Abstract
Toluene is an industrial solvent widely used as a drug of abuse, which can produce sudden sniffing death due to cardiac arrhythmias. In this paper, we tested the hypothesis that toluene inhibits cardiac sodium channels in Xenopus laevis oocytes transfected with Nav1.5 cDNA and in isolated rat ventricular myocytes.
In oocytes, toluene inhibited sodium currents (INa+) in a concentration-dependent manner, with an IC50 of 274 μM (confidence limits: 141–407μM). The inhibition was complete, voltage-independent, and slowly reversible.
Toluene had no effect on: (i) the shape of the I–V curves; (ii) the reversal potential of Na+; and (iii) the steady-state inactivation.
The slow recovery time constant from inactivation of INa+ decreased with toluene exposure, while the fast recovery time constant remained unchanged.
Block of INa+ by toluene was use- and frequency-dependent.
In rat cardiac myocytes, 300 μM toluene inhibited the sodium current (INa+) by 62%; this inhibition was voltage independent.
These results suggest that toluene binds to cardiac Na+ channels in the open state and unbinds either when channels move between inactivated states or from an inactivated to a closed state.
The use- and frequency-dependent block of INa+ by toluene might be responsible, at least in part, for its arrhythmogenic effect.
Keywords: Inhalants, toluene, Na+ cardiac channels, Xenopus oocytes, voltage clamp, sudden sniffing death
Introduction
The intentional inhalation of volatile solvents to achieve a state of intoxication is a worldwide problem that is more prevalent among children and adolescents (Anderson et al., 1985; Kozel et al., 1995; Kurtzman et al., 2001). Among inhalants, toluene is readily available in many commercial products, such as paint thinners and adhesives (Arlien-Soborg, 1992), and is widely used for intoxicating purposes. The abuse of inhalants constitutes an important public health problem because of the behavioural deficits associated with acute and chronic intoxication and the well-documented hazard of sudden sniffing death, even in sporadic abusers (Bass, 1970; Shepherd, 1989; Anderson, 1990; Bowen et al., 1999).
There is sound evidence indicating that toluene shares many behavioural effects with ethanol and other central nervous system depressants (for a review, see Evans & Balster, 1991). Based on these similarities, in 1998, our group initiated the study of the cellular actions of abused solvents on ligand-gated channels, showing that toluene was able to dose-dependently inhibit recombinant N-methyl-D-aspartic acid (NMDA) receptors at low millimolar concentrations in oocytes from Xenopus laevis (Cruz et al., 1998). Later on, Beckstead et al. (2000) reported that several inhalants, including toluene, potentiate GABAA and glycine receptor-activated currents, and enhance 5-HT3 receptor function (Lopreato et al., 2003) at similar concentrations than those found to be effective for the inhibition of NMDA receptors. Recently, Bale et al. (2002) demonstrated that toluene also inhibits nicotinic acetylcholine receptors expressed in Xenopus oocytes. All these studies were performed using concentrations of solvents that do not compromise cell membrane integrity and allow complete recovery of receptor function after washout. Although there are clear advances in this rapidly evolving field, many questions are yet to be answered. For example, available evidence in the literature indicates that exposure to high concentrations of solvents in general, and to toluene in particular, can lead to the occurrence of cardiac arrhythmias and sudden sniffing death, but the mechanisms by which these effects are produced are still unclear (Taylor & Harris, 1970; Shepherd, 1989; Wilcosky & Simonsen, 1991; Einav et al., 1997).
Voltage-gated sodium channels are responsible for membrane depolarisation and action potential conduction in the heart. Extensive evidence indicates that inhibition of these channels can result in cardiac arrhythmias (Balser, 2001) and anticonvulsant effects (Köhling, 2002). Based on this and on the previously reported anticonvulsant effects of toluene (Wood et al., 1984; Cruz et al., 2003) in the present work, we tested the hypothesis that toluene inhibits cardiac sodium channels. To this purpose, we carried out an initial characterisation of the effects of toluene in human cardiac sodium channels transfected into Xenopus oocytes. After this, toluene was tested in ventricular rat myocytes to confirm its inhibitory effects in naturally expressed cardiac sodium channels. Our results show that toluene is a potent inhibitor of voltage-gated cardiac sodium channels in both preparations.
Methods
Materials
Toluene (99.75%) was bought from Baker (Xalostoc, Mexico), alkamuls EL-620 (emulphor, ethoxylated castor oil) from Rhone-Poulenc (Princeton, NJ, U.S.A.), and trypsine from Boehringer Mannheim (Frankfurt, Germany). MS-222 (tricaine methanesulphonate), collagenase (type I), and other reagents were purchased from Sigma (St Louis, MO, U.S.A.). Dr Peter Backx and Dr Eduardo Marban kindly provided the α subunit Nav1.5 cDNA (pCDNA3, Invitrogen; Carlsbad, CA, U.S.A.) and β1 subunit cDNA (pGW1H, British Biotechno-logy, London, U.K.).
Preparation of oocytes and microinjections
Adult Xenopus laevis female frogs (Xenopus I, Ann Arbor, MI, U.S.A.) were anaesthetised by immersion in 0.2% MS-222. Stage V and VI oocytes were surgically removed and placed in OR-2 buffer containing (in mM): 82.5 NaCl, 2.5 KCl, 1 MgCl2, and 5 N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid] (HEPES), pH 7.6, and treated with collagenase (1.3 mg ml−1) to remove the follicular membrane. Oocytes were coinjected into the nucleus with a nanolitre automatic injector (model A203XVY; WPI, Sarasota, FL, U.S.A.) with 2–10 ng of Nav1.5 human α subunit and β1 subunit cDNA clones at a ratio of 1 : 5 (Li et al., 1999). Eggs were then maintained at 18°C in ND-96 solution (in mM): 96 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, and 1 CaCl2, pH 7.6, supplemented with 0.5 mM theophylline, 0.5 mM pyruvate, and 50 μg ml−1 gentamycin, for up to 3 days before recording.
Electrophysiological recording in oocytes
Oocytes were placed in a 1.6-ml recording chamber and continuously superfused with a barium-containing solution at a flow rate of approximately 1 ml min−1. Two-electrode voltage-clamp recordings were performed at room temperature (20–22°C), using an OC-725C amplifier (Warner, New Haven, CT, U.S.A.). Electrodes were pulled on a horizontal puller (P-97, Sutter Instruments, Novato, CA, U.S.A.). Agarose-cushion electrodes filled with 3 M KCl (Schreibmayer et al., 1994) were used to achieve a final resistance of 0.6–1.2 MΩ. Sodium current signals were digitised at a sampling rate of 10 kHz by an analog-to-digital converter (Digidata 1200, Axon Instruments, Foster City, CA, U.S.A.) and stored on a computer for analysis with pClamp software (Version 7.01, Axon Instruments, Foster City, CA, U.S.A.). Sodium currents (INa+) were elicited by step depolarisations from a holding potential of −110 mV at a 0.1 Hz, unless otherwise indicated. The amplitude of expressed INa+ was typically 1–10 μA. Only oocytes with peak INa+ lower than 7 μA were used in the present study to minimise voltage-clamp errors (Li et al., 1999; Orta et al., 2002). Current–voltage (I–V) relationships were determined from peak currents elicited by 30 ms, 10 mV steps from a holding potential of −120 up to +50 mV. The voltage dependence of steady-state inactivation of Na+ channels was determined by a two-pulse protocol. A first variable voltage-conditioning pulse that lasted 500 ms was applied to inactivate different fractions of sodium channels. After 2 ms, a second 20-ms-long test pulse was applied to −30 mV. Data were normalised to the maximal INa+ recorded at the test pulse. Recovery from inactivation was examined by applying a 30-ms conditioning pulse to −30 mV from a holding potential of −110 mV (to inactivate sodium channels), followed by a recovery interval of variable duration (Δt: 1–1000 ms) and a test pulse to −30 mV. Use-dependent block of Na+ channels was evaluated by a train of 20, 20-ms-long pulses to −30 mV at 1, 2 or 4 Hz from a holding potential of −120 mV. The current amplitudes of INa+ in each pulse in the train were normalised to peak INa+ from the first pulse in each train.
Isolation of myocytes
Isolated rat cardiac myocytes were prepared according to a standard enzymatic perfusion method (Bouchard et al., 1993; Clark et al., 1993). Briefly, adult Sprague–Dawley rats (200–250 g) were heparinised (1000 UI kg−1) and anaesthetised with sodium pentobarbital (35 mg kg−1, i.p.). Hearts were excised and perfused for 5 min through the aorta in a Langendorff system at 37°C, with normal Tyrode solution of the following composition (in mM): 140 NaCl, 4.5 KCl, 1.8 CaCl2, 1.0 MgCl2, 10.0 HEPES, 10 glucose, and 5.0 Na-pyruvate at pH 7.4. Thereafter, the hearts were perfused with a nominally zero-calcium Tyrode solution for further 5 min. The perfusate was then changed to a nominally zero-calcium solution containing collagenase (1 mg ml−1) and trypsine (0.1 mg ml−1) for approximately 20 min. The enzymes were washed out by perfusion with Tyrode solution containing 0.1 mM Ca2+ for 5 min. Small pieces of ventricular free wall (1 mm3) from both the left and right ventricles were dissected out and put into separate flasks containing the storage solution. Single cells were obtained by mechanical agitation with a pipette and stored in Tyrode solution with 0.1 mM Ca2+ at room temperature (22°C).
Electrophysiological recording of single myocytes
Isolated single cells were placed in a small-volume (0.2 ml) recording chamber on the stage of an inverted microscope (IM35, Zeiss, Germany). Macroscopic current recordings under voltage-clamp conditions were obtained using the whole-cell recording method (Hamill et al., 1981) and an Axopatch 1D amplifier (Axon Instruments, Foster City, CA, U.S.A.). Glass pipettes were pulled from borosilicate capillary tubing (TW 150, World Precision Instruments, Sarasota, FL, U.S.A.) using a horizontal puller (P-97, Sutter Instruments, Novato, CA, U.S.A.). Membrane potentials were corrected for the liquid junction potential (∼4.8 mV). Currents were filtered with an eight-pole Bessel filter at 2 kHz, digitised at 5 kHz, and stored on a computer hard disk. Data analysis was performed on leak-subtracted current traces using a P/4 leak subtraction protocol (Armstrong & Bezanilla, 1977). In a typical voltage-clamp experiment, the time required for the membrane potential to reach 95% of its final value following a step-in command potential is equivalent to the product of three times the membrane time constant (τm). Prior to the point at which the membrane capacitance is fully charged, membrane currents measured by a voltage-clamp amplifier will be due partly to charging of the membrane capacitance and partly to the flow of ions through channels. Indeed, in a previous work, we (Bouchard et al., 1993) and others (Brown et al., 1984; Isenberg & Klockner, 1983) have shown that serious voltage-clamp errors can occur, both at 18 and 37°C, when large and fast ionic currents, such as INa+, are not adequately controlled. Such errors can lead to erroneous measurements of the voltage dependence of INa+. It was therefore necessary to carefully control the membrane potential during voltage-clamp of INa+ during these experiments. In the present study, the seal resistance was typically between 2 and 5 GΩ, and the series resistance was compensated between 80 and 90% in each cell, in order to provide for optimal voltage clamp integrity. In a total of seven myocytes, the average (±s.e.m.) DC pipette resistance was 3.26±0.02 MΩ, the compensated series resistance was 1.85±0.01 MΩ, and cell capacitance was 82±12 pF, yielding an average τm of 148 μs.
Toluene and recording solutions
Toluene was mixed with alkamuls EL-620 (emulphor, ethoxylated castor oil) at a 1 : 1 ratio (v v−1) as previously described (Cruz et al., 1998). This mixture was then diluted as needed with extracellular recording solution. For oocytes, the composition of this solution was (mM): 96 NaCl, 2.5 KCl, 1 MgCl2, 5 HEPES, and 1 BaCl2; pH 7.6. Barium was used as the divalent cation to prevent activation of any endogenous calcium-dependent chloride currents (Lupu-Meiri et al., 1988). The final toluene concentrations tested in oocytes were 1, 10, 100, 300, and 2400 μM. This range of concentrations was selected because it has been proven to be effective in modulating various physiologically relevant channels without producing a nonspecific disruption of the oocyte membrane (Cruz et al., 1998,2000; Beckstead et al., 2000; Tillar et al., 2002; Bale et al., 2002; Lopreato et al., 2003). For myocytes, a low-Na+ recording Tyrode solution was used with the following composition (in mM): 10 NaCl, 120 CsCl, 1 CaCl2, 2 MgCl2, 3 CoCl2, 10 HEPES, and 10 glucose, pH 7.4. The pipette solution had the following composition (in mM): 118 CsCl, 22 aspartic acid, 6.4 MgCl2, 5 EGTA, 0.5 GTP, 4.2 ATP-Na+, 2.7 CaCl2, 5 HEPES; pH was adjusted to 7.2 using CsCl. A single concentration of toluene (300 μM) was tested in rat ventricular myocytes.
Statistics and data analysis
Results are expressed as the mean±s.e.m. Differences between mean data were analysed with a paired or unpaired Student's t-test as appropriate. The dose–response curve for toluene's effects was adjusted by the Win-nonlin program (Pharsight Co., version 2.1) to calculate its IC50 value and 95% confidence limits (CL) according to the model INa+=1/(1+([toluene]/IC50)n). Normalised inactivation–voltage curves were fitted to Boltzmann relationships of the form y=1/{1+exp[V−Vm1/2]/k}+A, where y is normalised INa+, A, the baseline, Vm, the membrane potential, Vm1/2, the voltage of half-maximal inactivation, and k a slope factor. A P-value <0.05 was used to denote the statistical difference between groups.
Results
Figure 1 (panel a) shows an experiment to exemplify the inhibitory effects of 300 μM toluene on INa+ (for a rationale to select this concentration, see below). Each point represents the normalised current recorded at each depolarising pulse using a 0.1 Hz stimulation frequency. The total time of this experiment was 13 min. After an initial recording of several control pulses, toluene was superfused for 5 min (bar). At this time, a steady-state inhibition of approximately 60% was observed. The calculated time constant of block (τon) was 2.8 min after adjustment with a monoexponential function. Based on these findings, the effects of toluene in the following experiments were measured considering steady-state inhibition. After completing the exposure time, oocytes were changed to normal barium-perfusion solution to determine the time course of recovery after washout. Under our experimental conditions, INa+ recovered to approximately 85% of its control value in approximately 7 min. Panel b of Figure 1 shows typical currents obtained in a same oocyte initially perfused with normal barium-containing solution (1), then with toluene-containing solution (2), and after washout with normal perfusion solution (3).
Figure 1.
Effects of toluene on INa+. (a) Time course of toluene effects. Each point represents the maximum current elicited by a 30-ms pulse to −30 mV, from a holding potential of −110 mV. The stimulation frequency was 0.1 Hz. The bar indicates the time of perfusion with the drug. Note the slow time course of installation of the new steady state after drug perfusion. (b) Original currents under three different conditions: control (1), with 300 μM toluene (2), and after washout (3).
Figure 2 shows the effects of increasing concentrations of toluene on peak INa+. The analysis of the INa+ elicited in six oocytes revealed that exposure to 300 μM toluene resulted in a 57±8.5% reduction of the peak currents with a slow establishment of the effect (∼5 min). The complete dose–response curve for INa+ block by toluene is shown. The best fit (r=0.998) for toluene block yielded an IC50 of 274 μM (95% CL: 141–407 μM), with a Hill coefficient=0.95.
Figure 2.
Concentration–response curve showing the blocking effects of toluene on INa+. Each point represents the mean±s.e.m. of six oocytes from at least two different frogs. INa+ was elicited by 30 ms-pulses to −30 mV, from a holding potential of −110 mV. The stimulation frequency was 0.1 Hz. Lines through the points correspond to the best fit using the Hill equation (see Methods).
To assess the voltage dependence of the inhibitory actions of toluene on sodium channels, current–voltage (I–V) relationships were studied in the absence and in the presence of 300 μM toluene (Figure 3, panel a). Under control experimental conditions, INa+ activated at about −60 mV, reached a maximum value at −30 mV, and attained its reversal potential at approximately +30 mV. Toluene blocked INa+ proportionally throughout the entire I–V relationship, and had no effect on the reversal potential for Na+. Under the experimental conditions used, the recovery was not complete after a 10-min washout period. Panel b shows sample tracings of selected original currents to illustrate the nature of toluene's block of inward sodium currents.
Figure 3.
(a) Mean (±s.e.m.) I–V relationships of six oocytes from different frogs before toluene, perfused with toluene (300 μM), and after washout. (b) A family of whole-cell Na+ currents from a typical oocyte superfused with control solution (left) or 300 μM toluene (right). Data shown are for test potentials between −80 and +20 mV. The stimulation frequency was 0.1 Hz.
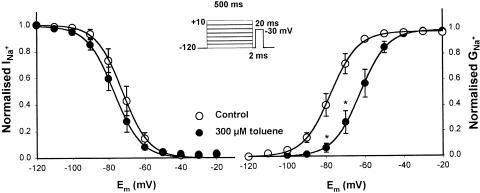
The effects of toluene on the voltage dependence of steady-state Na+ channel inactivation and activation, using a conventional double-pulse protocol, are shown in Figure 4. Toluene produced a slight leftward shift of the steady-state-inactivation curve of Nav1.5 channels, which, however, was not statistically different from the control curve (Vm1/2=−78±1.1 mV, k=−7.3; in toluene-treated oocytes versus Vm1/2=−72.3±1.2 mV, k=−7.2 in control eggs; n.s., Student's t-test). However, the activation curve after perfusion with toluene was shifted to the right by 16 mV (Vm1/2 varied from −77±0.8 to −62.5±0.2 mV), without changes in the slope factor (7.3 and 7.0, respectively), for control- and drug-perfused oocytes; these changes probably indicate that toluene alters the gating function of the channels.
Figure 4.
Effects of toluene on the voltage dependence of steady-state inactivation and activation of Na+ cardiac channels. Left: normalised data under control conditions and following toluene exposure are plotted against membrane potential. Right: normalised INa+–voltage relationship expressed as a function of maximal Na+ conductance (gNa+-max) under control conditions and in the presence of 300 μM of toluene. Each point represents the mean±s.e.m. of six oocytes from different frogs. Lines through the points correspond to the best fit using the Boltzmann equation.
Figure 5 illustrates the time course of recovery from INa+ inactivation in control conditions and after 300 μM toluene. The parameters obtained after adjusting data with a double-exponential function were as follows: τfast=45.7±2.78 ms, τslow=454±110 ms in toluene-treated oocytes versus a τ value of 43.3±1.5 ms obtained after adjustment with a monoexponential function in control oocytes. Toluene produced a slow recovery from inactivation, suggesting that drug-bound channels required more time than unbound channels to recover after depolarisation.
Figure 5.
Effects of 300 μM toluene on the time course of recovery from inactivation of INa+. Recovery from inactivation was measured using a conventional double-pulse protocol (a first conditioning pulse followed by a test pulse), as shown in the inset. The recovery interval (Δt) varied from 1 to 1000 ms. Peak current levels during the test pulse were normalised to peak current levels during conditioning pulse, and plotted against the recovery interval. Each point represents the mean±s.e.m. of six oocytes. Lines through the points correspond to the best fit using exponential equations.
The effects of toluene on I–V curves on the voltage dependence of steady-state inactivation of sodium channels and the time course of recovery from inactivation are similar to those reported for several local anaesthetics such as lidocaine and benzocaine (Li et al., 1999). Since these drugs show use-dependent block in addition to Na+ current inhibition (An et al., 1996; Li et al., 1999; Orta et al., 2002), we considered of interest to determine if toluene could produce a similar effect.
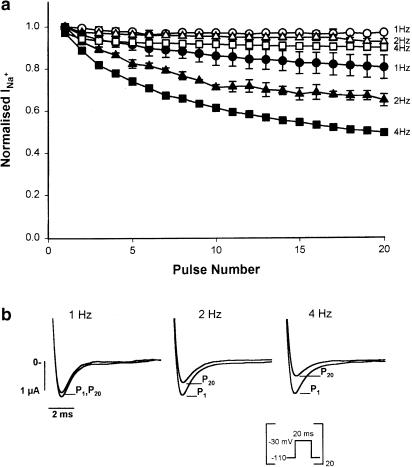
Use-dependent block of sodium channels was addressed using trains of depolarising pulses at 1, 2, and 4 Hz (Figure 6). Oocytes were perfused with normal barium solution and stimulated at each frequency. After a short resting period of 3 min stimulation was resumed, but this time in the continued presence of 300 μM toluene. Under control conditions, only a slight decrease in the amplitude of INa+ was observed towards the end of the 4-Hz stimulation train due to the cumulative inactivation given by high stimulation frequencies (Benitah et al., 1999). Toluene blocked Na+ channels in a use- and frequency-dependent fashion; that is, the higher the frequency of depolarising pulses, the greater the inhibition of sodium currents produced by toluene. It is important to keep in mind that the inhibition observed at the 20th pulse with a 1 Hz stimulation corresponds to that achieved after 20 s of toluene exposure, while that registered at the 20th pulse of oocytes stimulated at 4 Hz corresponds to the inhibition seen after 5 s of toluene exposure. Panel b of Figure 6 shows sample tracings of the 1st and the 20th pulse in toluene-treated oocytes under different conditions. In control oocytes, no difference was found between the first and the last pulse.
Figure 6.
Use-dependent block of INa+ by toluene. (a) A train of 20 20-ms long pulses to −30 mV from a holding potential of −110 mV was applied at three different frequencies (1, 2, and 4 Hz) under control conditions (empty symbols) and in the presence of 300 μM toluene (filled symbols). The means (±s.e.m.) of maximal current values during the 20 pulses at the three different frequencies are shown. (b) Superimposed Na+ original currents of a typical experiment. For reasons of clarity, only the first (P1) and the last (P20) currents are shown.
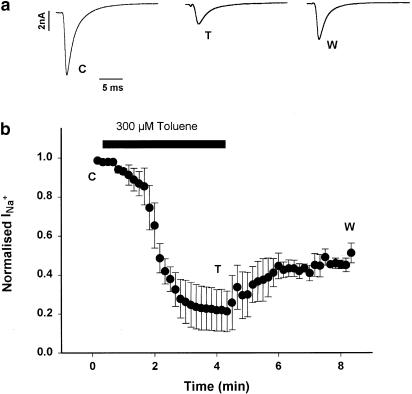
The results reported thus far suggest that toluene has an open-channel-blocking mechanism in human cardiac sodium channels expressed in Xenopus oocytes. To determine if this effect could be replicated in naturally expressed cardiac channels, the inhibitory effects of toluene on sodium channels were studied in rat ventricular myocytes. The results are shown in Figure 7. Toluene (300 μM) produced an inhibition of approximately 60% of the peak current that reached its maximum after 3 min of toluene exposure. This inhibition remained stable for the following minute in the presence of toluene, and no recovery was seen after 3-min washout. Original currents are shown to illustrate the inhibitory effects of toluene.
Figure 7.
Effects of toluene on rat cardiac myocytes. (a) Original records taken from right ventricle myocytes. Currents were elicited by 10 mV depolarising steps to −20 mV from a holding potential of −110 mV, lasting 30 ms. Traces under basal conditions (C), with 300 μM toluene (T), and after washout (W). (b) Time course of toluene effects. Normalised peak currents are shown under control conditions and after perfusion of the solvent. Note that the drug reaches a new steady state after 2 min of perfusion. Temperature was 18°C.
Discussion
In the present study, we used human cardiac channels (Nav1.5) transfected into oocytes from Xenopus laevis to characterise the electrophysiological effects of the abused inhalant toluene on INa+. This is the first biophysical report providing evidence that toluene may produce a deleterious effect on Na+ channel function.
The present results show that, in oocytes: (a) toluene blocks INa+ with an IC50 of 274 μM and a relatively slow time block constant (τon) of 2.8 min at 0.1 Hz; (b) the inhibitory effect of toluene on INa+ is ∼85% reversible after a 10-min washout; (c) toluene does not change steady-state inactivation curves; (d) toluene produces a shift to the right of the activation curve, probably by changing the gating of the channel; (e) toluene produces a slow recovery of inactivation, suggesting that drug-bound channels require more time than unbound channels to recover after depolarisation; and (f) toluene blocks INa+ in a frequency-dependent manner. In myocytes, toluene also inhibits the sodium current, but shows very little recovery after washout.
The fact that toluene inhibition strongly depends on the frequency of stimulation implies that there can be a different τon for each experimental condition. That this is indeed the case is evidenced by the finding that an inhibition of approximately 50% was seen after only 5 s at 4 Hz (Figure 6), but it took 4–5 min to achieve a similar degree of inhibition when the stimulation frequency was 0.1 Hz (Figure 1).
The results obtained in rat ventricular myocytes are included to provide evidence that the effects of toluene could be relevant to cardiac function in naturally expressed sodium channels. However, further experiments are needed to completely characterise toluene effects on this experimental preparation, and this is the matter of ongoing investigation.
Toluene inhalation for recreational purposes (‘huffing' or ‘bagging') can lead to sudden sniffing death. The main toxicological findings include bradycardia, tachyarrhythmias, A–V block, central nervous system depression, anoxia, and respiratory failure (Bass, 1970; Shepherd, 1989; Einav et al., 1997). In spite of this evidence, the basic mechanisms of toluene cardiac effects are poorly understood. In an early report, Bass (1970) proposed that the main effect of toluene could be a myocardium sensitisation to endogenous cathecholamines that could, in turn, produce asystolia. Our results show that relatively low concentrations of toluene (in the micromolar range) can block sodium channels, both transfected into oocytes and naturally expressed in rat ventricular myocytes. We propose that this effect can be responsible, at least in part, for toluene-induced arrhythmias.
In a recent paper, Tillar et al. (2002) showed that toluene inhibits the rise of calcium currents in pheochromocytoma cells that express L-type channels only when these cells were depolarised by 50 mM KCl (a condition under which Na+ channels are inactivated), but not in well-polarised cells. Other authors have found that toluene modifies GABA and catecholamine release in neurones, and that these effects were increased when cells were preincubated with tetrodotoxin (Westerink & Vijverberg, 2002), a very specific sodium channel blocker. These data suggest that the effects of toluene can be potentiated by block of Na+ channels, either by depolarisation or TTX administration, and support the role of these voltage-gated ion channels in toluene's actions.
The electrophysiological effects of toluene share some of the effects of local anaesthetics (LA) (Balser et al., 1996; Li et al., 1999; Nuss et al., 2000; Lee et al., 2001). A number of these compounds such as lidocaine and procaine act by blocking sodium channels in a use- and frequency-dependent fashion, in a similar way to what we saw with toluene. Since tertiary amine LA and toluene are lipophilic compounds, the possibility that toluene could reach an intracellular binding site by first penetrating the lipid phase of the membrane as tertiary amines do (Starmer et al., 1991; Narahashi, 2000; Lee et al., 2001), cannot be excluded. Further experiments are needed in order to clarify this point.
In a previous study of the effects of toluene on seizures elicited by NMDA in mice, we found that acute exposure to high concentrations of inhaled toluene (2000–6000 parts per million) resulted in anticonvulsant effects (Cruz et al., 2003). Although an inhibition of NMDA receptors seems to play a role in toluene's anticonvulsant effects, the block of Na+ channels by toluene observed in the present study might also contribute to the anticonvulsant effect of this solvent.
Toluene shares many pharmacological actions with other CNS depressants such as ethanol (Evans & Balster, 1991). According to several in vitro studies (Cruz et al., 1998; Beckstead et al., 2000; Bale et al., 2002), toluene is, in general, more potent than ethanol. Our results support this notion because, while in the present study, a concentration close to 300 μM toluene decreased peak Na+ currents by 50%, a reversible inhibition of approximately 12% was produced in the dorsal root ganglion tetrodotoxin-sensitive Na+ channels with 200 mM ethanol (Wu & Kendig, 1998). Furthermore, it is likely that toluene and ethanol differ in the way they interact with sodium channels because, as evidenced by the I–V curves obtained in this study, toluene did not change the reversal potential of Na+ channels in oocytes. In contrast, 10–100 mM ethanol applied to Na+ channels in rat sensory neurones significantly decreased the reversal potential (Krylov et al., 2000).
In conclusion, our results show that toluene blocks human cardiac sodium channels as a function of concentration and in a use- and frequency-dependent manner. These effects may lead to serious side effects of toluene abuse, such as the occurrence of cardiac arrythmias and sudden sniffing death.
Acknowledgments
This work was partially taken from Marcia Y. Gauthereau's doctoral dissertation and was supported by grants II-77G01 (E.S.) and 30571M (S.L.C.), and scholarships 153252 (M.Y.G.) and 122184 (G.O.) from Conacyt.
Abbreviations
- CL
confidence limits
- gNa+
sodium conductance
- gNa+max
maximum sodium conductance
- INa+
whole-cell sodium current
- IC50
inhibitory concentration at 50%
- k
slope factor
- Nav1.5
human cardiac sodium channels
- τf
fast recovery time constant
- τon
time constant for block
- τs
slow recovery time constant
- Vm
membrane potential
- Vm1/2
half-activation or inactivation potential
- Vt
test potential
References
- AN R.H., BANGALORE R., ROSERO S.Z., KASS R.S. Lidocaine block of LQT-3 mutant human Na+ channels. Circ. Res. 1996;79:103–108. doi: 10.1161/01.res.79.1.103. [DOI] [PubMed] [Google Scholar]
- ANDERSON H.R. Increase in deaths from deliberate inhalation of fuel gases and pressurised aerosols. Br. Med. J. 1990;301:41. doi: 10.1136/bmj.301.6742.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON H.R., BLOOR K., MACNAIR R.S., RAMSEY J. Recent trends in mortality associated with abuse of volatile substances in the UK. Br. Med. J. 1985;293:1472–1473. doi: 10.1136/bmj.293.6560.1472-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARLIEN-SOBORG P. Solvent Neurotoxicity. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- ARMSTRONG C.M., BEZANILLA F. Inactivation of the sodium channel. Gating current experiments. J. Gen. Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALE A.S., SMOTHERS C.T., WOODWARD J.J. Inhibition of neuronal nicotinic acetylcholine receptors by the abused solvent, toluene. Br. J. Pharmacol. 2002;137:375–383. doi: 10.1038/sj.bjp.0704874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALSER J.R. The cardiac sodium channel: gating function and molecular pharmacology. J. Mol. Cell. Cardiol. 2001;33:599–613. doi: 10.1006/jmcc.2000.1346. [DOI] [PubMed] [Google Scholar]
- BALSER J.R., NUSS H.B., ORIAS D.W., JOHNS D.C., MARBAN E., TOMASELLI G.F. Local anesthetics as effectors of allosteric gating. Lidocaine effects on inactivation-deficient rat skeletal muscle Na+ channel. J. Clin. Invest. 1996;98:2874–2886. doi: 10.1172/JCI119116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASS M. Sudden sniffing death. J. Am. Med. Assoc. 1970;212:2075–2079. [PubMed] [Google Scholar]
- BECKSTEAD M.J., WEINER J.L., EGER E.I., II, GONG D.H., MIHIC J.S. Glycine and gamma-aminobutyric acidA receptor function is enhanced by inhaled drugs of abuse. Mol. Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- BENITAH J.P., CHEN Z., BALSER G.F., TOMASELLI G.F., MARBAN E. Molecular dynamics of the sodium channel pore vary with gating: interactions between the P-segment motions and inactivation. J. Neurosci. 1999;19:1577–1585. doi: 10.1523/JNEUROSCI.19-05-01577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHARD R.A., CLARK R.B., GILES W. Role of sodium–calcium exchange in activation of contraction in rat ventricle. J. Physiol. 1993;472:391–413. doi: 10.1113/jphysiol.1993.sp019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWEN S.E., DANIEL J., BALSTER R.L. Deaths associated with inhalant abuse in Virginia from 1987 to 1996. Drug Alcohol Depend. 1999;53:239–245. doi: 10.1016/s0376-8716(98)00139-2. [DOI] [PubMed] [Google Scholar]
- BROWN H.F., KIMURA J., NOBLE F.S.R.D., NOBLE S.J., TAUPIGNON A. The ionic currents underlying pacemaker activity in rabbit sino-atrial node: experimental results and computer simulations. Proc. R. Soc. Lond. 1984;B222:329–347. doi: 10.1098/rspb.1984.0067. [DOI] [PubMed] [Google Scholar]
- CLARK R.B., BOUCHARD R.A., SALINAS-STEFANON E.M., SANCHEZCHAPULA J.A., GILES W.R. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc. Res. 1993;27:1795–1799. doi: 10.1093/cvr/27.10.1795. [DOI] [PubMed] [Google Scholar]
- CRUZ S.L., BALSTER R.L., WOODWARD J.J. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 2000;131:1303–1308. doi: 10.1038/sj.bjp.0703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUZ S.L., GAUTHEREAU M.Y., CAMACHO-MUNOZ C., LOPEZRUBALCAVA C., BALSTER R.L. Effects of inhaled toluene and 1,1,1-trichloroethane on seizures and death produced by N-methyl-D-aspartic acid in mice. Behav. Brain Res. 2003;140:195–202. doi: 10.1016/s0166-4328(02)00323-6. [DOI] [PubMed] [Google Scholar]
- CRUZ S.L., MIRSHAHI T., THOMAS B., BALSTER R.L., WOODWARD J.J. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1998;286:334–340. [PubMed] [Google Scholar]
- EINAV S., AMITAI Y., REICHMAN J., GEBER D. Bradycardia in toluene poisoning. Clin. Toxicol. 1997;35:295–298. doi: 10.3109/15563659709001214. [DOI] [PubMed] [Google Scholar]
- EVANS E.B., BALSTER R.L. CNS depressant effects of volatile organic solvents. Neurosci. Biobehav. Rev. 1991;15:233–241. doi: 10.1016/s0149-7634(05)80003-x. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., KLOCKNER A. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1983;395:30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- KÖHLING R. Voltage-gated sodium channels in epilepsy. Epilepsia. 2002;43:1278–1295. doi: 10.1046/j.1528-1157.2002.40501.x. [DOI] [PubMed] [Google Scholar]
- KOZEL N., SLOBODA Z., DE LA ROSA M. Epidemiology of inhalant abuse: an international perspective. Rockville, MD, US Department of Health and Human Services; NIDA Res. Monograph Series. 1995;148 [Google Scholar]
- KRYLOV B.V., VILIN Y.Y., KATINA I.E., PODZOROVA S.A. Ethanol modulates the ionic permeability of sodium channels in rat sensory neurons. Neurosci. Behav. Physiol. 2000;30:331–337. doi: 10.1007/BF02471787. [DOI] [PubMed] [Google Scholar]
- KURTZMAN T.L., OTSUKA K.N., WAHL R.A. Inhalant abuse by adolescents. J. Adolesc. Health. 2001;28:170–180. doi: 10.1016/s1054-139x(00)00159-2. [DOI] [PubMed] [Google Scholar]
- LEE P.J., SUNAMI A., FOZZARD H.A. Cardiac-specific external paths for lidocaine, defined by isoform-specific residues, accelerate recovery from use-dependent block. Circ. Res. 2001;89:1014–1021. doi: 10.1161/hh2301.100002. [DOI] [PubMed] [Google Scholar]
- LI A.R., TSUSHIMA R.G., HIMMELDIRK K., DIME D.S., BACKX P.H. Local anesthetic anchoring to cardiac sodium channels. implications into tissue-selective drug targeting. Circ. Res. 1999;85:88–98. doi: 10.1161/01.res.85.1.88. [DOI] [PubMed] [Google Scholar]
- LOPREATO G.F., PHELAN R., BORGHESE C.M., BECKSTEAD M.J., MIHIC S.J. Inhaled drugs of abuse enhance serotonin-3 receptor function. Drug Alcohol Depend. 2003;79:11–15. doi: 10.1016/s0376-8716(02)00330-7. [DOI] [PubMed] [Google Scholar]
- LUPU-MEIRI M., SHAPIRA H., ORON Y. Hemispheric asymmetry of rapid chloride responses to inositol triphosphate and calcium in Xenopus oocytes. FEBS Lett. 1988;240:83–87. doi: 10.1016/0014-5793(88)80344-2. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T. Neuroreceptors and ion channels as the basis for drug action: past, present and future. J. Pharmacol. Exp. Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- NUSS H.B., KAMBOURIS N.G., MARBAN E., TOMASELLI G.F., BALSER J.R. Isoform-specific lidocaine block of sodium channels explained by differences in gating. Biophys. J. 2000;78:200–210. doi: 10.1016/S0006-3495(00)76585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORTA S.G., BOUCHARD R.A., MORALES S.F., SALINAS-STEFANON E.M. Inhibition of cardiac Na+ current by primaquine. Br. J. Pharmacol. 2002;135:226–238. doi: 10.1038/sj.bjp.0704460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHREIBMAYER W., LESTER H.A., DASCAL N. Voltage clamping of Xenopus laevis oocytes utilizing agarose-cushion electrodes. Pflugers Arch. 1994;426:453–458. doi: 10.1007/BF00388310. [DOI] [PubMed] [Google Scholar]
- SHEPHERD R.T. Mechanism of sudden death associated with volatile substance abuse. Human Toxicol. 1989;8:287–292. doi: 10.1177/096032718900800406. [DOI] [PubMed] [Google Scholar]
- STARMER C.F., NESTERENKO V.V., UNDROVINAS A.I., GRANT A.O., ROSENSHTRAUKH L.V. Lidocaine blockade of continuously and transiently accessible sites in cardiac sodium channels. J. Mol. Cell. Cardiol. 1991;23 S1:73–83. doi: 10.1016/0022-2828(91)90026-i. [DOI] [PubMed] [Google Scholar]
- TAYLOR G.J., HARRIS W.S. Glue sniffing causes heart block in mice. Science. 1970;170:866–868. doi: 10.1126/science.170.3960.866. [DOI] [PubMed] [Google Scholar]
- TILLAR R., SHAFER T.J., WOODWARD J.J. Toluene inhibits voltage-sensitive calcium channels expressed in pheochromo-cytoma cells. Neurochem. Int. 2002;41:391–397. doi: 10.1016/s0197-0186(02)00048-7. [DOI] [PubMed] [Google Scholar]
- WESTERINK R.H., VIJVERBERG H.P. Toluene-induced, Ca(2+)-dependent vesicular catecholamine release in rat PC12 cells. Neurosci. Lett. 2002;326:81–84. doi: 10.1016/s0304-3940(02)00315-4. [DOI] [PubMed] [Google Scholar]
- WILCOSKY T.C., SIMONSEN N.R. Solvent exposure and cardiovascular disease. Am. J. Ind. Med. 1991;19:569–586. doi: 10.1002/ajim.4700190503. [DOI] [PubMed] [Google Scholar]
- WOOD R.W., COLEMAN J.B., SCHULER R., COX C. Anticonvulsant and antipunishment effects of toluene. J. Pharmacol. Exp. Ther. 1984;230:407–412. [PubMed] [Google Scholar]
- WU J.V., KENDIG J.J. Differential sensitivities of TTX-resistant and TTX-sensitive sodium channels to anesthetic concentrations of ethanol in rat sensory neurons. J. Neurosci. Res. 1998;54:433–443. doi: 10.1002/(SICI)1097-4547(19981115)54:4<433::AID-JNR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]