Abstract
Capsaicin, the active substance in chilli peppers, activates the vanilloid type 1 receptor (VR1) rather than the vanilloid-like receptor (VRL1) in the trigeminal ganglion and nucleus of small and medium C- and Aδ-fibres.
Capsaicin induces calcitonin gene-related peptide (CGRP) release when VR1 receptors are activated, and this can be reversed by both the VR1 receptor antagonist capsazepine and the CGRP blocker αCGRP8–37 in vitro.
In this study we used intravital microscopy to look at the possible role of the VR1 receptor in the trigeminovascular system in producing dilation of dural blood vessels. Capsazepine (3 mg kg−1) was given to study the effect of the VR1 receptor in dural vessel dilation produced by either electrical stimulation, CGRP (1 μg kg−1) or capsaicin (7 μg kg−1) bolus injection. We also looked at the effect of the CGRP blocker αCGRP8–37 (300 μg kg−1) on capsaicin-induced dilation so that we could see if the results found in vitro could also be found in vivo.
Electrical stimulation of the dura mater produced a robust vasodilator response between 130 and 137% of baseline diameter that was no different across four repeat stimuli (F3,18=0.6, P=0.61). CGRP similarly produced a dilatation of 99–111% that was no different across four baseline infusions (F3,15=2.4, P=0.113). Capsaicin also produced a consistent dilation of between 112 and 120% of baseline across three injections (F2,10=0.6, P=0.567).
Capsazepine did not inhibit the dilation brought about by either electrical stimulation or CGRP injection, while it was able to inhibit the dilation brought about by capsaicin (t5=3.4, P<0.05). αCGRP8–37 also inhibited the capsaicin-induced dilation (t5=7.4, P<0.05) probably inhibiting the action of released CGRP at the CGRP receptor.
The study demonstrates that capsaicin can repeatedly induce dural vessel dilation in vivo, presumably through inducing CGRP release from trigeminal sensory nerve fibres, while C-fibres may have been desensitised. The data imply that the VR1 receptor plays only a minor role in trigeminovascular-induced dural vessel dilation.
Keywords: Vanilloid receptors, capsaicin, CGRP, intravital microscopy, trigeminovascular
Introduction
Capsaicin is the pungent ingredient in hot chilli peppers that activates the vanilloid type 1 receptor (VR1) (Caterina et al., 1997). VR1 receptors are located on small- and medium-sized neurons that are either unmyelinated C-fibres or thinly myelinated Aδ-fibres (Joo et al., 1969). VR1 receptors are found on neurons in the trigeminal and dorsal root ganglia (Guo et al., 1999; Ichikawa & Sugimoto, 2001). Intravenous capsaicin promotes the release of the proinflammatory neuropeptides, substance P (SP) and neurokinin A from trigeminal neurons and causes dural extravasation in the rat (Markowitz et al., 1987). It is thought that this action is C-fibre dependent as the destruction of C-fibres in neonate rats prevents this action of capsaicin as an adult (Markowitz et al., 1987) and because SP is predominantly contained within C-fibres, and also, almost always colocalised with calcitonin gene-related peptide (CGRP) (Lee et al., 1985; O'Connor & van der Kooy, 1988). The potent vasodilator, CGRP is also released, probably via the activation of both C- and Aδ-fibres in the trigeminal region (Lee et al., 1985; O'Connor & van der Kooy, 1988; Escott et al., 1995a). The trigeminal nerve innervates pain-producing intracranial structures, so that the physiology and pharmacology of the trigeminovascular system are pivotal to understanding primary headaches (Goadsby et al., 2002).
VR1 receptors are also activated by acid and moderate heat, with a threshold ∼43°C (Caterina et al., 1997). The VR1 receptor belongs to a subgroup of the transient receptor potential (TRP) superfamily of ion channels called TRPV, so named because the VR1 receptor was the first mammalian member of the subgroup identified (Montell et al., 2002). They contain six transmembrane segments, with a re-entrant pore separating the last two segments (Gunthorpe et al., 2002; Montell et al., 2002). There are other members of the TRP superfamily that are also temperature sensitive covering a range of temperatures from cool to hot. They tend to be initially categorised by their sensitivity to capsaicin (a brief overview of the heat-sensitive TRP superfamily of ion channels is presented in Table 1).
Table 1.
Summary of the temperature-sensitive TRP superfamily of ion channels
| Nomenclature from the TRP superfamily (Montell et al., 2002) | Former receptor name | Temperature threshold (°C) | Agonist | Antagonist | References |
|---|---|---|---|---|---|
| TRPV1 | VR1 | ∼43 | Capsaicin, anandamide | Capsazepine (specific) | Caterina et al. (1997) |
| TRPV2 | VRL-1 | ∼52 | Capsaicin insensitive | Ruthenium red (nonspecific TRPV antagonist) | Caterina et al. (1999) |
| Insulin-like growth factor | |||||
| TRPV3 | VRL-3 | ∼39 | Capsaicin insensitive | Ruthenium red | Peier et al. (2002b), Smith et al. (2002) Xu et al. (2002) |
| TRPV4 | OTROC4, VRL-2, VR-OAC, TRP 12 | 27–42 | Capsaicin insensitive | Ruthenium red | Guler et al. (2002) |
| Hypotonic solution | |||||
| Phorbol derivatives | |||||
| ANKTM1 (mammalian TRP-like receptor) | 12–24 | Icilin (potent cooling compound) | Ruthenium red | Story et al. (2003) | |
| TRPM8 | CMR1 | 18–28 | Capsaicin-insensitive menthol, icilin | Not determined | McKemy et al. (2002), Peier et al. (2002a) |
Recently, VR1-immunoreactivity has been found in 16% of total neuronal cell bodies in human trigeminal ganglia; of this 16%, there was a small proportion that showed colocalisation with CGRP (Hou et al., 2002). CGRP is believed to be a marker of small- to medium-sized neurons in sensory ganglia (Ju et al., 1987), and it is on this type of neuron that VR1 receptors are expressed. Therefore, it seems likely that VR1 receptors are colocalised with CGRP in the trigeminovascular system and cause the release of CGRP when they are activated by capsaicin.
Neurogenic dural inflammation uses high intensity, sustained electrical stimulation that activates C-fibres and causes the release of SP and CGRP (Lee et al., 1985; Markowitz et al., 1987; O'Connor & van der Kooy, 1988). Relative to this, neurogenic dural vasodilatation is induced by much lower electrical intensity stimulation for only a brief period, and it is believed to only recruit trigeminal Aδ sensory nerve fibres (Escott et al., 1995b; Williamson et al., 1997a). The evidence for this comes from the fact that the SP/NK1 antagonist, RP 67580, was unable to attenuate neurgenic dural vasodilatation, and we report above that SP is predominantly contained within C-fibres, indicating the C-fibres are probably not activated (Williamson et al., 1997b). Trigeminal sensory nerve fibres innervate the cranial blood vessels and they contain the neuropeptide, CGRP (Edvinsson et al., 1989; Jansen et al., 1991). When these trigeminal sensory nerve fibres become activated, they release CGRP into the cranial circulation (Goadsby et al., 1988). Indeed, it is thought that neurogenic dural vasodilatation is caused by the release of CGRP from activated Aδ-fibres, and CGRP then acts on CGRP receptors on the smooth muscle of dural arteries to cause the dilation (Williamson et al., 1997a).
Capsaicin causes potent vasodilation by activating VR1 receptors in vitro (Sams-Nielsen et al., 2001), and the literature indicates that VR1 receptors are present on Aδ and C-fibres in the trigeminovascular system (Guo et al., 1999; Ichikawa & Sugimoto, 2001). Neurogenic dural vasodilatation, a model that is believed to activate Aδ-fibres solely, has been found to be a highly predictive model for identifying acute antimigraine drugs and helps dissect migraine pathophysiology. The purpose of the study was firstly to see if capsaicin could cause vasodilatation in our in vivo model, in the same way that it is able to cause vasodilation in vitro, presumably by activating C-fibres, and provoking CGRP release (Saito & Goto, 1986; Markowitz et al., 1987; Maggi et al., 1988; Martling et al., 1988; Wardle et al., 1997; Flores et al., 2001). It was the second aim of this study, having observed the response of capsaicin in vivo, to examine whether the VR1 receptor is also involved in Aδ-fibre specific mediated neurogenic dural vasodilatation. We used the in vivo model of intravital microscopy that allows direct study of dural vessel diameter. We examined the effect of a VR1 receptor antagonist, capsazepine, on neurogenically dilated dural blood vessels, and on dilation caused by both capsaicin and CGRP. We also looked at the effect of a CGRP blocker, CGRP8–37, on the activated trigeminal VR1 receptor response with capsaicin.
Methods
Surgical preparation
All experiments were conducted under UK Home Office (Scientific Procedures) Act (1986). Male Sprague–Dawley rats (285–430 g) were anaesthetised throughout the experiments with sodium pentobarbitone (60 mg kg−1 i.p. and then 18 mg kg−1 h i.v. infusion). The left femoral artery and vein were cannulated for blood pressure recording, and intravenous infusion of anaesthetic and test compounds, respectively. Temperature was maintained throughout using a homeothermic blanket system. The rats were placed in a stereotaxic frame and ventilated with oxygen-enriched air, 3–5 ml, 60–80 strokes per minute (Small Rodent Ventilator – Model 683, Harvard Instruments, U.K.). End-tidal CO2 was monitored (Capstar-100, CWE Inc., U.S.A.) and kept between 3.5 and 4.5% and blood pressure was monitored continually. This allows continuous monitoring of respiration and blood pressure for changes due to medium-term anaesthetic. The skull was exposed and the right or left parietal bone thinned by drilling with a saline-cooled drill until the blood vessels of the dura were clearly visible through the intact skull.
Intravital microscopy
The cranial window was covered with mineral oil (37°C) and a branch of the middle meningeal artery was viewed using an intravital microscope (Microvision MV2100, U.K.) and the image displayed on a television monitor. Dural blood vessel diameter was continuously measured using a video dimension analyser (Living Systems Instrumentation, U.S.A.) and displayed with blood pressure on an online data analysis system (Spike4 v2, Cambridge Electronic Design).
Experimental protocols
Defining electrical stimulation parameters
Electrical stimulation was used to evoke dilation of the dural blood vessels with a bipolar stimulating electrode (NE 200X, Clark Electromedical) that was placed on the surface of the cranial window approximately 200 μm from the vessel of interest. The surface of the cranial window was stimulated at 5 Hz, 1 ms for 10 s (Grass Stimulator S88, Grass Instrumentation) with increasing voltage until maximal dilation was observed. Subsequent electrically induced responses in the same animal were then evoked using that voltage (Williamson et al., 1997b; Akerman et al., 2002b).
CGRP and capsaicin-induced dilation
In the preparations where CGRP was used to dilate dural blood vessels, CGRP was given as an intravenous bolus of 1 μg kg−1, which has been shown to produce a maximal dilation (Williamson et al., 1997a). In those preparations where capsaicin was used to dilate the dural blood vessels, capsaicin was given as an intravenous bolus of up to 7 μg kg−1 until a maximal dilation was observed.
Effects of the vanilloid antagonist capsazepine on evoked dilation in rats
The effects of the VR1 receptor antagonist capsazepine were studied. Capsazepine (1 mg kg−1) was administered intravenously at least 10 min after the control response to either electrical stimulation, bolus of CGRP or capsaicin. The electrical stimulation, bolus of CGRP or capsaicin was then repeated after 10 min. Then at least a further 10 min later an increased dosage of capsazepine (3 mg kg−1) was administered followed by another electrical stimulation, bolus of CGRP or capsaicin after 10 min.
Effects of αCGRP8–37 on capsaicin-induced dilation
The effects of the CGRP peptide fragment αCGRP8–37, a CGRP blocker, was studied. αCGRP8–37 (300 μg kg−1) was administered intravenously at least 10 min after the control response to capsaicin, and then the control response to capsaicin was repeated 2 min later. At 20 min after the dose of αCGRP8–37 the control response to capsaicin was again repeated.
Data analysis
The effects of electrical stimulation, bolus of CGRP or capsaicin on dural vessel diameter were calculated as a percentage increase from the prestimulation baseline diameter. During the experimental set-up selecting the optimal vessel results in the magnification, as visualised, being different in each animal making it impossible to standardise the dural vessel measurement. The dural vessel diameter was, therefore, measured in arbitrary units. All data are expressed as mean±s.e.m. Statistical analysis was performed using an ANOVA for repeated measures and post hoc comparison made with a Student's paired t-test (SPSS v10.0). The reproducibility of the neurogenic vasodilator and CGRP responses has been tested previously using four consecutive saline-controlled stimuli (Akerman et al., 2002b) to determine whether there was any systematic effect of test compounds over time in the meningeal circulation. We also completed a series of saline-controlled vasodilator repetitions for comparison with capsaicin. For these baseline studies, the consecutive determinations of the neurogenic dilator response, CGRP or capsaicin-induced dilations were compared to the control dilation used for the pharmacological interventions using an ANOVA with repeated measures and a two-factor design, responses across consecutive stimuli and between the two groups. Significance was assessed at the P<0.05 levels.
Drugs
The infusion of anaesthetic and experimental drugs were all via the same catheter; however, the line was always flushed with saline first, several minutes before administering the different compound. Capsazepine (Tocris Cookson, U.K.) was dissolved in a couple of drops of DMSO (Sigma-Aldrich, U.K.) and further diluted in a 1 : 1 : 8 solution of Tween 80 (polyoxyethylene-sorbitan mono-oleate, Sigma-Aldrich, U.K.); ethanol: 0.9% NaCl. (E)-capsaicin (Tocris Cookson, U.K.) was also made up in the solution of Tween 80, ethanol and saline. CGRP and αCGRP8–37 (Sigma-Aldrich, U.K.) were both dissolved in deoxygenated water and aliquoted and frozen until required and then redissolved further in 0.9% NaCl.
Results
Baseline group
The results for the series of control dilations where saline was used as a pretreatment to electrical stimulation and CGRP-induced dilation showed no difference across the cohort (Akerman et al., 2002b). Similarly, three consecutive bolus capsaicin injections produced mean dilations that showed no difference across the cohort, 119.6±11, 115.3±16 and 111.6±12%, respectively (F2,10=0.6, P=0.567). These control effects were compared using an ANOVA to the control dilations used in the pharmacological studies, and there were no significant differences.
Effect of a vanilloid receptor antagonist on electrical stimulation, bolus of CGRP and capsaicin-induced dilation
In rats treated with capsazepine (1 and 3 mg kg−1, n=6) there was no difference in dural blood vessel diameter after electrical stimulation of the cranial window when compared with the control dilation, 119±6 to 126±12% (1 mg kg−1) and 134±19% (3 mg kg−1, F1,5=1.19, P=0.325). Increases in dural blood vessel diameter evoked by CGRP (1 μg kg−1, i.v.) also showed no significant difference between the control dilation and CGRP-induced dilation after capsazepine (1 and 3 mg kg−1, n=6) intervention, 121±12 to 122±13% (1 mg kg−1) and 128±12% (3 mg kg−1, F1,5=0.148, P=0.716). Dural blood vessel dilation brought about by capsaicin showed a significant reduction after pretreatment with capsazepine (3 mg kg−1) when compared to the control dilation, 134±11 to 102±11% (P<0.05, n=7, t5=3.4). There was no significant reduction with 1 mg kg−1 capsazepine (Figure 1). Capsazepine injections (n=31) caused a significant increase in blood pressure, 8±2 mmHg (P<0.05) that was accompanied by a significant drop in blood vessel diameter of 28±5% (P<0.05), both were restored to preinjection levels before control dilations were repeated.
Figure 1.
Effects of repeated capsaicin (up to 7 μg kg−1) injection with capsazepine treatment (grey) on dural blood vessel diameter. Following control responses rats were injected capsazepine (1 and 3 mg kg−1) and capsaicin injection repeated. *P<0.05 significance compared to the control response.
Effect of a CGRP blocker on capsaicin-induced dilation
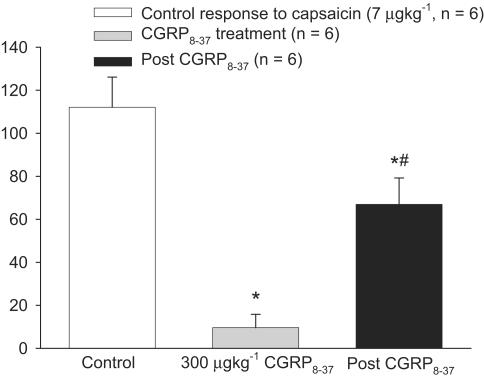
In rats treated with αCGRP8–37 (1 μg kg−1, i.v., n=6) there was significant inhibition of the capsaicin (7 μg kg−1)-induced dilation when compared with the control dilation, 112±14 to 10±6% (P<0.05, t6=7.3). The capsaicin-induced dilation returned significantly after a delay of 20 min, 10±6 to 67±12% (P<0.05, t5=7.4). Although there is a significant return of the dilation after a gap of 20 min, this dilation is still significantly different from the control dilation, 112±14 and 67±12% (P<0.05, t5=5.9) see Figure 2.
Figure 2.
Effects of repeated capsaicin (7 μg kg−1) injection with CGRP8–37 (grey) on dural blood vessel diameter. Following control responses rats were injected with CGRP8–37 (300 μg kg−1) and capsaicin injection repeated. *P<0.05 significance compared to the control response. ♯P<0.05 significance compared to the inhibitory response of CGRP8–37.
Effect of capsazepine on the blood pressure changes induced by CGRP and capsaicin
The four consecutive CGRP injections as previously described (Akerman et al., 2002b) and the control group of the Results section produced decreases in mean blood pressure of 41±7, 38±5, 37±7 and 33±3 mmHg, respectively. There was no difference across the cohort (F3,12=1.492, P=0.283). Our previous control effects (Akerman et al., 2002b) were compared with an ANOVA for repeated measures to the control dilations used in the pharmacological studies, and there were no significant difference. These blood pressure changes were not altered by pretreatment for capsazepine (F2,6=5.48, P=0.8). The blood pressure response to capsaicin has a triphasic effect, as has been previously shown (Chahl & Lynch, 1987), and the blood pressure decreases that accompanied the vasodilations, as stated above in the ‘Control Groups' of the results section, were 44±5, 38±4 and 31±3 mmHg, respectively. The blood pressure drop that accompanied the second and third capsaicin injections was significantly less than the first injection (t5=3.6 and 3.152, P<0.05, respectively). Similarly, when capsazepine was used as a pretreatment for capsaicin, the decrease in blood pressure was significantly less for the third capsaicin injection compared to the first (t6=2.973, P<0.05).
Discussion
Electrical stimulation, CGRP and capsaicin bolus injections were each capable of creating reproducible dural vessel dilation measured with intravital microscopy in rat, but only the capsaicin-induced dilation was inhibited by the VR1 antagonist capsazepine. Capsaicin-induced dilation was also inhibited by the CGRP receptor blocker, CGRP8–37 which has been shown previously to inhibit both CGRP and electrically induced dilation of dural blood vessels (Williamson et al., 1997a,1997b). These data suggest that VR1 receptor activation plays a minor role in the Aδ-fibre trigeminovascular-mediated dural vasodilation and predicts there is little or no role in migraine pathophysiology.
Capsaicin was able to cause dural vessel dilation repeatedly when given as an intravenous bolus in our in vivo model. The VR1 receptor antagonist capsazepine and CGRP receptor blocker, CGRP8–37, were able to inhibit capsaicin-induced dural blood vessel dilation. Capsaicin administration potently causes the release of CGRP from trigeminal sensory nerve terminals, in a capsazepine (VR1 receptor antagonist) reversible manner both in vitro and in vivo (Saito & Goto, 1986; Maggi et al., 1988; Martling et al., 1988; Wardle et al., 1997; Flores et al., 2001). In another in vitro study CGRP8–37, a CGRP blocker, as well as capsazepine, was found to inhibit the capsaicin-induced vasodilation in guinea-pig basilar artery (Sams-Nielsen et al., 2001). Although capsaicin administration also causes the release of SP and neurokinin A, it is CGRP release that causes the vasodilation of arteries (Franco-Cereceda & Rudehill, 1989; Jansen-Olesen et al., 1996). Capsaicin binding to VR1 receptors triggers calcium influx, and elevated intracellular calcium levels stimulate exocytosis and CGRP release (Caterina et al., 1997). In the in vivo model of trigeminovascular activation used here, it was demonstrated that a capsaicin-induced dilation of dural blood vessels is blocked by both capsazepine and CGRP8–37, while capsazepine was unable to block the CGRP-induced dural vessel dilation. It seems likely that the dilation brought about by capsaicin is caused by activation of VR1 receptors on trigeminovascular C-fibres, causing the release of CGRP from perivascular trigeminal sensory fibres and consequent dilation. Evidence from the neurogenic dural vasodilatation study will highlight any Aδ-fibre component to the action of VR1 receptors on dural vessel calibre. Capsazepine probably inhibits capsaicin-induced dilation prejunctionally by inhibiting the release of CGRP from C-fibres, while CGRP8−37 is likely to be acting postsynaptically to block the CGRP from CGRP receptors on the smooth muscle of the dura mater.
Neurogenic dural vasodilatation, as monitored by intravital microscopy, is likely to be a result of CGRP release via activation of perivascular trigeminal sensory Aδ-fibres (Escott et al., 1995b; Williamson et al., 1997a) and the distension of the dural blood vessels results in activation of trigeminal afferents that project to the trigeminal nucleus caudalis, a key relay centre in pain transmission (Moskowitz, 1984). The CGRP receptor antagonist, CGRP8–37, inhibits the effects of neurogenic dural vasodilatation by 85% and the ‘Triptans', acute antimigraine compounds, also significantly attenuate neurogenic dural vasodilatation response (Williamson et al., 1997a,1997b,1997c). Our results show that the VR1 receptor antagonist, capsazepine was unable to significantly inhibit the neurogenic dural vasodilatation despite VR1 receptor presence in the trigeminovascular pathway. Therefore, it seems that the VR1 receptors do not play a major role in causing CGRP release via Aδ-fibre activation from an electrical stimulus.
Direct intravenous administration of CGRP causes dilation through CGRP receptors located on the smooth muscle of dural blood vessels (Jansen et al., 1992; Edvinsson et al., 1998; Williamson et al., 1997a,1997b). The VR1 receptor antagonist capsazepine was unable to inhibit or attenuate the dilation brought about by CGRP administration. This lack of effect suggests that capsazepine does not act on CGRP1 receptors, and that there is not a postsynaptic component to the action of capsazepine on these neurons.
There is recent evidence indicating that VR1 receptors are present in the trigeminovascular system. VR1-immunoreactive sensory neurons are present in the rat trigeminal ganglion and trigeminal nucleus (Guo et al., 1999; Ichikawa & Sugimoto, 2000; Mezey et al., 2000) and the human trigeminal ganglion (Hou et al., 2002). Approximately 16% of total neuronal cell bodies in the human trigeminal ganglion had VR1 receptor-like immunoreactivity and that, of those cells, 10% were coexpressed with CGRP (Hou et al., 2002). Despite this recent evidence of localisation in the trigeminal region, it would appear that activation of VR1 receptors does not contribute to neurogenic dural vasodilatation, or that the few VR1 receptors that are located in the trigeminal region on Aδ sensory nerve fibres do not contribute significantly to neurogenic dural dilation. The low intensity electrical stimulation that was used in these studies is not capable of significantly recruiting C-fibres (Williamson et al., 1997b). It seems likely that the majority of VR1 receptors in the trigeminal region are situated on C-fibres, and these fibres were not activated by the electrical stimulus used in this study, therefore, capsazepine had little effect as the VR1 receptors were not sufficiently activated. It is important to note that the dose of capsazepine used was biologically effective in the rat as it was able to inhibit the capsaicin-induced dilation.
In our studies, dural vessel dilation can be readily reproduced with repeated injection of a capsaicin bolus over time. A reproducible dilation with capsaicin was also shown in the guinea-pig basiliar artery in vitro (Sams-Nielsen et al., 2001). Capsaicin administration produces an activation of primary afferent C and Aδ-fibres and the release of sensory neuropeptides, such as SP, neurokinin A and CGRP (Jancso et al., 1967; Holzer, 1988; Dray et al., 1989; Dray, 1992a,1992b;1996), through calcium entry in the cells (Dray et al., 1989). Repeated administration of capsaicin leads to a desensitisation and inactivation of C-fibre sensory neurons and contributes to the antinociceptive properties of capsaicin, as there is prolonged inactivation of transmitter release (Jancso et al., 1967; Jancso-Gabor et al., 1970; Dray et al., 1989; Lynn, 1990). Why then is it that repeated capsaicin administration in this study was still able to produce dural vessel dilation on each occasion? Reproducible dilation implies that no desensitisation of dural or basilar arteries occurs to capsaicin administration. VR1 receptors have been found to be present on small- and medium-sized neurons with unmyelinated C-fibres and thinly myelinated Aδ-fibres (Joo et al., 1969) in the trigeminal and dorsal root ganglia (Guo et al., 1999; Ichikawa & Sugimoto, 2001). CGRP is believed to be a marker of small- to medium-sized neurons in sensory ganglia that the VR1 receptors are expressed on (Ju et al., 1987). C-fibres that are normally activated with capsaicin, and contribute to plasma extravasation, may be desensitised by repeated administration. In contrast, the amount of capsaicin administered in the dural vessel dilation in our study and the basilar artery dilation previously reported (Sams-Nielsen et al., 2001) may have been sufficient to mediate the activation of the small amount of VR1 receptors on the Aδ-fibres that are known to release CGRP when activated (Lee et al., 1985; McCarthy & Lawson, 1990; Williamson et al., 1997b). It may be interesting in the future to compare whether C-fibre desensitisation, perhaps measured by markers of inflammation, can occur concurrently with Aδ-fibre activation, resulting in dural vessel dilation continues in the same animal. It may also be useful to electrical stimulate the dura to invoke dural vasodilatation after the desensitisation of C-fibres to show again that there is no C-fibre contribution to this vasodilation.
Both vasodilators that were administered had effects on blood pressure. CGRP is known to cause a dose-dependent drop in blood pressure (Brain et al., 1993; Williamson et al., 1997a), while capsaicin has been shown to cause a triphasic change in blood pressure, a fall, a big increase and then a fall again to baseline, which is thought to be partially mediated by the vagal nerve (Chahl & Lynch, 1987). Both of these effects were observed in this study. The response of both CGRP and capsaicin on blood pressure is short-lived compared to the dural vasodilator response, but it raises the question whether the blood pressure effect causes the vasodilation, or whether the vasodilation is a direct action on receptors in the trigeminal system? In a study that looked at the effect of CGRP in the cardiovascular system, it showed that CGRP caused greater increases in blood flow in the heart than the brain. However, when the same studies were conducted in the presence of β-adrenoceptor blockade, the changes in heart and brain blood flow were similar, indicating that the myocardial blood flow increases result from a reflex-mediated mechanism rather than from a direct effect at the CGRP receptors located in the coronary vasculature (Shen et al., 2001). This implies that the vasodilatory action is via CGRP, acting at the CGRP receptor on the smooth muscle, rather than a compensatory effect of the cardiovascular changes. Similarly, the increase in blood pressure with capsaicin appears to be predominantly mediated by adrenergic mechanisms, α-adrenoceptor antagonists blocked the blood pressure response and propranolol blocked the drop in heart rate (Chahl & Lynch, 1987). This would indicate a sympathetic basis for these changes. Although it cannot be ruled out that the vasodilatation is caused by a response to these blood pressure changes without replicating the blocking of the cardiovascular changes whilst observing dilation, it is likely that the vasodilatation is caused by action on receptors in the trigeminovascular system, rather than a response to the cardiovascular changes which are a result of a sympathetic reflex.
In summary, the new study demonstrates that capsaicin can produce repeated dural vessel dilation in vivo inducing the release of CGRP, probably by activating VR1 receptors on trigeminal sensory nerve C-fibres. The nature of this CGRP release is unclear, since we do not know whether VR1 receptors activate CGRP release directly, or via an indirect mechanism. VR1 receptors play a minor role in Aδ-fibre trigeminovascular-mediated dural vasodilatation as they are unable to inhibit neurogenic dural vasodilatation. It is possible that VR1 receptors on both Aδ- and C-fibres are involved in capsaicin-induced CGRP release as C-fibres become desensitised with repeated capsaicin administration, therefore, VR1 receptors on Aδ-fibres may play a role when this occurs. The data do not support a significant role for VR1 receptor modulation of the trigeminovascular system.
Acknowledgments
We thank Thorsten Bartsch, Kevin Shields, Yolande Knight, James Storer and Paul Hammond of the Headache Group at the Institute of Neurology for both assistance and technical support during these experiments. The work has been supported by the Wellcome Trust. PJG is a Wellcome Senior research Fellow. This work was presented in part at the 14th Migraine Trust International Symposium, London, September 2002 (Akerman et al., 2002a).
Abbreviations
- ANOVA
analysis of variance
- CGRP
calcitonin gene-related peptide
- DMSO
dimethyl sulfoxide
- SP
substance P
- TRP
transient receptor potential
- VR1
vanilloid type 1 receptor
- VRL1
vanilloid-like receptor
References
- AKERMAN S., KAUBE H., GOADSBY P.J. Vanilloid receptor 1 (VR1) evoked CGRP release plays a minor role in causing dural vessel dilation via the trigeminovascular system. Cephalalgia. 2002a;22:572. [Google Scholar]
- AKERMAN S., WILLIAMSON D.J., KAUBE H., GOADSBY P.J. Nitric oxide synthase inhibitors can antagonise neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002b;137:62–68. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAIN S.D., HUGHES S.R., CAMBRIDGE H., O'DRISCOLL G.The contribution of calcitonin gene-related peptide (CGRP) to neurogenic vasodilator responses Agents Actions 199338C19–C21.Spec No [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., ROSEN T.A., TOMINAGA M., BRAKE A.J., JULIUS D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHAHL L.A., LYNCH A.M. The acute effects of capsaicin on the cardiovascular system. Acta Physiol. Hung. 1987;69:413–419. [PubMed] [Google Scholar]
- DRAY A. Mechanism of action of capsaicin-like molecules on sensory neurons. Life Sci. 1992a;51:1759–1765. doi: 10.1016/0024-3205(92)90045-q. [DOI] [PubMed] [Google Scholar]
- DRAY A. Neuropharmacological mechanisms of capsaicin and related substances. Biochem. Pharmacol. 1992b;44:611–615. doi: 10.1016/0006-2952(92)90393-w. [DOI] [PubMed] [Google Scholar]
- DRAY A. Neurogenic mechanisms and neuropeptides in chronic pain. Prog. Brain Res. 1996;110:85–94. doi: 10.1016/s0079-6123(08)62566-2. [DOI] [PubMed] [Google Scholar]
- DRAY A., HANKINS M.W., YEATS J.C. Desensitization and capsaicin-induced release of substance P-like immunoreactivity from guinea-pig ureter in vitro. Neuroscience. 1989;31:479–483. doi: 10.1016/0306-4522(89)90390-4. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., GULBENKIAN S., BARROSO C.P., SA M.C.E., POLAK J.M., MORTENSEN A., JORGENSEN L., JANSENOLESEN I. Innervation of the human middle meningeal artery: immunohistochemistry, ultrastructure, and role of endothelium for vasomotility. Peptides. 1998;19:1213–1225. doi: 10.1016/s0196-9781(98)00066-7. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., HARA H., UDDMAN R. Retrograde tracing of nerve fibers to the middle cerebral artery with true blue: co-localization with different peptides. J. Cereb. Blood Flow Metabol. 1989;9:212–218. doi: 10.1038/jcbfm.1989.31. [DOI] [PubMed] [Google Scholar]
- ESCOTT K.J., BEATTIE D.T., CONNOR H.E., BRAIN S.D. Trigeminal ganglion stimulation increases facial skin blood flow in the rat: a major role for calcitonin gene-related peptide. Brain Res. 1995a;669:93–99. doi: 10.1016/0006-8993(94)01247-f. [DOI] [PubMed] [Google Scholar]
- ESCOTT K.J., CONNOR H.E., BRAIN S.D., BEATTIE D.T. The involvement of calcitonin gene-related peptide (CGRP) and substance P in feline pial artery diameter responses evoked by capsaicin. Neuropeptides. 1995b;29:129–135. doi: 10.1016/0143-4179(95)90014-4. [DOI] [PubMed] [Google Scholar]
- FLORES C.M., LEONG A.S., DUSSOR G.O., HARDING-ROSE C., HARGREAVES K.M., KILO S. Capsaicin-evoked CGRP release from rat buccal mucosa: development of a model system for studying trigeminal mechanisms of neurogenic inflammation. Eur. J. Neurosci. 2001;14:1113–1120. doi: 10.1046/j.0953-816x.2001.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., RUDEHILL A. Capsaicin-induced vasodilatation of human coronary arteries in vitro is mediated by calcitonin gene-related peptide rather than substance P or neurokinin A. Acta Physiol. Scand. 1989;136:575–580. doi: 10.1111/j.1748-1716.1989.tb08704.x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Release of vasoactive peptides in the extracerebral circulation of man and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., LIPTON R.B., FERRARI M.D. Migraine – current understanding and treatment. N. Eng. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- GULER A.D., LEE H., IIDA T., SHIMIZU I., TOMINAGA M., CATERINA M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTHORPE M.J., BENHAM C.D., RANDALL A., DAVIS J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- GUO A., VULCHANOVA L., WANG J., LI X., ELDE R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- HOU M., UDDMAN R., TAJTI J., KANJE M., EDVINSSON L. Capsaicin receptor immunoreactivity in the human trigeminal ganglion. Neurosci. Lett. 2002;330:223–226. doi: 10.1016/s0304-3940(02)00741-3. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA H., SUGIMOTO T. Vanilloid receptor 1-like receptor-immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience. 2000;101:719–725. doi: 10.1016/s0306-4522(00)00427-9. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA H., SUGIMOTO T. VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res. 2001;890:184–188. doi: 10.1016/s0006-8993(00)03253-4. [DOI] [PubMed] [Google Scholar]
- JANCSO N., JANCSO-GABOR A., SZOLCSANYI J. Direct evidence for neurogenic inflamation and it's prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCSO-GABOR A., SZOLCSANYI J., JANCSO N. Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J. Physiol. 1970;208:449–459. doi: 10.1113/jphysiol.1970.sp009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN I., ALAFACI C., MCCULLOCH J., UDDMAN R., EDVINSSON L. Tachykinins (substance P, neurokinin A, neuropeptide K, and neurokinin B) in the cerebral circulation: vasomotor responses in vitro and in situ. J. Cereb. Blood Flow Metab. 1991;11:567–575. doi: 10.1038/jcbfm.1991.105. [DOI] [PubMed] [Google Scholar]
- JANSEN I., UDDMAN R., EKMAN R., OLESEN J., OTTOSSON A., EDVINSSON L. Distribution and effects of neuropeptide Y, vasoactive intestinal peptide, substance P, and calcitonin gene-related peptide in human middle meningeal arteries: comparison with cerebral and temporal arteries. Peptides. 1992;13:527–536. doi: 10.1016/0196-9781(92)90084-g. [DOI] [PubMed] [Google Scholar]
- JANSEN-OLESEN I., MORTENSEN A., EDVINSSON L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- JOO F., SZOLCSANYI J., JANCSO-GABOR A. Mitochondrial alterations in the spinal ganglion cells of the rat accompanying the long-lasting sensory disturbance induced by capsaicin. Life Sci. 1969;8:621–626. doi: 10.1016/0024-3205(69)90023-x. [DOI] [PubMed] [Google Scholar]
- JU G., HOKFELT T., BRODIN E., FAHRENKRUG J., FISCHER J.A., FREY P., ELDE R.P., BROWN J.C. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res. 1987;247:417–431. doi: 10.1007/BF00218323. [DOI] [PubMed] [Google Scholar]
- LEE Y., KAWAI Y., SHIOSAKA S., TAKAMI K., KIYAMA H., HILLYARD C.J., GIRGIS S., MACINTYRE I., EMSON P.C., TOHYAMA M. Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: immunohistochemical analysis. Brain Res. 1985;330:194–196. doi: 10.1016/0006-8993(85)90027-7. [DOI] [PubMed] [Google Scholar]
- LYNN B. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. Pain. 1990;41:61–69. doi: 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., GEPPETTI P., PATACCHINI R., FRILLI S., ASTOLFI M., FUSCO B., MELI A. Simultaneous release of substance P- and calcitonin gene-related peptide (CGRP)-like immunoreactivity from isolated muscle of the guinea pig urinary bladder. Neurosci. Lett. 1988;87:163–167. doi: 10.1016/0304-3940(88)90163-2. [DOI] [PubMed] [Google Scholar]
- MARKOWITZ S., SAITO K., MOSKOWITZ M.A. Neurogenically mediated leakage of plasma proteins occurs from blood vessels in dura mater but not brain. J. Neurosci. 1987;7:4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTLING C.R., SARIA A., FISCHER J.A., HOKFELT T., LUNDBERG J.M. Calcitonin gene-related peptide and the lung: neuronal coexistence with substance P, release by capsaicin and vasodilatory effect. Reg. Peptides. 1988;20:125–139. doi: 10.1016/0167-0115(88)90046-8. [DOI] [PubMed] [Google Scholar]
- MCCARTHY P., LAWSON S.N. Cell type and conduction-velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34:623–632. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- MCKEMY D.D., NEUHAUSSER W.M., JULIUS D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- MEZEY E., TOTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GUO A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTELL C., BIRNBAUMER L., FLOCKERZI V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A. The neurobiology of vascular head pain. Ann. Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- O'CONNOR T.P., VAN DER KOOY D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in trigeminal sensory projection to the intracranial arteries. J. Neurosci. 1988;8:2468–2476. doi: 10.1523/JNEUROSCI.08-07-02468.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEIER A.M., MOQRICH A., HERGARDEN A.C., REEVE A.J., ANDERSSON D.A., STORY G.M., EARLEY T.J., DRAGONI I., MCINTYRE P., BEVAN S., PATAPOUTIAN A. A TRP channel that senses cold stimuli and menthol. Cell. 2002a;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- PEIER A.M., REEVE A.J., ANDERSSON D.A., MOQRICH A., EARLEY T.J., HERGARDEN A.C., STORY G.M., COLLEY S., HOGENESCH J.B., MCINTYRE P., BEVAN S., PATAPOUTIAN A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002b;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- SAITO A., GOTO K. Depletion of calcitonin gene-related peptide (CGRP) by capsaicin in cerebral arteries. J. Pharmacobiodyn. 1986;9:613–619. doi: 10.1248/bpb1978.9.613. [DOI] [PubMed] [Google Scholar]
- SAMS-NIELSEN A., ORSKOV C., JANSEN-OLESEN I. Pharmacological evidence for CGRP uptake into perivascular capsaicin sensitive nerve terminals. Br. J. Pharmacol. 2001;132:1145–1153. doi: 10.1038/sj.bjp.0703910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y.T., PITTMAN T.J., BUIE P.S., BOLDUC D.L., KANE S.A., KOBLAN K.S., GOULD R.J., LYNCH JR J.J. Functional role of alpha-calcitonin gene-related peptide in the regulation of the cardiovascular system. J. Pharmacol. Exp. Ther. 2001;298:551–558. [PubMed] [Google Scholar]
- SMITH G.D., GUNTHORPE M.J., KELSELL R.E., HAYES P.D., REILLY P., FACER P., WRIGHT J.E., JERMAN J.C., WALHIN J.P., OOI L., EGERTON J., CHARLES K.J., SMART D., RANDALL A.D., ANAND P., DAVIS J.B. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- STORY G.M., PEIER A.M., REEVE A.J., EID S.R., MOSBACHER J., HRICIK T.R., EARLEY T.J., HERGARDEN A.C., ANDERSSON D.A., HWANG S.W., MCINTYRE P., JEGLA T., BEVAN S., PATAPOUTIAN A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- WARDLE K.A., RANSON J., SANGER G.J. Pharmacological characterization of the vanilloid receptor in the rat dorsal spinal cord. Br. J. Pharmacol. 1997;121:1012–1016. doi: 10.1038/sj.bjp.0701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene – related peptide on dural blood vessel diameter in the anaesthetized rat. Cephalalgia. 1997a;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat- intravital microscope studies. Cephalalgia. 1997b;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., HILL R.G., HARGREAVES R.J. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur. J. Pharmacol. 1997c;328:61–64. doi: 10.1016/s0014-2999(97)83028-2. [DOI] [PubMed] [Google Scholar]
- XU H., RAMSEY I.S., KOTECHA S.A., MORAN M.M., CHONG J.A., LAWSON D., GE P., LILLY J., SILOS-SANTIAGO I., XIE Y., DISTEFANO P.S., CURTIS R., CLAPHAM D.E. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]