Abstract
Diabetic vessels undergo structural changes that are linked to a high incidence of cardiovascular diseases. Reactive oxygen species (ROS) mediate cell signalling in the vasculature, where they can promote cell growth and activate redox-regulated transcription factors, like activator protein-1 (AP-1) or nuclear factor-κB (NF-κB), which are involved in remodelling and inflammation processes. Amadori adducts, formed through nonenzymatic glycosylation, can contribute to ROS formation in diabetes.
In this study, we analysed whether Amadori-modified human oxyhaemoglobin, glycosylated at either normal (N-Hb) or elevated (E-Hb) levels, can induce cell growth and activate AP-1 and NF-κB in cultured human aortic smooth muscle cells (HASMC).
E-Hb (1 nM–1 μM), but not N-Hb, promoted a concentration-dependent increase in cell size from nanomolar concentrations, although it failed to stimulate HASMC proliferation. At 10 nM, E-Hb stimulated both AP-1 and NF-κB activity, as assessed by transient transfection, electromobility shift assays or immunofluorescence staining. The effects of E-Hb resembled those of the proinflammatory cytokine tumour necrosis factor-α (TNF-α). E-Hb enhanced intracellular superoxide anions content and its effects on HASMC were abolished by different ROS scavengers.
In conclusion, E-Hb stimulates growth and activates AP-1 and NF-κB in human vascular smooth muscle by redox-sensitive pathways, thus suggesting a possible direct role for Amadori adducts in diabetic vasculopathy.
Keywords: Diabetic vasculopathy, Amadori adducts, reactive oxygen species, human vascular smooth muscle
Introduction
It is well known that diabetes mellitus leads to a high incidence of vascular diseases, like atherosclerosis, myocardial infarction or hypertension (Stamler et al., 1993; Stratton et al., 2000). Diabetic vessels initially undergo functional alterations, which are later on followed by structural changes, including remodelling processes, as observed in both experimental models (Rumble et al., 1997) or human patients (Giannattasio et al., 2001). Among the causes leading to such vascular complications, a growing body of evidence has pointed at oxidative stress as a pivotal factor in developing diabetic vascular disease (Tesfamariam, 1994; Giugliano et al., 1996; Di Mario & Pugliese, 2001; Spitaker & Graier, 2002). Indeed, diabetic vessels exhibit a pro-oxidant status, due to either reduced antioxidant defenses and/or increased production of different reactive oxygen species (ROS), including superoxide anions, hydrogen peroxide or hydroxyl radicals (Giugliano et al., 1996; Di Mario & Pugliese, 2001; Spitaker & Graier, 2002), from early stages of the disease. Indeed, oxidative stress is increased in diabetic vessels, not only when complications are already set up, but also in early stages of diabetes prior to the onset of vascular alterations (Dominguez et al., 1998). Recently, using a monkey model of diabetes, a hydroxyl-like radical species has been shown to damage wall proteins in early diabetic vascular disease (Pennathur et al., 2001).
In the last years, ROS have emerged as key mediators in cell signalling (Irani, 2000). In the vasculature, ROS can regulate the activation of different pathways involved in signal transduction, and they are involved in different processes, like cell growth or apoptosis, that contribute to designing the vessel structure (Irani, 2000; Spitaker & Graier, 2002; Wolin et al., 2002). At the nuclear level, ROS can modulate the expression of a certain number of redox-regulated genes in vascular cells, among which activator protein-1 (AP-1) and nuclear factor-κB (NF-κB) are the best characterized (Kunsch & Medford, 1999; Spitaker & Graier, 2002).
AP-1, which is formed by homodimers and heterodimers of members of the c-jun and the c-fos families, is involved in vascular remodelling and it has been directly implicated in the control of vascular smooth muscle cell growth, either proliferative or hypertrophic, in response to a wide variety of stimuli (Kunsch & Medford, 1999).
NF-κB is a transcriptional factor complex, which is composed of homodimeric or heterodimeric complexes of the Rel family (Kunsch & Medford, 1999). Like AP-1, NF-κB has also been involved in controlling cell growth and apoptosis in the vascular wall, and it closely regulates the expression of many genes involved in immune and inflammatory responses (Barnes & Karin, 1997). In addition, it frequently acts by crosscoupling with other transcription factors, such as AP-1 (Stein et al., 1993). NF-κB appears to be overexpressed in several human diseases, particularly those characterized by a chronic inflammatory state, including diabetes mellitus (Baldwin, 2001).
During diabetes, hyperglycaemia can lead to the formation of ROS-releasing compounds through processes of nonenzymatic glycosylation. Thus, glucose and reactive protein amino groups can undergo a condensation reaction, yielding Schiff bases that rearrange in Amadori adducts within days or weeks, which can, in turn, undergo irreversible changes to form the so-called advanced glycosylation end products (AGEs) after longer periods of time (Cerami et al., 1988). To date, different studies have focused on the possible role of AGEs in diabetic vascular remodelling (Vlassara, 1997; Di Mario & Pugliese, 2001), but only few reports have analysed the possible participation of early products of protein glycosylation, like Amadori adducts. However, several research groups, including ours, support the hypothesis that these compounds may have a relevant role in producing either functional or structural alterations associated with diabetic vasculopathy (Rodríguez-Mañas et al., 1993; Cohen & Ziyadeh, 1994; Angulo et al., 1996; Peiró et al., 1998; Schalkwijk et al., 1999a).
Therefore, in the present study, we aimed to investigate whether a typical Amadori adduct, like nonenzymatically glycosylated oxyhaemoglobin, used at both a concentration and a percentage of glycosylation that may be found in pathophysiological situations, can activate growth- and inflammation-related mechanisms in cultured aortic human smooth muscle cells (HASMC). In addition, the possible participation of ROS in HASMC activation by Amadori-oxyhaemoglobin was also analysed.
Methods
Materials
Culture plasticware was obtained from Corning-Costar (New York, NY, U.S.A.). Dulbecco's modified Eagle's medium (DMEM), fetal calf serum (FCS) and trypsin – EDTA were from Biological Industries (Beit-Hamek, Israel). Antibodies against c-jun/AP-1 and NF-κB/p-65 were purchased from Transduction Laboratories (Lexington, KY, U.S.A.) and Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.), respectively. Secondary antibodies were from Chemicon International (Temecula, CA, U.S.A.). Human tumour necrosis factor-α (TNF-α) was from Peprotech (London, U.K.). Unless otherwise stated, all other reagents were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.).
Preparation of haemoglobins
Lyophilized human haemoglobins, glycosylated nonenzymatically at either elevated or normal levels, containing 11.1% (catalogue no. G-1012) and 5.4% (catalogue no. G-2012) HbA1, respectively, were purchased from Sigma Chemical Co. Before use, haemoglobins were prepared as previously described (Angulo et al., 1996). Briefly, haemoglobins were dissolved in deionized water and subsequently reduced by incubation with an excess of sodium dithionite. The haemoglobin solutions were then extensively dialysed using a 0.25 Å pore diameter (approximately 12 kDa mol. wt.) dialysis membrane (Visking®, Serva, Heidelberg, Germany) against deionized water containing 10 mg l−1 EDTA and continuously bubbled with N2. Oxyhaemoglobins were then aliquoted and stored at −70°C until used. The absence of AGEs in the oxyhaemoglobin solutions was assessed by measuring fluorescence in a Fluostar fluorometer (BMG Labtechnologies, Offenburg, Germany) at an excitation maximum of 370 nm and emission maximum of 440 nm, which allows to quantify total AGEs (Sell & Monnier, 1989). A standard curve (r=0.99) was carried out using AGE-modified BSA (0.5–5 μg ml−1), prepared following a previously described method (Bucala et al., 1991).
Cell culture
HASMC were obtained by enzymatic dissociation from the aortas of five organ donors, according to Spanish legal dispositions, as previously described (Peiró et al., 2001). HASMC were routinely cultured in DMEM containing 1 g l−1 D-glucose and supplemented with 10% FCS, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin and 2.5 μg ml−1 Amphotericin B. At confluence, HASMC were passaged using a 0.02% EDTA – 0.05% trypsin solution and split in a 1 : 2 ratio. In the present study, cultures between passages 2 and 10 were used. Cell characterization was performed based on both cell morphology and indirect immunofluorescence staining of α-smooth muscle actin, as described previously (Peiró et al., 2001). In the present work, HASMC obtained from the different donors were pooled together and then used for experimental work.
Western blotting
After washing with phosphate-buffered saline (PBS), HASMC were extracted in lysis buffer containing 10 mM Tris pH 7.4, 1% sodium dodecyl sulphfate (SDS), 10 mM sodium orthovanadate, 2 mM phenylmethylsulphonyl fluoride (PMSF) and 12.5 μg ml−1 aprotinin. Total protein extracts were diluted 3 : 1 in 4 × Laemmli's buffer and boiled for 5 min at 100°C. Proteins (10 μg/lane) were equally loaded and separated on 12% SDS – polyacrylamide gel electrophoresis (SDS – PAGE) gels and transferred onto a nitrocellulose membrane (BioRad Laboratories, Madrid, Spain). After blocking overnight at 4°C in 0.2% Tween-20 and 5% nonfat dry milk, the membrane was incubated for 1 h at room temperature with a monoclonal antibody against either c-jun/AP-1 (dilution 1/1000) or NF-κB/p65 (1/2000), followed by incubation for 45 min with a horseradish peroxidase-conjugated secondary antibody (dilution 1/10,000). Immunoreactive bands were detected using an enhanced chemiluminiscence detection kit (Amersham, Arlington Hills, IL, U.S.A.) and quantified by densitometry using NIH Image software.
Indirect immunofluorescence
HASMC were fixed with 4% paraformaldehyde and blocked in a PBS solution containing 4% goat serum and 0.1% Triton X-100. Cells were then incubated at 4°C overnight with a monoclonal antibody against either c-jun (dilution 1/50) or the NF-κB p65 subunit (dilution 1/50), followed by incubation with an anti-mouse FITC-conjugated secondary antibody (dilution 1/50) at room temperature for 45 min. HASMC were observed with an Eclipse TE300 epifluorescence microscope (Nikon, Tokyo, Japan).
Transient transfection
For transient transfection experiments, HASMC were grown in six-well plates to 80% confluence. The culture medium was then replaced by vehicle medium, that is, serum-free medium supplemented with 0.1% BSA, for 18–20 h. The transfection mixture consisted of 3–4 μg of either a firefly luciferase-reporter plasmid containing four tandem repeats of the NF-κB binding site (Clontech, Paloalto, CA, U.S.A.) or a reporter plasmid containing a human collagenase promoter containing one binding site for AP-1 at −73 bp fused to the firefly luciferase gene (Deng & Karin, 1993), together with 100 μl of Lipovec™ (InvivoGen; San Diego, CA, U.S.A.) in vehicle medium. After incubation at room temperature for 20 min, the transfection mixture was added to cell cultures for further 18 – 20 h. Afterwards, the transfection mixture was replaced by fresh vehicle medium containing the different compounds to be tested. At the indicated time points, HASMC were extracted with 1 × Reporter Lysis Buffer (Promega, Madison, WI, U.S.A.) for analysis of reporter gene expression. Luciferase activity was expressed as relative luciferase units/μg of protein.
Preparation of nuclear extracts and electrophoretic mobility shift assay
Nuclear extracts were prepared as previously described by others (Schreiber et al., 1989). For electrophoretic mobility shift assay (EMSA), nuclear extracts (5 μg of protein) were incubated in the presence of 3 μg poly-dIdC (Schreiber et al., 1989) together with commercial double-stranded 32P-labelled oligonucleotides (Promega) encoding either the AP-1 consensus sequence (5′-d(CGCTTGATGAGTCAGCCGGAA)-3′) or the NF-κB consensus sequence (5′-AGTTGAGGGGACTTTCCCAGGC-3′). DNA – protein complexes were electrophoretically separated and subjected to autoradiography. Specificity of binding was ascertained by competition with an excess of unlabelled consensus oligonucleotides.
Intracellular superoxide anions detection
To determine the presence of intracellular superoxide anions HASMC seeded onto 96-well microplates were grown to confluence and then serum deprived for 24 h. HASMC were then subjected to the different treatments and 1 h later, the fluorescent probe dihydroethidine (Molecular Probes Europe, Rinjnsburgerweg, The Netherlands) was added to cell cultures at a concentration of 160 μM. Fluorescence was followed up to 60 min, in a Fluostar fluorometer (BMG Labtechnologies, Offenburg, Germany), at an excitation maximum of 370 nm and emission maximum of 440 nm. In some experiments, oxidized dihydroethidine incorporation into nuclear DNA was visualized using an epifluorescence microscope (Nikon, Tokyo, Japan).
Determination of cell number
HASMC were cultured to confluence onto 24-well plates and then serum deprived for 24 h. After exposure to the different compounds to be tested for two consecutive periods of 24 h in vehicle medium, cell number per well was determined, using a 1% crystal violet dye to stain cell nuclei, as previously described (Peiró et al., 2001). In another set of experiments, cell number was determined on sparsely seeded cultures stimulated with 0.5% FCS either alone or supplemented with the different test compounds for 4 days.
Planar cell surface area and protein synthesis
To determine protein synthesis, HASMC were serum deprived for 24 h and then incubated in fresh vehicle medium containing 14C-leucine (0.5 μCi ml−1; 50 – 60 mCi mmol−1, Amersham) and the different compounds to be tested for additional 24 h. The uptake of 14C-leucine into HASMC was quantified by liquid scintillation, as previously described (Peiró et al., 1997).
Planar cell surface area was quantified by computer-assisted morphometry, as previously described (Peiró et al., 1998). Briefly, HASMC were sparsely seeded onto six-well culture plates in DMEM containing 10% FCS. After cell attachment, culture medium was switched to vehicle medium, either alone or containing the different compounds to be tested, and cultured for 48 h with medium renewal after the first 24 h. HASMC were then fixed with 1% glutaraldehyde and planar cell surface area was quantified by computer-assisted morphometry. Randomly selected images of VSMC were transmitted to a computer (Apple Macintosh Power 7100, Cupertino, California, U.S.A.) by a video camera (Sony Corporation, Tokyo, Japan) connected to the microscope (Nikon, Tokyo, Japan) and thereafter submitted to analysis with appropriate software (NIH Image). Measurements were performed in a blinded manner. In every experiment, at least 70 cells were counted for each treatment.
Statistical analysis
Results are expressed as means±s.e.m. The statistical analysis was evaluated by ANOVA followed by Fisher's protected least-significance-difference test, with the level of significance chosen at P<0.05.
Results
Effect of oxyhaemoglobins on HASMC growth
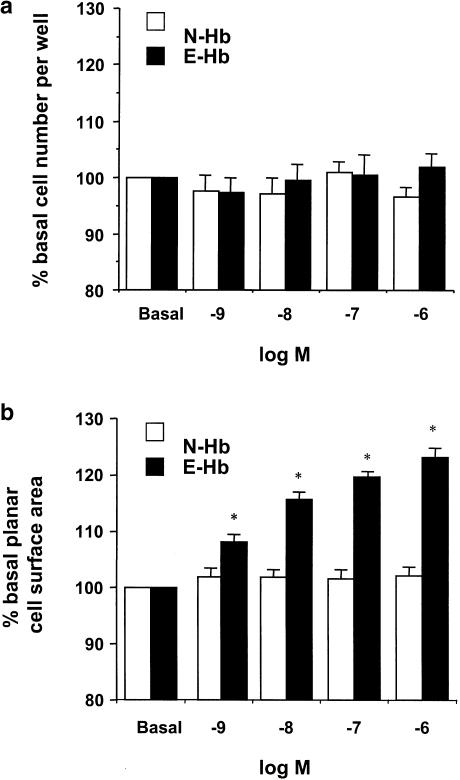
To assess whether oxyhaemoglobins could influence HASMC growth, confluent cultures were treated with human oxyhaemoglobin glycosylated at either normal (N-Hb) or elevated (E-Hb) levels (1 nM–1 μM) for two consecutive periods of 24 h. As shown in Figure 1a, neither N-Hb nor E-Hb significantly influenced cell proliferation at any of the concentrations used. Furthermore, neither N-Hb nor E-Hb modified final cell number of sparsely seeded cultures stimulated with 0.5% FCS for 4 days (2.07±0.35-, 2.14±0.33- and 2.16±0.26-fold increase over initial cell number for FCS alone, 10 nM N-Hb and 10 nM E-Hb, respectively).
Figure 1.
Effect of N-Hb and E-Hb on cultured HASMC growth. (a) Confluent HASMC were treated with 1 nM–1 μM of either N-Hb or E-Hb for two consecutive periods of 24 h and cell number per well was determined. (b) Planar cell surface area was measured in sparselly seeded HASMC cultures after exposure to the above-described conditions. Results are expressed as means±s.e.m. of four independent experiments. *P<0.05 vs N-Hb.
However, when planar cell surface area was quantified in subconfluent cultures (Figure 1b), a concentration-dependent increase in cell size was only observed in the presence of E-Hb. E-Hb significantly modified cell size from a threshold concentration of 1 nM, although the increase in planar cell surface area was quite modest (around 8–10%) at this concentration. For this reason, the 10 nM concentration, which yielded an increase in cell size around 15 – 20%, was chosen for further experiments.
In addition to increasing cell size, E-Hb promoted protein synthesis in HASMC. Indeed, when used at a concentration of 10 nM E-Hb, but not N-Hb, elicited an increase in protein synthesis by around 64% (P<0.05 vs basal untreated cultures). The values of 14C-leucine uptake were 16.995±1.696, 17.510±908, and 27.815±2.003 d.p.m. per well for basal untreated, N-Hb-treated and E-Hb-treated cultures, respectively.
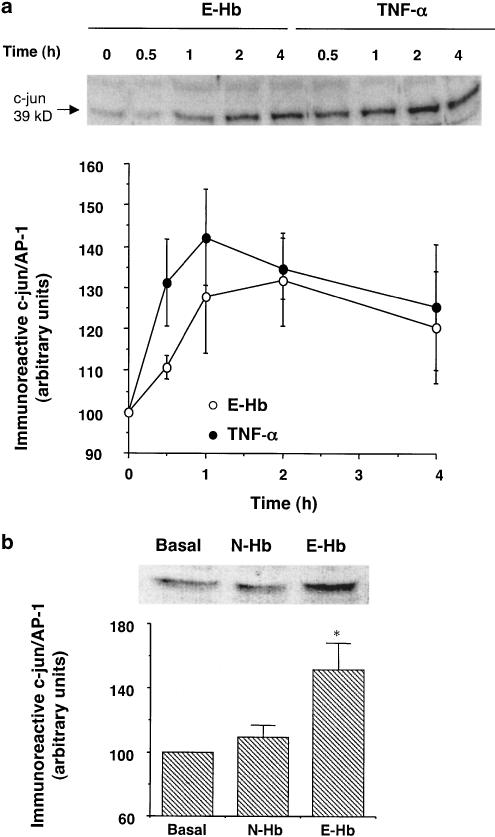
Effect of oxyhaemoglobins on AP-1 expression and activity
To assess whether Amadori-modified oxyhaemoglobin could promote AP-1 de novo synthesis, HASMC were incubated with 10 nM N-Hb or E-Hb. Figure 2a shows the time course of c-jun/AP-1 induction by 10 nM E-Hb. HASMC showed increased c-jun protein levels after 1 h of treatment (P<0.05 vs time 0). Maximal expression was reached after 2 h of exposure, followed by a slow decay of protein levels. Figure 2 also shows the effects of TNF-α in the same experimental conditions. TNF-α was used as a positive control, as this cytokine can stimulate cellular pathways leading to AP-1 and NF-κB activation in vascular smooth muscle (Madamanchi et al., 1998; Iseki et al., 2000). After 30 min of exposure to TNF-α (10 ng ml−1), total c-jun/AP-1 levels were already significantly enhanced in HASMC (P<0.05 vs time 0), with maximal protein expression observed 1 h after beginning of the treatment, again followed by a slow reduction in cellular c-jun/AP-1 content (Figure 2a). N-Hb did not share the effects of E-Hb on c-jun/AP-1 protein expression in HASMC. Indeed, after treating cell cultures with 10 nM N-Hb for 2 h (corresponding to the maximal stimulation time by E-Hb), no changes were observed in c-jun/AP-1 protein levels (Figure 2b).
Figure 2.
Effect of E-Hb on c-jun expression in HASMC. (a) After serum deprivation for 24 h, HASMC were treated with either 10 nM E-Hb or 10 ng ml−1 TNF-α and c-jun content was quantified by Western blotting at the indicated time periods. A representative immunoblot is shown on top. (b) Expression of c-jun in HASMC after a 2 h treatment with either N-Hb or E-Hb, both at a concentration of 10 nM. A representative immunoblot is also shown. Results are expressed as means±s.e.m. of three independent experiments. *P<0.05 vs basal.
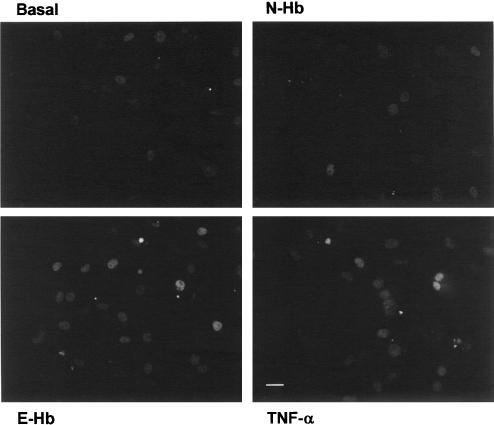
In accordance with these results, after treating HASMC with the different compounds for 2 h, followed by immunofluorescence staining with an appropriate monoclonal antibody, c-jun/AP-1 was visualized in the cell nuclei, although the fluorescence intensity was clearly higher in cultures treated with either 10 nM E-GHb or 10 ng ml−1 TNF-α (Figure 3).
Figure 3.
Indirect immunofluorescence staining of c-jun/AP-1 in serum-starved HASMC cultures treated for 2 h with basal vehicle medium, 10 nM of either N-Hb or E-Hb, or 10 ng ml−1 TNF-α. Scale bar=25 μm.
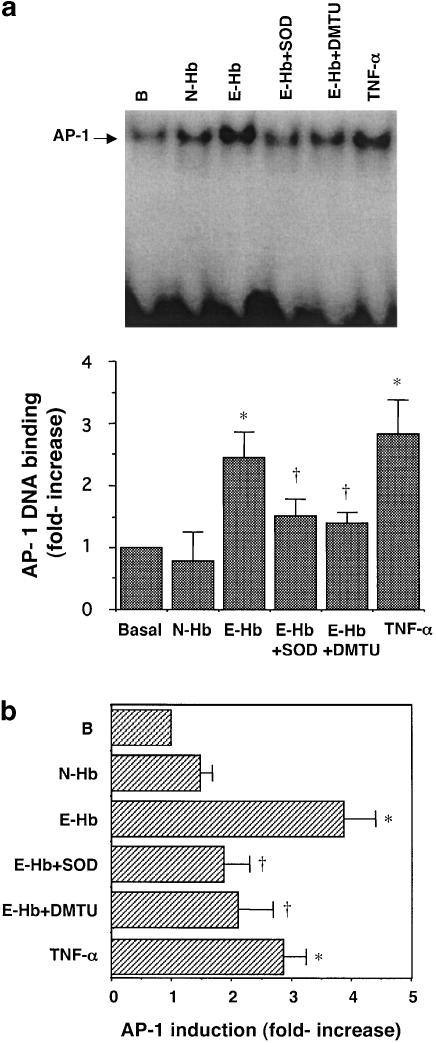
To assess whether overexpression of AP-1 induced by 10 nM E-Hb was accompanied by an increase in transcriptional activity, EMSA and transient transfection experiments were performed. Figure 4a shows that DNA-binding activity of AP-1 transcription factor was significantly increased by around 65% in HASMC treated with E-Hb compared to untreated cells, while no significant changes were observed in the presence of N-Hb. As expected, TNF-α also stimulated DNA-binding activity of AP-1 in this cell type (Figure 4a).
Figure 4.
Effect of E-Hb on AP-1 transcriptional activity. (a) DNA-binding activity of AP-1 was assessed after a 2 h treatment of HASMC with 10 nM of either N-Hb or E-Hb, or TNF-α (10 ng ml−1). A representative EMSA is shown at the top of the panel. (b) AP-1 activity was assessed in transiently transfected cells. The different compounds to be tested were added for 8 h, after which luciferase activity was measured and corrected for protein content. Results are expressed as means±s.e.m. of three independent experiments. SOD: 200 U ml−1; DMTU: 1 mM. *P<0.05 vs basal, †P<0.05 vs E-Hb.
As another approach to quantify AP-1 activity, HASMC were transiently transfected with a luciferase-reporter construct containing one binding site for AP-1 (Deng & Karin, 1993). Figure 4b shows that E-Hb enhanced AP-1 transcriptional activity in HASMC by around four-fold. Again, N-Hb did not substantially modify AP-1 activity compared to untreated cells. In accordance with EMSA experiments, TNF-α showed promoting effects on AP-1 transcriptional activity.
Effect of oxyhaemoglobins on NF-κB activity
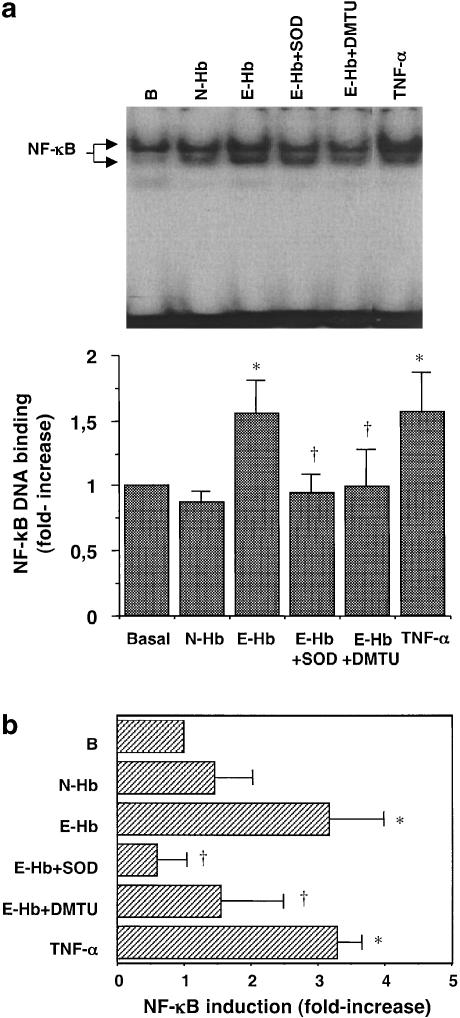
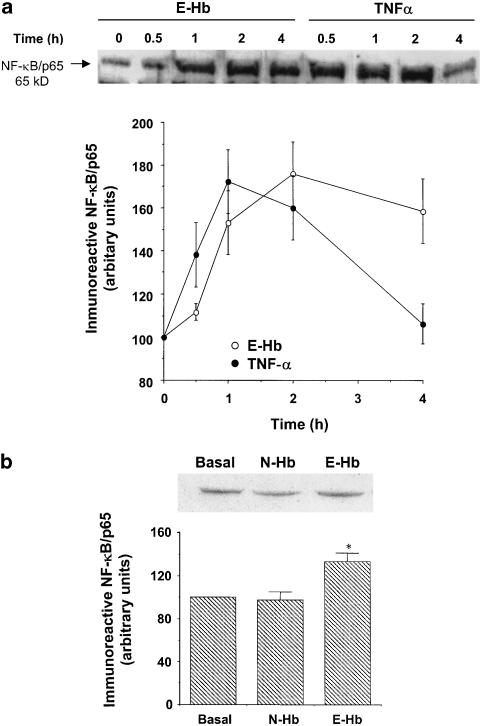
We observed that E-Hb also promoted NF-κB transcriptional activity. Indeed, 10 nM E-Hb enhanced DNA-binding activity of NF-κB by 60%, while this effect was not observed with N-Hb (Figure 5a). Similarly, using a luciferase-reporter construct containing four NF-κB binding sites, E-Hb, but not N-Hb, was shown to stimulate the transcriptional activity (Figure 5b). As expected, TNF-α significantly stimulated NF-κB activity, as assessed by both EMSA (Figure 5a) and transient transfection experiments (Figure 5b). Stimulation of HASMC also resulted in a time-dependent increase of NF-κB levels from 1 h (P<0.05 vs time 0) and 30 min (P<0.05 vs time 0) after stimulation with either E-Hb or TNF-α, respectively (Figure 6a). Again, N-Hb did not significantly modify NF-κB levels in HASMC (Figure 6b).
Figure 5.
Effect of E-Hb on NF-κB transcriptional activity. (a) After treating HASMC with 10 nM of N-Hb or E-Hb for 1 h, or 10 ng ml−1 TNF-α for 30 min, EMSA was performed. A representative assay is shown at the top of the panel. (b) Transient transfection experiments were performed to measure NF-κB transcriptional activity after a 2 h-exposure to the above-described treatments. Results are expressed as means±s.e.m. of three independent experiments. SOD: 200 U ml−1; DMTU: 1 mM. *P<0.05 vs basal, †P<0.05 vs E-Hb.
Figure 6.
Effect of E-Hb on NF-κB levels in HASMC. (a) After serum deprivation for 24 h, HASMC were treated with either 10 nM E-Hb or 10 ng ml−1 TNF-α, and NF-κB/p65 content was quantified by Western blotting at the indicated time periods. A representative immunoblot is shown on top. (b) Expression of NF-κB in HASMC after a 2 h treatment with either N-Hb or E-Hb, both at a concentration of 10 nM. A representative immunoblot is also shown. Results are expressed as means±s.e.m. of three independent experiments. *P<0.05 vs basal.
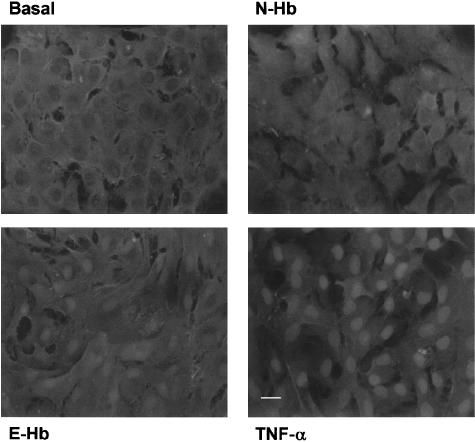
The transcriptional activity of NF-κB is accompanied by a translocation from the cell cytoplasm to the cell nucleus. Such translocation was visualized by indirect immunofluorescence staining using an anti-p65/NF-κB monoclonal antibody (Figure 7). HASMC nuclei appeared clearly stained after exposure to either E-Hb or TNF-α, whereas, in N-Hb-treated or untreated cultures, a positive stain was mainly seen in the cytoplasm.
Figure 7.
Indirect immunofluorescence staining of p65/NF-κB in HASMC cultures treated for 1 h with basal vehicle medium, 10 nM of either N-Hb or E-Hb, or 10 ng ml−1 TNF-α. Cytosolic-to-nuclear translocation was observed in HASMC exposed to either E-Hb or TNF-α. Scale bar=25 μm.
Participation of ROS on E-Hb-mediated effects
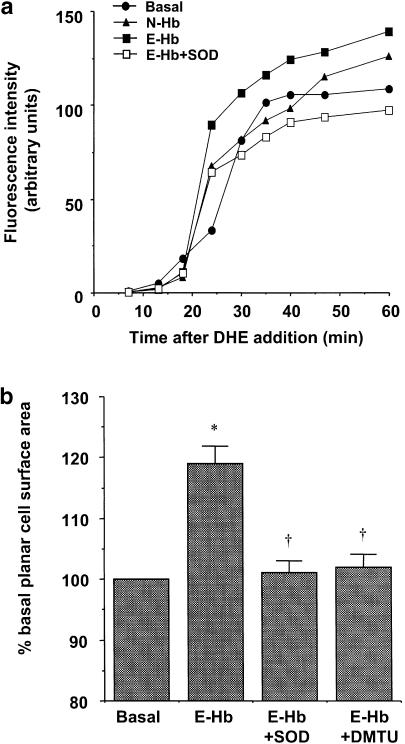
To elucidate whether ROS could be acting as mediators in the increased AP-1 and NF-κB transcriptional activity, we first measured intracellular superoxide production, by using the fluorescent probe dihydroethidium (DHE), after submitting HASMC to different treatments. Dihydroethidine-related fluorescence, measured over a 60 min period after the addition of the probe, was significantly higher in 10 nM E-Hb-treated cells, compared with basal untreated cultures (P<0.05, results from three independent experiments). The increase in fluorescence induced by E-Hb was totally prevented by 200 U ml of the superoxide anions scavenger superoxide dismutase (SOD; P<0.05 vs E-Hb alone, results from three independent experiments). Exposure of HASMC to 10 nM N-Hb did not produce any significant change in basal fluorescence. A representative experiment is shown in Figure 8a. SOD alone did not modify basal fluorescence levels (data not shown).
Figure 8.
(a) Representative experiment of the time-course detection of intracellular superoxide anions using the fluorescent probe DHE. Cultures were treated with either N-Hb or E-Hb (both at 10 nM) and 1 h later the fluorescent probe was added to HASMC. In some cases, E-Hb was added together with SOD (200 U ml−1). (b) Effect of the ROS scavengers SOD (200 U ml−1) and DMTU (1 mM) on the increase in planar cell surface area induced by 10 nM E-Hb. Results are expressed as means±s.e.m. of four independent experiments. *P<0.05 vs basal, †P<0.05 vs E-Hb.
Additionally, the EMSA and transient transfection experiments were performed in the presence of SOD and the intracellular hydroxyl radicals scavenger dimethylthiourea (DMTU). In the presence of either SOD (200 U ml−1) or DMTU (1 mM), the increases in AP-1 or NF-κB DNA-binding activity observed in EMSA experiments were abolished (Figures 4a and 5a, respectively). In the same manner, both scavengers prevented the induction of AP-1 and NF-κB transcriptional activity as assessed by transient transfection (Figures 4b and 5b, respectively). In the experimental conditions used, neither SOD nor DMTU did significantly modify AP-1 or NF-κB basal transcriptional levels (data not shown).
Furthermore, the increase in planar HASMC surface area elicited by 10 nM E-Hb alone was not observed when E-Hb was coincubated with either SOD or DMTU (Figure 8b). Neither SOD nor DMTU did modify basal planar cell surface area by themselves at the concentrations used (data not shown).
Discussion
Nonenzymatically glycosylated oxyhaemoglobin, and particularly HbA1c, has been used since the mid-1970s as a tool for diagnosis and glycaemic control of diabetic patients (Jovanovich & Peterson, 1981). HbA1c is formed by the nonenzymatic addition of a molecule of glucose to the N-terminal valine of one or both of the globin β chains, thus yielding a Schiff base that may undergo Amadori rearrangement to form a stable ketoamine adduct (Cohen, 1986). Indeed, the so-formed Amadori adducts are the prominent form of circulating glycosylated proteins in vivo (Cohen, 1986).
In addition to providing an excellent indicator of glycaemic control, HbA1c has also been proposed as a marker for the prognosis of long-term diabetic vascular complications (Stratton et al., 2000). Further beyond, in the last years our group has been interested in analysing whether HbA1c may represent not only a marker, but also a factor that directly participates in the development of diabetic vasculopathy. The fact that haemoglobin can be found free in plasma at nanomolar concentrations (Tietz, 1990) together with the finding that it can cross the endothelial barrier through a transcytosis mechanism, therefore reaching different cell types of the vascular wall (Faivre-Fiorina et al., 1999), has further raised our interest for a possible direct pathophysiological role of HbA1c in diabetes-associated vascular complications.
In this way, previous work from our laboratory provided evidence that nanomolar concentrations of human oxyhaemoglobin, glycosylated at a percentage of 9% or higher, impair endothelium-dependent relaxations, particularly those mediated by nitric oxide, in both rat aorta (Rodríguez-Mañas et al., 1993; Angulo et al., 1996) and human mesenteric microvessels (Vallejo et al., 2000). Other authors have also reported that, in diabetic patients, the presence of high levels of Amadori adducts, in particular glycosylated albumin, correlates with some markers of endothelial dysfunction and it is associated with the presence of diabetic nephropathy (Schalkwijk et al., 1999a).
In the present study, we aimed to analyse whether Amadori-modified oxyhaemoglobin could activate some cell mechanisms related to diabetic vasculopathy. We therefore studied the ability of glycosylated human oxyhaemoglobin to stimulate the expression and activity of the transcription factors AP-1 and NF-κB directly, as these factors are overexpressed in pathological conditions, like inflammation, hypertension or atherosclerosis (Baldwin, 2001), which are often associated with diabetes mellitus (Stamler et al., 1993; Stratton et al., 2000; Spitaker & Graier, 2002). Furthermore, NF-κB has even been proposed to be involved in diabetic vascular complications (Srivastava, 2002), as a sustained activation of NF-κB has been reported in vascular cells from diabetic rats (Bierhaus et al., 2001). AP-1 has also been shown to be overexpressed in the cardiovascular system of streptozotocin-induced diabetic rats (Nishio et al., 1998).
In the present work, we observed that exposure to nanomolar concentrations of oxyhaemoglobin resulted in increased AP-1 levels together with increased transcriptional activity of AP-1 in cultured human vascular smooth muscle. A similar activation was observed for NF-κB, as assessed by cytosolic-to-nuclear translocation together with enhanced DNA-binding and transcriptional activities. However, this stimulatory effect on AP-1 and NF-κB was observed only when oxyhaemoglobin presented elevated levels of glycosylation (∼11%), but not normal degrees of glycosylation that can be found in nondiabetic subjects (∼5%). As a positive control for transcription factor activation, TNF-α was used, as this proinflammatory cytokine has been shown to stimulate both AP-1 and NF-κB activation pathways in cultured vascular smooth muscle (Madamanchi et al., 1998; Iseki et al., 2000). Interestingly, circulating levels of TNF-α have been shown to be elevated in both type I and type II diabetic subjects (Lechleitner et al., 2000; Pickup et al., 2000), and a positive association has been found between plasma levels of TNF-α and cardiovascular risk factors in type I diabetes (Lechleitner et al., 2000). This elevation of circulating levels of proinflammatory cytokines in diabetes is in accordance with the proposal that diabetes mellitus is in some way a chronic inflammatory disease (Schalkwijk et al., 1999b). Indeed, NF-κB, which is activated in cultured HASMC by both TNF-α and human oxyhaemoglobin at elevated levels of glycosylation, is a pleiotropic transcription factor that can stimulate the expression of genes encoding from different chemokines and proinflammatory cytokines, together with some inflammatory enzymes, adhesion molecules and receptors, all of them involved in vascular inflammation and early steps of atherogenesis (Barnes & Karin, 1997).
On the other hand, AP-1 activation in vascular smooth muscle is associated with cell growth processes, which may take part in vascular remodelling (Kunsch & Medford, 1999). Indeed, alterations of the vessel wall structure are often seen in long-term diabetic vessels either from experimental models (Rumble et al., 1997) or human patients (Giannattasio et al., 2001). In this way, we wanted to analyse whether human E-Hbs, which had the ability to activate AP-1 in cultured human vascular smooth muscle, could also promote growth in this cell type. To analyse cell proliferation, we first used growth-arrested confluent cultures in order to further mimic in vivo conditions, as the proliferation rate of VSMC in the vessel wall is extremely low and no exponential proliferation rate is expected to be found. Under these conditions, both N-Hb and E-Hb, used at a wide concentration range, failed to promote cell division. Furthermore, we performed additional proliferation experiments in cultures stimulated with a low concentration of FCS, as some compounds exhibit their ability to promote cell division only when coincubated with another proliferative agent. Again, no significant influence on cell proliferation was observed after exposing HASMC to either N-Hb or E-Hb. On the contrary, E-Hb, but not N-Hb, elicited a concentration-dependent increase in cell size, as assessed by planar cell surface area quantification. E-Hb also stimulated protein synthesis in HASMC cultures. Thus, based on these findings, E-Hb seems to possess a growth-stimulating effect on HASMC, by promoting cell hypertrophy without directly affecting cell proliferation. Such effects were not shared by human oxyhaemoglobin when glycosylated at nonpathological levels.
Such a hypertrophic effect of E-Hb is in accordance with a previous study from our group, in which E-Hb was seen to promote cell growth of cultured vascular smooth muscle obtained from Sprague – Dawley rats (Peiró et al., 1998). Other authors have also reported that Amadori-modified albumin can stimulate cell activation, proliferation and migration of nonhuman mesangial and vascular smooth muscle cells (Cohen & Ziyadeh, 1994; Hattori et al., 2002). In the present study, however, we demonstrate that E-Hb may act as an activator and a hypertrophic factor for smooth muscle obtained, not from animal models, but from human vessels, which should be of greater relevance for the possible pathophysiological implications of the results obtained.
Concerning a possible common mechanism that could mediate the different effects of E-Hb observed in HASMC cultures, ROS appeared as the main candidates for different reasons. First of all, we have previously observed that the impairment of endothelial-dependent relaxations induced by E-Hb in different vascular beds was dependent on the release of ROS, particularly superoxide anions (Angulo et al., 1996; Vallejo et al., 2000). Indeed, we have previously shown that for a given concentration E-Hb releases a significantly higher amount of superoxide anions than N-Hb (Angulo et al., 1999). Similarly, other Amadori-modified proteins have been shown to release superoxide anions in aqueous solution (Sakurai & Tsuchiya, 1988; Mullarkey et al., 1990). In addition, both AP-1 and NF-κB are transcriptional factors with redox-regulated activity (Kunsch & Medford, 1999; Spitaker & Graier, 2002) and, in the last years, a growing body of evidence has emerged indicating that ROS can regulate different growth- or inflammation-related processes in vascular cells (Irani, 2000; Wolin et al., 2002).
We quantified the amount of superoxide anions present inside HASMC after exposure to different treatments. E-Hb, but not N-Hb (both at 10 nM), elicited a significant increase of intracellular superoxide anions, as compared with untreated cultures. As the effect of E-Hb was completely prevented by SOD, which does not cross the cell membrane, most of the superoxide anions are probably originated in the extracellular milieu. SOD also prevented different E-Hb-mediated effects on HASMC, like the activation of both AP-1 and NF-κB, as assessed by both EMSA and transient transfection experiments. We also used the intracellular hydroxyl radicals scavenger DMTU, based on data from a previous report (Peiró et al., 1998), and we observed a similar preventive effect on the activation of both transcription factors. Additionally, E-Hb-induced growth promoting effects could also be abolished in the presence of both ROS scavengers. These data suggest that E-Hb at 10 nM is releasing outside the cell superoxide anions, which are able to activate intracellular signals leading to early transcription factor activation and cell growth. The fact that the actions of E-Hb are mainly mediated by ROS could be in accordance with a central role for oxidative stress in the development of diabetic vasculopathy (Tesfamariam, 1994; Giugliano et al., 1996; Spitaker & Graier, 2002) and would point at E-Hb as a source of such oxidative stress in diabetic vessels.
In conclusion, physiological concentrations of human oxyhaemoglobin, when glycosylated at a high percentage, promote the transcriptional activity of AP-1 and NF-κB, and stimulate growth in human vascular smooth muscle through redox-sensitive pathways. This observation allows for proposing that E-Hb, in addition to representing as an early marker of diabetes mellitus evolution, can have a direct role in the development of diabetic vasculopathy.
Acknowledgments
This work was supported by grants from Ministerio de Ciencia y Tecnología (SAF2001-1328), Comunidad Autónoma de Madrid (08.4/0030/2001), Fondo de Investigaciones Sanitarias (99/0246 and 01/0579) and Instituto de Salud Carlos III RGDM (G03/212). We thank José Luis Llergo for excellent technical assistance.
Abbreviations
- AP-1
activator protein-1
- AGEs
advanced glycation end products
- DMTU
dimethylthiourea
- DMEM
Dulbecco's modified Eagle's medium
- EMSA
electrophoretic mobility shift assay
- FCS
fetal calf serum
- HASMC
human aortic smooth muscle cells
- NF-κB
nuclear factor-κB
- E-Hb
oxyhaemoglobin glycosylated at elevated levels
- N-Hb
oxyhaemoglobin glycosylated at normal levels
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TNF-α
tumour necrosis factor-α
References
- ANGULO J., RODRÍGUEZ-MAÑAS L., PEIRÓ C., VALLEJO S., SÁNCHEZFERRER A., SÁNCHEZ-FERRER C.F. Impairment of endothelial relaxations by glycosylated human oxyhemoglobin depends on the oxidative state of the heme group. Gen. Pharmacol. 1999;32:475–481. doi: 10.1016/s0306-3623(98)00251-1. [DOI] [PubMed] [Google Scholar]
- ANGULO J., SÁNCHEZ-FERRER C.F., PEIRÓ C., MARÍN J., RODRÍGUEZMAÑAS L. Impairment of endothelium-dependent relaxation by increasing percentages of glycosylated human hemoglobin. Hypertension. 1996;28:583–592. doi: 10.1161/01.hyp.28.4.583. [DOI] [PubMed] [Google Scholar]
- BALDWIN A.S., JR The transcription factor NF-κB and human disease. J. Clin. Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P., KARIN M. NF-κB: a pivotal transcription factor in chronic inflammatory disease. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BIERHAUS A., SCHIEKOFER S., SCHWANINGER M., ANDRASSY M., HUMPERT P.M., CHEN J., HONG M., LUTHER T., HENLE T., KLOTING I., MORCOS M., HOFMANN M., TRITSCHLER H., WEIGLE B., KASPER M., SMITH M., PERRY G., SCHMIDT A.M., STERN D.M., HARING H.U., SCHLEICHER E., NAWROTH P.P. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- BUCALA R., TRACEY K.J., CERAMI A. Advanced glycosylation products quench nitric oxide mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERAMI A., VLASSARA H., BROWNLEE M. Role of advanced glycosylation products in complications of diabetes. Diabetes Care. 1988;1:73–79. [PubMed] [Google Scholar]
- COHEN M.P. Diabetes and Protein Glycosylation: Measurement and Biological Relevance. New York, USA: Springer-Verlag; 1986. [Google Scholar]
- COHEN M.P., ZIYADEH F.N. Amadori glucose adducts modulate mesangial cell growth and collagen gene expression. Kidney Int. 1994;45:475–484. doi: 10.1038/ki.1994.62. [DOI] [PubMed] [Google Scholar]
- DENG T., KARIN M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 1993;7:479–490. doi: 10.1101/gad.7.3.479. [DOI] [PubMed] [Google Scholar]
- DI MARIO U., PUGLIESE G. 15th Golgi lecture: from hyperglycaemia to the disregulation of vascular remodelling in diabetes. Diabetologia. 2001;44:674–692. doi: 10.1007/s001250051676. [DOI] [PubMed] [Google Scholar]
- DOMINGUEZ C., RUIZ E., GUSSINYE M., CARRASCOSA A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care. 1998;21:1736–1742. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- FAIVRE-FIORINA B., CARON A., FASSOT C., FRIES I., MENU P., LABRUDE P., VIGNERON C. Presence of hemoglobin inside aortic endothelial cells after cell-free haemoglobin administration in guinea pigs. Am. J. Physiol. 1999;276:H766–H770. doi: 10.1152/ajpheart.1999.276.2.H766. [DOI] [PubMed] [Google Scholar]
- GIANNATTASIO C., FAILLA M., GRAPPIOLO A., GAMBA P.L., PALEARI F., MANCIA G. Progression of large artery structural and functional alterations in type I diabetes. Diabetologia. 2001;44:203–208. doi: 10.1007/s001250051600. [DOI] [PubMed] [Google Scholar]
- GIUGLIANO D., CERIELLO A., PAOLISSO G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., SUZUKI M., HATTORI S., KASAI K. Vascular smooth muscle cell activation by glycated albumin (Amadori adduct) Hypertension. 2002;39:22–28. doi: 10.1161/hy1201.097300. [DOI] [PubMed] [Google Scholar]
- IRANI K. Oxidant signalling in vascular cell growth, death, and survival. A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- ISEKI A., KAMBE F., OKUMURA K., NIWATA S., YAMAMOTO R., HAYAKAWA T., SEO H. Pyrrolidine dithiocarbamate inhibits TNFalpha-dependent activation of NF-kappaB by increasing intracellular copper level in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2000;276:88–92. doi: 10.1006/bbrc.2000.3452. [DOI] [PubMed] [Google Scholar]
- JOVANOVICH L., PETERSON C.M. The clinical utility of glycosylated hemoglobin. Am. J. Med. 1981;70:331–338. doi: 10.1016/0002-9343(81)90770-1. [DOI] [PubMed] [Google Scholar]
- KUNSCH C., MEDFORD R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 1999;85:753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- LECHLEITNER M., KOCH T., HEROLD M., DZIEN A., HOPPICHLER F. Tumor necrosis factor-alpha plasma level in type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J. Intern. Med. 2000;248:67–76. doi: 10.1046/j.1365-2796.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- MADAMANCHI N.R., BUKOSKI R.D., RUNGE M.S., RAO G.N. Arachidonic acid activates Jun N-terminal kinase in vascular smooth muscle cells. Oncogene. 1998;16:417–422. doi: 10.1038/sj.onc.1201551. [DOI] [PubMed] [Google Scholar]
- MULLARKEY C.J., EDELSTEIN D., BROWNLEE M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem. Biophys. Res. Commun. 1990;173:932–939. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- NISHIO Y., KASHIWAGI A., TAKI H., SHINOZAKI K., MAENO Y., KOJIMA H., MAEGAWA H., HANEDA M., HIDAKA H., YASUDA H., HORIIKE K., KIKKAWA R. Altered activities of transcription factors and their related gene expression in cardiac tissues of diabetic rats. Diabetes. 1998;47:1318–2135. doi: 10.2337/diab.47.8.1318. [DOI] [PubMed] [Google Scholar]
- PEIRÓ C., ANGULO J., RODRÍGUEZ-MAÑAS L., LLERGO J.L., VALLEJO S., CERCAS E., SÁNCHEZ-FERRER C.F. Vascular smooth muscle cell hypertrophy induced by glycosylated human oxyhaemoglobin. Br. J. Pharmacol. 1998;125:637–644. doi: 10.1038/sj.bjp.0702097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEIRÓ C., LAFUENTE N., MATESANZ N., CERCAS E., LLERGO J.L., VALLEJO S., RODRÍGUEZ-MAÑAS L., SÁNCHEZ-FERRER C.F. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br. J. Pharmacol. 2001;133:967–974. doi: 10.1038/sj.bjp.0704184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEIRÓ C., LLERGO J.L., ANGULO J., LÓPEZ-NOVOA J.M., RODRÍGUEZLÓPEZ A., RODRÍGUEZ-MAÑAS L., SÁNCHEZ-FERRER C.F. Effects of captopril, losartan, and nifedipine on cell hypertrophy of cultured vascular smooth muscle from hypertensive Ren-2 transgenic rats. Br. J. Pharmacol. 1997;121:1438–1444. doi: 10.1038/sj.bjp.0701261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNATHUR S., WAGNER J.D., LEEUWENBURGH C., LITWAK K.N., HEINECKE J.W. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J. Clin. Invest. 2001;107:853–860. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKUP J.C., CHUSNEY G.D., THOMAS S.M., BURT D. Plasma interleukin-6, tumor necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- RODRÍGUEZ-MAÑAS L., ARRIBAS S., GIRÓN C., VILLAMOR J., SÁNCHEZFERRER C.F., MARÍN J. Interference of glycosylated human hemoglobin with endothelium-dependent responses. Circulation. 1993;88:2111–2116. doi: 10.1161/01.cir.88.5.2111. [DOI] [PubMed] [Google Scholar]
- RUMBLE J.R., COOPER M.E., SOULIS T., COX A., WU L., YOUSSEF S., JASIK M., JERUMS G., GILBERT R.E. Vascular hypertrophy in experimental diabetes. Role of advanced glycation end products. J. Clin. Invest. 1997;99:1016–1027. doi: 10.1172/JCI119229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURAI T., TSUCHIYA S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 1988;236:406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- SCHALKWIJK C.G., LIGTVOET N., TWAALFHOVEN H., JAGER A., BLAAUWGEERS H.G., SCHLINGEMANN R.O., TARNOV L., PARVING H.H., STEHOUWER C.D., VAN HINSBERGH V.W. Amadori albumin in type 1 diabetic patients. Correlation with markers of endothelial function, association with diabetic nephropathy, and localization in retinal capillaries. Diabetes. 1999a;48:2446–2453. doi: 10.2337/diabetes.48.12.2446. [DOI] [PubMed] [Google Scholar]
- SCHALKWIJK C.G., POLAND D.C., VAN DIJK W., KOK A., EMEIS J.J., DRAGER A.M., DONI A., VAN HINSBERG V.W., STEHOUWER C.D. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999b;42:351–357. doi: 10.1007/s001250051162. [DOI] [PubMed] [Google Scholar]
- SCHREIBER E., MATTHIAS P., MULLER M.M., SCHAFFNER W. Rapid detection of octamer binding proteins with ‘mini-extracts' prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL D.R., MONNIER V.M. Structure elucidation of a senescence crosslink from human extracelular matrix. Implication of pentoses in the aging process. J. Biol. Chem. 1989;264:21597–21602. [PubMed] [Google Scholar]
- SPITAKER M.M., GRAIER W.F. Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476–494. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA A.K. High glucose-induced activation of protein kinase signaling pathways in vascular smooth muscle cells: a potential role in the pathogenesis of vascular dysfunction in diabetes (review) Int. J. Mol. Med. 2002;9:85–89. [PubMed] [Google Scholar]
- STAMLER J., VACCARO O., NEATON J.D., WENTWORTH D. The Multiple Risk Factor Intervention Trial Research Group Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trail. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- STEIN B., BALDWIN A.S., JR, BALLARD D.W., GREENE W.C., ANGEL P., HERRLICH P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRATTON I.M., ADLER A.I., NEIL H.A., MATTHEWS D.R., MANLEY S.E., CULL C.A., HADDEN D., TURNER R.C., HOLMAN R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–419. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESFAMARIAM B. Free radicals in diabetic endothelial cell dysfunction. Free Radic. Biol. Med. 1994;16:383–391. doi: 10.1016/0891-5849(94)90040-x. [DOI] [PubMed] [Google Scholar]
- TIETZ N.W. Clinical Guide to Laboratory Tests. Philadelphia, PA: WB Saunders Co; 1990. pp. 284–285. [Google Scholar]
- VALLEJO S., ANGULO J., PEIRÓ C., NEVADO J., SÁNCHEZ-FERRER A., PETIDIER R., SÁNCHEZ-FERRER C.F., RODRÍGUEZ-MAÑAS L. Highly glycated oxyhemoglobin impairs nitric oxide relaxations in human mesenteric microvessels. Diabetologia. 2000;43:83–90. doi: 10.1007/s001250050011. [DOI] [PubMed] [Google Scholar]
- VLASSARA H. Recent progress in advanced glycation end products and diabetic complications. Diabetes. 1997;46 Suppl. 2:S19–S25. doi: 10.2337/diab.46.2.s19. [DOI] [PubMed] [Google Scholar]
- WOLIN M.S., GUPTE S.A., OECKLER R.A. Superoxide in the vascular system. J. Vasc. Res. 2002;39:191–207. doi: 10.1159/000063685. [DOI] [PubMed] [Google Scholar]