are means±s.e.m.; n is the number of hearts.
Dose- and time-dependent changes in infarct size after preconditioning with U50,488H (UP) in the isolated perfused heart subjected to I/R
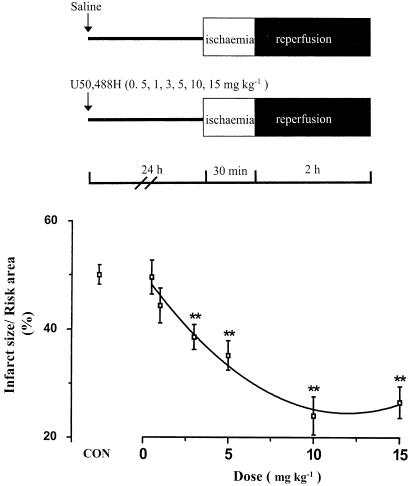
Myocardial ischaemia for 30 min and reperfusion for 2 h induced myocardial infarct as expected. A significant reduction in infarct size appeared when U50,488H was administered at the range of 3–15 mg kg−1 24 h before I/R (Figure 1). There was no significant reduction in infarct size when U50,488H was administered at 0.5 or 1 mg kg−1. A peak effect was reached at 10 mg kg−1 and the EC50 was 3.9 mg kg−1 (calculated by the Graphpad Prism Software).
Figure 1.
Infarct reducing effect of UP. Saline (CON group) or 0.5, 1, 3, 5, 10 and 15 mg kg−1 U50,488 H were given intravenously 24 h before the removal of the heart from the rat. The isolated heart was perfused and then subjected to I/R. n=8 in the CON group and n=6–8 in treatment groups. Values are expressed as mean±s.e.m. **P<0.01 vs CON.
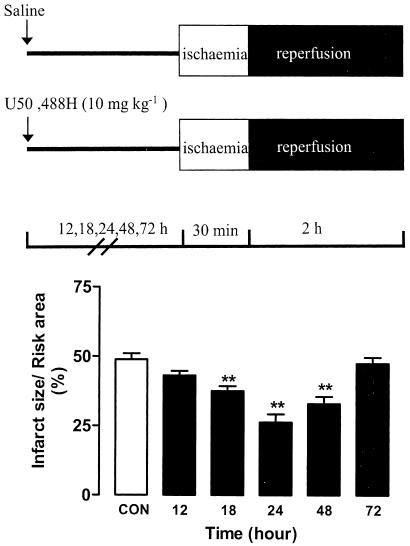
As shown in Figure 2, significant reductions occurred at 18, 24 and 48 h after administration of 10 mg kg−1 U50,488H with the greatest reduction at 24 h. There was no significant reduction in infarct size 12 or 72 h after administration of U50,488H. The observation is in agreement with our previous finding in an isolated ventricular myocytes preparation (Wu et al., 1999).
Figure 2.
Time course study of infarct reducing effect of UP. U50,488 H (10 mg kg−1) was given intravenously, and the heart was removed, perfused and subjected to I/R 12, 18, 24, 48 or 72 h later, n=8 in the CON group and n=5–8 in treatment groups. Values are expressed as mean±s.e.m. **P<0.01 vs CON.
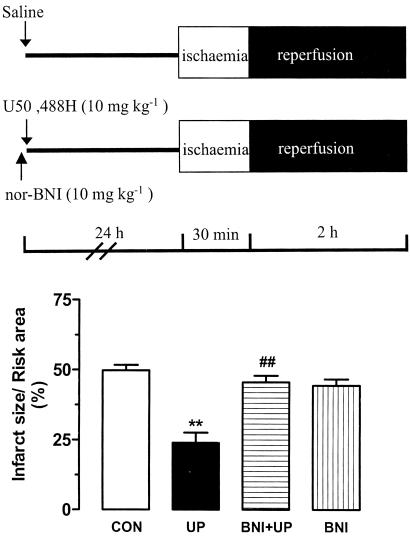
The effect of 10 mg kg−1 U50,488H was completely abolished when the selective κ-OR antagonist nor-BNI at 10 mg kg−1 was administered 10 min prior to administration of U50,488H, which took place 24 h before I/R (Figure 3), indicating that the effect of U50,488H was κ-OR mediated.
Figure 3.
Infarct reducing effect of UP in the presence or absence of nor-BNI in the isolated perfused heart of rats. nor-BNI (10 mg kg−1) was given intravenously 10 min before administration of saline or U50,488 H (10 mg kg−1). After 24 h, the heart was removed, perfused and then subjected to I/R. n=8 in the CON group and n=6 in treatment groups. Values are expressed as mean±s.e.m. **P<0.01 vs CON, ##P<0.01 vs UP.
Effects of blockade of KATP channels on UP-induced delayed cardioprotection
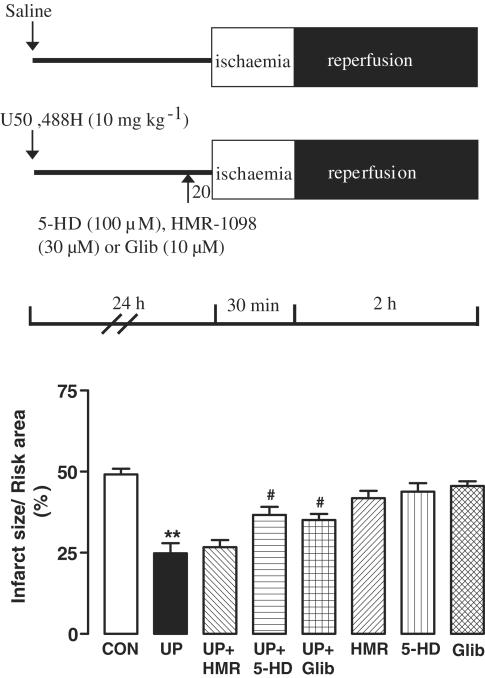
The roles of sarcKATP and mitoKATP channels in the delayed cardioprotection of U50,488 H were determined by the use of the selective blockers of sarcKATP and mitoKATP channels, HMR-1098 and 5-HD, respectively. To determine whether a channel was a trigger of protection, the blocker of the channel was coadministered with saline or U50,488H (10 mg kg−1) 24 h before I/R. HMR-1098 at 6 mg kg−1 (approximately 281–312 μM in plasma) and 5-HD at 10 mg kg−1 (approximately 1.10–1.22 mM in plasma) significantly attenuated the UP-induced reduction in infarct size (Figure 4). In another series of experiment, isolated hearts were removed at 24 h after administration of saline or U50,488H (10 mg kg−1), 30 μM HMR-1098 or 100 μM 5-HD was administered for 20 min before ischaemia. 5-HD, but not HMR-1098, significantly attenuated the reduction in infarct size induced by UP (Figure 5). In view of a recent finding that 5-HD may also interfere directly with the mitochondrial respiratory chain (Hanley et al., 2002), we determined the effect of 10 μM Glib, a nonselective KATP channel blocker and found that like 5-HD, Glib 20 min before ischaemia also significantly attenuated the reduction in infarct size induced by UP (35.1±1.9%), which was comparable to that of the group treated with 5-HD (36.7±2.5%). The finding indicates that the effects of 5-HD and Glib were due to blockade of the mitoKATP channel. In both series of experiments, 5-HD, HMR-1098 or Glib alone had no effect. These results indicate that mitoKATP channel act as both trigger and end effector, while sarcKATP channel as trigger only, in delayed cardioprotection of UP.
Figure 4.
Infarct reducing effect of UP with blockade of KATP channels during preconditioning. HMR-1098 (6 mg kg−1) or 5-HD (10 mg kg−1) was given intravenously simultaneously with saline or U50,488 H (10 mg kg−1). After 24 h, the heart was removed, perfused and then subjected to I/R. n=7 in CON and UP groups. Values are expressed as mean±s.e.m. **P<0.01 vs CON, #P<0.05 vs UP, ##P<0.01 vs UP.
Figure 5.
Infarct size reducing effect of UP with blockade of KATP channels before ischaemia. Saline or U50,488 H (10 mg kg−1) was given intravenously, 24 h later the heart was removed, perfused with Krebs–Ringer solution containing HMR-1098 (30 μM), 5-HD (100 μM) or Glib (10 μM) for 20 min before ischaemia. n=9 in the CON group and n=7 in the UP group. Values are expressed as mean±s.e.m. **P<0.01 vs CON, #P<0.05 vs UP.
Effects of blockade of KATP channels on alterations in [Ca2+]i induced by MI/A in isolated ventricular myocytes
To determine the roles of KATP channels in [Ca2+]i homeostasis in the heart subjected to ischaemic insults, we measured the resting [Ca2+]i and electrically induced [Ca2+]i transient in isolated ventricular myocytes at the end of MI/A. The former indicates the resting [Ca2+]i level, while the latter the release of Ca2+ during excitation–contraction (E–C) coupling (Janczewski & Lakatta, 1993). As shown in Figure 6, upon MI/A there was an elevation in [Ca2+]i and the elevation was significantly attenuated with UP, an effect abolished in the presence of nor-BNI. The effect of UP was also abolished by HMR-1098 or 5-HD administered together with U50,488 H 24 h before MI/A. On the other hand only 5-HD, but not HMR-1098, was able to abolish the effect of UP when administered just before MI/A.
Figure 6.
Effect of UP on resting [Ca2+]i in ventricular myocytes subjected to MI/A in the presence or absence of nor-BNI or with blockade of KATP channel blockers. Ventricular myocytes were isolated 24 h after intravenous administration of U50,488 H to the rat and loaded with fura/2-AM. The isolation of cells and loading of dye took about 3 h. The ventricular myocytes were superfused with Krebs solution for 100 s followed by glucose-free Krebs solution containing 10 mM 2-DOG and 10 mM Na2S2O4 for 10 min (MI/A). Then they were re-superfused with normal Krebs solution for 15 min reperfusion (RE). During the MI/A and RE, the resting [Ca2+]i or electrically induced [Ca2+]i transient were determined. Groups consist of (a) intravenous administration of saline about 27 h before MI/A (VP); (b) intravenous administration of U50,488 H (10 mg kg−1) in the presence or absence of nor-BNI (10 mg kg−1) about 27 h before MI/A (UP or UP+BNI group); (c) intravenous administration of HMR-1098 (6 mg kg−1) or 5-HD (10 mg kg−1) together with U50,488 H (10 mg kg−1) at about 27 h before MI/A (HMR+UP or 5-HD+UP group); (d) intravenous administration of U50,488 H (10 mg kg−1) about 27 h before MI/A and superfusion with HMR-1098 (10 μM) or 5-HD (100 μM) of ventricular myocytes 20 min before MI/A(UP+HMR or UP+5-HD group). Values are expressed as mean±s.e.m. n=6–7 rats in each group. If more than one myocyte was determined, values from all myocytes obtained from one rat were averaged and the mean was used as a single entity. *P<0.05 vs VP, #P<0.05 vs UP.
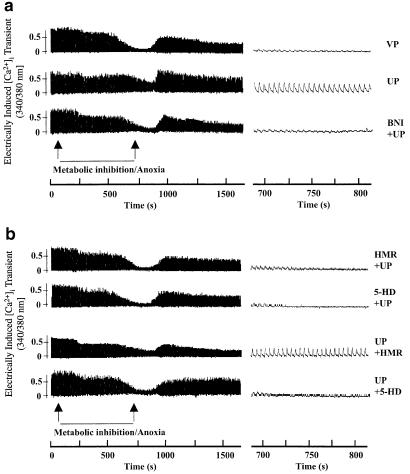
Representative tracings on the electrically induced [Ca2+]i transient in ventricular myocytes subjected to different treatments were shown in Figure 7. Table 2 summarizes the group results. In the vehicle-preconditioned (VP) group subjected to MI/A, only six out of 40 cells exhibit visible electrically induced [Ca2+]i transients at the end of MI/A. In the UP group, 40 out of 49 cells showed visible transients, which was significantly different from the VP group. The effect of UP was abolished in the presence of nor-BNI or with coadministration of HMR-1098 or 5-HD, which is similar to the resting [Ca2+]i responses. Also similar to the resting [Ca2+]i responses, 5-HD, but not HMR-1098, was able to abolish the effect of UP when administered before MI/A. The amplitude of the transients was similar in all groups (data not shown).
Figure 7.
Representative tracings showing effects of UP on electrically induced [Ca2+]i transient in the absence or presence of nor-BNI (a) or with blockade of KATP channel blockers (b). The experimental protocol was the same as in Figure 6. Left panel: recording of electrically induced [Ca2+]i during MI/A and RE. Right panel: recording of electrically induced [Ca2+]i at the end of MI/A.
Table 2.
Effect of UP on electrically induced [Ca2+]i transient
| n (with) | n (without) | |
|---|---|---|
| VP | 6 | 34 |
| UP | 40 | 9** |
| BNI+UP | 2 | 18## |
| HMR+UP | 3 | 22## |
| 5-HD+UP | 3 | 20## |
| UP+HMR | 20 | 5** |
| UP+5-HD | 4 | 21## |
Group results of UP on electrically induced [Ca2+]i transient in ventricular myocytes subjected to MI/A. The experimental protocol was described in Figure 6. n is the number of myocytes. The myocytes in this experiment were obtained from 7–10 rats in each group. Each myocyte was treated as a single entity as myocytes from the same rat responded differently. With: cells with electrically induced calcium transient at the end of MI/A. Without: cells without electrically induced calcium transient at the end of MI/A.
P<0.01 vs VP
P< 0.01 vs UP.
Discussion
In the present study, we have shown that the mitoKATP channel acts as both a trigger and a mediator, while sarcKATP channel acts as a trigger of delayed cardioprotection of UP. The most novel finding is that the abolition of the delayed cardioprotection of UP by blockade of these two channels were accompanied by attenuation of the [Ca2+]i overload in response to ischaemic insults. The findings indicate that both KATP channels contribute to delayed cardioprotection of preconditioning and [Ca2+]i homeostasis. They also suggest that the cardioprotective actions of these channels may be mediated via attenuation of [Ca2+]i overload.
In previous studies, it was shown that activation of the κ-OR with U50,488 H, a selective κ1-OR agonist, confers immediate cardioprotection both in vivo (Valtchanova-Matchouganska & Ojewole, 2002) and in vitro (Wang et al., 2001a; Ho et al., 2002). In contrast, preconditioning with bremazocine, an universal κ-OR agonist, acting preferentially to κ2-OR (Aitchison et al., 2000), or a κ-OR agonist with weak μ-agonist action, pentozocine (Coles Jr et al., 2003), further increased the infarct size induced by I/R in the rat and swine, respectively, and the effect was attenuated by nor-BNI. We have also shown previously that κ-OR activation with U50,488 H confers delayed cardioprotection in vitro (Wu et al., 1999; Zhou et al., 2001; Wang et al., 2001b). In the present study, we administered U50,488 H intravenously to the rat and found that after 24 h the infarct size in isolated perfused hearts was significantly reduced upon regional I/R. In another study in our lab, we also found that the infarct size in the intact heart of anaesthetized rat was also significantly reduced upon regional I/R (Chen & Wong, unpublished results). The observation demonstrates delayed cardioprotection of κ-OR stimulation in vivo. Interestingly, the width of the window of delayed cardioprotection was 18–48 h in the present study, which is similar to 16–48 h reported in the previous study (Wu et al., 1999). In the previous study U50,488 H was directly administered to the myocyte, while in the present study the κ-OR agonist was given intravenously. The observation therefore suggests that activation of the cardiac κ-OR may be the crucial event of cardioprotection.
It has been well established that δ-OR stimulation confers delayed cardioprotection (Fryer et al., 1999; Shinmura et al., 2002). The effect was attenuated when 5-HD, but not HMR-1098, was administered before ischaemia (Patel et al., 2002), indicating that the mitoKATP channel, but not sarcKATP channel, acts as an effector/mediator. It was also found that the delayed cardioprotection was affected by coadministration of HMR-1098, but not 5-HD, with the δ-OR agonist, indicating that the sarcKATP, but not mito KATP channel, is a trigger. The findings are not in agreement with our findings that both mito- and sarcKATP channels are triggers, while mitoKATP channel is also an effector/mediator of delayed cardioprotection of κ-OR stimulation. The observation indicates that the signalling mechanisms responsible for cardioprotection of activation of two types of opioid receptors are not the same.
It has also been shown that after administration of 5-HD at the time of ischaemic insult, the delayed cardioprotection of preconditioning was attenuated/abolished (Hoag et al., 1997; Fryer et al., 1999; Carroll & Yellon, 2000), indicating that the channel serves as a mediator. However, it was also shown that administration of 5-HD attenuated/abolished the effect of preconditioning when administered either before ischaemia or during preconditioning with ischaemia (Takashi et al., 1999), indicating that the channel acts as a mediator as well as a trigger. In the present study, we found that similar to the observation of Takashi et al. (1999), administration of 5-HD before ischaemic insults or during preconditioning abolished the delayed cardioprotection of UP. It is of interest to note that in three previous studies the mitoKATP channel was shown to act as a mediator, while in a previous (Takashi et al., 1999) and the present studies the channel was shown to act as both a mediator and a trigger. Similarly, the channel has been shown to act as a mediator (Gross & Auchampach, 1992; Yao et al., 2001) or a trigger (Baines et al., 1999; Fryer et al., 2000; Pain et al., 2000; Wang et al., 2001d) or as both mediator and trigger (Liang, 1997,1998; Wang et al., 2001c) in immediate cardioprotection of preconditioning. It seems that the role of the channel may be different in different types of preconditioning. On the other hand, it is well established that preconditioning with one insult may induce protection against other insults, a crosstolerance phenomenon, suggesting that preconditioning with different insults may activate a common signalling pathway. Further study is needed to clarify this.
The role of the SarcKATP channel in delayed cardioprotection is not as well studied as that of the mitoKATP channel. With the use of selective channel blocker, it was shown that the sarcKATP channel acts as a mediator (isoflurane; Tonkovic-Capin et al., 2002) and as a trigger (δ-OR agonist; Patel et al., 2002). In the present study, we showed that administration of HMR-1098 before preconditioning, but not before lethal ischaemia, abolished the cardioprotection of UP, indicating that the channel acts as a trigger, but not a mediator.
In the present study, we showed that UP attenuated the elevation in [Ca2+]i and prevented the reduction in the electrically induced [Ca2+]i transient upon ischaemic insults and blockade of the mitoKATP channel with its blocker, 5-HD, before ischaemia or during preconditioning, reversed the effect of UP. The result agrees with a previous finding that opening of the mitoKATP channel attenuated the [Ca2+]i overload induced by severe metabolic stress (Light et al., 2001). Similarly, blockade of the SarcKATP channel with its blocker, HMR-1098, during preconditioning, which confers delayed cardioprotection, also prevented/abolished the changes in [Ca2+]i and electrically induced [Ca2+]i transient. Since electrically induced [Ca2+]i transient results from the influx of Ca2+ and release of Ca2+ from sarcoplasmic reticulum (SR) upon electrical stimulation, the change in the transient suggests a reduction in influx of Ca2+ via the L-type Ca2+ channel or a reduction in release of Ca2+ from SR or both. So the change in the [Ca2+]i transient indicates changes in Ca2+ handling in the sarcolemma and SR, which may lead to alterations in [Ca2+]i. Further study is needed to delineate the alterations in Ca2+ handling responsible for changes in [Ca2+]i and the roles of channels in Ca2+ homeostasis in response to ischaemic insults.
We tried to compare the concentrations of the KATP channel blockers in the in vivo and in vitro preparations. According to Xu et al. (2002), the blood volume of the rat is 7.4% and the plasma volume is 52.6–58.5% of the blood volume. For a rat of 300 g, the plasma volume is approximately 11.7–13 ml. So when 5-HD at 10 mg kg−1 is administered intravenously, the concentration is approximately 1.10–1.22 mM, while 6 mg kg−1 of HMR-1098 is 281–312 μM. The concentrations are approximately 10 times those administered in vitro (100 and 30 μM for 5-HD and HMR-1098, respectively). It is difficult to compare the concentrations of these two blockers in in vivo and in vitro preparations because in the former, the heart is perfused by blood, while in the latter the heart is perfused with Krebs–Ringer solution. Since drugs are biologically active only in free form, information on binding of the two blockers to plasma proteins is needed in order to compare the effective concentrations in vivo and in vitro. Unfortunately, there is no report on the binding of these two blockers to plasma proteins in the rat.
In conclusion, the present study has demonstrated that administration of U50,488 H to the rat attenuated the infarct size induced by I/R in the isolated perfused heart and that the effect was abolished by nor-BNI. This is the first demonstration of delayed cardioprotection of κ-OR stimulation in the rat in vivo. Both SarcKATP and mitoKATP channels trigger, while the mitoKATP channel mediates, the delayed cardioprotection of κ-OR stimulation. The study has also provided first evidence that the protective actions of the KATP channels were accompanied by prevention/attenuation of the changes in [Ca2+]i homeostasis, namely [Ca2+]i overload and reduced Ca2+ release during E–C coupling induced by ischaemic insults, suggesting that the prevention/attenuation of the changes in [Ca2+]i homeostasis may contribute, at least partly, to the cardioprotective roles of the KATP channels.
Acknowledgments
This study was supported by CRCG, The University of Hong Kong and the Research Grants Council, Hong Kong. We thank Professor Gögelein and Dr Jürgen Pünter (Aventis Pharma Deutschland GmbH, Germany) for supplying the HMR-1098 as a generous gift. We also thank Dr I Bruce for advice and Mr C.P. Mok for technical assistance. Mai Chen was on leave from the Department of Cardiology, Xijing Hospital, The Fourth Military Medical University, Xi'an, China.
Abbreviations
- [Ca2+]i
intracellular Ca2+
- CF
coronary arterial flow
- 2-DOG
2-deoxy-D-glucose
- E–C coupling
excitation–contraction coupling
- Glib, glibenclamide: 5-HD
5-hydroxydecanoic acid
- IP
ischaemic preconditioning
- I/R
ischaemia/reperfusion
- IS/AAR
infarct size/area at risk
- KATP
ATP-sensitive potassium channel
- κ-OR
kappa-opioid receptor
- MI/A
metabolic inhibition and anoxia
- mitoKATP
mitochondrial KATP channel
- nor-BNI
nor-binaltorphimine
- PKC
protein kinase C
- sarcKATP
sarcolemmal KATP channel
- SR
sarcoplasmic reticulum
- TTC
2,3,5-triphenyltetrazolium chloride
- UP
preconditioning with U50,488H
- VP
vehicle-preconditioned
References
- AITCHISON K.A., BAXTER G.F., AWAN M.M., SMITH R.M., YELLON D.M., OPIE L.H.Opposing effects on infarction of delta and kappa opioid receptor activation in the isolated rat heart: implications for ischemic preconditioning Basic Res. Cardiol. 2000951–10.discussion 11 [DOI] [PubMed] [Google Scholar]
- BAINES C.P., LIU G.S., BIRINCIOGLU M., CRITZ S.D., COHEN M.V., DOWNEY J.M. Ischemic preconditioning depends on interaction between mitochondrial KATP channels and actin cytoskeleton. Am. J. Physiol. 1999;276:H1361–H1368. doi: 10.1152/ajpheart.1999.276.4.H1361. [DOI] [PubMed] [Google Scholar]
- CARROLL R., YELLON D.M. Delayed cardioprotection in a human cardiomyocyte-derived cell line: the role of adenosine, p38MAP kinase and mitochondrial KATP. Basic Res. Cardiol. 2000;95:243–249. doi: 10.1007/s003950050187. [DOI] [PubMed] [Google Scholar]
- COLES J.A., JR, SIGG D.C., IAIZZO P.A. The role of kappa-opioid receptor activation in pharmacological preconditioning of swine. Am. J. Physiol. Heart. Circ. Physiol. 2003;23:23. doi: 10.1152/ajpheart.00843.2002. [DOI] [PubMed] [Google Scholar]
- FRYER R.M., HSU A.K., EELLS J.T., NAGASE H., GROSS G.J. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ. Res. 1999;84:846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- FRYER R.M., HSU A.K., NAGASE H., GROSS G.J. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 2000;294:451–457. [PubMed] [Google Scholar]
- GROSS G.J., AUCHAMPACH J.A. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ. Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- GROSS G.J., FRYER R.M. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ. Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- HALESTRAP A.P. Regulation of mitochondrial metabolism through changes in matrix volume. Biochem. Soc. Trans. 1994;22:522–529. doi: 10.1042/bst0220522. [DOI] [PubMed] [Google Scholar]
- HANLEY P.J., MICKEL M., LOFFLER M., BRANDT U., DAUT J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J. Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO J.C., WU S., KAM K.W., SHAM J.S., WONG T.M. Effects of pharmacological preconditioning with U50488 H on calcium homeostasis in rat ventricular myocytes subjected to metabolic inhibition and anoxia. Br. J. Pharmacol. 2002;137:739–748. doi: 10.1038/sj.bjp.0704945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOAG J.B., QIAN Y.Z., NAYEEM M.A., D'ANGELO M., KUKREJA R.C. ATP-sensitive potassium channel mediates delayed ischemic protection by heat stress in rabbit heart. Am. J. Physiol. 1997;273:H2458–H2464. doi: 10.1152/ajpheart.1997.273.5.H2458. [DOI] [PubMed] [Google Scholar]
- JANCZEWSKI A.M., LAKATTA E.G. Buffering of calcium influx by sarcoplasmic reticulum during the action potential in guinea-pig ventricular myocytes. J. Physiol. 1993;471:343–363. doi: 10.1113/jphysiol.1993.sp019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOVANOVIC S., JOVANOVIC A. Pinacidil prevents membrane depolarisation and intracellular Ca2+ loading in single cardiomyocytes exposed to severe metabolic stress. Int. J. Mol. Med. 2001;7:639–643. doi: 10.3892/ijmm.7.6.639. [DOI] [PubMed] [Google Scholar]
- LIANG B.T. Protein kinase C-mediated preconditioning of cardiac myocytes: role of adenosine receptor and KATP channel. Am. J. Physiol. 1997;273:H847–H853. doi: 10.1152/ajpheart.1997.273.2.H847. [DOI] [PubMed] [Google Scholar]
- LIANG B.T. Protein kinase C-dependent activation of KATP channel enhances adenosine-induced cardioprotection. Biochem. J. 1998;336:337–343. doi: 10.1042/bj3360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHT P.E., KANJI H.D., FOX J.E., FRENCH R.J. Distinct myoprotective roles of cardiac sarcolemmal and mitochondrial KATP channels during metabolic inhibition and recovery. FASEB J. 2001;15:2586–2594. doi: 10.1096/fj.01-0188com. [DOI] [PubMed] [Google Scholar]
- LISHMANOV YU B., MASLOV L.N., NARYZHNAYA N.V., TAM S.W. Ligands for opioid and sigma-receptors improve cardiac electrical stability in rat models of post-infarction cardiosclerosis and stress. Life Sci. 1999;65:L13–L17. doi: 10.1016/s0024-3205(99)00226-x. [DOI] [PubMed] [Google Scholar]
- MACIANSKIENE R., MATEJOVIC P., SIPIDO K., FLAMENG W., MUBAGWA K. Modulation of the extracellular divalent cation-inhibited non-selective conductance in cardiac cells by metabolic inhibition and by oxidants. J. Mol. Cell. Cardiol. 2001;33:1371–1385. doi: 10.1006/jmcc.2001.1401. [DOI] [PubMed] [Google Scholar]
- MCKENNA C.E., GUTHEIL W.G., SONG W. A method for preparing analytically pure sodium dithionite. Dithionite quality and observed nitrogenase-specific activities. Biochim. Biophys. Acta. 1991;1075:109–117. doi: 10.1016/0304-4165(91)90082-r. [DOI] [PubMed] [Google Scholar]
- OLDENBURG O., COHEN M.V., YELLON D.M., DOWNEY J.M. Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc. Res. 2002;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- OTTER D., AUSTIN C. Hypoxia, metabolic inhibition, and isolated rat mesenteric tone: influence of arterial diameter. Microvasc. Res. 2000;59:107–114. doi: 10.1006/mvre.1999.2212. [DOI] [PubMed] [Google Scholar]
- OVEREND C.L., EISNER D.A., O'NEILL S.C. Altered cardiac sarcoplasmic reticulum function of intact myocytes of rat ventricle during metabolic inhibition. Circ. Res. 2001;88:181–187. doi: 10.1161/01.res.88.2.181. [DOI] [PubMed] [Google Scholar]
- PAIN T., YANG X.M., CRITZ S.D., YUE Y., NAKANO A., LIU G.S., HEUSCH G., COHEN M.V., DOWNEY J.M. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- PATEL H.H., HSU A.K., PEART J.N., GROSS G.J. Sarcolemmal K(ATP) channel triggers opioid-induced delayed cardioprotection in the rat. Circ. Res. 2002;91:186–188. doi: 10.1161/01.res.0000029085.69891.f2. [DOI] [PubMed] [Google Scholar]
- SHINMURA K., NAGAI M., TAMAKI K., TANI M., BOLLI R. COX-2-derived prostacyclin mediates opioid-induced late phase of preconditioning in isolated rat hearts. Am. J. Physiol. Heart. Circ. Physiol. 2002;283:H2534–H2543. doi: 10.1152/ajpheart.00209.2002. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA S., SATOH H., NOMURA N., TERADA H., WATANABE H., HAYASHI H. The importance of glycolytically-derived ATP for the Na+/H+ exchange activity in guinea pig ventricular myocytes. Mol. Cell Biochem. 2001;217:153–161. doi: 10.1023/a:1007261322878. [DOI] [PubMed] [Google Scholar]
- TAKASHI E., WANG Y., ASHRAF M. Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ. Res. 1999;85:1146–1153. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- TATEISHI N., SUZUKI Y., CICHA I., MAEDA N. O(2) release from erythrocytes flowing in a narrow O(2)-permeable tube: effects of erythrocyte aggregation. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H448–H456. doi: 10.1152/ajpheart.2001.281.1.H448. [DOI] [PubMed] [Google Scholar]
- TONKOVIC-CAPIN M., GROSS G.J., BOSNJAK Z.J., TWEDDELL J.S., FITZPATRICK C.M., BAKER J.E. Delayed cardioprotection by isoflurane: role of K(ATP) channels. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H61–H68. doi: 10.1152/ajpheart.01040.2001. [DOI] [PubMed] [Google Scholar]
- VALTCHANOVA-MATCHOUGANSKA A., OJEWOLE J.A. Involvement of opioid delta (delta)- and kappa (kappa)-receptors in ischemic preconditioning in a rat model of myocardial infarction. Methods Find. Exp. Clin. Pharmacol. 2002;24:139–144. doi: 10.1358/mf.2002.24.3.802298. [DOI] [PubMed] [Google Scholar]
- WANG G.Y., WU S., PEI J.M., YU X.C., WONG T.M. Kappa- but not delta-opioid receptors mediate effects of ischemic preconditioning on both infarct and arrhythmia in rats. Am. J. Physiol. Heart Circ. Physiol. 2001a;280:H384–H391. doi: 10.1152/ajpheart.2001.280.1.H384. [DOI] [PubMed] [Google Scholar]
- WANG G.Y., ZHOU J.J., SHAN J., WONG T.M. Protein kinase C-epsilon is a trigger of delayed cardioprotection against myocardial ischemia of kappa-opioid receptor stimulation in rat ventricular myocytes. J. Pharmacol. Exp. Ther. 2001b;299:603–610. [PubMed] [Google Scholar]
- WANG S., CONE J., LIU Y. Dual roles of mitochondrial K(ATP) channels in diazoxide-mediated protection in isolated rabbit hearts. Am. J. Physiol. Heart. Circ. Physiol. 2001c;280:H246–H255. doi: 10.1152/ajpheart.2001.280.1.H246. [DOI] [PubMed] [Google Scholar]
- WANG Y., TAKASHI E., XU M., AYUB A., ASHRAF M. Downregulation of protein kinase C inhibits activation of mitochondrial K(ATP) channels by diazoxide. Circulation. 2001d;104:85–90. doi: 10.1161/01.cir.104.1.85. [DOI] [PubMed] [Google Scholar]
- WU S., LI H.Y., WONG T.M. Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of kappa-opioid receptor. Circ. Res. 1999;84:1388–1395. doi: 10.1161/01.res.84.12.1388. [DOI] [PubMed] [Google Scholar]
- XU S.Y., BIAN R.L., CHEN X. The Method of Pharmacology Experiment 2002Beijing: People's Health Publisher; 3rd edn [Google Scholar]
- YAO Z., MCPHERSON B.C., LIU H., SHAO Z., LI C., QIN Y., VANDEN HOEK T.L., BECKER L.B., SCHUMACKER P.T. Signal transduction of flumazenil-induced preconditioning in myocytes. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1249–H1255. doi: 10.1152/ajpheart.2001.280.3.H1249. [DOI] [PubMed] [Google Scholar]
- YLITALO K.V., ALA-RAMI A., LIIMATTA E.V., PEUHKURINEN K.J., HASSINEN I.E. Intracellular free calcium and mitochondrial membrane potential in ischemia/reperfusion and preconditioning. J. Mol. Cell Cardiol. 2000;32:1223–1238. doi: 10.1006/jmcc.2000.1157. [DOI] [PubMed] [Google Scholar]
- ZHOU J.J., PEI J.M., WANG G.Y., WU S., WANG W.P., CHO C.H., WONG T.M. Inducible HSP70 mediates delayed cardioprotection via U-50488H pretreatment in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H40–H47. doi: 10.1152/ajpheart.2001.281.1.H40. [DOI] [PubMed] [Google Scholar]