Abstract
Cholecystokinin (CCK) is a postprandial hormone that elicits a satiating effect and regulates feeding behaviour. CCK has been shown to enhance the effect of leptin in several experimental paradigms.
The goal of this work was to characterize the effect of endogenous CCK on plasma leptin content by using CCK receptor antagonists. Therefore, we administered SR-27,897, a selective CCK1 receptor antagonist, and L-365,260, a selective CCK2 receptor antagonist, to fed and food-deprived rats and determined plasma leptin concentration by enzyme immunoassay. Plasma insulin and glucose concentration as well as food intake were also determined.
Under our conditions, SR-27,897 increased plasma concentration of leptin both in fed and food-deprived rats. It also increased food intake as well as plasma concentration of insulin in fed animals. L-365,260 increased plasma leptin concentration only in fed rats.
In animals receiving exogenous leptin, CCK-8 increased the ratio between the concentration of leptin in cerebrospinal fluid and plasma.
These results show that CCK receptor antagonists increases plasma concentration of leptin and suggest that endogenous CCK may facilitate the uptake of plasma leptin to the cerebrospinal fluid.
Keywords: Cholecystokinin; insulin; leptin; L-365,260; SR-27,897; food intake
Introduction
Cholecystokinin (CCK) is a gastrointestinal hormone that plays a key role in the regulation of both endo- and exocrine pancreas, and also a neurotransmitter in mammalian central nervous system (CNS). CCK-related peptides bind to two different receptors: the CCK1 receptor (CCK1R), which mediates most peripheral and hormonal actions of CCK; and the CCK2 receptor (CCK2R), which is the most abundant form in the brain and identical to the receptor of gastrin in peripheral organs (Crawley & Corwin, 1994; Noble et al., 1999). CCK is a postprandial hormone that elicits a satiating effect involving both CCK1Rs (Moran & McHugh, 1992) and CCK2Rs (Dourish et al., 1989). This effect seems to be linked to the activation of vagal afferents that synapse in brain stem areas with neurons projecting to the hypothalamus (Corp et al., 1993; Rinaman et al., 1995). Although the effect of CCK in reducing meal size is well documented (Smith et al., 1981), the importance of this hormone on both body weight and metabolism regulation remains to be characterized. Interestingly, Otsuka Long – Evans Tokushima Fatty (OLETF) rats, which are spontaneous mutants having a disrupted CCK1R gene, are obese (Funakoshi et al., 1995) and exhibit an altered feeding behaviour (Moran et al., 1998; Moran, 2000). These antecedents suggest that CCK could play a role in energy balance (Matson & Ritter, 1999) and be integrated in a larger regulatory system. In this context, the interaction between CCK and other hormones also involved in regulating satiety and energy expenditure, such as insulin or leptin, has been the goal of several number of studies (Verspohl et al., 1986; Barrachina et al., 1997).
CCK has been shown to stimulate in vitro insulin release from rat pancreatic islets through CCK1Rs (Verspohl et al., 1986; Karlsson et al., 1998). In accordance, CCK1R antagonists decrease insulin immunoreactivity in rats treated with exogenous CCK-8 that were challenged with oral glucose (Verspohl et al., 1988) or with an intraduodenal meal (Rossetti et al., 1987). More recently, a large body of literature focused on OLETF rats show that these animals develop a noninsulin-dependent diabetes-like syndrome associated with hyperinsulinaemia and hyperleptinaemia (Funakoshi et al., 1995; 1996; Takiguchi et al., 1998).
Concerning the interaction between leptin and CCK, pharmacological studies show a synergic interaction between these hormones on the control of food intake (Barrachina et al., 1997) and metabolic activity (Matson et al., 2002). The effect of CCK on circulating leptin has been extensively studied by Bado's group. These authors have reported that exogenous CCK-8, administered to fasted rats, increases plasma leptin in a dose-dependent manner and enhances leptin release from isolated perfused rat stomach (Bado et al., 1998).
The goal of this work was therefore to clarify the effect of endogenous CCK on circulating concentrations of both insulin and leptin. Since CCK is a postprandial hormone, our study has been performed by administering CCK1R (SR-27,897)- and CCK2R (L-365,260)-selective antagonists to rats just before the food-intake period occurring during the early dark phase of the circadian cycle (Akana et al., 1994). SR-27,897 and L-365,260 were used at a single dose assessed as selective in previous works (Ruiz-Gayo et al., 2000 and references cited therein). We have compared the effect of this treatment with a group of animals that were food deprived during this period.
Methods
Animals
Male Wistar rats (CRIFA, Spain), weighing 180 – 230 g at the time of the experiment, were housed under a light/dark cycle (12/12 h) in a temperature-controlled room (22±1°C), with standard rat chow (Panlab, Spain) and water available ad libitum. Lights were switched off at 1700. Animals were handled daily, for at least 1 week, to avoid stress by manipulation on the day of the experiment. All experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC) for the care and use of laboratory animals.
Chemicals
The CCK1R antagonist, SR-27,897 (1-[[2-4-(2-chlorophenyl)thiazol-2-yl-aminocarbony]-indolyl]-acetic acid), was kindly provided by Sanofi-Synthelabo, France (Poncelet et al., 1993). The CCK2R antagonist, L-365,260 ((3R)-(+)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3-yl)-3-methylphenylurea)), was a gift of Merck Sharp and Dohme Research Laboratories, U.S.A. (Chang et al., 1989). CCK-8, leptin and other chemicals and solvents were from Sigma, U.S.A.
Food-deprivation conditions
Animals were food deprived during 210 min. Briefly, food was withdrawn 120 min before the lights were switched off and rats were maintained food deprived until the end of the experiment, 90 min after the lights were switched off. Water was available ad libitum. Food-deprived rats were maintained in the same room than ad libitum fed rats.
Drug treatment and procedure for experiments with CCKR antagonists
Experiments were carried out simultaneously in fed and food-deprived rats. SR-27,897 and L-365,260 were administered subcutaneously at 0.3 and 1 mg kg−1, respectively, 120 min before the lights were switched off. Vehicle was 4% carboxymethylcellulose. Animals were killed by decapitation 90 min after the lights were switched off and trunk blood was collected in chilled tubes containing heparin, then centrifuged at 4°C for 20 min at 3000 r.p.m. and plasma stored at −20°C until assay.
Drug treatment and procedure for experiments with exogenous leptin and CCK-8
These experiments were carried out only in fed rats. Leptin (0.1 mg kg−1) was administered in saline buffer to all animals 120 min before the lights were switched off. CCK-8 (10 μg kg−1) was administered in saline 30 min after leptin. A sample of blood was obtained from the caudal artery 90 min after the lights were switched off and handled as described below. Rats were then immediately anaesthetized and cerebrospinal fluid (CSF) was obtained by puncturing the cisterna magna.
Plasma samples analysis
Plasma leptin concentration was analysed by using a specific enzyme immunoassay (EIA) kit for rat leptin (Assay Designs Inc., U.S.A). Intra- and interassay variations were 11.6 and 11%, respectively. For leptin assay, plasma was diluted 1/5 in buffer assay. Leptin concentration was within the detection range of the kit, that is, 0.06 – 3.6 ng ml−1. Insulin was determined by using a specific EIA kit for rat insulin (Mercodia, Denmark), based on the direct sandwich technique in which two monoclonal antibodies are directed against separated antigenic determinants on the insulin molecule. Plasma samples were within the detection range of the assay 0.7 – 5.5 μg ml−1 (1.8% intraassay variation, 3.8% interassay variation). Glucose was measured by a spectrophotometric method (Glucose Trinder Method, Roche, Spain) (Barham & Trinder, 1972).
Feeding experiments
Food was weighed 120 min before the lights were turned off. Drugs were administered at the same time. Food intake was determined during a period of 210 min. Food intake was expressed as % of the control group.
Statistical analysis
Individual group comparisons were made using a two-way ANOVA. The factors of variation were treatment and food availability. The effect of drugs within a given group (fed or food deprived) were analysed by using a one-way ANOVA, followed by a Newman – Keuls' test.
Results
Effect of the CCKR antagonists on plasma concentration of insulin
In the case of SR-27,897, two-way ANOVA indicated a significant effect of fasting (F(1,31)=32.503; P<0.01). The effect of treatment as well as the interaction between treatment and food deprivation were not significant. As illustrated in Figure 1a, treatment of fed rats with SR-27,897 (0.3 mg kg−1) raised plasma insulin concentration from 1.9±0.2 to 3.2±0.8 μg dl−1. In the case of L-365,260 (1 mg kg−1), two-way ANOVA also detected an effect of fasting (F(1,28)=27.469; P<0.01) but not of treatment. The interaction between them was not significant (Figure 1b).
Figure 1.
(a) Effect of SR-27,897 (0.3 mg kg−1) on insulin plasmatic concentration. Vehicle (open columns) or SR-27,897 (hatched columns) were administered 120 min before the lights were off. Values are means±s.e.m. of 8 – 10 animals. (b) Effect of L-365,260 (1 mg kg−1) on insulin plasma concentration. Values are means± s.e.m. of 8 – 10 animals.
Effect of CCKR antagonists on plasma glucose concentration
Table 1 resumes the effect of both SR-27,897 (0.3 mg kg−1) and L-365,260 (1 mg kg−1) on glucose plasma concentration. In the case of SR-27,897, two-way ANOVA revealed a significant effect of both treatment (F(1,35)=6.826; P<0.05) and food deprivation (F(1,35)=5.665; P<0.05) without significant interaction between them. In fed rats, SR-27,897 decreased plasma glucose concentration (one-way ANOVA, F(1,21)=9.536; P>0.01). In the case of L-365,260, two-way ANOVA only indicated a significant effect of food deprivation (F(1,35)=17.105; P>0.01).
Table 1.
Effect of SR-27,897 (0.3 mg kg−1) and L-365,260 (1 mg kg−1) on glucose (mg dl−1) plasma concentration determined 210 min after food deprivation
| Group | Glucose (mg dl−1) |
|---|---|
| Fed+vehicle | 149±3.2 |
| Fed+SR-27,897 | 131.5±4.3** |
| Fed+L-365,260 | 148.0±7.0 |
| Fasted+vehicle | 132.4±3.7# |
| Fasted+SR-27,897 | 128.6±4.6 |
| Fasted+L-365,260 | 130.0±3.5 |
Vehicle, SR-27,897 or L-365,260 were given 120 min before the lights were off.
P<0.01, compared to the fed+vehicle group.
P<0.05, compared to the fed+vehicle group (Newman – Keuls' test).
Effect of the CCKR antagonists on plasma concentration of leptin
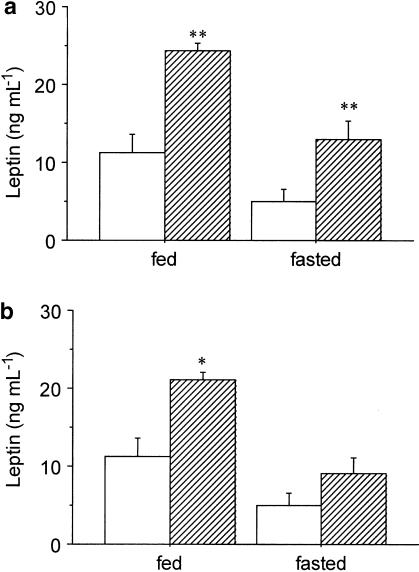
Figure 2a illustrates the effect of SR-27,897 (0.3 mg kg−1) on plasma leptin concentration, both in fed and fasted rats. Two-way ANOVA revealed significant effect of treatment (F(1,34)=19.797; P<0.01) and food deprivation (F(1,34)=13.869; P<0.01) without significant interaction between treatment and fasting. SR-27,897 significantly increased plasma leptin concentration both in fed and fasted rats from 11.2±2.2 to 25.0±1.3 ng ml−1 (one-way ANOVA, F(1,18)=11.193; P<0.01) and from 5.1±1.4 to 12.9±2.3 ng ml−1 (F(1,16)=9.539; P<0.01), respectively.
Figure 2.
(a) Effect of SR-27,897 (0.3 mg kg−1) on leptin plasmatic concentration. Vehicle (open columns) or SR-27,897 (hatched columns) were administered 120 min before the lights were off. Values are means±s.e.m. of 8 – 10 animals. **P<0.01, compared to the control group (Newman – Keuls' test). (b) Effect of L-365,260 (1 mg kg−1) on leptin plasma concentration. Values are means± s.e.m. of 8 – 10 animals. *P<0.05, compared to the control group (Newman – Keuls' test).
In the case of the CCK2R antagonist, L-365,260 (1 mg kg−1), two-way ANOVA also indicated a significant effect of treatment (F(1,35)=8.407; P<0.01) and fasting (F(1,35)= 14.652; P<0.01) without significant interaction between them. The increase of plasma leptin elicited by L-365,260 was from 11.2 to 20.5±1.1 ng ml−1 in fed rats (one-way ANOVA, F(1,19)=6.104; P<0.05) and from 5.0±1.4 to 9.1±1.9 ng ml−1 in fasted rats (F(1,16)=12.756; P<0.01) (Figure 2b).
Food intake measurement
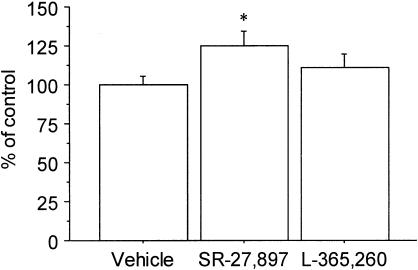
Figure 3 illustrates the effect of SR-27,897 (0.3 mg kg−1) and L-365,260 (1 mg kg−1) on food intake in a set of animals that were not fasted before the experiment. Under these conditions, one-way ANOVA revealed a significant effect of treatment (F(2,21)=6.263; P<0.01) in the case of animals treated with SR-27,897. In this experimental group, the increase of food intake was 47.3% over the control group.
Figure 3.
Effect of SR-27,897 (0.3 mg kg−1) and L-365,260 (1 mg kg−1) on food intake. Values are means±s.e.m. of 8 – 10 animals. *P<0.05, compared to the control group (Newman – Keuls' test).
Effect of CCK-8 on exogenous leptin distribution
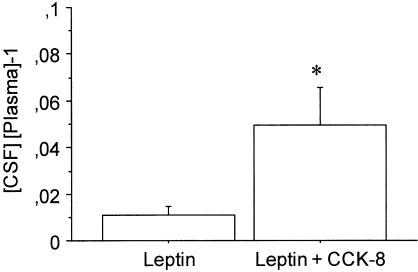
CCK-8 decreased the concentration of leptin in plasma (33.6±7.1 vs 22.1±0.5 ng ml−1; P=0.1) and increased leptin concentration in CSF (0.4±0.1 vs 1.1±0.3 ng ml−1; P=0.8). Figure 4 illustrates the effect of CCK-8 (10 μg kg−1) on the ratio between the concentration of leptin in CSF and plasma. One-way ANOVA revealed that the ratio was about four to five times higher in the group treated with CCK-8 (F(1,10)=5.513; P<0.05). Data are expressed as a relative value to minimize variability between different animals.
Figure 4.
Effect of CCK-8 on the ratio between CSF and plasma leptin concentration. Values are means±s.e.m. of five to six animals. *P<0.05 (Newman – Keuls' test).
Discussion
In this work, we describe the effect of CCKRs blockade on plasma concentrations of both insulin and leptin. We used selective CCK1R (SR-27,897) and CCK2R (L-365,260) antagonists, administered just before the onset of the dark phase of the circadian cycle, that is, a physiological period of food intake in rodents. These conditions were used to model the typical hormonal/metabolic situation of an early postprandial or feeding period in rats. The variables of our study were pharmacological treatment and food availability. This work was carried out simultaneously in (i) ad libitum fed rats and in (ii) rats that were food deprived from 2 h before the dark period. Under these conditions, SR-27,897 and L-365,260 significantly increased leptin plasma concentration both in fed and food-deprived animals, whereas insulin was only slightly increased in fed animals treated with SR-27,897. Such an increase was accompanied by a decrease of plasma concentration of glucose, which could be the consequence of the slight increase of plasma insulin, as food intake was higher in these animals.
A direct effect of CCK on insulin release would be rather unexpected as CCK, by acting on CCK1Rs, seems to stimulate, rather than inhibit, insulin release (Verspohl et al., 1986; 1988; Karlsson et al., 1998). Furthermore, the increase in insulin concentration was not observed in food-deprived animals treated with the CCK1R antagonist. Thus, in the present study, the observed effect on insulin (Figure 1) might be related to the increase of food intake elicited by SR-27,897. In contrast to that, the effect detected on leptin concentration (Figure 2) seems to reflect a direct action of endogenous CCK as leptin increased both in fed and food-deprived rats. These data are in apparent contradiction with previous results in the literature, suggesting a synergistic potentiation between both CCK and leptin (Barrachina et al., 1997; Matson & Ritter, 1999; Matson et al., 2002). CCK has been shown to increase plasma leptin concentration in fasting/refeeding paradigms with a concomitant decrease of leptin content in the stomach fundus epithelium (Bado et al., 1998), indicating a secretagogue effect of CCK on gastric stores of leptin. Under similar conditions, CCK2R blockade decreases the level of circulating leptin (Attoub et al., 1999). Nevertheless, to our knowledge, the effects of CCKRs antagonists under more physiological conditions, such as those used in this study, have not been investigated. As an alternative mechanism CCKRs antagonists might facilitate the release of leptin from fat stores. However, such a possibility seems to be also unlikely as CCK2R antagonists inhibit rather than stimulate leptin release from rat adipocytes (Attoub et al., 1999). In consequence, the effect reported in this study would not be linked to an inhibitory effect of endogenous CCK on the release of leptin from fat or from stomach. Interestingly, SR-27,897 increased plasma leptin levels both in fed and food-deprived rats, suggesting that the effect of this drug would be mediated by a mechanism independent of food intake. Thus, the effect of SR-27,897 in increasing food intake (Ruiz-Gayo et al., 2000; this study) is insufficient to explain its effect on plasma leptin concentration.
The effect of CCKR antagonists may therefore be due to the blockade of an eventual regulatory role of CCK on leptin kinetics, that is, metabolism and/or distribution. To test this hypothesis, we administered exogenous leptin and determined the effect of CCK-8 on the concentration of leptin both in plasma and in CSF. The dose of CCK-8 used in this work (10 μg kg−1) might increase CCK immunoreactivity in plasma until approximately 40 pM (Linden, 1989), which is below the KD value of CCK-8 for CCK receptors (Durieux et al., 1992; Attoub et al., 1999), but probably enough to occupy a significant number of CCKRs. Under these conditions, we observed a decrease of plasma leptin concentration together with an increase of leptin concentration in the CSF (P≈0.1). These results taken together with the significant increase of the ratio (leptin)CSF/(leptin)plasma in CCK-8-treated rats, strongly suggest that CCK-8 enhances the permeability of CNS barriers to leptin. Alternatively, the increase of leptin immunoreactivity in CSF might also be due to an eventual effect of CCK-8 on neuronal stores of leptin. This possibility has to be considered as leptin has been recently identified in circumventricular areas of the hypothalamus lacking blood – brain barrier (BBB) (Ur et al., 2002).
Leptin enters into the CNS by means of a specific transport system located both in the BBB and in the choroid plexus (Devos et al., 1996; Kastin et al., 1999; Zlokovic et al., 2000). If CCK facilitates the entry of leptin into the CNS, blockade of CCK receptors might increase plasma leptin content by hindering leptin distribution. Such an effect of CCK-8 would be compatible with the secretagogue effect of CCK observed by Bado's group. In this context one could also interpret other studies, which suggest that the hypothalamus is the target for a functional synergy between CCK and leptin (Barrachina et al., 1997; Wang et al., 1998; Matson et al., 2002). Interestingly, experiments carried out in OLETF rats, which lack CCK1Rs, demonstrate that these animals are resistant to the effect of peripheral leptin, but respond to i.c.v. administered leptin, suggesting a role for CCK in the regulation of the accessibility of leptin to the CNS (Niimi et al., 1999).
In summary, our results suggest that CCK may be a mediator for regulating leptin distribution and allow to hypothesize that the synergy between CCK and leptin could be, in part, due to a facilitatory role of CCK on leptin entry into the CNS. In fact, we have detected a robust effect of CCKR antagonist, which points to a physiological role of CCK in regulating plasma leptin concentration. In addition, as intraperitoneal CCK-8 slightly increases plasma concentration of CCK (Linden, 1989; Linden & Södersten, 1990), it can be argued that CCKRs occupancy would not be dramatically higher in animals treated with exogenous CCK than in controls. In consequence, although further studies are necessary, it can be proposed that the interaction between CCK and leptin described in this study can be endowed with physiological sense and may have clinical implications for obese patients.
Acknowledgments
This work was supported by grants from CICYT – Ministerio de Ciencia y Tecnología (PM 99-0010, PM 99-0011) and FSP-CEU (2/01 USP). MVC is a fellow of FUSP-CEU. SR-27,897 and L-365,260 were a generous gift of Sanofi Synthélabo (France) and MSD (U.S.A.), respectively.
Abbreviations
- BBB
blood – brain barrier
- CCK
cholecystokinin
- CCKR
cholecystokinin receptor
- CSF
cerebrospinal fluid
References
- AKANA S.F., STRACK A.M., HANSON E.S., DALLMAN M. Regulation of activity in the hypothalamo – pituitary – adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135:1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- ATTOUB S., LEVASSEUR S., BUYSE M., GOIOT H., LAIGNEAU J.P., MOIZO L., HERVATIN F., LE MARCHAND-BRUSTEL Y., LEWIN J.M.M., BADO A. Physiological role of cholecystokinin B/gastrin receptor in leptin secretion. Endocrinology. 1999;140:4406–4410. doi: 10.1210/endo.140.10.7079. [DOI] [PubMed] [Google Scholar]
- BADO A., LEVASSEUR S., ATTOUB S., KERMORGANT S., LAIGNEAU J.P., BORTOLUZZI M.N., MOIZO L., LEHY T., GUERRE-MILLOS M., LE MARCHAND-BRUSTEL Y., LEWIN M.J.M. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- BARHAM D., TRINDER P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- BARRACHINA M.D., MARTÍNEZ V., WANG L., WEI J.Y., TACHÉ Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R.S., CHEN T.B., BOCK M.G., FREIDINGER R.M., CHEN R., ROSEGAY A., LOTTI V.J. Characterization of the binding of [3H]-L-365,260: a new potent and selective brain cholecystokinin (CCK-B) and gastrin receptor antagonist radioligand. Mol. Pharmacol. 1989;35:803–808. [PubMed] [Google Scholar]
- CORP E.S., McQUADE J., MORAN T.H., SMITH G.P. Characterization of type A and type B CCK receptor binding sites in rat vagus nerve. Brain Res. 1993;623:91–95. doi: 10.1016/0006-8993(93)90024-h. [DOI] [PubMed] [Google Scholar]
- CRAWLEY J.N., CORWIN R.L. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- DEVOS R., RICHARDS J.G., CAMPFIELD L.A., TARTAGLIA L.A., GUISEZ Y., VAN DER HEYDEN J., TRAVERNIER J., PLAETINCK G., BURN P. OB protein binds specifically to the choroid plexus of mice and rats. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5668–5673. doi: 10.1073/pnas.93.11.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOURISH C.T., RYCROFT W., IVERSEN S. Postponement of satiety by blockade of brain cholecystokinin (CCK-B) receptors. Science. 1989;245:1509–1511. doi: 10.1126/science.2781294. [DOI] [PubMed] [Google Scholar]
- DURIEUX C., RUIZ-GAYO M., CORRINGER P.J., BERGERON F., DUCOS B., ROQUES B.P. [3H]pBC264, a suitable probe for studying cholecystokinin-B receptors: binding characteristics in rodent brains and comparison with [3H]SNF 8702. Mol. Pharmacol. 1992;41:1089–1095. [PubMed] [Google Scholar]
- FUNAKOSHI A., MIYASAKA K., KANAI S., MASUDA M., YASUNAMI Y., NAGAI T., IKEDA S., JIMI A., KAWANAMI T., KONO A. Pancreatic endocrine dysfunction in rats not expressing the cholecystokinin-A receptor. Pancreas. 1996;12:230–236. doi: 10.1097/00006676-199604000-00004. [DOI] [PubMed] [Google Scholar]
- FUNAKOSHI A., MIYASAKA K., SHINOZAKI H., MASUDA M., KAWANAMI T., TAKATA Y., KONO A. An animal model of congenital defect of gene expression of cholecystokinin (CCK)-A receptor. Biochem. Biophys. Res. Commun. 1995;210:787–790. doi: 10.1006/bbrc.1995.1728. [DOI] [PubMed] [Google Scholar]
- KARLSSON S., SUNDLER F., AHRÉN B. CCK receptor subtype in insulin-producing cells: a combined functional and in situ hybridization study in rat islets and a rat insulinoma cell line. Regul. Peptides. 1998;78:95–103. doi: 10.1016/s0167-0115(98)00136-0. [DOI] [PubMed] [Google Scholar]
- KASTIN A.J., PAN W., MANESS L.M., KOLETSKY R.J., ERNSBERGER P. Decreased transport of leptin across the blood – brain-barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–1453. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- LINDEN A. Role of cholecystokinin in feeding and lactation. Acta Physiol. Scand. 1989;585:1–49. [PubMed] [Google Scholar]
- LINDEN A., SÖDERSTEN P. Relationship between the concentration of cholecystokinin-like immunoreactivity in plasma and food intake in male rats. Physiol. Behav. 1990;48:859–863. doi: 10.1016/0031-9384(90)90240-5. [DOI] [PubMed] [Google Scholar]
- MATSON C.A., REID D.F., RITTER R.C. Daily CCK injection enhances reduction of body weight by chronic intracerebroventricular leptin infusion. Am. J. Physiol. 2002;282:R1368–R1373. doi: 10.1152/ajpregu.00080.2001. [DOI] [PubMed] [Google Scholar]
- MATSON C.A., RITTER R.C. Long-term CCK-leptin synergy suggest a role for CCK in the regulation of body weight. Am. J. Physiol. 1999;45:R1038–R1045. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- MORAN T.H. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16:858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., KATZ L.F., PLATA-SALAMAN C.R., SCHWARTZ G.J. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am. J. Physiol. 1998;43:R618–R625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., MCHUGH P.R.Gastric mechanisms in CCK satiety Multiple Cholecystokinin Receptors in the CNS 1992Oxford: Oxford University Press; 183–205.eds. Dourish, C.T., Cooper, S.J., Iversen S.D. & Iversen, L.L. pp [Google Scholar]
- NIIMI M., SATO M., YOKOTE R., TADA S., TAKAHARA J. Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long – Evans Tokushima Fatty rat with hyperleptinaemia. J. Neuroendocrinol. 1999;11:605–611. doi: 10.1046/j.1365-2826.1999.00368.x. [DOI] [PubMed] [Google Scholar]
- NOBLE F., WANK S.A., CRAWLEY J.N., BRADWEJN J., SEROOGY K.B., HAMON M., ROQUES B.P. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- PONCELET M., ARNONE M., HEAULME M., GONALONS N., GUEDET C., SANTUCCI V., THURNEYSSEN O., KEANO P., GULLY D., LE FUR G., SOUBRIÉ P. Neurobehavioral effects of SR-27,897, a selective cholecystokinin type A (CCK-A) receptor antagonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:102–107. doi: 10.1007/BF00168544. [DOI] [PubMed] [Google Scholar]
- RINAMAN L., HOFFMAN G.E., DOHANICS J., LE W.W., STRICKER E.M., VERBALIS J.G. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innevate the paraventricular nucleus of the hypothalamus in rats. J. Comp. Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- ROSSETTI L., SHULMAN G.I., ZAWALICH W.S. Physiological role of cholecystokinin in meal-induced insulin secretion in conscious rats. Studies with L-365,718, a specific inhibitor of CCK-receptor binding. Diabetes. 1987;36:1212–1215. doi: 10.2337/diab.36.10.1212. [DOI] [PubMed] [Google Scholar]
- RUIZ-GAYO M., GARRIDO M.M., FUENTES J.A. Inhibition of the hypothalamic – pituitary – adrenal axis in food-deprived rats by a CCK-A receptor antagonist. Br. J. Pharmacol. 2000;129:839–842. doi: 10.1038/sj.bjp.0703117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH G.P., JEROME C., CUSHIN B.J., ETERNO R., SIMANSKY K.J. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- TAKIGUCHI S., TAKATA Y., TAKAHASHI N., KATAOKA K., HIRASHIMA T., KAWANO K., MIYASAKA K., FUNAKOSHI A., KONO A. A disrupted cholecystokinin A receptor gene induces diabetes in obese rats synergistically with ODB1 gene. Am. J. Physiol. 1998;274:E265–E270. doi: 10.1152/ajpendo.1998.274.2.E265. [DOI] [PubMed] [Google Scholar]
- UR E., WILKINSON D.A., MORASH B.A., WILKINSON M. Leptin immunoreactivity is localized to neurons in rat brain. Neuroendocrinology. 2002;75:264–272. doi: 10.1159/000054718. [DOI] [PubMed] [Google Scholar]
- VERSPOHL E.J., AMMON H.P.T., WILLIAMS J.A., GOLDFINE I.D. Evidence that cholecystokinin interacts with specific receptors and regulates insulin release in isolated rat islets of Langerhans. Diabetes. 1986;35:38–43. doi: 10.2337/diab.35.1.38. [DOI] [PubMed] [Google Scholar]
- VERSPOHL E.J., WUNDERLE G., AMMON H.P.T. Proglumide antagonizes cholecystokinin effects on plasma glucose and insulin rats in vivo. Eur. J. Pharmacol. 1988;152:121–128. doi: 10.1016/0014-2999(88)90842-4. [DOI] [PubMed] [Google Scholar]
- WANG L., MARTINEZ V., BARRACHINA M.D., TACHÉ Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 1998;791:157–166. doi: 10.1016/s0006-8993(98)00091-2. [DOI] [PubMed] [Google Scholar]
- ZLOKOVIC B.V., JOVANOVIC S., MIAO W., SAMARA S., VERMA S., FARELL C.L. Differential regulation of leptin transport by the choroid plexus and blood – brain-barrier and high affinity transport systems for entry into hypothalamus and across the blood – cerebrospinal fluid barrier. Endocrinology. 2000;141:1434–1441. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]