Abstract
We previously reported that acute incubation with tetrahydrobiopterin (BH4) or sepiapterin, a cofactor for endothelial nitric oxide synthase and a stable precursor of BH4, respectively, enhanced the acetylcholine (Ach)-induced relaxation of isolated small mesenteric arteries (SMA) from diabetic (db/db) mice. In this study, we investigated the effect of chronic oral supplementation of sepiapterin (10 mg kg−1 day−1) to db/db mice on endothelium function, biopterin levels and lipid peroxidation in SMA.
Oral dietary supplementation with sepiapterin had no effect on glucose, triglyceride, cholesterol levels and body weight. SMA from db/db mice showed enhanced vascular reactivity to phenylephrine, which was corrected with sepiapterin supplementation. Furthermore, Ach, but not sodium nitroprusside-induced relaxation, was improved with sepiapterin supplementation in db/db mice.
BH4 levels and guanosine triphosphate cyclohydrolase I activity in SMA were similar in db/+ and db/db mice. Sepiapterin treatment had no effects on BH4 or guanosine triphosphate cyclohydrolase I activity. However, the level of dihydrobiopterin+biopterin was higher in SMA from db/db mice, which was corrected following sepiapterin treatment.
Thiobarbituric acid reactive substance, malondialdehyde, a marker of lipid peroxidation, was higher in SMA from db/db mice, and was normalized by sepiapterin treatment.
These results indicate that sepiapterin improves endothelial dysfunction in SMA from db/db mice by reducing oxidative stress. Furthermore, these results suggest that decreased biosynthesis of BH4 may not be the basis for endothelial dysfunction in SMA from db/db mice.
Keywords: Endothelial dysfunction, type II diabetes, tetrahydrobiopterin, sepiapterin, oxidative stress, db/db mice
Introduction
(6R)-5,6,7,8-tetrahydro-L-biopterin (BH4) is an essential cofactor required for the biosynthesis of nitric oxide (NO) by nitric oxide synthase (NOS). Although the exact mechanism whereby BH4 regulates NOS activity is not known, several different hypotheses have been proposed viz., BH4 exerts an allosteric action to stabilize the active dimeric state of NOS, it plays a redox active role in stimulating NOS, it increases the binding of L-arginine to NOS and scavenges reactive free radicals or it plays a role in the electron transfer to the ferrous-dioxy intermediate by forming a pterin radical, thus enabling the formation of a heme-based oxidant that rapidly hydroxylates L-arginine (Klatt et al., 1994; Kojima et al., 1995; Mayer & Werner, 1995; Hurshman et al., 1999; Wei et al., 2001).
In situations where there are suboptimal concentrations of BH4, endothelial NOS (eNOS) will generate superoxide in addition to NO. For instance, biochemical studies with purified constitutive NOS have demonstrated that in the presence of suboptimal concentrations of BH4, there is an uncoupling of NOS and subsequent formation of superoxide and hydrogen peroxide (Pou et al., 1992; Wever et al., 1997; Vasquez-Vivar & Kalyanaraman, 2000). Blockade of BH4 synthesis with 2,4-diamino-6-hydroxypyrimidine (DAHP), a selective guanosine triphosphate (GTP) cyclohydrolase I inhibitor, results in impaired endothelial function in isolated aorta, coronary, mesenteric and cerebral arteries (Cosentino & Katusic, 1995; Kinoshita et al., 1997; Tiefenbacher et al., 2000; Pannirselvam et al., 2002). Compared to control values, levels of BH4 have been shown to be decreased in aorta from insulin-resistant rats, in coronary endothelial cells from biobreeding (BB) diabetic rats and in aorta from spontaneously hypertensive (SHR) rats (Shinozaki et al., 1999; Meininger et al., 2000; Hong et al., 2001). Thus, supplementation with BH4 would be expected to restore impaired endothelium function and improve cardiovascular function in diabetes and other cardiovascular pathological conditions.
BH4 has been shown to improve endothelium-dependent vasorelaxation in isolated aorta from streptozotocin-induced diabetic rat, fructose-fed insulin-resistant rats and coronary arteries following reperfusion injury (Tiefenbacher et al., 1996; Pieper, 1997; Shinozaki et al., 1999). BH4 has also been shown to improve relaxation in blood vessels from patients with coronary artery disease, type II diabetes, smokers and atherosclerosis (Heitzer et al., 2000; Maier et al., 2000; Tiefenbacher et al., 2000; Heitzer et al., 2001). Chronic treatment with BH4 has been shown to improve endothelial dysfunction in aorta from fructose-fed insulin-resistant rats and decrease the elevated blood pressure in SHR rats (Shinozaki et al., 2000; Hong et al., 2001). We have reported endothelial dysfunction in isolated small mesenteric arteries (SMA) from diabetic (db/db) mice and also shown that acute incubation with BH4, sepiapterin or with a combination of polyethylene glycol superoxide dismutase (PEG-SOD) and catalase significantly improved acetylcholine (Ach)-induced relaxation (Pannirselvam et al., 2002). The objective of the present study was to investigate the effects of the chronic oral supplementation of sepiapterin on endothelial function, biopterin contents and lipid peroxidation in SMA from diabetic (db/db) mice.
Methods
Animals
Male C57BL/KsJ diabetic mice (db/db) and nondiabetic controls (db/+), 6-week-old, were purchased from The Jackson Laboratory (Bar Harbour, ME, U.S.A.). At 8 weeks of age, animals were divided into four groups – group 1: db/+ mice receiving powder chow for 8 weeks, group 2: db/+ mice receiving sepiapterin (10 mg kg−1 day−1) in powder chow for 8 weeks, group 3: db/db mice receiving powder chow for 8 weeks and group 4: db/db mice receiving sepiapterin (10 mg kg−1 day−1) in powder chow for 8 weeks. In accordance with a protocol approved by the University of Calgary animal care committee, mice were killed by cervical dislocation. Heparinized blood samples were collected for blood biochemistry. The mesenteric arcade was removed and first-order branches of the mesenteric artery (measuring approximately 150–200 μm in diameter) were dissected in cold Kreb's solution of the following composition (in mM): NaCl 120, NaHCO3 25, KCl 4.8, NaH2PO4 1.2, MgSO4 1.2, dextrose 11.0, CaCl2 1.8, aerated with 95% O2 and 5% CO2. The rest of the arterial bed was immediately stored at −70°C for biochemical measurements.
Wire myograph studies
Isometric tension studies using wire myograph were performed as described previously (Pannirselvam et al., 2002). SMA were cut into 2 mm ring and mounted on a Mulvany–Halpern myograph. The passive tension-internal circumference was determined by stretching to achieve an internal circumference equivalent to 90% of that of the blood vessel under a transmural pressure of 100 mmHg. All the experiments were performed at 37°C. Concentration–response curves to phenylephrine (PE) were constructed and normalized relative to the contraction induced by 120 mM KCl. Subsequently, endothelium-dependent and independent relaxations to Ach and sodium nitroprusside (SNP), respectively, were studied in tissues precontracted with a submaximal concentration of PE.
Determination of GTP cyclohydrolase I activity and biopterin content in SMA
GTP cyclohydrolase I activity in SMA was measured as previously described (Vann et al., 2000). Homogenized SMA was incubated with Tris-HCl buffer (pH 7.4), 10 mM dithiothreitol, 10 mg ml−1 bovine serum albumin, 10 mM GTP for 2 h at 37°C. The reactions were terminated with 1 M HCl and oxidized with iodine reagent (1% I2/2% KI, 1 : 1, wv−1). Neopterin triphosphate was dephosphorylated by incubating with 10 U of alkaline phosphatase for 30 min at 37°C. the reactions were terminated by adding 1 M phosphoric acid. Samples were centrifuged and the amount of neopterin formed was measured by reverse-phase high-performance liquid chromatography with fluorometric detection as described earlier. Neopterin levels were normalized to the amount (mg) of protein.
The biopterin contents of the SMA were measured by reverse-phase high-performance liquid chromatography as described previously (Howells et al., 1986). SMA were homogenized in 25 mM triethanolamine-HCl (pH 7.4) containing 1 mg ml−1 dithioerythritol and diethylenetriaminepentacetic acid and separated using ultrafree-MC tubes (Millipore Co., U.S.A.) by centrifuging at 5000 r.p.m. for 15 min at 4°C. The filtrates were immediately used for BH4 and biopterin assays. BH4 was measured directly using an ESA Coulochem electrochemical detector, dihydrobiopterin (BH2) was measured by fluorescence (358/447 nm) after oxidation on the conditioning cell electrode and biopterin was measured by its natural fluorescence (358/447 nm). BH4 and biopterin measured were normalized relative to the amount (mg) of protein.
Determination of lipid peroxidation in plasma and SMA
Thiobarbituric acid reactive substance, malondialdehyde, a marker for lipid peroxidation, was measured in plasma and SMA, using a commercial kit (OXI-TEK, ZeptoMetrix Co. U.S.A.) according to the manufacturer's instructions. The levels of malondialdehyde were normalized to milligrams of protein.
Protein assay
Protein content was determined by the method of bicinchoninic acid using a commercial kit from Pierce Co., U.S.A.
Drugs
L-Sepiapterin, a precursor of BH4, was obtained from Schircks Laboratories (Switzerland). All other chemicals were purchased from Sigma Chemicals (St Louis, MO, U.S.A.). Biochemical measurement of plasma glucose, triglyceride and cholesterol were performed using commercial kit from Sigma Diagnostics (St Louis, MO, U.S.A.).
Statistics
All values are expressed as mean±s.e.m.. The relaxation is expressed as mean percentage (±s.e.m.). In all experiments, n equals the number of animals used in the protocol. The concentration–response curves among the four groups were compared using a repeated measures ANOVA test. Statistical significance of difference in biochemical parameters among four groups was performed using one-way ANOVA. Multiple comparisons of the paired groups were performed using Student–Newman–Keuls method. A P-value of less than 0.05 was considered statistically significant.
Results
Metabolic characteristics in db/db mice
The db/db mice showed higher body weight compared to db/+ mice. Oral treatment with sepiapterin had no effect on body weight in either db/db or db/+ mice (Table 1). The db/db mice showed elevated levels of plasma glucose, cholesterol and triglyceride compared to db/+ mice. However, sepiapterin treatment had no effect on the biochemical parameters in either db/+ or db/db mice (Table 1).
Table 1.
Effect of sepiapterin on biochemical characteristics in db/db mice
| Parameters | db/+ | db/++ sepiapterin | db/db | db/db+ sepiapterin |
|---|---|---|---|---|
| Body weight (g) | 30±0.3 | 30±0.7 | 50±1*† | 49±0.4*† |
| Glucose (mg dl−1) | 168±19 | 179±24 | 349±21*† | 345±27*† |
| Cholesterol (mg dl−1) | 70±11 | 84±5 | 143±7*† | 142±8*† |
| Triglycerides (mg dl−1) | 69±16 | 72±3 | 119±13*† | 115±8*† |
db/++ sepiapterin, db/db+sepiapterin are the groups that received sepiapterin 10 mg kg−1 day−1 for 8 weeks in diet.
P<0.01 compared to db/+ group
P<0.01 compared to db/+ + sepiapterin group. The values represent mean±s.e.m.
Effect of sepiapterin treatment on vascular reactivity
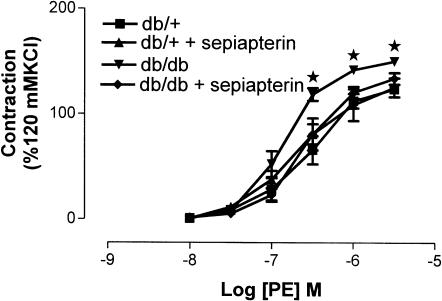
The PE-induced maximum contraction was significantly higher in SMA from db/db mice compared to db/+ mice. Treatment with sepiapterin significantly reduced the maximum contraction of SMA to PE in db/db mice, but not in SMA from db/+ mice (Figure 1). PE-induced maximum contractions were 124±4, 125±9, 150±4 and 134±6 (% 120 mM KCl) for db/+, db/+ treated with sepiapterin, db/db and db/db treated with sepiapterin, respectively.
Figure 1.
Effect of oral supplementation of sepiapterin on vascular reactivity to PE of SMA from db/+ and db/db mice. The PE-induced contraction was normalized to 120 mM KCl. The data are expressed as mean±s.e.m. based on seven to eight experiments. *P<0.05 compared to db/+ and db/+ treated with sepiapterin group.
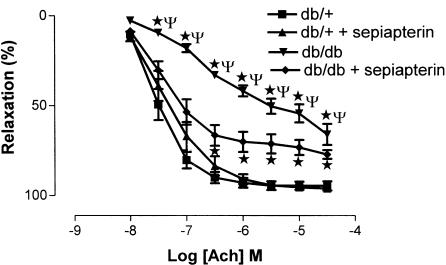
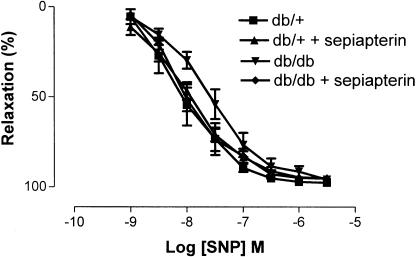
Ach induced a concentration-dependent relaxation of SMA from db/+ and db/db mice; however, maximum relaxations were significantly reduced in SMA from db/db mice compared to db/+ mice. Sepiapterin treatment significantly improved the relaxation to Ach in SMA from db/db mice but not in SMA from db/+ mice (Figure 2). Sensitivity (expressed as pD2) and maximum relaxation (expressed as %) to Ach were 7.5±0.1 and 95±2, 7.3±0.1 and 96±1, 6.4±0.1 and 66±6 and 7.2±0.2 and 77±2 for db/+, db/+ treated with sepiapterin, db/db and db/db treated with sepiapterin, respectively. SNP induced a concentration-dependent relaxation of SMA from db/+ and db/db mice, which were comparable and not altered in sepiapterin-treated groups (Figure 3). Sensitivity (expressed as pD2) and maximum relaxation (expressed as %) to SNP were 8.1±0.2 and 98±1, 8.0±0.1 and 95±1, 7.6±0.1 and 96±0.5 and 8.0±0.1 and 95±1 for db/+, db/+ treated with sepiapterin, db/db and db/db treated with sepiapterin, respectively.
Figure 2.
Effect of oral supplementation of sepiapterin on endothelium-dependent relaxation to Ach of SMA from db/+ and db/db mice. Ach-induced relaxations are expressed as percentage. The data are expressed as mean±s.e.m. based on seven to eight experiments. *P<0.05 compared to db/+ and db/+ treated with sepiapterin group, ΨP<0.05 to db/db treated with sepiapterin group.
Figure 3.
Effect of oral supplementation of sepiapterin on endothelium-independent relaxation to SNP of SMA from db/+ and db/db mice. SNP-induced relaxations are expressed as percentage. The data are expressed as mean±s.e.m. based on seven to eight experiments.
Effect of sepiapterin treatment on GTP cyclohydrolase I activity and biopterin contents
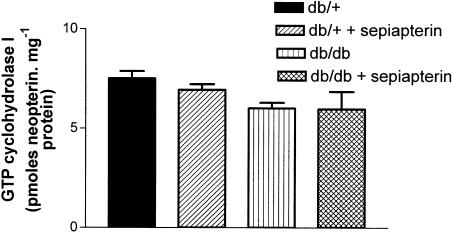
The level of neopterin, as a measure of GTP cyclohydrolase I activity, was not significantly different in SMA from db/+ and db/db mice. Sepiapterin treatment had no effect on GTP cyclohydrolase I activity in SMA from either db/+ or db/db mice (Figure 4).
Figure 4.
Effect of oral supplementation of sepiapterin on GTP cyclohydrolase activity in SMA from db/+ and db/db mice. The values are mean±s.e.m. neopterin formed in pmol mg−1 of protein based on seven to eight experiments.
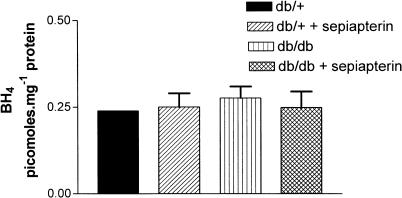
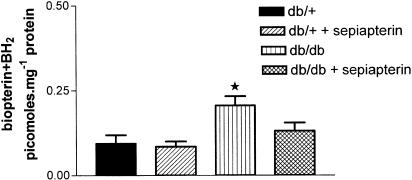
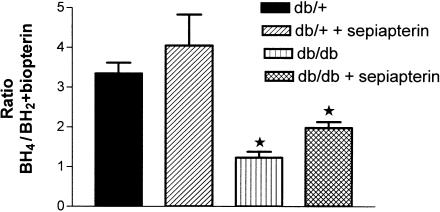
The level of BH4 in SMA was not significantly different between db/+ and db/db mice (Figure 5). Sepiapterin treatment had no effect on BH4 levels in SMA from either db/+ or db/db (Figure 5). The BH2+biopterin content in mesenteric arteries was significantly higher in db/db mice compared to db/+ mice (Figure 6). Sepiapterin treatment reduced the content of BH2+biopterin in SMA from db/db mice to control values (Figure 6). When the biopterin contents were expressed as a ratio of BH4 to BH2+biopterin, the value was significantly lower in SMA from db/db mice compared to db/+ mice. Sepiapterin treatment had no effect on the ratio of BH4 to BH2/biopterin in either db/db or db/+ mice (Figure 7).
Figure 5.
Effect of oral supplementation with sepiapterin on BH4 levels in SMA from db/+ and db/db mice. The values are mean±s.e.m. in pmol mg−1 of protein based on five to six experiments.
Figure 6.
Effect of oral supplementation with sepiapterin on BH2+biopterin levels in SMA from db/+ and db/db mice. The values are mean±s.e.m. pmol mg−1 of protein based on five to six experiments. *P<0.05 compared to db/+ and db/+ treated with sepiapterin group.
Figure 7.
Effect of oral supplementation with sepiapterin on the ratio of BH4 to BH2+biopterin in SMA from db/+ and db/db mice. The values are mean±s.e.m. pomoles mg−1 of protein based on five to six experiments. *P<0.05 compared to db/+ and db/+ treated with sepiapterin group.
Effect of sepiapterin treatment on lipid peroxidation levels in plasma and SMA
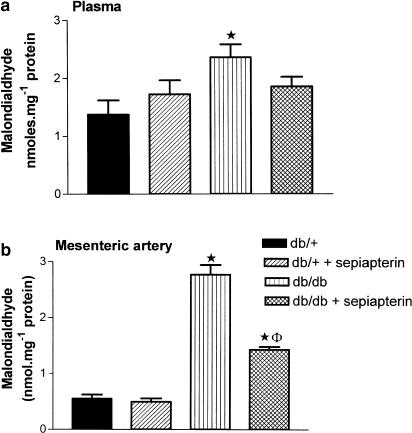
Malondialdehyde, a marker for lipid peroxidation, was significantly elevated in plasma and SMA from db/db mice compared to db/+ mice (Figure 8a and b). Sepiapterin treatment had no effect on malondialdehyde levels in plasma from db/db and db/+ mice (Figure 8a). Sepiapterin treatment significantly reduced the elevated levels of malondialdehyde in SMA from db/db mice, but not in db/+ mice (Figure 8b).
Figure 8.
Effect of oral supplementation of sepiapterin on lipid peroxidation in plasma (a) and SMA (b) from db/+ and db/db mice. The values are malondialdehyde in nmol mg−1 protein and expressed as mean±s.e.m. based on five to six experiments. *P<0.05 compared to db/+ and db/+ treated with sepiapterin, and §P<0.05 compared to db/db mice.
Discussion
Chronic oral supplementation of sepiapterin prevented the enhanced vascular contractility to PE and improved relaxation to Ach in SMA from db/db mice. Sepiapterin treatment lowered the levels of BH2+biopterin and malondialdehyde in SMA from db/db mice, but did not affect the levels of either BH4 or GTP cyclohydrolase activity. These results suggest that increased oxidative stress and oxidized products of BH4 (BH2+biopterin) contribute to endothelial dysfunction in SMA from db/db mice. Sepiapterin treatment restores endothelial function in SMA from db/db mice by decreasing the levels of lipid peroxidation and BH2+biopterin contents.
We previously reported improved relaxation to Ach following acute treatment with BH4 or sepiapterin in SMA from db/db mice (Pannirselvam et al., 2002). In the current study, we showed that chronic oral supplementation with sepiapterin improved endothelium-dependent relaxation of SMA to Ach and reduced vascular reactivity to PE. Treatment with sepiapterin, however, did not alter SNP-induced endothelium-independent relaxation. These findings are in agreement with previous reports in fructose-fed insulin-resistant rats and spontaneously hypertensive rats (Shinozaki et al., 2000; Hong et al., 2001). These data also support the hypothesis that a decreased bioavailability of BH4, either due to a decreased synthesis or increased oxidation of BH4, contributes to endothelial dysfunction in SMA from db/db mice.
BH4 is synthesized by two pathways. The de novo synthesis pathway that uses GTP as a precursor where GTP cyclohydrolase I is the rate-limiting enzyme, and by a salvage pathway where BH4 is regenerated from quinonoid form of the BH2 using dihydropteridine reductase (DHPR, Mayer & Werner, 1995). GTP cyclohydrolase I and DHPR activity were reduced in aorta from the fructose-fed insulin-resistant rat model and exhibited endothelial dysfunction with decreased levels of BH4, decreased production of NO and an increased production of superoxide anion (Shinozaki et al., 2000). Similar results were reported in coronary artery endothelial cells from BB diabetic rats (Meininger et al., 2000). However, in the current study, we did not observe any change in the BH4 level or GTP cyclohydrolase I activity in SMA from db/db mice. These data indicate that an impaired biosynthetic pathway for BH4 is not the cellular basis for endothelial dysfunction in the db/db mouse model. The level of BH2+biopterin was elevated in SMA from db/db mice, thus decreasing the ratio of BH4 to BH2 and biopterin. Increased oxidation of BH4 to BH2 and biopterin would result in decreased levels of BH4. Surprisingly, we did not observe a change in the BH4 levels in SMA from db/db mice. Based on the recent report, we speculate that BH4 is oxidized to trihydrobiopterin (BH3*) radical as well as BH2 and biopterin (Kuzkaya et al., 2003). BH3* radical is converted back to BH4 by ascorbate, maintaining levels of BH4, whereas BH2 and biopterin accumulates in the system probably because BH2 cannot be converted to BH4 due to decreased expression or activity of DHPR. Vasquez-Vivar et al. (2002b) reported that the relative proportion of the oxidized to the reduced BH4 metabolites regulate eNOS activity and the generation of superoxide. Thus, in the present study, increased levels of BH2+biopterin may competitively antagonize the binding of BH4 to eNOS and thus uncouple eNOS. However, further experiments are required to investigate this hypothesis.
Increased oxidization of BH4 in SMA from diabetic mice may result from an increased reactive oxygen species (ROS) and oxidative stress (Giugliano et al., 1996). ROS may impair endothelium-dependent relaxation by rapidly inactivating NO resulting in the formation of peroxynitrite. BH4 is a powerful reducing agent and it is possible that excess peroxynitrite formation may lead to oxidation and depletion of BH4 (Milstien & Katusic, 1999). In the present study, malondialdehyde levels were elevated in plasma and SMA from db/db mice and were restored to within the control range by sepiapterin treatment, suggesting that the restoration of the endothelial function by sepiapterin could in part be explained by an antioxidant action of sepiapterin. Direct antioxidant properties of BH4 have also been documented by Kojima et al. in xanthine/xanthine oxidase free-radical generating system and phorbol myristate acetate stimulated rat macrophage generated free radicals (Kojima et al., 1995). Oral supplementation with BH4 normalized vascular superoxide production, membrane lipid peroxidation and prevented the activation of nuclear factor kappa B, activating protein-1 in insulin-resistant rats (Shinozaki et al., 2000). Patel et al. (2002) reported that the scavenging efficiency of BH4 was comparable to ascorbate and could be a biologically viable antioxidant (Patel et al., 2002). This will also explain the effect of sepiapterin treatment on enhanced vascular reactivity to PE in SMA from db/db mice. Free radicals are known to impair endothelium integrity, enhance alpha-adrenergic receptor-mediated turnover of phosphoinositol and augment contractility through calcium channels (Chang et al., 1993). Paradoxical negative reports, however, have been reported on the effects of sepiapterin on endothelial dysfunction. Sepiapterin attenuated Ach- and A23187-induced relaxation of isolated aorta from cholesterol-fed rabbits although increased the levels of BH4 (Vasquez-Vivar et al., 2002a). It is possible that prolonged exposure to high concentration of sepiapterin has a direct effect to uncoupled eNOS, resulting in the production of superoxide instead of NO.
In conclusion, increased oxidative stress through oxidation of BH4 uncouples eNOS leading to endothelial dysfunction in SMA from db/db mice, and oral treatment with sepiapterin improves endothelial dysfunction by reducing oxidative stress.
Acknowledgments
We acknowledge the financial support of an operating grant from Norman D. MacDougall Canadian Diabetes Association (to TJA & C.R.T.) and a graduate fellowship award from Pfizer/CHS/CIHR and AHFMR (to M.P.).
Abbreviations
- Ach
acetylcholine
- BB
biobreeding
- BH2
dihydrobiopterin
- BH3*
trihydrobiopterin radical
- BH4
(6R)-5,6,7,8-tetrahydrobiopterin
- DAHP
2,4-diamino-6-hydroxypyrimidine
- DHPR
dihydropteridine reductase
- eNOS
endothelial nitric oxide synthase
- GTP
guanosine triphosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- PE
phenylephrine
- PEG-SOD
polyethylene glycol-superoxide dismutase
- ROS
reactive oxygen species
- SMA
small mesenteric artery
- SNP
sodium nitroprusside
- SHR
spontaneous hypertensive rats
References
- CHANG K.C., CHUNG S.Y., CHONG W.S., SUH J.S., KIM S.H., NOH H.K., SEONG B.W., KO H.J., CHUN K.W. Possible superoxide radical-induced alteration of vascular reactivity in aortas from streptozotocin-treated rats. J. Pharmacol. Exp. Ther. 1993;266:992–1000. [PubMed] [Google Scholar]
- COSENTINO F., KATUSIC Z.S. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- GIUGLIANO D., CERIELLO A., PAOLISSO G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- HEITZER T., BROCKHOFF C., MAYER B., WARNHOLTZ A., MOLLNAU H., HENNE S., MEINERTZ T., MUNZEL T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ. Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- HEITZER T., SCHLINZIG T., KROHN K., MEINERTZ T., MUNZEL T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- HONG H.J., HSIAO G., CHENG T.H., YEN M.H. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension. 2001;38:1044–1048. doi: 10.1161/hy1101.095331. [DOI] [PubMed] [Google Scholar]
- HOWELLS D.W., SMITH I., HYLAND K. Estimation of tetrahydrobiopterin and other pterins in cerebrospinal fluid using reversed-phase high-performance liquid chromatography with electrochemical and fluorescence detection. J. Chromatogr. 1986;381:285–294. doi: 10.1016/s0378-4347(00)83594-x. [DOI] [PubMed] [Google Scholar]
- HURSHMAN A.R., KREBS C., EDMONDSON D.E., HUYNH B.H., MARLETTA M.A. Formation of a pterin radical in the reaction of the heme domain of inducible nitric oxide synthase with oxygen. Biochemistry. 1999;38:15689–15696. doi: 10.1021/bi992026c. [DOI] [PubMed] [Google Scholar]
- KINOSHITA H., MILSTIEN S., WAMBI C., KATUSIC Z.S. Inhibition of tetrahydrobiopterin biosynthesis impairs endothelium-dependent relaxations in canine basilar artery. Am. J. Physiol. 1997;273:H718–H724. doi: 10.1152/ajpheart.1997.273.2.H718. [DOI] [PubMed] [Google Scholar]
- KLATT P., SCHMID M., LEOPOLD E., SCHMIDT K., WERNER E.R., MAYER B. The pteridine binding site of brain nitric oxide synthase. Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J. Biol. Chem. 1994;269:13861–13866. [PubMed] [Google Scholar]
- KOJIMA S., ONA S., IIZUKA I., ARAI T., MORI H., KUBOTA K. Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic. Res. 1995;23:419–430. doi: 10.3109/10715769509065263. [DOI] [PubMed] [Google Scholar]
- KUZKAYA N., WEISSMANN N., HARRISON D.G., DIKALOV S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid and thiols: implications for uncoupling endothelial nitric oxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- MAIER W., COSENTINO F., LUTOLF R.B., FLEISCH M., SEILER C., HESS O.M., MEIER B., LUSCHER T.F. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J. Cardiovasc. Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- MAYER B., WERNER E.R. In search of a function for tetrahydrobiopterin in the biosynthesis of nitric oxide. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:453–463. doi: 10.1007/BF00171035. [DOI] [PubMed] [Google Scholar]
- MEININGER C.J., MARINOS R.S., HATAKEYAMA K., MARTINEZ-ZAGUILAN R., ROJAS J.D., KELLY K.A., WU G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem. J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILSTIEN S., KATUSIC Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- PANNIRSELVAM M., VERMA S., ANDERSON T.J., TRIGGLE C.R. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br. J. Pharmacol. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL K.B., STRATFORD M.R., WARDMAN P., EVERETT S.A. Oxidation of tetrahydrobiopterin by biological radicals and scavenging of the trihydrobiopterin radical by ascorbate. Free Radic. Biol. Med. 2002;32:203–211. doi: 10.1016/s0891-5849(01)00777-8. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J. Cardiovasc. Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- POU S., POU W.S., BREDT D.S., SNYDER S.H., ROSEN G.M. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- SHINOZAKI K., KASHIWAGI A., NISHIO Y., OKAMURA T., YOSHIDA Y., MASADA M., TODA N., KIKKAWA R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2-imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- SHINOZAKI K., NISHIO Y., OKAMURA T., YOSHIDA Y., MAEGAWA H., KOJIMA H., MASADA M., TODA N., KIKKAWA R., KASHIWAGI A. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ. Res. 2000;87:566–573. doi: 10.1161/01.res.87.7.566. [DOI] [PubMed] [Google Scholar]
- TIEFENBACHER C.P., BLEEKE T., VAHL C., AMANN K., VOGT A., KUBLER W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation. 2000;102:2172–2179. doi: 10.1161/01.cir.102.18.2172. [DOI] [PubMed] [Google Scholar]
- TIEFENBACHER C.P., CHILIAN W.M., MITCHELL M., DEFILY D.V. Restoration of endothelium-dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circulation. 1996;94:1423–1429. doi: 10.1161/01.cir.94.6.1423. [DOI] [PubMed] [Google Scholar]
- VANN L.R., TWITTY S., SPIEGEL S., MILSTIEN S. Divergence in regulation of nitric-oxide synthase and its cofactor tetrahydrobiopterin by tumor necrosis factor-alpha. Ceramide potentiates nitric oxide synthesis without affecting GTP cyclohydrolase I activity. J. Biol. Chem. 2000;275:13275–13281. doi: 10.1074/jbc.275.18.13275. [DOI] [PubMed] [Google Scholar]
- VASQUEZ-VIVAR J., DUQUAINE D., WHITSETT J., KALYANARAMAN B., RAJAGOPALAN S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler. Thromb. Vasc. Biol. 2002a;22:1655–1661. doi: 10.1161/01.atv.0000029122.79665.d9. [DOI] [PubMed] [Google Scholar]
- VASQUEZ-VIVAR J., KALYANARAMAN B. Generation of superoxide from nitric oxide synthase. FEBS Lett. 2000;481:305–306. doi: 10.1016/s0014-5793(00)02001-9. [DOI] [PubMed] [Google Scholar]
- VASQUEZ-VIVAR J., MARTASEK P., WHITSETT J., JOSEPH J., KALYANARAMAN B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem. J. 2002b;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI C.C., WANG Z.Q., WANG Q., MEADE A.L., HEMANN C., HILLE R., STUEHR D.J. Rapid kinetic studies link tetrahydrobiopterin radical formation to heme-dioxy reduction and arginine hydroxylation in inducible nitric-oxide synthase. J. Biol. Chem. 2001;276:315–319. doi: 10.1074/jbc.M008441200. [DOI] [PubMed] [Google Scholar]
- WEVER R.M., VAN DAM T., VAN RIJN H.J., DE GROOT F., RABELINK T.J. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 1997;237:340–344. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]